Abstract
Increasing evidence indicates that sirtuin 3 (Sirt3) has neuroprotective effects in regulating oxidative stress and energy metabolism, both of which are involved in the pathogenesis of Alzheimer’s disease (AD). However, it is unclear whether Sirt3 is associated with cognitive performance and pathological changes in AD. We conducted a case-control study of the postmortem brains of AD (n = 16), mild cognitive impairment (n = 13), and age- and education-matched cognitively normal (CN, n = 11) subjects. We measured the mRNA and protein levels of Sirt3 and assessed their association with cognitive performance and AD pathology. In an ex vivo model of cortical neurons from transgenic mice that carry human tau protein, we modified Sirt3 expression by genetic knockdown and knock-in to investigate the cause-effect relationship between Sirt3 and tau. Sirt3 levels were reduced in the entorhinal cortex, the middle temporal gyrus, and the superior frontal gyrus of AD subjects compared to those of CN. This reduction was associated with poorer test scores of neuropsychological evaluation and the severity of tau pathology. Further study with genetic manipulation of Sirt3 revealed that amyloid-β increased levels of total tau acetylated tau through its modulation of Sirt3. These data suggest that reduction of Sirt3 is critically involved in pathogenesis of AD.
Keywords: Alzheimer’s disease, Mild cognitive impairment, Sirtuin, Acetylation, Tau, Amyloid
Background
Amyloid plaques and neurofibrillary tangles (NFTs) are the pathological hallmarks that define Alzheimer’s disease (AD). Virtually all non-demented people develop NFTs in the mesial temporal lobe by age 70 [1, 2], but generally lack widespread neocortical tangles if neocortical amyloid plaques are absent [3]. Neocortical amyloid plaques develop in up to 80% of non-demented elderly people by age 90 [4], and in the subset of those who progress to mild cognitive impairment (MCI) and dementia, this appears up to 20 years or more prior to the clinical change [5]. Neocortical plaque load plateaus soon after MCI, although continuing to spread to diencephalic areas, brain stem, and cerebellum [6]. The appearance of neocortical NFTs is closely associated with neocortical synaptic loss and dementia severity [5]. Neocortical amyloid-β (Aβ) is considered to trigger neocortical tau pathology, but the mechanistic link between the two remains unknown [7, 8].
We propose that sirtuin 3 (Sirt3) may be the mediator that connects Aβ and tau pathology. In amouse model of AD (APP/ PS1) that overproduces A β, mRNA and protein levels of Sirt3, a deacetylase, are reduced in the cortex [9]. Reduction in deacetylases causes an increase in acetylation. Drastic enhancement of tau acetylation has been observed in AD brain at early Braak stages and is involved in regulating tau early accumulation [10, 11]. In human AD cases, both Sirt1 and 3 levels are reduced in the entorhinal cortex and hippocampal subregions [12]. We have shown that Sirt3, along with its upstream activator PACAP, is reduced in postmortem AD neocortex compared to that in cognitively normal (CN) subjects [13]. But, the relationship between Sirt3 levels and cognitive performance remains unclear. In this pilot study, we test the hypothesis that Sirt3 is an essential mediator in the Aβ-tau nexus.
Methods
Study Subjects
All subjects were recruited from the Arizona Alzheimer’s Disease Center (ADC) Clinical Core [15]. Clinical and neuropsychological assessment is performed annually according to the National Alzheimer’s Coordinating Center protocol. Subjects designated as “cognitively normal” have no cognitively based limitations in daily living activities and a Clinical Dementia Rating (CDR) score of 0. The diagnosis of mild cognitive impairment (MCI) was given according to published consensus criteria [14] and a CDR of 0.5. A diagnosis of probable AD by the National Institute of Neurological Disorders and Stroke (NINDS) criteria [15] was given to subjects with a CDR score of ≥ 1.0 and a neuropsychological profile showing disproportionately severe impairment of learning and delayed recall.
Postmortem human cerebral cortex was obtained from the Banner Sun Health Research Institute Brain and Body Donation Program (BBDP) [14]. The median postmortem interval is 2.75 h for the entire collection. Briefly, frozen cerebral cortex postmortem brain tissues were obtained from 16 subjects with diagnosis of AD, 13 MCI, and 11 CN. In addition, they were free of other neurodegenerative disorders. Cortical Aβ neuritic plaque number (plaque score) and Braak tangle stage were determined by a single neuropathologist (T.G.B). Frozen human brain tissues were homogenized and centrifuged, and supernatants were collected as sample solution.
Since Sirt3 levels may change with age, only the final antemortem assessment score within 1 year prior to death, if available, was included for Sirt3 correlation analysis. The raw scores from Mattis dementia rating scale (DRS), auditory verbal learning test total learning (AVLT-TL), 60-item Boston naming test (BNT), and Stroop color-word interference test were converted to age- and education-corrected Z scores.
Sirt3 Protein Assay
Sirt3 levels were quantified using sandwich enzyme-linked immunosorbent assay (ELISA) in the middle temporal gyrus (MTG), superior frontal gyrus (SFG), primary visual cortex (PVC), and entorhinal cortex (ENT). Frozen tissues were homogenized and sonicated in lysis buffer (700 μl per 100 mg brain tissues) on ice. They were centrifuged at 14,000g for 15 min. The supernatant was incubated with rabbit unlabeled capture antibody (Cell signaling, Davers, MA) in a refrigerated room (4 °C) overnight. After washing 4 times using phosphate buffered saline/0.05% Tween 20 (PBST), 1% casein (Life technologies, Carlsbad, CA) was used as the blocking buffer for 1 h at room temperature. Human Sirt3 protein (Sigma, St. Louis, MO) was used as the standard control with the following dilution: 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, and 0 ng/ml. Samples and standards were incubated at 4 °C overnight. Unlabeled goat antibody (Sigma, St. Louis, MO) was used as the detection antibody and incubated at room temperature for 2 h. Anti-goat secondary antibody conjugated horseradish peroxidase (Life technologies, Carlsbad, CA) and 3,3′,5,5′-tetramethylbenzidine (Sigma, St. Louis, MO) were used to magnify the signal. Color development was finally stopped by adding 2 N sulfuric acid and the plate was read immediately at 450 nm on a Tecan Infinite M200 Pro Spectrometry. Sirt3 protein levels (pg/μg) were normalized with total protein.
ELISA was validated by Western blot. Frozen postmortem brain tissues were homogenized and sonicated in lysis buffer (700 μl per 100 mg brain tissues). Supernatant was collected for Western blot. Antibodies used were the following: anti-Sirt3 (Cell Signaling, Danvers, MA, USA), anti-synapsin 1 (Abcam, Cambridge, MA), and anti-β-actin (Santa Cruz, Dallas, TX, USA). Protein levels were presented relative to β-actin as an internal control.
Sirt3 mRNA Assay
Another set of frozen postmortem brain samples from the same brain bank was used for a neurotranscriptome study as described previously [15, 16]. Briefly, brain sections were stained with 1% neutral red, and pyramidal neurons were identified by their characteristic size, shape, and location. Total RNA was isolated from the cell lysate with the Arcturus PicoPure RNA Isolation Kit with DNase I treatment using Qiagen’s RNase-free DNase Set. Isolated total RNA from each sample of ~ 500 neurons was double-round amplified, cleaned, and biotin labeled with Affymetrix’s GeneChip Two-Cycle Target Labeling kit with a T7 promoter and Ambion’s MEGAscript T7 High Yield Transcription Kit per the manufacturer’s protocol. Amplified and labeled cRNA was quantitated on a spectrophotometer and run on a 1% Tris-acetate-EDTA gel to check for an evenly distributed range of transcript sizes.
Vector Construction and Transfection
We constructed a short sequence of 19 nucleotides targeting Sirt3 location 764 into OmicsLink shRNA expression clone (no. MSH032833, Genecopoeia, Rockville, MD) to knock down the expression of Sirt3 [13]. We subcloned exogenous Sirt3 cDNA with a mitochondrial targeting sequence (a gift from Drs. Jin and Westphal [17]) into Lenti-CMV-GFP vector to overexpress Sirt3. After the mouse cortical neurons matured in a petri dish in 10 days, we added the transfecting viral particles at a multiplicity of infection of 250. All vectors contained a sequence of eGFP so the result of transfection could be visualized after adding virus particles for 4 days. The levels of knockdown and overexpression were confirmed by Western blotting.
Animals and Primary Neuron Culture
Transgenic mice that carry human tau protein (hTau) were obtained from the Jackson Laboratory (Bar Harbor, ME). This strain B6.Cg-Mapttm1 (EGFP)kit Tg 8cPdav/J is hemizygous for the transgene expressing all six isoforms, including both 3R and 4R forms of human microtubule-associated protein tau [18]. All mice were housed in a temperature- and humidity-controlled vivarium, kept on a 12-h dark/light cycle, and had free access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Barrow Neurological Institute and performed according to the Revised Guide for the Care and Use of Laboratory Animals.
Primary cortical neurons were cultured from hTau and wild-type (B6) mice. They were prepared from newborn mouse pups according to standard procedures [19] with minor modifications. Cortical neurons were plated on poly-D-lysinecoated glass coverslips in neurobasal media supplemented with 0.5% (w/v) L-glutamine, 1% penicillin-streptomycin, 5% fetal bovine serum, and 2% B27 supplement (Invitrogen) and medium was partially replaced every 4 days. On the 14th day, cultured neurons were used for experiments as described in the text.
Oligo Aβ-42 Preparation
The preparation of soluble oligomeric Aβ42 was described previously [20]. Briefly, human synthetic Aβ42 (rPeptide, Watkinsville, GA) was treated in 20 μM ammonium acetate in distilled water (pH = 8, ionic strength = 0.25 M) and incubated for 30 min at room temperature, lyophilized (− 80 °C), and stored in − 80 °C. The pretreated Aβ42 was dissolved in artificial cerebrospinal fluid or culture media at room temperature immediately prior to use. The presence and stability of oligomers were tested by electron microscopy and Western blot techniques.
ATP Levels, NAD+ and NADH Measurement
Cortical neurons (n = 6 per group) were seeded in a 96-well plate (2.5 × 104 cells per well) and treated with oligo Aβ-42 (1000 ng/ml) for 24 h before ATP measurement. ATP levels were tested using a Luminescent ATP Detection Assay Kit (no. ab113849, Abcam, Cambridge, MA) according to the manufacturer’s protocol. The ATP value of vector cells without Aβ-42 treatment was normalized to 100%. Total NAD and NADH were tested using an NAD+/ NADH assay kit (no. ab65348, Abcam, Cambridge, MA) according to the manufacturer’s protocol. NAD+/NADH ratio was calculated based on the value of total NAD and NADH (NAD+ = total NAD – NADH).
Immunoprecipitation
Immunoprecipitation was run according to the manufacturer’s protocol using Dynabeads Protein G (no. 10007D, Thermo Fisher Scientific Inc., Gilbert, AZ). Briefly, 50 μl of completely resuspended Dynabeads was transferred into 1.5-ml tubes, and then placed on a magnet to remove supernatant. Dynabeads were resuspended in 200 μl antibody binding washing buffer. Mouse anti-total Tau monoclonal antibody (no. ab80579, Abcam, 2 μg/mg of supernatant) was added and incubated for 10 min with rotation at room temperature. Supernatant was removed by the magnet. The Dynabeads-Ab complex was resuspended in 200 μl antibody binding washing buffer again. Of the sample supernatant, 400 μl (1μg/μl) was added and incubated for 10 min at room temperature with rotation. The Dynabeads-Ab-Ag complex was washed 3 times using 200 μl washing buffer on magnet. Of the washing buffer, 100 μl was added for the last wash and the supernatant was removed by the magnet. Dynabeads-Ab-Ag complex was gently resuspended by adding 20 μl elution buffer and incubated for 2 min at room temperature. Final supernatant was transferred to a clean tube and mixed with 10 μl loading buffer. Samples were loaded on a gel for Western blot. Antiacetylated lysine antibody (no. 9441, Cell Signaling, Danvers, MA) was used to detect acetylated tau.
Western Blot
Cortical neurons (n = 6 per group) were pretreated with oligo Aβ-42 (0,10,100,1000,5000 ng/ml) for 24 h. Fifty micrograms of total proteins was used for Western blotting. Antibodies used were the following: anti-Sirt3 (no. 5490S, Cell Signaling, Danvers, MA), anti-total tau (Tau-5, no. 556319, BD Pharmingen, San Diego, CA), anti-β-actin (Santa Cruz, Dallas, TX), and IRDye 800CW and IRDye 680CW antibodies (LICOR, Lincoln, NE). Primary antibodies were omitted to serve as negative controls. Immunoreactive signals were quantified using Odyssey CLx. Protein levels were presented percentage relative to β-actin, an internal control.
Statistics
We preselected particular cognitive tests to reflect the brain regions being studied, thus reducing the need for multiple comparisons adjustment. For comparing values across multiple groups, one-way ANOVA with post hoc Tukey’s test was used in GraphPad Prism version 5.03. Pearson’s correlation analysis was applied for correlation analyses in IBM SPSS statistics version 23. Statistical significance was set at p < 0.05.
Results
Demographic, Cognitive, and Pathological Data
Subjects’ demographic information, final antemortem cognitive evaluation scores, and postmortem pathological scores are summarized in Table 1. In total, 16 AD, 13 MCI, and 11 CN subjects were enrolled in the study. The mean age and education were similar in AD, MCI, and CN. The scores of neuropsychological tests were consistent with the disease state. In terms of pathology, CN and MCI had similar amyloid plaque scores throughout the brain, which were much less than those found in the AD group. Similarly, all CN cases and the majority of MCI cases were at Braak stages of III-IV; while more than half of the AD cases were at V-VI.
Table 1.
Subject demographic, cognitive, and pathological data
| Characteristic | CN (n =11) | MCI (n = 13) | AD (n =16) |
|---|---|---|---|
| Age (years), mean (SD) | 88.6 (6.4) | 89.4 (5.6) | 82.9 (8.1) |
| Education (years), mean (SD) | 15.3 (3.4) | 14.4 (2.4) | 15.2 (2.8) |
| Sex, no. (%) | |||
| Male | 9 (81.8) | 8 (61.5) | 7 (43.8) |
| Female | 2 (18.2) | 5 (38.5) | 9 (56.3) |
| APOE4, no. (%) | |||
| Carrier | 5 (45.5) | 3 (23.1) | 8 (50) |
| Non-carrier | 6 (54.5) | 10 (76.9) | 8 (50) |
| Cognitive performance (Z scorea), mean (SD) | |||
| Mattis dementia rating scale | 0.33 (0.60) | − 0.50 (0.50) * | − 2.02 (0.82)*** |
| AVLT total learning | 0.03 (1.30) | − 0.62 (0.92) * | − 2.00 (1.04) *** |
| Boston naming total, 60 items | 1.39 (0.58) | 0.13 (0.96) * | − 1.59 (1.23) *** |
| Stroop color-word interference test | 0.10 (0.83) | − 0.30 (1.33) | − 1.24 (1.14)* |
| Postmortem pathological score, mean (SD) | |||
| Plaques in the temporal cortex | 1.73 (1.40) | 1.10 (1.24) | 2.72 (0.58) |
| Plaques in the frontal cortex | 1.41 (1.36) | 1.19 (1.23) | 2.72 (0.68) ** |
| Plaques in the whole brain | 6.68 (5.85) | 4.88 (5.68) | 12.84 (3.00) ** |
| Tangles in the temporal cortex | 0.50 (0.50) | 0.73 (0.44) | 2.56 (0.70) *** |
| Tangles in the frontal cortex | 0.09 (0.20) | 0.17 (0.24) | 2.00 (1.21) *** |
| Tangles in the whole brain | 5.64 (1.49) | 5.83 (2.00) | 12.31 (3.24) *** |
| Braak stage | |||
| I-II no. (%) | 0(0) | 1 (7.7) | 0(0) |
| III-IV no. (%) | 11(100) | 12 (92.3) | 6 (37.5) |
| V-VI no. (%) | 0 (0) | 0(0) | 10 (62.5) |
CN cognitive normal controls, MCI mild cognitive impairment, AD Alzheimer’s disease, AVLT auditory verbal learning test
Cognitive testing was done within 1 year of death. All scores were converted to Z scores based on age- and education-corrected norms
p < 0.05,
p <0.01, and
p < 0.001 compared with the CN group using post hoc tests (Tukey HSD) of one-way analysis
Sirt3 Levels Were Reduced in AD Brain
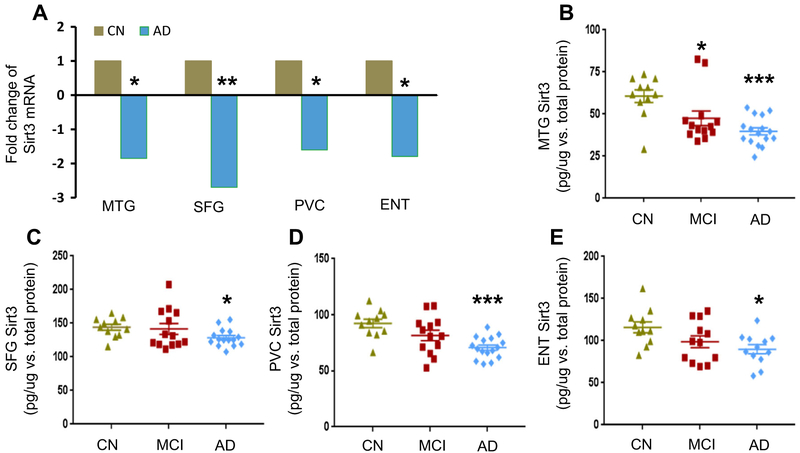
To measure Sirt3 mRNA expression, neurons were laser captured and microdissected from AD and CN cases. In the middle temporal gyrus (MTG), the superior frontal gyrus (SFG), the primary visual cortex (PVC), and the entorhinal cortex (ENT), Sirt3 mRNA levels were reduced in AD compared to those in CN (Fig. 1a). We assessed Sirt3 protein levels using ELISA. Similar to its mRNA, Sirt3 protein levels were reduced in all four regions in AD compared to those in CN. Sirt3 levels in MTG were 60.7 ±3.8 pg/μg vs. 39.8 ±2.1 pg/μg (CN vs. AD); in SFG, they were 144.0±4.4 pg/μg vs. 128.6 ±3.3 pg/μg; in PVC, they were 92.4 ±3.8 pg/μg vs. 70.7 ± 2.4 pg/μg; and in ENT, they were 116.0± 6.5 pg/μg vs. 89.8 ±5.3 pg/μg (Fig. 1b-e). In MCI cases, there was a significant reduction in Sirt3 protein in MTG compared to that in CN (60.7 ±3.8 pg/μg vs. 47.5 ± 4.3 pg/μg). In the other three regions, there was a reduction but did not reach significance. To validate ELISA results, we analyzed Sirt3 protein expression in MTG by Western blot. They correlated well (r = 0.583, p = 0.011, Supplemental Fig. 1a and b). Loss of neurons and synapses is a common feature in AD, so we ran parallel Western blot gels on Sirt3 and synapsin 1, a marker of synapses. Sirt3 to synaptic loss ratios were lower in AD cases (0.86 ± 0.20 vs. 1.85 ± 0.38, p < 0.05, Supplemental Fig. 1c and d), underscoring the significance of Sirt3 loss in AD. These findings suggest that Sirt3 levels in MTG are more sensitive to the progression of dementia.
Fig. 1.
Sirt3 expression in human AD brain. Sirt3 mRNA levels were analyzed in four brain regions. a Fold changes were compared in AD (n = 16) with those in CN cases (n = 11) which were set at 1. Sirt3 protein levels were quantified by ELISA. They were normalized with total protein (pg/μg). Sirt3 levels in CN, MCI, and AD groups were shown in b MTG, c SFG, d PVC, and e ENT. Data were reported mean ± SEM. AD Alzheimer’s disease, MCI mild cognitive impairment, CN cognitive normal controls, MTG middle temporal gyrus, SFG superior frontal gyrus, PVC primary visual cortex, ENT entorhinal cortex. *p < 0.05 and ***p < 0.001, compared to CN group
Sirt3 Levels Were Associated with Cognitive Function
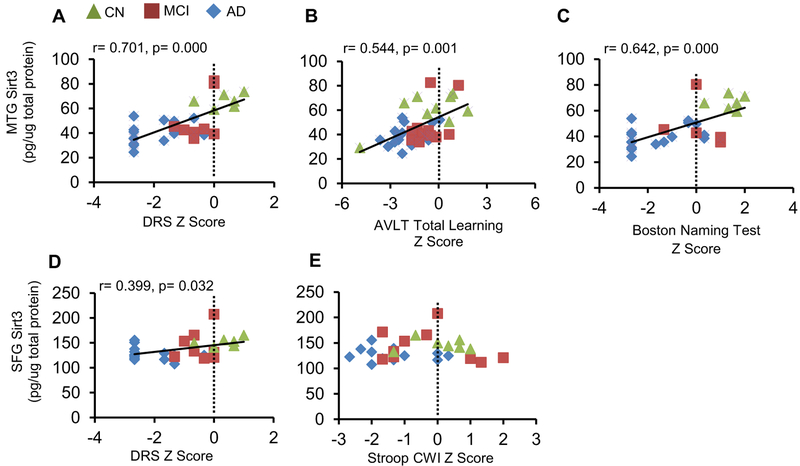
We focused on cognitive tests that reflect global and frontal lobe and temporal lobe functions. DRS assesses global cognitive function, AVLT total learning temporal lobe function, and Stroop color-word interference test frontal lobe function [21]. The 60-item BNT is a widely used naming task which is associated with the left triangularis in the frontal lobe and superior temporal lobe regions (including the planum temporale) [22]. Sirt3 expression in MTG was closely associated with the Z scores of DRS (Pearson r = 0.701, p <0.001), AVLT (Pearson r = 0.544, p = 0.001), and BNT (Pearson r =0.642, p <0.001) (Fig. 2a-c). The upwards trend indicated that higher levels of Sirt3 were associated with better cognitive performance. By contrast, although Sirt3 levels in SFG were associated with the Z scores of DRS (Fig. 2d), they were not associated with the Z score of Stroop color-word interference test (Fig. 2e). The flat line suggested that Sirt3 in SFG did not reflect the performance of these tests, implying that Sirt3 in SFG may already be reduced to the bottom level in the disease state.
Fig. 2.
Correlation of Sirt3 levels with neuropsychological tests. Sirt3 levels in selected cortical regions were correlated with respective neuropsychological tests. All neuropsychological test scores were converted to Z scores. Pearson’s correlation curve was depicted. In MTG, Sirt3 levels were correlated with a DRS, b AVLT total learning, and c Boston naming test. In SFG, the correlations with Sirt3 levels were assessed in d DRS and e Stroop color-word interference test
Sirt3 Levels Were Inversely Correlated with Neurofibrillary Tangles
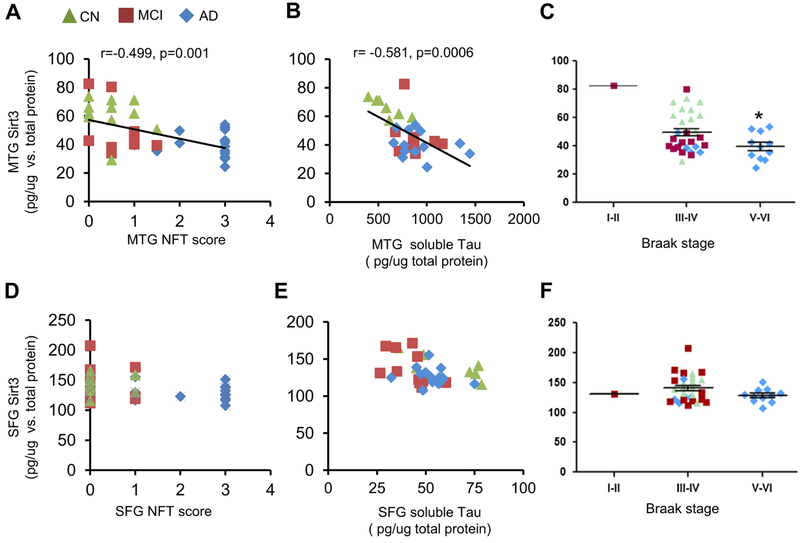
We looked into the relationship between Sirt3 and two pathological hall markers of AD. As the disease progresses from CN to MCI to AD, amyloid burden and total tau increase along with the progression. In MTG, Sirt3 levels were inversely correlated with the neurofibrillary tangle score (Pearson r = − 0.499, p = 0.001) (Fig. 3a) and total tau protein (Pearson r = − 0.581, p <0.001, Fig. 3b). The Sirt3 level in MTG was reduced at Braak stages VI-V compared to that at Braak stages III-IV (p < 0.05, Fig. 3c). However, in SFG, the Sirt3 level did not correlate with the NFT score or total tau protein (Fig. 3d and e), nor did it correlate with Braak stage (Fig. 3f). In addition, there was no correlation between Sirt3 levels and neuritic plaques.
Fig. 3.
Sirt3 levels were reversely related to AD tau pathology in MTG. Sirt3 levels were analyzed for correlation with tau pathology. Pearson’s correlation curve was shown. In MTG, Sirt3 levels were correlated with a NFT score in MTG, b soluble tau protein level in MTG, and c Braak stage. In SFG, the correlations with Sirt3 levels were assessed in d NFT score in SFG, e soluble tau protein level in SFG, and f Braak stage
Oligo Aβ-42-Induced Tau Increase Was in Parallel with Sirt3 Reduction
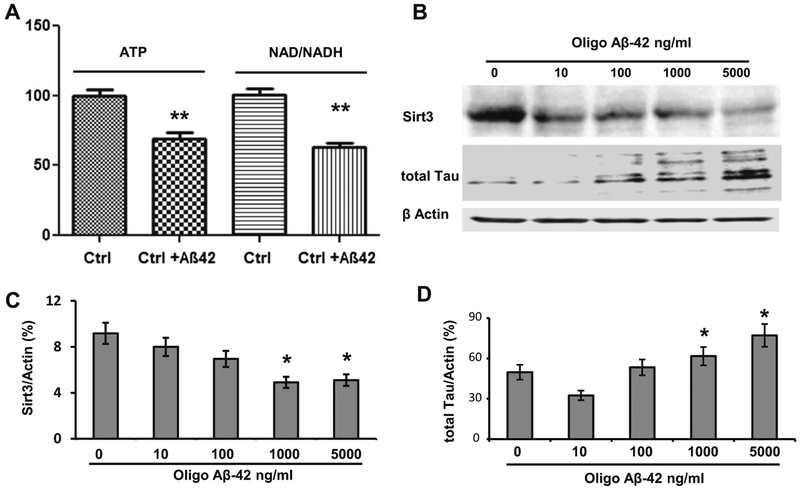
We have shown that oligo Aβ-42 enters the neuron and damages the mitochondria [20]. Unlike typical amyloid plaques, oligo Aβ-42 can move around freely and interact with both Sirt3 and tau. Therefore, we chose oligo Aβ-42 to serve as a messenger between Sirt3 and tau in order to exemplify the relationship between the two. After cortical neuron cells were treated with oligo Aβ-42, both ATP and NAD+/NADH ratio were reduced to 62.8 ± 5.5% and 69.2 ±4.5% of the controls (p <0.01, n = 6 per group, Fig. 4a). Sirt3 is NAD+-dependent and was expected to react to the alteration in NAD+/NADH ratio, so we measured Sirt3 expression after oligo Aβ-42 treatment. It showed a dose-dependent reduction in Sirt3 expression (n = 6, Fig. 4b and c). In the meantime, the total tau expression was increased in a dose-dependent manner by the same oligo Aβ-42 treatment (Fig. 4b and d).
Fig. 4.
Oligo Aβ-42-induced Tau increase was in parallel with Sirt3 reduction in primary neurons. a Intracellular ATP levels and NAD+/ NADH ratio in mouse cortical neuron cells were measured after oligo Aβ-42 treatment. The control group was normalized to 100%. b Cortical neuron cells were treated with serial concentrations of oligo Aβ-42 for 24 h. A representative Western blot of Sirt3 and total tau protein were shown. β-actin was used as an internal control. c Sirt3 levels were measured after oligo Aβ-42 treatment. It showed a dose-dependent reduction. d From the same samples, total tau was measured after oligo Aβ-42 treatment. It showed a dose-dependent increase
Tau Expression Was Increased by Oligo Aβ-42via Sirt3 Modulation
Because mouse tau protein varies from human tau protein, we cultured primary neurons from hTau transgenic mice. Similarly, after treatment with oligo Aβ-42, total tau protein showed a dose-dependent increase while Sirt3, a dose-dependent decrease (Fig. 5a, b, and d). Since Sirt3 is a deacetylase, we measured the levels of the acetylated tau (Ac-tau) protein after immunoprecipitation of total tau protein. Ac-tau paralleled total tau in a dose-dependent manner (Fig. 5a and c), suggesting Sirt3 exerted effects on tau acetylation. To test if the increase in tau is dependent on Sirt3, we genetically manipulated Sirt3 gene in the hTau primary neuron cells (Fig. 6a) [13]. After pretreating hTau neurons with oligo Aβ-42 (1000 ng/ml), Sirt3 levels were reduced (Fig. 6b and e). This reduction in Sirt3 was translated into an increase in total tau and Ac-tau. Neurons with knockdown of Sirt3 (shRNA) increased total tau expression (555.8 ±44.7 vs. 361.1 ± 31.9 in vector, n = 6, p < 0.01) and Ac-tau (422.4± 22.8 vs. 299.2 ± 24.0 in vector, n = 6, p < 0.01); whereas, the overexpression of Sirt3 (Sirt3 cDNA) reduced total tau (221.8 ±23.1, p < 0.05, n = 6) and Ac-tau (200.1 ± 18.5, p < 0.05, n = 6, Fig. 6c and d). These results suggested that oligo Aβ-42 treatment increased total tau through its mediation of Sirt3.
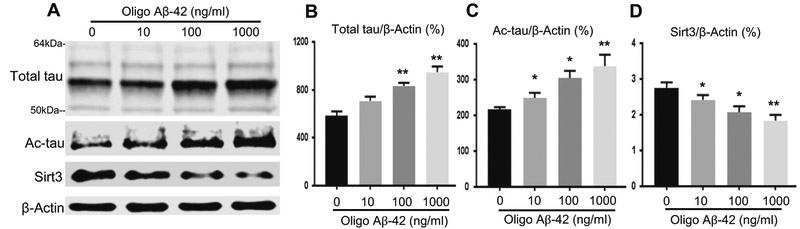
Fig. 5.
Oligo Aβ-42 induced an increase in total tau and Ac-tau and a decrease in Sirt3 in hTau primary neurons. Primary cortical neurons from newborn hTau mice were treated with various doses of oligo Aβ-42 for 24 h. a A representativeWestern blot of total tau (multiple bands ranging from 50 to 64 kDa), Ac-tau, and Sirt3 proteins was shown. b Total tau, c Ac-tau, and d Sirt3 levels were normalized with β-actin, an internal control. N = 6 per group; *p < 0.05 and **p < 0.01
Fig. 6.
Tau expression was increased by oligo Aβ-42via Sirt3 modulation. Primary cortical neurons from newborn hTau mice were transfected with vector, Sirt3 shRNA (knockdown), or Sirt3 cDNA (overexpression). a Neurons were shown being transfected with the vector (green). About 90% neurons were successfully transfected. The transfected neurons were then treated with oligo Aβ−42 (1000 ng/ml) for 24 h. b A representative Western blot of total tau (multiple bands ranging from 50 to 64 kDa), Ac-tau, and Sirt3 proteins was shown. c Total tau, d Ac-tau, and e Sirt3 levels were normalized with β-actin, an internal control. N = 6 per group; *p< 0.05 and **p< 0.01
Discussion
Sirt3 is expressed at high levels in the brain and other nervous system tissues [23,24]. Most previous studies showed a downregulation of Sirt3 in AD [12,13]. In addition, there is no research evidence recording the association of Sirt3 with cognition in the elderly. In this study, we found that higher levels of Sirt3 quantified by standard ELISA in MTG from postmortem human brains were associated with better cognitive performance. This includes global cognitive ability, naming, verbal learning and memory. These findings provide further evidence that Sirt3 plays an important role in slowing cognitive decline which may underscore its close association with tau pathology.
Amyloid plaques and NFT have different spatiotemporal patterns of distribution and progression. Amyloid plaques start in the precuneus and frontal lobe and spread inward to the temporal lobe and hippocampus, whereas NFT follows the Braak staging in an inside out pattern: from the entorhinal cortex, hippocampus, association cortex, and finally primary neocortex [7]. Although Aβ as an initiator or accelerator is considered the main factor in the pathogenesis of AD based on current evidence [25, 26], the primary pathologic change caused by Aβ is still a subject of debate [27-30]. As for NFT, neuropathological studies of AD show a strong association between tau deposits and decreased cognitive function [31]. With regard to the relationship between these two major pathological signs of AD, it is thought that tau-mediated neurodegeneration and amyloid deposition may develop independently and in parallel [32]. In this study, we introduced Sirt3 as a novel mediator between Aβ and tau and found that reduced Sirt3 was correlated with the severity of tau pathology, suggesting that Sirt3 has an influence on tau pathology.
Sirt3 levels were also markedly reduced in 3× Tg AD mice (APP, PSEN1, MAPT) [13]. However, there is no mouse model that mimics the sequential evolution of both amyloid and tau pathology. Human tau transgenic mice are appropriate models to test tau phosphorylation and its pathological consequence [18]. In the strain we used, the intrinsic mouse tau was disrupted by inserting eGFP sequence to the first exon of mouse tau, but all six isoforms of human tau were knocked in. The hTau mice show age-dependent accumulation of hyperphosphorylated and aggregated tau in the cortex and hippocampus, neuronal loss, and cognitive decline [18]. We used an ex vivo model to treat hTau primary neurons with oligo Aβ. This helps us assess the direct relationship between amyloid and tau.
Recent evidence supports soluble tau species as the major toxic species [33, 34]. Like phosphorylation, acetylation is also an important step in posttranslational modification of proteins. Researchers reported that tau acetylation at different lysine sites inhibited tau function and promoted pathological tau aggregation [10, 35]. We found Sirt3 levels were inversely associated with tau pathology. It suggests that tau acetylation due to lack of Sirt3-induced deacetylation might happen at an early stage or all stages in AD brains and may be a critical determinant in tau homeostasis and toxicity. Thus, reducing tau acetylation by promoting Sirt3 could be a new disease-modifying target for drug discovery and biomarker development in AD. Some aspects of this study must be taken into account in the interpretation of the findings. We used a relatively homogenous cohort in this cross-section study. Because of the relatively small number of patients investigated and the lack of longitudinal follow-ups, our results should be further validated in other cohorts and interpreted in the appropriate clinical context. For example, Sirt3 may reduce with age. After Sirt1 was found to extend life spans of yeast, and later in drosophila and mice, it has generated great interest in linking the sirtuin family and aging. Single nucleotide polymorphisms (SNPs) in the SIRT3 gene were discovered in centenarians and Sirt3 genetic variants were found to be relevant in human longevity in the Italian TRELONG cohort. Since we enrolled patients with advanced age at the time of study (mean age in the 80s), we did not find statistical significant correlation between Sirt3 levels with aging (data not shown). In a population setting, we would see a change in Sirt3 expression association with age to determine the generalizability of our findings. Nonetheless, the fact that a difference in Sirt3 levels was found despite the age-related accumulation of amyloid in CN brains suggests this finding is specific for disease and not simply a correlate of normal age-related AD-like amyloid accumulation. Second, it would be interesting to see longitudinal changes in Sirt3. It would help to validate the finding and to address the question of conversion from MCI to dementia. Third, we had fewer numbers of female samples in the control group.Estrogen suppresses the production of mitochondrial ROS, specifically in strains where females live longer [36,37]. The estrogen receptors α and β are in the mitochondria and have direct action on SOD2 [38]. SIRT1 has been shown to ameliorate oxidative stress induced by neural cell death and is downregulated in Parkinson’s disease [39] and to protect against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis [40, 41]. Similar to SIRT1, SIRT3 regulates oxidative stress by modulating FoxO3a and SOD2 [42]. However, the interaction between Sirt3 and estrogen or its receptors remains unknown. It would be interesting to include more gender-balanced subjects to address this issue. The fourth caveat relates to the ex vivo model. We provide data of a cause-effect in the mouse neuron cell culture. Future new animal models would be essential to clarify the temporal course when amyloid triggers tau tangle formation and how Sirt3 is involved in the process. (Howitz et al. 2003; Wood et al. 2004; Baur et al. 2006; Bellizzi et al. 2005; Rose et al. 2003; Albani et al. 2014) is cited in the body but its bibliographic information is missing. Kindly provide its bibliographic information. Otherwise, please delete it from the text/body.I added citations and reformated the ref list. please see attachment
Conclusions
We found that Sirt3 mRNA and protein expression in multiple brainregions were significantly low in AD compared to those in CN subjects. We also identified a strong association between Sirt3 and cognition along with a clear inverse correlation between Sirt3 and tau pathology. These results suggest that Sirt3 may be a novel player in the pathogenesis of AD. Strategies to modulate Sirt3 expression could disrupt Aβ-induced overproduction of tau and be useful in the treatment of AD.
Supplementary Material
Acknowledgements
The authors are deeply grateful for the subjects and families who have participated in our brain donation program.
Funding Information This work is funded by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson’s Disease Consortium), and Barrow Neurological Foundation (3032226); the National Science Foundation of China (81671050 to JS) and the Alzheimer Association (NIRG 14-322078). The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Compliance with Ethical Standards
The operations of the Arizona Alzheimer’s Disease Core Center and Banner Sun Health Research Institute Brain and Body Donation Program are approved by their individual Institutional Review Boards. We thus received approval for any experiments using human subjects. Written informed consent was obtained from all subjects (or guardians of subjects) participating in the study [43, 44].
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12035-018-0977-0) contains supplementary material, which is available to authorized users.
References
- 1.Price JL, McKeel DW Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC et al. (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 4:138–150 [DOI] [PubMed] [Google Scholar]
- 3.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ et al. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL (1988) A4 amyloid protein deposition and the diagnosis of Alzheimer’s disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology 38:1688–1693 [DOI] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P et al. (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A betadeposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Braak E (1991) Neuropathological stageing of Alzheimerrelated changes. Acta Neuropathol 82:239–259 [DOI] [PubMed] [Google Scholar]
- 8.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Zou Y, Zhang M, Zhao N, Tian Q, Gu M, Liu W, Shi R et al. (2015) Mitochondrial Sirt3 expression is decreased in APP/ PS1 double transgenic mouse model of Alzheimer’s disease. Neurochem Res 40:1576–1582 [DOI] [PubMed] [Google Scholar]
- 10.Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS et al. (2015) Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21:1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracy TE, Sohn PD, Minami SS, Wang C, Min SW, Li Y, Zhou Y, Le D et al. (2016) Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron 90:245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG (2014) Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. NeuroMolecular Med 16:405–414 [DOI] [PubMed] [Google Scholar]
- 13.Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM, Shi J (2014) Pituitary adenylate cyclase-activating polypeptide protects against beta-amyloid toxicity. Neurobiol Aging 35:2064–2071 [DOI] [PubMed] [Google Scholar]
- 14.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L et al. (2004) Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256:240–246 [DOI] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA et al. (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics 33: 240–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin L, Galonek H, Israelian K, Choy W, Morrison M, Xia Y, Wang X, Xu Y et al. (2009) Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci 18:514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86:582–590 [DOI] [PubMed] [Google Scholar]
- 19.Rinetti GV, Schweizer FE (2010) Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J Neurosci 30:3157–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin JX, Maalouf M, Han P, Zhao M, Gao M, Dharshaun T, Ryan C, Whitelegge J et al. (2016) Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol Aging 39:25–37 [DOI] [PubMed] [Google Scholar]
- 21.Ottowitz WE, Dougherty DD, Savage CR (2002) The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry 10:86–99 [DOI] [PubMed] [Google Scholar]
- 22.Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro A 3rd, Hyun J, Kim DS, Goral M et al. (2010) Bilateral brain regions associated with naming in older adults. Brain Lang 113:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kincaid B, Bossy-Wetzel E (2013) Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonnell E, Peterson BS, Bomze HM, Hirschey MD (2015) SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab 26:486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vishnu VY (2013) Can tauopathy shake the amyloid cascade hypothesis? Nat Rev Neurol 9:356. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau SM, Frosch MP (2014) Tracking the earliest pathologic changes in Alzheimer disease. Neurology 82:1576–1577 [DOI] [PubMed] [Google Scholar]
- 28.Attems J, Jellinger KA (2013) Amyloid and tau: neither chicken nor egg but two partners in crime! Acta Neuropathol 126:619–621 [DOI] [PubMed] [Google Scholar]
- 29.Mann DM, Hardy J (2013) Amyloid or tau: the chicken or the egg? Acta Neuropathol 126:609–613 [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121:171–181 [DOI] [PubMed] [Google Scholar]
- 31.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC (2015) Tau imaging: early progress and future directions. Lancet Neurol 14:114–124 [DOI] [PubMed] [Google Scholar]
- 32.Chetelat G (2013) Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat Rev Neurol 9:123–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR, Kayed R (2012) Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J 26:1946–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren Y, Sahara N (2013) Characteristics of tau oligomers. Front Neurol 4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM (2011) The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun 2:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanz A, Hiona A, Kujoth GC, Seo AY, Hofer T, Kouwenhoven E, Kalani R, Prolla TA et al. (2007) Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol 42:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vina J, Borras C (2010) Women live longer than men: understanding molecular mechanisms offers opportunities to intervene by using estrogenic compounds. Antioxid Redox Signal 13:269–278 [DOI] [PubMed] [Google Scholar]
- 38.Germain D (2016) Sirtuins and the estrogen receptor as regulators of the mammalian mitochondrial UPR in cancer and aging. Adv Cancer Res 130:211–256 [DOI] [PubMed] [Google Scholar]
- 39.Singh P, Hanson PS, Morris CM (2017) SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci 18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA et al. (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braidy N, Jayasena T, Poljak A, Sachdev PS (2012) Sirtuins in cognitive ageing and Alzheimer’s disease. Curr Opin Psychiatry 25:226–230 [DOI] [PubMed] [Google Scholar]
- 42.Yin J, Han P, Tang Z, Liu Q, Shi J (2015) Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35:1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE et al. (2015) Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35:354–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han P, Liang W, Baxter LC, Yin J, Tang Z, Beach TG, Caselli RJ, Reiman EM et al. (2014) Pituitary adenylate cyclase-activating polypeptide is reduced in Alzheimer disease. Neurology 82:1724–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.