Abstract
Objectives
We aimed to explore whether weight loss may improve central hemodynamics in obesity
Background
Hemodynamic abnormalities in obese HFpEF patients are correlated with the amount of excess body mass, suggesting a possible causal relationship.
Methods
We systematically searched relevant databases from inception to May 2018, without language restriction. Studies reporting invasive hemodynamic measures prior to and following therapeutic weight loss interventions in patients with obesity but no clinically-overt HF were extracted.
Results
A total of 9 studies were identified, providing data for 110 patients. Six studies tested dietary intervention and 3 studies tested bariatric surgery. Over a median duration of 9.7 months [range 0.75 to 23], median weight loss of 43 kg [range 10–58] was associated with significant reductions in heart rate [−9 bpm, 95% CI −12 to −6, p<0.001], mean arterial pressure [−7 mmHg, 95% CI −11 to −3, p<0.001], and resting oxygen consumption [VO2, −85 mL/min, 95% CI −111 to −60, p<0.001]. Central cardiac hemodynamics improved, manifest by reductions in pulmonary capillary wedge pressure [−3 mmHg, 95% CI −5 to −1, p<0.001] and mean pulmonary artery pressure [−5 mmHg, 95% CI −8 to −2, p=0.001]. Exercise hemodynamics were assessed in a subset of patients (n=49), where there was significant reduction in exercise pulmonary artery pressure (p=0.02).
Conclusion
Therapeutic weight loss in obese patients without HF is associated with favorable hemodynamic effects. Randomized controlled trials evaluating strategies for weight loss in obese patients with HF such as the obese phenotype of HFpEF are needed.
Keywords: heart failure, obesity, weight loss, invasive hemodynamics, meta-analysis
Introduction
Obesity is a major risk factor for development of heart failure (HF), and in particular, HF with preserved ejection fraction (HFpEF).(1–5) Among patients with unexplained dyspnea, the mere presence of obesity increases the odds that HFpEF is the cause of symptoms by more than 3-fold.(3) As compared to non-obese HFpEF patients, those with obesity present with more severe HF symptoms, adverse hemodynamics, greater systemic inflammation, and impaired exercise capacity.(6–8)
Abnormal hemodynamics play a central role in the pathophysiology of HFpEF, directly contributing to symptoms of dyspnea, worsening functional capacity, and increasing morbidity and mortality.(9–11) Among patients with obesity and HFpEF, the magnitude of elevation in filling pressures is directly related to the amount of excess body mass, suggesting a possible causal relationship.(6) Modest, therapeutic weight loss induced by caloric restriction in patients with obesity and HFpEF improves aerobic capacity,(12) but its effects on directly measured invasive cardiac hemodynamics have not been examined.
In order to explore whether therapeutic, purposeful weight loss might be a viable treatment for obese patients with HF, we conducted a systematic review and meta-analysis of prior studies reporting the effects of weight loss on central hemodynamics assessed invasively in patients with obesity but without frank HF.
Methods
Study and patient selection
Randomized and observational studies evaluating the effects of weight loss on invasive hemodynamic measurements before and following weight loss in obese adults (age>18 years) were identified up to May 1, 2018. (The detailed search strategy is included in the supplementary appendix). Studies were required to report pre and post weight loss invasive hemodynamics as mean and standard deviation, such that mean differences (MD) could be calculated for each variable. Studies were included regardless of method of weight loss (caloric restriction or bariatric surgery). All abstracts were screened by a single investigator (YNR) to form the list for full text review. Full text reviews were performed by YNR and MAN in duplicate. Any discrepancy between the two reviewers was resolved by consulting the third reviewer (BAB).
Data analysis
The mean and standard deviation of central hemodynamic variables of interest were extracted from each study before and after weight loss to calculate mean differences. In the subset of studies that also evaluated exercise hemodynamics before and after weight loss, mean differences between peak exercise hemodynamics were also compared before and after weight loss. Individual patient data was available from tables in some studies and was used to summarize statistics when not directly reported in the original publication.
For studies that did not report the necessary raw data for calculation, this was calculated from included bar graphs and figures when available after creating a digitized curve using Engauge digitizer 4.1.(13) Detailed baseline characteristics for age, sex, body mass index, weight before and after weight loss and duration of follow up were also extracted. A random effects model was used to summarize differences following weight loss among subjects who underwent therapeutic weight loss intervention. Risk of bias was assessed by one reviewer independent from study selection (MO) using the New Castle Ottawa Scale for cohort studies (details in supplemental appendix).
Statistical analysis
Data were pooled in a random effects model with the pooled effect size represented as mean difference after weight loss, with 95% confidence interval (CI) limits. For ease of reporting, differences were calculated as post minus pre weight loss values, such that a negative difference in means indicates a reduction in value after weight loss. Baseline hemodynamics were summarized across studies as weighted means and Cochrane’s Q-statistics were used to determine the heterogeneity of included studies for each outcome. Heterogeneity was calculated using prediction intervals as recommended by Borenstein et al.(14) I2 values of <25%, 25–50%, and 50–75% were considered as low, moderate, and high heterogeneity, respectively. A p-value of <0.05 was considered statistically significant. Analyses were performed by MAN and YNR using the software Comprehensive Meta-analysis (version 3.3).
Results
Study selection
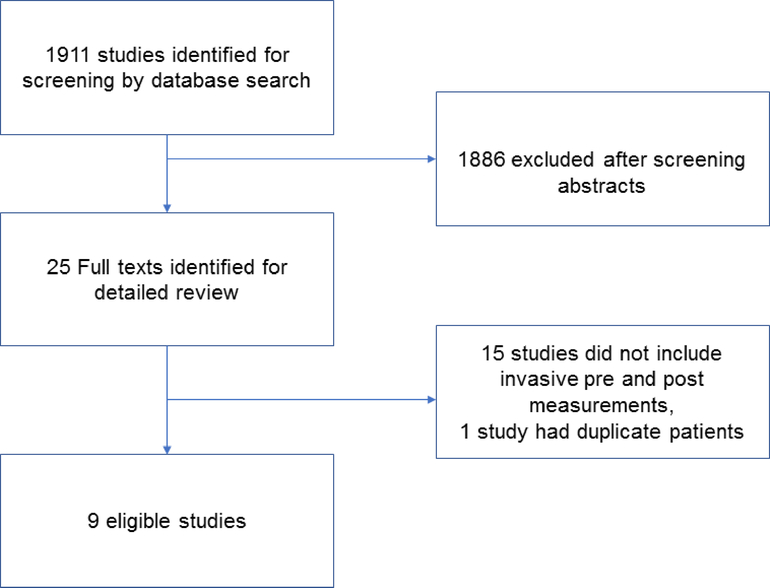
On the initial library search, 1911 studies were identified for abstract screening (Figure 1 and Supplemental Appendix). After manual review of all abstracts, 25 studies were selected for full text review and were evaluated by two authors, who independently identified the same 9 studies for inclusion. No studies including patients with overt obesity related heart failure were identified that reported pre and post weight loss hemodynamics. Among 200 randomly evaluated abstracts, no disagreements on exclusion were noted between the two reviewing authors. Data was extracted by YNR from individual studies and then verified in duplicate by MAN. Three of the studies (22–24) required some summary data extraction from bar graphs when not included in the text or tables.
Figure 1: Identification of Studies.
Screening strategy resulting in 1911 abstracts screened for 9 eligible studies
Fifteen studies did not include the necessary invasive assessment of central hemodynamics before and after weight loss, and were therefore excluded (Figure 1). One study by Andersson et al(15) included duplicate patients with another study by the same authors(16) and was therefore excluded. The included Andersson study contained one arm of patients treated with both weight loss and salt loading; and the data from those subjects was therefore not included.(16)
Of the 9 studies identified, no randomized trials were identified; all were prospective observational studies reporting invasive hemodynamic, metabolic and neurohormonal changes following therapeutic weight loss interventions (Table 1).(16–24)
Table 1:
Study Details and Patient Demographics
| Study | Sample Size | Age (years) | Female (%) | Intervention | Body weight Pre (kg) | Body weight Post (kg) | Follow-up (months) | Hemodynamic and Metabolic Variables Assessed |
|---|---|---|---|---|---|---|---|---|
| Alaud-din A et al 1990(17) | 12 | NA | 87 | BS | 132±17 | 77±5 | 13±4 | CO, SV, HR |
| Alexander et al, 1972(18) | 9 | 38±13 | 78 | CR | 166±31 | 111±28 | 13 (range 4–34) | PCWP, PAm, MAP, CO, SV, HR, SVR, BV, VO2, AVO2D |
| Andersson et al, 1984(16) | 10 | 51±4 | NA | CR | 98±8 | 89±9 | NA | MAP, CO, SV, HR, SVR, BV |
| Backman et al, 1979(19) | 22 | 35±2 | 77 | BS | 146±4 | 88±3 | 23 (range 10–38) | PCWP, PAm, PASP, MAP, CO, SV, HR, VO2, AVO2D |
| Kaltman et al, 1975(20) | 4 | 32±9 | 50 | CR | 155±33 | 116±17 | 4–20 | PCWP, RAP, MAP, CO, SV, HR, VO2, AVO2D, |
| Reisin et al, 1983(21) | 12 | NA | NA | CR | 96±4 | 86±4 | 9±3 | MAP, CO, SV, HR, SVR |
| Slany et al, 1974(22) | 12 | 43±13 | 58 | CR | 115±18 | 104±17 | 0.5–1 | PCWP, PAm, MAP, CO, HR, |
| Slany et al, 1975(23) | 11 | 38±9 | 79 | CR | 116±15 | NA* | 0.5–0.7 | PCWP, PAm, RAP, MAP, CO, SV, HR |
| Sugerman et al, 1988(24) | 18 | NA | NA | BS | 224±59 (%IBW) | 167±57 (%IBW) | 3–9 | PCWP, PAm |
NA indicates data not available
although absolute body weight post intervention was not reported in the Slany et al (1975) study, the range of weight loss was 8–15 kg; BS, bariatric surgery; CR, caloric restriction; AVO2D, arterial-venous oxygen content difference; BV, blood volume; CO, cardiac output; HR, heart rate; IBW, ideal body weight; MAP, mean arterial pressure; PAm, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; SV, stroke volume; SVR, systemic vascular resistance; VO2, oxygen consumption.
Study characteristics
The studies included represent data from a total of 110 patients with obesity. Six studies evaluated effects following caloric restriction (diet intervention) and 3 studies evaluated changes following bariatric surgery (Table 1). The median of study level mean age was 37 years [range 32–43], with baseline weight of 124 kg [range 96–166] and included mostly women (median 81% [range 50–87]). Hemodynamic evaluation was performed at baseline before intervention and following weight loss at a median follow up of 9.7 months [range 0.75 to 23]. The magnitude of weight loss achieved across studies was 43 kg [range 10–58] which represented an approximate 25% weight loss [range 9–42].
Baseline Oxygen Delivery, Metabolism and Hemodynamics
The weighted mean heart rate and mean arterial pressures prior to weight loss were 76 bpm [range 63–82 bpm] and 104 mmHg [range 94–114 mmHg], respectively (Table 2). Resting oxygen consumption (VO2) prior to weight loss was 362 ml/min [range 360–381 ml/min], ~50% higher than published normative values in non-obese adults (241±57 ml/min),(25) in keeping with the increased metabolic rate associated with excess body mass. Plasma volume was 6.1 L/min [range 4.9–7.8], coupled with a borderline increase in resting cardiac output at 6.4 L/min [range 5.7–7.6 L/min]. There were also borderline elevations in left sided cardiac filling pressures [pulmonary capillary wedge pressures (PCWP) 12 mmHg, range 10–17] and mean pulmonary artery (PA) pressures [23mmHg, range 19–36 mmHg] (Table 2).
Table 2:
Estimates of Hemodynamic and Metabolic Parameters before and after Weight Loss
| Number of studies (n) | Weighted Mean Pre WL | Mean Difference Following WL | 95% CI | p value | I2 (%) | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| Heart rate (min−1) | 8 (93) | 76 | −9 | −11.7 to −5.7 | <0.001 | 57* |
| Mean arterial pressure (mmHg) | 7 (81) | 104 | −7 | −11.3 to −3.3 | <0.001 | 41 |
| Oxygen consumption (ml/min) | 3 (35) | 362 | −85 | −111 to −60 | <0.001 | 0 |
| Right atrial pressure (mmHg) | 3 (27) | 5 | −2.4 | −4.8 to −0.1 | 0.04 | 81* |
| Mean pulmonary artery pressure (mmHg) | 4 (60) | 23 | −5.2 | −8.1 to −2.2 | 0.001 | 45 |
| Pulmonary wedge pressure (mmHg) | 6 (76) | 12 | −3.2 | −5.0 to −1.4 | <0.001 | 40 |
| Cardiac output (l/min) | 8 (93) | 6.4 | −0.6 | −0.8 to −0.5 | <0.001 | 0 |
| Arterial-venous O2 difference (ml/ml) | 3 (35) | 4.6 | −0.4 | −0.9 to −0.01 | 0.04 | 0 |
| Exercise | ||||||
| Heart rate (min−1) | 5 (61) | 119 | −4 | −7.8 to +0.2 | 0.07 | 23 |
| Mean arterial pressure (mmHg) | 4 (49) | 124 | −13 | −21.6 to −5.2 | 0.001 | 26 |
| Oxygen consumption (ml/min) | 3 (35) | 1237 | −231 | −361 to −100 | <0.001 | 0 |
WL, weight loss. P value reflects the change with weight loss, (I2) reflects the heterogeneity for the change in each parameter with weight loss
heterogeneity p value<0.05
Effects of Weight Loss on Oxygen Delivery and Metabolism
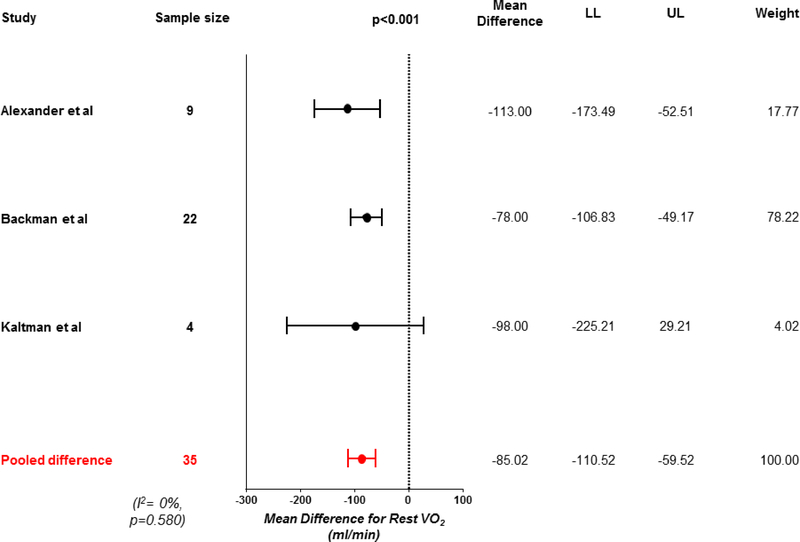
Following weight loss, there was a 24% reduction in resting VO2 (−85 mL/min, 95% CI −111 to −60, p<0.001; Figure 2, Central Illustration). This was coupled with a 9% decrease in cardiac output (−0.64 L/min, 95% CI −0.79 to −0.50, p<0.001; Table 2, Supplementary Figures). Resting arterial venous O2 difference decreased, indicating less O2 extraction in the tissues (−0.43 mL/dL, 95% CI −0.85 to −0.01, p=0.04), even as cardiac output decreased. There was an 11% reduction in heart rate (−9 beats per minute, 95% CI −12 to −6, p<0.001), with no change in stroke volume (−1 mL, 95% CI −9 to +6, p=0.70). Heterogeneity was high for changes in heart rate (I2=57%, p=0.022, Table 2). There was a trend towards a reduction in blood volume following weight loss (−0.44 L 95% CI −1.05 to 0.17, p=0.16, Table 2). Weight loss was associated with a 7% reduction in mean arterial pressure (−7mmHg, 95% CI −11 to −3, p<0.001; Table 2).
Figure 2: Change in resting oxygen consumption with weight loss.
Forest plot showing reduction in resting oxygen consumption (VO2) with weight loss. LL, lower limit; UL, upper limit.
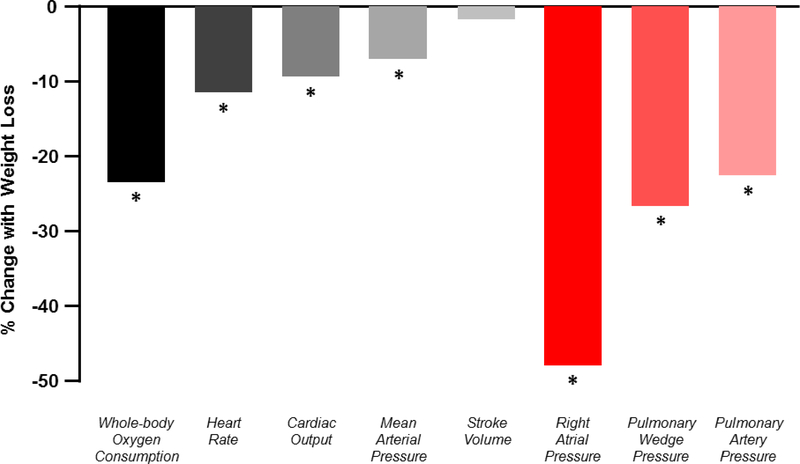
Central illustration: Changes in central hemodynamics and metabolism following weight loss.
Mean percent changes in resting metabolism, vital signs and hemodynamics. *p<0.05
Effects of Weight loss on Hemodynamics
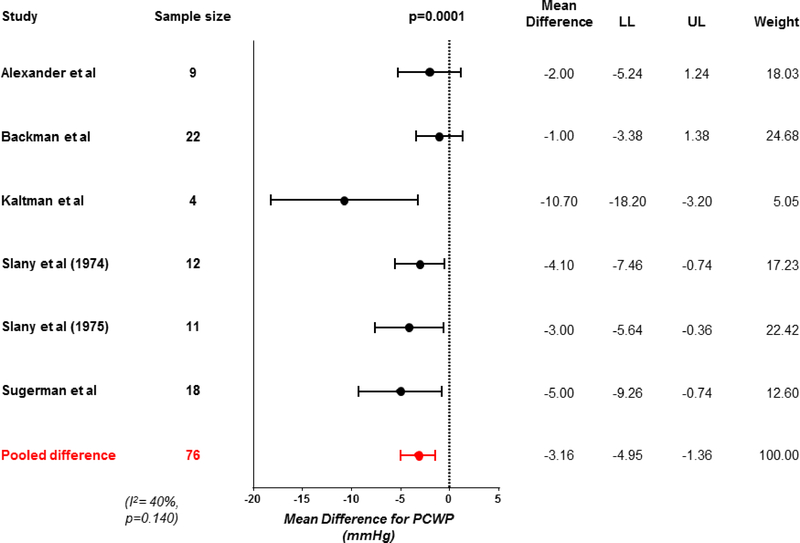
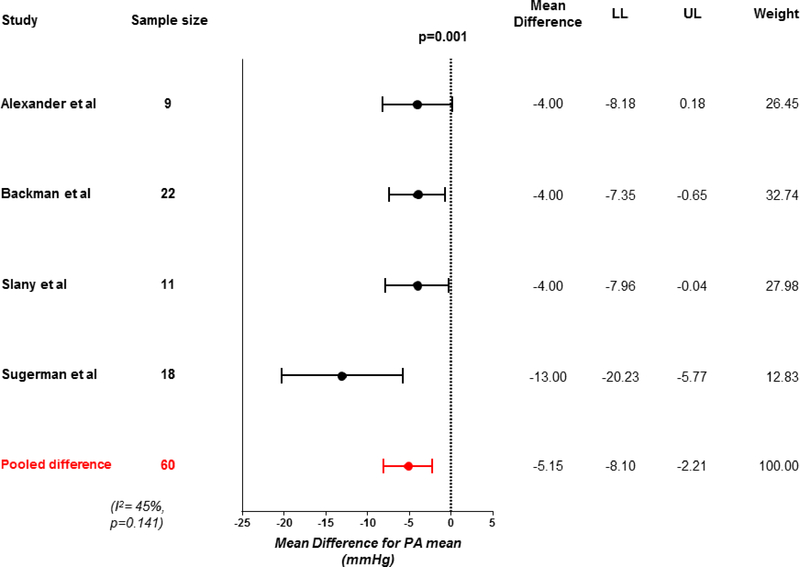
Weight loss was associated with significant improvements in biventricular filling pressures, with a 26% reduction in pulmonary capillary wedge pressure (−3 mmHg, 95% CI −5 to −1, p<0.001; Figure 3) and 46% reduction in mean right atrial pressure (−2 mmHg, 95% CI −5 to −0.1, p=0.042). Heterogeneity was high for the changes in right atrial pressure (Table 2, Supplementary Figures). Concordant with the reduction in left sided filling pressures, mean pulmonary artery pressures were also reduced by 22% following weight loss (−5 mmHg, 95% CI −8 to −2, p=0.001; Figure 4).
Figure 3: Change in resting pulmonary capillary wedge pressure with weight loss.
Forest plot showing reduction in resting pulmonary capillary wedge pressure (PCWP) with weight loss. Abbreviations as in Figure 2.
Figure 4: Change in resting pulmonary artery pressure with weight loss.
Forest plot showing effect of weight loss on resting mean pulmonary artery (PA) pressure. Abbreviations as in Figure 2.
Effects of Weight loss on Gas Exchange and Neurohormones
One study of patients with obesity-hypoventilation syndrome reported changes in the partial pressure of arterial CO2 in 18 patients, and there was a significant reduction with weight loss from 52±7 to 42±4 mmHg (p<0.0001).(24) This change was coupled with an increase in partial pressure of arterial O2 from 50±10 to 69±14 mmHg (p<0.0001). Two studies reported plasma norepinephrine levels and plasma renin activity.16, 19 Point estimates suggested reduction in norepinephrine that was not statistically significant (−205, 95% CI −451.31 to 40.63, p=0.1), but heterogeneity was high (I2=98%, p<0.0001).(16,21) There was a small reduction in plasma renin activity in the same two studies of borderline significance (−0.08 ng/ml.h, 95% CI −0.16 to 0.001, p=0.04)
Effects of Weight Loss on Exercise Hemodynamics
Five studies reported invasive hemodynamics at peak exercise before and after weight loss, or included necessary raw data to calculate means and standard deviations using data from a subset of 49 patients (Table 2, Supplementary Figures). The weighted mean of peak exercise wedge pressure was 22 mmHg [range 20–34] and pulmonary artery mean pressure was 33 mmHg [range 28–37]. Following weight loss, there was a reduction in peak pulmonary artery mean pressure (−5 mmHg, 95% CI −8 to −1, p=0.02) and mean arterial pressure (−13 mmHg, 95% CI −22 to −5, p=0.001). There was a trend to lower pulmonary capillary wedge pressure with exercise (−3 mmHg, 95% CI −6 to +1, p=0.19).
Consistent with the reduction in resting VO2, there was similar reduction in peak exercise VO2 (−230 ml/min, 95% CI −361 to −100, p<0.001; Table 2, Supplementary Figures), which was related to reductions in both exercise cardiac output (−1.45 L/min, 95% CI −2.60 to −0.31, p=0.01), and arterial-venous O2 content difference (−0.78 mL/dL, 95% CI −1.35 to −0.21, p=0.008). Peak ergometric workloads achieved during exercise were not reported in the studies.
Study quality and Risk of Bias
Quality assessment was performed using a modified New Castle Ottawa Scale for cohort studies. Included studies demonstrated high quality with low risk of bias (Supplemental Table). One of the included studies (16) evaluated the effects of weight loss achieved through both caloric and sodium restriction, but exclusion of this study in a sensitivity analysis did not alter the results (Supplemental Table).
Discussion
This systematic review and meta-analysis summarizes the existing evidence regarding the effects of therapeutic weight loss on invasive hemodynamics in patients with obesity, but no clinical HF. We observed that weight loss is associated with significant reductions in biventricular filling pressures and pulmonary artery pressure, heart rate, cardiac output, systemic blood pressure, and whole-body oxygen consumption (Central Illustration). In a smaller sub-analysis, there were improvements or trends to improvement in exercise hemodynamics with weight loss. These data, derived exclusively from observational cohort studies, raise the possibility that therapeutic weight loss interventions may be effective to mitigate the hemodynamic derangements that contribute to morbidity and mortality in people with the obese phenotype of HFpEF, and potentially even obese patients with HFrEF.
Obesity and its effect on central hemodynamics
Obesity has become one of the most important causes of HF in the modern era, particularly HFpEF.(1–8) Abnormal hemodynamics play a central role in the pathophysiology of HFpEF.(9–11) In HFpEF patients with obesity, the magnitude of PCWP elevation is directly related to the amount of excess body mass.(6) In contrast, this relationship is absent among non-obese HFpEF patients. This suggests that excess adipose may be an important promoter of adverse hemodynamics in the obese phenotype of HFpEF. If this is true, then weight loss would be expected improve these hemodynamic derangements.
Consistent with this hypothesis, we observed significant improvements in cardiac filling pressures and pulmonary artery pressures in patients with obesity but no clinical diagnosis of HF. These data support, but do not prove, a causative role for excess adiposity in the pathogenesis of elevated filling pressures and pulmonary pressures in obesity, even as hemodynamic findings at baseline (prior to weight loss) were only borderline abnormal.
Potential Mechanisms of Benefit
The consistent improvement in biventricular filling pressures, lowering of cardiac output and blood pressure collectively suggest that relief of volume overload from weight loss may have been a contributor to the improvements in PCWP.39−41 While the point estimates of blood volume reduction with weight loss were not statistically significant, only 3 of the included studies directly measured blood volume, which may have compromised power. The observed reductions in systemic blood pressure and cardiac output would be expected to ameliorate the excessive ventricular workload in obesity, and metabolic improvements associated with weight loss may improve myocardial substrate utilization and efficiency.(26)
The favorable hemodynamic effects observed in the current study are consistent with salutary effects noted in prior noninvasive echocardiographic studies, where reductions in ventricular mass with weight loss have variably been associated with improvements in myocardial function.(27–29) However, noninvasive estimates of hemodynamics are imprecise, particularly among obese patients where image quality may be compromised. For this reason, we restricted the analysis to studies where invasive central hemodynamics were measured directly rather than using noninvasive surrogates.
The reduction in cardiac output following weight loss was driven by a decrease in heart rate rather than stroke volume. This suggests a reduction in sympathetic tone, which is often elevated in obesity, and is consistent with the trend to reductions in plasma norepinephrine and renin activity in the two studies that reported these measures.16, 19 The potential neurohormonal basis for obesity-related HFpEF represents an area of intense interest, but remains poorly understood.(30)
At first glance, one might consider a reduction in cardiac output and VO2 (an indicator of fitness) to imply a detrimental impact of weight loss. However, it must be remembered that cardiac output increases relative to metabolic demand,(31) and excessive increases in VO2, as are observed in obesity, may be detrimental or even lead to high output HF.(32) The excess body mass in obesity increases the metabolic work (VO2) required for locomotion, decreasing efficiency.(6,8)
Even as cardiac output at rest and with exercise was reduced, we observed that weight loss in obese patients reduced the AVO2 difference, both at rest and at peak exercise. This suggests more favorable O2 delivery relative to metabolic demand, since venous O2 content is greater despite lower total cardiac output. Viewed in this light, the tandem reductions in VO2, cardiac output and AVO2 difference together suggest improved total efficiency and net oxygen delivery to exercising muscle following weight loss.
Potential for Weight Loss to treat Heart Failure
Weight loss in obese patients without HF mitigates age-related ventricular stiffening (33) and decreases incidence of new-onset HF, yet the mechanisms remain unclear.(34,35) Only one clinical trial has been published to date examining the effects of weight loss intervention as a treatment for HFpEF. In an elegant trial, Kitzman and colleagues demonstrated that weight loss achieved through caloric restriction (400 kcal/day), or the combination of caloric restriction and exercise training, improved aerobic capacity and quality of life assessed by the Kansas City Cardiomyopathy Questionnaire.(12) Kitzman and colleagues did not measure direct hemodynamic effects of weight loss, but it is notable that the amount of weight loss achieved over 20 weeks with caloric restriction (mean −7 kg) was substantially lower than that observed in this review (−43 kg), which included a number of patients that underwent bariatric surgery, which produces greater magnitude of weight loss, as well as more profound secondary benefits on insulin sensitivity, inflammation, lipids, and mechanical complications of obesity.(34,35) The studies included in this review tested weight loss alone and not the combination of weight loss and increases in physical activity, as is currently recommended. Isolated weight loss without concomitant resistance and aerobic exercise training may result in loss of lean mass, which could prove harmful in older patients with HFpEF. Considering the current data together with the study from Kitzman and colleagues, it is clear that randomized trials testing more aggressive weight loss interventions, together with appropriate lifestyle and physical activity interventions are necessary in HFpEF, as well as HFrEF, where favorable effects on hemodynamics might also be effective.
Limitations
Because the studies were performed at different centers and across different eras, hemodynamic evaluations were not standardized, which in addition to the modest sample size across studies, may contribute to wider variability and greater heterogeneity. However, this limitation would only have been expected to bias the results towards the null. Given the need for repeat invasive assessments, the number of subjects for whom data is available was modest and follow up assessments were not uniformly performed, which may limit the ability to properly estimate the benefits of weight loss on hemodynamics. Individual-level patient data were not available in the majority of studies precluding attempts at patient-level meta-analyses, and baseline characteristics such as age, sex and magnitude of weight loss were not uniformly reported. Most of the included studies are 20–40 years old, and we did not identify any more contemporary studies in our search. This likely relates to the greater utilization of echocardiography in the current era, particularly for repeat assessments, which was not available when most of these studies were reported. While this limitation may affect the applicability of these data to the current era, it seems unlikely that the hemodynamic effects of weight loss in humans would be likely to differ in a meaningful way across eras. There were no randomized trials identified in our systematic review, and accordingly there is no control group for comparison, making it difficult to determine whether observed hemodynamic changes were causally related to weight loss. This observation emphasizes the need for well performed prospective randomized trials testing the hypothesis that weight loss might improve hemodynamics and other clinical endpoints in obese patients with heart failure. The weight loss interventions administered were not uniform across study groups and thus we cannot comment on the specific efficacy of particular interventions of weight loss to improve central hemodynamics. The studies did not also provide sufficient data to understand if the maximum weight loss had been observed at the post-intervention measurement period, and there was variability in the time of follow up which may also confound interpretation. Gas exchange data was only available from one study evaluating weight loss in patients with obesity hypoventilation syndrome, and it is not clear whether similar changes would be observed in other obese populations, or whether favorable effects on gas exchange might be related to hemodynamic changes following weight loss. The number of studies does not provide adequate power to evaluate for potential dose-response relationships between weight loss and hemodynamic benefits. The mean age of patients included in these studies is much lower than typical HFpEF patients, and it is unclear how age and disease state may modify the hemodynamic response to aggressive weight loss.
Conclusion
In obese patients without heart failure, weight loss is associated with improved biventricular filling pressures, lower systemic and pulmonary artery pressures, reduction in ventricular work, and more favorable cardiac perfusion relative to metabolism. Because each of these hemodynamic derangements play a central role in the pathophysiology of the obese phenotype of HFpEF, future studies, preferably through randomized control trials, are need to define the potential role and optimal methods to achieve weight loss in this large and growing cohort of patients for whom few treatment options exist.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
In this meta-analysis of observational studies of invasive hemodynamic changes in obesity, we found that weight loss was associated with improved biventricular filling pressures, lower systemic and pulmonary artery pressures, reduction in ventricular work, and more favorable cardiac perfusion relative to metabolism.
Translational Outlook
There is an urgent need for prospective randomized controlled trials testing whether weight loss can improve cardiac hemodynamics and outcomes in patients with the obese phenotype of HFpEF
Acknowledgments
Funding: BAB is supported by R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262, all from the National Institute of Health
Abbreviations
- HFpEF
Heart Failure with preserved Ejection Fraction
- VO2
Oxygen consumption
Footnotes
Disclosures and relationship with industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 2.Ndumele CE, Matsushita K, Lazo M, et al. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc. 2016;5: pii: e003921. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savji N, Meijers WC, Bartz TM, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 6.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalos D, Mascherbauer J, Zotter-Tufaro C, et al. Functional Status, Pulmonary Artery Pressure, and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:189–99. [DOI] [PubMed] [Google Scholar]
- 8.Reddy YN, Lewis GD, Shah SJ, et al. Characterization of the Obese Phenotype of Heart Failure with Preserved Ejection Fraction: A RELAX Trial Ancillary Study Mayo Clin Proc. 2019;in press. [DOI] [PubMed] [Google Scholar]
- 9.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, Dyspnea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 11.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. [DOI] [PubMed] [Google Scholar]
- 15.Andersson OK. Relative importance of sodium and energy restriction for the hemodynamic adaptation in obesity hypertension. Scand J Clin Lab Invest Suppl. 1985;176:15–24. [PubMed] [Google Scholar]
- 16.Andersson OK, Fagerberg B, Hedner T. Importance of dietary salt in the hemodynamic adjustment to weight reduction in obese hypertensive men. Hypertension. 1984;6:814–9. [DOI] [PubMed] [Google Scholar]
- 17.Alaud-din A, Meterissian S, Lisbona R, MacLean LD, Forse RA. Assessment of cardiac function in patients who were morbidly obese. Surgery. 1990;108:809–18; discussion 818–20. [PubMed] [Google Scholar]
- 18.Alexander JK, Peterson KL. Cardiovascular effects of weight reduction. Circulation. 1972;45:310–8. [DOI] [PubMed] [Google Scholar]
- 19.Backman L, Freyschuss U, Hallberg D, Melcher A. Reversibility of cardiovascular changes in extreme obesity. Effects of weight reduction through jejunoileostomy. Acta Med Scand. 1979;205:367–73. [DOI] [PubMed] [Google Scholar]
- 20.Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med 1976;60:645–53. [DOI] [PubMed] [Google Scholar]
- 21.Reisin E, Frohlich ED, Messerli FH, et al. Cardiovascular changes after weight reduction in obesity hypertension. Ann Intern Med. 1983;98:315–9. [DOI] [PubMed] [Google Scholar]
- 22.Slany J, Mosslacher H, Bodner P, et al. [Cardiovascular changes during therapeutic starvation in obese subjects (author’s transl)]. Wien Klin Wochenschr. 1974;86:423–8. [PubMed] [Google Scholar]
- 23.Slany J, Mosslacher H, Irsigler K. [Does obesity have an effect on heart function?]. Z Kardiol. 1975;64:851–62. [PubMed] [Google Scholar]
- 24.Sugerman HJ, Baron PL, Fairman RP, Evans CR, Vetrovec GW. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg. 1988;207:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narang N, Thibodeau JT, Levine BD, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation. 2014;129:203–10. [DOI] [PubMed] [Google Scholar]
- 26.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The Effects of Bariatric Surgery on Cardiac Structure and Function: a Systematic Review of Cardiac Imaging Outcomes. Obes Surg. 2016;26:1030–40. [DOI] [PubMed] [Google Scholar]
- 28.Owan T, Avelar E, Morley K, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol. 2011;57:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer M, Kitzman DW. Obesity-Related Heart Failure With a Preserved Ejection Fraction: The Mechanistic Rationale for Combining Inhibitors of Aldosterone, Neprilysin, and Sodium-Glucose Cotransporter-2. JACC Heart Fail. 2018;6:633–639. [DOI] [PubMed] [Google Scholar]
- 31.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. J Am Coll Cardiol. 2016;68:473–82. [DOI] [PubMed] [Google Scholar]
- 33.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Failure. 2014;2:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benotti PN, Wood GC, Carey DJ, et al. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.