Abstract
Background
The EU Paediatric Regulation was introduced in 2007. In the United States, specific paediatric legislation has existed for even longer. This overview describes the similarities and differences in the legislation and provides input on how to achieve a global, harmonized pediatric development plan.
Objectives
The overview aims to investigate, through discussions and case examples, how to achieve pediatric medicines development fulfilling the expectations of the authorities as well as sponsors.
Methods
The pediatric legislation used in the European Union and United States are compared, and case studies for pediatric development plans where a global harmonized plan was eventually achieved are discussed.
Results
The case studies demonstrate some difficulties in getting to the goal of globally aligned pediatric plan development; however, recent initiatives from EMA and FDA are to a large degree addressing such challenges.
Conclusions
Global pediatric drug development is a evolving field, and with recent initiatives from the European Medicines Agency and US Food and Drug Administration, this goal is definitively attainable. (Curr Ther Res Clin Exp. 2019; 80:XXX–XXX).
Key words: European Medicines Agency, Food and Drug Administration, Globally aligned pediatric drug development, Pediatric study plan, Pediatrics, Paediatric Investigation Plan
Introduction
The EU Paediatric Regulation was introduced in 2007 and has had its 11-years birthday. In the United States, pediatric legislation has existed for even longer, and the introduction of the EU Paediatric Regulation was heavily inspired by the US pediatric legislation.
Pediatric legislation in the United States was most recently updated in 2013 with the Food and Drug Administration (FDA) Safety and Innovation Act, which among other things made 2 pediatrics-specific acts—the Pharmaceutical Research Equity Act (PREA) and Best Pharmaceuticals for Children Act (BPCA)—permanent.
Whereas some notable differences exist in the pediatric legislation between the 2 regions, there are still more similarities. The experience that is building up is that it is definitively possible in the vast majority of cases to make a global pediatric program that is acceptable to both the Eurpoean Medicines Agency/Paediatric Committee (EMA/PDCO) and the FDA, thereby avoiding the risk of duplicate or meaningless trials in the vulnerable pediatric population.
The EMA and FDA have a close collaboration on pediatric development plans and pediatric strategies and have been holding monthly pediatric cluster teleconferences since 2007. This cooperation is constantly evolving and new initiatives from EMA and FDA for a harmonized approach on pediatric development plans make the common goal of a single, aligned pediatric plan feasible. This overview will discuss similarities and differences in EMA and FDA handling of pediatric development plans and will go through a few case examples from actual Paediatric Investigation Plan (PIP) procedures that were cumbersome but offer important things for us to learn. Furthermore, the authorities’ initiatives for global alignment of pediatric development plan requirements will be discussed. For list of abbreviations, see Table 2.
Table 2.
List of abbreviations.
| BPCA | Best Pharmaceuticals for Children Act |
| CDER | Center for Drug Evaluation and Research |
| CHMP | Committee for Human Medicinal Products |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FDASIA | FDA Safety and Innovation Act |
| iPSP | initial Pediatric Study Plan |
| PDCO | Paediatric Committee |
| PeRC | Pediatric Review Committee |
| PIP | Paediatric investigation plan |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PPSR | Proposed Pediatric Study Request |
| PREA | Pharmaceutical Research Equity Act |
| PRIME | Priority medicines |
| PSP | Pediatric study plan |
| SAWP | Scientific Advice Working Party |
| TGA | Therapeutic Goods Administration |
| T2DM | Type 2 diabetes mellitus |
Background
The EU Paediatric Regulation1 came into force on January 26, 2007, and has now celebrated its 11th birthday. A 10-year report on the accomplishments of the EU Paediatric Regulation was issued by the EU Commission in October 2017.2 The objective of the Regulation is to improve the health of children in Europe by facilitating the development and availability of medicines for children from birth to age 18 years. The Paediatric Regulation requires PIPs to be submitted to the EMA for evaluation and approval by the PDCO.
In the United States, pediatric legislation has existed for many more years, and the introduction and content of the EU Paediatric Regulation was heavily inspired by US pediatric legislation.
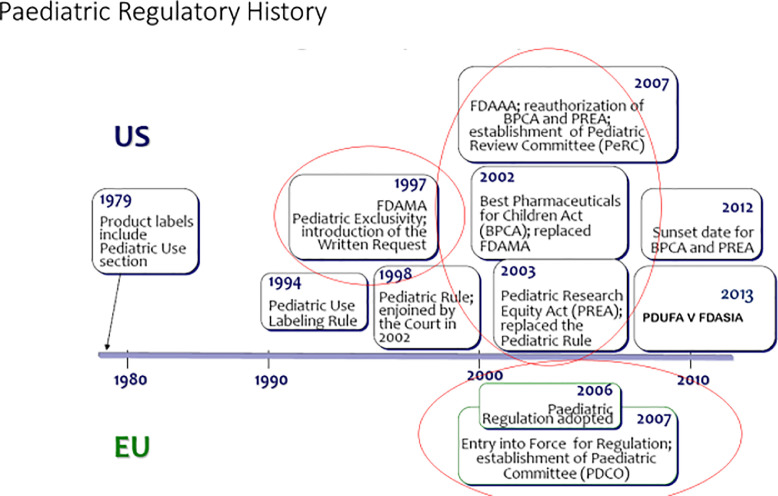
Pediatric legislation in the United States was most recently updated in 2012 with the Safety and Innovation Act, which includes pediatric provisions that among other things makes 2 pediatrics-related acts—PREA and BPCA—permanent3 (Figure 1). Some of the main similarities and differences of the US and EU pediatric legislations are depicted in Table 1.
Figure 1.
Paediatric regulatory history in EU and US.
Table 1.
Similarities and differences in the pediatric legislation in the United States and European Union. Table provided by European Medicines Agency (EMA) and/or US Food and Drug Administration (FDA).
| US Best Pharmaceuticals for Children Act | US Pharmaceutical Research Equity Act | European Union | |
|---|---|---|---|
| Development | Optional | Mandatory | Mandatory* |
| Instrument | Written Request | Pediatric Study Plan | Paediatric Investigation Plan |
| Waiver | N/A | 3 grounds | 3 grounds |
| Timing | Any time adequate information available | End of Phase II | > End of Phase I |
| Reward | 6-mo exclusivity | – | Main: 6-month SPC extension (patent) |
| Orphan products | Included | Excluded | Included |
| Decision | FDA | FDA | EMA [not European Commission (EC)] Opinion: Paediatric Committee |
| Scope of pediatric development | Not limited to adult indication | = adult indication | = adult indication |
| Scientific advice | Normally in global fee | Normally in global fee | Free for paediatrics |
Optional for off-patent.
In the European Union, the Paediatric Regulation is applicable for new active substances and for already authorised products still covered by a patent whenever a new indication, pharmaceutical form, or route of administration is being developed. In these cases, a PIP or a waiver must be submitted to EMA for evaluation by the PDCO.
Whereas in the European Union, there is a sinle unified Paediatric Regulation, paediatric legislation in the United States consists of 2 separate acts, the PREA and the BPCA.
For drugs and biologics falling under PREA, a mandatory initial Pediatric Study Plan (iPSP) must be submitted to the FDA in case of development for a new active ingredient, new indication, new dosage form, new dosing regimen, or a new route of administration. Orphan products are exempt from the requirement for submitting an iPSP. The PREA does not provide for any reward (eg, exclusivity).
The BPCA provides a financial incentive to companies to voluntarily conduct pediatric studies. Under the BPCA, a sponsor may request the FDA to issue a written request (WR) by submitting a Proposed Pediatric Study Request (PPSR). Although the PPSR is voluntary and can be retracted at any time, the reward (ie, 6-month extra exclusivity added to the patent) will only be granted if the sponsor completes the FDA requirements in the issued WR (ie, submits completed studies to fulfill the WR). The studies suggested in the PPSR may be the same studies included in the PSP, new studies, or even for new indications.
Much like the situation for the EU PIP, where the PDCO may request development for an indication not pursued in adults (yet still a part of the overall condition, as explained in the policy on determination of scope of PIPs4), the FDA may also request development of a new indication in the WR, if an unmet need in the pediatric population is identified and the product may fulfill this unmet need.
If a mandatory PSP is made under PREA, the sponsor may anyway become eligible for the reward (ie, 6 months exclusivity) by proposing studies in a BPCA WR (PPSR) procedure.
Content of pediatric development plans: Differing expectations on level of detail?
It is a common misperception that the FDA requires less-detailed information in the iPSP than does EMA/PDCO in the PIP. In particular, since the Safety and Innovation Act update of the pediatric legislation in the United States, which also introduced more structured requirements for the timing and content of the iPSPs, as further detailed in the draft guidance on PSPs,5 the expectations have become very similar. The experience from PSPs completed in recent years is definitively that the FDA requires as much detailed information on the product, on justifications for the planned development, on formulations, nonclinical studies and pediatric clinical trials, and on timelines as does the PDCO. For this reason, and to enhance the chances for an aligned global development (discussed in the later section on Pediatric Cluster Meetings), it is highly recommended that applicants include exactly the same information in the iPSP as in the PIP.
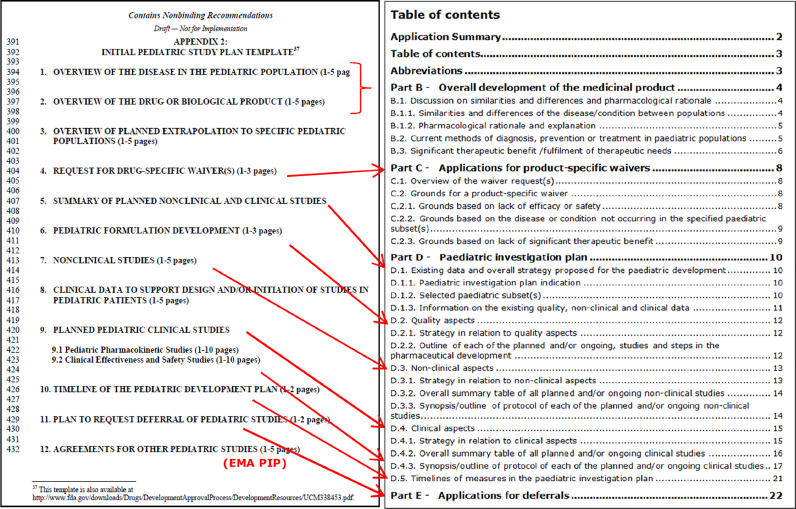
Figure 2 compares the table of contents taken from the PSP and PIP template issued by FDA and EMA, respectively. The requested content in the 2 documents is very similar. The similarity is not a coincidence, because EMA was consulted for comments before FDA releasing the draft 2016 guidance,5 which also includes the iPSP template.
Figure 2.
Table of contents derived from the iPSP (left) and PIP (right) templates. As can be seen, the requested content is very similar. Figure provided by EMA and/or FDA.
The PPSR should contain a rationale for studies, a detailed study design, and a discussion on nonclinical studies and on whether there are appropriate formulations for each age group. Experience with recent PPSRs is that the FDA expects inclusion of an extended trial outline/protocol submitted with the PPSR. The requested format for the PPSR is less structured than for the PSP; however, the FDA WR letter template6 includes guiding questions. The format of the PPSR may be done in a similar way to the PSP and PIP to ensure that the level of information is sufficient.
Timing of submission
The EU Commission is following the evolvement of late PIP submissions. The consequence of submitting a PIP late is generally that the company will be named in the yearly EU Commission report as having submitted a PIP late. Evidently, a late PIP submission may also confer a risk to the company of delay of the marketing authorisation or of additional costs caused by execution of a program that turned out not to be what the PDCO expected.
According to Article 16 of the Paediatric Regulation,1 the PIP should be submitted, unless duly justified, “not later than upon completion of the human pharmacokinetic (PK) studies,” which is seen as about the time of end of Phase I.
In the United States, for drugs and biologics falling under the purview of the FDA Center for Drug Evaluation and Research, submission of an iPSP is required by, at the latest, 60 days after the end-of-Phase II meeting, or if no such meeting is held, before the Phase III/combined Phase II and III study and at least 210 days before submission of the marketing application.5
This discrepancy in timing of submission requirements between the 2 regions has caused concern in the pharmaceutical industry because submitting the plans to the 2 agencies at different points in time reduces the chance of obtaining a sinlge, harmonized pediatric plan agreed to by both agencies without having to spend considerable resources in laborious PIP modifications/PSP amendments.
However, in practice, the difference in the EU and US legislation with regard to timing of pediatric plan submission has not caused any major problems, and submissions can very well be aligned.
Legal input from the EU Commission to when PIP submissions would be considered late led to the following statement on the EMA website regarding questions and answers for PIP submissions: “Submitting a PIP application for a new active substance during confirmatory or phase-III trials in adults, or after starting clinical trials in children, is likely to be considered unjustified” (https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans/paediatric-investigation-plans-questions-answers#applying-for-a-pip,-waiver-or-deferral-section, Accessed February 18, 2019). This should be interpreted in the way that submission of the PIP before start of Phase III or before start of clinical trials in children will be acceptable and will not legally be regarded as a late PIP submission. Therefore, it is possible to align the PIP and PSP by submitting both plans at about the same time; for example, by the end of Phase II.
A recommended way to do it would be, by the end of Phase II (or even earlier if it makes sense and is accepted by the FDA), first to submit the PIP to EMA/PDCO, and then, after the PDCO Day 60 Request for Modification has been received, to submit the iPSP to FDA during PIP clock-stop, updated according to the comments received from EMA/PDCO. The PSP approval procedure may then be finalized while the PIP is still in clock-stop. This way of doing it is much more practical than getting a PIP agreed upon early and then submitting an iPSP to FDA much later, with high risk of the need for a later PIP modification due to FDA not agreeing to the content.
If completed in the above-described way, it will be secured that the PIP and PSP approval procedures are open and running simultaneously and particularly during PIP clock-stop. This will allow sponsors to adjust a pediatric plan to both agencies’ expectations, and will also allow the possibility of discussions between FDA and EMA, as described later. Thereby, the sponsor optimizes the conditions for reaching consensus from the 2 agencies on the requirements for the pediatric development plan and will make the process as uncomplicated as possible.
Pediatric cluster meetings to facilitate global alignment of paediatric plans
The so-called pediatric cluster meetings were established in 2007 shortly after the first PDCO meeting took place. The cluster meetings are monthly teleconferences held between EMA and FDA, and also representatives from the Canadian, Japanese, and Australian agencies participate. The objective of the cluster is to help supporting global development plans for pediatric medicinal products and also to exchange information on applications and topics related to pediatric medicines development.
The agencies have mutual confidentiality agreements and each month, exchange documentation on ongoing procedures. Ongoing PIP, iPSP, and PPSR applications are discussed at the meetings, where the FDA and EMA strive to seek a harmonized approach and, whenever possible, consensus on their requirements for the individual plans. In this way, the pediatric cluster meetings can assist in creating a true global development program for the pediatric population. Within the pediatric cluster setting, 438 products and 138 more general topics have been discussed in the period from 2007 to 2017 (personal communication with Irmgard Eichler, EMA; May 15, 2018).
Common commentary
The common commentary is a tool to inform sponsors in written of products discussed about which the FDA and EMA have come to an agreement on the suggested strategy at the pediatric cluster meeting. Until now, the common commentary has mostly been on products for life-threatening diseases or plans with major FDA–EMA differences. The process works such that EMA and FDA identify issues for discussions that could be, for example, study design or timing or even more fundamentally the whole pediatric plan strategy. After the pediatric cluster discussion, an approved 1- to 2-page common commentary document will be sent to the sponsor for information. The agencies are stressing that the comments are not binding. Between 2012 and 2015, 25 issues were discussed in the common commentary process (personal communication with Irmgard Eichler, EMA; May 15, 2018).
Some recent experience from industry side with the common commentary process is that it is may appear somewhat bureaucratic, at least for cases where the common commentary has been requested by the sponsor for a full pediatric development program. In a particular case, the written common commentary took a month to be issued from the time the pediatric cluster meeting took place, despite the fact that it had been requested early—this caused a 1-month delay for the PIP procedure restart.
Other regions
The European Union and United States are the only 2 regions with dedicated complete pediatric legislation. In other regions, some pediatric provisions exist. In Canada, a 6-month extension for data protection is granted to innovator companies providing evidence to support a pediatric label indication. In Japan, there is no comprehensive legislation on pediatric medicines. There are some incentive schemes, priority for scientific advice by the Pharmaceuticals and Medical Devices Agency, and there is a national network for pediatric clinical studies. In Australia, there is no formal legislative and regulatory legislation addressing pediatric medicines. In Switzerland, a 6-month pediatric supplementary protection certificate extension is becoming possible. Furthermore, a new law relating to pediatric development will be enforced in 2019, with a requirement for sponsors submitting marketing authorization applications to have a pediatric plan agreed upon. PIPs and PSPs will be accepted for such purpose.
Discussion
How to facilitate global alignment
From an applicant's side, several things can be done to facilitate the process and do the best to ensure a global, aligned review with an optimal outcome.
-
•
If time allows, going through an EMA–FDA parallel scientific advice procedure7 before finally drafting and submitting the PIP and iPSP will facilitate streamlining of the pediatric development program. In particular for pediatric plans for medicines that are first in class or where an innovative or controversial approach is suggested, it may be of benefit. The EU Paediatric Regulation allows for free scientific advice for all questions relating to the pediatric drug development. It should be remarked that pediatric scientific advice agreed by the Scientific Advice Working Party and endorsed by the Committee for Human Medicinal Products (responsible for assessing marketing authorization applications), is regarded as important in the PDCO PIP assessment. There is a close collaboration between PDCO and Scientific Advice Working Party, and PDCO is involved in pediatric scientific advice, including the parallel scientific advice.
-
•
It is important to include exactly the same information in the PIP and iPSP, and to be aware of informing both agencies of all ongoing activities, ensuring full transparency and not risking giving the agency the impression that one is hiding important information. This will set optimal conditions for the EMA–FDA collaboration on the pediatric plan.
-
•
One should aim to submit the PIP and iPSP at about the same time (eg, submit PIP first and then iPSP in clock-stop), thereby planning that the EMA/PDCO and FDA approval procedures are open and running simultaneously. This enables the FDA– EMA pediatric cluster discussion (which in most cases will take place during PIP clock-stop), and enhances the chance of the agencies agreeing between each other on the pediatric plan. If, on the other hand, the PSP for some reason or another is made after the PIP has already been approved, then one should beware and do the utmost to keep compliance with the PIP; for example, by informing the FDA on the already agreed PIP measures. If compliance cannot be kept, a future PIP modification will have to be submitted to the EMA, with the inherent risk that the PDCO will not accept the modification request.
-
•
To ensure that EMA and FDA are discussing the specific pediatric plan, it is advisable to propose to the EMA coordinator that the PIP is taken up for discussion at the FDA–EMA pediatric cluster meeting, and to request this as early as possible, as soon that the need has been identified. After the cluster discussion of the pediatric plan, EMA will include the outcome of the discussion in the PIP summary report. During PIP clock-stop, meetings may also be arranged with the individual agencies to get input on how to solve discrepant viewpoints between the agencies.
Even more EMA–FDA alignment needed
Although it is clear that there is close collaboration between EMA and FDA on pediatric drug development, even more alignment is needed. The below cases illustrate some of the many obstacles that one can run into in the attempt to align the PIP and PSP/WR to both PDCO and FDA expectations.
Case 1
A product for type 2 diabetes mellitus (T2DM) that had an already agreed-upon PIP and WR had to be amended due to severe recruitment difficulties in an ongoing pediatric trial.
For T2DM, EMA/PDCO and FDA generally agree to grant waivers for the population younger than age 10 years, due to the disease not occurring (or extremely rare), whereas in children aged 10 years and older, they request at least the conduct of a safety/efficacy pediatric trial.
Disease control is not deemed sufficient in T2DM children with the current therapeutic options, with products approved in the paediatric population mostly limited to metformin and insulin. Whereas various new treatment options exist for adult populations, the same is still not seen for children.
Conduct of T2DM pediatric trials is challenging. Many simultaneous drug development programs are ongoing, causing competition for patients in these programs. There are few available patients in total for all the studies; although the prevalence of T2DM in pediatric populations is rising, there are still many fewer pediatric than adult patients.8, 9 There are considerable compliance difficulties with such trials. T2DM disproportionally affects children who are difficult to recruit or unwilling to participate in clinical research studies.10 There appears in many regions to be a lack of access to trained pediatric endocrinologists, and no adequately developed pediatric clinical research infrastructure.11
Because of all this, ongoing paediatric T2DM trials have been challenged by recruitment difficulties and the resulting delays.
FDA and EMA are aware of the recruitment challenges in pediatric T2DM trials and have made a number of initiatives to address this. A workshop was held within EMA, with FDA invited, on T2DM PIPs during May 2013.12 The workshop resulted in several proposals for enhancing recruitment in pediatric T2DM trials.
Suggestions raised were, among others, a broadened role for extrapolation (for efficacy) and for sample size reduction; for example, by use of Bayesian methods with adult priors, by having lower significance levels, or even by conducting a safety study only. Also, broadening of inclusion criteria was suggested, for among other things insulin treatment and for age, where it was suggested to include young adults up to age 22 or 25 years in the pediatric trials. Furthermore, multicompany studies testing several products from different companies against a single placebo group was suggested, as well as single-company studies with multiple agents.
The reasons for screening ineligibility hat contributed to recruitment challenges in the pediatric efficacy/safety trial were identified. This led to a regulatory decision to amend the protocol, broadening the inclusion criteria. This had to be negotiated with the FDA and EMA via amendment of the WR and a PIP modification, and afterward to amend the protocol and get it approved by FDA and by the national competent authorities in European Union and other areas. Although many things were tried to speed up the process and not loose more time, the regulatory activities for modifications to the pediatric T2DM trial to improve recruitment took altogether 1.5 years. The sequence of the regulatory activities were:
-
•
Due to recruitment challenges, it was proposed to amend the protocol to FDA,
-
•
Agreement with FDA on trial changes plus deferral,
-
•
EMA PIP modification presubmission meeting,
-
•
PIP modification submission,
-
•
FDA WR amendment submitted with updated protocol,
-
•
PIP modification agreed by PDCO,
-
•
FDA 120-day protocol review final and agreed, and finally
-
•
Clinical trial application (CTA) amendments.
While the process was ongoing, the sponsor negotiated a PIP for another T2DM product with the PDCO. In the day 60 response to this other PIP, PDCO did on its own initiative suggest an innovative approach that could lead to a reduced sample size, stating that this was in the of the scarce patient population and the acknowledged competition for patients. Stating that pediatric T2DM patients are quite similar to adult patients with regard to weight and general pathophysiology, and that young adults behave more like adolescents than adults with T2DM, PDCO was open to an alternative study design, such as a Bayesian approach with adult priors. It was suggested by PDCO to include patients of a broader age range (eg, 10–25 years) to make the study more feasible.
Inspired by PDCO feedback for this other T2DM PIP, the sponsor made a proposal to the FDA for the ongoing pediatric efficacy/safety trial to change the current patient age range from “10 to 16 years, subjects cannot turn 17 years and 11 months before the end of treatment (52 weeks)” to: “Children, adolescents and young adults between the ages of 10 to less than 25 years at the time of randomization.” However, the clear response to this proposal was that FDA did not agree to use young adult data to inform safety in the pediatric population. This was based on both scientific and legal discussions concerning that a young adult cannot be considered a child, among other issues. The end result was that the initiatives from EMA/PDCOs side for broadening the inclusion criteria with regard to age could in reality not be executed due to the global pediatric program needing to comply with requirements from both regions.
Case 2
A product with a new, innovative approach submitted a PIP to EMA, and after receipt of the day 60 request for modification from PDCO, the iPSP document was updated with fulfilment of the largely uncontroversial PDCO requests and then submitted to FDA during PIP clock-stop.
After 90 days, the initial FDA response to the iPSP was received. It differed considerably from the PDCO viewpoint: among other things the waiver was challenged, the safety/efficacy trial design was substantially challenged, and a proposed pharmacokinetic study was suggested to be omitted.
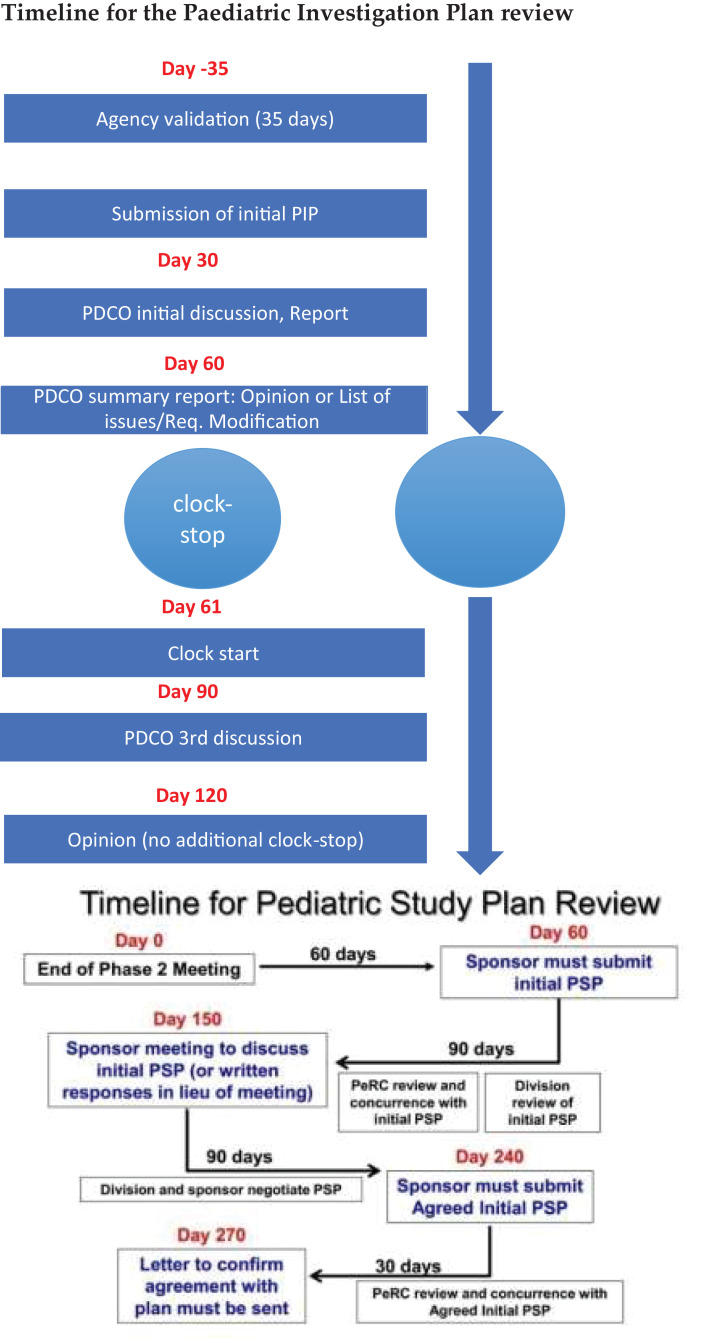
Due to the PSP timelines, which in contrast to the PIP timelines do not include any formal clock-stop (see Figure 3), and due to short time to complete the PSP process, FDA provided the applicant with rather short time—30 days—to respond to their comments and requested changes. However, the quite substantial change requests needed to be confirmed with the PDCO to ensure that they would agree on such changes. Because 30 days was not sufficient time to get into agreement with the PDCO on this, the sponsor had to request to the FDA that the iPSP be put on hold while PDCO was consulted. This request was granted.
Figure 3.
Timelines for PIP and PSP process. PSP review slide provided from the FDA.
The sponsor then submitted a suggestion for a revised compromise study design to EMA and requested that it be discussed at the next EMA–FDA pediatric cluster meeting. EMA agreed to discuss the revised design with FDA, and following the EMA–FDA cluster discussion, a teleconference was arranged with EMA, the PDCO rapporteur, and peer reviewer to discuss the outcome. PDCO representatives expressed that they and FDA representatives were amenable to the suggested trial design changes, so the sponsor updated the iPSP accordingly and resubmitted it to the FDA. After 3 months, an FDA response was received, saying that PeRC review of the iPSP was still awaited.
PeRC is an internal FDA expert committee established to carry out activities related to PREA and BPCA. As seen in Figure 3, PeRC review is an integral part of the PSP process, and will typically discuss such questions as, What is the public health benefit? Are the study designs feasible and sufficient to support dosing, safety, and efficacy? Have all populations and conditions been addressed? and, Are there other products already approved for the condition? In contrast to the PDCO, which has an approving role, PeRC is an advisory committee, and PDCO has the final authority to decide on the PSP.
Returning back to Case 2, the PeRC review comments were received an additional 1-month later, and they substantially changed the whole program. Despite the earlier discussion of the proposal at the pediatric cluster meeting, the PeRC comments did not seem to take this fully into account with regard to waiver and study design. At that point in time, there was a misalignment between the sponsor's suggestions, what PDCO had expressed that they could agree on, and what FDA requested. To solve this, the applicant proposed a joint teleconference between all 3 parties, and even suggested that PeRC members participate; however, a joint teleconference was unfortunately not agreed to by FDA. Therefore, teleconferences had to be arranged with FDA and EMA separately to discuss the program.
A new compromise suggestion was submitted to both agencies, and finally, 1.5 years after the initial PIP submission, FDA agreed to the PSP. Following that, the PIP could be updated and resubmitted for restart of procedure. At this point, the time to submit the marketing application was fast approaching and it was critical that the PSP and PIP be finally approved because the marketing applications would otherwise not have been accepted by either agency.
Case 3
This case concerns a pediatric development program where the sponsor proactively attempted to align the global pediatric development program to both EMA and FDA expectations by requesting parallel FDA–EMA scientific advice on their pediatric plan before the PIP and iPSP were prepared. In this way, the sponsor anticipated that potential difficult discussions, in particular on the pediatric formulation and the nonclinical program, could be solved proactively and thus ease the PIP and iPSP procedures, minimizing the risk of the PIP and iPSP taking divergent directions.
However, in this case, EMA agreed to parallel advice, whereas FDA, due to being too busy, did not agree. The sponsor then decided instead to run separate but concurrent scientific advice procedures with both agencies.
Based on the above cases, the following conclusions were drawn:
-
•
Consider having parallel FDA–EMA scientific advice before PIP/iPSP preparation, if possible, and in particular for pediatric plans with anticipated complex issues.
-
•
Agencies may talk amongst each other when asked; however, there seems to be a certain risk of further requests coming afterward.
-
•
Try to align with meetings FDA–EMA and company–EMA. However, one should wait with these meetings until after PeRC review, which may change the full program.
-
•
FDA accepted in several instances an PSP hold for alignment with the PIP. This can evidently not usually be expected because it is not part of the official timelines. However, it shows that FDA may show flexibility in cases where alignment becomes difficult.
EMA–FDA alignment initiatives
A European Union–United States strategic meeting on the future of pediatric medicine was held at EMA offices in September 2016 with the participation of the EU Commission, EMA, and FDA. Minutes can be found on the EMA website.13 Many of the issues discussed at the meeting relate directly to wishes that industry has expressed. Furthermore, many of the difficulties experienced in the above-discussed cases could have been avoided if the below initiatives had already been introduced. Among the issues discussed were direct interactions with sponsor/joint tripartite meetings and implementing a common commentary that includes input from Canada, Japan, and Australia.
Among the complicating factors in Case 2 described above was the apparent lack of clear communication between the agencies and, in particular, that a tripartite meeting could not be held among sponsor, EMA, and FDA. If this can be solved in the future, it will make the process much easier, especially in cases where the FDA and EMA requirements for the individual pediatric development plan have moved in divergent directions. It was also decided at the strategy meeting that the common commentary process going forward will incorporate input from regulators in Canada, Japan, and Australia. This will aid in making the pediatric drug development truly global.
As a follow-up to the strategy meeting, new initiatives have been implemented:
-
•
Members of PDCO and the Paediatric Medicines Office have the opportunity to call into the weekly PeRC meeting and vice-versa for PeRC members to remotely participate in PDCO discussions.
-
•
Joint EMA–FDA collaboration during early pediatric interactions within a pediatric cluster, to issue a common commentary with the goal to mitigate the risk of pediatric plan assessments by EMA and FDA moving in divergent directions.
-
•
Jointly organized EMA–FDA workshops (the first, on pediatric pulmonary arterial hypertension was held during June 2017 at the EMA). Jointly arranged workshops is a great initiative for reaching consensus and looking forward together.
Conclusions
The collaboration between EMA and FDA on the pediatric medicines development is very intense and is becoming more elaborate. The goal of a common, global pediatric development plan is possible. However, as the described cases illustrate, it can also be cumbersome. The many initiatives taken by the authorities for further aligning the collaboration on pediatric medicines development is certainly making this goal more easily attainable.
Acknowledgments
I wrote and revised it all by myself, and I was not supported by any monetary support.
Conflicts of Interest
The author has indicated that she has no conflicts of interest regarding the content of this article.
References
- 1.Regulation (EC) No. 1901/2006, of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use. https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed 4 May 2018.
- 2.Report from the Commission to the European Parliament and the Council. State of Paediatric Medicines in the EU. 10 years of the EU Paediatric Regulation. COM (2017) 626. https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf. Accessed 8 May 2018.
- 3.FDA Fact Sheet: Pediatric provisions in the Food and Drug Administration Safety and Innovation Act (FDASIA). https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/ucm311038.htm. Accessed 7 May 2018.
- 4.EMA/PDCO Policy on the determination of the condition(s) for a Paediatric Investigation Plan/Waiver. 30 July 2012 EMA/272931/2011 Human Medicines Development and Evaluation. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/09/WC500133065.pdf. Accessed 8 May 2018.
- 5.FDA Guidance for industry. Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Initial Pediatric Study Plans Guidance for Industry. Draft. 2016 [Google Scholar]
- 6.FDA: WR letter template. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM570144.pdf. Accessed 7 May 2018.
- 7.GENERAL PRINCIPLES EMA-FDA PARALLEL SCIENTIFIC ADVICE (HUMAN MEDICINAL PRODUCTS). EMA, FDA 2017. https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/OfficeofInternationalPrograms/UCM557100.pdf. Accessed 8 May 2018.
- 8.Dabelea D., Mayer-Davis E.J., Saydah S. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency European Medicines Agency (EMA) Report of the Pediatric Workshop on Pediatric Investigation Plans (PIP) in type 2 diabetes mellitus. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/05/WC500143022.pdf Available from.
- 10.Ortiz B., Guerrero S. Recruitment and Retention of Minority Populations in Clinical Trials. In: Mulberg A.E., Murphy D., Dunne J., Mathis L.L., editors. Pediatric Drug Development: Concepts and Applications. John Wiley& Sons Ltd.; Chichester, U.K.: 2013. [Google Scholar]
- 11.Karres J., Pratt V., Guettier J.M., Temeck J., Tamborlane W.V., Dunger D., Bejnariu C., Beaufort C.D., Tomasi P. Joining Forces: A Call for Greater Collaboration To Study New Medicines in Children and Adolescents With Type 2 Diabetes. Diabetes Care. 2014;37:2665–2667. doi: 10.2337/dc14-0494. [DOI] [PubMed] [Google Scholar]
- 12.Workshop on paediatric investigation plans in type-2 diabetes mellitus. EMA 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2013/01/event_detail_000695.jsp&mid=WC0b01ac058004d5c3. Accessed 7 May 2018.
- 13.EU-USA strategic meeting on the future of paediatric medicines. Meeting report. 26 October 2016 EMA/662807/2016. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2016/12/WC500218004.pdf. Accessed 8 May 2018.