Abstract
The lateral habenula (LHb) is activated by a range of aversive states including those related to alcohol withdrawal and has glycine receptors (GlyRs), a sensitive target of alcohol. However, whether GlyRs in the LHb contribute to alcohol-related behaviors is unknown. Here, we report that rats experiencing withdrawal from chronic alcohol consumption showed higher anxiety and sensitivity to stress compared to their alcohol-naïve counterparts. Intra-LHb injection of glycine attenuated these aberrant behaviors and reduced alcohol intake upon alcohol re-access. Glycine’s effect was blocked by strychnine, a GlyR antagonist, indicating that it was mediated by strychnine-sensitive GlyRs. Conversely, intra-LHb strychnine elicited anxiety- and depression-like behaviors in Naïve rats but not in withdrawal rats. Additionally, both the frequency and the amplitude of the spontaneous IPSCs were lower in LHb neurons in slices of withdrawal rats compared to naïve rats. Also, there were sporadic strychnine-sensitive synaptic events in some LHb neurons. Bath perfusion of strychnine induced a depolarizing inward current and increased action potential firings in LHb neurons. By contrast, bath perfusion of glycine or sarcosine, a glycine transporter subtype 1 inhibitor, inhibited LHb activity. Collectively, these data reveal that LHb neurons are under the tonic glycine inhibition both in physiological and pathological conditions. Activation of GlyRs reverses LHb hyperactivity, alleviates aberrant behaviors, and reduces alcohol intake, thus highlighting the GlyRs in the LHb as a potential therapeutic target for alcohol-use disorders.
Keywords: alcohol exposure and withdrawal, aversion, electrophysiology, elevated plus maze test, forced swimming test, strychnine
1. Introduction
Anxiety disorders and depression often occur in alcohol-dependent individuals (Kushner et al., 1990b; Weissman et al., 1980) and may contribute to excessive alcohol consumption, and the development of alcohol dependence (Heilig et al., 2010; Koob, 2003). However, the neurobiological mechanisms underlying these negative emotions are not entirely understood.
The lateral habenula (LHb) is critical for coding negative emotions, including those related to alcohol. Immunohistochemical (Glover et al., 2016) and electrophysiological (Tandon et al., 2017) experiments revealed increased LHb activity in ethanol-induced conditioned taste aversion (CTA); lesions of the LHb increased ethanol consumption (but see Donaire et al. (2019), and reversed the development of CTA (Haack et al., 2014). Consistent with this, we have shown that ethanol stimulated LHb neurons and led to conditioned place aversion (Zuo et al., 2017; Zuo et al., 2016a). Moreover, rats withdrawn from chronic excessive alcohol consumption showed depression- and anxiety-like behaviors, an increase in the release of glutamate, and in the activity of LHb neurons. Inhibition of glutamate transmission or LHb neuronal activity rescued anxiety- and depression-like behaviors in alcohol-withdrawal rats and decreased alcohol intake upon ethanol reaccess (Gregor et al., 2019; Kang et al., 2018; Kang et al., 2017; Li et al., 2017; Li et al., 2016). Thus, the LHb is a critical brain region in the pathophysiology of alcohol-related neuropsychiatric disorders (Shah et al., 2017).
Glycine is a major inhibitory neurotransmitter in the CNS (Aprison and Werman, 1965). Glycine receptors (GlyRs) are throughout the brain and are tonically activated by ambient levels of endogenous GlyR agonists and play a significant role in neuronal excitability. Glycine transporters regulate the extracellular glycine concentration. The glycinergic system controls a broad spectrum of physiological and pathological conditions, including various psychiatric and neurological disorders (Maguire et al., 2014), such as pain signaling (Perkins et al., 2010; Xiong et al., 2011; Xiong et al., 2012), hyperekplexia (Xiong et al., 2014), schizophrenia, depression, and anxiety (Cioffi and Guzzo, 2016; Harvey and Yee, 2013).
The glycinergic system is an important target of alcohol (Soderpalm et al., 2017). Alcohol could modulate glycine-evoked currents (Aguayo and Pancetti, 1994; Badanich et al., 2013; Forstera et al., 2017; Lu and Ye, 2011; Tao and Ye, 2002; Xie et al., 2013; Ye et al., 1998), alter glycine levels (Richardson and Rossi, 2017; Yamada et al., 2018) or agonist affinity (Welsh et al., 2009). Behavioral studies have demonstrated that targeting the glycinergic system could interfere with alcohol consumption (Li et al., 2012; Molander et al., 2007) and alcohol-withdrawal symptoms (Gonzalez, 1993; Harvey and Yee, 2013). Our prior electrophysiological study found that ethanol-sensitive GlyRs were expressed in the LHb (Xie et al., 2013), which raises the possibility that these GlyRs might be a relevant target for alcohol’s effects on the brain. In this report, we provide compelling evidence that strychnine-sensitive GlyRs in the LHb regulate alcohol consumption and withdrawal-related anxiety/depression-like behaviors.
2. Materials and Methods
2.1. Animals
Experiments were on male, adult Sprague-Dawley (SD) rats (7–8 weeks old at the start of the experiments). All procedures were performed according to the National Institute of Health’s guidelines and with the approval of the Animal Care and Utilization Committee of Rutgers, the State University of New Jersey. Rats were housed with a standard 12-h light/dark cycle in the animal center: lights were turned off at 10:00 am. They were housed single per cage, with food and water available ad libitum, unless otherwise indicated.
2.2. Experimental Outline
A total of 446 rats were assigned randomly into the water group (Naive, n = 190) and the alcohol group (n = 256). Rats in the alcohol group drank ethanol in the intermittent access to 20% ethanol two bottle free choice (IA2BC) paradigm (see 2.3. for details) for 8 weeks (24 ethanol-access sessions) before the tests/surgery. As listed in Table 1, these rats were divided into 7 groups. Rats in Groups 1–3 were used for the measurement of anxiety and depression levels in vivo, and for electrophysiological recording ex vivo. Rats in Group 4–7 received intra-LHb/ mediodorsal thalamic nucleus (MD) cannula implantation 1–2 weeks before local chemical infusion (artificial cerebrospinal fluid (aCSF), Glycine, Strychnine, or Strychnine plus Glycine). Each rat received 2–4 intracranial injections, with 1-week or 2-week intervals. Sucrose preference test (SPT) and forced swim test (FST) were conducted in the same groups of rats (group 2,5,7), while marble burying test (MBT) and elevated plus test (EPM) were conducted in the other groups (group 3,6,8). The experimenters were blinded to the treatment history of the animals.
Table 1.
List of experimental groups. Rats started to drink water (Naïve group) or ethanol (EtOH group) one week after arrival at the facility. Rats in groups 4–8 received cannula implantation to the LHb/MD at week 8. Anxiety-, depression-like behaviors were measured at weeks 10–16. Intra-LHb/MD infusion was conducted once per 14 days in each rat. Abbreviations: LHb: lateral habenula, MD: mediodorsal thalamic nucleus, /: not applicable, SPT: sucrose preference test, FST: forced swimming test, MBT: marble burying test, EPM: elevated-plus maze test, LMA: locomotor activity test. Stry, strychnine; Gly, glycine
| Group | Numbers of rats | Cannula location | Intra-LHb/MD fusion of Drugs | Experiments | |
|---|---|---|---|---|---|
| Naïve | EtOH | ||||
| 1 | 50 | 55 | / | Electrophysiology | |
| 2 | 12 | 13 | SPT & FST | ||
| 3 | 13 | 12 | MBT & EPM | ||
| 4 | 13 | LHb | aCSF, Glycine | Ethanol intake, LMA | |
| 5 | 60 | 59 | LHb | aCSF, Glycine, Strychnine, | Ethanol intake (EtOH Rats), |
| Stry+Gly | SPT & FST | ||||
| 6 | 55 | 55 | LHb | aCSF, Glycine, Strychnine, | Ethanol intake (EtOH Rats), |
| Stry+Gly | MBT & EPM | ||||
| 7 | 19 | MD | aCSF, Glycine | Ethanol intake (EtOH Rats), | |
| SPT & FST | |||||
| 8 | 20 | MD | aCSF, Glycine | Ethanol intake (EtOH Rats), | |
| MBT & EPM | |||||
2.3. Intermittent access to 20% ethanol in two-bottle free choice drinking procedure
Rats were trained to drink alcohol under the IA2BC paradigm, as described (Fu et al., 2016a; Fu et al., 2015; Li et al., 2012). Briefly, animals were given 24-h concurrent access to one bottle of 20% (v/v) ethanol in water and one bottle of water, starting at 10:00 am on Monday. After 24 h, the ethanol bottle was replaced with a second water bottle that was available for the next 24 h. This pattern was repeated on Wednesdays and Fridays, and the placement of the ethanol bottle was alternated to control for side preferences. On all other days, the rats had unlimited access to two bottles of water. Animal body weight was determined once per week on Wednesdays. The amount of ethanol or water consumed was determined by weighing the bottles before access and after 2h or 24h of access. Ethanol intake was measured by calculating grams of alcohol consumed per kilogram of body weight. Water and total fluid intake were also present by per kilogram of body weight.
2.4. Implantation of cannula
Ethanol-naïve or ethanol-drinking rats, stereotaxic surgery for the intra-LHb or intra- MD cannula implantation was performed using a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) under anesthesia, as described (Fu et al., 2016b; Zuo et al., 2017). Bilateral guide cannula (FIT 5MM C232G-1.5W-1MM PROJ, 22 gauge; Plastics One, Roanoke, VA) were inserted dorsally to the LHb (in mm: −3.9 AP, ±0.75 ML, −5.2 DV) or MD (−1.8 AP, ±0.75 ML, −5.2 DV). Histological verification was performed as described (Li et al., 2011). Seven rats with injection sites outside the LHb were excluded from further analysis.
2.5. Intra-LHb/MD injections of drugs
Behavioral tests performed to evaluate the anxious- and depression-like behaviors in 24h ethanol withdrawn rats and naïve rats were carried out between 10:00 and 18:00 at an illuminated room. Habituation to conditions of experimentation was performed the day preceding the experimental procedures. Drugs were administered into the LHb/MD as described (Zuo et al., 2017): the injector extended 1.0 mm beyond the guide cannula tip, infusion lasted for 120 sec, and injectors were left in place for an additional 60 sec to allow for diffusion. The compounds include: 200 nl/side aCSF, 0.15 ng/200 nl/side of glycine, 1.5 ng/200 nl/side of glycine, 0.37 ng/200 nl/side of strychnine, or 1.5 ng glycine plus 0.37 ng strychnine/200 nl/side of cocktail.
2.6. Measurement of spontaneous locomotor activity
Ten minutes after the intra-LHb infusion of aCSF or glycine, the rats were placed in the locomotor chambers (TruScan Photobeam Activity Monitors, 41 cm × 41 cm × 41 cm) for 120 min sessions. Movement in terms of total distance traveled (cm) was recorded automatically using TruScan 2.0 software, as described (Li et al., 2012).
2.7. Sucrose preference test (SPT)
Ethanol-naïve and ethanol-drinking rats were habituated in the home cage to two bottles of 1% (w/v) sucrose solution for 24 h on Tuesday (Li et al., 2017). On Thursday, these rats were water deprived for 22 h and then accessed to two identical bottles for 2 h in the dark phase, one containing water and the other 1% sucrose solution. The position of the bottles was counterbalanced. Sucrose preference was determined by the consumption of sucrose/ (consumption of water + consumption of sucrose).
2.8. Porsalt Forced swim test (FST)
The procedure was a modification of the technique described by Detke et al. (1995). Before the test, animals were housed in their home cages in a quiet testing room. Forced swim sessions were performed by placing rats in individual acrylic cylinders (height, 50 cm; diameter, 20 cm) containing water at 24°C to a depth of 28 cm (Fu et al., 2019; Kang et al., 2018). The FST paradigm includes two sections: a first 15-min pretest followed by a 5-min test 24 h later. The raters scored the behavior for each 5-s period (60 times for the 5-min test) as one of the following: (i) immobility - making only those movements necessary to keep its head above water; (ii) swimming - making active swimming movements; and (iii) climbing - making vigorous movements with the forepaws in and out of the water, usually against the cylinder walls.
2.9. Elevated plus maze (EPM) test
Anxiety-like behavior was assessed using a standard EPM apparatus, as described (Kang et al., 2017). Briefly, after 24h withdrawal from the last ethanol session, rats were placed in the central platform of the EPM with their heads directed toward the open arms. Their behavior during 5 min was then recorded by using Smart 3.0 (Pan lab Harvard Apparatus, Barcelona, Spain). The percent of open-arm entries (100×open/total entries) and of time spent in the open arms (100× open/total time) were calculated for each rat as standard anxiety indices. Travel distances were used as indices of locomotor activity.
2.10. Marble burying test (MBT)
Animals were individually housed in a Plexiglas cage (47 × 25 × 30 cm) and were left undisturbed before the marble-burying test. During the test, the animals were transferred to a fresh cage of the same size that contained 20 glass marbles (1.5 cm in diameter, arranged in a 4 × 5 grid) on top of the 10-cm thick bedding. After a 30-min test, the animals were returned to their home cages, and the number of marbles buried (to 2/3 their depth) with bedding was counted.
2.11. Brain Slice Preparation and Electrophysiology
Rats were sacrificed under deep anesthesia with ketamine/xylazine (80 mg/5 mg/kg, i.p.), and the brain was rapidly removed and placed in standard artificial cerebrospinal fluid (aCSF) containing (in mM): 126 mM NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 0.3 L-ascorbate, 11 glucose, and carbogenated (95% O2/5% CO2). Coronal slices (250 μm thick) containing the LHb were cut with a Compresstome VF-200 slicer (Precisionary Instruments Inc., Greenville, NC, USA), then incubated for 1 hour at room temperature (24–25°C) in carbogenated aCSF.
LHb neurons were visualized using infrared differential contrast and fluorescence microscopy (Leica Microsystems). Electrical signals were recorded with an Axon 700B amplifiers, a Digidata 1440A A/D converter, and Clampfit 10.4 software (Molecular Devices Co., Union City, CA, USA). Throughout the experiments, the bath was continually perfused with warm (33°C) carbogenated aCSF (2.0 ml/min). For IPSC (inhibitory postsynaptic current) recording, patch pipettes (6–8 MΩ) were filled with a solution containing (in mM): 135 KCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 5 MgATP, 1 GTP, pH 7.2. Patch pipettes were also filled with internal solutions (in mM) 140 cesium methanesulfonate (Cs-Me), 5 KCl, 2 MgCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 MgATP, 0.2 Guanosine-5’-triphosphate (GTP) for recordings under voltage-clamp. The spontaneous firing was recorded by the loose-patch cell-attached technique.
A liquid junction potential correction was subtracted off-line from all recordings (Pages 123–131 at Neher (1992). The calculated junction potential was +3.7 mV for the KCl based internal solution, and +15.1 mV for Cs-ME-based intracellular solution. Thus, with KCl-based pipette solution, the voltage membrane potential (Vm) was (−70.0 – 3.7=) −73.7 mV for the holding potential (VH) of −70 mV. With Cs-ME-based solution, the Vm was (+40 −15.1=) +24.9 mV for the VH of +40 mV and (−70 −15.1=) −85.1 mV for the VH of −70 mV.
Electrophysiological recordings were divided into three recording epochs, including a 5–10 min baseline, 6–10 min treatment, and 10–15 min washout session, as described (Zuo et al., 2013; Zuo et al., 2016b). Firing/IPSC data recorded during the initial control period were averaged and normalized to 100%. Postsynaptic current traces induced by chemicals were filtered at 10 Hz. The means of 30-s baseline before chemical application and 30 s during the maximum effect were calculated and subtracted to give the magnitude of the baseline.
2.12. Reagents and Drugs
We purchased glycine, strychnine, sarcosine, 6, 7-dinitroquinoxaline-2, 3-dione (DNQX), DL-2-amino-5-phosphono-valeric acid (DL-AP5), SCH50911, gabazine, tetrodotoxin (TTX), and other common salts from Sigma-Aldrich (St Louis, MO); ethanol, made from grains, 190 proof, stored in a glass bottle, from Pharmco Products (Brookfield, CT).
2.13. Data Analysis and Statistics
All data were expressed as mean ± S.E.M. All statistical calculations were carried out using SigmaPlot 14.0 (SYSTAT Software, USA). Behavioral data were analyzed by experimenters who were blinded to the treatment history. No statistical power calculation was conducted prior to the study. The sample size was based on our previous experience with this design. Behavioral data were analyzed with one-way (Treatment) analysis of variance (ANOVA), two-way (Dose or Treatment × time point) repeated-measures ANOVA, or by Student’s unpaired two-tailed t-test. Electrophysiology data were analyzed with two-way ANOVA (Subtype or Dose × Group) followed by Turkey post hoc comparisons, one-sample t-test, or unpaired two-tailed t-test, as appropriate. A Kolmogorov- Smirnov test was used to evaluate statistical significance for cumulative data. Linear regression was used to determine the relationship between firing rate and sIPSC frequency or the ratio of sIPSCs/sEPSCs. A value of z was applied to assess the significance of the difference between two correlation coefficients using the Fisher r-to-z transformation. p < 0.05 was considered significant.
3. Results
3.1. Intra-LHb infusion of glycine reduces the intake of and the preference for ethanol
To determine whether LHb GlyRs contribute to ethanol-related behaviors, we bilaterally infused glycine (0.15 and 1.5ng/200nl/side, Fig.1A1–2) into the LHb. This treatment significantly and dose-dependently reduced ethanol intake (main effect of treatment: F2,24 = 39.45, p < 0.001; Fig.1A1) and slightly increased the water intake (F2,24 = 4.93, p = 0.016; Fig. 1A2), without changing the total fluid intake (F2,24 = 2.56, p = 0.1) or the general locomotor activity (F1,17 = 0.025, p = 0.876; Fig. 1D). Co-infusion of strychnine, a selective antagonist of GlyRs, reversed the effects of glycine (Ethanol intake: F3,218 = 28.8, p < 0.001, post hoc p < 0.001 glycine vs. strychnine + glycine; Water intake: F3,218 = 6.09, p < 0.001, post hoc p = 0.005; Fig. 1C1–2), indicating that the effects of glycine are mediated by strychnine-sensitive GlyRs. Intra-LHb strychnine alone did not alter ethanol consumption (p > 0.5 strychnine vs. aCSF, Fig. 1C1) or water intake (p > 0.5, Fig. 1C2). To determine whether the effect of glycine is selective for the LHb, in a separate cohort of rats, we microinjected glycine into the mediodorsal thalamic nucleus (MD), a brain region that borders the habenular complex dorsally. Intra-MD glycine administrations did not significantly change ethanol intake (p > 0.5, Fig. 1B). As intra-LHb infusion of the moderate dose (1.5 ng/200 nl/side) but not the low dose (0.15 ng/200 nl/side) of glycine regulated ethanol consumption, in the following behavioral experiments, we microinjected glycine (1.5 ng/200 nl/side) into the LHb (Fig. 1E).
Figure 1.
Intra-LHb infusion of glycine reduces ethanol intake and preference. (A) Intra-LHb infusion of glycine of moderate dose (1.5 ng/200 nl/side) but not low dose (0.15 ng/200 nl/side) significantly reduced ethanol intake (A1) and slightly increased water intake (A2) on 2h and 24h after the onset of drinking. Data are means ± SEM. For all figures, numbers inside columns indicate the number of rats or neurons. *p < 0.05, **p < 0.01, ***p < 0.001 vs. aCSF. #p < 0.05, ###p < 0.001 vs. low dose of glycine. Two-way repeated measures (RM) ANOVA followed by Tukey multiple comparisons test. (B) Intra-MD infusion of glycine did not alter ethanol intake. (C) Co-application of strychnine reversed glycine-induced reduction on ethanol intake and enhancement on water intake. *p < 0.05, ***p < 0.001 vs. aCSF. &p < 0.05, &&&p < 0.001, glycine vs. Gly + Stry. @@@p < 0.001, glycine vs. strychnine. Two-way ANOVA. (D) Intra-LHb glycine did not affect the locomotion activity in rats withdrawn from alcohol (EtOH-WD). (E) Left, Cresyl violet-stained brain sections show the accurate guide cannula placements above the LHb. Scale bar = 1000 μm. Right, Dots show the injector tip placements. Numbers at right show the distance from bregma (in mm).
3.2. Intra-LHb infusion of glycine alleviates depression-like behaviors in ethanol-withdrawal (EtOH-WD) rats
Psychiatric disorders such as depression have high comorbidity with alcoholism (Driessen et al., 2001). Having found that LHb glycine regulates ethanol intake, we next assessed whether LHb GlyRs play a role in the depression-like behaviors. Consistent with our earlier observation, a depression-like phenotype was clear in rats at 24h withdrawal from chronic ethanol consumption, as reflected by the significant reduction in the preference for 1% sucrose in the sucrose preference test (SPT) compared to ethanol-naive rats (t = 2.5, p = 0.02; Fig. 2A). Additionally, forced swim tests (FST) showed that rats at 24h withdrawal from ethanol (EtOH-WD) had a significantly shorter climbing time (t = 4.13, p < 0.001; Fig. 2D) and longer immobile time (t = 3.35, p = 0.003), without a significant change in the swimming time (t = 0.75, p = 0.461), compared to the naïve rats. These depression-like behaviors in EtOH-WD rats were reversed by intra-LHb injections of glycine, including the deficit of sucrose preference (F3,54 = 6.15, p = 0.001, post hoc p = 0.008 vs. aCSF; Fig. 2B), and the performance in FST (Climbing: F3,54 = 16.43, p < 0.001, post hoc p < 0.001 vs. aCSF; Immobility: F3,54 = 13.79, p < 0.001, post hoc p < 0.001; Fig. 2E1–2). The effects of glycine were blocked by the co-application of strychnine (p < 0.01 vs. glycine; Fig. 2B, E1–2), while intra-LHb strychnine alone did not induce notable changes in EtOH-WD rats (p > 0.5 vs. aCSF). In contrast, naïve rats receiving intra-LHb strychnine displayed reduced sucrose preference (F3,54= 4.72, p = 0.005, post hoc p = 0.021 vs. aCSF; Fig. 2B) in the SPT, and decreased climbing (F3,54= 8.05, p < 0.001, post hoc p = 0.002; Fig. 2E1) while enhanced immobility (F3,54= 7.92, p < 0.001, post hoc p = 0.002; Fig. 2E2) in the FST. Thus, inhibition of LHb GlyRs could incite a typical depression-like phenotype in ethanol-naïve rats. On the other hand, modulation of GlyRs in the MD did not affect depression-like behaviors in EtOH-WD rats (p > 0.5 glycine vs. aCSF; Fig. 2C, F).
Figure 2.
Intra-LHb, but not intra-MD infusion of glycine produces antidepressant-like effects in EtOH-WD rats. (A-C) Sucrose preference test (SPT). (A), The sucrose preference was lower in EtOH-WD rats than naïve rats. $p < 0.05, Two-tailed unpaired t-test. (B) Effects of intra-LHb infusion of aCSF, glycine, strychnine, and strychnine + glycine on sucrose preference in naïve (left panel) and EtOH-WD rats (right panel). Strychnine reduced sucrose preference in Naïve rats, while glycine increased sucrose preference in EtOH-WD rats. (C) Intra-MD infusion of glycine did not alter sucrose preference in EtOH-WD rats. (D-F) Forced swimming test (FST). (D) EtOH-WD rats exhibited decreased climbing time but increased immobility time in the FST related to Naïve rats. (E) Effects of intra-LHb infusion of aCSF, glycine, strychnine, and strychnine + glycine on climbing time (E1) and immobility time (E2) in Naïve and EtOH-WD rats. The climbing time was decreased by strychnine in Naïve rats but was increased by glycine in EtOH-WD rats. *p < 0.05, **p < 0.01, ***p < 0.001 vs. aCSF, &p < 0.05, &&&p < 0.001 vs. strychnine + glycine. @p < 0.05, @@p < 0.01, @@@p < 0.001, glycine vs. strychnine. One-way ANOVA. (F) Intra-MD infusion of glycine did not affect the climbing and immobility time in EtOH-WD rats.
3.3. Intra-LHb infusion of glycine alleviates anxiety-like behaviors in EtOH-WD rats
Anxiety often occurs during alcohol withdrawal and may contribute to relapse drinking (Driessen et al., 2001; Kushner et al., 1990a; Sinha, 2001). Consistent with this observation, the current study showed a significant increase in anxiety-levels, indicated by the shorter time (t = 4.74, p < 0.001) and fewer entries to the open arms (t = 2.62, p = 0.015; Fig. 3A), without any changes in travel distance (t = 0.89, p = 0.381) in the elevated plus maze (EPM) in 24h EtOH-WD rats compared to their Naïve counterparts. These anxiety-like behaviors at EtOH-WD rats were rescued by intra-LHb infusion of glycine. Intra-LHb glycine significantly enhanced the percentage of time spent in the open arms (F3,51 = 10.67, p < 0.001, post hoc p < 0.001 vs. aCSF; Fig. 3B1). Moreover, intra-LHb glycine, regardless of the presence of strychnine, did not change open arm entries (F3,51 = 2.09, p = 0.113; Fig. 3B2) or the movement (F3,51 = 1.25, p = 0.302). The anxiolytic effect of intra-LHb glycine was also supported by the results of the marble burying test (MBT). Compared to the ethanol-naïve counterparts, EtOH-WD rats buried significantly more marbles (t = 3.27, p = 0.003; Fig. 3E), which was reversed by intra-LHb glycine (F3,51 = 6.96, p < 0.001, post hoc p = 0.003 vs. aCSF; Fig. 3F); individual comparisons also revealed that glycine’s effect was completely reversed by the co-application of strychnine (p < 0.001 glycine vs. strychnine plus glycine); whereas intra-LHb glycine did not alter anxiety levels in the ethanol-naïve counterparts (all p > 0.5 vs. aCSF; Fig. 3B, F). Notably, intra-LHb strychnine induced anxiety-like behaviors in the naïve counterparts, as reflected by that these rats reduced the time spent in the open arms in the EPM (F3,49 = 3.93, p = 0.014, post hoc p =0.019 vs. aCSF; Fig. 3B1), without altering the open arm entries (F3,49 = 1.66, p = 0.188; Fig. 3B2) or locomotion activity (F3,49 = 1.17, p = 0.333); and buried more marbles in the MBT (F3,49 = 8.56, p < 0.00, post hoc p < 0.001 vs. aCSF; Fig.3F). Moreover, neither intra-LHb strychnine (all p > 0.5 vs. aCSF; Fig. 3B, F) nor intra-MD glycine (Fig. 3C, F) significantly affected anxiety-like behaviors in the EPM, and MBT in EtOH-WD rats. Collectively, these data show a critical role for GlyRs in the LHb in anxiety-like behaviors in both EtOH-WD and Naïve animals.
Figure 3.
Intra-LHb infusion of glycine induces anxiolytic-like effects in EtOH-WD rats. (A-C) Elevated plus maze (EPM). (A) Compared with the ethanol-naïve control rats, EtOH-WD rats displayed anxiety-like behaviors in EPM by decreasing total time spent on open arms and the percentage of open arm entries. $p < 0.05, $$$p < 0.001, Two-tailed unpaired t-test. (B) Effects of intra-LHb infusion of aCSF, glycine, strychnine, and strychnine + glycine on EPM in Naïve and EtOH-WD rats. (C) Intra-MD infusion of glycine did not change the time spent on open arms in EtOH-WD rats. Two-tailed unpaired t-test. (E) EtOH-WD rats buried more marble than Naïve rats. $$p < 0.01. (F) Effects of intra-LHb infusion of aCSF, glycine, strychnine, and strychnine + glycine on the number of marbles buried in Naïve and EtOH-WD rats. *p < 0.05, **p < 0.01, ***p < 0.001 vs. aCSF, &p < 0.05 vs. strychnine + glycine. @p < 0.05, @@@p < 0.001, glycine vs. strychnine. One-way ANOVA. (G) Intra-MD infusion of glycine did not alter the number of buried marbles in EtOH-WD rats.
3.4. GABAergic transmission is reduced in LHb neurons of EtOH-WD rats
In LHb neurons voltage clamped at a Vm of −73.7 mV using the KCl-based internal solution, we found that the spontaneous inhibitory postsynaptic currents (sIPSCs) were mediated by both GABAARs and GlyRs. Both the frequency and the amplitude of these sIPSCs were significantly lower in the LHb neurons in slices of 24h EtOH-WD rats compared to naïve rats (frequency: t = 2.7, p = 0.009; amplitude: t = 2.5, p = 0.015; Fig. 4A1–3). A similar result was obtained with the cs-methanesulfonate-based internal solution (Fig. 4B). These data showed a decreased inhibitory tone on LHb neurons of EtOH-WD rats. To determine the relationship between glutamatergic transmission (spontaneous excitatory postsynaptic current, sEPSC), inhibitory transmission and neuronal activity, we calculated correlation coefficients between the initial rate of spontaneous firing and sIPSCs and the ratio of sIPSC/sEPSC frequencies from the same individual LHb neurons. The initial firing rate was negatively correlated with the initial sIPSC frequency of EtOH-WD rats but not Naïve rats (Fig. 4C). There was no significant difference in correlation coefficients between the 2 groups of animals (z = 1.19, p = 0.234; Fig. 4C). The initial firing rate of LHb neurons correlated negatively with the ratio of sIPSC/sEPSC frequencies; and the correlation coefficient was significantly higher in LHb neurons of EtOH-WD rats compared to that of Naïve rats (z = 2.07, p = 0.039; Fig. 4D). These data suggest that the balance of IPSC/EPSC signaling shifted towards reduced IPSCs, which may contribute to the hyperactivity of the LHb neurons of EtOH-WD rats.
Figure 4.
GABAergic transmission is reduced in the LHb neurons of EtOH-WD rats. (A1) Sample traces of sIPSCs of LHb neurons in slices from Naïve rats and EtOH-WD rats recorded at the voltage membrane potential (Vm) of −73.7 mV using the KCl-based internal solution. (A2) Representative graphs of the averaged cumulative probability of frequencies and amplitudes of sIPSCs demonstrating a significant decrease in LHb neurons of EtOH-WD rats vs. of Naïve rats. K-S test. (A3) The averaged initial sIPSC frequency and amplitude of LHb neurons of EtOH-WD rats were higher than that of Naïve rats. $p < 0.05, $ $p < 0.01. Two-tailed unpaired t-test. (B) Sample traces of spontaneous spiking (left), sEPSCs (middle), and sIPSCs (right) recorded from the same LHb neuron of Naïve or EtOH-WD rats using the cs-methanesulfonate based internal solution. (C) A plot of initial firing rate against the initial sIPSC frequency of Naïve and EtOH-WD rats; r: Pearson’s correlation coefficients. (D) A plot of initial firing rate against the initial sIPSC/sEPSC frequency ratio of Naïve and EtOH-WD rats.
3.5. Functional glycinergic synaptic events are sparse in LHb neurons
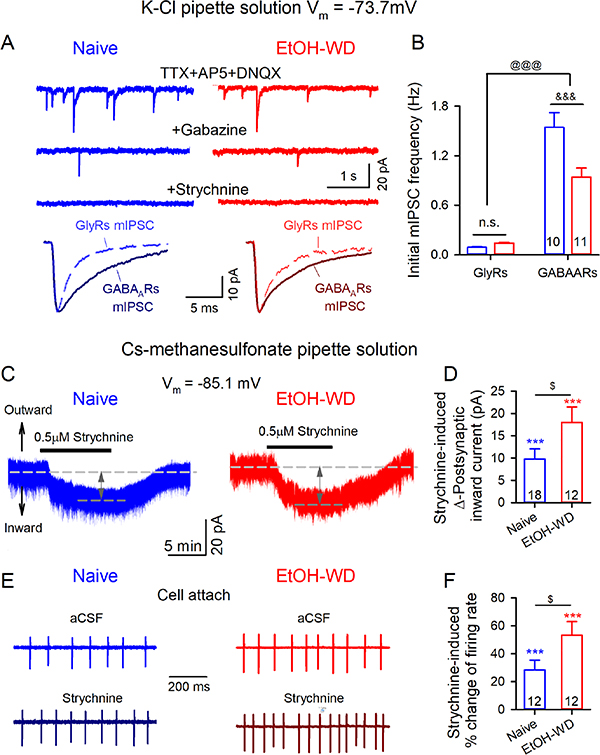
Next, we investigated whether glycinergic synaptic currents exist in the LHb. Gabazine (10 μM), in the presence of TTX and glutamate receptor antagonists (AP5 plus DNQX), completely abolished the spontaneous events in 12/22 and 12/23 LHb neurons in slices of Naïve and EtOH-WD rats, respectively, indicating that these events were mediated by GABAARs. In the other neurons (n = 10, 11 for Naïve and EtOH-WD rats, respectively), gabazine blocked about 90% of the events (Fig. 5A–B). The remaining events had a significantly faster decay time (τ) compared to the those blocked by gabazine and were eliminated by strychnine (Fig. 5A), indicating that they were mediated by strychnine-sensitive GlyRs. To determine whether a history of ethanol consumption and withdrawal would affect the extra-synaptic GlyRs, in the presence of TTX, gabazine, SCH50911, AP5 and DNQX, which block action potentials, GABAA, GABAB, NMDA and AMPA receptors respectively, bath application of strychnine induced a depolarizing inward current on 94.7% (18/19) and 100% (12/12) of LHb neurons of Naïve and EtOH-WD rats, respectively (Fig. 5C, D). Strychnine also significantly increased the ongoing firing rate (Fig. 5E, F). Furthermore, the effect of strychnine was greater in LHb neurons of EtOH-WD rats compared to naïve rats (inward current: t = 2.1, p = 0.047; firing: t = 2.1, p = 0.048; Fig. 5D, F). Together, these data show the presence of functional glycinergic synapses and extrasynaptic GlyRs on the LHb neurons, and the potentiation of the current mediated by extrasynaptic GlyRs by chronic ethanol consumption and withdrawal.
Figure 5.
LHb neurons are under tonic inhibition of strychnine-sensitive GlyRs. (A) Sample traces of miniature IPSCs recorded at a Vm of −73.7 mV using the KCl-based internal solution in the presence of TTX (0.5 μM) and glutamate antagonists (50 μM AP5 plus 20 μM DNQX), before and after bath application of the GABAAR antagonist gabazine (10 μM) or the cocktails of gabazine plus strychnine (0.5 μM) in slices from Naïve and EtOH-WD rats. Normalized (to peak amplitude) ensemble averages of exemplar GABAAR and GlyR mIPSCs superimposed on the same scale to illustrate the difference in their decay kinetics. (B) Graphs summarize the initial frequency of mIPSCs, showing a significant decrease in GABAergic but not glycinergic transmission on the LHb neurons of EtOH-WD rats. &&&p < 0.001, Naïve vs. EtOH-WD. @@@p < 0.001, GABAARs vs. GlyRs. n.s. no significant. Two-way ANOVA. (C) Sample traces of depolarized inward currents induced by strychnine (0.5 μM) at a Vm of −85.1 mV using the cs-methanesulfonate based internal solution, and in the presence of the Inhibitor Cocktail containing 0.5 μM TTX, 50 μM AP5, 20 μM DNQX, 10 μM gabazine and 10 μM SCH50911. (D) Summary graph of strychnine-induced inward currents in Naïve and EtOH-WD rats. ***p < 0.001 vs. baseline (aCSF). One-sample t-test. $p < 0.05, Naïve vs. EtOH-WD, unpaired t-test. (E) Strychnine accelerated ongoing discharges of LHb neuron. (F) Summary of strychnine-induced potentiation of firing.
3.6. Glycine inhibits LHb neurons via strychnine-sensitive GlyRs
To determine the influence of GlyRs on LHb activity, we applied glycine to the brain slices. Glycine (50–1000 μM) concentration-dependently inhibited ongoing spontaneous action potential firing of LHb neurons (main effect of dose: F3,94 = 17.51, p < 0.001), with a greater effect on LHb neurons from EtOH-WD rats than those from naïve rats (main effect of group: F1,91 = 23.82, p < 0.001), especially at 50 and 100 μM glycine (Group × Dose interaction: F3,94 = 4.46, p = 0.006; Fig. 6A–B). Strychnine reversed glycine-induced inhibition (F1,48 = 43.67, p < 0.001; Fig. 6A, C). In the presence of TTX, gabazine, SCH50911, AP5 and DNQX, glycine concentration-dependently elicited a hyperpolarizing outward current (F3,109 = 8.71, p < 0.001; Fig. 6D–E), which was larger in LHb neurons of EtOH-WD than naïve rats (F1,109 = 10.72, p = 0.001). There was no significant difference between the Group × Dose interaction (F3,109 = 0.59, p = 0.621). Strychnine abolished glycine-induced current in LHb neurons (F1,58 = 11.46, p = 0.001; Fig. 6F).
Figure 6.
Glycine inhibits LHb activity via activating strychnine-sensitive GlyRs. (A) Representative traces of spontaneous firing in the absence and presence of glycine (50, 300 μM) in Naïve ( ) and EtOH-WD (
) and EtOH-WD ( ) rats. (B) Summary graph of inhibition induced by glycine (50–1,000 μM). *p < 0.05, ***p < 0.001 relative to 50 μM glycine, @@@p < 0.001, Naïve vs. EtOH-WD. &&&p < 0.001, Naïve in comparison with EtOH-WD rats that underwent the same dose of glycine. Two-way ANOVA. (C) Mean inhibition of firing rate induced by 300 μM glycine in the absence and presence of 0.5 μM strychnine. ^^p < 0.01, ^^^p < 0.001, glycine vs. strychnine + glycine. (D) Glycine-induced hyperpolarizing outward currents at a Vm of −85.1 mV and in the presence of the Inhibitor Cocktail. (E) Summary graph of postsynaptic currents induced by glycine. (F) Mean hyperpolarizing outward currents induced by 300 μM glycine in the absence and presence of 0.5 μM strychnine. (G-H) Sample traces (left) and summary graph (right) of sarcosine (500 μM), a selective blocker of type 1 glycine transporters, induced changes in spontaneous firing (E) and postsynaptic currents (F) in slices from Naïve and EtOH-WD rats. Numbers inside column indicate the number of cells tested. **p < 0.01, ***p < 0.001 related to baseline. $p < 0.05, Naïve vs. EtOH-WD.
) rats. (B) Summary graph of inhibition induced by glycine (50–1,000 μM). *p < 0.05, ***p < 0.001 relative to 50 μM glycine, @@@p < 0.001, Naïve vs. EtOH-WD. &&&p < 0.001, Naïve in comparison with EtOH-WD rats that underwent the same dose of glycine. Two-way ANOVA. (C) Mean inhibition of firing rate induced by 300 μM glycine in the absence and presence of 0.5 μM strychnine. ^^p < 0.01, ^^^p < 0.001, glycine vs. strychnine + glycine. (D) Glycine-induced hyperpolarizing outward currents at a Vm of −85.1 mV and in the presence of the Inhibitor Cocktail. (E) Summary graph of postsynaptic currents induced by glycine. (F) Mean hyperpolarizing outward currents induced by 300 μM glycine in the absence and presence of 0.5 μM strychnine. (G-H) Sample traces (left) and summary graph (right) of sarcosine (500 μM), a selective blocker of type 1 glycine transporters, induced changes in spontaneous firing (E) and postsynaptic currents (F) in slices from Naïve and EtOH-WD rats. Numbers inside column indicate the number of cells tested. **p < 0.01, ***p < 0.001 related to baseline. $p < 0.05, Naïve vs. EtOH-WD.
To test the hypothesis that endogenous glycine mediates LHb activity, we inhibited glycine reuptake with sarcosine (500 μM) (McCracken et al., 2017), a selective blocker of type 1 glycine transporters. Sarcosine decreased the firing in 60% (6/10) and 82% (9/11) of LHb neurons from Naïve and EtOH-WD rats, respectively. The inhibition was stronger in LHb neurons of EtOH-WD rats compared to that of Naïve rats (t = 2.36, p = 0.035; Fig. 6G). Also, sarcosine induced a larger outward current in 71% (10/14) of LHb neurons of EtOH-WD rats and a small outward current in only 29% (6/17) of LHb neurons of Naïve rats (t = 2.2, p = 0.048, Naïve vs. EtOH-WD; Fig. 6H). These data show that glycine reuptake was altered in the LHb of EtOH-WD rats.
4. Discussion
Intra-LHb administration of glycine attenuated anxiety- and depression-like behaviors in rats experiencing withdrawal from chronic ethanol consumption and reduced ethanol intake upon ethanol reaccess. By contrast, intra-LHb strychnine induced anxiety- and depression-like behaviors in otherwise experimentally naïve rats. Bath perfusion of glycine and the glycine reuptake blocker inhibited LHb firing. On the other hand, bath perfusion of strychnine excited LHb neurons. Thus, GlyRs in the LHb are tonically activated under physiological and pathological conditions and play a significant role in the modulation of psychiatric status.
A net predominance of excitation over inhibition influencing LHb neuronal activity was observed in rodent models of depression (Shabel et al., 2014; Tchenio et al., 2017), drug addiction (Tan et al., 2018; Zuo et al., 2016a), and withdrawal from chronic alcohol consumption (Fig. 4B). Previous studies from others and our own have demonstrated that inhibition of LHb glutamatergic transmission or of LHb activity reduced ethanol consumption (Kang et al., 2018; Kononoff et al., 2018; Li et al., 2017). Here we showed that while blockade of GlyRs increased LHb activity (Fig. 5E), activation of GlyRs suppressed LHb activity (Fig. 6A). A single administration of glycine into the LHb reduced ethanol intake (Fig. 1A). Our data thus show that normalization of the balance between inhibitory/excitatory signaling, by activation of GlyRs in the LHb, may have therapeutic value for alcohol use disorders.
The LHb is involved in the depression-like behaviors seen in EtOH-WD rats (Kang et al., 2018; Li et al., 2017). In the present study, intra-LHb glycine decreased immobility in the FST without changing the swimming time, suggesting that glycine exerts antidepressant-like effects without altering motor function. Intra-LHb glycine also prevented the reduction in sucrose preference. Our data are in line with the previous observations that suppression of LHb hyperexcitability reversed deficits of sucrose preference (Zhang et al., 2018) and that the rescue of inhibitory transmission produced anti-depressive-like effects in rodents (Lecca et al., 2016). The EPM and MBT have been widely employed in evaluating anxiety-like behaviors in rodents (Calhoon and Tye, 2015; Kang et al., 2017). Withdrawal from chronic alcohol consumption reduced the exploratory activity in the EPM and increased the number of buried marbles in the MBT, indicating an anxiogenic effect of ethanol consumption and withdrawal. In the EPM, intra-LHb glycine induced anxiolytic-like responses, as reflexed by increasing the time spent on the open quadrants without altering locomotion activity. Interestingly, a recent rodent study showed that the inhibition of the LHb by muscimol, a GABAergic agonist, did not affect open field exploratory activity or the time spent on the open arms in the EPM (Tomaiuolo et al., 2014). Based on this finding and the data in the current study, we propose that targeting the GlyRs, instead of the GABAA receptors in the LHb, might modulate anxiety-like behaviors. Notably, LHb hyperactivity contributes to the anxiogenic behaviors induced by various stressful stimuli including withdrawal from chronic alcohol consumption (Kang et al., 2018; Park et al., 2017; Purvis et al., 2018). Activation of LHb GlyRs produced the anxiolytic-like/anti-compulsive-like effects, which were blocked by strychnine, indicating that they were mediated by strychnine-sensitive GlyRs. By contrast, intra-MD injection of glycine did not alter anxiety- or depression-like behaviors in EtOH-WD rats, suggesting that the effects of glycine are selective for the LHb.
In Naïve rats, intra-LHb glycine did not significantly change the behaviors we tested. In contrast, intra-LHb strychnine reduced exploration in the EPM and increased marble burying activity in the MBT and immobility in the FST. These data suggest that GlyRs in the LHb are tonically activated and play a significant role in the psychiatric status of the animals. In support of this view, systemic administration of strychnine has been reported to induce depression-like behaviors (Soegnen, 1965). Collectively, these data showed an essential role of LHb GlyRs in the regulation of anxiety and depression phenotypes.
The habenula is a crucial relay station between the forebrain and the midbrain. The glycinergic signaling in the LHb may target multiple downstream systems. Activation of LHb neurons inhibits the monoaminergic (dopaminergic, serotonergic, and noradrenergic) systems through GABAergic rostromedial tegmental (RMTg) neurons or local GABAergic interneurons in the midbrain (Fu et al., 2017; Fu et al., 2019; Lecourtier et al., 2008; Purvis et al., 2018; Zhou et al., 2017). Diminished monoamine neurotransmission is the primary causal factor in precipitating a depression/anxiety episode. Based on the data of our current and previous work (Xie et al., 2013), we propose that activation of LHb GlyRs will inhibit LHb neurons, which in turn restores the activity of the neurons in the midbrain monoamine nuclei and rescue the aberrant behaviors associated with chronic alcohol consumption and withdrawal.
The reduction in GABAergic signaling contributes to the imbalance of inhibitory/excitatory transmission and the hyperactivity of LHb neurons in EtOH-WD rats. In the LHb, both GABAergic and glycinergic neurotransmissions produce the phasic inhibition, and glycinergic transmission contributes to about 5–10% of the mIPSCs. Though the number of glycinergic events in LHb neurons is insignificant compared to GABAergic IPSCs, our data suggest that the GlyRs functionally modulate LHb excitability and play a critical role in withdrawal symptoms of alcohol-dependent animals. We found strychnine induced a larger depolarizing inward current and greater stimulation of LHb neurons from EtOH-WD rats than those from naïve rats, indicating that the formers are under a stronger tonic inhibition than the latter due to the continuous activation of extrasynaptic GlyRs. This inhibitory effect of glycine may partly counteract LHb hyperactivity that is resulted from hyper-glutamatergic and hypo-GABAergic transmission. Also, glycine could directly control LHb cell activity by eliciting hyperpolarizing outward currents and decreasing spontaneous spikes. Moreover, LHb GlyRs of EtOH-WD rats are more sensitive to glycine, via enhancing GlyR protein level or remodeling GlyR composition. Additional studies are needed to test this possibility.
Glycine is released by glial cells and glycinergic neurons in the CNS and is removed by two specific transporters, subtype 1 (GlyT1) and subtype 2 (GlyT2). GlyT1 is predominantly expressed by astrocytes and partly expressed in some glutamatergic terminals (Cubelos et al., 2005; Zafra et al., 1995). Rodent studies have shown that pharmacological blockade of GlyT1 decreased alcohol consumption and relapse-like behavior (Molander et al., 2007; Vengeliene et al., 2018). However, a previous clinical trial using systemic administration of the GlyT1 inhibitor org 25935 failed to observe a significant effect (de Bejczy et al., 2014). The reasons for failure are unclear but may indicate that the role of GlyT1 in regulating alcohol consumption is more complicated than we expected. In the current study, sarcosine, a GlyT1 inhibitor, suppressed LHb neuronal activity, most likely by increasing glycine levels, which activated GlyRs. These data support the presence of GlyT1 in the LHb and corroborate a previous histological study that showed the existence of intense GlyT1 immunoperoxidase staining in this region (Cubelos et al., 2005; Zeilhofer et al., 2005). Studies in several brain regions have demonstrated that acute or chronic ethanol exposure can alter synaptic glycinergic events (Richardson and Rossi, 2017; Yamada et al., 2018), modulate the expression of GlyT1 (Nimitvilai et al., 2016; Nunez et al., 2000), and regulate the function and composition of GlyRs (Cui and Koob, 2017; McCool et al., 2003), leading to various pathological and behavioral effects observed during ethanol consumption and withdrawal. In our case, activation of GlyRs by administering exogenous glycine or blocking GlyT1 activity elicited a more significant inhibition on LHb activity in EtOH-WD rats than in ethanol-naive rats. Based on the evidence of the presence of functional GlyRs and the tonic glycine conductance, we found glycine transmission in the LHb as a new therapeutic target for alcohol use disorders. Conversely, ethanol may have a dual effect on the glycinergic system in the LHb, potentiating the extrasynaptic GlyR function/expression, altering GlyR subunit composition, or increasing the membrane expression levels and the activity of GlyT1. These adaptations may be significant in the development of comorbid alcohol-related psychiatric disorders.
Conclusion
A reduction of the inhibitory signaling in LHb neurons contributes to LHb hyperactivity and the aversive symptoms in EtOH-WD rats. The results of the current study provide convincing evidence that glycine, by normalizing this imbalance, robustly decreases alcohol intake and alleviates the aversive behaviors (depression and anxiety) associated with ethanol withdrawal. These findings highlight the importance of better understanding the in vivo functions of GlyRs in the brain to unravel their roles in pathophysiological and behavioral processes.
Highlights.
Intra-LHb glycine rescues anxiety and depression in alcohol-withdrawal rats
Intra-LHb glycine reduces alcohol intake upon alcohol re-access
Intra-LHb strychnine induces anxiety- and depression-like behaviors in naïve rats
Strychnine induces depolarizing currents and excitation of LHb neurons
Glycine or sarcosine, a glycine transporter subtype 1 inhibitor inhibit LHb neurons
Authorship and Acknowledgements
J.H.Y., and W.Z. designed the research. W.Z., W.L., W.W., H.Z., and Q.K.Z. performed the electrophysiology and behaviors. W.Z., L.W., R.F., and M.N. analyzed the data and prepared the figures. W.Z., Q.K.Z., and J.H.Y. wrote the paper. All authors read over the manuscript. The authors thank Dr. Somdatta Gupta and Sharon Sebastian, who read over the manuscript. This research is supported by NIH-NIAAA AA021657, AA022292 (JHY). The authors declare no conflicts of interest, financial or otherwise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aguayo LG, Pancetti FC, 1994. Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther 270, 61–69. [PubMed] [Google Scholar]
- Aprison MH, Werman R, 1965. The distribution of glycine in cat spinal cord and roots. Life Sci 4, 2075–2083. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ, 2013. Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38, 1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM, 2015. Resolving the neural circuits of anxiety. Nat Neurosci 18, 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi CL, Guzzo PR, 2016. Inhibitors of Glycine Transporter-1: Potential Therapeutics for the Treatment of CNS Disorders. Curr Top Med Chem 16, 3404–3437. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Gimenez C, Zafra F, 2005. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex 15, 448–459. [DOI] [PubMed] [Google Scholar]
- Cui C, Koob GF, 2017. Titrating Tipsy Targets: The Neurobiology of Low-Dose Alcohol. Trends Pharmacol Sci 38, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bejczy A, Nations KR, Szegedi A, Schoemaker J, Ruwe F, Soderpalm B, 2014. Efficacy and safety of the glycine transporter-1 inhibitor org 25935 for the prevention of relapse in alcohol-dependent patients: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 38, 2427–2435. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I, 1995. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121, 66–72. [DOI] [PubMed] [Google Scholar]
- Donaire R, Moron I, Blanco S, Villatoro A, Gamiz F, Papini MR, Torres C, 2019. Lateral habenula lesions disrupt appetitive extinction, but do not affect voluntary alcohol consumption. Neurosci Lett 703, 184–190. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K, 2001. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol 36, 249–255. [DOI] [PubMed] [Google Scholar]
- Forstera B, Munoz B, Lobo MK, Chandra R, Lovinger DM, Aguayo LG, 2017. Presence of ethanol-sensitive glycine receptors in medium spiny neurons in the mouse nucleus accumbens. J Physiol 595, 5285–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Chen X, Zuo W, Li J, Kang S, Zhou LH, Siegel A, Bekker A, Ye JH, 2016a. Ablation of mu opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology 107, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J, 2015. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol 7, 136–144. [PMC free article] [PubMed] [Google Scholar]
- Fu R, Mei Q, Zuo W, Li J, Gregor D, Bekker A, Ye J, 2017. Low-dose ethanol excites lateral habenula neurons projecting to VTA, RMTg, and raphe. Int J Physiol Pathophysiol Pharmacol 9, 217–230. [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zuo W, Gregor D, Li J, Grech D, Ye JH, 2016b. Pharmacological Manipulation of the Rostromedial Tegmental Nucleus Changes Voluntary and Operant Ethanol Self-Administration in Rats. Alcohol Clin Exp Res 40, 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zuo W, Shiwalkar N, Mei Q, Fan Q, Chen X, Li J, Bekker A, Ye JH, 2019. Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ, 2016. Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol. Alcohol Clin Exp Res 40, 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LP, 1993. Sensitivity to strychnine seizures is unaltered during ethanol withdrawal. Alcohol Clin Exp Res 17, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Gregor DM, Zuo W, Fu R, Bekker A, Ye JH, 2019. Elevation of Transient Receptor Potential Vanilloid 1 Function in the Lateral Habenula Mediates Aversive Behaviors in Alcohol-withdrawn Rats. Anesthesiology 130, 592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA, 2014. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One 9, e92701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Yee BK, 2013. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov 12, 866–885. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC, 2010. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Li J, Bekker A, Ye JH, 2018. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology 129, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH, 2017. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-Type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononoff J, Kallupi M, Kimbrough A, Conlisk D, de Guglielmo G, George O, 2018. Systemic and Intra-Habenular Activation of the Orphan G Protein-Coupled Receptor GPR139 Decreases Compulsive-Like Alcohol Drinking and Hyperalgesia in Alcohol-Dependent Rats. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2003. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27, 232–243. [DOI] [PubMed] [Google Scholar]
- Kushner M, Nencini P, Reivich M, Rango M, Jamieson D, Fazekas F, Zimmerman R, Chawluk J, Alavi A, Alves W, 1990a. Relation of hyperglycemia early in ischemic brain infarction to cerebral anatomy, metabolism, and clinical outcome. Ann Neurol 28, 129–135. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD, 1990b. The relation between alcohol problems and the anxiety disorders. Am J Psychiatry 147, 685–695. [DOI] [PubMed] [Google Scholar]
- Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, Mameli M, 2016. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med 22, 254–261. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B, 2008. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci 27, 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH, 2011. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol 16, 600–614. [DOI] [PubMed] [Google Scholar]
- Li J, Kang S, Fu R, Wu L, Wu W, Liu H, Gregor D, Zuo W, Bekker A, Ye JH, 2017. Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology 126, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH, 2012. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J Pharmacol Exp Ther 341, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zuo W, Fu R, Xie G, Kaur A, Bekker A, Ye JH, 2016. High Frequency Electrical Stimulation of Lateral Habenula Reduces Voluntary Ethanol Consumption in Rats. Int J Neuropsychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH, 2011. Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain Res 1393, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EP, Mitchell EA, Greig SJ, Corteen N, Balfour DJ, Swinny JD, Lambert JJ, Belelli D, 2014. Extrasynaptic glycine receptors of rodent dorsal raphe serotonergic neurons: a sensitive target for ethanol. Neuropsychopharmacology 39, 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK, 2003. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res 963, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Lowes DC, Salling MC, Carreau-Vollmer C, Odean NN, Blednov YA, Betz H, Harris RA, Harrison NL, 2017. Glycine receptor alpha3 and alpha2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proc Natl Acad Sci U S A 114, E7179–E7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B, 2007. The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol 42, 11–18. [DOI] [PubMed] [Google Scholar]
- Neher E, 1992. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207, 123–131. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ, 2016. Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology 41, 1112–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Lopez-Corcuera B, Martinez-Maza R, Aragon C, 2000. Differential effects of ethanol on glycine uptake mediated by the recombinant GLYT1 and GLYT2 glycine transporters. Br J Pharmacol 129, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rhee J, Park K, Han JS, Malinow R, Chung C, 2017. Exposure to Stressors Facilitates Long-Term Synaptic Potentiation in the Lateral Habenula. J Neurosci 37, 6021–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL, 2010. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther 127, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis EM, Klein AK, Ettenberg A, 2018. Lateral habenular norepinephrine contributes to states of arousal and anxiety in male rats. Behav Brain Res 347, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Rossi DJ, 2017. Recreational concentrations of alcohol enhance synaptic inhibition of cerebellar unipolar brush cells via pre- and postsynaptic mechanisms. J Neurophysiol 118, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R, 2014. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 345, 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Zuo W, Kang S, Li J, Fu R, Zhang H, Bekker A, Ye JH, 2017. The lateral habenula and alcohol: Role of glutamate and M-type potassium channels. Pharmacol Biochem Behav 162, 94–102. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2001. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158, 343–359. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Lido HH, Ericson M, 2017. The Glycine Receptor-A Functionally Important Primary Brain Target of Ethanol. Alcohol Clin Exp Res 41, 1816–1830. [DOI] [PubMed] [Google Scholar]
- Soegnen E, 1965. Apparent Depression in the Absorption of Strychnine, Alcohol and Sulphanilamide after Oral Administration of Sodium Fluoride, Sodium Oxalate, Tetracemin and Sodium Phytate. Acta Pharmacol Toxicol (Copenh) 22, 8–18. [PubMed] [Google Scholar]
- Tan D, Nuno-Perez A, Mameli M, Meye FJ, 2018. Cocaine withdrawal reduces GABAB R transmission at entopeduncular nucleus - lateral habenula synapses. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon S, Keefe KA, Taha SA, 2017. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion. J Physiol 595, 1393–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Ye JH, 2002. Protein kinase C modulation of ethanol inhibition of glycine-activated current in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther 300, 967–975. [DOI] [PubMed] [Google Scholar]
- Tchenio A, Lecca S, Valentinova K, Mameli M, 2017. Limiting habenular hyperactivity ameliorates maternal separation-driven depressive-like symptoms. Nat Commun 8, 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo M, Gonzalez C, Medina JH, Piriz J, 2014. Lateral Habenula determines long-term storage of aversive memories. Front Behav Neurosci 8, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Rossmanith M, Takahashi TT, Alberati D, Behl B, Bespalov A, Spanagel R, 2018. Targeting Glycine Reuptake in Alcohol Seeking and Relapse. J Pharmacol Exp Ther 365, 202–211. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Myers JK, Harding PS, 1980. Prevalence and psychiatric heterogeneity of alcoholism in a United States urban community. J Stud Alcohol 41, 672–681. [DOI] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ, 2009. Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther 330, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Li G, Ye JH, 2013. Acute effects of ethanol on GABAA and glycine currents in the lateral habenula neurons of young rats. Open J Neurosci 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Chen SR, He L, Cheng K, Zhao YL, Chen H, Li DP, Homanics GE, Peever J, Rice KC, Wu LG, Pan HL, Zhang L, 2014. Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat Neurosci 17, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, Zhang L, 2011. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol 7, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, Zhang L, 2012. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J Exp Med 209, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Koga K, Kume K, Ohsawa M, Furue H, 2018. Ethanol-induced enhancement of inhibitory synaptic transmission in the rat spinal substantia gelatinosa. Mol Pain 14, 1744806918817969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA, 1998. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem 273, 3314–3319. [DOI] [PubMed] [Google Scholar]
- Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C, 1995. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci 7, 1342–1352. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM, 2005. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol 482, 123–141. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li K, Chen HS, Gao SQ, Xia ZX, Zhang JT, Wang F, Chen JG, 2018. Dorsal raphe projection inhibits the excitatory inputs on lateral habenula and alleviates depressive behaviors in rats. Brain Struct Funct 223, 2243–2258. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu MZ, Li Q, Deng J, Mu D, Sun YG, 2017. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep 18, 3018–3032. [DOI] [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye JH, 2013. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology 70, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH, 2017. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol 22, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Wang L, Chen L, Krnjevic K, Fu R, Feng X, He W, Kang S, Shah A, Bekker A, Ye JH, 2016a. Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula. Neuropharmacology 113, 178–187. [DOI] [PubMed] [Google Scholar]
- Zuo W, Xiao C, Gao M, Hopf FW, Krnjevic K, McIntosh JM, Fu R, Wu J, Bekker A, Ye JH, 2016b. Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms. Sci Rep 6, 32937. [DOI] [PMC free article] [PubMed] [Google Scholar]