Abstract
Background
To evaluate the association of liver fibrosis scores with PSA level among US adult men overall and by race/ethnicity.
Methods
Data from the National Health and Nutrition Examination Survey (NHANES), 2001–2010, were used. Males aged ≥40 years without a prostate cancer diagnosis and who had serum PSA, liver enzymes, albumin, and platelet counts measured as part of NHANES protocol were included. Liver fibrosis was measured using three scores: AST to platelet ratio index (APRI), fibrosis 4 index (FIB-4) and NAFLD fibrosis score (NFS). We assessed overall and race/ethnicity-stratified geometric mean PSA by fibrosis score using predictive margins by linear regression, and the association of abnormal fibrosis scores (APRI>1, FIB-4>2.67, NFS>0.676) and elevated PSA (>4 ng/mL) by logistic regression.
Results
6,705 men were included. Abnormal liver fibrosis scores were present in 2.1% (APRI), 3.6% (FIB-4), and 5.6% (NFS). Men with higher fibrosis scores had lower geometric mean PSA (all p-trend<0.02). Men with abnormal APRI had a lower odds of PSA>4ng/mL (adjusted odds ratio [aOR]=0.33, 95% confidence interval [CI] 0.11–0.96). Compared with men with 0 abnormal scores, those with 2 or 3 abnormal fibrosis scores had a lower odds of PSA>4ng/mL (aOR=0.55, 95% CI 0.33–0.91). The patterns were similar by race/ethnicity.
Conclusions
Men of all race/ethnicities with higher liver fibrosis scores had lower serum PSA and men with advanced fibrosis scores had a lower odds of an elevated PSA.
Impact
These findings support further research to inform the likelihood of delay in prostate cancer detection in men with abnormal liver function.
Keywords: liver fibrosis, liver enzymes, PSA, men, prostate cancer
Introduction
Studies of men with cirrhosis link poor liver function to reduced serum prostate-specific antigen (PSA) concentration.(1,2) The liver plays a key role in PSA production and clearance.(3) Reduced PSA clearance by an impaired liver could result in a higher PSA concentration. Alternatively, with chronic poor liver function, testosterone levels drop, which can result in reduced PSA production, which is under androgenic regulation in the prostate.(4,5) No universally accepted PSA cutpoint exists; a threshold >4 ng/mL is commonly considered for prostate biopsy, including in a US trial, (6) although >3 ng/mL has also been used, including in a European trial.(7) A shift in PSA distribution toward a higher level could result in some men without prostate cancer being sent for biopsy unnecessarily. Alternatively, a shift in the distribution of PSA concentration toward lower levels could result in some men with yet undiagnosed prostate cancer from being detected with the disease while it is still asymptomatic. Moreover, most PSA circulates bound to alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin; the liver synthesizes these proteins.
Since the early 1990s, though there has been controversy over the benefits and risk of over-diagnosis, PSA testing has been adopted for prostate cancer screening in the US.(8) PSA concentration that is lower due to comorbidities may lead to a delay in detection, including of lethal prostate cancer. Black men experience a profound disparity in lethal prostate cancer rates compared with White men.(9,10) Non-Hispanic Black men have more than twice the risk of dying from this disease than White or Hispanic/Latino men.(11) Meanwhile, previous studies in the US nationally representative National Health and Nutrition Examination Survey (NHANES) document a racial/ethnic disparity in liver diseases. For example, non-Hispanic Blacks have a higher prevalence of cirrhosis and infectious hepatitis,(12–14) while nonalcoholic fatty liver disease (NAFLD) is more common in Mexican Americans.(15) For men with reduced liver function due to liver fibrosis but not a significantly reduced life expectancy, early detection may be beneficial for prostate cancer treatment and survival, (7,16,17) especially possibly for population subgroups with a higher underlying risk of aggressive prostate cancer.
Thus, we determined the association between liver fibrosis scores and serum PSA concentration overall and by race/ethnicity in a nationally representative sample of men in a target age range for prostate cancer screening. We addressed this association to inform the likelihood of delay in prostate cancer detection in men with liver conditions.
Materials and Methods
Study Population
We used data from five cycles of continuous NHANES (2001–2010). NHANES is a nationally representative survey aiming to provide knowledge on the health status of the US population through collecting data from interviews, physical examinations, and laboratory measurements.(18) Written informed consent was obtained. All NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention.
We restricted to men ≥40 years given that PSA was only measured in them. We excluded men with missing demographic (age, race/ethnicity) or clinical (liver fibrosis scores biomarkers, body mass index (BMI), diabetes status, smoking, alcohol drinking history, or viral hepatitis) data. We excluded men with extreme BMI (<18.5, >50 kg/m2) or prostate cancer or liver cancer. The analytical, unweighted sample size was 6,705.
PSA Measurement
As part of the NHANES protocol, from 2001 to 2010, serum total PSA concentration was measured by Hybritech immunoassay (Beckman Coulter, Fullerton, CA) for men aged ≥40 years.(19) PSA was not measured for men with a recent prostate biopsy, prostate infection or inflammation, rectal examination, or with a prostate cancer history.
Liver Fibrosis Assessment
As part of the NHANES protocol, biochemical markers related to liver function – aspartate aminotransferase (AST), alanine aminotransferase (ALT) – and albumin were measured using a Beckman Synchron LX20. Platelet count was measured using the Beckman UniCel® DxC800 Synchron. Details are described elsewhere.(19) To assess liver fibrosis, we calculated (see Supplement for details) three non-invasive fibrosis scores: AST/platelet ratio index (APRI), fibrosis 4 index (FIB-4) and NAFLD fibrosis score (NFS). APRI and FIB-4 indices were originally developed to predict fibrosis and cirrhosis among patients with hepatitis C,(20,21) but have been validated in other chronic liver diseases to identify patients with significant fibrosis.(22–25) The NFS was developed to identify advanced fibrosis in patients with NAFLD and showed 0.84 of the area under the ROC curve.(26)
Statistical analysis
To account for the clustered design and differential sampling probabilities, sample weights reflecting the probability of selection and response were used to produce unbiased national estimates. The Taylor linearization method and masked variance units (MVU) were used for variance estimation to account for the complex NHANES sample design. Participant characteristics were compared by normal/abnormal fibrosis score and PSA concentration categories (<1, 1-<2, 2-<4, 4-<10, ≥10 ng/mL).
PSA concentration was transformed using the natural logarithm to achieve a normal distribution. Overall and race-stratified geometric mean PSA concentration and 95% confidence intervals (CIs) were estimated using predictive margins (model-based standardization) from multivariable linear regression. In the models, we entered quintiles of each fibrosis score or clinical categories (normal vs. abnormal). P-trends were estimated using linear regression with the median of each fibrosis score category as continuous variable. Logistic regression was used to estimate the odds ratio (OR) of elevated PSA level (>3, >4, >10 ng/mL) associated with abnormal liver fibrosis scores. Model covariate details are provided in the Supplement.
We conducted sensitivity analyses restricting to men with BMI <30 kg/m2; without a diagnosis of diabetes or glycated hemoglobin >6.4%; with self-reported liver disease; with self-reported liver disease and/or current or previous viral hepatitis; with self-reported liver disease and/or chronic viral hepatitis; or aged 55 years and older. To improve classification of abnormal and normal liver fibrosis scores, we re-classified the men by the number of the three abnormal APRI, FIB-4 and NFS fibrosis scores (0, 1, or 2 or 3 abnormal fibrosis scores), and analyzed the association between the joint indicator and elevated PSA concentration by logistic regression.
P-values were 2-sided and p<0.05 was used as the threshold for statistical significance. Statistical analyses were performed using STATA 14.2.
Results
Study Population Characteristics
Abnormal liver fibrosis scores were present in 2.1% (APRI), 3.6% (FIB-4), and 5.6% (NFS) of the men (Table 1). The proportions slightly differed by race (APRI, FIB-4, NFS: 1.8%, 3.6%, 5.8% in non-Hispanic Whites; 3.5%, 4.5%, 5.4% in non-Hispanic Blacks; 2.8%, 2.7%, 3.9% in Mexican American/other Hispanics). Overall, the mean age was 55.1 years, and 55.7, 53.3 and 52.1 years among non-Hispanic Whites, non-Hispanic Blacks and Mexican American/other Hispanics, respectively. Men with abnormal scores tended to be older, to be non-Hispanic White, to have a lower educational level, to be heavy drinkers, to have ever smoked, to be obese, to have diabetes or prediabetes, to have self-reported liver disease, and to be seropositive for hepatitis C virus (HCV) or current or previous hepatitis B virus (HBV) infection. PSA levels increased with age, and as serum PSA concentration increased, the proportion of men who are non-Hispanic Black, who have a lower educational level, or who have a current or previous HBV infection increased, while the proportion of men with obesity, self-reported liver disease, or HCV infection decreased (Supplement Table 1).
Table 1.
Characteristics of Participants by Liver Fibrosis Scores Among Men 40 Years and Older, NHANES 2001–2010
| Overall | APRI | FIB-4 | NFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormala | Normal | Abnormal | Normal | Abnormal | ||||
| Unweighted Participants | 6,705 | 6,541 | 164 | 6,319 | 386 | 6,139 | 566 | ||
| Age group, % (95% CI) | |||||||||
| 40 – 49 years | 38.0 (36.2–39.9) |
37.9 (36.1–39.8) |
42.4 (32.3–53.2) |
39.0 (37.2–40.9) |
11.6 (7.3–17.7) |
40.0 (38.1–41.9) |
5.2 (3.8–7.1) |
||
| 50 – 59 years | 31.1 (29.4–32.8) |
31.1 (29.5–32.7) |
28.6 (19.1–40.3) |
31.6 (30.0–33.4) |
15.3 (10.5–21.7) |
32.0 (30.3–33.8) |
14.4 (10.5–19.5) |
||
| 60 – 69 years | 17.4 (16.5–18.4) |
17.4 (16.4–18.4) |
18.9 (13.8–25.3) |
17.3 (16.3–18.3) |
20.2 (15.5–25.9) |
16.8 (15.8–17.9) |
27.8 (23.4–32.6) |
||
| 70 – 79 years | 9.6 (9.0–10.3) |
9.7 (9.0–10.4) |
6.5 (3.7–11.1) |
9.0 (8.4–9.7) |
25.2 (21.1–29.7) |
8.2 (7.6–8.9) |
32.8 (29.0–36.7) |
||
| ≥80 years | 3.9 (3.5–4.4) |
3.9 (3.5–4.4) |
3.7 (2.0–6.5) |
3.0 (2.6–3.5) |
27.8 (23.7–32.3) |
3.0 (2.6–3.4) |
19.7 (17.0–22.8) |
||
| Race/ethnicity, % (95% CI) | |||||||||
| White (non-Hispanic) | 77.1 (74.5–79.7) |
77.3 (74.7–79.9) |
67.4 (57.4–77.4) |
77.2 (74.6–79.7) |
76.4 (71.0–81.9) |
77.0 (74.2–79.5) |
79.8 (75.4–83.6) |
||
| Black (non-Hispanic) | 8.6 (7.3–9.8) |
8.5 (7.2–9.7) |
14.4 (8.6–20.1) |
8.5 (7.3–9.7) |
10.6 (7.3–13.8) |
8.6 (7.4–9.9) |
8.3 (6.2–11.0) |
||
| Mexican-American/Other Hispanic | 9.6 (7.7–11.5) |
9.5 (7.6–11.4) |
12.5 (6.5–18.6) |
9.7 (7.8–11.6) |
7.1 (4.1–10.1) |
9.8 (8.0–11.9) |
6.6 (4.8–9.0) |
||
| Other | 4.7 (3.8–5.6) |
4.7 (3.7–5.6) |
5.7 (0.7–10.7) |
4.7 (3.7–5.6) |
5.9 (2.7–9.1) |
4.7 (3.8–5.7) |
5.4 (3.5–8.1) |
||
| Education level, % (95% CI) | |||||||||
| Less than 9th grade | 7.4 (6.5–8.2) |
7.3 (6.4–8.1) |
11.0 (5.9–16.2) |
7.1 (6.3–7.9) |
14.3 (10.7–17.9) |
6.9 (6.1–7.8) |
15.0 (11.3–19.6) |
||
| 9–11th grade (includes 12th grade, no diploma) | 10.2 (9.2–11.3) |
10.1 (9.0–11.1) |
18.5 (10.3–26.7) |
10.1 (9.0–11.1) |
15 (10.1–19.9) |
10.1 (9.1–11.3) |
11.6 (9.2–14.6) |
||
| High school graduate/GED or equivalent | 25.2 (23.8–26.6) |
25.0 (23.6–26.4) |
33.1 (24.7–41.6) |
25.2 (23.8–26.6) |
24.6 (18.6–30.6) |
25.5 (23.9–26.6) |
24.8 (21.0–29.0) |
||
| Some college or associates degree | 27.4 (26.0–28.9) |
27.5 (26.1–29.0) |
22.4 (14.4–30.4) |
27.6 (26.2–29.1) |
21.8 (16.0–27.7) |
27.4 (26.0–29.0) |
27.3 (21.5–34.1) |
||
| College graduate or above | 29.8 (27.6–32.0) |
30.1 (27.9–32.3) |
14.9 (7.2–22.6) |
30.0 (27.7–32.2) |
24.3 (18.1–30.5) |
30.3 (28.0–32.6) |
21.3 (16.9–26.4) |
||
| Drinking status, % (95% CI) | |||||||||
| Non-drinker or non-heavy drinker | 91.2 (90.3–92.1) |
91.6 (90.8–92.5) |
70.2 (62.3–78.1) |
91.4 (90.5–92.3) |
85.7 (81.4–90.1) |
91.0 (90.0–91.9) |
94.2 (91.6–96.0) |
||
| Heavy drinker | 8.8 (7.9–9.7) |
8.4 (7.5–9.2) |
29.8 (21.9–37.7) |
8.6 (7.7–9.5) |
14.3 (9.9–18.6) |
9.0 (8.1–10.0) |
5.8 (4.0–8.4) |
||
| Smoking history, % (95% CI) | |||||||||
| Never | 41.7 (39.7–43.6) |
42.1 (40.1–44.1) |
23.9 (14.6–33.3) |
41.9 (39.9–43.9) |
35.2 (29.7–40.7) |
42.1 (40.0–44.1) |
35.3 (31.1–39.8) |
||
| Ever | 58.3 (56.4–60.3) |
57.9 (55.9–59.9) |
76.1 (66.7–85.4) |
58.1 (56.1–60.1) |
64.8 (59.3–70.3) |
57.9 (55.9–60.0) |
64.7 (60.2–68.9) |
||
| BMI category, % (95% CI) | |||||||||
| Normal/overweight (<30 kg/m2) | 65.4 (63.7–67.2) |
65.5 (63.8–67.3) |
62.0 (52.9–71.1) |
65.3 (63.5–67.1) |
69.1 (63–75.2) |
67.4 (65.6–69.1) |
32.5 (28.8–36.4) |
||
| Obese (≥30 kg/m2) | 34.6 (32.8–36.3) |
34.5 (32.7–36.2) |
38.0 (28.9–47.1) |
34.7 (32.9–36.5) |
30.9 (24.8–37.0) |
32.6 (30.9–34.4) |
67.5 (63.6–71.2) |
||
| Diabetes status, % (95% CI) | |||||||||
| No diabetes | 63.5 (62.1–65.0) |
63.6 (62.2–65.0) |
61.9 (52.8–71.0) |
64.0 (62.5–65.4) |
51.7 (44–59.5) |
65.9 (64.4–67.3) |
24.5 (20.6–28.9) |
||
| At risk for diabetes | 22.5 (21.4–23.7) |
22.6 (21.4–23.8) |
17.5 (9.6–25.3) |
22.4 (21.2–23.7) |
24.8 (19.2–30.4) |
22.3 (21.1–23.6) |
25.4 (21.2–30.1) |
||
| Controlled diabetes | 5.8 (5.0–6.6) |
5.7 (4.9–6.6) |
7.7 (2.9–12.5) |
5.5 (4.7–6.3) |
12.5 (7.0–18.0) |
4.5 (3.8–5.4) |
26.7 (22.1–32.0) |
||
| Uncontrolled diabetes | 8.2 (7.3–9.1) |
8.1 (7.2–9.0) |
12.9 (6.1–19.7) |
8.1 (7.2–9.0) |
10.9 (7.4–14.4) |
7.3 (6.4–8.2) |
23.4 (19.1–28.3) |
||
| Self-reported liver disease, % (95% CI) | |||||||||
| Never | 95.2 (94.5–95.8) |
95.6 (95.0–96.2) |
75.7 (64.7–84.1) |
95.6 (94.9–96.2) |
85.1 (78.8–89.8) |
95.4 (94.6–96.0) |
92.4 (88.4–95.1) |
||
| Ever | 4.8 (4.2–5.5) |
4.4 (3.8–5.0) |
24.3 (15.9–35.3) |
4.4 (3.8–5.1) |
14.9 (10.2–21.2) |
4.6 (4.0–5.4) |
7.6 (4.9–11.6) |
||
| HCV status, % (95% CI) | |||||||||
| HCV negative | 96.7 (96.0–97.3) |
97.4 (96.7–97.9) |
65.3 (55.6–74.0) |
97.1 (96.4–97.6) |
86.2 (80.4–90.4) |
96.7 (96.0–97.3) |
96.1 (93.4–97.7) |
||
| HCV infected | 3.3 (2.7–4.0) |
2.6 (2.1–3.3) |
34.7 (26.0–44.4) |
2.9 (2.4–3.6) |
13.8 (9.6–19.6) |
3.3 (2.7–4.0) |
4.0 (2.3–6.6) |
||
| HBV status, % (95% CI) | |||||||||
| Negative | 92.2 (91.2–93.0) |
92.4 (91.5–93.3) |
79.2 (70.0–86.1) |
92.4 (91.4–93.3) |
85.6 (79.8–89.9) |
92.3 (91.3–93.2) |
90.1 (86.6–92.7) |
||
| Current or previous HBV infected | 7.8 (7.0–8.8) |
7.6 (6.7–8.5) |
20.8 (13.9–30.0) |
7.6 (6.7–8.6) |
14.4 (10.1–20.2) |
7.7 (6.8–8.7) |
9.9 (7.3–13.4) |
||
| Chronic HBV infected | 0.5 (0.3–0.7) |
0.5 (0.3–0.7) |
1.2 (3.5–4.0) |
0.5 (0.3–0.7) |
0.5 (0.2–1.7) |
0.5 (0.3–0.7) |
0.3 (0.1–1.2) |
||
Cutpoint for abnormal liver fibrosis score equates to advanced fibrosis (APRI >1, FIB-4 >2.67 and NFS >0.676).
Liver fibrosis scores and geometric mean PSA
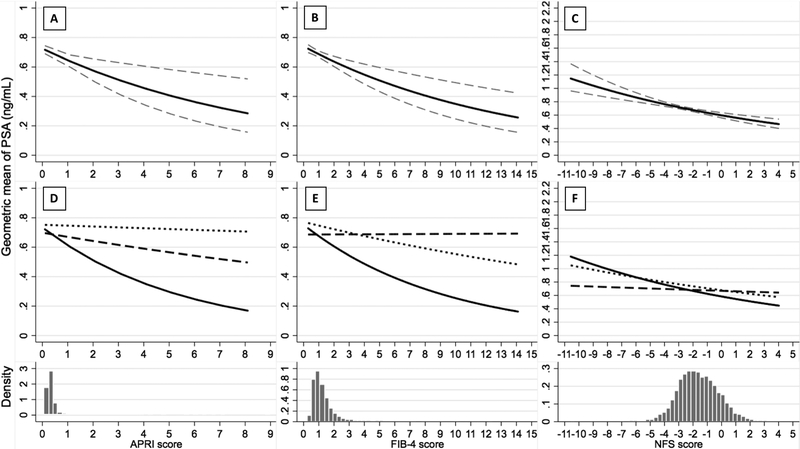
Geometric mean PSA decreased with increasing APRI, FIB-4, and NFS scores (Figure 1. A–C). Age-adjusted geometric mean PSA significantly decreased across APRI, FIB-4 and NFS quintiles (p-trend <0.001; Table 2). This trend persisted after multivariable adjustment. Similar trends were observed among men with BMI <30 kg/m2, among men without a diagnosis of diabetes or elevated glycated hemoglobin, and among men aged 55 years and older, respectively (Supplement Tables 2–4).
Figure 1. Association between Fibrosis Scores and Geometric Mean PSA Serum Concentration (ng/mL) Overall (Panel A, B, C) and By Race/Ethnicity (Panel D, E, F) Among Men 40 Years and Older, NHANES 2001–2010.
In panels A, B and C, the solid line indicates the estimated geometric mean of PSA, while the dash line indicates the 95% CI of the estimated geometric mean of PSA. The histogram below each panel displays the density distribution of APRI, FIB-4, and NFS score, respectively. In panels D, E and F, the solid line indicates the estimated geometric mean of PSA among non-Hispanic Whites, the dash line indicates non-Hispanic Blacks, and the dot line indicates Mexican American/other Hispanics. The x-axis ranges represent the true ranges of fibrosis scores among the analyzed population. The p values for the interaction between race/ethnicity and APRI, FIB-4 and NFS are 0.235, 0.002 and 0.004, respectively.
Table 2.
Geometric Mean Serum PSA Concentration (ng/mL) and 95% CIs by Quintile of Fibrosis Scores Among Men 40 Years and Older, NHANES 2001–2010
| APRI | ||||||
|---|---|---|---|---|---|---|
| Q1 (<0.24) | Q2 (0.24 to <0.29) | Q3 (0.29 to <0.35) | Q4 (0.35 to <0.44) | Q5 (≥0.44) | Per 1 unit increase in fibrosis score (p trend) |
|
| Model 1a | 1.03 (0.98,1.09) | 0.96 (0.91,1.02) | 0.94 (0.89,0.99) | 0.97 (0.93,1.01) | 0.89 (0.84,0.95) | −0.36 (<0.001) |
| Model 2b | 1.04 (0.99,1.10) | 0.96 (0.91,1.02) | 0.93 (0.89,0.98) | 0.97 (0.93,1.01) | 0.89 (0.84,0.95) | −0.38 (<0.001) |
| FIB4 | ||||||
| Q1 (<0.79) | Q2 (0.79 to <0.99) | Q3 (0.99 to <1.23) | Q4 (1.23 to <1.62) | Q5 (≥1.62) | Per 1 unit increase in fibrosis score (p trend) |
|
| Model 1 | 1.03 (0.97,1.08) | 0.96 (0.92,1.01) | 0.97 (0.91,1.03) | 0.94 (0.89,0.99) | 0.90 (0.84,0.96) | −0.09 (0.007) |
| Model 2 | 1.04 (0.99,1.10) | 0.97 (0.93,1.02) | 0.97 (0.92,1.03) | 0.94 (0.89,0.99) | 0.88 (0.83,0.95) | −0.11 (0.001) |
| NFS | ||||||
| Q1 (<0.17) | Q2 (0.17 to <0.89) | Q3 (0.89 to <1.58) | Q4 (1.58 to <2.42) | Q5 (≥2.42) | Per 1 unit increase in fibrosis score (p trend) |
|
| Model 1 | 1.05 (1.00,1.11) | 0.99 (0.94,1.04) | 1.01 (0.96,1.05) | 0.92 (0.86,0.98) | 0.84 (0.78,0.91) | −0.06 (<0.001) |
| Model 2 | 1.02 (0.96,1.08) | 0.96 (0.92,1.01) | 1.00 (0.95,1.04) | 0.93 (0.87,0.99) | 0.89 (0.82,0.96) | −0.04 (0.017) |
Model 1 adjusted for age and race;
Model 2 adjusted for the age, race, BMI category, diabetes status, alcohol drinking status and smoking status.
Stratifying by race, geometric mean PSA decreased more steeply with increasing fibrosis scores in non-Hispanic Whites than in non-Hispanic Blacks and in Mexican-Americans/other Hispanics (Figure 1. D–F). Trends among each race/ethnicity became more similar to each other after excluding potentially influential points (Supplement Figure 1. D–F). Dividing fibrosis scores into quintiles, all three fibrosis scores were inversely associated with PSA among non-Hispanic Whites and among Mexican-American/other Hispanics, whereas among non-Hispanic Blacks, fibrosis scores quintiles were not significantly associated with PSA concentration (Supplement Table 5).
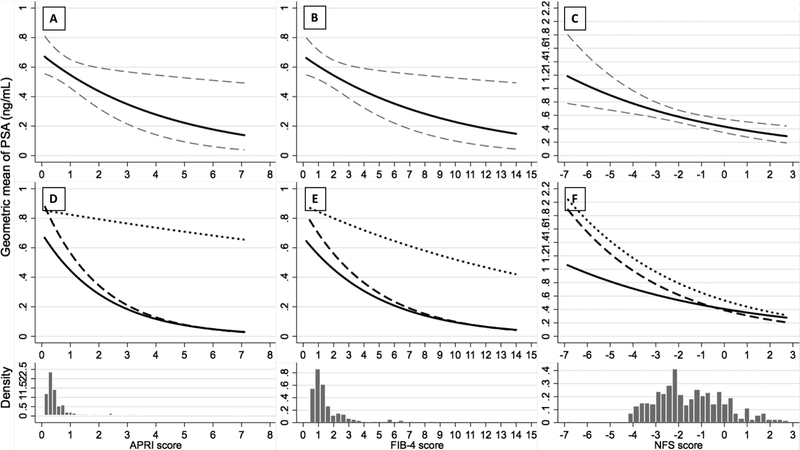
Restricting to men who reported a liver disease diagnosis, the results were similar to the main analysis, geometric mean PSA decreased with increasing fibrosis scores in each racial/ethnic group (Figure 2). Geometric mean PSA decreased across quintiles of fibrosis scores overall and this pattern was observed in each racial/ethnic group (Supplement Table 6). Restricting to men with self-reported liver disease and/or viral hepatitis infection confirmed by serum test, there was a decreasing trend of geometric mean PSA with increasing fibrosis scores among non-Hispanic Whites, but no association among non-Hispanic Blacks or Mexican American/other Hispanics (Supplement Table 7, Supplement Figure 2 and 3).
Figure 2. Association between Fibrosis Scores and Geometric Mean PSA Serum Concentration (ng/mL) Overall and By Race/Ethnicity Among Men 40 Years and Older and With Self-reported Liver Disease, NHANES 2001–2010.
In panels A, B and C, the solid line indicates the estimated geometric mean of PSA, while the dash line indicates the 95% CI of the estimated geometric mean of PSA. The histogram below each panel displays the density distribution of APRI, FIB-4, and NFS score, respectively. In panels D, E and F, the solid line indicates the estimated geometric mean of PSA among non-Hispanic Whites, the dash line indicates non-Hispanic Blacks, and the dot line indicates Mexican American/other Hispanics. The x-axis ranges represent the true ranges of fibrosis scores among the analyzed population. The p values for the interaction between race/ethnicity and APRI, FIB-4 and NFS are 0.038, 0.261 and 0.632, respectively.
We also determined geometric mean PSA concentration within clinical categories of the fibrosis scores (Table 3). Regardless of score used, men with abnormal scores suggestive of advanced fibrosis had significantly lower geometric mean serum PSA (0.15–0.21 ng/mL). This pattern was also observed after multivariable adjustment, and was observed in each race/ethnicity across all three scores.
Table 3.
Geometric Mean Serum PSA Concentration (ng/mL) and 95% Confidence Intervals by Abnormal versus Normal Fibrosis Scores Overall and By Race/Ethnicity Among Men 40 Years and Older, NHANES 2001–2010
| Overall | White (non-Hispanic) | Black (non-Hispanic) | Mexican-American /Other Hispanic |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| APRI | |||||||||||
| Normal | Abnormalc | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | ||||
| Model 1a | 0.96 | 0.75 | 0.97 | 0.69 | 1.00 | 0.86 | 0.95 | 0.89 | |||
| (0.94,0.99) | (0.64,0.88) | (0.94,1.00) | (0.55,0.86) | (0.95,1.06) | (0.66,1.12) | (0.89,1.01) | (0.72,1.09) | ||||
| Model 2b | 0.96 | 0.76 | 0.97 | 0.71 | 1.00 | 0.82 | 0.95 | 0.9 | |||
| (0.94,0.99) | (0.64,0.89) | (0.94,1.00) | (0.57,0.89) | (0.95,1.07) | (0.63,1.07) | (0.89,1.01) | (0.72,1.13) | ||||
| FIB4 | |||||||||||
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | ||||
| Model 1 | 0.96 | 0.81 | 0.97 | 0.79 | 1.00 | 0.92 | 0.95 | 0.88 | |||
| (0.94,0.99) | (0.70,0.93) | (0.94,1.00) | (0.66,0.95) | (0.94,1.06) | (0.72,1.18) | (0.89,1.01) | (0.67,1.15) | ||||
| Model 2 | 0.96 | 0.80 | 0.97 | 0.79 | 1.00 | 0.89 | 0.95 | 0.88 | |||
| (0.94,0.99) | (0.70,0.93) | (0.94,1.00) | (0.66,0.95) | (0.95,1.06) | (0.69,1.13) | (0.89,1.01) | (0.66,1.17) | ||||
| NFS | |||||||||||
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | ||||
| Model 1 | 0.97 | 0.77 | 0.97 | 0.78 | 1.00 | 0.98 | 0.95 | 0.82 | |||
| (0.95,1.00) | (0.71,0.84) | (0.94,1.00) | (0.71,0.86) | (0.94,1.06) | (0.79,1.22) | (0.89,1.01) | (0.66,1.01) | ||||
| Model 2 | 0.97 | 0.83 | 0.97 | 0.85 | 1.00 | 1.02 | 0.95 | 0.84 | |||
| (0.94,0.99) | (0.77,0.90) | (0.94,1.00) | (0.77,0.94) | (0.94,1.06) | (0.81,1.29) | (0.89,1.01) | (0.69,1.04) | ||||
Model 1 adjusted for age and race, numbers with bold font implies p<0.05;
Model 2 adjusted for the age, race, BMI category, diabetes status, alcohol drinking status and smoking status;
Cutpoint for abnormal liver fibrosis equates to advanced fibrosis (APRI >1, FIB-4 >2.67 and NFS >0.676).
Liver fibrosis scores and elevated PSA concentration
The multivariable-adjusted OR of elevated PSA for abnormal fibrosis score defined by APRI was 0.75 (95% CI: 0.37, 1.49) for PSA >3 ng/mL and 0.33 (95% CI: 0.11, 0.96) for PSA >4 ng/mL, for those with elevated FIB-4 the corresponding OR (95% CI) were 0.74 (0.47, 1.15) and 0.67 (0.44, 1.03), and for those with elevated NFS were 1.01 (0.73, 1.39) and 0.71 (0.50, 1.00) (Table 4). Each SD interval of APRI was associated with 9% (95%CI 0.79, 1.04) and 24% (95%CI 0.62, 0.92) decrease in risk of PSA > 3 ng/mL and > 4ng/mL, respectively. For FIB4 and NFS, each SD interval was associated with 15% (95%CI 0.77, 0.94) and 12% (95%CI 0.78, 0.99) decrease in risk of PSA > 3 ng/mL, and 16% (95%CI 0.75, 0.94) and 9% (95%CI 0.78, 1.07) decrease in risk of PSA > 4 ng/mL, respectively. Stratifying by race, similar trends were observed but were not statistically significant among each race/ethnicity group. For FIB-4 and NFS, men who had an abnormal score were less likely to have a PSA >10 ng/mL, albeit 95% CIs were wide (data not shown); the analysis was too sparse for APRI (Supplement Table 8).
Table 4.
Odds Ratios and 95% CIs for the Association between Fibrosis Scores and Elevated Serum PSA Concentration Overall Among Men 40 Years and Older, NHANES 2001–2010
| Odds Ratio (95% CI)a | ||
|---|---|---|
| PSA > 3 ng/mL | PSA > 4 ng/mL | |
| APRI | ||
| Normal | 1 [reference] | 1 [reference] |
| Abnormal | 0.75 (0.37,1.49) | 0.33 (0.11,0.96) |
| Per 1 SD (0.31) change | 0.91 (0.79,1.04) | 0.76 (0.62,0.92) |
| FIB4 | ||
| Normal | 1 [reference] | 1 [reference] |
| Abnormal | 0.74 (0.47,1.15) | 0.67 (0.44,1.03) |
| Per 1 SD (0.74) change | 0.85 (0.77,0.94) | 0.84 (0.75,0.94) |
| NFS | ||
| Normal | 1 [reference] | 1 [reference] |
| Abnormal | 1.01 (0.73,1.39) | 0.98 (0.66,1.46) |
| Per 1 SD (1.37) change | 0.88 (0.78,0.99) | 0.91 (0.78,1.07) |
Adjusted for the age, BMI category, diabetes status, alcohol drinking status and smoking status.
As shown in Supplemental Table 9, overall, compared with men with 0 abnormal scores, those with 1 abnormal and those with 2 or 3 abnormal fibrosis scores had a lower odds of PSA >2 ng/mL (OR: 0.83 and 0.59, respectively), PSA >4 ng/mL (OR: 0.80 and 0.55, respectively), and PSA > 10 ng/mL (OR: 0.76 and 0.45, respectively). After stratifying by race/ethnicity, the association between number of abnormal fibrosis scores and elevated PSA among each race/ethnicity was inverse as for overall, although was less strong among non-Hispanic Black and Mexican-American/other Hispanic men than that among the Whites (p for interaction: 0.089, 0.005 and 0.0004 for PSA >2, 4 and 10 ng/mL, respectively).
Discussion
In this nationally representative study of men without a prostate cancer diagnosis, we found that men with higher liver fibrosis scores had a relatively lower serum PSA concentration. This association was similar among non-obese and non-diabetic men, and among men with self-reported liver disease. Furthermore, results were similar across racial/ethnic groups. Though the PSA level difference corresponding to per unit increasing of liver fibrosis score is relatively small, men with advanced fibrosis scores had lower odds of a PSA level above the commonly used prostate biopsy cutpoints. Given the increasing prevalence of liver fibrosis resulting from the rise in nonalcoholic fatty liver disease, our findings may have implications for early detection of prostate cancer.
No consensus exists about the influence of liver fibrosis on PSA concentration. To our knowledge, our study is the first to examine this association among men ≥40 years old in the overall US population, and results are consistent with previous studies observing that PSA tended to be lower in men with cirrhosis than without.(1,2,4,27,28) These studies were based on fewer cases (<250) and showed 0.4–1.0 ng/mL lower total PSA level in men with cirrhosis. With respect to liver function, a study of 38,157 healthy Korean males also found that higher serum concentration of the liver enzyme ALT was correlated with lower PSA concentration.(5) Additionally, a small number of studies conducted in the US found that PSA concentration was lower among patients with liver diseases than in men with normal function, although these findings were based on fewer cases and were not statistically significant. (29–31)
Over the last 25 years, testing for elevated PSA has been widely used for the early detection of prostate cancer in the US.(8) Following changed screening recommendations from the US Preventive Services Task Force (USPSTF) in 2012 uptake decreased.(32,33) Early detection by PSA testing coupled with appropriate treatment likely reduces risk of death for a subset of men with occult prostate cancer with an aggressive phenotype.(17) From a clinical perspective, whether to screen a man with liver fibrosis for elevated PSA may be dependent on life expectancy, as for any man. For men with significant liver fibrosis, the harms of PSA screening may outweigh the benefits especially if life expectancy is shorter than 10 years. In contrast, if men with slight to moderate liver fibrosis have more than a 10-year life expectancy, they could still potentially benefit from PSA testing. Thus, for men with slight to moderate liver fibrosis, consequent lower PSA concentration could potentially delay detection and treatment of occult aggressive prostate cancer, possibly increasing risk of death. In our study, we observed that men in the fourth and fifth quintiles of the liver fibrosis scores had a lower PSA concentration. The cutpoints for the fifth quintile was much lower than the cutpoints we used to define abnormal fibrosis scores. Based on previous validation studies of the fibrosis scores among liver disease patients, both patients with biopsy-documented no to mild fibrosis (stage 0–2) and advanced fibrosis (stage 3–4) could fall into the fourth and fifth-quintile score categories.(25,34,35) This observation suggests that both men with mild and advanced fibrosis could experience the reduced PSA that we observed in quintiles 4 and 5 of the liver fibrosis scores. However, it should also be noted that the PSA level difference between the highest and lowest quintile of fibrosis score is relatively small, and its impact on prostate cancer detection or mortality could be slight. The 2018 revised USPSTF guidelines now indicate that providers should discuss the benefits and risk of this test for men 55–69 years old.(33) Whether liver disease should be a component of that discussion and also considered in the interpretation of the PSA results remains to be determined, as does how reduced life expectancy due to liver disease affects the screening benefit to risk ratio.
We found that the inverse association between liver fibrosis score and PSA level was present in each US racial/ethnic group studied. Compared with White men, Black men have a significantly higher incidence of prostate cancer, are more likely to develop prostate cancer at younger ages, and are more likely to die of prostate cancer.(36) Although disparities are not consistent across liver diseases (i.e., Blacks have a higher risk of infectious hepatitis, while Mexican Americans have a higher prevalence of NAFLD), including in NHANES (14,15), and as seen in this NHANES analysis, the proportion with an abnormal liver fibrosis score was higher among non-Hispanic Black than among non-Hispanic White or Mexican-American men. Given our results, men with abnormal liver fibrosis scores were less likely to have PSA >3–4 ng/mL and may not be referred to prostate biopsy. Therefore, Black men may be more likely to have delayed detection of prostate cancer attributable to a higher prevalence of liver fibrosis and a higher risk of prostate cancer than their non-Hispanic White or Mexican-American counterparts.
Study strengths include use of a nationally representative sample of men in the age range often targeted for early detection of prostate cancer. Liver enzyme and PSA measurements were not performed for clinical indication and the men were not enriched for liver disease. Instead, measurements were performed on all eligible men as part of the NHANES protocol to understand the health and nutritional status of Americans. Thus, the findings are likely generalizable to US men in these age ranges. Secondly, each biomarker was measured under the same protocol in the same laboratory. Moreover, this is the first and largest study to examine the association between liver fibrosis and PSA in different US races/ethnicities.
One possible limitation is that we used non-invasive indicators of liver fibrosis, which may result in misclassification compared with the gold standard liver biopsy or modern imaging elastography methods. We used three fibrosis scores that were primarily developed for monitoring liver conditions, but they have been validated in different populations.(25,34) Given that each is purported to measure liver fibrosis, we performed a sensitivity analysis in which cross-categorized the men with respect to all three of the scores. In doing so, we expected that men with liver fibrosis would be more likely score high on two or three of these scores, while those without liver fibrosis would be more likely to score low on all three of these scores. The results (i.e., lower PSA in those most likely to have liver fibrosis than in those unlikely to have liver fibrosis) when using the cross-classification were consistent with those when using the three scores separately.
Another study limitation is that it was cross-sectional, and we are not able to evaluate how progression to fibrosis influences PSA concentration. We could not study the mechanism by which liver fibrosis influences serum PSA level. We had initially hypothesized that liver fibrosis is associated with higher serum PSA because the liver is the primary site for PSA metabolism and clearance.(3,37) However, we also recognized that men with liver cirrhosis have lower testosterone levels,(38) and given that PSA is under androgenic regulation,(39) we alternatively hypothesized that men with fibrosis may have lower serum PSA due to reduced testosterone level including intraprostatic.
We cannot rule out that the inverse association that we observed is explained by men with abnormal liver function being less likely to develop prostate cancer, and thus have lower PSA levels. As prostate growth is dependent on androgens, levels of which can be influenced by hepatic function, liver disease may impact prostate cancer risk.(39,40) To address this alternative explanation, a prospective study of men with and without liver disease followed for a decade or more for the development of clinically-detectable prostate cancer or for prostate cancer detected by biopsy performed irrespective of PSA level is needed. Such a study is unlikely to be conducted in the future.
In conclusion, men with an abnormal liver fibrosis score may be less likely to have an elevated PSA. Further research is needed to determine whether liver disease could result in a delayed of prostate cancer, and whether it should be considered in the PSA-based prostate cancer screening.
Supplementary Material
Funding:
This work was funded in part by the National Cancer Institute (P30 CA006973, Nelson).
Footnotes
Conflict of interest disclosure statement: The authors have no conflict of interests.
References
- 1.Vicentini FC, Botelho LAA, Hisano M, Ebaid GX, Lucon M, Lucon AM, et al. Are Total Prostate-specific Antigen Serum Levels in Cirrhotic Men Different From Those in Normal Men? Urology 2009;73(5):1032–5 doi 10.1016/j.urology.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Akdogan M, Hassoun BS, Gurakar A, El-Sahwi K, Jazzar A, Wright H, et al. Prostate-specific antigen levels among cirrhotic patients. The International journal of biological markers 2002;17(3):161–4. [DOI] [PubMed] [Google Scholar]
- 3.Agha AH, Schechter E, Roy JB, Culkin DJ. Prostate specific antigen is metabolized in the liver. The Journal of urology 1996;155(4):1332–5. [PubMed] [Google Scholar]
- 4.Inci M, Rifaioglu MM, Inci M, Celik M, Demir M, Ulutas T, et al. The investigation of total PSA, free PSA, and free/total PSA ratio in patients with liver cirrhosis patients according to Child-Pugh score. Urology 2013;81(3):617–22 doi 10.1016/j.urology.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Han JH, Chang IH, Ahn SH, Kwon OJ, Bang SH, Choi NY, et al. Association between serum prostate-specific antigen level, liver function tests and lipid profile in healthy men. BJU international 2008;102(9):1097–101 doi 10.1111/j.1464-410X.2008.07774.x. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. The New England journal of medicine 2009;360(13):1310–9 doi 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. The New England journal of medicine 2009;360(13):1320–8 doi 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 8.Stark JR, Mucci L, Rothman KJ, Adami HO. Screening for prostate cancer remains controversial. BMJ (Clinical research ed) 2009;339:b3601 doi 10.1136/bmj.b3601. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer causes & control : CCC 2010;21(7):1071–80 doi 10.1007/s10552-010-9535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 2017;123(12):2312–9 doi 10.1002/cncr.30687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 12.Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. Journal of clinical gastroenterology 2015;49(8):690–6 doi 10.1097/mcg.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 13.Flores YN, Yee HF, Leng M, Escarce JJ, Bastani R, Salmerón J, et al. Risk Factors for Chronic Liver Disease in Blacks, Mexican Americans, and Whites in the United States: Results From NHANES IV, 1999–2004. The American journal of gastroenterology 2008;103(9):2231–8 doi 10.1111/j.1572-0241.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of internal medicine 2014;160(5):293–300 doi 10.7326/m13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. American journal of epidemiology 2013;178(1):38–45 doi 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidenreich A, Abrahamsson PA, Artibani W, Catto J, Montorsi F, Van Poppel H, et al. Early detection of prostate cancer: European Association of Urology recommendation. European urology 2013;64(3):347–54 doi 10.1016/j.eururo.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 17.Schroder FH, Hugosson J, Carlsson S, Tammela T, Maattanen L, Auvinen A, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). European urology 2012;62(5):745–52 doi 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 18.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital and health statistics Ser 1, Programs and collection procedures 2013(56):1–37. [PubMed] [Google Scholar]
- 19.National Institutes of Health. Laboratory Procedure Manual. Hyattsville, MD2011. [Google Scholar]
- 20.Wai C-T, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md) 2003;38(2):518–26 doi 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md) 2006;43(6):1317–25 doi 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 22.Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). Journal of viral hepatitis 2014;21(12):917–20 doi 10.1111/jvh.12279. [DOI] [PubMed] [Google Scholar]
- 23.Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. Journal of hepatology 2016;64(4):773–80 doi 10.1016/j.jhep.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Castera L Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Seminars in liver disease 2015;35(3):291–303 doi 10.1055/s-0035-1562948. [DOI] [PubMed] [Google Scholar]
- 25.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2009;7(10):1104–12 doi 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, Md) 2007;45(4):846–54 doi 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 27.Mittal RD, Singh MK, Selvaraju C, Choudhuri G. Total PSA and free PSA in patients with severe liver dysfunction. Indian Journal of Urology 2003;19(2):117–9. [Google Scholar]
- 28.Kubota Y, Sasagawa I, Sinzawa H, Kunii T, Itoh K, Miura H, et al. Serum levels of free and total prostate-specific antigen in males with liver cirrhosis. European urology 1999;36(5):409–12 doi 20022. [DOI] [PubMed] [Google Scholar]
- 29.Williams PB, Eastham JA, Culkin DJ, Mata JA, Venable DD, Sartor O. Influence of hepatic function on serum levels of prostate specific antigen. The Journal of urology 1997;158(5):1867–9. [DOI] [PubMed] [Google Scholar]
- 30.Kilic S, Guntekin E, Danisman A, Kukul E, Suleymanlar I, Sevuk M. Serum free and total prostate-specific antigen levels in patients with liver disease. Urology 1998;52(5):825–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 31.Malavaud B, Miedouge M, Payen JL, Izopet J, Rischmann P, Pascal JP, et al. Prostate-specific antigen in acute hepatitis and hepatocellular carcinoma. The Prostate 1999;41(4):258–62. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman RM, Volk RJ, Wolf AMD. Making the grade: The newest US Preventive Services Task Force prostate cancer screening recommendation. Cancer 2017;123(20):3875–8 doi 10.1002/cncr.30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Preventive Services Task Force. Screening for prostate cancer: US preventive services task force recommendation statement. Jama 2018;319(18):1901–13 doi 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 34.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59(9):1265–9 doi 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 35.Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, van der Poorten D, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology 2011;26(10):1536–43 doi 10.1111/j.1440-1746.2011.06774.x. [DOI] [PubMed] [Google Scholar]
- 36.American Cancer Society. Cancer facts and figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 37.Kilic S, Yalcinkaya S, Guntekin E, Kukul E, Deger N, Sevuk M. Determination of the site of metabolism of total, free, and complexed prostate-specific antigen. Urology 1998;52(3):470–3. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. Journal of gastroenterology and hepatology 2015;30(2):244–51 doi 10.1111/jgh.12695. [DOI] [PubMed] [Google Scholar]
- 39.Young CY, Andrews PE, Tindall DJ. Expression and androgenic regulation of human prostate-specific kallikreins. Journal of andrology 1995;16(2):97–9. [PubMed] [Google Scholar]
- 40.Bañez LL, Loftis RM, Freedland SJ, Presti JC, Aronson WJ, Amling CL, et al. The Influence of Hepatic Function on Prostate Cancer Outcomes Following Radical Prostatectomy. Prostate cancer and prostatic diseases 2010;13(2):173–7 doi 10.1038/pcan.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.