Abstract
Background:
Population-based screening programs are credited with earlier colorectal cancer (CRC) diagnoses and treatment initiation which reduce mortality rates and improve patient health outcomes. However, recommended screening methods are unsatisfactory as they are invasive, resource intensive, suffer from low uptake, or have poor diagnostic performance. Our goal was to identify a urine metabolomic-based biomarker panel for the detection of CRC that has the potential for global population-based screening.
Methods:
Prospective urine samples were collected from study participants. Based upon colonoscopy and histopathology results, 342 participants (CRC, 171; healthy controls, 171) from two study sites (e.g., Canada, United States) were included in the analyses. Targeted liquid chromatography-mass spectrometry was performed to quantify 140 highly valuable metabolites in each urine sample. Potential biomarkers for CRC were identified by comparing the metabolomic profiles from CRC versus controls. Multiple models were constructed leading to a good separation of CRC from controls.
Results:
A panel of 17 metabolites was identified as possible biomarkers for CRC. Using only two of the selected metabolites, namely diacetylspermine and kynurenine, a predictor for detecting CRC was developed with an AUC of 0.864, a specificity of 80.0% and a sensitivity of 80.0%.
Conclusions:
We present a potentially “universal” metabolomic biomarker panel for CRC independent of cohort clinical features based on a North American population. Further research is needed to confirm the utility of the profile in a prospective, population-based CRC screening trial.
Impact:
A urinary metabolomic biomarker panel was identified for CRC with the potential of clinical application.
Keywords: Colorectal Cancer Screening, Mass Spectrometry, Urine (Human), Diacetylspermine, Kynurenine
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths in the world. Based on 2018 estimates, the 2040 incidence rates for CRC are projected to increase by 72% to 3.1 million new cases while mortality rates will increase by 82% to 1.5 million deaths (https://gco.iarc.fr/tomorrow). Mortalities due to CRC are largely preventable through regular screening and early detection using fecal-based tests and colonoscopy (1). To be effective, population-based screening must be programmatic rather than opportunistic to ensure a high rate of compliance (2). Such programs have been instituted nationally or regionally within many countries in Europe (e.g., UK, Ireland, Germany, France), United States, Japan, and Australia as reviewed by Navarro and colleagues (3).
The most commonly used population-based screening modalities are the fecal immunochemical test (FIT) and colonoscopy (4). FIT detects hidden blood in stool which occurs mostly in the later stages of cancer and has low sensitivity for detecting the precursors to CRC, adenomatous polyps (9). A new fecal DNA test detects DNA mutations in addition to hidden blood in stool with improved sensitivity (5), but it is costly and only available in a few countries. To date, fecal-based tests are limited to CRC detection not prevention, and have low adherence rates due to the need for stool collection and manipulation (6–10). Colonoscopy has a superior sensitivity and specificity to non-invasive screening tests, but is costly in terms of direct and indirect health care dollars, has a higher risk of procedural-related complications, and, like fecal-based tests, has low rates of screening compliance (11).
To increase screening compliance rates, programs have largely focused on CRC education and sending reminders to eligible participants (12,13). An alternative approach for improving CRC screening rates is to use a biosample other than stool (14). A blood-based screening test has been shown to have higher patient uptake than FIT (15), but its cost-effectiveness is debatable for population-based screening (16). Urine is commonly used for many clinical tests, can be readily collected, and is more acceptable to patients (17,18). Recently, putative biomarkers of CRC were identified in urine in the forms of volatile organic compounds (19), modified cytosine nucleosides (20), and polyamines (21,22). As well, we have reported a urine-based screening test specific for colorectal adenomatous polyps (23,24) developed in a Canadian population and its subsequent validation in a homogenous Asian cohort to demonstrate its clinical relevance transcending both diet and ethnicity (25).
In the current multicenter study, the potential utility of urine-based metabolomics for detecting CRC was investigated. This was done by analyzing metabolites in urine samples from colonoscopy- and histopathology-confirmed cases of CRC and healthy controls (e.g., polyp- and CRC-free). Our findings highlight the predictive potential of urinary metabolites for CRC and we discuss the clinical relevance of a proposed screening test.
Materials and Methods
Study Participants and Sample Collection
Adult patients with newly diagnosed CRC (based on preoperative imaging, colonoscopies, and pathology reports of biopsies) were eligible for study inclusion provided they had not received CRC-related treatment. Canadian recruitment (October 2008–2010) was conducted at four tertiary hospitals in the Edmonton region (Grey Nuns Hospital, Misericordia Hospital, University of Alberta Hospital, and the Royal Alexandra Hospital) and included patients from across the prairie provinces (i.e., CRC-CAD cohort). American patients were recruited (February - July 2018) from the Memorial Sloan Kettering Cancer Center (MSK) in New York City, New York (i.e., CRC-MSK cohort).
Patients diagnosed with CRC provided a urine sample prior to any operation, chemotherapy, radiation, or other cancer-related treatment. Clinical features, such as age, gender, and smoking status, were also collected at this time. Each urine sample was transferred to labelled 1 ml tubes (5x) and frozen at −80oC within 1 hour of collection. Frozen urine was shipped on dry ice in a standard insulated Styrofoam shipper and immediately transferred to a −80oC freezer upon arrival at the University of Alberta in Edmonton, Alberta. Pathology reports were reviewed to abstract cancer stage.
The healthy controls were selected from a previous population-based study (n=1,000) called Stop COlorectal cancer through Prevention and Education (SCOPE) (23,26–28) The SCOPE program, regional colon cancer screening program (Edmonton, Alberta, Canada) where over 1000 urine samples were collected from April 2008 to October 2009. Study participants (40–74 years of age) of average or increased risk for CRC were recruited. On day of entry, participants provided informed written consent, a midstream urine sample, and completed a demographic survey. Urine was aliquoted and frozen at −80oC within 1 hour of collection. Colonoscopy was performed 2–6 weeks after the urine collection confirmed that the individuals were classified as normal based upon endoscopy findings and pathology reports. Urine samples from the healthy controls were matched 1:1 to the CRC cases based on gender. A study design chart was shown in Figure S1 (Supporting Information).
Ethics approval was obtained from the Health Research Ethics Boards at the University of Alberta (Pro0000514 and Pro00074045) and MSK (IRB #06–107 and #15–209).
Metabolite Analysis
Targeted liquid chromatography-mass spectrometry (LC-MS) was performed to quantify urinary metabolites in each sample using the LC-MS kit TMIC00UJ designed and prepared by The Metabolomics Innovation Centre (TMIC) at the University of Alberta in Edmonton, Alberta. Calibration solutions (Cal 1 - Cal 7), isotopically labeled standard mix (ISTD), quality control solutions (QC 1 - QC 3), LC-MS methods, and standard operating procedures were provided by TMIC. The TMIC00UJ kit was a combination of three assays to identify 140 unique urinary metabolites (see Table S1) indexed by the Human Metabolome Database (www.hmdb.ca). The phenyl isothiocyanate (PITC) assay quantified 47 biologic amines in the LC mode while 75 lipids were semi-quantified in the flow-injection analysis (FIA) mode. The organic acid assay quantified 17 compounds while ascorbic acid was quantified independently.
The TMIC00UJ kit components were run on an API4000 Qtrap® tandem mass spectrometry instrument (AB Sciex, Framingham, MA) coupled with a Waters UPLC system (Waters Limited, Mississauga, ON). Urine samples were thawed on ice, vortexed, then centrifuged at 13,000 × g. Each plate contained 82 unique urine samples as well as 1 solvent blank solution, 3 matrix solutions, 7 calibration solutions (Cal 1 – Cal 7), and 3 quality control (QC) samples. Phosphate-buffered saline (PBS 1×, pH 7.4) was used as the matrix solution. Metabolite quantification was achieved using the AB Sciex Analyst® software, version 1.6.2. During quantification, each metabolite was identified using the internal standard and compared against the established calibration curve. The lower limits of detection (LLOD) were calculated as three times the value of the matrix solutions. The upper limit of detection was not reached for any metabolite.
Statistical Analysis
Data pre-processing was performed using code written in R, version 3.4.3. Metabolites that were lower than the LLOD or not detected in more than half of the urine samples were removed from the initial list of 140 metabolites. For the remaining metabolites on the list, if a sample had a metabolite concentration that was less than the LLOD, it was replaced with half the value of the LLOD. Statistical analyses were conducted with MetaboAnalyst, version 4.0 for the web (29). Metabolite concentration was normalized against creatinine, log-transformed, and auto-scaled. Potential biomarkers for CRC were identified (30) by comparing the metabolomic profiles of the CRC and control groups for both fold-change analyses and Student t tests. One-way ANOVA was performed on the independent sample groups (e.g., CRC-CAD, CRC-MSK, and control) to identify statistically significant metabolite differences (31–33). The metabolites with concentration changes in the same direction for both the CRC-CAD and CRC-MSK groups were considered consistent CRC markers. Furthermore, multivariate models, using principal component analysis (PCA), partial least squares - discriminant analysis (PLS-DA), and sparse PLS-DA (sPLS-DA) (34) were constructed. Finally, predictors were built using the logistic regression with selected biomarkers. Leave-out approach was used to evaluate the built models. 171 Controls were randomized to form 121 Controls for training and 50 Controls for testing with balanced age, gender and smoking status. 121 CRC-CAD and 121 Controls were used as training set to build a model, and 50 CRC-MSK and 50 Controls were used as testing set to validate the model.
All code for statistical analyses was also written in R, version 3.4.3 (https://www.R-project.org). The glmnet package was used for logistic regression (35). Receiver operating characteristic (12) curves were generated and reported using the ROCR package.
Results
Patient Characteristics
In Canada, a total of 161 participants were enrolled of which 40 were excluded due to missing clinical information. A total of 50 samples were collected from patients at MSK and used for this study. The 171 CRC samples were matched with 171 urine samples from colonoscopy-confirmed healthy controls. See Table 1 for a summary of clinical characteristics for the participants. Statistical analysis was performed on Control group vs. CRC group. The p-value for gender was 0.63 indicating there was no significant difference in gender between CRC and controls. The p-value for smoking was 0.02 with more current smokers in the CRC group. The p-value for age was 2.83×10−13 indicating there was a significant difference in age between CRC and controls where the mean age in CRC group was ~ 7 years older than the control group.
Table 1.
Patient characteristics
| Controls | CRC Cases | |||
|---|---|---|---|---|
| CRC-All | CRC-CAD | CRC-MSK | ||
| Mean Age, yrs (SD) | 58.9 (5.6) | 66.4 (11.5) | 67.4 (10.9) | 63.8 (12.5) |
| Gender, n (%) | ||||
| Male | 100 (58.5%) | 89 (52.0%) | 68 (53.7%) | 24 (48.0%) |
| Female | 71 (41.5) | 82 (48.0%) | 59 (46.3%) | 26 (52.0%) |
| Smoking, n (%) | ||||
| Current | 12 (7.0%) | 29 (17.0%) | 24 (19.8%) | 5 (10.0%) |
| Prior | 66 (38.6%) | 56 (32.7%) | 38 (31.4%) | 18 (36.0%) |
| Never | 87 (50.9%) | 86 (50.3%) | 59 (48.8%) | 27 (54.0%) |
| By Stage, n (%) | ||||
| 0 | - | 3 (1.8%) | 3 (2.5%) | 0 (0.0%) |
| I | - | 30 (17.5%) | 16(13.2%) | 14 (28.0%) |
| II | - | 50(29.2%) | 30 (24.8%) | 20 (40.0%) |
| III | - | 57 (33.3%) | 51(42.1%) | 6 (12.0%) |
| IV | - | 31 (18.1%) | 21 (17.4%) | 10 (20.0%) |
| Total, n | 171 | 171 | 121 | 50 |
Metabolite analysis
A total of 140 metabolites were quantified in each urine sample by three LCMS assays. In the PITC assay, a total of 47 biologic amines were quantified in LC mode and a total of 75 lipids were semi-quantified in the FIA mode. In the organic acid assay, a total of 17 valuable organic acids were quantified. Ascorbic acid was quantified using a specific assay. For each assay, a total of 382 samples including both the CRC and control samples were randomized and analyzed using 5 plates in 96-well plate format. For each plate, a set of calibration curves was generated and used for quantification. Linear regression (R2) for the calibration curves of each metabolite were > 0.99 for all plates. For each plate, the LOQs were calculated to be three times the values of the matrix solutions and an average of LODs from 5 plates were reported in Table S1 and used for later analysis. Metabolites concentration that is lower than the LOD was unreliable and classified as missing value. A total of 46 metabolite features (including methyl-histidine, propionic acid, isobutyric acid, and 43 lipids) were removed as > 50% of the information was missing (Table S1). Three QC samples at different concentration levels were included in each 96-well plate to assess the coefficient of variation (CV%) across the 5 different plates. The CV% of QC samples for each metabolite was calculated as the standard deviation divided by the average. Notably, the CV% for each metabolite across was < 15% indicating a robust analytical method.
Potential biomarkers for CRC
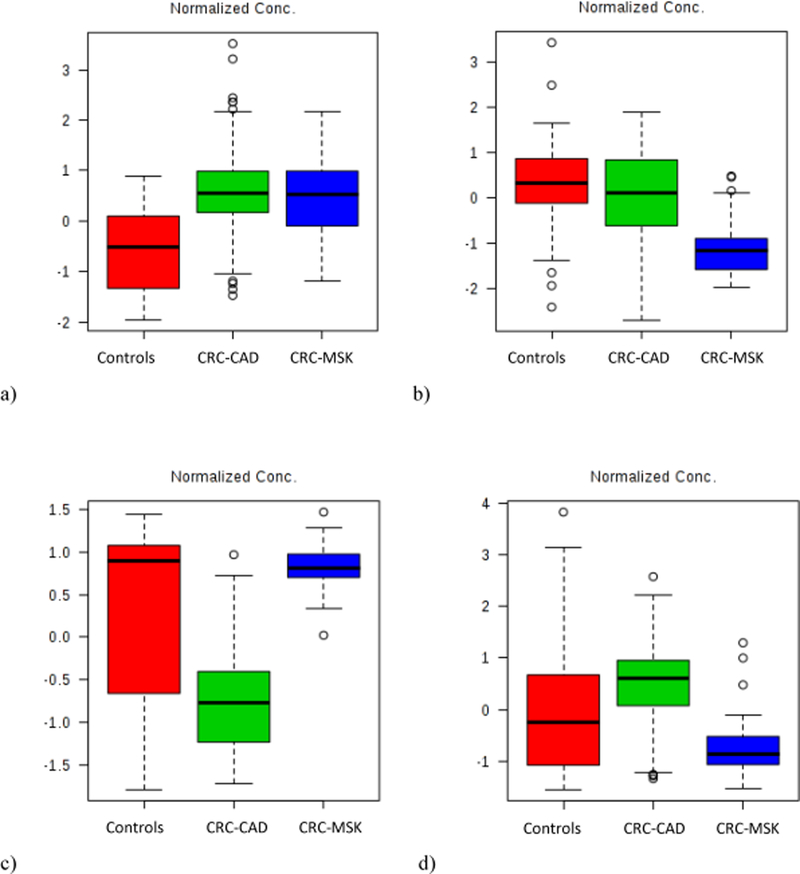
Potential biomarkers for CRC were identified by comparing the metabolomic profile from CRC versus controls for both the fold change analyses and T-tests. A total of 17 metabolites were identified by volcano plot with a threshold for fold change (FC) either > 2 or < 0.5 and p-value < 0.05 (Table 2). Results from the one-way ANOVA analyses for the three study groups identified consistent markers for CRC. For each of the 17 metabolites, the concentration change in either CRC group (e.g., CRC-CAD or CRC-MSK) compared to the control group were analyzed. Diacetylspermine (Figure 1a), proline, kynurenine, and glucose were upregulated in both CRC groups compared to controls and classified as consistent biomarkers. Although they were identified as potential markers according to the volcano plot for CRC cases versus controls, the concentrations of 3-(3-Hydroxyphenyl)-3-hydroxypropanoic acid (HPHPA, Figure 1b), beta-hydroxybutyric acid, 3,4-dihydroxyl phenylalanine (DOPA), 4-hydroxyproline, aminoadipic acid, putrescine, indole acetic acid, hippuric acid, citric acid, and sarcosine did not significantly change when CRC-CAD were compared to controls. Similarly, there were no significant changes in the concentrations of Tetradecenoyl carnitine (C14:1), and aspartic acid (Figure 1c), and sarcosine when CRC-MSK was compared to controls. When compared against the control group, the concentration of butyric acid (Figure 1d) increased in CRC-CAD and decreased in CRC-MSK. The concentration changes of 13 metabolites were dependent on the cohort rather than CRC status and were discarded from future analyses (Table 2).
Table 2.
Potential CRC biomarkers
| Metabolite | HMDB ID | Fold Change | p-value | Metabolite concentration change relative to controls | Consistent biomarker | |
|---|---|---|---|---|---|---|
| CRC-CAD | CRC-MSK | |||||
| 1. Diacetylspermine | HMDB02172 | 10.75 | 3.61E-31 | + | + | Yes |
| 2. Proline | HMDB00162 | 2.53 | 4.04E-31 | + | + | Yes |
| 3. C14:1 | HMDB62588 | 3.20 | 3.19E-22 | + | NC | No |
| 4. Kynurenine | HMDB00684 | 3.50 | 6.53E-16 | + | + | Yes |
| 5. Glucose | HMDB00122 | 3.06 | 1.90E-15 | + | + | Yes |
| 6. HPHPA | HMDB02643 | 0.33 | 9.44E-11 | NC | - | No |
| 7. Aspartic acid | HMDB00191 | 0.32 | 5.73E-10 | - | NC | No |
| 8. Beta-hydroxybutyric acid | HMDB00357 | 17.56 | 2.55E-09 | NC | + | No |
| 9. DOPA | HMDB00181 | 14.63 | 5.57E-09 | NC | + | No |
| 10. 4-Hydroxyproline | HMDB00725 | 2.53 | 1.31E-08 | NC | + | No |
| 11. Aminoadipic acid | HMDB00510 | 0.47 | 2.70E-08 | NC | - | No |
| 12. Putrescine | HMDB01414 | 3.78 | 1.36E-05 | NC | + | No |
| 13. Indole acetic acid | HMDB00197 | 0.21 | 2.06E-04 | NC | - | No |
| 14. Hippuric acid | HMDB00714 | 0.39 | 4.42E-04 | NC | - | No |
| 15. Citric acid | HMDB00094 | 3.07 | 1.18E-03 | NC | + | No |
| 16. Sarcosine | HMDB00271 | 14.68 | 1.82E-03 | NC | NC | No |
| 17. Butyric acid | HMDB00039 | 0.19 | 9.72E-03 | + | - | No |
Abbreviations: “+” indicates a significant metabolite concentration increase; “-” indicates a significant metabolite concentration decrease; “NC” means that the metabolite concentration was not significantly changed.
Figure 1 (a, b, c, and d).
Normalized concentrations of metabolites for controls, CRC-CAD, and CRC-MSK study groups for: a) diacetylspermine; b) HPHPA; c) aspartic acid; and, d) butyric acid.
Prediction models
To construct an effective diagnostic model for CRC, we conducted multivariate analysis using MetaboAnalyst. Among the PCA, PLS-DA, and sPLS-DA model options, sPLS-DA provided the best separation between the groups with the least number of metabolites. Figure 2a shows the separation plot from sPLS-DA with component 1 and component 2. The classification error rate was 11.4%. The metabolites selected by the sPLS-DA model for component 1 and component 2 with their loading value are shown in Figures 2b and 2c. Notably, diacetylspermine, proline, kynurenine, and glucose were among the top 6 selected features based on loading values for component 1. This confirms their selection as consistent markers.
Figure 2 (a, b, and c).
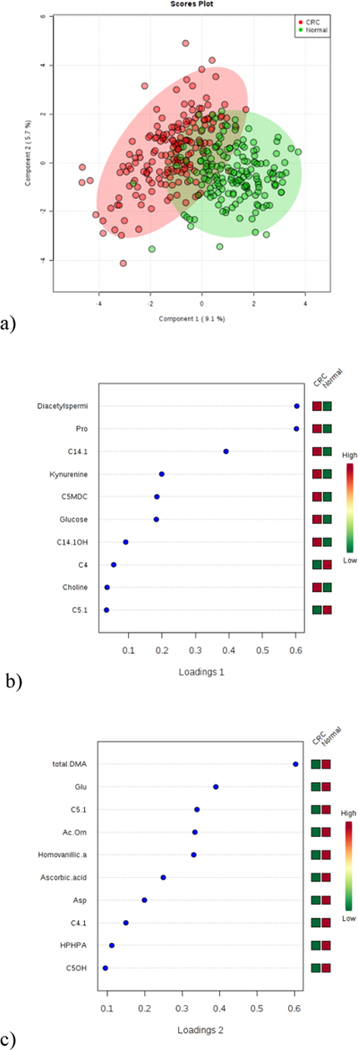
Results showing: a) separation plot from sPLS-DA with component 1 and component 2; b) variables selected by the sPLS-DA model for a component 1; and c) variables selected by the sPLS-DA model for a component 2.
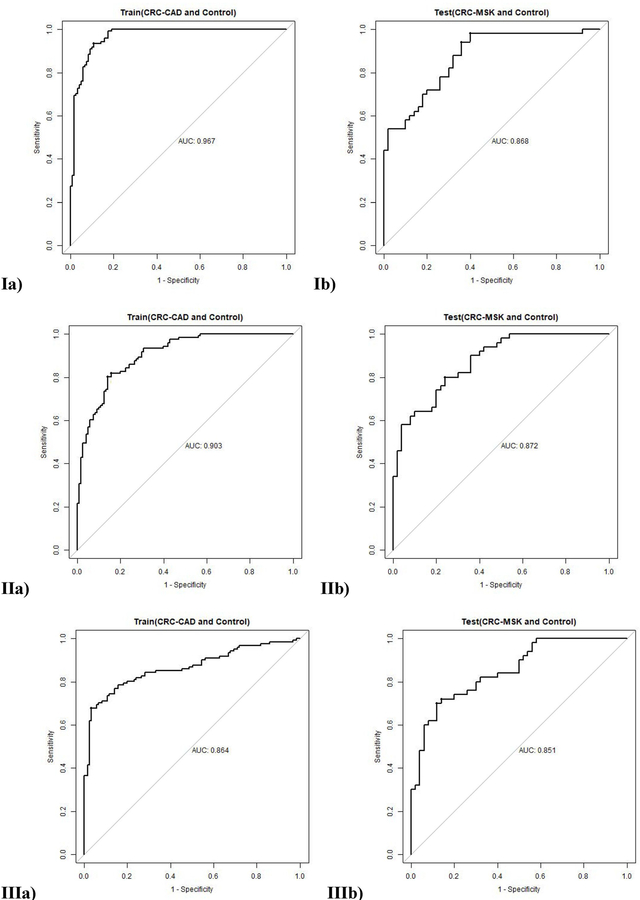
Finally, logistic regression models were constructed in R with selected metabolites. We used a leave-out approach to build and evaluate models as it is most rigorous. 121 CRC-CAD and 121 Controls were used as training set to build a model and 50 CRC-MSK and 50 Controls were used as testing set to validate the model. The first model (I) used the 17 metabolites listed in Table 2 selected according to the volcano plot of CRC versus control. This model had an area under the curve (AUC) value of 0.967 for training set and 0.868 for testing set (Figures 3.Ia and 3.Ib). At specificity of 80%, the model’s sensitivity were 99.2% for training set and 74.0% for testing set, respectively (Table 3). The second model (II) was limited to the four metabolites (e.g., proline, diacetylspermine, kynurenine, and glucose) identified as robust CRC biomarkers from the ANOVA analysis. The model had an AUC of 0.903 for training set and an AUC of 0.873 on testing set (Figures 3.IIa and 3.IIb) with a training sensitivity of 82.6% and a testing sensitivity of 72.% at specificity of 80% (Table 3). The last logistic regression model (III) incorporated only diacetylspermine and kynurenine. Proline and glucose were excluded due to their potential association with diet (36), a feature that was not controlled during the 24 hours prior to urine sample collection. With an AUC of 0.868 on training set and an AUC of 0.851 on testing set (Figures 3.IIIa and 3.IIIb), model III has the least AUC drop from training to testing among 3 models which confirmed the robustness of the selected biomarkers. At specificity of 80%, model III’s sensitivity were 80.0% for training set and 74.0% for testing set, respectively (Table 3).
Figure 3 (Ia, Ib, IIa, IIb, IIIa, IIIb).
The ROC curve of: Ia) Model I on training set using 17 metabolites, Ib) Model I on testing set using 17 metabolites, IIa) Model II on training set using 4 metabolites, IIb) Model II on testing set using 4 metabolites, IIIa) Model III on training set using diacetylspermine and kynurenine, and IIIb) Model III on testing set using diacetylspermine and kynurenine.
Table 3.
The AUC of the ROC curve, sensitivity, and specificity for each model
| Logistic regression models | Features | AUC | Sensitivity at specificity of 80% | ||||
|---|---|---|---|---|---|---|---|
| Train | Test | Delta (Train-Test) | Train | Test | Delta (Train-Test) | ||
| I | Proline, Diacetylspermine, C14.1, Kynurenine, Glucose, Aspartic acid, Glutamate, Beta-Hydroxybutyric acid, HPHPA, DOPA, c4-OH.Proline, Putrescine, Indole acetic acid, Citric acid, Hippuric acid, Sarcosine, and Butyric acid | 0.967 | 0.868 | 0.099 | 99.2% | 74.0% | 25.2% |
| II | Proline, Diacetylspermine, Kynurenine, and Glucose | 0.903 | 0.873 | 0.030 | 82.6% | 72.0% | 10.6% |
| III | Diacetylspermine and Kynurenine | 0.864 | 0.851 | 0.013 | 80.0% | 74.0% | 6.0% |
Discussion
We have identified a discrete subset of common urinary metabolites that may serve as potential biomarkers for CRC when used in combination based upon modelling to separate CRC and control samples. An sPLS-DA model with two components was built with a classification error rate of 11.4%. For logistic models, the AUC varied from 0.965 to 0.868 highlighting the predictive power of urinary metabolomics for CRC screening. However, given the sample size (n = 342), one needs to be conscientious about error due to overfitting the model. To guard against this, further analyses were performed by building a model that only used consistent biomarkers regardless of the cohorts. Finally, a metabolomic predictor for CRC was built with two metabolites: diacetylspermine and kynurenine. At its optimal cut-off value of 0.498, the predictor’s specificity and sensitivity values were 90.6% and 74.3%, respectively.
The mechanism of diacetylspermine and kynurenine being CRC markers still needs to be investigated. Here, we plotted the trend of their changes from control, to stage 0, to stage I, to stage II, to stage III, to stage IV in Figure 4. For both diacetylspermine and kynurenine, the biggest change was observed from control to stage 0 confirming the usage of these two markers for early screening. There was a continuous increase in diacetylspermine as the cancer progresses. The final metabolites, diacetylspermine and kynurenine, have been associated with cancer detection in the past. For instance, increased urinary kynurenine concentrations were first identified in patients with different malignancies by Spacek in 1955 (37). Urine samples were collected without dietary modifications, and the kynurenine levels increased from 1 to 7-fold in patients with CRC. Several teams have identified diacetylspermine’s presence in urine in association with hepatocellular carcinoma (sensitivity of 65.5%, specificity versus cirrhosis of 76.0%) (38), breast and colorectal cancers (sensitivity was 60.2% and 75.8%, respectively) (39), pancreatobiliary cancer (sensitivity of 75%) (40), and non-small cell lung cancer recurrence following resection (sensitivity 62.2%) (41). In spite of its utility, urinary diacetylspermine was unable to discriminate between patients with and without bladder cancer (42). Enrichment of proline has been identified as a biomarker for CRC based upon serum, tissue, urine, exhaled breath, and plasma (43). The urinary metabolite glucose is typically associated with reduced concentrations in samples from cancer patients compared to healthy controls while we report increased levels in both CRC groups (43).
Figure 4 (a,b).
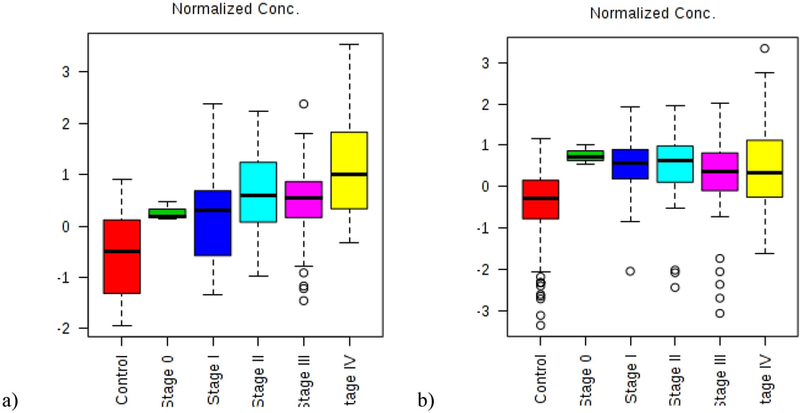
The normalized concentration trend for a) diacetylspermine and b) kynurenine from controls, to Stage 0, to Stage I, to Stage II, to stage III and to Stage IV.
Although our approach to diagnosing CRC is novel and promising, there were several limitations to this study. Smoking and age are known contributors to CRC (44). As such, we tried to match controls and CRC cases based upon smoking status and age, however this was not possible due to higher than expected rates of smoking (current, prior, never) and age in the two CRC groups. This may have impacted the selection of metabolites in a negative way. Urinary metabolites are waste products, it is unclear the upstream metabolic role of either diacetylspermine or kynurenine in cancer pathogenesis. Knowing more about the metabolic cycles and degradation pathways involved in CRC will be helpful to identify additional biomarkers. The specificity of the metabolic profile must also be evaluated by comparing with samples from patients with other cancer types. Though promising results were obtained, the metabolomic profile obtained cannot yet be considered definitive and need to be tested in clinical setting, ideally within a pragmatic study setting to make the findings relevant and generalizable to others. Testing the predictive performance of the metabolite profile against other cancers is especially relevant as diacetylspermine has been included in many non-CRC panels. In addition, it may be beneficial to make a more comprehensive metabolomic assessment. This could be done using additional analytical assays, such as gas chromatography-mass spectrometry (GC-MS), which will enable the detection of more metabolites (45). A more comprehensive metabolomic profile may improve diagnostic accuracy. It is possible that we could derive a better understanding of the underlying metabolic processes associated with CRC. We intentionally did not have patients follow a controlled diet or fast before providing a urine sample. Appreciating the diurnal changes in urinary metabolite concentrations (46), all collections were completed during daytime business hours. Dietary controls place unreasonable burdens on patients and believed that this would decrease the value of this or any urinary biomarker panel intended for use as a screening tool for CRC. Further, it is highly probably that differences in the intestinal microbiota between healthy individuals and those with CRC impact urinary metabolites more so than diet (47). A limitation of any large multicentre study is the need to handle, ship, and store the biosamples over time. To minimize metabolite degradation, all specimens were handled similarly regardless of collection date and aliquoting prior to the first freeze at −80oC prevented exposure to multiple free-thaw cycles (48,49).
In conclusion, this metabolomic-based predictor for CRC has potential clinical application for population-based CRC screening using urine; a preferred biosample that is readily available, straightforward to collect as part of any physician’s clinic visit, and acceptable to patients in most cultures. Further supporting the use of urine is availability of collection, handling, shipping, and storage protocols many of which have been instituted by major biobanks and repositories. A 2018 systematic review of 16 urinary metabolomic studies in CRC listed metabolites independently reported three or more times (47); none of which were the same as those we reported. As the largest, multicentre urine-based metabolomics study conducted to date (43,47), there were insufficient samples at each cancer stage to analyze them independently or in sequence to understand the disease trajectory. Larger data sets supported by comprehensive clinicodemographic characteristics will be valuable to discern the discrete shifts in metabolites associated with real time changes in cellular metabolism associated with disease. This will also facilitate external validation of putative biomarker panels such as that reported herein.
Supplementary Material
Acknowledgements:
We would like to express our deep gratitude to Dr. Richard N. Fedorak who contributed to this project and passed away on Nov 8, 2018.
Financial Support: This work was funded in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH) (Kathleen P. Ismond, Olusegun I. Alatise, and P.T. Kingham are supported by grant number UG3EB024965,) and Mitacs (Zhengjun Liu was supported by grant IT10425).
Conflict of Interest: L.D., K.P.I., and D.C. are employees, H.W. is the co-founder of Metabolomic Technologies Inc., Edmonton, Alberta, Canada. Metabolomic Technologies Inc. is commercializing a metabolomic-based urine test for the screening of colorectal cancer.
References
- 1.Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018;155(5):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube C Organized screening is better than opportunistic screening at decreasing the burden of colorectal cancer in the United States. Gastroenterology 2018;155(5):1302–4. [DOI] [PubMed] [Google Scholar]
- 3.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World journal of gastroenterology 2017;23(20):3632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64(10):1637–49. [DOI] [PubMed] [Google Scholar]
- 5.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. New England Journal of Medicine 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 6.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. The New England journal of medicine 2012;366(8):697–706. [DOI] [PubMed] [Google Scholar]
- 7.van Roon AH, Goede SL, van Ballegooijen M, van Vuuren AJ, Looman CW, Biermann K, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013;62(3):409–15. [DOI] [PubMed] [Google Scholar]
- 8.Zubero MB, Arana-Arri E, Pijoan JI, Portillo I, Idigoras I, Lopez-Urrutia A, et al. Population-based colorectal cancer screening: comparison of two fecal occult blood test. Frontiers in pharmacology 2014;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh H, Bernstein CN, Samadder JN, Ahmed R. Screening rates for colorectal cancer in Canada: a cross-sectional study. CMAJ open 2015;3(2):E149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Corley DA, Kamineni A, Garcia M, Zheng Y, Doria-Rose PV, et al. Patterns and predictors of repeat fecal immunochemical and occult blood test screening in four large health care systems in the United States. The American journal of gastroenterology 2018;113(5):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church J Complications of Colonoscopy. Gastroenterology Clinics of North America 2013;42(3):639–57. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis. JAMA internal medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossu G, Saba L, Minerba L, Mascalchi M. Colorectal Cancer Screening: The Role of Psychological, Social and Background Factors in Decision-making Process. Clinical practice and epidemiology in mental health : CP & EMH 2018;14:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborne JM, Flight I, Wilson CJ, Chen G, Ratcliffe J, Young GP. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient preference and adherence 2018;12:1825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liles EG, Coronado GD, Perrin N, Harte AH, Nungesser R, Quigley N, et al. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treatment and Research Communications 2017;10:27–31. [Google Scholar]
- 16.Lamb YN, Dhillon S. Epi proColon((R)) 2.0 CE: A Blood-Based Screening Test for Colorectal Cancer. Molecular diagnosis & therapy 2017;21(2):225–32. [DOI] [PubMed] [Google Scholar]
- 17.Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN endocrinology 2013;2013:893913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oboler SK, Prochazka AV, Gonzales R, Xu S, Anderson RJ. Public expectations and attitudes for annual physical examinations and testing. Annals of internal medicine 2002;136(9):652–9. [DOI] [PubMed] [Google Scholar]
- 19.Widlak MM, Neal M, Daulton E, Thomas CL, Tomkins C, Singh B, et al. Risk stratification of symptomatic patients suspected of colorectal cancer using faecal and urinary markers. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 2018;20(12):O335–O42. [DOI] [PubMed] [Google Scholar]
- 20.Guo C, Xie C, Chen Q, Cao X, Guo M, Zheng S, et al. A novel malic acid-enhanced method for the analysis of 5-methyl-2’-deoxycytidine, 5-hydroxymethyl-2’-deoxycytidine, 5-methylcytidine and 5-hydroxymethylcytidine in human urine using hydrophilic interaction liquid chromatography-tandem mass spectrometry. Analytica chimica acta 2018;1034:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima T, Katsumata K, Kuwabara H, Soya R, Enomoto M, Ishizaki T, et al. Urinary Polyamine Biomarker Panels with Machine-Learning Differentiated Colorectal Cancers, Benign Disease, and Healthy Controls. International journal of molecular sciences 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venalainen MK, Roine AN, Hakkinen MR, Vepsalainen JJ, Kumpulainen PS, Kiviniemi MS, et al. Altered Polyamine Profiles in Colorectal Cancer. Anticancer research 2018;38(6):3601–7. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Tso V, Wong C, Sadowski D, Fedorak RN. Development and validation of a highly sensitive urine-based test to identify patients with colonic adenomatous polyps. Clin Transl Gastroenterol 2014;5:e54.24646506 [Google Scholar]
- 24.Deng L, Chang D, Foshaug RR, Eisner R, Tso VK, Wishart DS, et al. Development and Validation of a High-Throughput Mass Spectrometry Based Urine Metabolomic Test for the Detection of Colonic Adenomatous Polyps. Metabolites 2017;7(3):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Fang H, Tso VK, Sun Y, Foshaug RR, Krahn SC, et al. Clinical validation of a novel urine-based metabolomic test for the detection of colonic polyps on Chinese population. International journal of colorectal disease 2017;32(5):741–3. [DOI] [PubMed] [Google Scholar]
- 26.Tso V ER, Macleod S, Ismond KP, Foshaug RR, Wang H, Joseph R, Chang D, Taylor N and Fedorak RN. Consistency of Metabolite Determination from NMR Spectra over Time and Between Operators. Metabolomics 2015;5(3):151. [Google Scholar]
- 27.Eisner R, Greiner R, Tso V, Wang H, Fedorak RN. A machine-learned predictor of colonic polyps based on urinary metabolomics. Biomed Res Int 2013;2013:303982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CK, Fedorak RN, Prosser CI, Stewart ME, van Zanten SV, Sadowski DC. The sensitivity and specificity of guaiac and immunochemical fecal occult blood tests for the detection of advanced colonic adenomas and cancer. International journal of colorectal disease 2012;27(12):1657–64. [DOI] [PubMed] [Google Scholar]
- 29.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research 2018;46(W1):W486–W94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altobelli E, Angeletti PM, Latella G. Role of Urinary Biomarkers in the Diagnosis of Adenoma and Colorectal Cancer: A Systematic Review and Meta-Analysis. Journal of Cancer 2016;7(14):1984–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu G, Zheng Y, Wang H, Sun J, Ma H, Xiao Y, et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. International Journal of Epidemiology 2016;45(5):1507–16. [DOI] [PubMed] [Google Scholar]
- 32.Stoessel D, Stellmann J-P, Willing A, Behrens B, Rosenkranz SC, Hodecker SC, et al. Metabolomic Profiles for Primary Progressive Multiple Sclerosis Stratification and Disease Course Monitoring. Frontiers in human neuroscience 2018;12:226-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delplancke TDJ, de Seymour JV, Tong C, Sulek K, Xia Y, Zhang H, et al. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Scientific reports 2018;8(1):36-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lê Cao K-A, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics 2011;12(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman JH, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Cifuentes A Foodomics: Advanced Mass Spectrometry in Modern Food Science and Nutrition Wiley; 2013. [DOI] [PubMed] [Google Scholar]
- 37.Spacek M Kynurenine in disease, with particular reference to cancer. Canadian Medical Association journal 1955;73(3):198–201. [PMC free article] [PubMed] [Google Scholar]
- 38.Enjoji M, Nakamuta M, Arimura E, Morizono S, Kuniyoshi M, Fukushima M, et al. Clinical significance of urinary N1,N12-diacetylspermine levels in patients with hepatocellular carcinoma. The International journal of biological markers 2004;19(4):322–7. [DOI] [PubMed] [Google Scholar]
- 39.Hiramatsu K, Takahashi K, Yamaguchi T, Matsumoto H, Miyamoto H, Tanaka S, et al. N(1),N(12)-Diacetylspermine as a sensitive and specific novel marker for early- and late-stage colorectal and breast cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11(8):2986–90. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi K, Nakamura M, Shirahane K, Konomi H, Torata N, Hamasaki N, et al. Urine diacetylspermine as a novel tumour maker for pancreatobiliary carcinomas. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2005;37(3):190–4. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y, Sakaguchi K, Horio H, Hiramatsu K, Moriya S, Takahashi K, et al. Urinary N1, N12-diacetylspermine is a non-invasive marker for the diagnosis and prognosis of non-small-cell lung cancer. British journal of cancer 2015;113(10):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stejskal D, Humenanska V, Hanulova Z, Fiala R, Vrtal R, Solichova P, et al. Evaluation of urine N1,N12-Diacetylspermine as potential tumor marker for urinary bladder cancer. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 2006;150(2):235–7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang F, Zhang Y, Zhao W, Deng K, Wang Z, Yang C, et al. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: a systematic review. Oncotarget 2017;8(21):35460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. International journal of cancer 2009;124(10):2406–15. [DOI] [PubMed] [Google Scholar]
- 45.Bathe OF, Shaykhutdinov R, Kopciuk K, Weljie AM, McKay A, Sutherland FR, et al. Feasibility of identifying pancreatic cancer based on serum metabolomics. Cancer Epidemiology Biomarkers & Prevention 2011;20(1):140–7. [DOI] [PubMed] [Google Scholar]
- 46.Ni Y, Xie G, Jia W. Metabonomics of Human Colorectal Cancer: New Approaches for Early Diagnosis and Biomarker Discovery. Journal of Proteome Research 2014;13(9):3857–70. [DOI] [PubMed] [Google Scholar]
- 47.Erben V, Bhardwaj M, Schrotz-King P, Brenner H. Metabolomics biomarkers for detection of colorectal neoplasms: a systematic review. Cancers 2018;10(246):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotter M, Brandmaier S, Prehn C, Adam J, Rabstein S, Gawrych K, et al. Stability of targeted metabolite profiles of urine samples under different storage conditions. Metabolomics 2017;13(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laparre J, Kaabia Z, Mooney M, Buckley T, Sherry M, Le Bizec B, et al. Impact of storage conditions on the urinary metabolomics fingerprint. Analytica chimica acta 2017;951:99–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.