Abstract
Background:
Embryonic ethanol exposure is known to increase alcohol drinking later in life and have long-term effects on neurochemical systems in the brain. With zebrafish having marked advantages for elucidating neural mechanisms underlying brain disorders, we recently tested and showed in these fish, similar to rodents, that low-dose embryonic ethanol stimulates voluntary consumption of ethanol while increasing expression of hypocretin/orexin (hcrt) neurons, a neuropeptide that promotes consummatory and reward-related behaviors. The goal of the present study was to characterize how embryonic ethanol affects early development of the hcrt system and produces persistent changes at older ages that may contribute to this increase in ethanol consumption.
Methods:
We utilized live imaging and Imaris software to investigate how low-dose embryonic ethanol (0.5%), administered from 22-24 hours post-fertilization, affects specific properties of hcrt neurons in hcrt:EGFP transgenic zebrafish at different ages.
Results:
Time-lapse imaging from 24-28 hpf showed that embryonic ethanol increased the number of hcrt neurons, reduced the speed, straightness and displacement of their migratory paths, and altered their direction early in development. At older ages up to 6 dpf, the embryonic ethanol-induced increase in hcrt neurons was persistent, and the neurons became more widely dispersed. These effects of embryonic ethanol were found to be asymmetric, occurring predominantly on the left side of the brain, and at 6 dpf, they resulted in marked changes in the anatomical location of the hcrt neurons, with some detected outside their normal position in the anterior hypothalamus again primarily on the left side.
Conclusions:
Our findings demonstrate that low-dose embryonic ethanol has diverse, persistent and asymmetric effects on the early development of hypothalamic hcrt neurons, which lead to abnormalities in their ultimate location that may contribute to behavioral disturbances, including an increase in ethanol consumption.
Keywords: hypocretin, orexin, prenatal ethanol exposure, fetal alcohol syndrome, zebrafish
Introduction
Alcohol use disorder is a major health problem resulting in great personal and societal cost (World Health Organization, 2018), with severe alcohol abuse and dependence impacting as many as 80 million people globally. There is strong evidence demonstrating that maternal consumption of alcohol during pregnancy increases the risk of offspring for developing alcohol use disorder (Alati et al., 2006). With about 10% of women known to drink alcohol during pregnancy and an additional 3% reporting binge drinking (Tan et al., 2015), there is a large volume of clinical and preclinical literature describing the effects of moderate-to-high levels of alcohol on neurochemical systems in the brain (Glass et al., 2014, Petrelli et al., 2018). There is also clinical evidence showing even low levels of fetal alcohol exposure to increase alcohol use and produce behavioral dysfunction in the offspring (Murray et al., 2016, Goldschmidt et al., 2019), suggesting that no amount of alcohol during pregnancy is safe. This is supported by animal studies from our laboratory (Chang et al., 2015, Sterling et al., 2016) and others (Chotro et al., 2007) showing low-dose ethanol during gestation in rats to increase intake of ethanol during adolescence. These alterations in behavior are persistent and occur in association with long-term neuronal changes, including an increased expression of orexigenic peptides (Chang et al., 2015, Chang et al., 2012).
Hypocretin/orexin (hcrt), an orexigenic neuropeptide expressed in a population of hypothalamic neurons, is known to be involved in promoting substance abuse and related behaviors such as increased ethanol consumption, arousal, anxiety, motivation and stress (Li et al., 2014), suggesting that this neuropeptide is a potential therapeutic target for preventing the development of alcohol use disorder (Moorman, 2018). Studies in rats demonstrate that maternal ethanol consumption at low doses stimulates drinking in offspring and increases the expression and density of hcrt neurons (Chang et al., 2015, Chang et al., 2012), and hypothalamic injection of this neuropeptide in adults increases ethanol intake and related emotional behaviors (Barson et al., 2011).
The zebrafish is an especially advantageous model for investigating the mechanisms of low-dose ethanol-induced neuronal changes, primarily due to its optical transparency, genetic tractability, small size, rapid and easy testing, and external development that permits precise control of ethanol dose and exposure time without the influence of maternal physiology (Gerlai, 2011). In addition, zebrafish have a comparable CNS that contains neuropeptide systems (Berman et al., 2009, Faraco et al., 2006), including a well-conserved hcrt system (Elbaz et al., 2017, Kaslin et al., 2004). Using a behavioral model developed in our lab, we have shown voluntary consumption of ethanol-gelatin by adult zebrafish to stimulate hcrt expression (Sterling et al., 2015) and central injection of hcrt/orexin-A to increase ethanol consumption (Sterling et al., 2016), similar to our findings in rodents (Chang et al., 2015, Chang et al., 2012). Although there are few reports of hcrt’s reward-related functions in zebrafish (Sterling et al., 2015, Sterling et al., 2016), there is evidence in this species that optogenetic activation (Singh et al., 2015) and overexpression (Prober et al., 2006) of hcrt neurons stimulate arousal behaviors, which are behaviors associated with and sometimes predictive of excess consumption of ethanol (Barson et al., 2013). Studies of other systems in the brain show that moderate-to-high doses (0.75-3.0%) of embryonic ethanol in zebrafish which produce morphological abnormalities (Ali et al., 2011) cause a decrease in neuronal differentiation (Joya et al., 2014) and reduce the number of neurons and neural precursor cells (Yin et al., 2014), and lower concentrations of ethanol (0.25-0.5%) reduce dopamine, serotonin and their metabolite levels in whole brain (Buske and Gerlai, 2011) while having no effect on levels of glutamate, aspartate, glycine and GABA (Mahabir et al., 2018a). Our studies in the anterior hypothalamus, in contrast, demonstrate that embryonic ethanol at low concentrations (0.25-0.5%), while producing no observable physical malformations, actually stimulates neurogenesis and expression of the orexigenic neuropeptides, galanin as well as hcrt, and increases voluntary ethanol consumption during adulthood (Sterling et al., 2016).
To advance our understanding of these neuronal changes induced by ethanol, we used in the present study live imaging of transgenic hcrt:EGFP zebrafish to characterize the effects of low-dose embryonic ethanol on early development of these hcrt neurons, including their number, migration and direction, which are crucial to the formation of synapses, neural circuits, and proper functioning. We also tested at older ages whether embryonic ethanol, in addition to affecting the number of hcrt neurons, alters their dispersion and position, and ultimately their anatomical location identified using brain registration and the annotated Zebrafish Brain Browser (Marquart et al., 2015). Our results in this report reveal marked and diverse effects of embryonic ethanol on early development of hcrt neurons, which lead them to migrate toward areas outside of their normal location in the anterior hypothalamus (AH), an effect likely to impact their neurocircuitry and cause behavioral disturbances later in life.
Materials and Methods
Animals and Housing
Transgenic hcrt:EGFP (Appelbaum et al., 2009) zebrafish (Danio rerio) of an AB/wildtype genetic background, and transgenic vglut2a:DsRed (Satou et al., 2012) of a wildtype genetic background, lines that were generously gifted to us (see Acknowledgements), were used in the present study. These hcrt:EGFP zebrafish were selected due to their strong EGFP labelling of hcrt neurons and projections and their colocalization with hcrt mRNA, albeit this colocalization was not quantified, allowing us to view the development of hcrt neurons using live imaging. The vglut2a:DsRed zebrafish, which exhibit a broad expression pattern of vesicular glutamate transporter 2a throughout the entire brain, were selected for crossing with hcrt:EGFP zebrafish to perform brain registration and alignment to the Zebrafish Brain Browser, in order to evaluate the anatomical location of hcrt neurons.
Adult zebrafish were group housed in 3 L tanks (Aquatic Habitat, Apopka, FL) with recirculating water flow at a temperature between 28-29°C and a pH between 6.9-7.4. Water was first filtered by reverse osmosis followed by the addition of CrystalSea Marinemix (Baltimore, MD) Bioassay Laboratory Formula salts, which primarily contains chloride, sodium, sulfate, magnesium, calcium, potassium and bicarbonate, in addition to other minor and trace elements. Conductivity of zebrafish housing water was maintained at 0.5-0.6 mS/cm.
All animals were maintained on a 12:12 hour light-dark cycle (8 AM lights on and 8 PM lights off) within an AAALAC accredited facility. Embryos were produced by natural spawning and raised in embryo media (E2) (29.2g NaCl, 1.27g KCl, 4.86 g CaCl2, 8.14g MgSO4 dissolved in 1 L of deionized water with 250 μl of Methylene Blue). They were maintained in 6 well cell culture plates in an incubator at 28-29°C and on a 12:12 light cycle. At 24 hpf, embryos were transferred to E2 + 0.003% phenothiourea (PTU) to inhibit melanin pigmentation and retain optical transparency for live imaging. All protocols were approved by the Rockefeller University Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals.
Embryonic Ethanol Treatment
The ethanol exposure procedure was conducted as described previously (Sterling et al., 2016) and is briefly summarized here. At 22 hpf, embryos were removed from the incubator, placed in a fresh solution of either 0.0% or 0.5% (vol/vol%) ethanol and then immediately returned to the incubator for 2 hours. After this 2 hour period, embryos were washed in E2 and either returned to the incubator or prepared for live-imaging.
Image Acquisition and Analysis
Following embryonic ethanol exposure, 24-28 hpf and 30 dpf zebrafish were mounted ventral side down within 1% low-melting-point agarose containing 15 mM Tricaine Methanesulfonate (Pentair, Cary, USA), similar to previous reports (Hall et al., 2009), within a 15-mm diameter well of a metal slide with a 24 x 50 x 1 mm glass coverslip adhered to the bottom via high vacuum grease. Time-lapse images were taken with an inverted Zeiss Axiovert 200 spinning disk confocal microscope equipped with a 25x water immersion lens. Z-stack images were acquired every 10-min with a 2 μm step size over the course of 4 hours between 24-28 hpf. Zebrafish at 3 and 6 dpf were mounted dorsal side down and imaged using an inverted Leica SP5 laser scanning confocal microscope with a 20x or 40x water immersion lens. Deconvolution of confocal images was performed using AutoQuant X3 software (Media Cybernetics, Rockville, USA), and images were analyzed using Imaris software (Bitplane, Zurich, Switzerland). Hcrt neuronal migration was tracked using the ‘Spot’ function in Imaris. Hcrt neurons were automatically identified by the software using a 6 μm threshold for cell size, and neurons not accounted for by the software were manually added. Spots were tracked over time using the autoregressive algorithm and edited to ensure each track corresponded to the correct neuron, and track displacement, speed, straightness and direction were calculated using Imaris. The dispersion of hcrt neurons within the XYZ planes at 30 hpf, 3 and 6 dpf was determined by positioning a reference frame at the midline and immediately ventral and posterior to the hcrt neurons, and by averaging the distance of each hcrt neuron from the reference frame along each axis.
Zebrafish Brain Registration and Zebrafish Brain Browser
Hcrt:EGFP zebrafish were crossed with the vglut2a:DsRed transgenic line and they were imaged using confocal microscopy at 6 dpf. We used Advanced Normalization Tools (ANTs) software (Avants et al., 2011) on the Rockefeller University’s High-Performance Computing cluster to perform registrations and align brains using parameters as previously described (Marquart et al., 2015). The registered brains for control and ethanol zebrafish were averaged and then aligned to the Zebrafish Brain Browser (Marquart et al., 2015) to evaluate anatomical location. The reference brain within the Zebrafish Brain Browser is from a 6 dpf zebrafish brain, and thus requires the registered images to be of 6 dpf brains for proper alignment. The ‘Spot’ function in Imaris software was used to quantify the number of hcrt neurons.
Statistical Analyses
Data from experiments evaluating hcrt neuron number, migration, direction, and dispersion were analyzed using a two-way ANOVA, which tested the within-subject main effect of age, the between subject main effect of ethanol, and the interactions between age and ethanol. We calculated the difference for each measure between control and ethanol treated fish on the left and right sides of the brain and performed paired-sample t-tests to determine if there were differences between the two sides of the brain in ethanol-induced changes of hcrt development. Significant interactions were followed by a post-hoc Holm-Sidak’s multiple comparisons test. To simplify the statistical analysis, we binned migratory data from 24-28 hpf for each hour of imaging. Comparisons of hcrt volume between control and embryonic ethanol zebrafish were analyzed using two-tailed Student’s t-test. Statistical analysis was performed using GraphPad Prism (GraphPad, La Jolla, CA). All data are presented as mean ± SEM.
Results
Embryonic Ethanol Exposure Increases the Number of Hcrt Neurons
Our first aim was to determine whether 2 hour (22-24 hpf) ethanol exposure to low-dose ethanol (0.5% v/v) alters the number of hcrt neurons early in development when they are known to be present in tight bilateral clusters (Faraco et al., 2006). In transgenic hcrt:EGFP zebrafish, time-lapse live imaging from 24-28 hpf revealed strong and consistent effects of ethanol on the number of hcrt neurons. Analyses of these bilateral hcrt clusters at each hour of imaging revealed a significant main effect of ethanol (F(1,40) = 61.24, p < 0.001) (Fig. 1A), reflecting an increased number of hcrt neurons from 24-28 hpf as shown by the representative photomicrographs at 24 hpf (Figs. 1B–C), as well as a significant main effect of age (F(4,40) = 4.08, p < 0.01), indicating an increased number of hcrt neurons over the four hours of imaging. There was no significant ethanol x age interaction (p = 0.34). While increasing the number of neurons, ethanol produced no change in their volume at 28 hpf (control: 321.9 ± 28.8 μm3, ethanol: 317 ± 24.7μm3, p = 0.89), indicating the lack of apoptosis and necrosis that are characteristically produced by higher ethanol doses (Kunzelmann, 2016). This increased number of hcrt neurons was confirmed at later ages, with tests at 30 hpf, 3 dpf and 6 dpf revealing significant main effects of ethanol (F(1,73) = 17.3, p < 0.001) and age (F(2,73) = 31.2, p < 0.001) (Fig. 1D), with no significant ethanol x age interaction (p = 0.95) and with no change in cell volume analyzed at 3 dpf (control: 452.9 ± 15.8 μm3, ethanol: 436.2 ± 14.8 μm3, p = 0.4). These results indicate a strong and persistent stimulatory effect of embryonic ethanol on the total number of bilateral hcrt neurons.
Figure 1.
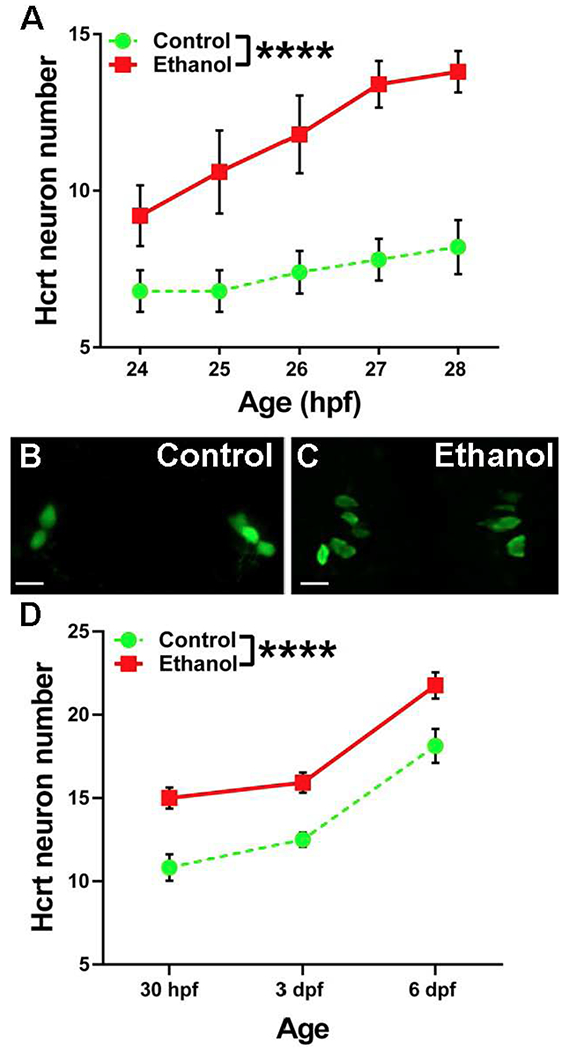
Effects of embryonic ethanol on the number of hcrt neurons from 24-28 hpf and 30 hpf, 3 dpf and 6 dpf. (A) Ethanol increases the number of hcrt neurons from 24-28 hpf. Two-way ANOVA main effect of ethanol. ****p < 0.001. (B) Representative image of a control 24 hpf hcrt:EGFP zebrafish. Scale bar = 10 μm. (C) Representative image of a 0.5% ethanol treated 24 hpf hcrt:EGFP zebrafish. Scale bar = 10 μm. (D) Ethanol increases the number of hcrt neurons from 30 hpf to 6 dpf. Two-way ANOVA main effect of ethanol. ****p < 0.001.
Embryonic Ethanol Exposure Alters the Migration, Direction and Dispersion of Hcrt Neurons.
We next tested if embryonic ethanol alters the migration, direction and dispersion of these bilateral hcrt neurons. Time-lapse imaging from 24-28 hpf showed in both control and ethanol exposed zebrafish that hcrt neurons at 24 hpf were located in the anterior region of the developing hypothalamus, adjacent to the forebrain ventricle, and were found to migrate in the dorsal and lateral directions. While initially moving in the anterior direction from 24-28 hpf, these neurons became progressively more posterior at the older ages, consistent with prior reports of their general location (Faraco et al., 2006). Analysis of the straightness of hcrt migratory tracks revealed a significant main effect of ethanol (F(1,434) = 19.08, p < 0.001) (Fig. 2A), with no main effect of age (p = 0.83) and no significant interaction (p = 0.43). There was also a significant main effect of ethanol on the speed of migration (F(1,434) = 6.77, p < 0.01) (Fig. 2B), with no main effect of age (p = 0.55) and no significant interaction (p = 0.06), indicating a reduction in migratory speed and straightness produced by ethanol. Analysis of displacement length (Fig. 2C) showed a significant ethanol x age interaction (F(3,434) = 6.24, p < 0.001) indicating a reduction in displacement from 27-28 hpf (p < 0.001), as illustrated in the photomicrographs (Figs. 2D–E). The migratory direction of these bilateral hcrt neurons was also affected by embryonic ethanol. While lateral track displacement of the total population was unaffected by ethanol (p = 0.33) (Fig. 2F), a significant main effect of ethanol (F(1,434) = 4.4, p < 0.05) and main effect of age (F(3,434) = 8.94, p < 0.0001), with no interaction (p = 0.46) on anterior track displacement revealed an overall reduction in anterior migration from 24-28 hpf (Fig. 2G). There was also a significant ethanol x age interaction (F(3,434) = 19.25, p < 0.0001) on dorsal/ventral track displacement which reflected a reduction in the dorsal migration from 26-27 hpf (p < 0.01) and 27-28 hpf (p < 0.001) (Fig. 2H). The dispersion of the bilateral hcrt neurons was also affected by ethanol. While there was a significant main effect of age (F(2,1254) = 105.2 , p < 0.001) with no effect of ethanol (p = 0.36) and no ethanol x age interaction (p = 0.10) on the lateral/medial dispersion of hcrt neurons indicating that they became more tightly clustered across this plane over time (Fig. 2I), we observed significant main effects of ethanol (F(1,1254) = 13.4, p < 0.001) and age (F(2,1254) = 28.43, p < 0.0001) on hcrt dispersion across the anterior/posterior plane (Fig. 2J) and a main effect of ethanol (F(1,1254) = 10, p < 0.01) on dorsal/ventral dispersion (Fig. 2K) with no effect of age (p = 0.07), which in ethanol-treated zebrafish reflected an increase in the spread of these neurons over a wider area along these planes. There were no significant ethanol x age interactions in hcrt dispersion across the anterior/posterior (p = 0.81) or dorsal/ventral (p = 0.52) planes. These findings suggest that the early effects from 24-28 hpf of ethanol on the migration and direction of the bilateral hcrt neurons causes them to become more widely dispersed and misplaced at older ages.
Figure 2.

Effects of embryonic ethanol on hcrt migration, direction and dispersion. (A) Embryonic ethanol reduces the straightness of hcrt migratory tracks from 24-28 hpf. Two-way ANOVA main effect of ethanol. ****p < 0.001. (B) Ethanol reduces the speed of hcrt migration from 24-28 hpf. Two-way ANOVA main effect of ethanol. **p < 0.01. (C) Ethanol reduces track displacement length of hcrt neurons at 28 hpf. Post-hoc Holm-Sidak’s test. ****p < 0.0001. (D-E) Representative hcrt migratory track in a control zebrafish (left) and ethanol zebrafish (right). The color of the track from blue to red represents time from 24-28 hpf, the white arrows show track displacement, and the white sphere in center of each hcrt neuron is Imaris ‘spot’ labeling. Scale bar = 2 μm. (F) ethanol has no effect on the lateral displacement of hcrt neurons from 24-28 hpf. (G) Ethanol reduces anterior displacement from 24-28 hpf. Two-way ANOVA main effect of ethanol.*p < 0.05. (H) Ethanol reduces the dorsal displacement of hcrt neurons at 26-27 hpf and 27-28 hpf. Post-hoc Holm-Sidak’s test. **p < 0.01, ****p < 0.0001 (I) Ethanol has no effect on lateral/medial dispersion of hcrt neurons at 30 hpf, 3 dpf and 6 dpf. (J) Hcrt neurons in ethanol zebrafish are more dispersed across the anterior/posterior plane at 30 hpf, 3 dpf and 6 dpf. Two-way ANOVA main effect of ethanol. **p < 0.01 (J) ethanol increases the dispersion of hcrt neurons across the dorsal/ventral plane at 30 hpf, 3 dpf and 6 dpf. Two-way ANOVA main effect of ethanol. ***p < 0.001.
Embryonic Ethanol Has Asymmetric Effects on the Early Development of Hcrt Neurons
With these analyses of the bilateral hcrt populations revealing marked ethanol-induced changes in the number, migration, direction and dispersion of the neurons, we next examined these measures separately on the two sides of the brain to determine whether any of these effects of ethanol were asymmetric. While the measures in control fish were symmetric and similar on both sides, we found ethanol exposure to produce strongly asymmetric effects, almost exclusively on the left side of the brain. Analyses using time-lapse imaging of the number of hcrt neurons from 24-28 hpf showed a significant main effect of ethanol on the left (F(1,40) = 51.2, p < 0.0001) with no effect of age (p = 0.11) and no interaction (p = 0.54), and a significant main effect of ethanol on the right side (F(1,40) = 11.8, p < 0.01), also with no main effect of age (p = 0.15) or interaction (p = 0.8), indicating an ethanol-induced increase of hcrt neurons on both sides. Comparison of the two sides of the brain revealed a significantly greater ethanol-induced change in hcrt neuron number on the left side compared to the right (t(4) = 7.7, p < 0.01) (Fig. 3A). This asymmetry in ethanol’s effect on hcrt number was confirmed at the older ages (30 hpf, 3 dpf and 6 dpf), with a significant main effect of ethanol (F(1,72) = 35.18, p < 0.0001) and age (F(2,72) = 31.36, p < 0.0001) on the left, and no effect of ethanol on the right (F(1,72) = 3.3, p = 0.07) with a main effect of age (F(2,72) = 22.64, p < 0.0001), thus confirming the greater increase of hcrt neurons on the left at these later ages (Fig. 3B). There were no significant ethanol x age interactions on the number of hcrt neurons on the left (p = 0.51) or the right (p = 0.7) at these older ages. While the measures from 24-28 hpf of straightness, displacement length, and migration in the dorsal and anterior directions were similarly reduced by ethanol on both sides of the brain, the speed of migration of hcrt neurons was asymmetrically altered with significant main effects of ethanol (F(1,207) = 10, p < 0.01) and age (F(3,207) = 2.89, p < 0.05) on the left with no ethanol x age interaction (p = 0.24), and no main effect of ethanol (p = 0.3), age (p = 0.6) and no interaction (p = 0.27) on the right (Table 1).
Figure 3.
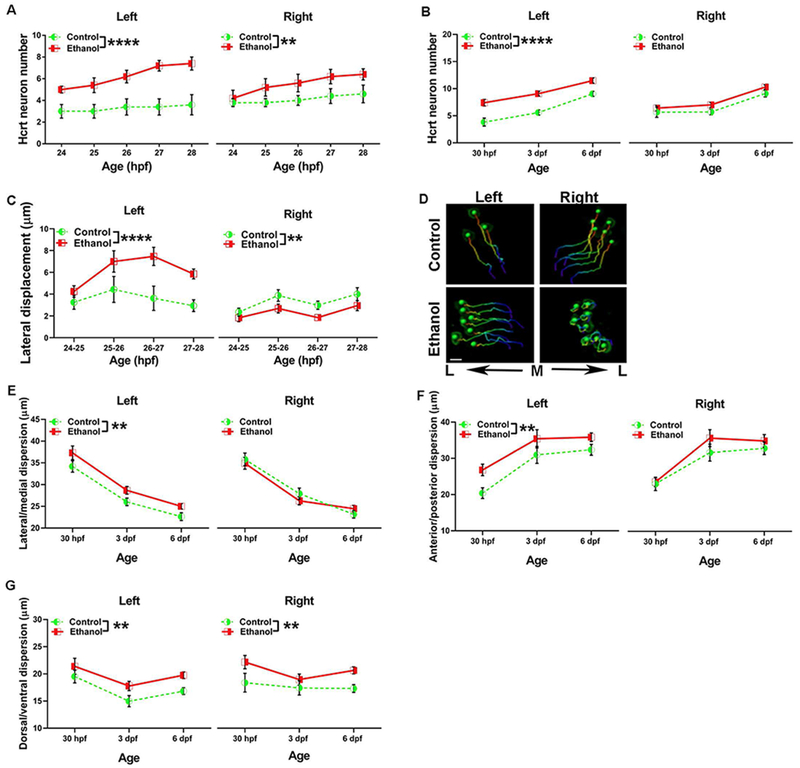
Asymmetric effects of embryonic ethanol on hcrt neuron number, lateral displacement and dispersion. (A) Embryonic ethanol produces an increase in the number of hcrt neurons from 24-28 hpf on the left side of the brain and on the right side. Two-way ANOVA main effects of ethanol. **p < 0.01, ****p < 0.0001. (B) This ethanol-induced increase in hcrt neurons persists at 30 hpf, 3 dpf and 6 dpf on the left but not on the right side. Two-way ANOVA main effect of ethanol. ****p < 0.0001. (C) Ethanol increased the lateral displacement of hcrt neurons from 24-28 hpf on the left, and had an opposite effect on the right, reducing lateral displacement. Two-way ANOVA main effect of ethanol. **p < 0.01, ****p < 0.0001. (D) Representative bilateral hcrt migratory tracks, with the color of the tracks from blue to red represents time from 24-28 hpf. The green spheres in the center of each hcrt neuron is Imaris ‘spot’ labeling at the end of the migratory track at 28 hpf. While control tracks show symmetrical migration between the left and right sides, lateral displacement of hcrt neurons in ethanol-treated fish is increased on the left and decreased on the right side. Scale bar = 5 μm. M: medial. L: lateral (E) Ethanol increases the lateral/medial dispersion of hcrt neurons at 30 hpf, 3 dpf, and 6 dpf on the left, but not the right. Two-way ANOVA main effect of ethanol. **p < 0.01. (F) Ethanol increases the anterior/posterior dispersion of hcrt neurons at 30 hpf, 3 dpf, and 6 dpf on the left but not the right. Two-way ANOVA main effect of ethanol. **p < 0.01. (G) Ethanol increases the dorsal/ventral dispersion of hcrt neurons at 30 hpf, 3 dpf, and 6 dpf on both the left and the right. Two-way ANOVA main effect of ethanol. **p < 0.01.
Table 1.
Hcrt neuronal migration and direction from 24-28 hpf.
| Left | Right | ||||
|---|---|---|---|---|---|
| Measure | Hpf | Control | Ethanol | Control | Ethanol |
| Straightness | 24-25 | 0.76 ± 0.04 | 0.64 ± 0.04 | 0.66 ± 0.03 | 0.51 ± 0.03 |
| 25-26 | 0.76 ± 0.04 | 0.64 ± 0.04 | 0.67 ± 0.06 | 0.56 ± 0.04 | |
| 26-27 | 0.68 ± 0.05 | 0.61 ± 0.05 | 0.68 ± 0.04 | 0.6 ± 0.04 | |
| 27-28 | 0.73 ± 0.04 | 0.61 ± 0.04 | 0.71 ± 0.05 | 0.53 ± 0.05 | |
| Speed | 24-25 | 2.9 ± 0.20 | 2.7 ± 0.18 | 2.5 ± 0.21 | 2.7 ± 0.3 |
| 25-26 | 3.3 ± 0.23 | 2.4 ± 0.17 | 2.4 ± 0.21 | 2.2 ± 0.22 | |
| 26-27 | 2.9 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.16 | 2.5 ± 0.22 | |
| 27-28 | 2.6 ± 0.29 | 2.02 ± 0.11 | 3.0 ± 0.33 | 2.26 ±0.28 | |
| Displacement length | 24-25 | 10.25 ± 1.07 | 10.44 ± 0.81 | 8.3 ± 0.83 | 9.67 ± 0.86 |
| 25-26 | 13.26 ± 1.9 | 7.33 ± 1.04 | 8.8 ± 1.44 | 7.54 ± 0.86 | |
| 26-27 | 13.16 ± 1.3 | 10.23 ± 1.32 | 9.9 ± 0.94 | 8.6 ± 1.05 | |
| 27-28 | 12.22 ± 1.647 | 7.18 ± 0.54 | 14.2 ± 1.8 | 7.0 ± 0.92 | |
| Lateral displacement | 24-25 | 3.25 ± 0.53 | 4.25 ± 0.62 | 2.41 ± 0.37 | 1.87 ± 0.37 |
| 25-26 | 4.44 ± 0.98 | 7.00 ± 1.19 | 3.99 ± 0.55 | 2.75 ± 0.42 | |
| 26-27 | 3.62 ± 0.87 | 7.46 ± 1.06 | 3.06 ± 0.39 | 1.89 ± 0.27 | |
| 27-28 | 2.94 ± 0.42 | 5.85 ± 0.58 | 4.12 ± 0.61 | 3.03 ± 0.47 | |
| Anterior displacement | 24-25 | 3.23 ± 1.67 | 2.65 ± 2.92 | 2.01 ± 1.29 | 3.57 ± 2.16 |
| 25-26 | 10.93 ± 1.08 | 6.39 ± 0.77 | 6.60 ± 1.53 | 5.62 ± 1.04 | |
| 26-27 | 9.44 ± 0.98 | 6.96 ± 1.41 | 7.83 ± 0.98 | 6.52 ± 1.34 | |
| 27-28 | 5.14 ± 1.05 | 1.87 ± 0.87 | 7.18 ± 1.75 | 2.82 ± 1.12 | |
| Dorsal displacement | 24-25 | 5.41 ± 0.78 | 4.92 ± 0.39 | 4.84 ± 0.67 | 3.10 ± 0.38 |
| 25-26 | 1.72 ± 0.47 | 1.70 ± 0.44 | 0.47 ± 0.32 | 0.74 ± 0.36 | |
| 26-27 | 3.18 ± 0.68 | 0.20 ± 0.45 | 1.79 ± 0.77 | 0.20 ± 0.39 | |
| 27-28 | 7.58 ± 1.66 | 0.01 ± 0.67 | 7.94 ± 1.57 | 0.41 ± 0.63 | |
Hours post-fertilization (hpf). Data are expressed as mean ± SEM; n = 5/group.
Whereas analyses of the combined bilateral clusters failed to reveal any effect of ethanol on lateral displacement (see above), this measure analyzed separately on the two sides was strongly asymmetrically altered, with a significant main effect of ethanol on the left (F(1,207) = 19.1, p < 0.0001), no main effect of age (p = 0.07) and no ethanol x age interaction (p = 0.4), showing hcrt neurons to migrate more laterally than in control fish. On the right, there was a significant main effect of ethanol (F(1,217) = 10.3, p < 0.01) and age (F(3,217) = 4.72, p < 0.01), with no interaction (p = 0.87), showing the neurons to remain more medially, reflecting a reduction in lateral migration (Fig. 3C), as illustrated by representative photomicrographs (Fig. 3D). This change in lateral displacement produced by ethanol was also greater on the left compared to the right side (t(3) = 4.9, p < 0.05). Analyses of the dispersion of these neurons at the older ages revealed main effects of ethanol on the left side in the lateral/medial plane (F(1,621) = 9, p < 0.01), but not on the right side (p = 0.7), with main effects of age on both the left (F(2,621) = 60.1, p < 0.0001) and the right (F(2,627) = 49.73, p < 0.0001) and no ethanol x age interactions on the left (p = 0.9) or right (p = 0.32) (Fig. 3E). Similarly, there was a main effect of ethanol on hcrt dispersion on the left side in the anterior/posterior plane (F(1,621) = 8.5, p < 0.01), but not on the right side (F(1,627) = 1.4, p = 0.24) (Fig. 3F), indicating asymmetric changes. There were main effects of age on both the left (F(2,621) = 14.79, p < 0.0001), and the right (F(2,627) = 11.9, p < 0.0001) side on anterior/posterior dispersion, with no significant ethanol x age interactions on the left (p = 0.76) or the right (p = 0.25). There were however symmetric changes along the dorsal/ventral plane with significant main effects of ethanol on both the left (F(1,602) = 9.7, p < 0.01) and right (F(1,614) = 10.8, p < 0.01) sides (Fig. 3G), with no differences in the change produced by ethanol between brain sides (t(2) = 0.3, p = 0.8). There was a main effect of age on the left side (F(2,621) = 6.65, p < 0.01) with no significant interaction (p = 0.86), and no main effect of age (p = 0.01) or ethanol x age interaction on the right side (p = 0.56). These results demonstrate that embryonic ethanol asymmetrically increases only on the left side the number of hcrt neurons, their lateral migration, and their medial/lateral and anterior/posterior dispersion, effects that may lead to changes in the ultimate location of hcrt neurons predominantly on the left.
Embryonic Ethanol Exposure Asymmetrically Alters the Anatomical Location of Hcrt Neurons
Our findings of marked and asymmetrical disturbances induced by embryonic ethanol on the number, migration, direction, and dispersion of hcrt neurons led us to our ultimate goal of determining more accurately in 6 dpf zebrafish whether ethanol ultimately causes these neurons to be located in abnormal anatomical positions. To do this, we registered and aligned brain scans of hcrt:EGFP zebrafish crossed with vglut2a:DsRed transgenic zebrafish to the annotated Zebrafish Brain Browser (Marquart et al., 2015), and averaged these scans (n=3) of hcrt neurons as illustrated by green or yellow colored neurons in control and red or yellow colored neurons in ethanol zebrafish in the dorsal view (Fig. 4A), lateral view (Fig. 4B), and posterior view (Fig. 4C). In control zebrafish, we found all hcrt neurons to be located in relatively tight bilateral clusters in the AH and to overlap with a previously scanned hcrt:RFP line (Singh et al., 2015), indicating their correct alignment. In the ethanol zebrafish, in contrast, we found the location of their hcrt neurons to be markedly altered, with approximately 25% (red color) found in areas outside their normal site in the AH. Some were located more anterior and dorsal in the preoptic area (POA) (Figs. 4A–C), while others were located more dorsal in the posterior tuberculum (PT) (Figs. 4B–4C) or more posterior, ventral, and lateral in the intermediate zone of the hypothalamus (IH) (Figs. 4A–C). These abnormally located hcrt neurons were invariably found on the left side of the POA and IH (Figs. 4A–C), although on the left as well as right in the PT (Fig. 4C). These results demonstrate that embryonic ethanol, after affecting the number, migration and dispersion of hcrt neurons early in development, asymmetrically alters their anatomical location primarily on the left side of the brain.
Figure 4.

Zebrafish Brain Browser results illustrating hcrt neurons in control (green color), ethanol (red color) and neurons with a shared location between control and ethanol (yellow color). (A) Dorsal view showing hcrt neurons in ethanol zebrafish abnormally located in the POA and IH on the left side as indicated by the arrows. Scale bar = 100 μm (B) Lateral view showing hcrt neurons in ethanol zebrafish abnormally located in the POA, PT and IH, indicated by arrows. (C) Posterior view showing hcrt neurons in ethanol zebrafish abnormally located in the POA and IH on the left side of the brain, and in the PT on both the left and right sides. POA: preoptic area. PT: posterior tuberculum. IH: intermediate zone of the hypothalamus.
Discussion
The findings of this study show that embryonic exposure to a relatively low concentration of ethanol markedly alters the early development of hypothalamic hcrt neurons, producing effects that persist at least until 6 dpf and that alter their ultimate location. Analyses of the total bilateral population of neurons on both sides of the brain during early development demonstrate that low-dose embryonic ethanol exposure for 2 hours increases the number of hcrt neurons and reduces the speed, straightness and displacement of their migration while decreasing their anterior and dorsal migration. At older ages, embryonic ethanol increases hcrt neuron number and their dispersion across the anterior/posterior and dorsal/ventral planes. In addition, separate analyses of the two sides of the brain show some effects of ethanol on early hcrt development to be asymmetric occurring only on the left, including the increase in number, lateral migration, and dispersion of hcrt neurons. These effects lead to changes in the anatomical location of some hcrt neurons, which were detected in regions outside the AH again predominantly on the left side. This study reveals for the first time the dramatic, persistent and asymmetric alterations induced by low-dose embryonic ethanol in the early development of hcrt neurons which ultimately affect the location of these neurons and may have lasting functional consequences.
Whereas embryonic ethanol exposure is found to reduce whole-brain levels of dopamine and serotonin in zebrafish (Buske and Gerlai, 2011), as well as reduce the number of cells expressing the pan-neuronal marker elavl3:gfp (Buckley et al., 2019) and HuC+ neurons in spinal cord (Joya et al., 2014), effects similarly observed in children with FASD (Riikonen et al., 2005) and rodents (Gil-Mohapel et al., 2010; Livy et al., 2003), our results here in the anterior hypothalamus reveal a very different effect, with 0.5% ethanol exposure found to increase the number of hcrt neurons, not only at 24-28 hpf but also at 30 hpf, 3 dpf and 6 dpf. This stimulatory effect of low-dose ethanol on the neurogenesis of orexigenic neuropeptides is consistent with our prior reports in both zebrafish (Sterling et al., 2016) and rodents (Chang et al., 2015, Chang et al., 2012) and with another study showing ethanol consumption in rats to increase prepro-orexin mRNA in the lateral hypothalamus (Lawrence et al., 2006). Using in situ hybridization and immunohistochemistry, we further demonstrated that 0.5% embryonic ethanol exposure in zebrafish increases at 4-5 dpf the density of hcrt-expressing as well as galanin-expressing neurons and the density of HuC/D+ neurons that co-label the proliferation marker BrdU, suggesting an increase in neurogenesis of neuropeptides (Sterling et al., 2016). The possibility that this increase in the number of hcrt neurons likely affects behavior later in development is supported by our further evidence that 0.5% embryonic ethanol exposure in zebrafish increases voluntary ethanol-gelatin intake during adulthood (Sterling et al., 2016). This is consistent with our report in rats showing prenatal ethanol exposure at low doses to stimulate neurogenesis and increase expression of orexigenic neuropeptides, such as melanin-concentrating hormone, enkephalin, and galanin similar to hcrt, in conjunction with increased voluntary ethanol consumption during adolescence (Chang et al., 2015, Chang et al., 2012).
Disturbances in neuronal migration early in development are known to cause aberrant neuron positioning and synaptic connectivity, which in turn are linked to neurodevelopmental disorders in humans (Moffat et al., 2015). Our results in ethanol fish reveal marked disturbances from 24-28 hpf, showing alterations in the migration and direction of the bilateral hcrt neurons, including reduced displacement length, speed, and straightness and reduced migration in the dorsal and anterior directions. These results are consistent with a study of cranial neural crest cell migration in zebrafish showing ethanol to reduce the directionality of migration from 4 to 20 hpf (Boric et al., 2013). They also agree with a report of prenatal ethanol exposure in rodents, showing ethanol to inhibit the migration of serotonin neurons in the midline raphe of mouse embryos (Zhou et al., 2001) and to delay cortical neuron migration in rat embryos (Miller, 1993). In addition, our z-stack images from ethanol-treated zebrafish at the older ages of 30 hpf and 3 and 6 dpf show hcrt neurons at these ages to be more widely dispersed along the different planes. This effect may be attributed to a reduction in cell adhesion molecules which is known to be caused by ethanol (Charness et al., 1994), with neurons positive for neuronal cell adhesion molecule reported to be reduced in the brain of adult zebrafish exposed from 24-26 hpf to 1% ethanol (Mahabir et al., 2018b). Together with this evidence, our results demonstrate that low-dose embryonic ethanol, while initially reducing neuronal migration, subsequently causes an increase in the dispersion of hcrt neurons, which persists across the older ages at least until 6 dpf and may impact the ultimate location of these neurons and perhaps their projections and functioning.
While in control zebrafish these different measures of hcrt development were found to be symmetric at all ages and similar on each side of the brain, many of the changes induced by low-dose embryonic ethanol were found to be asymmetric. Left-right asymmetries are known to occur naturally in a number of peripheral organs and CNS structures (Blackiston and Levin, 2017). For instance, the pineal and parapineal organs located in the epithalamus region of zebrafish develop asymmetrically, with a left bias that is seen in 95% of the population (Halpern et al., 2003). Also, located in the epithalamus, the habenula which is made up of lateral and medial subnuclei also exhibits asymmetry, with sensory neurons that respond to visual stimuli lateralized in the left habenula and those that respond to olfactory stimuli located in the right habenula (Taylor et al., 2010, Alqadah et al., 2018). Disturbances in these natural asymmetries or the presence of irregular left-right differences are known to cause developmental and behavioral abnormalities (Blackiston and Levin, 2017). This is evident in zebrafish, with disturbances in the naturally asymmetric epithalamus leading to reduced exploratory behavior and increased cortisol levels (Facchin et al., 2015). It is also seen in adult mice, with gestational exposure to ethanol showing an enlargement of the left hippocampus (Marjonen et al., 2015), and in humans, with abnormal asymmetries linked to a number of disorders including alcohol use disorder (Hanson et al., 2010). Additionally, meta-analyses of neuroimaging studies in humans report greater left hemisphere activity in response to approach-related emotions and drug cue-induced cravings and greater right hemisphere activity in response to withdrawal-related emotions and impulsivity (Murphy et al., 2003, Gordon, 2016).
Interestingly, we observed markedly asymmetric effects of embryonic ethanol on the lateral migration of hcrt neurons, which was increased on the left while decreased on the right. This result has similarities to a study in zebrafish, showing ethanol to initially increase the lateral migration of neural crest cells on the left side toward the right and also the migration of cells located on the right side towards the left (Boric et al., 2013). Although the functional implication of these ethanol-induced asymmetries in lateral migration remains to be investigated, there are studies in the rat suggesting that hcrt neurons in the lateral and medial hypothalamus exist in topographically dichotomous subpopulations that may have different functions. For example, there is evidence that the lateral hcrt neurons function in motivation and reward-seeking while more medial neurons regulate arousal and stress (Harris and Aston-Jones, 2006), with lateral hcrt neurons activated by drug-associated stimuli which in turn increase drug-seeking behavior (Harris et al., 2005) and with selective knockdown of lateral but not medial hcrt neurons blocking the motivation for cocaine (James et al., 2018). Also, hcrt neurons are shown to be genetically heterogeneous in zebrafish (Yelin-Bekerman et al., 2015) and neurochemically heterogeneous in rodents (Harris et al., 2005), suggesting that these neurons may exhibit functionally heterogeneity as well. Our results in ethanol-treated zebrafish, demonstrating increased hcrt neuron number, dispersion and lateral migration on the left side of the brain along with abnormally located neurons on the left, suggest that ethanol may create subpopulations of these neurons that are functionally and topographically dichotomous and may in turn contribute to disturbances in ethanol consummatory behavior.
In our analysis of the anatomical location of hcrt neurons, we in fact found that some hcrt neurons in ethanol exposed fish, which in control fish normally exist in tight bilateral clusters within the AH, were located outside of their normal location at 6 dpf. Some of these abnormally located hcrt neurons in the POA and IH were found only on the left side of the brain, consistent with the asymmetric changes in hcrt number, migration and dispersion observed only on the left across age, whereas the additional neurons in the PT were found on both the left and right sides. Whereas ethanol initially reduced the migration of hcrt neurons from 24-28 hpf as revealed by time-lapse imaging, the ultimate location of these neurons at 6 dpf in the POA and PT suggests that they continued to migrate more anteriorly and dorsally relative to the AH mostly on the left. With an ex vivo study of fetal mouse cortical epithelial cells showing ethanol to increase their migration during differentiation (Camarillo and Miranda, 2007), it is possible that migration of the newly differentiated hcrt neurons produced by ethanol on the left may have been stimulated at the older ages, directly contributing to their increased dispersion and abnormal anatomical locations. Also, the abnormally located hcrt neurons in the IH on the left may possibly result from the early increase in lateral migration and dispersion induced by ethanol on the left.
With hcrt neurons known to interact with a number of different neurotransmitter systems (Elbaz et al., 2017), these ethanol-induced changes in anatomical location are likely to alter the functional connectivity with these other systems. The fibers of hcrt neurons are found in zebrafish (Kaslin et al., 2004) and rats (Baimel and Borgland, 2015, Moorman, 2018) to project toward clusters of dopaminergic neurons, a circuit shown to mediate reward-related behaviors (Baimel and Borgland, 2015). With the IH (Ren et al., 2013) and PT (Tay et al., 2011) in zebrafish known to contain dopaminergic neurons that regulate reward and are functionally homologous to the mammalian mesodiencephalic dopamine system (Kastenhuber et al., 2010), the increased presence and close proximity of hcrt neurons in these two areas in ethanol fish may facilitate their signaling with dopaminergic neurons, possibly more on the left side of the brain where positive stimuli are believed to promote reward behaviors (Davidson, 1984). In both mammals (Leinninger et al., 2011) and zebrafish (Levitas-Djerbi et al., 2015), hcrt neurons are also shown to be innervated by neurotensin, a neuropeptide that in zebrafish is expressed in the PT, POA and hypothalamus and is known to stimulate hcrt neuronal activity (Furutani et al., 2013) and regulate reward-related behaviors (Ferraro et al., 2016). Our evidence that hcrt neurons in ethanol fish are located in these same brain areas as neurotensin neurons, primarily on the left side, suggests that there may be more interaction between these two systems and increased hcrt signaling that is shown to promote ethanol consumption (James et al., 2016).
In conclusion, our results provide new information on how embryonic ethanol exposure at a low concentration for only 2 hours produces marked, persistent and asymmetric changes in the development of the hcrt system. This low dose of ethanol and short duration of exposure employed here may more closely model the most prevalent and mild forms of FASD, such as alcohol-related neurodevelopmental disorder (O’Malley, 2015), which is typically non-dysmorphic and characterized by disturbances in neural development, functioning and behavior. The asymmetric changes in hcrt neurons we observed may be important factors in the development of alcohol use disorder, as shown by an increased risk for this disorder in adolescents with asymmetrical brain morphology (Sharma and Hill, 2017) and by the asymmetries across brain regions produced by prenatal ethanol exposure (Gordon, 2017, Gordon, 2016). While further research is needed to determine if the function of the hcrt neurons and their connectivity with other systems are disturbed, our findings here that embryonic ethanol markedly alters the number, migration, direction and dispersion of the hcrt neurons, and ultimately their anatomical location primarily on the left, supports the idea that ethanol produces asymmetric subpopulations of hcrt neurons that may be functionally distinct and contribute to disturbances in behavior, including an increase in ethanol consumption later in life.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health Award R01AA024798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Harold Burgess and Gregory Marquart (National Institute of Child Health and Human Development) for their assistance with Advanced Normalization Tools and the Zebrafish Brain Browser, Drs. Lior Appelbaum (Bar-Ilan University) and Shin-ichi Higashijima (National Institute for Basic Biology) who kindly shared their transgenic hcrt:EGFP and vglut2a:DsRed zebrafish with us, respectively, and Rockefeller University’s Bio-Imaging Resource Center and High Performance Computing Cluster for the use of their equipment. We also thank Aja Evans for her help with this project. The authors declare no conflict of interest.
This research was supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA024798
References
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W (2006). In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch. Gen. Psychiatry, 63, 1009–1016. [DOI] [PubMed] [Google Scholar]
- Ali S, Champagne DL, Alia A, Richardson MK (2011). Large-scale analysis of acute ethanol exposure in zebrafish development: a critical time window and resilience. PLoS One, 6, e20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqadah A, Hsieh YW, Morrissey ZD & Chuang CF (2018). Asymmetric development of the nervous system. Dev. Dyn, 247, 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum L, Wang GX, Maro GS, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ & Gothilf Y (2009). Sleep–wake regulation and hypocretin–melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. U.S.A, 106, 21942–21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54, 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Borgland SL (2015). Orexin signaling in the VTA gates morphine-induced synaptic plasticity. J. Neurosci, 35, 7295–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF (2013). Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol. Clin. Exp. Res, 37, E141–E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF (2011). Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol. Behav, 104, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JR, Skariah G, Maro GS, Mignot E, Mourrain P (2009). Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J. Comp. Neurol, 517, 695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Levin M (2017). Reversals of bodies, brains, and behavior, in Lateralized Brain Functions. Neuromethods, (Rogers L, Vallortigara G eds), vol 122, pp 667–694. Humana Press, New York. [Google Scholar]
- Boric K, Orio P, Viéville T, Whitlock K (2013). Quantitative analysis of cell migration using optical flow. PLoS One, 8, e69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R (2011). Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol. Teratol, 33, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DM, Sidik A, Kar RD, Eberhart JK (2019). Differentially sensitive neuronal subpopulations in the central nervous system and the formation of hindbrain heterotopias in ethanol-exposed zebrafish. Birth Defects Research. doi: 10.1002/bdr2.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarillo C, Miranda RC (2008). Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expr., 14, 159–171. [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz S (2015). Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience, 310, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Liang S, Barson J, Leibowitz S (2012). Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience, 222, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME, Safran RM, Perides G (1994). Ethanol inhibits neural cell-cell adhesion. J. Biol. Chem, 269, 9304–9. [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G (2007). Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci. Biobehav. Rev, 31, 181–191. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1984). Affect, cognition, and hemispheric specialization, in Emotions, Cognition, and Behavior (Izard CE, Kagan J, Zajonc RB eds), pp 320–365. Cambridge University Press, New York. [Google Scholar]
- Elbaz I, Levitas-Djerbi T, Appelbaum L (2017). The hypocretin/orexin neuronal networks in zebrafish. Behavioral Neuroscience of Orexin/Hypocretin, 33, 75–92. [DOI] [PubMed] [Google Scholar]
- Facchin L, Duboué ER, Halpern ME (2015). Disruption of Epithalamic Left–Right Asymmetry Increases Anxiety in Zebrafish. J. Neurosci, 35, 15847–15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco JH, Appelbaum L, Marin W, Gaus SE, Mourrain P, Mignot E (2006). Regulation of hypocretin (orexin) expression in embryonic zebrafish. J. Biol. Chem, 281, 29753–29761. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M & Gerlai R (2015). Embryonic alcohol exposure impairs the dopaminergic system and social behavioral responses in adult zebrafish. Int. J. Neuropsychopharmacol, 18, pyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tiozzo Fasiolo L, Beggiato S, Borelli AC, Pomierny-Chamiolo L, Frankowska M, Antonelli T, Tomasini MC, Fuxe K, Filip M (2016). Neurotensin: A role in substance use disorder? Journal of psychopharmacology (Oxford, England), 30, 112–27. [DOI] [PubMed] [Google Scholar]
- Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M, Shioda S, Sakurai T (2013). Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness states. PLoS One, 8, e62391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R (2011). Using zebrafish to unravel the genetics of complex brain disorders, in Behavioral Neurogenetics. Current Topics in Behavioral Neurosciences, (Cryan J, Reif A eds), vol 12, pp 3–24. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Glass L, Ware AL, Mattson SN (2014). Neurobehavioral, neurologic, and neuroimaging characteristics of fetal alcohol spectrum disorders. Handb. Clin. Neurol, 125, 435–62. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L & Christie BR (2010). Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain research reviews, 64, 283–303. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, De Genna NM, Cornelius MD, Day NL (2019). Prenatal alcohol exposure and offspring alcohol use and misuse at 22 years of age: A prospective longitudinal study. Neurotoxicol. Teratol, 71, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW (2016). Laterality of brain activation for risk factors of addiction. Current drug abuse reviews, 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW (2017). Hemispheric Asymmetry of Development Due to Drug Exposure. J. Syst Integr. Neurosci., 3, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores MV, Crosier K, Crosier P (2009). Live cell imaging of zebrafish leukocytes, in Zebrafish. Methods in Molecular Biology, (Lieschke G, Oates A, Kawakami K eds), vol 546, pp 255–271. Humana Press, New York. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Liang JO & Gamse JT (2003). Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci., 26, 308–13. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF (2010). Hippocampal volumes in adolescents with and without a family history of alcoholism. Am. J. Drug Alcohol Abuse, 36, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G (2006). Arousal and reward: a dichotomy in orexin function. Trends Neurosci., 29, 571–577. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437, 556–559. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2016). A decade of orexin/hypocretin and addiction: Where are we now?, in Behavioral Neuroscience of Orexin/Hypocretin. Current Topics in Behavioral Neurosciences, (Lawrence A, de Lecea L eds), vol 33, pp 247–281. Springer, Cham, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G (2018). Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya X, Garcia-Algar O, Vall O, Pujades C (2014). Transient exposure to ethanol during zebrafish embryogenesis results in defects in neuronal differentiation: an alternative model system to study FASD. PLoS One, 9, e112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Nystedt JM, Östergård M, Peitsaro N, Panula P (2004). The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J. Neurosci, 24, 2678–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W (2010). Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol, 518, 439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K (2016). Ion channels in regulated cell death. Cell. Mol. Life Sci., 73, 2387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006). The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol, 148, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG Jr., (2011). Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab., 14, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitas-Djerbi T, Yelin-Bekerman L, Lerer-Goldshtein T, Appelbaum L (2015). Hypothalamic leptin-neurotensin-hypocretin neuronal networks in zebrafish. J. Comp. Neurol, 523, 831–48. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, Lecea L (2014). The hypocretins/orexins: integrators of multiple physiological functions. Br. J. Pharmacol, 171, 332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE & West JR (2003). Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol. Teratol, 25, 447–58. [DOI] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D & Gerlai R (2018a). Short exposure to low concentrations of alcohol during embryonic development has only subtle and strain- dependent effect on the levels of five amino acid neurotransmitters in zebrafish. Neurotoxicol. Teratol, 68, 91–96. [DOI] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D, Misquitta K, Chatterjee D & Gerlai R (2018b). Lasting changes induced by mild alcohol exposure during embryonic development in BDNF, NCAM and synaptophysin positive neurons quantified in adult zebrafish. Eur. J. Neurosci, 47, 1457–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjonen H, Sierra A, Nyman A, Rogojin V, Grohn O, Linden AM, Hautaniemi S, Kaminen-Ahola N (2015). Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLoS One, 10, e0124931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart GD, Tabor KM, Brown M, Strykowski JL, Varshney GK, Lafave MC, Mueller T, Burgess SM, Higashijima S-I, Burgess HA (2015). A 3D searchable database of transgenic zebrafish Gal4 and Cre lines for functional neuroanatomy studies. Frontiers in neural circuits, 9, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW (1993). Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol. Clin. Exp. Res, 17, 304–14. [DOI] [PubMed] [Google Scholar]
- Moffat JJ, Ka M, Jung EM, Kim WY (2015). Genes and brain malformations associated with abnormal neuron positioning. Mol. Brain, 8, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE (2018). The hypocretin/orexin system as a target for excessive motivation in alcohol use disorders. Psychopharmacology (Berl.), 235, 1663–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD (2003). Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci, 3, 207–233. [DOI] [PubMed] [Google Scholar]
- Murray J, Burgess S, Zuccolo L, Hickman M, Gray R, Lewis SJ (2016). Moderate alcohol drinking in pregnancy increases risk for children’s persistent conduct problems: causal effects in a Mendelian randomisation study. J Child Psychol Psychiatry, 57, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley K (2015). Alcohol-related neurodevelopmental disorder, in Encyclopedia of Psychopharmacology, (Stolerman IP, Price LH eds), pp 83–91. Springer, Berlin, Heidelberg. [Google Scholar]
- Petrelli B, Weinberg J, Hicks GG (2018). Effects of prenatal alcohol exposure (PAE): insights into FASD using mouse models of PAE. Biochem. Cell Biol., 96, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF (2006). Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci, 26, 13400–13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Li S, Zhong H, Lin S (2013). Zebrafish tyrosine hydroxylase 2 gene encodes tryptophan hydroxylase. J. Biol. Chem, 288, 22451–22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, Valkonen K., Kolehmainen AI, Kumpulainen KI, Kononen M, Vanninen RL, Kuikka JT (2005). Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol. Psychiatry, 57(12), 1565–1572. [DOI] [PubMed] [Google Scholar]
- Satou C, Kimura Y & Higashijima S (2012). Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J. Neurosci, 32, 1771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Hill SY (2017). Differentiating the effects of familial risk for alcohol dependence and prenatal exposure to alcohol on offspring brain morphology. Alcohol. Clin. Exp. Res, 41, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C, Oikonomou G, Prober DA (2015). Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling Chang G-Q, Karatayev O, Chang S, Leibowitz S (2016). Effects of embryonic ethanol exposure at low doses on neuronal development, voluntary ethanol consumption and related behaviors in larval and adult zebrafish: role of hypothalamic orexigenic peptides. Behav. Brain Res, 304, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling M, Karatayev O, Chang G-Q, Algava D, Leibowitz S (2015). Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav. Brain Res., 278, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D (2015). Alcohol use and binge drinking among women of childbearing age - United States, 2011-2013. MMWR Morb. Mortal. Wkly. Rep., 64, 1042–6. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W (2011). Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nature Commun., 2, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Hsieh YW, Gamse JT & Chuang CF (2010). Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development, 137, 681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018). Global status report on alcohol and health. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yelin-Bekerman L, Elbaz I, Diber A, Dahary D, Gibbs-Bar L, Alon S, Lerer-Goldshtein T & Appelbaum L (2015). Hypocretin neuron-specific transcriptome profiling identifies the sleep modulator Kcnh4a. Elife, 4, e08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Yao F, Chen X, Wang N, Wang H, Chang HE, Yuan Z & Wu B (2014). Ethanol reduces neural precursor cells and inhibits neuronal and glial differentiation in zebrafish embryos. Nan Fang Yi Ke Da Xue Xue Bao, 34, 1555–61. [PubMed] [Google Scholar]
- Young EJ, Williams CL (2010). Valence dependent asymmetric release of norepinephrine in the basolateral amygdala. Behav. Neurosci, 124, 633–44. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T (2001). Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res. Dev. Brain Res., 126, 147–55. [DOI] [PubMed] [Google Scholar]


