Abstract
Autism Spectrum Disorder (ASD) is characterized by early onset of behavioural and cognitive alterations. Low plasma levels of oxytocin have also been found in ASD patients and recently, a critical role for the enzyme CD38 in the regulation of oxytocin release was demonstrated. CD38 is important in regulating several Ca2+ dependent pathways, but beyond its role in regulating oxytocin secretion, it is not known whether a deficit in CD38 expression leads to functional modifications of the prefrontal cortex, a structure involved in social behaviour. Here, we report that CD38-/- male mice show an abnormal cortex development, an excitation-inhibition balance shifted towards a higher excitation, and impaired synaptic plasticity in the prefrontal cortex such as those observed in various mouse models of ASD. We also show that a lack of CD38 alters social behaviour and emotional responses. Finally, examining neuromodulators known to control behavioural flexibility, we found elevated monoamine levels in the prefrontal cortex of CD38-/- adult mice. Overall our study unveiled major changes in prefrontal cortex physiological mechanisms and provides new evidence that the CD38-/- mouse could be a relevant model to study pathophysiological brain mechanisms of mental disorders such as ASD.
Keywords: Prefrontal cortex, anxiety, plasticity, oxytocin, calcium, autism, ASD, serotonin, dopamine, norepinephrine, excitation
Introduction
Autism Spectrum Disorder (ASD) covers a group of neurodevelopmental disorders characterized by behavioural and cognitive disturbances with early-onset deficits in language acquisition and social interaction. ASD is highly inherited but the genetic determinants are poorly understood. The genetic basis of ASD has been associated with copy-number variations (CNV) or single-nucleotide polymorphism (SNP) in genes involved in brain development, synapse formation or cell signalling (1). ASD is frequently associated with excessive cortical growth (2) and a current hypothesis is that the behavioural defects observed in ASD are due to impairments in synaptic functioning and plasticity (1, 3–5). For example, some mutations affect glutamatergic synaptic transmission such as for the SHANK proteins (4–6) or the neuroligins (3, 7). One important consequence of these mutations is that the mechanisms of synaptic plasticity are altered and affects ionotropic NMDA receptors and/or metabotropic glutamate receptors regulating learning processes and cognitive functions (8, 9). Recently the hypothesis of an increase in the excitation-inhibition (E-I) ratio in neuronal circuits emerged as a common pathophysiological principle that could explain the genesis of cognitive and social-behaviour deficits in ASD (10). Remarkably, experimental perturbation of the E-I balance in the prefrontal cortex (PFC) of mice reproduces autistic-like behavioural defects and therefore supports this hypothesis (11). Some mouse models of ASD have changes in the E-I balance in favour of a greater excitation (1, 10, 12–14). Recently oxytocin (OT) have been shown to play a crucial role in synapse maturation and in synaptic transmission (14–17).
Several studies have pointed toward a deficit in OT signalling in patients with ASD (18–22). Low plasma levels of OT have been found in ASD patients and, although a deficit in OT may not be a primary cause in ASD cases, several studies have suggested that such deficit likely contribute and worsen the social deficit observed in these patients (18, 21–24). The enzyme CD38, known for catalysing the production of second messengers upon G-protein-coupled receptor (GPCRs) (25–28), is an important player in social behaviour and is one of the major signalling components controlling the Ca2+-dependent secretion of OT (29, 30). Moreover, CD38 has been associated with familial forms of ASD with common single-nucleotide polymorphism (SNP; CD38 rs3796863) (21).
Identifying whether and how genetic defects associated with ASD alter the E-I balance of the PFC, cognitive functions and social behaviour constitutes an important challenge in the search of common therapeutic targets for this neurodevelopmental disorder. Considering that in patients the risk-factor SNP, CD38 rs3796863, results in low expression of CD38 and low plasma level of OT, we focused our present study on CD38-/- mice to determine whether CD38 loss of function, which reduces OT secretion, affects brain development, E-I balance and plasticity of the PFC, as well as cognitive, emotional and social processes. In the present study, we report that CD38-/- mice display macrocephaly and larger cortical layers, a shift of the E-I balance towards higher excitation, impaired synaptic plasticity and altered monoamines levels in the PFC. This is associated with enhanced anxiety-like responses, altered vocal communication and social behaviour with enhanced aggressiveness during social interactions.
Methods
Animals
Male C57BL/6 mice (CD38 sufficient) were purchased from CER Janvier. CD38-/- mice holding a deletion of exon 2 and 3 in CD38 gene were obtained from Frances Lund’s laboratory (Trudeau Institute, New York). The CD38-/- mice have been backcrossed 10 times onto the C57BL/6 inbred strain background and were shown to exhibit no residual enzymatic activity in vitro (31). The CD38-/- mice received from Frances Lund’s laboratory were crossed on regular basis with C57BL/6 mice to generate our colony. For genotyping, genomic DNA was isolated from mice tails using the Nucleospin tissue kit (Macherey Nagel). Exon 2 was amplified by PCR and products were analysed in agarose gel electrophoresis. C57BL/6 mice and CD38-/- mice were bred in our animal facility. For behavioural studies adult mice were > 3 months old and juvenile mice were P28-P30 days old. For each behavioural experiment, the apparatus was gently washed before each animal testing. Animal care and experimental procedures complied with the European Communities Council Directive (CEE 86/609/EEC), EU Directive 2010/63/EU, and local ethic committee (Paris Centre et Sud, N°59).
Magnetic Resonance Imaging
Mice were imaged under isoflurane anaesthesia (induction 2%; flow rate 0.8% in 50% O2, 50% N2O) controlled on the basis of respiratory parameters. The body temperature was maintained at 37 °C using a heated mattress. Magnetic Resonance Imaging (MRI) measurements were performed on a 7T horizontal bore magnet (Oxford, UK) driven by Paravision (Bruker®, Wissembourg, France) and equipped with a 300 mT/m actively shielded gradient device (internal diameter [ID] = 90 mm, Bruker). For MRI examination, the animal's head was introduced in a “bird-cage” 1-H coil (ID = 22mm). The mouse brain was positioned using scouting gradient-echo images in the 3 orthogonal directions. After the shimming process, three Turbo-RARE (Rapid Acquisition with Relaxation Enhancement) sequence with fat suppression were acquired: one perpendicular (axial) to the brain anteroposterior axis (TR/TE eff = 5000/40 ms [repetition time/effective echo time]; rare factor = 8; 2 averages; 37 contiguous 0.5-mm-thick sections; 20 × 20 mm field of view; 256 × 256 matrix; pixel size = 78 μm2] and two parallel (coronal and sagittal) to the brain anteroposterior axis [TR/TE eff = 5000/40 ms; rare factor = 4; 2 averages; 40*20 mm field of view; 512 × 256 matrix; voxel size = 78 μm2 and respectively 43 (sagittal MRI) and 35 (coronal MRI) contiguous 0.25 mm-thick sections]. The total time spent by the mouse in the magnet was around 25 min. After each experiment, mice were released from anaesthesia and returned to their home cages with free access to food and water.
Slice preparation and electrophysiological recordings
Coronal slices (250 μm thickness) of the PFC were obtained from P21 to P28 male mice. They were incubated for at least 1 h at 33 °C in an extracellular solution containing (in mM): NaCl, 126; NaHCO3, 26; glucose, 10; CaCl2, 2; KCl, 1.5; KH2PO4, 1.25; MgCl2, 2 (pH 7.4, 310-330 mOsm) and oxygenated continuously with a mixture of 95% O2 and 5% CO2. Electrical stimulations (1-10 μA, 0.2 ms duration) were delivered in layer 2-3 using 1 MΩ impedance bipolar tungsten electrodes (TST33A10KT; WPI). The intensity of the stimulation corresponded to 50 % of the maximum response. Somatic whole-cell recordings were performed in pyramidal neurons of layer 5 (L5PyNs) at 33 °C using borosilicate glass pipettes (3-5 MΩ resistance in bath) filled with a solution containing (in mM): K-gluconate, 140; HEPES, 10; ATP, 4; MgCl2, 2; GTP, 0.4; EGTA, 0.5 (pH 7.3 adjusted with KOH; 270-290 mOsm). Voltage-clamp recordings were performed using an Axopatch 1D (Axon Instruments), filtered by a low-pass Bessel filter with a cut-off frequency set at 2 kHz, and digitally sampled at 4 kHz. The membrane potential was corrected off-line by −10 mV to account for junction potential. The firing profile of patched neurons and their membrane resistance were obtained using 1 s depolarizing steps ranging from −100 to 200 pA. Only cells having a resting membrane potential more negative than –55 mV and with an access resistance lower than 25 MΩ were kept for further analysis. The access resistance was compensated off-line in voltage clamp mode and neurons exhibiting more than 10 % of access resistance were rejected. Analysis of the synaptic response and decomposition of synaptic conductance changes were performed as extensively described in previous publications (32–37).
Synaptic plasticity
A High Frequency Stimulation (HFS) protocol was applied in the PFC layer 2-3 with theta-burst stimulation (three trains of 13 bursts at 5 Hz, each burst containing four pulses at 100 Hz, for a total duration of 2 min, see Fig. 2D), while the L5PyN was under current clamp condition (I=0). Recordings of current responses in L5PyNs were then done as described above (frequency of stimulation 0.05 Hz), at 15, 30, 45 and 60 min after the beginning of the HFS protocol, for comparison with control recordings. Changes above 20% of control conductance before HFS protocol were considered as reflecting long-term potentiation (LTP), a long-lasting enhancement of neurotransmission that could be blocked by an NMDA-receptor antagonist (D-L-AP5, data not shown), thus demonstrating that LTP depended on activation of these receptors in our experimental conditions.
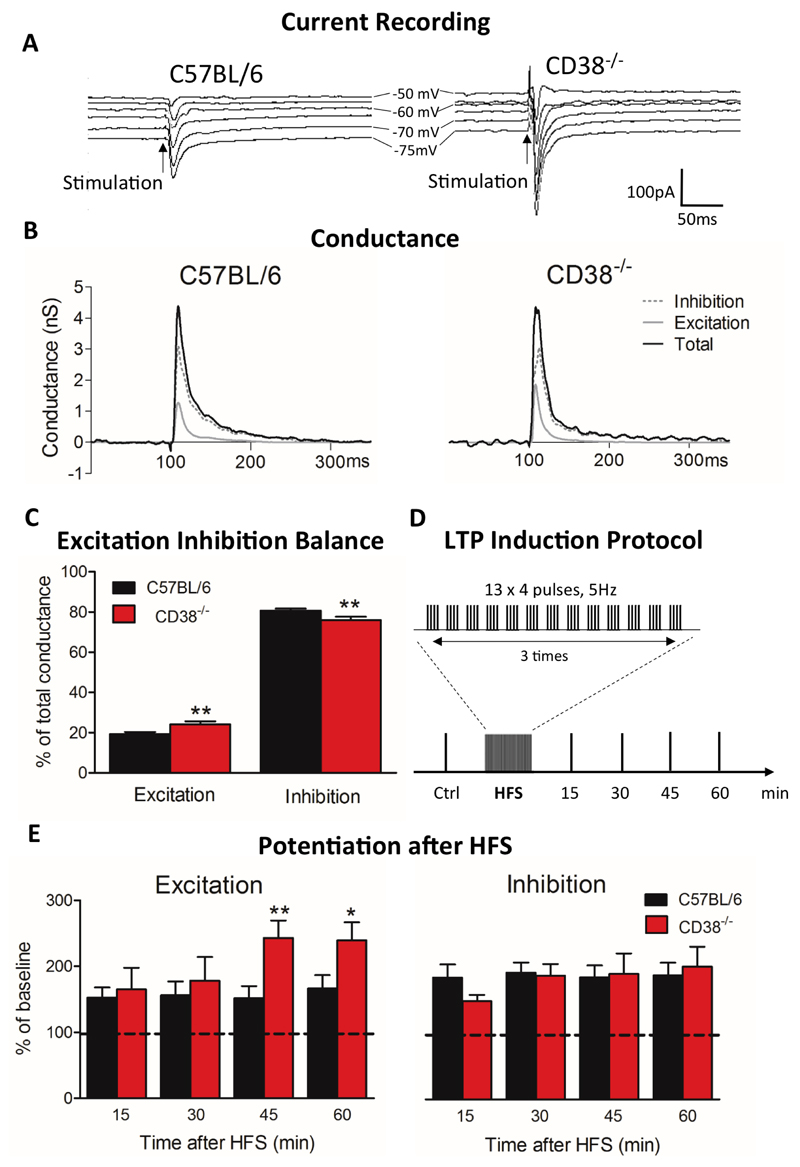
Figure 2. Impaired E-I balance in the PFC of CD38-/- male mice.
(A) Representative current responses in a L5PyN following layer 2/3 stimulation in PFC of a C57BL/6 mouse (left column) and of a CD38-/- mouse (right column), recorded under voltage-clamp at various holding potentials (each response is the mean of 5 recordings). Vertical arrows indicate stimulation onset. (B) Corresponding total conductance changes (black line). Changes in excitatory conductance (light grey line) and inhibitory conductance (dotted dark grey line) derived from total conductance decomposition (see Material and Methods). (C) Histograms of the E-I balance. The E-I balance changed significantly in CD38-/- mice (n=25 cells, 13 mice) compared with C57BL/6 mice (n=43 cells, 21 mice). Mann-Whitney, two tailed (**p<0.01). (D) LTP induction protocol by HFS. (E) LTP induced in L5PyNs from C57BL/6 mice (n=11 cells, 8 mice) and CD38-/- mice (n=8 cells, 5 mice) at 15 min, 30 min, 45 min and 60 min after the HFS protocol. Histograms represent relative changes compared to control (0 min, before HFS) in excitatory (IntgE, left panel) and inhibitory (IntgI, right panel) conductances. Error bars represent S.E.M., Mann-Whitney, one-tailed (*p<0.05; **p<0.01).
Social Interaction Task
The social interaction task was previously described in details (38). Briefly, it took place in a transparent Plexiglas cage (50 cm long × 25 cm wide × 30 cm deep) located in a non-familiar experimental room with a 100 lux illumination. It contained a handful of clean sawdust for each mouse. The experimental cage was located below a camera connected to a computer that recorded video of social behaviour for subsequent off-line analyses. One mouse, either of the CD38-/- or C57BL/6 genotype, was placed alone in the experimental cage and was allowed to freely explore for 30 min. A novel mouse (same sex, age, weight and C57BL/6 genotype) was then gently introduced in the cage for an 8-min test during which we recorded ultrasonic vocalizations (USVs) (see below). For the experiment with juvenile mice, the experimental design is identical to the one described above for the adult mice except that the novel mouse is from the same genotype of the tested mouse. Multiple social parameters were analysed offline, such as the duration of contact between the two mice and the number of aggressive attacks. Adult male mice were isolated 3 weeks before testing whereas juveniles were not.
Ultrasonic Vocalization recording
USVs were recorded during the social interaction task in dyads of mice (39). A condenser ultrasound microphone Polaroid/CMPA was placed above the experimental chamber, high enough so that the receiving angle of the microphone covered the whole area of the test cage. It was connected to an ultrasound recording interface (UltraSoundGate 416H) plugged to a computer equipped with the Avisoft Recorder USG (sampling frequency: 250 kHz; FFT-length: 1024 points; 16-bit format). All recording hardwares and softwares were from Avisoft Bioacoustics (Berlin, Germany). Spectrograms were generated for each detected call to measure the number of calls and their duration (39, 40) with the following characteristics: Blackman window, overlap: 87.5%, time resolution: 0.512 ms, frequency resolution 244 Hz.
Three-chamber social behaviour test
In a first part of the test, a C57BL/6 or CD38-/- mouse was placed in the three-chambered apparatus (transparent Plexiglas cage separated in three different and accessible rooms, see fig. 3C left panel) and was allowed to freely explore the environment for 10 min (habituation period). Then, the mouse was put back in its home cage for 1 min. A “stranger” C57BL/6 mouse was placed in one of the two cups (the social cup), the other remaining empty cup called “unknown object” (or non-social cup). In the second part, the tested C57BL/6 or CD38-/- mouse was placed in the centre zone and allowed to explore all chambers for 10 min (social motivation test). Contacts were scored when the mouse sniffed and touched the unknown object or the social cup. The experimental apparatus was located in a room under 100 lux illumination and below a camera connected to a computer for video recording of social behaviour for subsequent off-line analyses (Any-maze software).
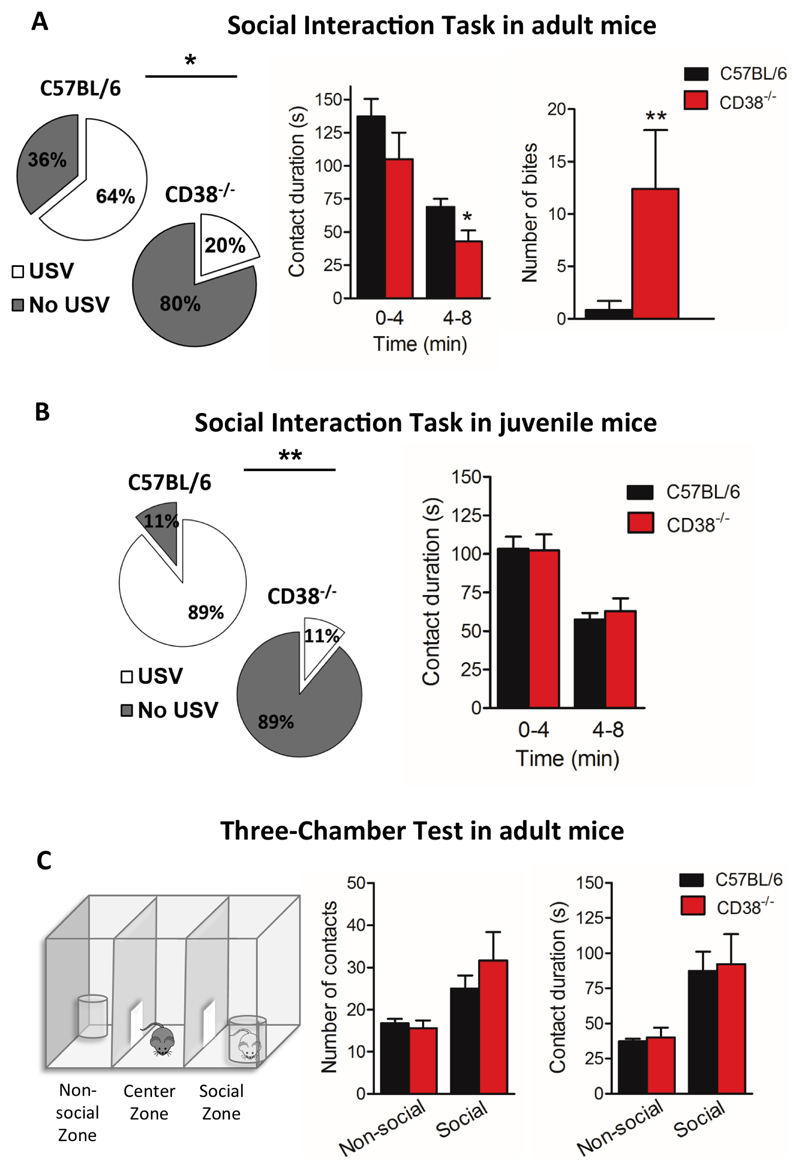
Figure 3. CD38-/- adult male mice display aggressive behaviour in a social interaction task.
(A) Left panel shows the analysis of the proportion of mice emitting USVs in the C57BL/6 (n=14 mice), and CD38-/- groups (n=10 mice). White sections represent the proportion of vocalizing mice and grey section represent the proportion of non-vocalizing mice. Central histogram shows the time spent in social contact between tested mice (C57BL/6, n=14 mice; CD38-/-, n=10 mice) and visitors in an 8 min-session. Right histogram shows the number of bites recorded during the session for C57BL/6 mice (n=14) and CD38-/- mice (n=10). (B) Left panel shows the proportion of juvenile mice emitting USVs (C57BL/6, n=9 mice; CD38-/-, n=9 mice). Right histogram shows the time spent in social contact between host juvenile tested mice (C57BL/6, n=19 mice; CD38-/-, n=13 mice) and juvenile visitors in a session of 8 min. (C). Left panel shows a drawing of the three-chamber test apparatus. The histogram in the centre shows the number of social and non-social contacts made by C57BL/6 adult mice and CD38-/- adult mice. Right histogram shows contact duration during social and non-social contacts in C57BL/6 mice and CD38-/- (C57BL/6, n=15 mice; CD38-/-, n=10 mice). Error bars represent S.E.M., Mann-Whitney, two-tailed (*p<0.05; **p < 0.01).
Light-dark choice anxiety test
The apparatus had 20 cm-high Plexiglas walls and consisted of a brightly lit white compartment (40 x 15 cm; illumination: 600 lux) connected by a trap door (6 x 6 cm) to a dark compartment (15 x 15 cm; illumination < 10 lux). Each mouse was placed in the dark compartment for 20 s, the trap door was then opened and mice were allowed to freely explore the whole apparatus for 5 min. Step-through latency, number of entries and total time spent in the lit compartment were scored.
Elevated plus-maze anxiety test
The maze had two facing arms enclosed with high walls (20 x 8 x 25 cm), two open arms (20 x 8 cm) and a central area (8 x 8 cm) forming a plus sign 65 cm above the floor. Illumination was 140 lux in open arms and 30 lux in enclosed arms. Mice were individually placed at the centre of the maze with the head facing an open arm. The number of entries and time spent in open or enclosed arms were recorded for 5 min. Head-dipping over sides of open arms were counted and classified as protected head dips when the rest of the mouse’s body remained in a closed arm, and as unprotected head dips when the whole mouse’s body was located in the open arm.
Open-field exploratory activity
The test box was a square open-field (50 x 50 x 50cm) with black walls and white floor under homogeneous illumination (100 lux in centre area). Each mouse was released near the wall and video-tracked for 40 min using the Any-maze software. Recorded x-y positions were used to generate tracking plot of the exploration paths and to calculate the distance travelled, speed and time spent in distinct zones of the box, i.e. in the whole apparatus, in a virtual corridor (width: 10 cm) along the walls and in the remaining central area, referred to as the centre area. Latency of the first entry, number of entries, percent distance travelled in centre area and along walls were calculated as relative measures of anxiety.
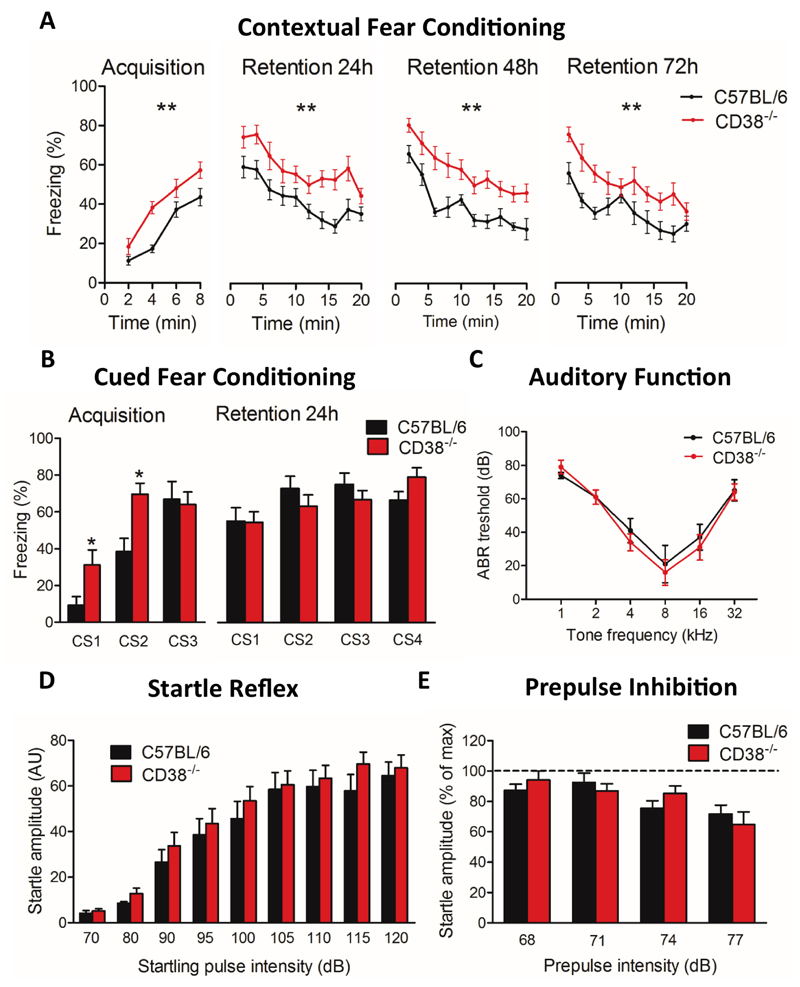
Contextual fear conditioning
The conditioning box consisted of a grid floor (30 x 30 cm) enabling delivery of electric footshocks as unconditioned stimuli (US) and clear Plexiglas walls (45 cm in height) with no ceiling in order to allow full observation and videotracking. Experiments were performed under moderate illumination (150 lux). During acquisition (day 1, duration: 8 min), each mouse was allowed to freely explore the box for 2 min; three footshocks (0.4 mA, 2 s) were then successively delivered with a two-min interval between shocks (2, 4, 6 min) and the mouse remained in the box for 2 min after the last shock. Retention and extinction of conditioned fear was measured during the next 3 days (24h, 48h, 72h), by placing the mouse in the same context for 20 min during each daily session without any footshock. Tonic immobility (freezing) was analysed from videotracking plots (Any-maze, Stoelting) and used to quantify expression of fear responses and fear memory.
Auditory-cued fear conditioning
Conditioning procedure was performed using the StartFear system (Panlab S.L., Barcelona). The conditioning chamber (25 x 25 x 25 cm) had three black methacrylate walls, a transparent front door, a grid floor connected to a shock (US) scrambler and a speaker on the ceiling to deliver audible tones as Conditioned Stimulus (CS). It stood on a high-sensitivity weight transducer system generating analogical signals reflecting animal’s movement and was confined in a ventilated soundproof enclosure on an anti-vibration table with a surrounding 60-dB white noise. Interchangeable floors and walls were used to analyse fear memory in a novel context. On the first day (acquisition), a 2-min baseline period was recorded before delivery of three CS–US pairs (Tone: 80 dB, 10 kHz, 30 s; footshocks: each at 0.2 mA for 2 s). On the next day (retention), the mouse was placed in a different context for 2 min (baseline) before delivery of four CS (80 dB, 10 kHz, 30 s). In both sessions stimuli were separated by pseudo-randomly distributed intervals (60-180 s). Animal’s movements were sampled at 50 Hz for quantitative analysis (Freezing software, Panlab S.L., Barcelona). Freezing was analysed during delivery of the CS (periods of 30 s) to specifically reflect associative learning performance.
Auditory brainstem responses
Thresholds for the averaged Auditory brainstem response (ABR) were used here as an electrophysiological measure of auditory sensitivity (41, 42). ABRs were recorded in response to acoustic stimuli under deep anaesthesia (95 mg/kg ketamine, 24 mg/kg xylazine, I.P.). Mice were placed on a heating blanket to avoid hypothermia. Auditory stimuli were presented monaurally using an insert earphone (Etymotic Research ER-1, USA) ending with a 17 mm polyethylene tubing inserted into external auditory meatus to avoid sound reverberation on ear lobe. ABRs were recorded using two subcutaneous electrodes (SC25, Neuro-Services, France) located just above the tympanic bulla and skull dorsal midline and one ground electrode placed in the thigh. Insertion of subcutaneous electrodes was not associated with any sign of discomfort. ABRs were collected by a Centor-USB interface (DeltaMed, France). Pure tone bursts (duration: 10 ms; rise-fall time: 2 ms) were delivered at specific frequencies (8, 2, 16, 24, 4, and 32 kHz) and presented at 70, 50, 40, 30, 20, 10, 5 and 0 dB SPL (decibel in sound pressure level). Stimuli were presented at 15 Hz. The signal was filtered at 0.2–3.2 kHz with a sampling rate of 100 kHz and waveforms were averaged (500–1000 waveforms depending on the stimulus intensity) and stored for off-line analyses. All sound intensities are expressed in dB SPL (sound pressure level).
Acoustic startle reflex and auditory gating
Testing was conducted in the same conditioning chamber used for auditory-cued fear conditioning. Mice were individually placed in a small non-restraining Plexiglas cylinder (5 x 10 x 3.5 cm) mounted on the grid floor 5 min prior to testing (acclimation period) and throughout the experiment. A background white noise (65 dB) was present throughout testing. The acoustic startle reflex was detected by a piezoelectric accelerometer plugged to the grid floor and evaluated in response to 4 blocks of 9 startling tones (pulses of 70, 80, 90, 95, 100, 105, 110, 115 and 120 dB; 10 kHz, 40 ms) presented in a pseudo-random order with inter-trial intervals of 10, 15 or 20 s. In a second experiment (24h later), the maximal startle amplitude induced by 120 dB pulses was modulated by the presentation of non-startling low-intensity prepulses (68-77 dB, i.e., 0–12 dB above background noise, 10 kHz, 20 ms) pseudo-randomly delivered 100 ms before the 120 dB startling pulse (prepulse inhibition or PPI). In both experiments, individual amplitudes recorded in distinct blocks of trials were averaged. PPI was normalized to the startle amplitude induced by the 120 dB startling pulses.
Plasma oxytocin levels measurement
Mouse blood samples were centrifuged (5 min, 5 000 rpm, 4 °C), and plasma samples (50 μl) were kept at −20 °C until the extraction step performed at 4 °C as follows: 20 mg of heat-activated (700 °C) LiChroprep Si 60 (Merck) in 1 ml distilled water was added to each sample, mixed for 30 min and centrifuged; the pellet was mixed in 60% acetone to elude the neuropeptide; the evaporated extracts were kept at −20 °C. The plasma OT content was estimated using a highly sensitive and specific radioimmunoassay (RIAgnosis, Munich) (43, 44). In brief, 0.05 ml of rabbit anti-OT antibody was applied for 60 min and 0.01 ml of 125I-labeled tracer (Perkin Elmer) were then added to each aliquot. After an incubation period of 3 days at 4 °C, unbound radioactivity was precipitated by activated charcoal (Sigma Aldrich).
Measurements of monoamines and associated metabolites
Amounts of norepinephrine (NE), serotonin (5-HT) dopamine (DA) and their metabolites were quantified by Ultra Performance Liquid Chromatography (UPLC). Briefly, the PFC was quickly removed and homogenized in 0.3 M perchloric acid and centrifuged at 22 000 g for 30 min at 4 °C. Supernatants were collected and filtered through a 10 kDa membrane (Nanosep, Pall). Then 50 μl of each sample was analysed through a four-channel electrochemical array detector (ThermoFisher). Analysis, data collection and peak identification were fully automated (Chromeleon 7). The results were expressed as fmoles per milligram of fresh tissue.
Statistical analysis
Normality was tested with the Kolmogorov-Smirnov test and the d’Agostino and Pearson test. When data did not follow a normal distribution, we used the non-parametric test Mann-Whitney two-tailed. For multiple comparison, 2-way ANOVA was used. Each values are expressed as mean ± SEM and significance was illustrated as the following conventions: ns p>0.05, *p<0.05, **p<0.01, ***p<0.001.
Results
1). Brain morphological alterations
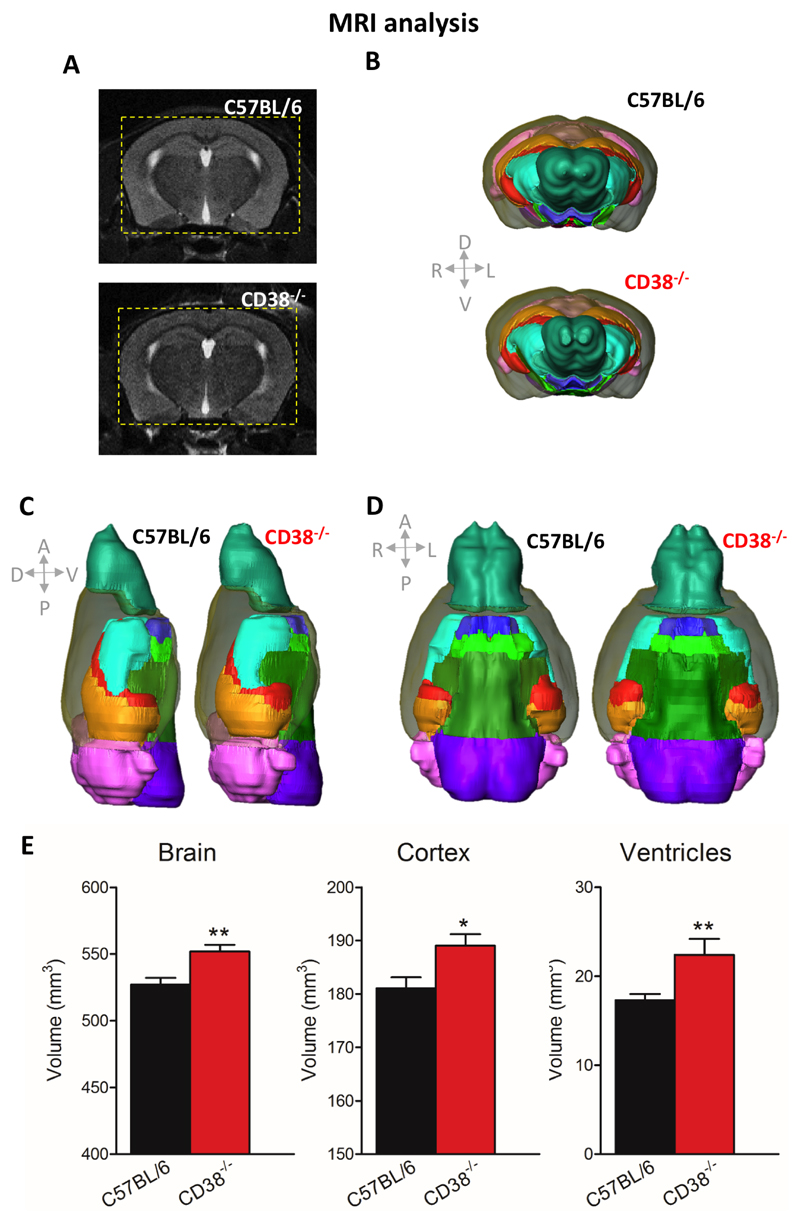
We examined whether adult CD38-/- male mice could exhibit abnormal cortical development knowing that CD38 plays a critical role in cell differentiation. We performed in vivo MRI to assess volumetric estimation of different brain structures in CD38-/- mice. Figure 1 shows MRI evaluation of brain volumetric integrity with images corresponding to both 2D cutting plane (Fig. 1A) and 3D reconstruction of mouse brains (Fig. 1B, C, D). The whole brain volume was significantly larger in CD38-/- mice compared to C57BL/6 mice (Fig. 1E, 551.7 ± 5.2 mm3 for CD38-/- vs 527.2 ± 4.9 mm3 in control mice, **p=0.0030). Volumes of different structures were analysed and we found that the cortex and ventricles were significantly larger in CD38-/- mice compared to C57BL/6 mice (Fig. 1E, Cortex: 189.1 ± 2.1 mm3 for CD38-/- mice vs 181.1 ± 2.0 mm3 for controls, *p=0.0199; ventricles: 22.4 ± 1.8 mm3 for CD38-/- mice vs 17.3 ± 0.7 mm3 in controls, **p=0.0057). In contrast, the volumes of other brain structures such as hippocampus, cerebellum and olfactory bulbs did not differ between genotypes (not shown). We also examined whether juvenile CD38-/- male mice, juvenile CD38-/- female mice and adult CD38-/- female mice could also display abnormal brain development. No difference was observed between the different groups compared with their respective C57BL/6 controls (Fig. S1).
Figure 1. Alteration of brain volumetric integrity in CD38-/- male mice evaluated by MRI.
(A) Sample MRI coronal images (top, C57BL/6 mouse; bottom, CD38-/- mouse), corresponding to 2D cutting planes. To better highlight the difference between genotypes, dotted yellow squares were drawn on both images to outline the size of the CD38-/- mice brain compared with the C57BL/6 brain size. (B, C, D) 3D reconstructions of mouse brains with brain regions delineated according to mouse brain atlas (left C57BL/6; right CD38-/- mice), showing significant differences in brain gross anatomy between C57BL/6 mice and CD38-/- mice. Brain regions considered in the analysis are represented by distinct colours as follows: ventricles, red; cortex, grey ; hippocampi, orange; ventral structures, green ; striatum, turquoise ; olfactory bulbs, blue-green ; cerebellum/colliculus, pink and purple. (E) Histograms of estimated volumes in cubic millimetres of the total brain, cortex and ventricles (C57BL/6, n = 9 mice; CD38-/- mice, n= 10 mice). Error bars represent S.E.M., Mann-Whitney, two tailed (*p<0.05; **p<0.01).
2). Enhanced excitation-inhibition balance in prefrontal cortex networks
We investigated whether the balance between the excitatory and inhibitory inputs, which directs the pyramidal response, might be impaired in CD38-/- mice, knowing that CD38 is normally expressed in pyramidal neurons (45). The L5PyNs mostly elaborate the output signals of the cortex, realizing the spatiotemporal dendritic integration of excitatory and inhibitory signals transmitted by upstream microcircuits (46). To determine the E-I balance, stable somatic voltage-clamp recordings of L5PyNs subthreshold postsynaptic responses evoked by layer 2-3 electrical stimulation at various holding potentials were obtained in the PFC of CD38-/- and C57BL/6 mice (Fig. 2A). The evoked synaptic conductance was extracted (see Material and Methods) and decomposed into excitatory and inhibitory conductances (Fig. 2B). This allowed us to better evaluate the relative contribution of evoked excitatory and inhibitory inputs reaching the soma of the recorded neurons. Layer 2-3 electrical stimulation typically produced a fast excitatory conductance followed by a long lasting inhibitory conductance. Integrals of excitatory (IntgE) and inhibitory (IntgI) conductances were expressed as a percentage of the integral of the total conductance (IntgT) to determine the E-I balance (32, 34, 36). Quantification of these somatic conductances showed that the evoked composite signal at the soma was composed of 19.3 ± 1.1 % of excitation and 80.7 ± 1.1 % of inhibition in the PFC of C57BL/6 mice (Fig. 2C). In contrast, the E-I balance in CD38-/- mice was significantly shifted towards enhanced excitation (excitation: 24 ± 1.6 %; inhibition: 76 ± 1.6%, **p=0.0098).
3). Impaired synaptic plasticity in prefrontal cortex networks
Synaptic plasticity such as LTP in the brain is essential for learning and memory (47–49), but to keep functional neuronal networks (i.e. to ensure the stability of the E-I balance) the inhibitory plasticity has to adjust to the excitatory plasticity (50, 51). Here we applied a HFS protocol in layer 2-3 of the PFC to induce LTP of both excitatory and inhibitory conductances in L5PyNs (Fig. 2D). A control experiment in C57BL/6 mice showed that excitatory and inhibitory conductances were enhanced 45 min after HFS by around 50% and 90%, respectively (Fig. 2E). Importantly, we found that the potentiation of excitation was greater in CD38-/- mice than in C57BL/6 mice and became significantly more potentiated 45 min after HFS (CD38-/- mice: 143 ± 26.4 %; C57BL/6 mice: 51.4 ± 18.6 %, **p=0.0074), whereas potentiation of inhibition was comparable between genotypes (Fig. 2E).
4). Impaired social interactions
Although in ASD various cerebral structures are affected, it appears that the PFC is particularly important. The medial PFC (mPFC) is crucial for the management of social interactions and social cues and recent MRI studies have shown that the PFC is one of the brain structures associated with autistic social behaviour (52). Social behaviour was first assessed in a specific social interaction task that requires integrity of the PFC (53, 54), and in which male mice establish a novel and free interaction when placed together in a novel environment (55, 56). We have previously shown that during the social interaction task, mice exchange acoustic communication signals (39), while this does not occur when mice cannot interact freely (57). In addition, we showed that acoustic communication is correlated to specific social sequences in the social interaction task and can reflect affiliative behaviours, dominance, or aggressiveness (40). Here we showed that the proportion of adult CD38-/- mice vocalizing during this task was significantly lower (20%) than in C57BL/6 mice (64.3%, *p=0.0387) (Fig. 3A, left panel). As shown in Fig. 3A (centre panel), CD38-/- mice spent less time in contact with a stranger conspecific male (42.8 ± 8.4 s) as compared with C57BL/6 mice (69 ± 5.9 s; *p=0.0223). Importantly, CD38-/- mice showed massive aggressiveness compared to C57BL/6 mice, as reflected by an increased number of bites (Fig. 3A, right panel: 12.4 ± 5.6 for CD38-/- mice vs 0.9 ± 0.8 for C57BL/6 mice, **p=0.0063). To test whether theses alterations occurred earlier in the development, we performed a similar experiment in juvenile mice (P28-30). Interestingly, juvenile CD38-/- mice displayed a deficit of vocalization during the social task, even though they exhibited unaltered social behaviour at this age (Fig. 3B). The proportion of CD38-/- juvenile mice vocalizing during the task was also significantly lower (11.1%) than in C57BL/6 mice (88.9%, **p=0.0016) (Fig. 3B, left panel). This suggests that impairment in vocal communication precedes deterioration of social behaviour, particularly the emergence of an aggressive behaviour at adulthood.
We also tested adult mice in a three-chamber social test, as a control to examine social motivation. In this task, mice with normal social motivation show a preference for a stranger mouse over an unknown object (empty cup) when submitted to a choice between these two options. As shown in Fig. 3C, in this test CD38-/- and control mice behaved similarly, spending more time interacting with the stranger mouse than with the unknown object, indicating intact interest for social stimuli (contact duration and numbers with a stranger mouse are similar for CD38-/- mice and for C57BL/6 mice).
5). Enhanced anxiety-like responses
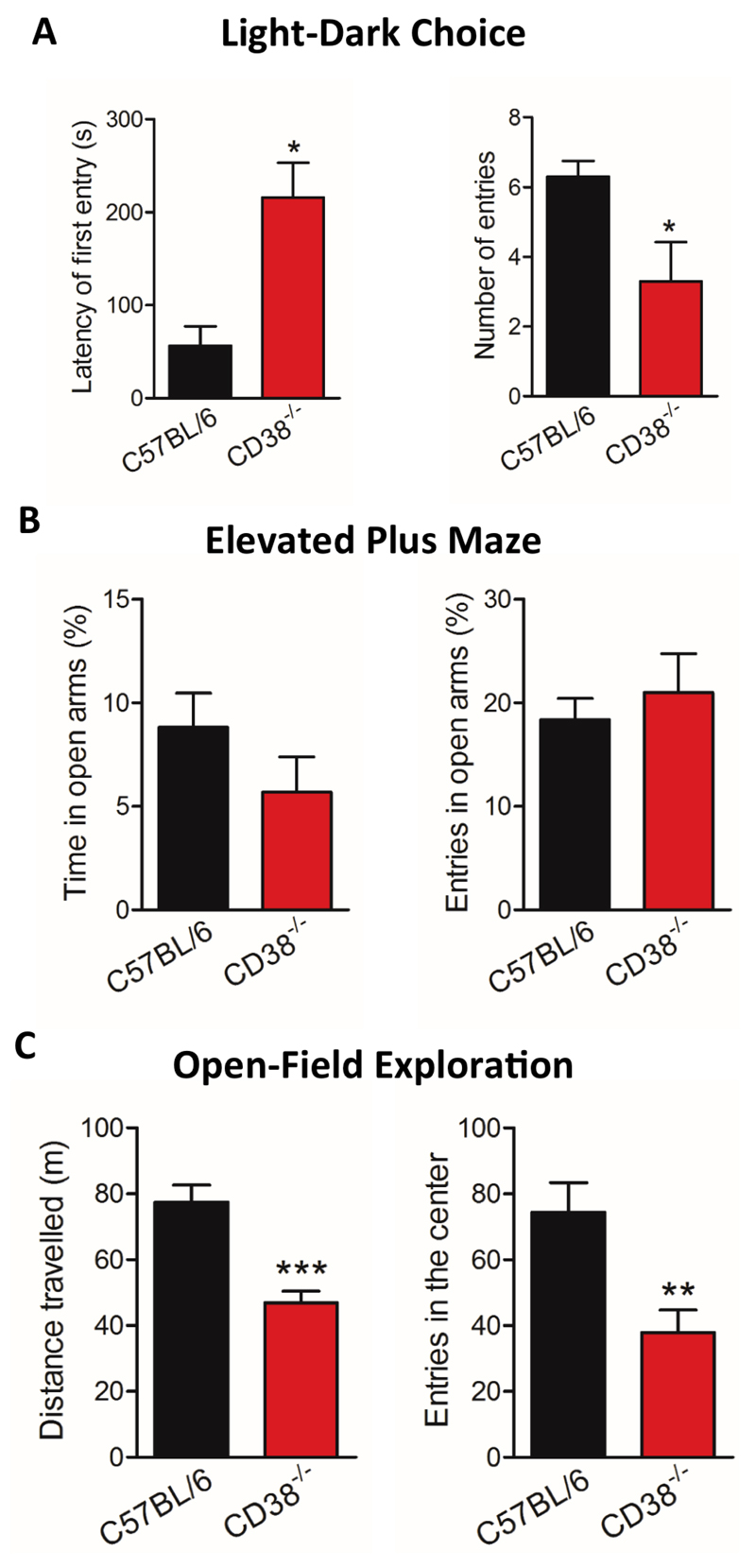
Aggressive behaviours observed in CD38-/- mice during social interactions prompted us to further characterize emotional reactivity and emotional learning in this model. We first evaluated anxiety-related behavioural responses in these mice using three different tests typically used to evaluate anxiety levels in mice. In the light-dark box test, mice had the choice to explore a brightly lit anxiogenic compartment or to stay in a more secure dark compartment. As shown in Fig. 4A, CD38-/- mice showed a significant preference for the dark compartment, as reflected by their longer latencies to enter the lit compartment (56.4 ± 20.9 s for C57BL/6 mice and 216 ± 37.2 s for CD38-/- mice, *p=0.0357) and reduced number of entries in the lit compartment (6.3 ± 0.4 for C57BL/6 mice and 3.3 ± 1.1 for CD38-/- mice, *p=0.0478), suggesting a higher level of anxiety in CD38-/- mice. In the elevated plus maze, in which anxiety is induced by the void in elevated open arms, the percentage number of entries and time spent in open arms were not significantly different between CD38-/- mice and C57BL/6 mice (Fig. 4B). Moreover, the number of protected and unprotected head dips was also comparable between genotypes (data not shown), indicating unaltered risk assessment behaviour. Finally, we also analysed locomotion and anxiety-related parameters during exploration of an open field (Fig. 4C). CD38-/- mice showed a significant reduction in the distance travelled in the open field over the 40 min recording period (46.8 ± 3.6 m for CD38-/- mice and 77.4 ± 5.2 m for C57BL/6, ***p=0.0003), associated with a reduced number of entries in the centre area (37.9 ± 6.8 for CD38-/- and 74.5 ± 8.8 for C57BL/6, **p=0.0072), suggesting alterations of both exploration and anxiety-related behavioural responses in CD38-/- mice in this test.
Figure 4. CD38-/- male mice show high levels of anxiety.
(A) Latency of first entry in the lit compartment and number of entries in the lit compartment during the light-dark test. (B) Behavioural responses in the elevated plus-maze test expressed as the percent time spent and number of entries in the open arms. (C) Distance travelled and number of entries in the centre in the open-field test. C57BL/6, n=10 mice; CD38-/- mice, n=10 mice. Error bars represent S.E.M., Mann-Whitney, two-tailed (*p<0.05; **p<0.01; ***p<0.001).
6). Enhanced emotional reactivity in CD38-/- mice but unaltered fear learning and memory
Emotional learning and memory were first evaluated in a contextual fear conditioning paradigm, which depends on the integrity of both amygdala and hippocampus (Fig. 5A). In this task, CD38-/- mice consistently showed longer durations of freezing following electric footshock delivery compared to control mice (Fig. 5A, 57,2% at 8 min in CD38-/- mice vs 43,6% in C57BL/6 mice, **p=0.0061). Mice were placed back in the same context 24h, 48h and 72h after this acquisition session for 20 min each day without any footshock delivery. Mice of both genotypes showed long freezing responses at the beginning of each session, reflecting strong retention of contextual fear memory, and mice of both genotypes displayed a progressive and parallel decay of freezing behaviour during the 20 min sessions, suggesting comparable extinction of the conditioned response in the two genotypes. Remarkably, however, the amount of freezing was consistently higher in CD38-/- mice than in control mice throughout the experiment (all sessions, **p<0.01), suggesting stronger expression of fear responses rather than genuine differences in learning and memory performances. In an amygdala-dependent auditory-cued fear conditioning paradigm (Fig. 5B), the percentage of freezing was also significantly increased in CD38-/- mice compared with C57BL/6 mice during the first and second tone deliveries during acquisition session (Conditioning Stimulus, CS1, CS2, both *p<0.05). Increased freezing during first tone delivery, i.e., before any footshock delivery, suggests it reflected increased emotional reactivity rather than increased pain sensitivity. However, the amount of freezing was comparable in CD38-/- and control mice during delivery of the last tone of the acquisition session (CS3), as well as during delivery of the four conditioned stimuli during the retention session performed 24h later (CS1-CS4), suggesting comparable fear learning and memory in the two genotypes.
Figure 5. Enhanced emotional reactivity in CD38-/- male mice.
(A) Contextual fear conditioning. The plot shows the percent freezing recorded in C57BL/6 (n=10 mice) and CD38-/- mice (n=10 mice) during the acquisition session (8 min, 4 shocks delivered) and during successive retention sessions (24h, 48h and 72h). Statistical analysis using a 2-way ANOVA, (**p<0.01). (B) Auditory-cued fear conditioning (C57BL/6, n=9; CD38-/- mice, n=10). Percent freezing was calculated during presentation of the conditioned stimulus (CS, tone lasting 30 s) during acquisition (CS1-3 each followed by one footshock or unconditioned stimulus, (US) and during retention (CS1-4 not followed by US). Statistical analysis using Mann-Whitney, two-tailed (*p<0.05). (C) Auditory brainstem responses (ABR) recorded in C57BL/6 (n=5 mice) and CD38-/- mice (n=5 mice). ABR thresholds are presented as a function of pure tone frequencies. Statistical analysis using a 2-way ANOVA. (D) Acoustic startle reflex of C57BL/6 mice (n=9 mice) and CD38-/- mice (n=10 mice). The acoustic startle reflex response increased when raising startling pulse intensities from 70 dB to 120 dB. Statistical analysis using Mann-Whitney, two-tailed. (E) Prepulse inhibition of the acoustic startle reflex expressed as the amplitude of the startle response when a prepulse of 3, 6, 9 or 12 dB above background noise (65 dB) preceded the startling pulse. Data are normalized to the maximal amplitude recorded in the absence of prepulse (C57BL/6, n=9 mice; CD38-/-, n=10 mice). Mann-Whitney, two-tailed. Error bars represent S.E.M.
We then investigated auditory perception by comparing ABRs in mice of both genotypes (Fig. 5C). CD38-/- mice had normal ABRs thresholds in response to pure tones of different frequencies, showing a typical V-shape curve with an optimal threshold detected at 8 kHz, as in C57BL/6 mice, suggesting unaltered hearing capability. We further determined the acoustic startle reflex induced by tone intensities ranging from 70 to 120 dB (Fig. 5D), and did not detect any significant genotype effect, suggesting normal reflex responses in CD38-/- mice in response to auditory tones. We then investigated the prepulse inhibition of acoustic startle reflex, to evaluate auditory gating (Fig. 5E). Non-startling prepulse from 3 to 12 dB above 65 dB background noise (68, 71, 74 and 77 dB) induced a relative decrease in the startle amplitude, which was comparable in CD38-/- and C57BL/6 mice. Overall, CD38-/- mice had no impairment in fear learning and memory tasks and their general enhancement in freezing duration during conditioning could not be attributed to overt alterations in the perception and/or processing of auditory stimuli.
7). Neurochemical levels impairment in CD38-/- mice
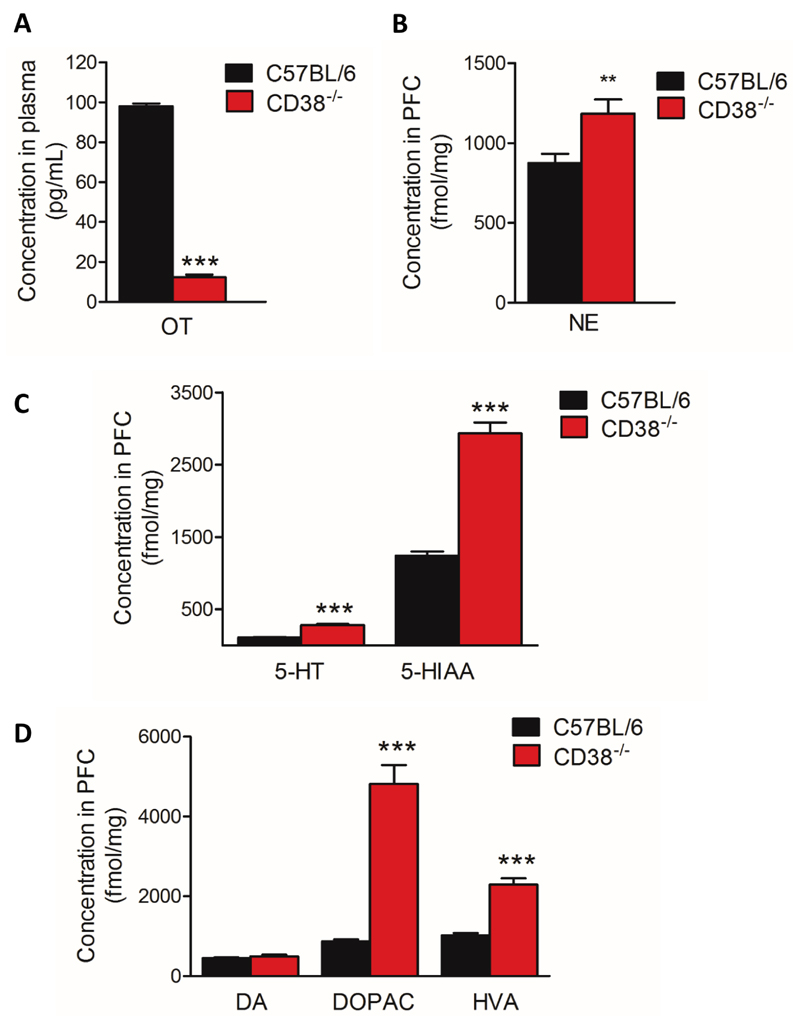
A seminal study from Higashida’s group revealed that CD38-/- mice on an ICR (Institute of Cancer Research) genetic background display low plasma levels of OT (29). Using a radioimmunoassay, we investigated whether CD38-/- mice under a C57BL/6 genetic background display similar characteristic. We found that adult male CD38-/- mice have lower levels of plasma OT compared with adult C57BL/6 control mice (Fig 6A, 12.4 ± 1.2 pg/ml for CD38-/- mice vs 98 ± 1.4 pg/ml for C57BL/6 mice, ***p=0.0002). We found similar reductions in OT levels in juvenile CD38-/- male mice, juvenile CD38-/- females and adult CD38-/- females (Fig. S2A, B, C, D). However, OT may not be the sole neuromodulator altered in CD38-/- mice and it is known that monoamines contribute to flexible behaviours mainly via prefrontal modulation. Neurotransmitters such as NE, 5-HT and DA have been shown to be associated with aggressive- and anxiety-related behaviours. However, there is currently no information on the levels of any monoamine and their metabolites in CD38-/- mice. We used UPLC to measure the content of endogenous monoamines and their respective metabolites in frozen PFC samples. We found that the basal levels of NE (Fig. 6B, + 35%, **p=0.0068) as well as that of 5-HT (+ 151 % ***p=0.0002), and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) (Fig. 6C, + 136 % ***p<0.0001) were significantly increased in CD38-/- mice compared with C57BL/6 mice in the PFC. Finally, we found that the basal level of DA was unchanged in CD38-/- whereas 3,4-dihydroxyphenylacetic acid (DOPAC) (+ 453%, ***p<0.0001) and homovanillic acid (HVA) (+ 124%, ***p<0.0001), two main metabolites of DA, were found significantly increased in CD38-/- mice (Fig. 6D) suggesting a higher DA turn over in CD38-/- mice compared with control mice. We also measured monoamine levels in juvenile CD38-/- male, juvenile CD38-/- female and adult CD38-/- female mice. We found very modest changes in levels of 5-HT and 5-HIAA in adult female and juvenile female, respectively (Fig. S2F, H). In the case of the juvenile CD38-/- male mice, only a very modest reduction in NE was observed (Fig. S2G).
Figure 6. Alteration of endogenous neurochemical levels in CD38-/- male mice.
(A) Plasma levels of OT. (B) PFC endogenous content of NE in C57BL/6 mice and CD38-/- mice. (C) PFC endogenous content of 5-HT and 5-HIAA in C57BL/6 and CD38-/- mice. (D) PFC endogenous content of DA, DOPAC and HVA in C57BL/6 and CD38-/- mice. C57BL/6 mice, n=10 mice; CD38-/- mice, n=10 mice. Error bars represent S.E.M. Statistical analysis using Mann-Whitney, two-tailed (*p<0.05; **p<0.01; ***p<0.001).
Discussion
Here we report that CD38-/- male mice show an abnormal development of the cortex and we also provide the first direct evidence that this is associated with an excitation-inhibition balance shifted towards higher excitation and altered synaptic plasticity in the PFC. Importantly, we also show that the PFC of these mice show alterations in monoamines levels involved in the control of flexible behaviours. At the behavioural level we demonstrate that a social interaction task relying on the PFC is compromised in CD38-/- mice. Acoustic communication is markedly altered in these mice, and we show that this impairment precedes development of enhanced aggressiveness in adult CD38-/- mice. Moreover, our data indicate that the behavioural disturbances displayed by CD38-/- mice cannot be attributed to changes in the perception or gating of auditory stimuli but are associated with higher levels of emotional reactivity in anxiety tests and fear conditioning tasks. Overall, our data bring new evidences that the CD38-/- mouse could be a relevant model to study pathophysiological brain mechanisms associated with ASD.
Previous studies in CD38-/- mice suggested that CD38 plays a critical role in social behaviour by regulating the secretion of OT by hypothalamus, and the deficits in these mice were also associated with observation of a decrease in maternal care and impaired social memory (29). These profound behavioural abnormalities make CD38-/- mice an important model candidate in the study of neurodevelopmental pathologies such as ASD. Indeed, numerous studies pointed toward a deficit in OT signalling in patients with ASD (18) and a common single-nucleotide polymorphism (SNP) has been associated with a familial form of autism (CD38 rs3796863). Additionally, individuals with the CD38 “risk allele” (CC) have lower expression of CD38 and lower plasma levels of OT. ASD patients with this CC risk allele are more severely affected than ASD patients with the AA allele (19, 21). However, OT deficit is currently not considered as the primary cause of ASD but has rather been suggested to worsen the social deficits.
Excessive cerebral growth during development has been reported in ASD patients, especially at the PFC level. Many studies have found an abnormally high number of neurons in human cortex (2, 58). Here, our in vivo MRI volumetric measurements clearly show a selective increase of cortical size in adult CD38-/- male mice not seen in adult female mice or juvenile mice. The mechanism of this gender-specific abnormal brain growth is not clear. However, this phenomenon is not restricted to the brain since it has also been reported that CD38-/- male mice show cardiac hypertrophy whereas female CD38-/- mice display normal heart development (59). In adult CD38-/- male mice the simplest explanation would be that CD38 deletion has altered early adult brain modifications such as either an on-going brain growth occurring in early adulthood or due to a reduced activity of pruning and/or neuronal apoptosis during this later period. Consistent with this hypothesis, CD38 is expressed at the embryonic stage (60, 61) and its expression is known to be involved in neural differentiation and microglia activation (62–64). Therefore, deleting CD38 may have promoted cell proliferation (62, 65) leading to excessive growth of cortical layers.
The cerebral cortex consists mainly of pyramidal excitatory glutamatergic neurons (about 80%) and inhibitory GABAergic interneurons (about 20%) (66). Intriguingly, the cerebral structures involved in ASD seem to be diverse, but among them, the PFC holds an important place. The mPFC plays a critical role in the treatment of information during social interactions (53, 67, 68) and recent functional MRI studies have shown that the mPFC is one of the brain structures associated with altered social behaviour in ASD patients (52). CD38 is involved in a variety of cellular processes, as it is widely expressed in pyramidal neurons (45), astrocytes (69) and microglia (69). One main goal of our study was to explore whether and how CD38 loss of function may impact the physiological control of neural networks in the PFC. The E-I balance determined at the level of the L5PyNs of C57BL/6 mice was around 19%-81%, which is not different from the balance determined in mPFC of another control mouse line (e.g., 129/SV line: 20%-80%; (70)) or in other cortical structures such as the rat visual cortex (20%-80%; (32)). These E-I balance values are within a narrow range, consistent with the existence of a specific set point required to maintain a coherent functioning of neural networks (50, 71). The increased E-I balance in CD38-/- mice may result from an increase in the number or weight of glutamatergic synapses and/or a decrease in the number or weight of GABAergic synapses. This hypothesis is consistent with the involvement of CD38 in a neurodevelopmental disease such as ASD, in which significant neuronal network remodelling has been observed (72, 73). The CD38 signalling pathway is recruited by several GPCRs which could normally participate in the establishment of neural networks within the mPFC and thereby directly or indirectly modulate the E-I balance. Interestingly, the PFC is one of the structures that have been reported to be rich in OT receptors (74) and therefore a reduction in OT signalling might affect the E-I balance. Supporting this view, recent articles have outlined the important role of OT in synapse maturation and in synaptic transmission and plasticity (14–17). However, OT is not the sole neuromodulator altered in CD38-/- mice, as we also report here an increased turnover of 5-HT and DA in the PFC of adult CD38-/- male mice. These biochemical changes have to be also considered knowing that, in our previous studies we have shown that the fine-tuning of the E-I balance is linked to the action of numerous neuromodulators such as DA and 5-HT (37, 70).
Genetic models of autism, such as Shank3-/- and Nlgn3-/- mice, and the pharmacological (valproic acid) mouse model, are often associated with changes in long-term plasticity and NMDA currents. Here, we found that LTP is increased in the mPFC of CD38-/- mice, which is the first report of altered cortical plasticity in this model. In CD38-/- mice the excitatory component was excessively potentiated while the potentiation of the inhibitory component was similar to that recorded in mPFC of control mice. This suggests that deletion of CD38 led to an increase of neuronal excitability and facilitated potentiation of AMPA receptor-dependent currents.
At the behavioural level, adult male CD38-/- mice displayed a deficit in social interaction and reduced emission of ultrasonic vocalization (USV), along with enhanced aggressiveness. Social interactions involve a circuit of brain structures that includes PFC, hippocampus, amygdala, or other cortical areas susceptible to be altered in ASD. The E-I balance of the PFC is important for both normal and pathological social interactions (75, 76) and we demonstrate here that both social interactions and the E-I balance of the PFC are dysfunctional in the absence of CD38. Our results in juvenile male mice indicate that a decrease in USV emission rate (77) preceded the social alterations seen in adults. It is thus plausible that an early decrease in communication abilities favoured emergence of non-adapted social behaviour and aggressiveness in adulthood that might be linked to OT deficits (78). This relationship between OT levels and social behaviour would remain to be fully investigated in CD38-/- females. In line with this hypothesis, our previous study comparing distinct mouse strains highlighted that aggressiveness is markedly promoted by low capability to vocalize during social interaction (40). The enhanced anxiety-like responses observed here in CD38-/- mice could also contribute to alteration of social behaviour. This view is largely supported by the strong comorbidity of anxiety disorders with social cognitive disorders and by the involvement of an integrated brain circuit including amygdala, hypothalamus and PFC in the control of innate social behaviours such as aggression and parenting responses ((79) for a review). Previous studies in CD38-/- mice reported variable effects of CD38 loss-of-function on anxiety and parenting behaviours (29, 80). In the present study, CD38-/- mice displayed enhanced anxiety-like responses during open-field and light-dark choice testing, but not in the elevated plus maze anxiety test. This variability may depend on the genetic background and/or on the specific pattern of neurotransmitter and OT signalling alterations (78, 81). This latter hypothesis may be particularly relevant in CD38-/- mice in which we detected increased levels of NE and 5-HT, which has been shown to be formerly associated with increased aggressiveness (54, 82) and impaired social and non-social decision-making processes (54, 83), respectively. We have evidence that appropriate integration of social cues and adequate response to an unknown social conspecific rely on the integrity of the PFC (53). The impaired ability of CD38-/- mice to integrate social cues, such as the vocal ones, may have favoured aggressive reactions, despite unaltered propensity to seek social contact. As suggested in earlier studies (84), it is also likely that PFC-dependent executive dysfunctions more importantly affected CD38-/- mice behaviour during direct social interactions, in which it is required to rapidly adapt to the encounter’s behavioural responses, than during simple approach responses in the three-chamber test which may involve lower PFC demand. The high levels of OT receptors in PFC (74) suggest that a neuronal OT signalling defect may also contribute to their aggressive behaviour, as reported in OT-null mutant mice (78).
In all, our results show that the CD38-/- mice are characterized by a modification of the E-I balance that could be explained by an alteration in the development of PFC neural networks. In another animal model (ß2-/- mice), also exhibiting marked social disorganisation, we previously showed the E-I balance alteration to be associated with impaired decision-making process (85). We confirm here that compromising the E-I balance of the PFC may be associated with extensive social defects. The presence of OT signalling impairment in CD38-/- mice and its association with critical behavioural, monoamine deregulations and neurobiological alterations bring strong support to consider CD38-/- mice as a mouse model for studying ASD. Whether the panel of behavioural deficits exhibited by CD38-/- animals are interrelated is currently unknown but we have paved the way here for novel avenues that could be investigated with the help of this model. In particular, this study suggests that future therapies for ASD patients with the common SNP (CD38 rs3796863) should also consider how OT and monoamines signalling interact and control neuronal network activity.
Supplementary Material
Acknowledgments
This research has been supported by grants from CNRS, INSERM, Paris-Sud University. Funding for this work was provided by the Fondation Jérôme Lejeune (JMC, PF) and by an international CNRS grant LIA (JMC, AG). LLM and AZ are recipients of a PhD fellowship from Paris-Sud University. The authors thank Frances Lund for providing CD38-/- mice. This work was also partly funded by France Life Imaging, grant ANR-11-INBS-0006.
Abbreviations
- ASD
Autism spectrum disorder
- ABRs
Auditory brainstem responses
- cADPR
cyclic ADP-Ribose
- CamKII
Calmodulin-dependent protein Kinase II
- CD38
Cluster Differentiation 38
- CNV
Copy-number variations
- CS
Conditioning Stimulus
- DA
Dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- E-I
Excitation-Inhibition
- GPCRs
G-protein-coupled receptors
- HFS
High frequency stimulation
- HVA
homovanillic acid
- ICR
Institute of Cancer Research
- IntgE
Integral of excitatory conductance
- IntgI
Integral of inhibitory conductance
- IntgT
Integral of total conductance
- L5PyNs
Pyramidal neurons of layer 5
- LTP
Long-Term Potentiation
- mPFC
medial PFC
- MRI
Magnetic resonance imaging
- NAADP
Nicotinic Acid Adenine Dinucleotide Phosphate
- NE
Norepinephrine
- OT
Oxytocin
- PFC
Prefrontal Cortex
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
Serotonin
- SNP
Single-nucleotide polymorphism
- UPLC
Ultra Performance Liquid Chromatography
- US
Unconditioned Stimulus
- USV
Ultrasonic vocalisation
Footnotes
Author Contributions
Conceived and designed the experiments: JMC, CV, SG, PF, SDP, AG. Performed experiments: LLM, MA, RC, GB, OB, JML, JC, CS, JME. Analyzed data: LLM, MA, RC, GB, OB, AZ, AN, JML, JC, CS, JME, SDP, PF, SG, CV, JMC. Wrote the paper: JMC, CV, SG, SDP, LLM, AG, PF.
References
- 1.Bourgeron T. Current knowledge on the genetics of autism and propositions for future research. C R Biol. 2016;339:300–307. doi: 10.1016/j.crvi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 3.Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, Sponheim E, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harony-Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi-Gunal O, Riad M, Daskalakis NP, Sonar S, Castillo PE, Hof PR, Shapiro ML, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife. 2017;6 doi: 10.7554/eLife.18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanda S, Aoto J, Lee S-J, Wernig M, Südhof TC. Pathogenic mechanism of an autism-associated neuroligin mutation involves altered AMPA-receptor trafficking. Mol Psychiatry. 2016;21:169–177. doi: 10.1038/mp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16:551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell K. Opening a window to the autistic brain. PLoS Biol. 2004;2:E267. doi: 10.1371/journal.pbio.0020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 15.Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J-J, Li S-J, Zhang X-D, Miao W-Y, Zhang D, Yao H, Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17:391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]
- 18.Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, Nurit Y, Ebstein RP. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res Off J Int Soc Autism Res. 2010;3:293–302. doi: 10.1002/aur.156. [DOI] [PubMed] [Google Scholar]
- 20.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 21.Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, Asaka T, Liu H-X, Jin D, Koizumi K, Islam MS, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H-F, Dai Y-C, Wu J, Jia M-X, Zhang J-S, Shou X-J, Han S-P, Zhang R, Han J-S. Plasma Oxytocin and Arginine-Vasopressin Levels in Children with Autism Spectrum Disorder in China: Associations with Symptoms. Neurosci Bull. 2016;32:423–432. doi: 10.1007/s12264-016-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A. 2017;114:8119–8124. doi: 10.1073/pnas.1705521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 26.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 27.Cosker F, Cheviron N, Yamasaki M, Menteyne A, Lund FE, Moutin M-J, Galione A, Cancela J-M. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem. 2010;285:38251–38259. doi: 10.1074/jbc.M110.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chini CCS, Tarragó MG, Chini EN. NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol. 2017;455:62–74. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 30.Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J Physiol Sci JPS. 2016;66:275–282. doi: 10.1007/s12576-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partida-Sánchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 32.Le Roux N, Amar M, Baux G, Fossier P. Homeostatic control of the excitation-inhibition balance in cortical layer 5 pyramidal neurons. Eur J Neurosci. 2006;24:3507–3518. doi: 10.1111/j.1460-9568.2006.05203.x. [DOI] [PubMed] [Google Scholar]
- 33.Lucas-Meunier E, Monier C, Amar M, Baux G, Frégnac Y, Fossier P. Involvement of nicotinic and muscarinic receptors in the endogenous cholinergic modulation of the balance between excitation and inhibition in the young rat visual cortex. Cereb Cortex N Y N 1991. 2009;19:2411–2427. doi: 10.1093/cercor/bhn258. [DOI] [PubMed] [Google Scholar]
- 34.Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex N Y N 1991. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- 35.Moreau AW, Amar M, Callebert J, Fossier P. Serotonergic modulation of LTP at excitatory and inhibitory synapses in the developing rat visual cortex. Neuroscience. 2013;238:148–158. doi: 10.1016/j.neuroscience.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Meunier CNJ, Amar M, Lanfumey L, Hamon M, Fossier P. 5-HT(1A) receptors direct the orientation of plasticity in layer 5 pyramidal neurons of the mouse prefrontal cortex. Neuropharmacology. 2013;71:37–45. doi: 10.1016/j.neuropharm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Meunier CNJ, Cancela J-M, Fossier P. Lack of GSK3β activation and modulation of synaptic plasticity by dopamine in 5-HT1A-receptor KO mice. Neuropharmacology. 2017;113:124–136. doi: 10.1016/j.neuropharm.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Granon S, Faure P, Changeux J-P. Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci U S A. 2003;100:9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, Granon S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PloS One. 2012;7:e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faure A, Pittaras E, Nosjean A, Chabout J, Cressant A, Granon S. Social behaviors and acoustic vocalizations in different strains of mice. Behav Brain Res. 2017;320:383–390. doi: 10.1016/j.bbr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Willott JF, Erway LC. Genetics of age-related hearing loss in mice. IV. Cochlear pathology and hearing loss in 25 BXD recombinant inbred mouse strains. Hear Res. 1998;119:27–36. doi: 10.1016/s0378-5955(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 42.Willott JF. Measurement of the auditory brainstem response (ABR) to study auditory sensitivity in mice. Curr Protoc Neurosci. 2006;Chapter8:Unit8.21B. doi: 10.1002/0471142301.ns0821bs34. [DOI] [PubMed] [Google Scholar]
- 43.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Yamada M, Mizuguchi M, Otsuka N, Ikeda K, Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997;756:52–60. doi: 10.1016/s0006-8993(97)00117-0. [DOI] [PubMed] [Google Scholar]
- 46.Binzegger T, Douglas RJ, Martin KAC. A quantitative map of the circuit of cat primary visual cortex. J Neurosci Off J Soc Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 49.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Roux N, Amar M, Moreau A, Baux G, Fossier P. Impaired GABAergic transmission disrupts normal homeostatic plasticity in rat cortical networks. Eur J Neurosci. 2008;27:3244–3256. doi: 10.1111/j.1460-9568.2008.06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe T, Yahata N, Abe O, Kuwabara H, Inoue H, Takano Y, Iwashiro N, Natsubori T, Aoki Y, Takao H, Sasaki H, et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PloS One. 2012;7:e39561. doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin J-C, Bourgeois J-P, Maskos U, Changeux J-P, Granon S. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:2145–2155. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- 54.Coura RS, Cressant A, Xia J, de Chaumont F, Olivo-Marin JC, Pelloux Y, Dalley JW, Granon S. Nonaggressive and adapted social cognition is controlled by the interplay between noradrenergic and nicotinic receptor mechanisms in the prefrontal cortex. FASEB J Off Publ Fed Am Soc Exp Biol. 2013;27:4343–4354. doi: 10.1096/fj.13-231084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Chaumont F, Coura RD-S, Serreau P, Cressant A, Chabout J, Granon S, Olivo-Marin J-C. Computerized video analysis of social interactions in mice. Nat Methods. 2012;9:410–417. doi: 10.1038/nmeth.1924. [DOI] [PubMed] [Google Scholar]
- 56.Nosjean A, Cressant A, de Chaumont F, Olivo-Marin J-C, Chauveau F, Granon S. Acute stress in adulthood impoverishes social choices and triggers aggressiveness in preclinical models. Front Behav Neurosci. 2014;8:447. doi: 10.3389/fnbeh.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chabout J, Cressant A, Hu X, Edeline J-M, Granon S. Making choice between competing rewards in uncertain vs. safe social environment: role of neuronal nicotinic receptors of acetylcholine. Front Hum Neurosci. 2013;7:468. doi: 10.3389/fnhum.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khundrakpam BS, Lewis JD, Kostopoulos P, Carbonell F, Evans AC. Cortical Thickness Abnormalities in Autism Spectrum Disorders Through Late Childhood, Adolescence, and Adulthood: A Large-Scale MRI Study. Cereb Cortex N. Y. N 1991. 2017;27:1721–1731. doi: 10.1093/cercor/bhx038. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi J, Kagaya Y, Kato I, Ohta J, Isoyama S, Miura M, Sugai Y, Hirose M, Wakayama Y, Ninomiya M, Watanabe J, et al. Deficit of CD38/cyclic ADP-ribose is differentially compensated in hearts by gender. Biochem Biophys Res Commun. 2003;312:434–440. doi: 10.1016/j.bbrc.2003.10.143. [DOI] [PubMed] [Google Scholar]
- 60.Ceni C, Pochon N, Brun V, Muller-Steffner H, Andrieux A, Grunwald D, Schuber F, De Waard M, Lund F, Villaz M, Moutin M-J. CD38-dependent ADP-ribosyl cyclase activity in developing and adult mouse brain. Biochem J. 2003;370:175–183. doi: 10.1042/BJ20020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ceni C, Pochon N, Villaz M, Muller-Steffner H, Schuber F, Baratier J, De Waard M, Ronjat M, Moutin M-J. The CD38-independent ADP-ribosyl cyclase from mouse brain synaptosomes: a comparative study of neonate and adult brain. Biochem J. 2006;395:417–426. doi: 10.1042/BJ20051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei W, Lu Y, Hao B, Zhang K, Wang Q, Miller AL, Zhang L-R, Zhang L-H, Yue J. CD38 Is Required for Neural Differentiation of Mouse Embryonic Stem Cells by Modulating Reactive Oxygen Species. Stem Cells Dayt Ohio. 2015;33:2664–2673. doi: 10.1002/stem.2057. [DOI] [PubMed] [Google Scholar]
- 63.Hattori T, Kaji M, Ishii H, Jureepon R, Takarada-Iemata M, Minh Ta H, Manh Le T, Konno A, Hirai H, Shiraishi Y, Ozaki N, et al. CD38 positively regulates postnatal development of astrocytes cell-autonomously and oligodendrocytes non-cell-autonomously. Glia. 2017;65:974–989. doi: 10.1002/glia.23139. [DOI] [PubMed] [Google Scholar]
- 64.Mayo L, Jacob-Hirsch J, Amariglio N, Rechavi G, Moutin M-J, Lund FE, Stein R. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol Baltim Md 1950. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei W-J, Sun H-Y, Ting KY, Zhang L-H, Lee H-C, Li G-R, Yue J. Inhibition of cardiomyocytes differentiation of mouse embryonic stem cells by CD38/cADPR/Ca2+ signaling pathway. J Biol Chem. 2012;287:35599–35611. doi: 10.1074/jbc.M112.392530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeFelipe J, Fariñas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- 67.Rudebeck PH, Walton ME, Millette BHP, Shirley E, Rushworth MFS, Bannerman DM. Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur J Neurosci. 2007;26:2315–2326. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W-T, Hsu W-Y, Chiu Y-C, Liang C-W. The hierarchical model of social interaction anxiety and depression: the critical roles of fears of evaluation. J Anxiety Disord. 2012;26:215–224. doi: 10.1016/j.janxdis.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Bruzzone S, Verderio C, Schenk U, Fedele E, Zocchi E, Matteoli M, De Flora A. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J Neurochem. 2004;89:264–272. doi: 10.1111/j.1471-4159.2003.02326.x. [DOI] [PubMed] [Google Scholar]
- 70.Meunier CNJ, Callebert J, Cancela J-M, Fossier P. Effect of dopaminergic D1 receptors on plasticity is dependent of serotoninergic 5-HT1A receptors in L5-pyramidal neurons of the prefrontal cortex. PloS One. 2015;10:e0120286. doi: 10.1371/journal.pone.0120286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joseph A, Turrigiano GG. All for One But Not One for All: Excitatory Synaptic Scaling and Intrinsic Excitability Are Coregulated by CaMKIV, Whereas Inhibitory Synaptic Scaling Is Under Independent Control. J Neurosci Off J Soc Neurosci. 2017;37:6778–6785. doi: 10.1523/JNEUROSCI.0618-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J Neurosci Off J Soc Neurosci. 2016;36:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal Cortex and Social Cognition in Mouse and Man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, Ramakrishnan C, Fenno LE, Davidson TJ, Wright M, Deisseroth K. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H-X, Lopatina O, Higashida C, Tsuji T, Kato I, Takasawa S, Okamoto H, Yokoyama S, Higashida H. Locomotor activity, ultrasonic vocalization and oxytocin levels in infant CD38 knockout mice. Neurosci Lett. 2008;448:67–70. doi: 10.1016/j.neulet.2008.09.084. [DOI] [PubMed] [Google Scholar]
- 78.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 79.Ko J. Neuroanatomical Substrates of Rodent Social Behavior: The Medial Prefrontal Cortex and Its Projection Patterns. Front Neural Circuits. 2017;11:41. doi: 10.3389/fncir.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S, Kim T, Lee H-R, Jang E-H, Ryu H-H, Kang M, Rah S-Y, Yoo J, Lee B, Kim J-I, Lim CS, et al. Impaired learning and memory in CD38 null mutant mice. Mol Brain. 2016;9:16. doi: 10.1186/s13041-016-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clément Y, Le Guisquet A-M, Venault P, Chapouthier G, Belzung C. Pharmacological alterations of anxious behaviour in mice depending on both strain and the behavioural situation. PloS One. 2009;4:e7745. doi: 10.1371/journal.pone.0007745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cambon K, Dos-Santos Coura R, Groc L, Carbon A, Weissmann D, Changeux JP, Pujol JF, Granon S. Aggressive behavior during social interaction in mice is controlled by the modulation of tyrosine hydroxylase expression in the prefrontal cortex. Neuroscience. 2010;171:840–851. doi: 10.1016/j.neuroscience.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Pittaras E, Callebert J, Chennaoui M, Rabat A, Granon S. Individual behavioral and neurochemical markers of unadapted decision-making processes in healthy inbred mice. Brain Struct Funct. 2016;221:4615–4629. doi: 10.1007/s00429-016-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miranda R, Nagapin F, Bozon B, Laroche S, Aubin T, Vaillend C. Altered social behavior and ultrasonic communication in the dystrophin-deficient mdx mouse model of Duchenne muscular dystrophy. Mol Autism. 2015;6:60. doi: 10.1186/s13229-015-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pittaras EC, Faure A, Leray X, Moraitopoulou E, Cressant A, Rabat AA, Meunier C, Fossier P, Granon S. Neuronal Nicotinic Receptors Are Crucial for Tuning of E/I Balance in Prelimbic Cortex and for Decision-Making Processes. Front Psychiatry. 2016;7:171. doi: 10.3389/fpsyt.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.