Abstract
OBJECTIVES:
The objective of this study was to access the prevalence of alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD) histology in the general population, which had been otherwise difficult to access because of inherent misclassification bias in surrogate marker studies and referral bias in patient case series. The interaction among rs738409, obesity, and alcohol remains controversial. This population-based autopsy study investigated the histological prevalence of ALD and NAFLD, and interactions among rs738409, obesity, and alcoholism.
METHODS:
A total of 170 alcoholic and 235 nonalcoholic cases were selected from 1,034 adult car accident autopsies in 17 Kansas and Missouri counties from 2000 to 2010. The nonalcoholic group had undetectable blood alcohol concentration, while the alcoholic group had a blood alcohol concentration ≥0.08%.
RESULTS:
The age-standardized prevalences of hepatic steatosis, steatohepatitis, and advanced fibrosis were 56, 6, and 18% among alcoholics and 36, 4, and 6% in nonalcoholics, respectively. The interaction terms among alcohol, body mass index (BMI), and genotype were not significant. rs738409 GC or GG genotype was associated with 1.9-fold odds (95% confidence interval (CI), 1.2–2.9) of a higher NAFLD Activity Score (NAS). Alcohol had 3.5-fold odds (95% CI, 2.0–5.9), while every 5-unit increase in BMI had 1.9-fold odds (95% CI, 1.7–2.5). A negative interaction between alcohol and BMI towards fibrosis had been observed (P = 0.045). Every 5-unit increase in BMI had 2.2-fold odds (95% CI, 1.5–2.5) of fibrosis among nonalcoholics, but not in alcoholics.
CONCLUSIONS:
This study assessed the prevalence of fatty liver histology in the general population from an autopsy study perspective. The finding of an additive interaction among rs738409, obesity, and alcohol towards NAS may be useful in targeting preventative care to patients at highest risk for ALD. The negative interaction between alcohol and obesity towards fibrosis supported previous findings and suggests the need for future research to explore potential mechanisms that may improve treatment of nonalcoholic steatohepatitis-related fibrosis.
INTRODUCTION
As many as half of the adults in the United States consume at least one alcoholic drink in a month, 5.1% are heavy drinkers, and 15.5% are binge drinkers (1). On the other hand, 33.8% of adults are obese (2). Excessive alcohol intake and obesity are known to cause alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD), respectively. The histological spectrum of the two diseases is identical, which includes steatosis, steatohepatitis, and cirrhosis. The exact prevalence of ALD and NAFLD, however, is unknown. Although histology represents the reference standard for diagnosis, it is generally not feasible in population-based screening (3). Most population-based cross-sectional studies have utilized aminotransferase levels (4) or ultrasound (5-7). These surrogate markers have limited sensitivity and specificity for ALD and NAFLD, and cannot accurately differentiate steatosis from steatohepatitis or cirrhosis. Using magnetic resonance spectroscopy, the most accurate noninvasive method, the Dallas Heart Study, estimated the prevalence of hepatic steatosis at 33.6% (8). However, the prevalence of steatohepatitis and cirrhosis could not be assessed, and the prevalence of ALD in excessive drinkers could not be estimated because of relatively few excessive drinkers in their sample. Liver biopsy case series are generally from patients with signs and symptoms of liver disease who are followed and biopsied by gastroenterologists, and therefore limited by referral bias. In this study, the first aim was to assess the prevalence of steatosis, steatohepatitis, and advance fibrosis among alcoholics and nonalcoholics, using a population-based autopsy study of patients who died in motor vehicle accidents.
There are conflicting data on whether alcohol increases obesity-associated steatosis in an additive (9,10) or a synergistic manner (11), or, alternatively, has the opposite effect of reducing the incidence of obesity-associated steatosis (3). Recently, the rs738409 C> G (I148M) single-nucleotide polymorphism in the PNPLA3 gene has been associated with hepatic steatosis. However, it remains unclear whether rs738409 is a simple risk factor for ALD and NAFLD, or it modifies the effects of obesity and alcoholism. Our second aim was to explore the interaction among obesity, alcohol, and rs738409 towards NAFLD Activity Score (NAS) and fibrosis. Our a priori hypothesis is that the interaction may be synergistic, additive, or negative. The inclusion of unbiased subjects with various body mass index (BMI), with and without alcoholism, allowed assessment of interactions of rs738409, obesity, and alcoholism.
METHODS
Study sample
This study included adult (age ≥18 years) subjects who died as a result of motor vehicle accidents and underwent autopsy in 13 counties in Kansas (Shawnee, Douglas, Lyon, Chase, Allen, Neosho, Wilson, Woodson, Leavenworth, Johnson, Miami, Bourbon, and Linn) and four counties in Missouri (Johnson, Henry, Clinton, and Livingston) between January 2000 and August 2010. Forensic pathologists in these counties routinely performed liver biopsies and permanently stored histological specimens from all autopsy cases. Cases with a blood alcohol concentration (BAC) ≥0.08% constituted the alcoholic group. A BAC ≥0.08% typically results from ≥5 drinks in men or ≥4 drinks in women, and represents the threshold for binge drinking (12) as defined by the National Institute on Alcohol Abuse and Alcoholism. Although the prevalence of heavy drinkers and binge drinkers in this group has not been studied directly, the Behavioral Risk Factor Surveillance System of 2006 (13) estimated that 90% of impaired driver episodes in the United States occurred in either heavy drinkers or binge drinkers. The nonalcoholic group, defined by an undetectable BAC, although not guaranteed to be free of alcoholics, represents a sample of the general population. Owing to the association between substance abuse and alcohol abuse, a negative drug screening is only required for inclusion in the nonalcoholic group. Subjects who died more than 24 h after hospital admission were excluded because their liver histology could have been affected by prolonged resuscitation (14). Subjects with severe burns or decomposition were also excluded because measurement of BMI was not possible. Half of the male subjects and two-thirds of the alcoholic subjects were randomly selected for inclusion because of limited resources and the excess of nonalcoholic and male subjects available for analysis. This study was categorized with a “Determination of Not Human Subjects Research” from the Human Research Protection Program in The University of Kansas Medical Center.
Clinical data, genotype, and liver histology
At the time of each autopsy, the county forensic pathologists recorded the age, gender, race/ethnicity, BMI, toxicology, cause of death, and the time of death relative to the accident. Liver biopsy slides were stained with hematoxylin and eosin at the time of autopsy. Formaldehyde-fixed and paraffin-embedded liver tissue blocks were recovered for additional staining and genotyping. CK-8/18 staining was prepared from a 1:1 mixture of monoclonal mouse K8 Ab-8 Clone K8.8 and monoclonal mouse K18 Ab-1 Clone DC 10 (NeoMarkers, Fremont, CA), both 1:50 in Dako REAL antibody diluent. Trichrome staining was prepared using Richard-Allan Gomori Trichrome Stain Kit (Thermo Fisher Scientific, Waltham, MA). Histology and immunohistochemistry were reviewed by a blinded pathologist (M.O.) and scored for NAS and fibrosis. Although there has not been any accepted pathological scoring system for alcohol-related steatosis, ballooning, and inflammation, we adopted the Brunt classification system (15) for grading both alcohol and nonalcohol cases. Steatosis was recorded as grade 1 (5–33%), grade 2 (34–66%), or grade 3 ( > 66%). Owing to the large size of autopsy specimens compared with needle biopsy, all specimens had some degree of lobular inflammation (i.e., ≥grade 1). Therefore, grade 1 was used as the reference in the ordinal logistic regression analysis. The aggregate NAS was calculated from the sum of steatosis, lobular inflammation, and ballooning degeneration. Cases without steatosis were assigned an NAS of 0. One case with grade 2 lobular inflammation without steatosis was excluded from the NAS analysis. None of the cases had ballooning without steatosis. Fibrosis staging was analyzed as stage 1a/b/c (zone 3 predominant perisinusoidal/pericellular fibrosis or portal fibrosis), stage 2 (plus periportal fibrosis), stage 3 (bridging fibrosis), and stage 4 (cirrhosis) in the ordinal logistic regression analysis. The diagnosis of steatohepatitis was based on the pathologist and hepatologist’s overall assessment of steatosis, lobular inflammation, ballooning degeneration, and perisinusoidal fibrosis (16) rather than a particular NAS cutoff. The pathologist and hepatologist were blinded to the clinical and demographic characteristics of the cases. Genomic DNA was extracted from formalin-fixed and paraffin-embedded tissue using phenol:chloroform:isopropyl alcohol at 25:24:1. A predesigned TaqMan probe (Assay ID: C_7241_10; Product number: 4351379; Applied Biosystems, Carlsbad, CA) was purchased for genotyping of rs738409. Real-time polymerase chain reaction was carried out using C1000 Thermal Cycler (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. The success rates of these assays were > 100.0%.
Statistical analysis
The prevalences of steatosis, steatohepatitis, and cirrhosis in the alcoholic and nonalcoholic groups were calculated. To account for the ordinal nature of steatosis, lobular inflammation, ballooning degeneration, and NAS score, ordinal logistic regression was used. Ordinal logistic regression cannot be used for fibrosis analysis owing to a violation of the proportional odds assumption. Therefore, logistic regression was used to compare stage 0–2 vs. 3–4 fibrosis. Owing to the small number of rs738409 GG genotype, the GC and GG genotypes were grouped together as the “G allele carrier” and compared with the CC genotype (17). The year 2000 census was used for direct age standardization (18). All multivariable models included age, age2, gender, race, alcohol, BMI, and rs738409. Interactions among alcohol, BMI, and rs738409 were tested using interaction cross-terms (alcohol×BMI, alcohol×rs738409, BMI×rs738409). SAS 9.13 (SAS Institute, Cary, NC) was used for statistical analysis.
RESULTS
Between January 2000 and August 2010, 1034 adult motor vehicle accident autopsies were performed within the aforementioned counties in Kansas and Missouri. After excluding nonalcoholics with positive drug screens, intermediate BAC, prolonged hospitalization, or severely burned and/or decomposed bodies, 736 cases were eligible for study inclusion. Of these cases, 68% were men and 63% were nonalcoholic. Half of the male cases and two-thirds of the alcoholic cases were randomly selected for inclusion (n = 471). The final analysis included 405 cases with tissue blocks available for staining and genotyping. There were 170 alcoholic cases and 235 nonalcoholic cases. The distribution of the rs738409 genotype was 233 subjects with CC, 141 CG, and 31 GG (Table 1). The distribution of the rs738409 genotype by race and ethnicity was shown in Table 2.
Table 1.
Demographics by alcohol use and obesity
| Nonobese nonalcoholic | Nonobese alcoholic | Obese nonalcoholic | Obese alcoholic | |
|---|---|---|---|---|
| n | 137 | 117 | 98 | 53 |
| Age (years) | 48 (23) | 36 (14) | 48 (18) | 34 (10) |
| Gender | ||||
| Male | 54% | 56% | 70% | 68% |
| Female | 46% | 44% | 30% | 32% |
| Race | ||||
| Caucasian | 75% | 82% | 77% | 76% |
| Black | 14% | 7% | 9% | 8% |
| Hispanic | 8% | 6% | 12% | 11% |
| Other | 3% | 4% | 2% | 5% |
| Driver | 71% | 75% | 68% | 78% |
| Positive drug screen | 19% | 24% | ||
| Blood alcohol concentration | 0.24% (0.26%) | 0.19% (0.07%) | ||
| rs738409 genotype | ||||
| CC | 55% | 57% | 65% | 53% |
| GC | 39% | 34% | 28% | 40% |
| GG | 6% | 9% | 7% | 7% |
Categorical variables are expressed as percentages and continuous variables are expressed as mean (s.d.).
Table 2.
Distribution of rs738409 by race and ethnicity
| Caucasian (n=326) | Black (n=38) | Hispanic (n=30) | Other (n=11) | |
|---|---|---|---|---|
| rs_738409 genotype | ||||
| CC | 188 (58%) | 28 (74%) | 9 (30%) | 8 (73%) |
| GC | 118 (36%) | 10 (26%) | 12 (40%) | 1 (9%) |
| GG | 20 (6%) | 0 (0%) | 9 (30%) | 2 (18%) |
Prevalence expressed as frequency (expressed as percent).
Potential bias
Although cases with hospitalization over 24 h have been excluded, the prevalence of steatosis does not differ if the victim died at the scene. The prevalence of steatosis and fibrosis did not differ if the victim is a driver, passenger, or pedestrian.
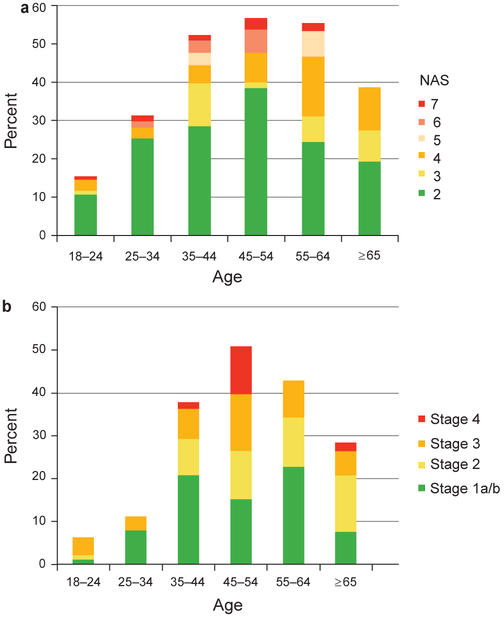
Effect of age and gender
The prevalence of steatosis, NAS, and fibrosis peaked between 45 and 54 years of age, and then decreased with advancing age (Figure 1a, b). Therefore, direct age standardization with the year 2000 census was used for prevalence estimates. The interaction term age2 was highly significant (< 0.0001) and therefore included in all multivariable ordinal logistic regression analyses. Women obesity, and genotype groups is shown in Figure 2. The interaction terms among alcohol, BMI, and genotype were not significant (alcohol×BMI, P = 0.56; alcohol×genotype, P = 0.67; BMI×genotype, P = 0.95). This study had 80% power to detect had similar NAS compared to men (odds ratio (OR), 0.84; 95% confidence interval (CI), 0.54–1.3) and less fibrosis (OR, 0.50; 95% CI, 0.30–0.83).
Figure 1.
(a) Distribution of hepatic steatosis and NAFLD Activity Score (NAS) score by age. Cases without steatosis were assigned an NAS score of 0. The denominator includes the entire study sample, with and without hepatic steatosis. (b) Distribution of fibrosis stage by age. The denominator includes the entire study sample, with and without hepatic steatosis.
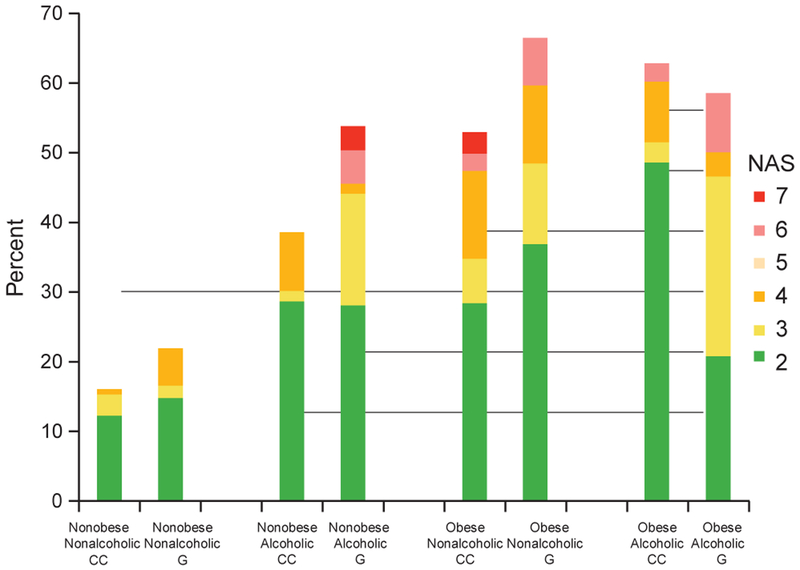
Figure 2.
Distribution of hepatic steatosis and NAFLD Activity Score (NAS) by alcohol, obesity, and genotype after direct age standardization using the year 2000 census. The denominator includes the entire study sample, with and without hepatic steatosis.
NAS
Overall, the age-standardized prevalence of hepatic steatosis was 56% in the alcoholic cases and 36% in the nonalcoholic cases. The prevalence of steatohepatitis was 6 and 4%, respectively. The distribution of hepatic steatosis and NAS in the alcoholic, obesity, and genotype groups is shown in Figure 2. The interaction terms among alcohol, BMI, and genotype were not significant (alcohol×BMI, P = 0.56; alcohol×genotype, P = 0.67; BMI×genotype, P = 0.95). This study had 80% power to detect an interaction if the interaction carried an additional fourfold odds. The rs738409 GC or GG genotypes were associated with 1.9-fold odds (95% CI, 1.2–2.9) of a higher NAS. When GC and GG were analyzed separately, there appears to be a small incremental increase in odds from GC (OR 1.8) to GG (OR 2.2). Alcohol had 3.5-fold odds (95% CI, 2.0–5.9), while every 5-unit increase in BMI had 1.9-fold odds (95% CI 1.7–2.5).
In terms of the point estimate of individual histological features comprising the NAS, alcohol was associated with 3.6-fold odds for macrovesicular steatosis, 2.9-fold odds for lobular inflammation and 3.1-fold odds for ballooning degeneration. Every 5-unit increase in BMI was associated with 2.2-fold odds for macrovesicular steatosis, 1.8-fold odds for lobular inflammation and 1.6-fold odds for ballooning degeneration. G allele was associated with 2.1-fold odds for macrovesicular steatosis, 1.8-fold odds for lobular inflammation and 1.1-fold odds for ballooning degeneration.
Fibrosis
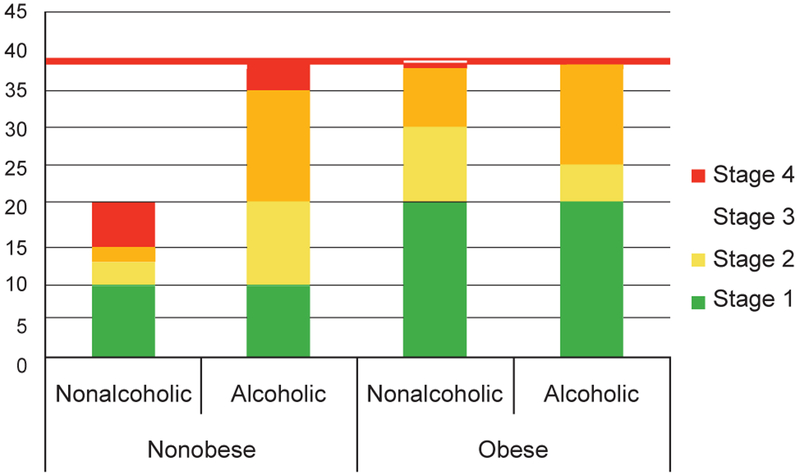
The age-adjusted prevalence of hepatic fibrosis of any stage was 42% in the alcoholic cases and 30% in the nonalcoholic cases, while the age-adjusted prevalence of advanced fibrosis was 18% and 6%, respectively. However, the rs738409 CC genotype was not associated with hepatic fibrosis (OR, 1.2; 95% CI, 0.76–2.0). The effect of alcohol and BMI was also similar across rs738409 genotypes.
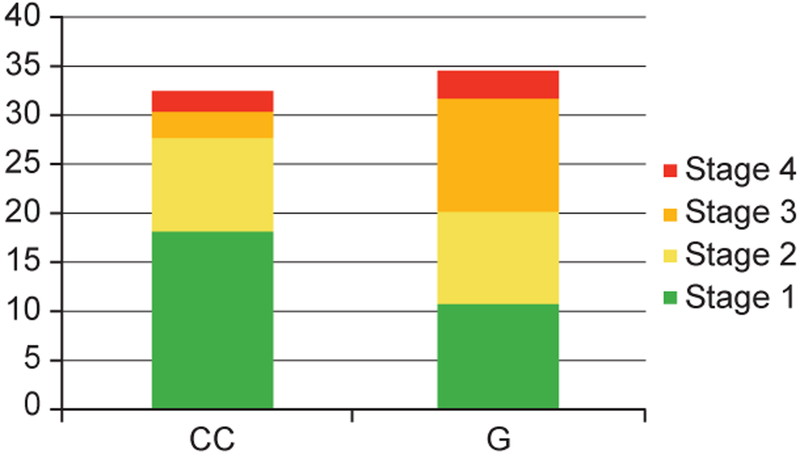
The interaction term alcohol×BMI was statistically significant (P = 0.036). Every 5-unit increase in BMI was associated with 2.2 times the odds (95% CI, 1.5–2.5) of fibrosis in the nonalcohol group, but was not associated with increased fibrosis in the alcohol group (OR, 0.84; 95% CI, 0.66–3.1). Figure 3 outlines the age-standardized prevalence of hepatic fibrosis by alcohol and obesity. The rs738409 GC/GG genotype was not associated with hepatic fibrosis (OR, 1.2; 95% CI, 0.76–2.0) owing to a higher prevalence of stage 1 and 2 fibrosis in CC genotype and stage 3 and 4 fibrosis in GC/GG genotype (Figure 4), which violated the proportional odds assumption of the ordinal regression model (cumulative model test, P = 0.0004). When compared across stage 0–2 vs. 3–4 fibrosis, the rs738409 GC/GG genotype was associated with 3.2 times the odds (95% CI, 1.4–7.3).
Figure 3.
Prevalence of hepatic fibrosis by alcohol and obesity. The denominator includes the entire study sample, with and without hepatic steatosis.
Figure 4.
Prevalence of hepatic fibrosis by rs738409 genotype. The denominator includes the entire study sample, with and without hepatic steatosis.
Other liver disease histologies
There were 12 cases with grade 1 portal inflammation without steatosis. Compared with normal liver, isolated portal inflammation was associated with rs738409 GC/GG genotype (P = 0.04), but not BMI or alcohol. These 12 cases were consistent with viral hepatitis. One patient had grade 2 lobular inflammation without steatosis. Forty-four patients had fibrosis without steatosis (stage 1a/b = 14; stage 1c = 12; stage 2 = 10; stage 3 = 5; and stage 4 = 3). Isolated fibrosis was not associated with rs738409 genotype, BMI, or alcohol. There were 19 cases of stage 1c portal-only fibrosis: 7 were associated with steatosis (grade 1 = 4; grade 2 = 2; and grade 3 = 1) and 12 were not. Stage 1c fibrosis was associated with BMI (P = 0.01), but not genotype or alcohol.
DISCUSSION
This study provided an assessment of fatty liver histology in the general population from an autopsy perspective, which was not possible with previous surrogate marker studies and patient case series. The prevalences of hepatic steatosis, steatohepatitis, and advanced fibrosis were 56, 6, and 18% in alcoholics, and 36, 4, and 6% among nonalcoholics, respectively. The association of NAS was different from that of fibrosis. For NAS, rs738409, BMI, and alcohol were simply additive. For fibrosis, alcohol and BMI demonstrated a statistically significant negative interaction. The rs738409 GC/GG genotype was associated with more advanced fibrosis and less early fibrosis. Both NAS score and fibrosis peaked between 45 and 54 years of age, and then decreased with advancing age.
In the Dallas Heart Study, the prevalence of hepatic steatosis estimated by magnetic resonance spectroscopy was found to be 34% (8), which was very similar to our estimate of 36% in nonalcoholics. In addition, our study estimated a steatohepatitis prevalence of 4% and advanced fibrosis at 6%. The prevalence in the excessive drinkers could not be estimated because excessive drinkers comprised only 6% of the Dallas Heart Study sample. Biopsy case series of heavy drinkers estimated the prevalences of steatosis, steatohepatitis, and cirrhosis to be 58–100, 10–34, and 10–34%, respectively (19-22), which were slightly higher than our estimates. In comparison, population-based screening with ultrasound typically finds a prevalence of < 20% for steatosis among heavy drinkers (3,5-7). The difference may result from selection bias in the biopsy case series, lack of sensitivity of the ultrasound examination, and the 10% misclassification bias in our autopsy study.
The interaction between obesity and alcohol in causing hepatic steatosis remains controversial. A cross-sectional study in Finland showed an additive interaction between alcohol consumption and obesity in the prevalence of liver enzyme abnormalities (9). A UK study showed an additive effect between heavy drinking and obesity in the incidence of cirrhosis and liver disease mortality (10). On the other hand, a cross-sectional study in northern Italy showed a negative interaction between alcohol and obesity in the prevalence of ultrasound and liver enzyme abnormalities (3). A US study showed that obesity may decrease the alcohol threshold for liver injury (4). This study showed an additive interaction between alcohol and obesity towards NAS (steatosis, inflammation, and cell injury), but a statistically significant negative interaction between alcohol and obesity towards fibrosis. The finding that obese alcoholics had less fibrosis than obese nonalcoholics suggested a possible beneficial effect of alcohol for this subgroup. Our finding of negative interaction should be interpreted as a mechanistic clue that alcohol may have antifibrotic properties. Along with other studies that have suggested an antifibrotic property of alcohol (23-25), our finding justifies a pharmacological investigation into the antifibrotic mechanism of alcohol, and how this mechanism can be activated without alcohol.
Recently, a genome-wide association scan of the nonsynonymous single-nucleotide polymorphisms in the Dallas Heart Study participants identified that the I148M allele (rs738409) in the PNPLA3 gene was strongly associated with increased hepatic triglycerides as measured by magnetic spectroscopy (26). Little information exists in the literature as to whether rs738409 simply represents a risk factor for steatosis, or the gene modifies the effects of obesity and alcoholism toward steatosis. In a case–control study of Hispanics with alcohol dependence (27), those with cirrhosis were more likely to have the G allele than those with apparently normal liver function. In another case–control study of European Caucasian (17) patients undergoing liver biopsy for suspected ALD vs. healthy social workers, the G allele was associated with ALD and cirrhosis in ALD. However, these two findings did not differentiate whether rs738409 is a simple risk for hepatic steatosis, or if it modifies the effects of obesity and alcoholism in causing hepatic steatosis. This study included an unbiased sample with subjects of various BMI, with and without alcoholism, to study the interactions of rs738409, obesity, and alcoholism. Our study showed an additive interaction among rs738409, obesity, and alcohol towards NAS. Those with the rs738409 G allele are especially at risk if they are obese or drink alcohol excessively.
By far, the most critical issue concerning the validity of this study is the misclassification bias in the alcoholic and nonalcoholic group. All cases in the alcoholic group fulfilled the criteria for binge drinking at the time of death, and the alcohol use was in a situation that was physically hazardous. According to the Diagnostic and Statistical Manual of Mental Disorders IV, the diagnosis of alcohol abuse can be made based on “recurrent alcohol use in situations in which it is physically hazardous.” The recurrent nature of the drinking pattern in this group has been supported by Behavioral Risk Factor Surveillance System 2006 data (13), which estimated that alcohol-impaired drivers in the United States comprised of 33.9% heavy drinkers with binge drinking, 49.4% nonheavy drinkers with binge drinking, 1% heavy drinkers without binge drinking, and 15.7% nonheavy drinkers without binge drinking. When weighted by alcoholimpaired drinking episodes, only 10.6% of alcohol-impaired driving episodes were attributed to nonheavy drinkers without binge drinking. Behavioral Risk Factor Surveillance System did not set a minimum alcohol threshold to be considered for alcohol-impaired drivers, while our study used BAC ≥0.08% as the inclusion criteria for the alcohol group. On the other hand, the Behavioral Risk Factor Surveillance System definition of heavy drinking (also the NIAAA definition) was > 1 drink per day for women and > 2 drinks per day for men (28), which was lower than the usual criteria for ALD. Still, we anticipate a misclassification bias of 10.6% in the alcoholic group. The nonalcoholic group should be similar to the general population, with 15.3% heavy and/or binge drinkers (1). Unlike case series, the alcohol frequency and amount cannot be quantified. Nevertheless, a BAC ≥0.08% typically results from ≥5 drinks in men or ≥4 drinks in women.
This study design had a number of important characteristics that allowed for an accurate assessment of the prevalence of hepatic steatosis. Histology is currently the reference standard for diagnosis of ALD and NAFLD. Autopsy studies with liver histology have been used to estimate the prevalence of fatty liver disease in West India (29), Toronto (30), and Northwestern Greece (31). In contrast to this study, nonaccidental death was included in previous studies, which may have resulted in selection bias as a large proportion of patients died of ischemic heart disease, and 70% of those had steatosis or steatohepatitis. In a San Diego county pediatric autopsy study (14), only cases of accidental death, homicide, and suicide were included. In an unpublished pilot autopsy study in Olmsted County, MN comparing accidental, homicidal, and suicidal cases, homicide and suicide cases were found to have a substantially higher prevalence of hepatic steatosis than the accidental cases. On the basis of these data, we chose to only include accidental cases in this study. Furthermore, this study excluded cases with hospitalization exceeding 1 day to eliminate the impact of prolonged resuscitation on liver histology. These features in the methodology allowed this study to circumvent the limitations of previous studies and to measure the prevalence of hepatic steatosis with minimal bias.
In conclusion, this study reported the prevalence of fatty liver histology in the general population, stratified by age, BMI, alcoholism, and rs738409 genotype. The finding of additive interaction among rs738409, obesity, and alcohol towards NAS may be useful in targeting preventative care to patients at highest risk for ALD. The negative interaction between alcohol and obesity towards fibrosis justifies future research to explore potentially antifibrotic mechanism of alcohol, and may lead to improved treatment to prevent fibrosis.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
The histological prevalence of fatty liver disease among alcoholics and nonalcoholics in the general populationremains unknown.
The interaction of alcohol, obesity, and rs738409 towards fatty liver remains controversial
WHAT IS NEW HERE
This study assessed the prevalence of hepatic steatosis, steatohepatitis, and advanced fibrosis among alcoholics and nonalcoholics using a population-based autopsy study.
Alcohol, obesity, and rs738409 are additive towards NAFLD activity score.
Alcohol and obesity has negative interaction towards fibrosis
Acknowledgments
Financial support: This work was supported by Clinical/Translational Research in Liver Disease Pilot Project.
Footnotes
Potential competing interests: None.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data GUSDoHaHS, Centers for Disease Control and Prevention: Atlanta, GA, 2008. [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000;132:112–7. [DOI] [PubMed] [Google Scholar]
- 4.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol 2005;3:1260–8. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Fukatsu M, Suzuki S et al. Alcohol drinking may not be a major risk factor for fatty liver in Japanese undergoing a health checkup. Dig Dis Sci 2010;55:176–82. [DOI] [PubMed] [Google Scholar]
- 6.Zhou YJ, Li YY, Nie YQ et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol 2007;13:6419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu XL, Luo JY, Tao M et al. Risk factors for alcoholic liver disease in China.World J Gastroenterol 2004;10:2423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczepaniak LS, Nurenberg P, Leonard D et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–8. [DOI] [PubMed] [Google Scholar]
- 9.Alatalo PI, Koivisto HM, Hietala JP et al. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr 2008;88:1097–103. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Balkwill A, Reeves G et al. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart CL, Morrison DS, Batty GD et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechsler H, Austin SB. Binge drinking: the five/four measure. J Stud Alcohol 1998;59:122–4. [DOI] [PubMed] [Google Scholar]
- 13.Flowers NT, Naimi TS, Brewer RD et al. Patterns of alcohol consumption and alcohol-impaired driving in the United States. Alcohol Clin Exp Res 2008;32:639–44. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Deutsch R, Kahen T et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–93. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM. Pathology of fatty liver disease. Mod Pathol 2007;20 (Suppl 1): S40–8. [DOI] [PubMed] [Google Scholar]
- 17.Trepo E, Gustot T, Degre D et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol 2011;55:906–12. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep 1998;47:1–16, 20. [PubMed] [Google Scholar]
- 19.Naveau S, Giraud V, Borotto E et al. Excess weight risk factor for alcoholic liver disease. Hepatology 1997;25:108–11. [DOI] [PubMed] [Google Scholar]
- 20.Lelbach W Epidemiology of alcoholic liver disease In: Popper HSF (eds). Progress in Liver Disease Vol 5 Grune and Stratton: New York, 1976, pp 494–515. [PubMed] [Google Scholar]
- 21.MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis 1986;6:221–32. [DOI] [PubMed] [Google Scholar]
- 22.Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic and clinical aspects. Alcohol Clin Exp Res 1991;15:45–66. [DOI] [PubMed] [Google Scholar]
- 23.Dunn W, Xu R, Schwimmer JB. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology 2008;47:1947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn WBE, Sanyal AJ, McCullough et al. Modest alcohol consumption is associated with a decreased prevalence of steatohepatitis in patients with nonalcoholic fatty liver disease. Hepatology 2009;50:390A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cynthia A, Moylan AD, Pang H. Modest alcohol consumption attenuates expression of fibrosis-associated genes in patients with non-alcoholic fatty liver disease (NAFLD). Gastroenterology 2011;140:S914–5. [Google Scholar]
- 26.Romeo S, Kozlitina J, Xing C et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian C, Stokowski RP, Kershenobich D et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet 2010;42:21–3. [DOI] [PubMed] [Google Scholar]
- 28.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide. NIH Publication No. 05-3769. . The Institute: Bethesda, MD: 2005. [Google Scholar]
- 29.Amarapurkar A, Ghansar T. Fatty liver: experience from western India. Ann Hepatol 2007;6:37–40. [PubMed] [Google Scholar]
- 30.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990;12:1106–10. [DOI] [PubMed] [Google Scholar]
- 31.Zois CD, Baltayiannis GH, Bekiari A et al. Steatosis and steatohepatitis in postmortem material from Northwestern Greece. World J Gastroenterol 2010;16:3944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]