Abstract
Compulsive drives for alcohol, where intake persists despite adverse consequences, are substantial obstacles to treating Alcohol Use Disorder (AUD). However, there are limited treatment options and thus considerable interest in identifying new, potent and safe pharmacotherapies. We found that non-canonical N-methyl-d-aspartate receptors (NMDARs), active at hyperpolarized potentials, drive compulsion-like alcohol drinking in rats without affecting regular, alcohol-only intake. Congruent human studies suggest that NMDAR inhibition reduces alcohol drinking in treatment-seekers but not non-treatment-seekers and suppresses craving. These cross-species studies of consumption under conflict indicate that inhibiting non-canonical NMDARs could be of clinical value for AUD. D-serine activates NMDARs overall, but actually inhibits non-canonical NMDARs. Also, D-serine has been widely tested in humans as a moderate NMDAR modulator, but some nephrotoxicity concerns remain, and thus any strategy that reduces D-serine exposure could be of broad utility. Here, co-administration of sodium benzoate (NaBenz), which reduces D-serine breakdown, allowed subthreshold D-serine levels to suppress compulsion-like alcohol drinking without altering normal alcohol-only consumption, providing a novel intervention for AUD and underscoring the importance of non-canonical NMDARs for compulsion-like intake. Low NaBenz doses alone had no average effect. NaBenz/D-serine reduced compulsion-like intake in nearly all animals, while higher D-serine alone decreased compulsion-like intake but less so in lower-drinking subjects. Thus, combining subthreshold NaBenz and D-serine suppressed compulsion-like intake, helping both to alleviate some D-serine concerns, and, importantly, to reduce consequence-resistant consumption across nearly all individuals. Therefore, NaBenz/D-serine likely represents an FDA-approved and immediately-accessible pharmacotherapy to help counteract compulsion-like drives and treat AUD.
Keywords: compulsive, addiction, alcohol, pathological, NMDA receptor, treatment
Graphical Abstract

1. Introduction
Compulsion-like drives, where intake persists despite adverse social, physical, and legal consequences, are a major obstacle to treating alcohol use disorder (AUD) and a strong contributor to the heavy social and personal costs of AUD (CDC, 2014; Epstein and Kowalczyk, 2017; Hopf, 2017; Hopf and Lesscher, 2014; Koob and Volkow, 2010; Naqvi et al., 2014). AUD also lacks broadly effective pharmacotherapies (Spanagel, 2009). Thus, there is considerable interest in identifying novel pharmacological targets to decrease alcohol intake, especially for consequence-resistant drives and their substantial contribution to problem drinking. Our rodent compulsion-like intake paradigms involve alcohol drinking that persists despite adulterating alcohol with aversive quinine, which has been considered to model aspects of compulsion-like intake in humans (see Hopf, 2017; Hopf and Lesscher, 2014).
Addiction is thought to be driven by brain adaptations, and N-methyl-D-aspartate receptors (NMDARs) exhibit many alcohol-related changes that could strongly influence alcohol responding (reviewed in Hopf, 2017). Our rodent work (Seif et al., 2013; Seif et al., 2015) and several human studies (Bisaga and Evans, 2004; Krishnan-Sarin et al., 2015; Krupitsky et al., 2007; Lee et al., 2018; Muhonen et al., 2008; but see Evans et al., 2007) suggest that NMDARs can be critical for driving conflict-resistant alcohol behavior, with much less contribution to “regular” alcohol-only intake (see also Discussion). This is consistent with NMDARs being implicated for more pathological alcohol behaviors, such as relapse, rather than regular drinking (Eisenhardt et al., 2015) (except perhaps for operant intake; reviewed in Hopf, 2017). Interestingly, we find that long-term alcohol drinking leads to intra-nucleus accumbens insertion of non-canonical NMDARs under cortical inputs (Hopf, 2017; Seif et al., 2013; Seif et al., 2015). This type of NMDAR is active at hyperpolarized potentials (Chatterton et al., 2002; Takarada et al., 2009; Takarada et al., 2012; Traynelis et al., 2010) and can significantly enhance neuronal firing even in the absence of strong glutamatergic drive (Seif et al., 2013; Zhang et al., 2009). In addition, GluN2C subunits are critical for compulsion-like alcohol drinking in rat (Seif et al., 2013), and a genetic variation in GluN2C predicts human alcohol risk (Bach et al., 2015). Also, memantine, another NMDAR blocker used in humans, may preferentially inhibit GluN2C- and 2D-containing NMDARs (Kotermanski and Johnson, 2009). Thus, non-canonical NMDARs likely play a central role in clinically-relevant pathological drives for alcohol.
Since NMDARs may contribute more selectively to pathological drinking, NMDAR blockers might serve as potent and selective inhibitors of compulsion-like drives for alcohol. However, stronger NMDAR inhibition can have significant adverse effects such as psychosis (Heresco-Levy et al., 2013; Tsai and Lin, 2010), and there is considerable interest in using moderate NMDAR modulators to alleviate these concerns. In this regard, D-serine is a canonical positive modulator of NMDARs at the glycine site (Traynelis et al., 2010), with potential for moderate NMDAR regulation. Indeed, D-serine has been tested in many human studies, ranging from Parkinson’s, schizophrenia and mood regulation to enhancing extinction of fear or drug stimuli, and is well tolerated with very few adverse effects (Avellar et al., 2016; Gelfin et al., 2012; Guercio and Panizzutti, 2018; Heresco-Levy et al., 2013; Kantrowitz et al., 2010; Levin et al., 2015; Tsai and Lin, 2010).
In addition to canonical enhancement of NMDAR function, D-serine can also actually inhibit non-canonical NMDARs containing the GluN3 subunit (Chatterton et al., 2002; Takarada et al., 2009; Takarada et al., 2012). Importantly, D-serine administration systemically or within the nucleus accumbens significantly reduces compulsion-like alcohol drinking but not alcohol-only intake (Seif et al., 2015). Together, our results suggest that D-serine-inhibited, GluN2C/3-containing NMDARs drive aversion-resistant alcohol intake in rats (Seif et al., 2013; Seif et al., 2015), with congruent studies in humans, and thus that D-serine-based strategies may represent novel, FDA-approved and immediately accessible therapies to counteract compulsion-like drives for alcohol and their harm. However, despite D-serine’s long track record of safety, tolerability, and efficacy in humans (see above), there exists a small but still potential risk of kidney damage (e.g. Krug et al., 2007). Thus, any strategy allowing lower D-serine exposure while maintaining efficacy could have broad utility. In this regard, inhibitors of D-amino acid oxidase (DAAO), an enzyme that breaks down D-serine, should allow subthreshold D-serine levels to have behavioral effects. Sodium benzoate (NaBenz) is a common preservative which inhibits DAAO (Hashimoto, 2011; Howley et al., 2017) and represents a possible FDA-approved, well-tolerated (Lane et al., 2013; Lin et al., 2014; Lin et al., 2017) method to increase the behavioral impact of exogenous D-serine.
Here, we examined whether co-administering D-serine with NaBenz would allow a lower D-serine dose to suppress compulsion-like alcohol consumption, in order to determine the efficacy of this proposed combination therapy. We identified D-serine and NaBenz doses that were largely ineffective for reducing alcohol consumption, and showed that combining these lower doses of D-serine and NaBenz significantly reduced compulsion-like alcohol intake. In addition, the higher D-serine dose overall inhibited aversion-resistant consumption but was less effective in moderate-drinking individuals, while NaBenz/D-serine together reduced compulsion-like drinking in nearly all individuals. Thus, we have identified a novel, combined-agent, NMDAR-based treatment strategy that is FDA-approved and immediately accessible, would help reduce possible side effects of D-serine, can suppress compulsion-like alcohol drinking, and may have broader utility across a range of neuropsychiatric conditions.
2. Materials and methods
2.1. Animals and alcohol drinking methods
All procedures were conducted in accordance with the Guide for Care and Use of Laboratory animals provided by the National Institutes of Health, and approved by the UCSF Institutional Animal Care and Use Committee. Adult, male Wistar rats were singly housed with ad lib food and water throughout. P45-50 rats (Harlan) acclimatized to the vivarium for two weeks before undergoing voluntary alcohol consumption procedures. Rats were first trained to consume alcohol (20% v/v in water) using the intermittent, two-bottle choice paradigm following our previously published methods (Hopf et al., 2010; Seif et al., 2013; Seif et al., 2015). Briefly, three days a week (starting Monday, Wednesday and Friday afternoons), rats had an ~24-hr period where alcohol was available concurrently with water under two-bottle choice. Bottle positions were alternated across days to prevent a position bias. Intermittent access continued for 10-12 weeks, as longer-term intake is necessary to facilitate development of aversion-resistant intake (Hopf et al., 2010; Loi et al., 2010; Seif et al., 2013; Seif et al., 2015; Spoelder et al., 2017). After ~3 months of intermittent access, rats were shifted to limited-daily access, with 20 min/day to two-bottle choice for 20% alcohol or water, Monday through Friday. We have used this method to assess mechanisms underlying compulsion-like alcohol intake (Darevsky et al., 2018; Hopf et al., 2010; Seif et al., 2013; Seif et al., 2015). After 3-4 weeks of limited-daily access, rats were habituated to systemic injection (i.p.) before intake. Rats then had 2-3 alcohol-quinine sessions (with 10 mg/L quinine) to habituate to the novelty of quinine in alcohol, and then returned to alcohol-only drinking.
After this pre-training, animals then had two experimental sessions per week (typically Tues and Thurs), where animals were injected with experiments compounds or vehicle, and drank either alcohol-quinine or alcohol-only. It is important to note that experimental conditions (e.g. in Fig. 3A) were randomized across animals and sessions in a Latin-square design. Also, animals were exposed to each experimental condition twice: all conditions were randomized for one round of injections, then all conditions were randomized again for the second round. Then, for each experimental condition from a given animal, drinking data from the two injection days were averaged to give a single intake value. We routinely utilize this approach to reduce variability in drinking measures (Lei et al., 2016a; Lei et al., 2016b; Seif et al., 2013; Seif et al., 2015). Animals drank alcohol-only on all days other than experimental sessions, and there was at least one drinking session between experimental injection days.
Fig. 3. NaBenz/D-serine together suppressed aversion-resistant alcohol intake.

(A) The combination of ineffective concentrations of D-serine and NaBenz (dark purple, n=27) significantly reduced compulsion-like drinking. (B) The NaBenz/D-serine combination did not reduce alcohol-only intake (light purple, n=12). Dser: D-serine; NaB: NaBenz. *** p<0.001 for NaBenz/D-serine versus other conditions.
One large cohort of rats was used first to screen D-serine alone (n=27). We tested NaBenz alone with n=18 because, at this time, some rats were older. A younger subset of these rats (n=15) were then used to examine whether quinine-resistant alcohol drinking was inhibited by 100 mg/kg D-serine alone, NaB alone, or NaBenz/D-serine in combination. In a separate cohort of rats (n=12), we tested (in a randomized manner across rats and test sessions) NaBenz/D-serine versus vehicle during alcohol-only and quinine-alcohol intake, and also including sessions examining the impact of NaBenz (60 mg/kg) alone or D-serine (100 mg/kg) alone during quinine-alcohol drinking.
For Figure 2, we first screened NaBenz under regular alcohol drinking, since we had previously (Seif et al., 2015) screened D-Serine during alcohol-only intake before testing the impact on quinine-alcohol drinking. Importantly, the goal was to identify approximate sub-threshold doses with minimal “non-specific” effects; specifically, we looked for doses that did not affect regular, alcohol-only intake, which could reflect more general effects on motivation or locomotion. We then used these lower (putatively sub-threshold) doses in quinine-alcohol drinking studies.
Fig. 2. Identifying a subthreshold dose of NaBenz.

30 or 60 mg/kg NaBenz did not reduce alcohol-only intake (orange for NaBenz treatment, except red for 60 mg/kg NaBenz, n=18), with 60 mg/kg being the highest dose not significantly different from the vehicle control (p=0.8641). Only the two highest doses of NaBenz (125, 250 mg/kg) reduced alcohol-only consumption. ** p<0.01 for two highest NaBenz doses versus all other doses.
2.2. Reagents
D-Serine and NaBenz were from Sigma-Aldrich and diluted (w/v) in molecular-biology grade water (Sigma). When drugs were administered in isolation, injections used a concentration of 100 mg/ml, and lower doses received smaller injection volume than higher doses; this addressed solubility concerns at higher concentrations and the desire to inject the lowest possible volume. Alternatively, when drugs were co-administered as a cocktail, injection volume was 1 ml/kg, since drug dosages were different between compounds making it necessary to adjust the concentration of the lower compound to achieve the correct dose in a single injection. Drug or vehicle conditions were injected (i.p.) 30 minutes prior to bottle presentation.
2.3. Statistics and Data Analysis
Alcohol consumption was determined through changes in bottle weight before and after a drinking session, and converted to grams ethanol/kilogram body weight. All statistical comparisons were performed within-subject; each rat was tested for all conditions in a given experiment. Data were analyzed primarily by one- or two-way ANOVA with repeated-measures followed by multiple comparison post-hoc with Bonferroni correction; some comparisons used paired t-test or Pearson’s correlation. Statistical analysis was performed using Graph Pad Prism or SPSS. All data are shown as mean±SEM.
3. Results
3.1. Lower doses of D-serine and NaBenz together suppress compulsion-like alcohol drinking
Despite D-serine’s potential as a safe and effective NMDAR modulator in humans, concerns for renal toxicity at higher doses necessitates the development of a treatment strategy that reduces D-serine dosage without decreasing efficacy. In order to determine whether subthreshold D-serine and NaBenz levels in combination could reduce compulsion-like alcohol drinking, we first looked for a dose of D-serine that was ineffective at reducing aversion-resistant intake. 300 mg/kg D-serine significantly reduced compulsion-like intake of alcohol (Fig. 1A; n=26; t25=3.558, p=0.0015), but did not reduce alcohol-only intake (Fig. 1B; n=11; t10=0.755, p=0.468), similar to our previous work (Seif et al., 2015). However, 100 mg/kg D-serine was not effective in reducing quinine-alcohol drinking (Fig. 1A; n=38; t37=0.9855, p=0.3308 paired t-test vs vehicle). Thus, 300 but not 100 mg/kg D-serine significantly suppressed compulsion-like alcohol drinking.
Fig. 1. Identifying a subthreshold dose of D-serine.

(A) 300 mg/kg D-serine (dark blue, n=26) but not 100 mg/kg D-serine (light blue, n=38) reduced compulsion-like, quinine-resistant alcohol drinking. In addition to statistics shown in Results, a one-way ANOVA in n=26 rats where both D-serine doses were tested in the same animal found a significant effect across doses (F2,50=12.19, p<0.0001; p<0.05 posthoc for 300 mg/kg versus vehicle or 100 mg/kg). (B) 300 mg/kg D-serine did not reduce alcohol-only intake (n=11). N.s.: not significant. ** p<0.01.
Next, in order to determine whether NaBenz itself had similar effects on alcohol intake, and to help identify a subthreshold NaBenz dose, we sought to examine the impact of NaBenz on alcohol-only drinking (see Methods). 60 mg/kg NaBenz was the highest dose tested (across 30-250 mg/kg) that did not significantly reduce alcohol-only intake (Fig. 2; n=18; F4,68=11.88, p<0.0001 across NaBenz doses; post-hoc p=0.864 for vehicle vs 60 mg/kg NaBenz). These studies are congruent with others (Hopf, 2017; Seif et al., 2013; Seif et al., 2015) indicating that non-canonical NMDARs are critical for driving compulsion-like but not regular alcohol drinking, and also identify a possible subthreshold dose of NaBenz.
We next examined whether NaBenz (60 mg/kg) reduced compulsion-like alcohol drinking when given alone, or in combination with a D-serine dose (100 mg/kg) that did not reduce compulsion-like drinking (Fig. 3A). These were performed in a subset of animals tested in Figure 1 (n=27, see Methods). Two-way, repeated-measures ANOVA found a significant interaction between D-serine and NaBenz (F1,26=7.899, p=0.009; also significant overall effects of D-serine, F1,26=9.085, p=0.006, and NaBenz, F1,26=38.276, p<0.001); NaBenz/D-serine was significantly different from other conditions (p<0.001), with no differences between NaBenz alone or D-serine alone when compared to vehicle (p>0.19). In contrast, the NaBenz/D-serine combination had no impact on alcohol-only intake (Fig. 3B; t11=0.806, p=0.437), indicating a specific effect on compulsion-like drive for alcohol. Thus, the NaBenz/D-serine combination significantly reduced aversion-resistant alcohol consumption, supporting the possibility that lower doses of D-serine and NaBenz, when used in combination, might represent an available, safer therapeutic intervention to target compulsion-like drives for alcohol in humans.
3.2. NaBenz/D-serine reduces compulsion-like alcohol intake across most individuals
Because there are individual differences in mechanisms that drive alcohol drinking, which could strongly impact a therapy’s effectiveness in a particular individual (see Discussion), we also examined whether the impact of NaBenz/D-serine on compulsion-like drinking was related to individual differences in basal (moderate vs higher) alcohol intake. First, basal alcohol-only intake (averaged for 4 days before testing) was significantly correlated with aversion-resistant drinking (vehicle injection days) across individuals (Suppl. Fig. 1; p=0.0024, R2=0.235, slope=0.374, n=37), consistent with the idea that compulsion-like intake is a state where a preferred level of drinking occurs regardless of any negative consequences (Darevsky et al., 2018; Hopf and Lesscher, 2014; Goltseker et al., 2019).
Basal quinine-alcohol drinking levels were significantly related to NaBenz/D-serine changes in intake (Fig. 4A; p=0.0313, R2=0.172, slope=−41.77), suggesting a differential effect in moderate and higher drinkers. However, paired t-tests in moderate- and higher-drinking individuals (divided by median split) showed that NaBenz/D-serine significantly decreased compulsion-like drinking in both moderate (Fig. 4B, t13=4.13, p=0.0012) and higher drinkers (Fig. 4C, t12=6.212, p<0.0001). Thus, while NaBenz and D-serine in combination had more pronounced effects in higher drinkers, our results suggest that NaBenz/D-serine reduced aversion-resistant intake across the majority of individuals.
Fig. 4. NaBenz in combination with D-serine reduced compulsion-like consumption across nearly all individuals.
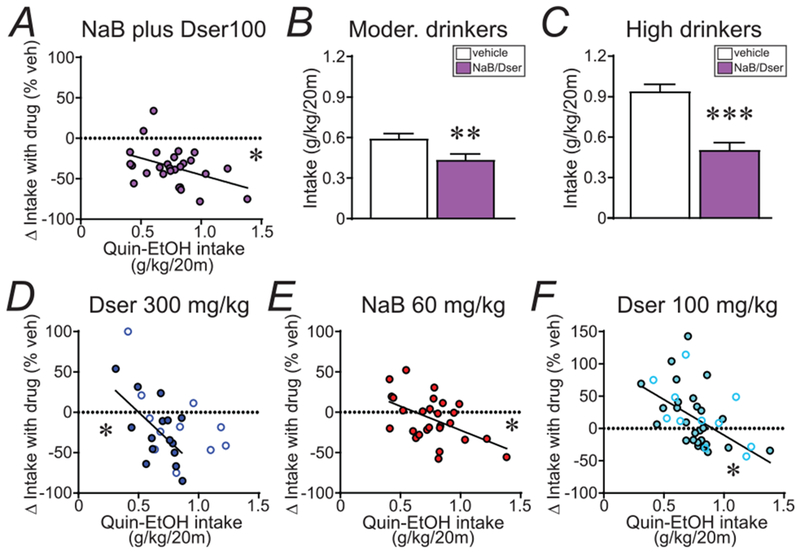
(A) Decreased quinine-alcohol drinking with NaBenz/D-serine was observed across individuals. While basal intake was correlated with the drop in drinking by NaBenz/D-serine, (B,C) paired t-tests found significant decreases in drinking with NaBenz/D-serine in both (B) moderate and (C) higher basal drinkers, divided by a median split (n=14 in moderate, 13 in higher). (D-F) Significant negative correlations (across animals) between basal aversion-resistant drinking level and the impact of treatment on intake, observed with (D) 300 mg/kg D-serine, (E) 60 mg/kg NaBenz, and (F) 100 mg/kg D-serine. In (D,F), filled circles and solid lines indicates results from rats used in Fig.3, while open circles indicate additional animals. Dser: D-serine; NaB: NaBenz. * p<0.05, ** p<0.01, *** p<0.001.
Other conditions also showed a significant relationship of basal drinking levels with intake change with drug. 300 mg/kg D-serine, which significantly reduced compulsion-like drinking (Fig. 1A), was less effective in individuals with more moderate basal quinine-alcohol consumption; this was observed when examining the 15 rats used for Fig.3A (Fig. 4D, filled circles; p=0.0207, R2=0.348, slope=−139.8) and in a larger data set (n=26) including Fig.3A rats and others (Fig. 4D, all circles; p=0.0138, R2=0.227, slope=−88.07). Also, a median split of the 300 mg/kg D-serine data found no significant change in moderate drinkers (vehicle: 0.56±0.03 g/kg; D-serine: 0.52±0.05 g/kg; t12=0.592, p=0.565) but a significant effect in higher basal drinkers (vehicle: 0.91±0.05 g/kg; D-serine: 0.58±0.08 g/kg; t12=4.768, p=0.0005). A significant correlation with basal intake level was also seen with 60 mg/kg NaBenz alone (Fig. 4E; p=0.008, R2=0.250, slope=−59.37) and 100 mg/kg D-serine alone (Fig. 4F; filled circles: n=27 rats from Fig.3A; p=0.0117, R2=0.229, slope=−110.2; all circles: n=38 with Fig.3A rats and others; p=0.0014, R2=0.250, slope=−103.5). Thus, as addressed in the Discussion, differences in D-serine regulation may differentially impact drinking in moderate and higher alcohol drinkers. Since D-serine and NaBenz was efficacious in reducing alcohol drinking amongst animals with both moderate and high basal levels of intake (Fig. 4A–C), the combination of the two drugs is uniquely positioned to treat a spectrum (from moderate to excessive) of compulsion-like drinking.
4. Discussion
Compulsive, consequence-resistant drives are substantial contributors to problem alcohol drinking in humans. Since non-canonical NMDARs can play an important role in driving conflict-related intake (see Introduction and below), our primary goal was to understand whether combining subthreshold doses of D-serine and NaBenz could suppress compulsion-like drives for alcohol. Here, we found that higher D-serine levels specifically reduced compulsion-like drinking, with no impact on alcohol-only intake. Importantly, lower D-serine levels that were minimally effective at reducing aversion-resistant intake did significantly reduce drinking when combined with an ineffective dose of NaBenz. In addition, NaBenz/D-serine reduced compulsion-like intake across animals with higher or moderate basal drinking levels, underscoring the critical importance of non-canonical NMDARs for consequence-resistant drinking. In contrast, while higher D-serine levels overall reduced aversion-resistant intake, there was little net effect in moderate-drinking individuals. Thus, this NaBenz/D-serine strategy allowed lower D-serine levels to suppress compulsion-like intake, which could alleviate some D-serine concerns and also increase confidence in the ability to reduce aversion resistance across individuals. Together, our findings support use of this FDA-approved and immediately accessible NaBenz/D-serine-based strategy to facilitate inhibition of compulsion for alcohol across the majority of individuals. This would not only reduce D-serine exposure and possible side effects, but also could have broad applicability across a range of D-serine-targeted neuropsychiatric conditions.
Compulsion-like drives are a major clinical obstacle, and there is considerable interest in understanding their circuitry in order to improve treatment. We found that cortical inputs to the nucleus accumbens, and non-canonical NMDAR activation within the nucleus accumbens, are essential for driving aversion-resistant alcohol intake (Seif et al., 2013; Seif et al., 2015). These results agree with several clinical groups (Naqvi et al., 2014; Tiffany and Conklin, 2000) (see Hopf, 2017) who propose that it is the conflict during compulsion (between desire for alcohol and desire to avoid bad consequences) that recruits cortical conflict-processing circuits; these circuits then help maintain responding in the face of “unavoidable” adversity. In contrast, drinking in the absence of overt conflict would have much less requirement for these cortical circuits. Thus, it is likely that accumbens non-canonical NMDARs are critical for aversion-resistant intake because they are activated by cortical inputs when a negative challenge is present during drinking. Interestingly, this possibility is supported by studies from human alcohol drinkers. The NMDAR inhibitor memantine reduces alcohol consumption in treatment-seeking human drinkers with comorbid depression or bipolar disorder (Lee et al., 2018; Muhonen et al., 2008; but see Evans et al., 2007). Memantine also reduces craving (Bisaga and Evans, 2004; Krishnan-Sarin et al., 2015; Krupitsky et al., 2007), which may reflect desire for alcohol when it is not available. In contrast, memantine does not reduce intake in non-treatment-seekers (Krishnan-Sarin et al., 2015), who are ostensibly not in conflict about their intake. Thus, human and rodent work concurs that aversion-resistant alcohol behaviors, but not regular alcohol intake, are driven by NMDARs. Furthermore, we identified GluN2C non-canonical NMDAR subunits as important for consequence-resistant consumption in rats (Seif et al., 2013), while a GluN2C genetic variant associates with higher problem drinking in humans (Bach et al., 2015), and memantine may preferentially inhibit GluN2C/2D-containing NMDARs (Kotermanski and Johnson, 2009). Along with D-serine inhibition of non-canonical NMDARs (Chatterton et al., 2002; Seif et al., 2015; Takarada et al., 2009; Takarada et al., 2012) and compulsion-like alcohol drinking in rats (Seif et al., 2015), these findings together support the possibility that non-canonical NMDARs mediate compulsion-like drives for alcohol in both rodent and human.
There is considerable interest in D-serine since it, like D-cycloserine, represent a therapy to moderately increase NMDAR activity in humans, and it has shown efficacy in humans for schizophrenia (e.g. Kantrowitz et al., 2010), mood disorders (Avellar et al., 2016; Levin et al., 2015), Parkinson’s disease (Gelfin et al., 2012), and cognitive enhancement in healthy controls (e.g. Guercio and Panizzutti, 2018; Levin et al., 2015). Nonetheless, despite being widely used with good tolerability and few adverse effects, concerns about nephrotoxicity remain (Krug et al., 2007), and lower exposure to exogenous D-serine would be preferable. NaBenz represents one such strategy, since NaBenz acts by inhibiting DAAO (Hashimoto, 2011; Howley et al.,2017) which should decrease D-serine breakdown. NaBenz is well tolerated (Lane et al., 2013; Lin et al., 2017), although it might be contraindicated for some conditions (Hashimoto, 2011). Also, some previous studies have used NaBenz to increase D-serine efficacy, but the inactive concentrations were much higher (400 mg/kg NaBenz, 600 mg/kg D-serine; Sershen et al., 2016). In contrast, our results strongly suggest that lower NaBenz/D-serine doses in combination could represent an effective and practically useful strategy to counteract compulsion-like drives for alcohol across humans. We note that we have not examined D-serine levels directly, since several factors make this complex. For example, DAAO knockout mice have the same peak plasma D-serine as wildtype after exogenous D-serine administration, but with a prolonged plateau of D-serine (Rais et al., 2012). Also, strong pharmacological inhibition of DAAO alters plasma D-serine with little effect on brain D-serine (Sershen et al., 2016). Thus, brain D-serine levels are likely under strong homeostatic regulation. In addition, D-serine concentrations within a synapse can be maintained at near-saturating levels, while extra-synaptic D-serine levels are lower (Mothet et al., 2000). Non-canonical NMDARs are likely to be localized extrasynaptically, since GluN2A- and GluN2B-containing NMDARs are anchored in the synapse while excess GluN2B and other subunits are extra-synaptic (Hopf, 2017; Traynelis et al., 2010). Therefore, while it may be very challenging to directly examine D-serine levels near non-canonical NMDARs, our results nonetheless strongly indicate that NaBenz exposure allows lower, normally ineffective levels of D-serine to become effective for suppressing compulsion-like alcohol intake.
Importantly, NaBenz/D-serine inhibition of compulsion-like alcohol consumption was observed in individuals with higher and moderate basal drinking level. This reinforces the hypothesis that compulsion-like intake is a cognitive state, involving a choice to act despite negative consequences, which all alcohol drinkers can exhibit. For example, some people report drinking problems and preoccupation even though their actual intake level is more moderate (Esser et al., 2014). However, our results contrast with some studies finding individual differences in level of compulsivity. As addressed at length in Hopf and Lesscher (2014), evidence overall suggests that compulsion after long-term intake can be exhibited by the majority of individuals, but with individual differences in aversion tolerance. In fact, as we have shown for alcohol (Hopf et al., 2010; Seif et al., 2013) and others for cocaine (see Hopf and Lesscher, 2014; Goltseker et al., 2019), nearly all animals will give up responding if the cost is high enough. Some differences may also reflect strains used, e.g. where the majority of Wistar and some alcohol-preferring rat strains develop compulsion with intermittent access (Hopf et al., 2010; Loi et al., 2010; Seif et al., 2013; Seif et al., 2015; Vengeliene et al., 2015), while Lister hooded and other alcohol-preferring strains show much greater individual diversity (Giuliano et al., 2018; Spoelder et al., 2017). However, with longer-term alcohol drinking, even the majority of Lister hooded rats exhibit substantial aversion-resistant intake (Spoelder et al., 2017). We also found that alcohol-only drinking levels in a given rat were correlated with compulsion-like intake levels, again indicating that compulsion-like drives can develop in the majority of individuals. Taken together, it is clear that individuals can vary in their proclivity for compulsion-like intake, e.g. depending of genetic background across rodents. However, with long enough intake, many individuals express the capacity for aversion-resistant motivation, opening the possible utility of NaBenz/D-serine therapy to reduce compulsion-like drives for alcohol.
We also note that, while NaBenz/D-serine reduced compulsion-like intake across animals, 300 mg/kg D-serine had a smaller impact on individuals with moderate basal drinking. One possibility is that higher intake individuals have lower DAAO or other regulatory mechanisms for D-serine, which is less effective at metabolizing D-serine when given without a DAAO blocker. However, a similar negative slope relationship between basal intake was also observed with 100 mg/kg D-serine or NaBenz alone. Considerable future work will be required to understand possible basal variation in D-serine regulation in moderate versus higher drinkers, in part because other explanations are possible including differences in NMDAR adaptations that drive compulsion-like intake. Another possibility is that D-serine alone can have a moderate pro-intake influence, perhaps through canonical NMDAR activation, in addition to inhibiting non-canonical NMDARs. In this regard, results in Fig.4D–F suggest that moderate drinkers may have exhibited more of such a pro-intake effect of D-serine or NaBenz alone. Also, although we did not see an average effect of 60 mg/kg NaBenz alone on quinine-alcohol drinking, our correlational analyses in Fig.4 perhaps suggested that NaBenz alone was effective in reducing compulsion-like alcohol drinking in higher-drinking individuals. Thus, in addition to supporting the use of NaBenz and D-Serine in combination to suppress compulsion-like drives for alcohol, our findings also indicate that NaBenz alone might be effective against compulsion-like intake in higher-drinking individuals (and sidestep any possible nephrotoxic concerns related to D-serine). Nonetheless, our results clearly demonstrate that ineffective doses of NaBenz and D-serine, when used in combination, reduced aversion-resistant drinking across the majority of individuals.
5. Conclusion
In summary, we found that combining NaBenz with D-serine allowed a lower D-serine dose to significantly suppress compulsion-like intake, with efficacy in both moderate and higher basal drinkers. Importantly, these pharmacological interventions did not alter regular, alcohol-only intake, indicating a specific use in targeting compulsion-like drives for alcohol, and perhaps that NaBenz/D-serine should have lower side effects on other motivated behaviors (as seen with D-serine; Seif et al., 2015). In addition, D-serine exposure would still likely have canonical effects of increasing NMDAR function, with the potential to increase memory; this could be favorable if preventing compulsion-like drives occurs concurrently with an increased learning about exercising better self-control. Together, our studies provide a new, FDA-approved and immediately available pharmacotherapy to improve AUD treatment by suppressing compulsion like, consequence-resistant drives for alcohol, with translational potential for other human D-serine uses.
Supplementary Material
Highlights.
Intake despite adverse consequences (compulsivity) is a substantial obstacle to treating Alcohol Use Disorder (AUD). Also, D-serine-inhibited, non-canonical N-methyl-d-aspartate receptors drive compulsion-like alcohol drinking in rats, with congruent studies in humans.
Here, co-administration of subthreshold levels of sodium benzoate, which reduces D-serine breakdown, allowed subthreshold D-serine to suppress compulsion-like alcohol drinking, and in nearly all individuals.
This combination therapy provides an FDA-approved and immediately-accessible pharmacotherapy to help counteract compulsion-like drives and treat AUD, with implications for other human D-serine-based strategies.
Acknowledgements
Supported by AA021445 (FWH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
None.
References
- Avellar M, Scoriels L, Madeira C, Vargas-Lopes C, Marques P, Dantas C, Manhaes AC, Leite H, Panizzutti R, 2016. The effect of D-serine administration on cognition and mood in older adults. Oncotarget 7, 11881–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Kirsch M, Hoffmann S, Jorde A, Mann K, Frank J, Charlet K, Beck A, Heinz A, Walter H, Rietschel M, Kiefer F, Vollstadt-Klein S, 2015. The effects of single nucleotide polymorphisms in glutamatergic neurotransmission genes on neural response to alcohol cues and craving. Addict. Biol 20, 1022–1032. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM, 2004. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology 172, 16–24. [DOI] [PubMed] [Google Scholar]
- CDC, 2014. Excessive Drinking Costs U.S. $223.5 Billion. Center for Disease Control, Atlanta, GA. [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D, 2002. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415, 793–798. [DOI] [PubMed] [Google Scholar]
- Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW, 2018. Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict Biol. Epub in press. [DOI] [PubMed] [Google Scholar]
- Eisenhardt M, Leixner S, Lujan R, Spanagel R, Bilbao A, 2015. Glutamate Receptors within the Mesolimbic Dopamine System Mediate Alcohol Relapse Behavior. J. Neurosci 35, 15523–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Kowalczyk WJ, 2018. Compulsive Seekers: Our take. Two Clinicians’ Perspective on a New Animal Model of Addiction. Neuropsychopharm. 43, 677–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS, 2014. Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Preventing Chronic Disease, CDC 11, 140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F, 2007. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin. Exp. Res 31, 775–782. [DOI] [PubMed] [Google Scholar]
- Gelfin E, Kaufman Y, Korn-Lubetzki I, Bloch B, Kremer I, Javitt DC, Heresco-Levy U, 2012. D-serine adjuvant treatment alleviates behavioural and motor symptoms in Parkinson’s disease. Int. J. Neuropsychopharm 15, 543–549. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Pena-Oliver Y, Goodlett CR, Cardinal RN, Robbins TW, Bullmore ET, Belin D, Everitt BJ, 2018. Evidence for a Long-Lasting Compulsive Alcohol Seeking Phenotype in Rats. Neuropsychopharm. 43, 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltseker K, Hopf FW, Barak S, 2019. Advances in behavioral animal models of alcohol use disorder. Alcohol 74, 73–82. [DOI] [PubMed] [Google Scholar]
- Guercio GD, Panizzutti R, 2018. Potential and Challenges for the Clinical Use of d-Serine As a Cognitive Enhancer. Front. Psychiatry 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, 2011. Food Coloring, Sodium Benzoate Preservative, and D-serine: Implications for Behavior Handbook of Behavior, Food and Nutrition Springer, Preedy VR eds., 577–612. [Google Scholar]
- Heresco-Levy U, Shoham S, Javitt DC, 2013. Glycine site agonists of the N-methyl-D-aspartate receptor and Parkinson’s disease: a hypothesis. Mov. Disord 28, 419–424. [DOI] [PubMed] [Google Scholar]
- Hopf FW, 2017. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 16, 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A, 2010. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self administration. Alcohol Clin. Exp. Res 34, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM, 2014. Rodent models for compulsive alcohol intake. Alcohol 48, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley E, Bestwick M, Fradley R, Harrison H, Leveridge M, Okada K, Fieldhouse C, Farnaby W, Canning H, Sykes AP, Merchant K, Hazel K, Kerr C, Kinsella N, Walsh L, Livermore DG, Hoffman I, Ellery J, Mitchell P, Patel T, Carlton M, Barnes M, Miller DJ, 2017. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756. Neurochem. Res 42, 3279–3288. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D’Souza C, Saksa J, Woods SW, Javitt DC, 2010. High dose D-serine in the treatment of schizophrenia. Schizophr. Res 121, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharm. 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW, 2009. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci 29, 2774–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, O’Malley SS, Franco N, Cavallo DA, Morean M, Shi J, Pittman B, Krystal JH, 2015. N-methyl-D-aspartate receptor antagonism has differential effects on alcohol craving and drinking in heavy drinkers. Alcohol Clin. Exp. Res 39, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug AW, Volker K, Dantzler WH, Silbernagl S, 2007. Why is D-serine nephrotoxic and alpha-aminoisobutyric acid protective? Am. J. Physiol. Renal Physiol 293, F382–390. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH, 2007. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am. J. Psychiatry 164, 519–523. [DOI] [PubMed] [Google Scholar]
- Lane HY, Lin CH, Green MF, Hellemann G, Huang CC, Chen PW, Tun R, Chang YC, Tsai GE, 2013. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry 70, 1267–1275. [DOI] [PubMed] [Google Scholar]
- Lee SY, Wang TY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang LJ, Lee IH, Chen KC, Yang YK, Hong JS, Lu RB, 2018. Add-on Memantine Treatment for Bipolar II Disorder Comorbid with Alcohol Dependence: A 12-Week Follow-Up Study. Alcohol Clin. Exp. Res 42, 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Hopf FW, 2016a. Orexin-1 receptor blockade suppresses compulsive-like alcohol drinking in mice. Neuropharmacology 110, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW, 2016b. Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front. Neurosci 10, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R, Dor-Abarbanel AE, Edelman S, Durrant AR, Hashimoto K, Javitt DC, Heresco-Levy U, 2015. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: initial findings. J. Psychiatr. Res 61, 188–195. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen PK, Chang YC, Chuo LJ, Chen YS, Tsai GE, Lane HY, 2014. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biol. Psychiatry 75, 678–685. [DOI] [PubMed] [Google Scholar]
- Lin CY, Liang SY, Chang YC, Ting SY, Kao CL, Wu YH, Tsai GE, Lane HY, 2017. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: A randomised, double-blind, placebo-controlled trial. World J. Biol. Psychiatry 18, 357–368. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G, 2010. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin. Exp. Res 34, 2147–2154. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO Jr., Linden DJ, Ferris CD, Rogawski MA, Snyder SH, 2000. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci 97, 4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Sinclair D, Lonnqvist J, Alho H, 2008. Treatment of alcohol dependence in patients with co-morbid major depressive disorder--predictors for the outcomes with memantine and escitalopram medication. Subst. Abuse Treat. Prev. Policy 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A, 2014. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann. NY Acad. Sci 1316, 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais R, Thomas AG, Wozniak K, Wu Y, Jaaro-Peled H, Sawa A, Strick CA, Engle SJ, Brandon NJ, Rojas C, Slusher BS, Tsukamoto T, 2012. Pharmacokinetics of oral D-serine in D-amino acid oxidase knockout mice. Drug Metab. Dispos 40, 2067–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW, 2015. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharm. 40, 2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Dunlop DS, Suckow RF, Cooper TB, Javitt DC, 2016. Modulating NMDA Receptor Function with D-Amino Acid Oxidase Inhibitors: Understanding Functional Activity in PCP-Treated Mouse Model. Neurochem. Res 41, 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, 2009. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev 89, 649–705. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Pol S, Janssen BSG, Baars AM, Vanderschuren L, Lesscher HMB, 2017. Loss of control over alcohol seeking in rats depends on individual vulnerability and duration of alcohol consumption experience. Behav. Pharmacol 28, 334–344. [DOI] [PubMed] [Google Scholar]
- Takarada T, Takahata Y, Iemata M, Hinoi E, Uno K, Hirai T, Yamamoto T, Yoneda Y, 2009. Interference with cellular differentiation by D-serine through antagonism at N-methyl-D-aspartate receptors composed of NR1 and NR3A subunits in chondrocytes. J. Cell. Physiol 220, 756–764. [DOI] [PubMed] [Google Scholar]
- Takarada T, Takarada-Iemata M, Takahata Y, Yamada D, Yamamoto T, Nakamura Y, Hinoi E, Yoneda Y, 2012. Osteoclastogenesis is negatively regulated by D-serine produced by osteoblasts. J. Cell. Physiol 227, 3477–3487. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA, 2000. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction 95 Suppl 2, S145–153. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Lin PY, 2010. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des 16, 522–537. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Olevska A, Spanagel R, 2015. Long-lasting effect of NMD A receptor antagonist memantine on ethanol-cue association and relapse. J. Neurochem 135, 1080–1085. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE, 2009. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Front. Neural Circuits 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


