Abstract
We discovered that 90.3% of patients with angiomyolipomas, LAM, and tuberous sclerosis complex (TSC) carry the arginine variant of codon 72 (R72) of TP53 and that R72 increases the risk for angiomyolipoma. R72 transactivates NOTCH1 and NODAL better than the proline variant of codon 72 (P72), therefore, the expression of NOTCH1 and NODAL is increased in angiomyolipoma cells that carry R72. The loss of Tp53 and Tsc1 within nestin expressing cells in mice resulted in development of RCC with high Notch1 and Nodal expression suggesting that similar downstream mechanisms contribute to tumorigenesis as a result of p53 loss in mice and p53 polymorphism in humans. The loss of murine Tp53 or expression of human R72 contribute to tumorigenesis via enhancing epithelial-to-mesenchymal transition and motility of tumor cells through the Notch and Nodal pathways.
Implications:
This work revealed unexpected contributions of the p53 polymorphism to the pathogenesis of TSC and established signaling alterations caused by this polymorphism as a target for therapy. We found that the codon 72 TP53 polymorphism contributes to TSC-associated tumorigenesis via Notch and Nodal signaling.
Introduction
Tuberous sclerosis complex (TSC) manifests as tumors in the central nervous system, renal angiomyolipomas, renal cell carcinomas (RCC), and pulmonary lymphangioleiomyomatosis (LAM), and is caused by germline mutations in the TSC1 or TSC2 tumor suppressor genes (1, 2). These mutations cause Rheb and mTORC1 hyperactivation, which contributes to TSC-tumorigenesis (2). Therefore, rapamycin and its analogs (rapalogs), which inhibit mTORC1, were approved for treatment of TSC and LAM (3). However, this treatment causes only partial tumor regression with disease progression upon treatment discontinuation (2), suggesting contributions of additional pathways to TSC-tumorigenesis.
The tumor suppressive role of p53 in TSC has emerged from the requirement of p53 inactivation for tumor development in tsc2+/− zebrafish (4) and increased levels of p53 in TSC-deficient cells and angiomyolipoma tumors (5, 6). However, no mutations were found in TP53 or other genes in TSC patients (1, 7), yet the alternative causes of alterations in p53 function were not considered. We hypothesized that dysfunction in TP53 impacts TSC tumorigenesis. The most common single-nucleotide germline polymorphism (SNP) in TP53 at codon 72, which affects the p53 proline-rich domain regulating apoptosis (8), alters p53 protein structure and the function (8-10). Therefore, we explore if this polymorphism alters TP53 tumor suppressive role in TSC, LAM and angiomyolipoma patients. There are two forms of codon 72: the proline variant (P72) and arginine variant (R72). This polymorphism was linked to increased susceptibility to cancer. In contrast, the genome-wide association studies (GWAs) revealed weak or lack of association with cancer risk (11, 12). However, none of these studies included patients with TSC and LAM. Importantly, GWAs identified the significant associations between R72 and obesity, insulin resistance, diabetes, metabolic dysfunction, and inflammation (13). The compelling data indicate increased survival of cells carrying R72 in response to nutrient deprivation (13, 14). Because dysregulation of metabolism and nutrient signaling are established players in TSC pathogenesis (2), these data support link of R72 to TSC.
Materials and Methods
Human and animal studies
Human samples from the National Disease Research Interchange and the Center for LAM Research at Brigham and Women’s Hospital were collected with IRBs’ approvals.
The animal studies approved by IACUC according to the NIH guidelines used B6.Cg-Tg(Nes-cre)1Kln/J, C57BL/6-Tg(Nes-cre/Esr1)1Kuan/J, Tsc1tm1Djk/J, B6.129P2-Trp53<tm1Brn>/J (The Jackson Laboratory), Hupki P72, and Hupki R72 mice (from Dr. Maureen Murphy). Tamoxifen in corn oil (40 mg/ml) was administered i.p. (120mg/kg/day) for two days.
CRISPR Cas9
The sgRNAs targeting genomic regions of interest were designed and subcloned into pSpCas9(BB)-2A-GFP (PX458) (15) Addgene vector as in Supplementary Material and Methods. 3μg pSpCas9(BB)-2A-GFP (PX458) and 3 μl of 10 μM of homologous repair DNA template were co-transfected into CRL 4004 R72 cells and then sorted as described in Supplementary Material and Method section.
Antibodies, immunohistochemistry (IHC) and immunofluorescence
Notch-C-20, Hes5 (Santa Cruz); hamartin, p53 (1C12), phospho-S6, phospho-S6K (T389), Hes1 and Gapdh (Cell Signaling); Nodal (Abnova). Secondary steps for IHC used a detection kit (Invitrogen). Secondary antibodies conjugated with fluorochromes (AF488) and DAPI were used for immunofluorescence. Images were captured with Nikon TE2000 microscope and analyzed using Nikon Elements Advanced Research software. In addition, Aperio digital pathology system (currently Leica Biosystems, IL, USA) algorithm-based software was used to locate and quantitate staining.
Western blotting
Cells were lysed using RIPA buffer, clarified and then subjected to gel electrophoresis.
Chromatin immunoprecipitation (ChIP)-quantitative PCR (qPCR) analyses
ChIP-qPCR was performed using EZ-ChIP (Millipore) or Simple ChIP Enzymatic Chromatin IP kit (Cell Signaling) and X-chip protocol (Abcam) per manufacturer’s instructions and as described in Supplementary Material and Methods.
q(RT)-PCR
q(RT)-PCR used High Capacity cDNA Synthesis Kit, Fast SybrGreen, StepOnePlus Applied Biosystems, as described in Supplementary Material and Methods. Relative expression was calculated using the 2-ΔΔCt method and RT2 profiler PCR Array Data Analysis (SAB Biosciences) and normalized to GAPDH.
Wound healing assays
The 0.7×106 cells were seeded in 6 well plate and scratched with a pipette tip. The images were captured every 2 hours for 10 hours and cell covered area was quantified using the Nikon microscope software. The wound healing efficiency (WHE) was calculated for each time point from 6 selected fields using the formula WHE= original wound area – wound area after migration/original wound area.
Boyden Chamber Cell Migration Assay
The modified Boyden chamber trans-well system (Corning Incorporated, NY, USA) was used as described in the Supplementary Material and Methods.
Sphere-forming and ALDH activity
The cells were seeded on the ultra-low attachment plates as described (16). The ALDH activity was measured using ALDEFLUOR™ Kit (StemCell Technologies, Canada).
Statistical analysis
Kolmogorov-Smirnov, un-paired t-tests, one-way or two-way ANNOVA, non-parametric Mann-Whitney tests, or the regression analysis of longitudinal data (repeated measures) were used. Standard error of means (S.E.M) was estimated. The variance was similar between groups that were compared. For normalized q(RT)-PCR, fold changes and densitometry analyses one-sample t-tests were used. Outliers were identified using Grubb’s test at α=0.05. Analyses were done using GraphPad Prism 6 and Statistica.
Supplementary information
Supplementary information includes five figures, three tables and supplementary material, methods and references.
Data and Materials Availability
The authors declare that data supporting the findings of this study are available within the paper and its supplementary information files. All additional relevant data and materials are available from the authors.
Results
R72 correlates with a higher incidence of angiomyolipomas.
The analysis of non-diseased tissue DNA revealed that 95.4% of TSC and 81.6% of LAM patients carry one or two R72 alleles (Supplementary Table S1). None of the sporadic angiomyolipoma patient carried P72 allele (Supplementary Table S1). The frequencies of R72 in TSC patients are similar to those in U.S. residents with inheritance from Northern and Western Europe but higher than in individuals with Hispanic, African–American and Pacific Rim heritage, (~60%, 50%, and 40%, respectively) (11). TSC affects people of all races and ethnicities equally (https://www.tsalliance.org/), therefore, R72 appears to be more frequent in TSC patients than in general population, although we do not have data on races of patients involved in this study. To determine whether there is an association between the occurrence of angiomyolipoma and R72, we performed the Fisher’s Exact (p=0.0469), Chi-Square (p=0.0461), and Likelihood Ratio (p=0.0437) tests on thirty-eight out of forty-three TSC patients, for whom we have detailed clinical information and were included in the Supplementary Table S1 (17). We did not use the P72 homozygous patients as a control cohort because of very low number of patients with this genotype. The 62% of subjects had a grade 1 or higher angiomyolipomas in the R72 category compared to 29% in the P72/R72 category. The odds ratio is not significantly different from 1, but it is marginally indicative of a difference in a risk of angiomyolipoma in R72 vs. P72/R72 individuals (Point Estimate: 0.256; 95% Wald Confidence Limits: 0.065–1.004). Twenty-two angiomyolipomas and four TSC-associated RCC and two angiomyolipoma-derived cell lines [621–101 (18) and CRL 4004 (19)] were either homozygous or heterozygous for R72 (Supplementary Table S2). The results from normal tissue from patients with TSC or sporadic angiomyolipomas (14 patients), included in the Supplementary Table 2, are also included in the Supplementary Table 1. No mutations in TP53 nor allelic loss in fourteen analyzed angiomyolipomas were found.
Loss of Tsc1 and p53 results in renal carcinogenesis in mice.
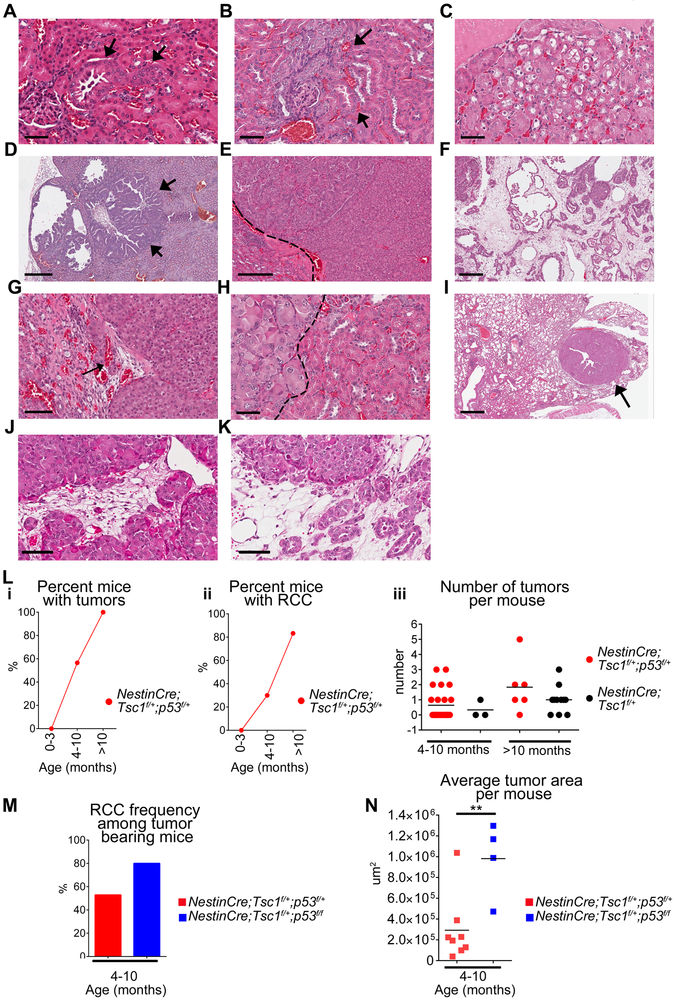
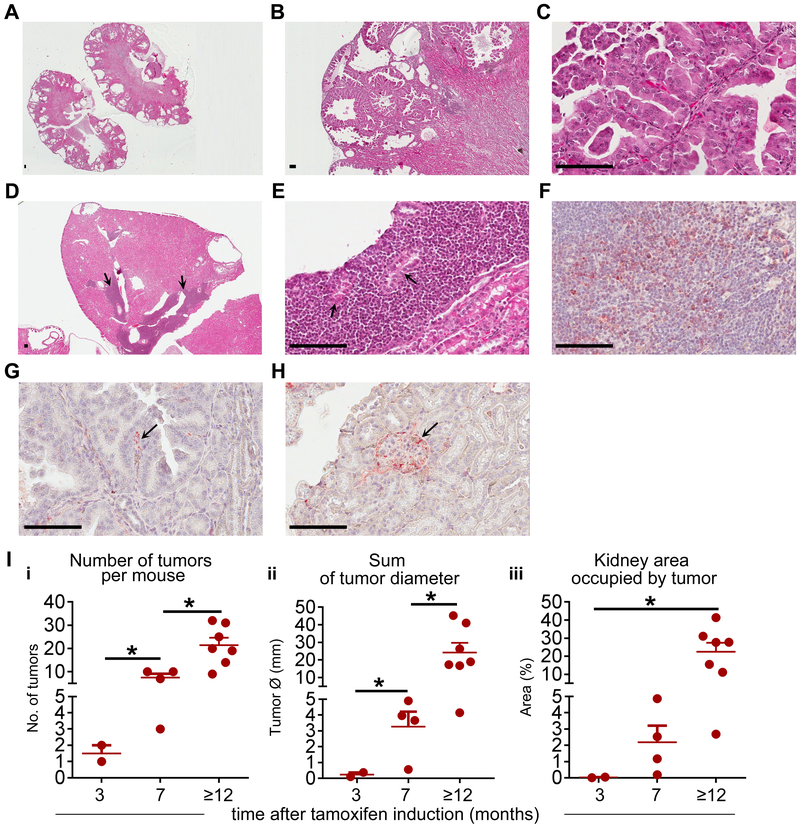
To determine a role of Tp53 in Tsc1-induced tumorigenesis, we generated a mouse with cell specific, partial and complete, loss of Tp53 with partial loss of Tsc1, which mimics inherited inactivating mutation(s) within one allele of TSC1/2 genes of TSC patients. We used the non-inducible nestin promoter, which facilitates Tsc1 and/or Tp53 recombination around E.8.5., because nestin is expressed in angiomyolipomas of TSC patients (18), urinary pole progenitors (20), podocytes of adult kidney (21), and has recently been identified as a surrogate marker of cancer stem cells. Importantly, nestin is unfavorable prognostic biomarker for sporadic RCC (Supplementary Figure S1A) but not for any other common human tumors (https://www.proteinatlas.org). The partial loss of only Tp53 (n=14) did not result in development of neoplastic renal lesions (Supplementary Fig. S1B, Supplementary Table S3). The partial loss of Tsc1 together with partial or complete loss of Tp53 in nestin expressing cells of NestinCre;Tsc1f/+;p53f/+ and NestinCre;Tsc1f/+;p53f/f mice led to development of cysts, low and high grade renal intraepithelial neoplasia ([RIN], Fig. 1 A-C, Supplementary Table S3), and the invasive papillary (Fig. 1D), chromophobe (Fig. 1E), and collecting duct RCC (Fig. 1F) similar to human sporadic or TSC-associated RCC (22). High grade RIN lesions, containing cells with clear cytoplasm, were found (Fig. 1C). The morphological features of malignancy: invasive border, invasion of blood vessels (Fig. 1G), and cytological atypia (Fig. 1H) were observed. In two out of four animals older than 1 year we found lung metastasis (Fig. 1I). In one tumor we found large polygonal cells with clear cytoplasm and hyperchromatic nuclei (Fig. 1J-K) resembling cells of epithelioid angiomyolipoma (23). The frequency of RIN and invasive carcinoma positively correlated with age, with first lesions noted in 4 month old mice (Fig. 1L-i-ii). NestinCre;Tsc1f/+ mice also developed RIN and RCC, however, these lesions were less frequent than in NestinCre;Tsc1f/+;p53f/+mice. There were less tumors per mouse (Fig. 1L-iii) and these lesions were smaller than tumors in NestinCre;Tsc1f/+;p53f/+ (Supplementary Figure S1C), suggesting that partial loss of p53 accelerates TSC-associated tumorigenesis in mice. In tumor bearing mice, the invasive RCC were found more often in NestinCre;Tsc1f/+;p53f/f than NestinCre;Tsc1f/+;p53f/+mice at age of 4–10 months (Fig. 1M) with larger tumors in NestinCre;Tsc1f/+;p53f/f mice (Fig. 1N). p53 and hamartin expression were reduced in these tumors (Supplementary Fig. S1D-G). Because complete loss of Tsc1 caused by the non-inducible nestin promoter, leads to embryonic lethality, we utilized inducible nestin promoter strain (CreEsr1) to determine impact of partial loss of p53 on tumorigenesis caused by complete loss of Tsc1 (18). Mice were induced at the age of 2–4 months. In all Nestin-TamCre;Tsc1f/f;p53f/+ mice older than 12 months we found the bilateral cysts that occupied most of kidneys (Fig. 2A) and invasive cystic RCC with papillary structures (Fig. 2B-C, Supplementary Table S3) similar to tumors in Nestin-TamCre;Tsc1f/f mice (18). We also found, in some tumors, large infiltrates of small undifferentiated cells (Fig. 2D, arrows; 2E, blastema-like areas by analogy to blastemal cells in nephroblastoma), with the tubular structures that may represent either entrapped renal tubules or abortive tubular structures (Fig. 2E, arrows), indicating the tendency for differentiation to adenocarcinomas. These undifferentiated tumor cells did not express markers for T lymphocytes or myeloid cells. They expressed nestin (Fig. 2F). In the well-differentiated areas only single small nestin positive cells were present (Fig. 2G). Nestin was expressed in the urinary pole progenitors and podocytes (Fig. 2H), as reported (21). The partial loss of only Tp53 in did not cause tumors. Therefore, we concluded that loss of both Tsc1 and Tp53 in nestin expressing adult but not embryonic cells contributes to the formation of blastema-like areas. The frequency of RIN and RCC (Fig. 2I-i) and the size of the RIN and RCC lesions (Fig. 2I-ii-iii) increased with mouse age. We verified the reduced expression of p53 and lack of hamartin in these tumors (Supplementary Fig. S1H-K). The age-matched 3–5 month old non-induced controls did not develop tumors with exception of one mouse with few small low grade RIN lesions (Supplementary Table S3).
Figure 1. Renal tumorigenesis induced by partial loss of Tsc1 and p53 in a non-inducible mouse model.
(A-K) Hematoxylin and eosin (H&E) stained kidney sections of (A-C, E-K) of NestinCre;Tsc1f/+;p53f/+ and (D) NestinCre;Tsc1f/+;p53f/f kidneys; scale bars 50μm.
(A-C) renal intraepithelial neoplasia (RIN)-arrows.
(D-F) (D) papillary (arrows), (E) chromophobe and (F) collecting duct RCC; scale bars 50μm
(G-I) (G) invasion of blood vessels (arrow), (H) high atypia; (I) lung metastasis; Dash lines-border between tumor and benign kidney; scale bars 50μm.
(J-K) Areas containing large polygonal cells with clear cytoplasm and hyperchromatic nuclei; scale bars 50μm.
(L-N) The tumor burden in mouse cohorts: (L-i) invasive RCC and RIN, (L-ii-iii, M-N) invasive RCC.
Data represent means±SEM. **P≤0.01,t-test; nNestinCre;Tsc1f/+;p53f/+=38, nNestinCre;Tsc1f/+;p53f/f =13, nNestinCre;Tsc1f/+=14.
Figure 2. Inducible loss of Tsc1 and partial loss of p53 in nestin expressing cells leads to the development of invasive papillary RCC.
(A-E) H&E stained kidney sections from induced Nestin-TamCre;Tsc1f/f;p53f/+ mice; (A) bilateral cysts, (B) invasive cystic RCC with (C) papillary structures. Arrows: (D) large infiltrates of undifferentiated small tumor cells, (E) abortive tubular structures; scale bars 50μm.
(F-H) Nestin expression (red) in (F) undifferentiated small tumor cells, (G) cells in the stalk of papilla in RCC (arrow) and (H) podocytes in normal kidney glomerulus (arrow); scale bars 50μm.
(I) The tumor burden in mouse cohorts (invasive RCC and RIN).
Data represent means±SEM. *P≤0.05. nNestin-TamCre;Tsc1f/f;p53f/+=7 (sacrificed 3 months after induction ), nNestin-TamCre;Tsc1f/f;p53f/+=4 (7 months), nNestin-TamCre;Tsc1f/f;p53f/+ =7 (12 months).
Our non-inducible model mimicking the genetic status of TSC patients, suggests that dysregulation of both Tsc1 and p53 in nestin expressing embryonic cells accelerates carcinogenesis resembling TSC-associated RCC (22), with the progression of the pre-invasive neoplastic lesions (RIN) to invasive metastatic carcinomas. In the inducible model, targeting both Tsc1 and p53 or Tsc1 alone in nestin expressing adult cells resulted in similar, however, not identical phenotypes, suggesting that the crosstalk between these two genes has different impact on tumorigenesis in an adult mouse vs. embryo. Therefore, alterations in p53 function may have different contributions to sporadic vs. TSC-associated RCC.
R72 increases transcription of NOTCH1 and Notch1 signaling.
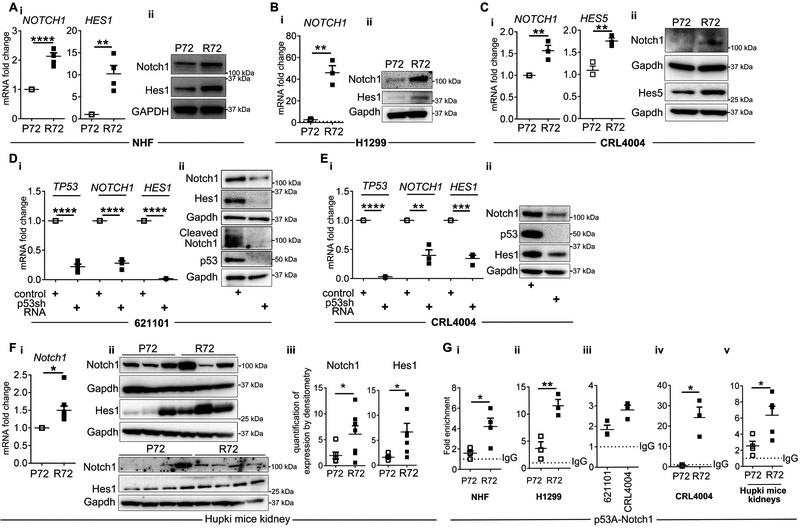
To identify downstream signaling alterations caused by R72 in human and TSC-null cells, we determined impact of R72 on the Notch pathway because this pathway is involved in TSC pathogenesis (18, 24), controls cancer initiation and cell differentiation, and the crosstalk between Notch and p53 involves p53-dependent regulation of Notch1 in human cells (25). We used non-isogenic normal human fibroblasts (NHF) that carry P72 or R72 (9), and isogenic H1299 non-small cell lung carcinoma cells, containing the doxycycline-inducible p53, encoding P72 or R72 (12). We generated the isogenic P72 CRL4004 angiomyolipoma-derived cells, in which arginine was replaced with proline at codon 72 using CRISPR-Cas9 (Supplementary Fig. S2A-C). We confirmed some results in mouse embryonic fibroblasts (MEF) derived from knock-in Hupki mice that carry chimeric human/murine P72 or R72 (26). In all non-isogenic and isogenic cell lines R72 cells had a higher level of Notch1, Hes1 or Hes5 mRNA and protein vs. P72 cells (Fig. 3A-C and Supplementary Fig. S2D-E, S2F-i-iii). In all R72 cells, the increased expression of Notch1 was associated with its increased activation demonstrated by the increased Hes1 or Hes5 (Fig. 3A, B-ii, C and Supplementary Figure S2F-ii-iii). To corroborate these findings, we depleted two angiomyolipoma-derived cell lines of R72 using shRNA. This depletion reduced expression of Notch1 and Hes1 mRNA and protein (Fig. 3D-E). We confirmed that similar mechanisms operate in vivo because the increased expression and activation of the Notch1 was observed in kidneys of R72 vs. P72 Hupki mice (Fig. 3F). These data suggest that codon 72 polymorphism impacts p53 ability to regulate Notch1. We examined expression of additional Notch1 (P21, MYC, CYCLIN D1, CYCLIN D3, HEY1) and p53 (P21, PUMA, BIM, GADD45A, CYCLIN D1, CYCLIN D3, HEY1) targets and found that only CYCLIN D1 is consistently affected by this polymorphism (Supplementary Figure S2G), which is consistent with published studies suggesting that this polymorphism impacts only some of p53 functions (14).
Figure 3. R72 has greater transcriptional activity toward Notch1.
(A) q(RT)-PCR of (i) NOTCH1 and HES1 relative to GAPDH in R72 and P72 NHF cells; (ii) Expression of Notch1 and Hes1 in P72 and R72 NHF cells by Western immunoblotting.
(B-C) Expression of NOTCH1 and HES1/5 in P72 and R72; (B) H1299 cells in the presence of doxycycline (dotted line-expression of Notch1 in non-treated H1299 cells) or in (C) CRL4004 angiomyolipoma-derived cells by (B-i, C-i) q(RT)-PCR relative to GAPDH and (B-ii, C-ii) Western immunoblotting.
(D-E) Expression of TP53, NOTCH1 and HES1 in (D) 621-101 and (E) CRL4004 cells depleted of p53 using shRNA by (D-i, E-i) q(RT)-PCR relative to GAPDH and (D-ii, E-ii) Western immunoblotting.
(F) Expression of Notch1 and Hes1 in kidneys of P72 and R72 mice by (i) q(RT)-PCR relative to GAPDH, or (ii) Western immunoblotting, (iii) Quantification of data in F-ii by densitometry.
(G) Binding of P72 and R72 to p53 responsive element (p53-RE, P53A) within the Notch1 promoter in (i) NHF, (ii) doxycycline treated H1299, (iii-iv) 621101 and CRL4004 cells and (v) in kidneys of P72 and R72 mice by ChIP-qPCR. Binding is shown as fold enrichment over the IgG (dotted line).
Data represent means±SEM. *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
Data are representative of four, n=4 (A-i), , n=14 (Hupki P n=7 mice; Hupki R n=7 mice) (F-ii), n=5 (Hupki P n=2 mice; Hupki R n=3 mice) (F-i), n=5 (G-v), five (A-ii), three, n=3 (B, C, D, E), and two (G-i, n=4 and G-ii-G-iv n=3) independent experiments.
R72 transactivates Notch1 better than P72
The human NOTCH promoter is a direct p53 target (25, 27). Therefore, we hypothesize that increased transcription of NOTCH1 in R72 cells is caused by increased binding of R72 to the NOTCH1 promoter. The increased binding of R72 vs. P72 was detected to p53A-one out of two tested p53-RE sites (Fig. 3G-i-iii). The substitution of arginine (R72) with proline (P72) in CRL4004 angiomyolipoma cells suppressed p53 binding to the Notch1 promoter (Fig. 3G-iv). Similar difference of Notch1 promoter regulation by both p53 variants was observed in vivo, as binding of p53 to the Notch1 promoter was stronger in the kidneys of R72 vs. P72 Hupki mice (Fig. 3G-v). Because increased binding of R72 to the NOTCH1 promoter correlated with the increased level of NOTCH1 and HES1 mRNA in R72 cells, we concluded that R72 polymorphism increases the ability of human p53 to activate Notch1.
R72 leads to Notch1-dependent overexpression of Nodal.
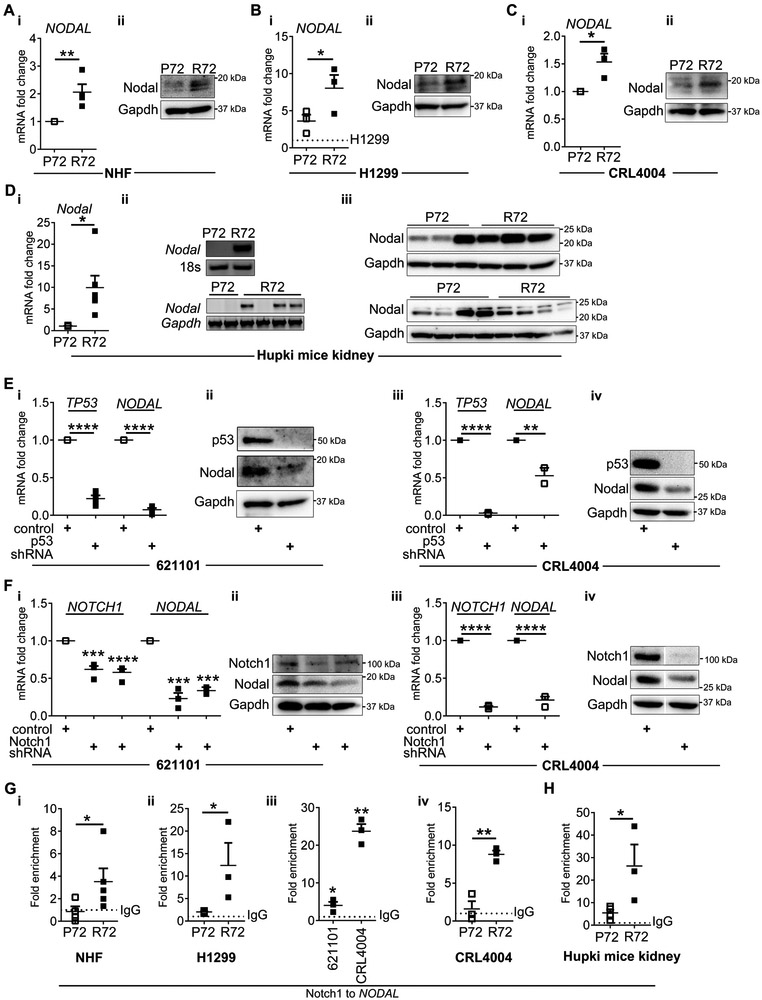
Because we found that R72 leads to p53 “gain-in-function” toward Notch1 and R72 is present in majority of LAM and angiomyolipoma patients, we hypothesized that signaling downstream of Notch1 and R72, contributes to TSC tumorigenesis. The p53 family members suppress neuroectodermal fate in human embryonic stem cells via p53-dependent integration of Wnt and Nodal pathways (28) and Nodal has similar to p53 effect on this fate (29). p53 binds Smad2/3 proteins, a downstream target of Nodal, in Xenopus embryos to regulate the transforming growth factor-β (TGF-β) target genes (30). The expression of Nodal is under transcriptional control of Notch1 (31) and Nodal emerged as an important player in tumorigenesis (32, 33). Thus, we hypothesized that p53 cross-talks to the Notch1-Nodal axis and that the codon 72 polymorphism impacts this function. We examined levels of Nodal and found several fold increase in its expression in R72 vs. P72 cells (Fig. 4A-B and Supplementary Figure S2F-iv). The substitution of arginine with proline in CRL4004 angiomyolipoma cells suppressed Nodal (Fig. 4C-i-ii) and the overexpression of Nodal was evident in kidneys of R72 vs. P72 mice (Fig. 4D). To confirm that Nodal expression is under control of R72, we depleted 621–101 and CRL4004 angiomyolipoma cells carrying R72 using shRNA. This resulted in suppression of Nodal (Fig. 4E). Next we determined whether regulation of Nodal in R72 angiomyolipoma cells is under control of Notch1. The depletion of Notch1 in R72 angiomyolipoma cells reduced expression of Nodal (Fig. 4F). To delineate the underlying mechanism of R72- and Notch-dependent overexpression of Nodal, we examined binding of Notch1 to the NODAL promoter. The increased binding of Notch1 to NODAL promoter was detected in R72 vs. P72 cells (Fig. 4G-i-iii). The substitution of arginine with proline in CRL4004 angiomyolipoma cells suppressed Notch1 binding to the promoter of NODAL (Fig. 4G-iv). The increased binding of Notch1 to the Nodal promoter was also detected in kidneys of R72 vs. P72 mice (Fig. 4H). These findings together with the elevated levels of NODAL mRNA in R72 cells suggest that R72, in contrast to P72, enables or increases expression of Nodal via increasing expression of Notch1 and facilitating its binding to the NODAL promoter.
Figure 4. R72- and Notch1-dependent expression of Nodal.
(A-D) Expression of Nodal in P72 and R72 (A) NHF, (B) doxycycline treated H1299 cells (dotted line- expression of Nodal in non-treated H1299 cells), (C) P72 and R72 CRL4004 angiomyolipoma cells and (D) kidneys of P72 and R72 mice by (A-i, B-i, C-i, D-i) q(RT)-PCR relative to GAPDH followed by (D-ii) gel detection and (A-B-C-ii, D-iii) Western immunoblotting. The loading control in D-iii is also shown in second panel of 3F-ii and 6A-ii.
(E-F) Expression of (E) TP53 and NODAL or (F) NOTCH1 and NODAL in (E-i-ii, F-i-ii) 621–101 and (E-iii-iv, F-iii-iv) CRL4004 angiomyolipoma cells, depleted of (E) p53 or (F) Notch by (E-i, iii, F-i, iii) q(RT)-PCR relative to GAPDH and (E-ii, iv, F-ii, iv) Western immunoblotting. The loading control (Gapdh) and p53 immunoblot in E-ii and E-iv are also shown in 3D-ii and 3E-ii, respectively. The same control cell line was used and run simultaneously for experiments in E-F-iv, therefore the same Nodal and Gapdh immunoblots are shown in E-F-iv.
(G-H) Binding of Notch1 to the NODAL promoter by ChIP-qPCR in (G-i) P72 and R72 NHF cells, (G-ii) P72 and R72 H1299 cells treated with doxycycline, (G-iii) 621–101 and CRL4004 angiomyolipoma cells, (G-iv) P72 and R72 CRL4004 angiomyolipoma cells, and in (H) kidneys of R72 and P72 mice shown as fold enrichment over the IgG (dotted line).
Data represent means±SEM. *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
Data are representative of four, n=4 (A), three, n=3 (B-C, E, F), n=8 (D-i-ii) and n=14 (D-iii) animals, two n=5 (G-i-ii), n=3 (G-iii-iv, H) independent experiments.
R72 and p53 deficiency in the background of TSC1/Tsc1 loss lead to similar dysregulations of the Notch1/Nodal axis, in human and mouse tumors, respectively.
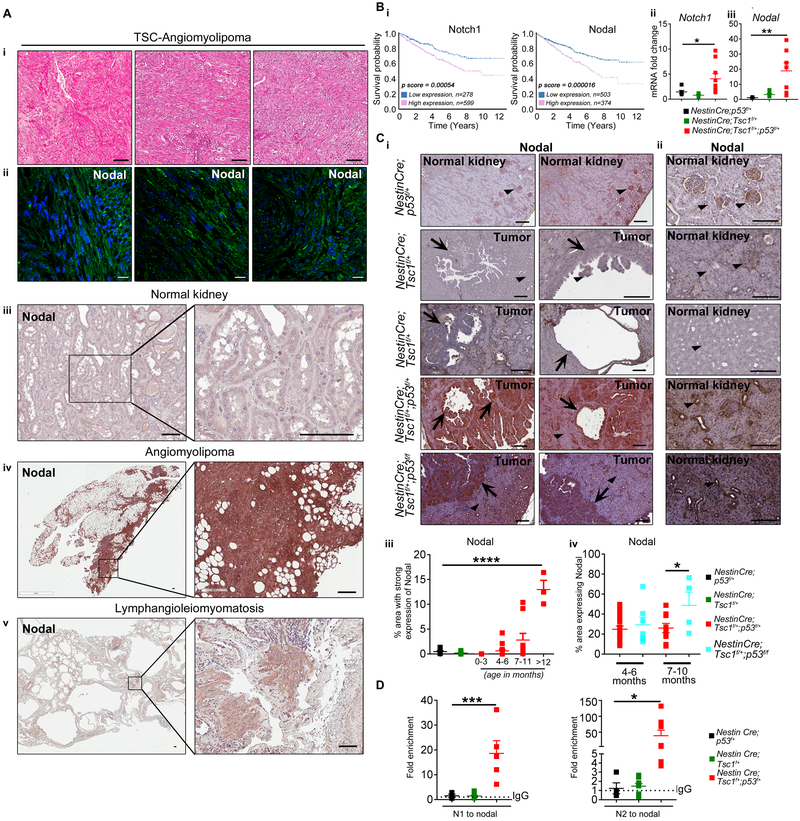
To determine Nodal contributions to TSC tumorigenesis, we examined its expression in TSC-associated angiomyolipoma and LAM. Nodal was overexpressed in TSC-associated angiomyolipomas and LAM compared to normal kidney or normal lung (Fig. 5A and Supplementary Figure S3). We analyzed expression of Notch1 and Nodal in sporadic RCC using https://www.proteinatlas.org and found that high expression of Notch1 and Nodal is associated with lower probability of 5-year survival of RCC patients (Fig. 5B-i). These data together with evidence of increased renal cell proliferation mediated by Nodal (32) led us to question whether the alterations of Notch1/Nodal axis, similar to those caused by the R72 in humans, are also responsible for tumorigenesis in mice with partial loss of p53 and/or Tsc1. We examined expression of Nodal and Notch1 in NestinCre;p53f/+, NestinCre;Tsc1f/+ vs. NestinCre;Tsc1f/+;p53f/+ and NestinCre;Tsc1f/+;p53f/f mice. qPCR revealed increased expression of Notch1 and Nodal only in NestinCre;Tsc1f/+;p53f/+ mice (Fig. 5B-ii-iii and Supplementary Fig. S4A-i) indicating that upregulation of these genes in a mouse requires partial loss of both Tsc1 and p53. Immunohistochemistry confirmed these findings as the expression of Nodal was weak in normal kidney of NestinCre;p53f/+ (Fig. 5 C-i-ii) and NestinCre;Tsc1f/+ (Fig. 5 C-i-ii) mice or in renal tumors of NestinCre;Tsc1f/+ mice (Fig. 5 C-i) in contrast to renal tumors of NestinCre;Tsc1f/+;p53f/f and NestinCre;Tsc1f/+;p53f/+ mice (Fig. 5 C-i). The renal tubules outside the tumors of NestinCre;Tsc1f/+;p53f/f and NestinCre;Tsc1f/+;p53f/+ mice had also increased Nodal expression (Fig. 5C-ii). We demonstrated the positive correlation between Nodal expression (area occupied by nodal positive cells) and age of NestinCre;Tsc1f/+;p53f/f and NestinCre;Tsc1f/+;p53f/+ mice (Fig. 5C-iii-iv). The expression of Nodal was higher in NestinCre;Tsc1f/+;p53f/f vs. NestinCre;Tsc1f/+;p53f/+ mice (Fig. 5C-iv). The increased expression of Nodal correlated also with increased binding of Notch1 to the Nodal promoter in NestinCre;Tsc1f/+;p53f/+ in contrast to NestinCre;Tsc1f/+ and NestinCre;p53f/+ mice, in which this binding was minimal (Fig. 5D). To determine whether similar alterations within the Notch-Nodal axis exist in Nestin-TamCre;Tsc1f/f;p53f/+ mice and to understand the impact of partial loss of p53 on renal tumorigenesis in adult mouse, we compared expression of Notch1 and Nodal within kidneys/tumors of non-induced Nestin-TamCre;Tsc1f/f;p53f/+ and tamoxifen-induced Nestin-TamCre;Tsc1f/f and Nestin-TamCre;Tsc1f/f;p53f/+ mice. Consistent with data from our non-inducible model, increased expression of Notch1 and Nodal mRNAs was observed only in tumors of tamoxifen-induced Nestin-TamCre;Tsc1f/f;p53f/+ mice (Supplementary Fig. S4B), suggesting that p53 contributes to TSC-associated tumorigenesis via regulation of Notch and Nodal expression in these mouse models. We confirmed overexpression of Nodal protein in Nestin-TamCre;Tsc1f/f;p53f/+ mice (Supplementary Fig. S4C-D) and in small nestin positive (Fig. 2F) undifferentiated tumor cells (Supplementary Fig. S4D). Although some published data suggest that human Notch1 is under positive regulation of p53, likely in cell-type specific manner (34), information on murine p53-dependent Notch1 regulation is limited. The two studies yielded conflicting results: indicating that either murine p53 is required for induction of Notch expression (35) or loss of murine p53 increases expression and activation of Notch1 in thymocytes (36), with the latter implicating inhibitory role of murine p53 toward Notch1. Our results align with this second study and indicate the partial loss of p53 and Tsc1 leads to increased expression of Notch1 and mimics R72 effect on Notch1. Thus, it appears that murine p53 has a different from human p53 role in regulating Notch1. To our knowledge there is no information if murine p53 affects the expression of Notch1 mRNA.
Figure 5. Overexpression of Nodal in TSC-associated renal tumors.
(A) (i) Hematoxylin and eosin staining in angiomyolipoma tumors from three TSC patients; (second to fifth panels) Expression of Nodal in (ii) TSC-associated angiomyolipomas, (iii) non-diseased normal kidneys, (iv) sporadic angiomyolipoma, (v) LAM by (ii) immunofluorescence and (iii-v) immunohistochemistry, scale bars 50μm.
(B-C) Expression of (B-i-ii) Notch1 and (B-i, B-iii, C) Nodal in (B-i) sporadic human RCC (from Human Protein Atlas), (B-ii-iii, C) kidneys (NestinCre;p53f/+ and NestinCre;Tsc1f/+) and/or RCC (NestinCre;Tsc1f/+;p53f/+ and NestinCre;Tsc1f/+;p53f/f), by (B-ii-iii) q(RT)-PCR relative to GAPDH, (C-i-ii) immunohistochemistry (arrows indicate renal carcinoma, arrowheads indicate normal kidney); scale bars 50μm. (C-iii) The correlation between the percentage of area with strong expression of Nodal (Aperio-Digital Pathology) and mouse age. (C-iv) The correlation between the percentage of area expressing Nodal and p53 status and mouse age.
(D) Binding of Notch1 to the NODAL promoter to two NRE sites (N1 and N2) by ChIP-qPCR in NestinCre;p53f/+, NestinCre;Tsc1f/+, NestinCre;Tsc1f/+;p53f/+ mice shown as fold enrichment over the IgG (dotted line).
Data represent means±SEM. *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
Data are representative of five (A), n=19, n=14 animals (B-ii-iii), n=65 (C), n=7 (D) independent experiments.
Expression of human R72 in mouse kidneys renders similar increase in the expression of Nodal as the partial loss of murine p53.
Given differences in the sequences of murine and human p53 and increased expression of Nodal upon partial loss of Tp53 and Tsc1 in our mouse models, we sought to determine whether R72, in a humanized mouse, causes similar alterations in the Notch1/Nodal signaling axis as concomitant and partial loss of Tp53 and Tsc1. We compared expression of Notch1 and Nodal and binding of Notch1 to the promoter of NODAL in the kidneys of R72 and P72 mice and kidneys or tumors of non-induced and induced Nestin-TamCre;Tsc1f/f;p53f/+ mice. The increased expression of Notch1 and Nodal was detected in kidneys of R72 mice and it was similar to the levels observed in kidney/tumors of induced Nestin-TamCre;Tsc1f/f;p53f/+ mice (Supplementary Fig. S4E-i). The expression of Notch1 and Nodal in kidneys of P72 Hupki mice was low and similar to the levels observed in kidneys of non-induced Nestin-TamCre;Tsc1f/f;p53f/+ (Supplementary Fig. S4E-i, first vs. fourth or sixth vs. eight lanes). The increased expression of Nodal associated with increased binding of Notch1 to the Nodal promoter in R72 Hupki and tamoxifen-induced Nestin-TamCre;Tsc1f/f;p53f/+ compared to P72 Hupki and non-induced mice (Supplementary Fig. S4E-ii, second and fourth graph vs. first and third graph). These results suggest that the downstream signaling alterations, caused by R72 in humans mirror signaling changes in mice with the partial loss of Tp53 and Tsc1, therefore, similar mechanisms appear to be responsible for tumorigenesis associated with loss of TSC1 and TP53 polymorphism in humans and loss of Tsc1 and Tp53 in mice. These mechanisms, although not fully understood, involve activation of the Notch/Nodal axis.
R72 promotes epithelial-to-mesenchymal transition and increases cell migration via the Notch and Nodal pathways.
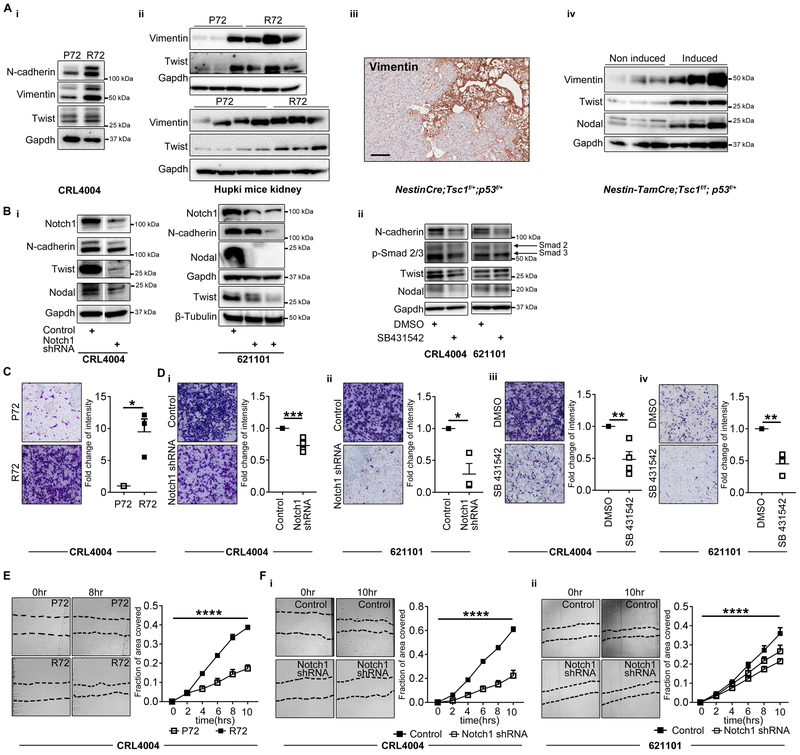
Angiomyolipoma and LAM cells express nestin and contain cell populations with neural stem cell like phenotypes related to increased activity of the Rheb-Notch-Rheb regulatory loop (18). The expression of nestin and dysregulation of Notch and Nodal signaling are common for both epithelial-to-mesenchymal (EMT) phenotypes and cancer stem cells (CSCs) (37). Therefore, we determined impact of R72-dependent overexpression of Notch and Nodal on EMT and on self-renewal of angiomyolipoma cells (16). The expression of vimentin, N-cadherin (EMT markers) and twist (EMT transcriptional factor) was increased in R72 vs. P72 angiomyolipoma cells (Fig. 6A-i) and in kidneys of R72 vs. P72 Hupki mice (Fig. 6A-ii). Consistent with these results and with published work, indicating expression of vimentin in sporadic angiomyolipoma tumors (38), increased expression of vimentin was detected within the tumors from NestinCre;Tsc1f/+;p53f/+ and induced Nestin-TamCre;Tsc1f/f;p53f/+mice (Fig. 6A-iii-iv). Silencing Notch using shRNA or suppressing Nodal signaling using Nodal inhibitor, reduced expression of N-cadherin and twist in R72 angiomyolipoma cells (Fig. 6Bi-ii). To suppress Nodal we used selective inhibitor of the TGF-β type I receptor activin receptor-like kinase ALK5 and its relatives ALK4/7 (SB 431542), which was also shown to inhibit Nodal signaling as Nodal is a member of the TGF-β family and signals via ALK4/5/7 (39). These results suggest that altered function of p53 promotes EMT phenotypes via dysregulation of Notch and Nodal signaling in TSC-null cells. We compared side by side the expression of vimentin in kidneys of p53f/f, NestinCre; p53f/+, Hupki P72 and HupkiR72 and found that partial loss of p53 increases the expression of vimentin similar to R72 (Supplementary Figure S5A-B). The increased expression of vimentin was evident in glomeruli of NestinCre;p53f/- mice (Supplementary Figure S5B) and in epithelial cells of renal tubules of R72 mice (Supplementary Figure S5B). This is consistent with the role of p53 in regulating EMT via additional mechanisms (40).
Figure 6. Notch1 increases migration of R72 cells via Nodal.
(A) The expression of N-cadherin, vimentin, twist, and nodal in (i) P72 and R72 CRL4004 angiomyolipoma cells, (ii) kidneys from P72 and R72 Hupki mice, (iii) NestinCre;Tsc1f/+;p53f/+ mice, (iv) non-induced and tamoxifen induced Nestin-TamCre;Tsc1f/f;p53f/+ mice. The loading control (Gapdh) in panel A-ii is also shown in 3F-i and 4D-iii.
(B) The expression of Notch1, N-cadherin, twist, nodal, and Smad2/3 phosphorylation (only in ii) in CRL4004 and 621101 angiomyolipoma cells, (i) depleted of Notch1 by shRNA or (ii) treated with SB431542 for 72 hours by Western immunoblotting.
(C-D) (left panels) representative images of the Boyden chamber inserts stained with crystal violet, showing migrated (C) P72 and R72 CRL4004 angiomyolipoma cells or (D); R72 (i, iii) CRL4004 and (ii, iv) 621101 angiomyolipoma cells (i-ii) depleted of Notch1 or (iii-iv) treated with SB431542; (right panels) quantification of migrated R72 cells shown as fold change in mean intensity relative to (C) P72 cells, or (D) cells (i-ii) depleted of Notch or (iii-iv) treated with SB431542.
(E-F) Images of migration assay and quantification of the area of scratch closure in (E) P72 and R72 CRL4004 angiomyolipoma cells, or (F) (i) CRL4004 and (ii) 621101 cells expressing control or Notch shRNA.
Data represent means±SEM. *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
Data are representative of two experiments (A, B), three n=3 (C, D-i-ii, iv), four, n=4 (D-iii), two, n=6 (E), four experiments, n=12 (F-i), five experiments, n=15 (F-ii).
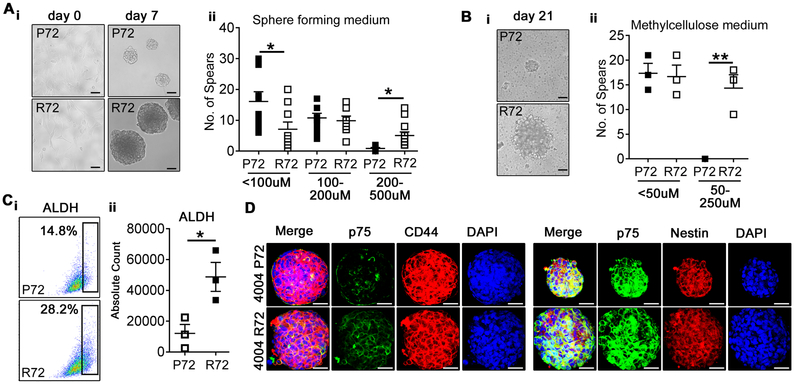
The metastatic potential of LAM suggests that LAM cells spread from angiomyolipoma to the lungs, or from unknown peripheral site to both lungs and kidneys (2). Therefore, we next determined migratory properties of R72 and P72 cells. Both The Boyden chamber and the “scratch-wound” healing assays revealed increased migration of R72 vs. P72 angiomyolipoma cells (Fig. 6C and E). These data are consistent with recent discovery that tumor cells carrying R72 form of mutant p53 have increased migration ability (41). The suppression of R72 reduced migration of CRL4004 and 621–101 angiomyolipoma cells to the levels observed in P72 CRL4004 cells expressing control shRNA (Supplementary Figure S5C). The suppression of P72 in CRL4004 cells resulted in mild reduction of these cells’ migration compared to cells expressing control shRNA (Supplementary Figure S5C). To determine if altered Notch and Nodal signaling contribute to increased migratory properties of R72 cells, we depleted Notch or treated these cells with a Nodal inhibitor SB 431542. The partial suppression of Notch1 through shRNA impaired 621–101 and CRL4004 angiomyolipoma cell migration (Fig. 6D-i-ii and F). The treatment with SB 431542 inhibited Nodal signaling, indicated by reduced phosphorylation of Smad2/3 (Fig. 6B-ii), and reduced migration of angiomyolipoma cells (Fig. 6D-iii-iv). SB 431542 did not impact cell proliferation (data not shown). Sphere-forming assay revealed that R72 angiomyolipoma cells form bigger spheres compared to P72 cells (Fig.7A-B), suggesting increased self-renewal properties of R72 vs. P72 cells. We also found increased number of R72 vs. P72 cells with high aldehyde dehydrogenase (ALDH) activity (Fig. 7C). Because high expression/activity of ALDH has been reported in stem cells (including cancer stem cells) and progenitor cells of various lineages (16), these data suggest that neural stem cell like properties of angiomyolipoma cells, reported in our recent study (18), are R72-dependent. However, the expression of CSCs surrogate markers including CD44, p75 and nestin was not affected by codon 72 polymorphism (Fig. 7D).
Figure 7. Increased clonogenic growth potential of R72 angiomyolipoma cells.
(A-B) (i) Bright field images of P72 and R72 CRL4004 angiomyolipoma cells grown in (A) DMEM (day 0) or in sphere forming medium (day 7), (B) methyl cellulose medium (day 21); (ii) Numbers of spheres at (A) day 7 and (B) day 21.
(C) (i-ii) FACS dot plots of ALDH positive P72 and R72 CRL4004 cells grown in sphere forming medium at day 7; (i) percentages and (ii) absolute counts of ALDH positive cells.
(D) Expression of p75, CD44 and nestin in P72 and R72 CRL4004-derived spheres at day7.
Data represent means±SEM. *P≤0.05, **P≤0.01.
Data are representative of three n=9 (A), n=3 (B, C), two (D) independent experiments, scale bar represents 50 μM.
Discussion
The significance of codon 72 polymorphism for cancer pathogenesis is not fully understood. This polymorphism likely influences apoptosis and tumor suppression in a tissue- and/or tumor-specific manner, perhaps explaining the conflicting results from human cancer studies. The very recent study, published when this manuscript was under review, demonstrated that R72 increases susceptibility to mammary tumorigenesis through chronic inflammation (42). Our data indicate higher risk for angiomyolipoma in TSC patients that are homozygous vs. heterozygous for R72, suggesting that either R72 increases risk for this tumor, or P72 has protective function. Since neural stem-cell like properties of angiomyolipoma cells and their EMT programs may be R72-dependent and both EMT and CSCs drive aggressive tumor phenotypes and treatment resistance, this study identifies polymorphic p53 and p53-dependent downstream signaling alterations as potential targets for TSC therapy, which can overcome resistance to rapamycin. R72-dependent-Notch-mediated EMT traits of angiomyolipoma cells are consistent with a currently broadly accepted concept of metastatic origin of LAM, potentially from angiomyolipoma (2). Our data implicate that partial loss of only murine Tp53 does not affect levels of Notch1, which is consistent with published results (36). Some studies suggest that human Notch1 is under positive regulation of p53, likely in cell-type specific manner (34), thus R72 might lead to gain of p53 function with respect to Notch signaling. Therefore, we concluded that the roles of murine vs. human p53 in regulating Notch1 are different and/or context dependent. Because the expression of Nodal was not affected by partial loss of only Tp53 or Tsc1, we propose that only concomitant partial/complete loss of murine Tp53 and Tsc1 mimics impact of R72 on the Notch1/Nodal axis, at least, in our models.
Since the partial loss of Tp53 in the background of loss of Tsc1 accelerated TSC tumorigenesis we propose that TP53 polymorphism in TSC contributes to formation of multiple tumors including RCC and enhances their growth. Although the partial loss of p53 only in NestinCre;p53f/+ mice did not affect markedly the expression of Nodal in contrast to increased expression of Nodal in R72 Hupki mice, none of these mice develop tumors. Therefore, the Notch- and Nodal-dependent tumorigenesis must involve additional not fully understood mechanisms triggered only upon concomitant loss of Tsc1, activation of mTOR, and alteration in some p53 function(s) that are difficult to dissect with the use of our current models because of unclear role of murine p53 toward Notch1. The humanized Hupki mouse model would be ideal to overcome these problems and to examine the role of P72 and R72 p53 variants in TSC pathogenesis. In summary, we propose that loss of TSC1/2 in the background of codon 72 polymorphism accelerates TSC tumorigenesis. The altered function of p53, caused by codon 72 polymorphism, appears to be an additional and novel mechanism contributing to TSC pathogenesis, and, perhaps, explaining conflicting results on a role of TP53 in pathogenesis of this malignancy (43). Importantly, our data are consistent with recent findings indicating that tumor cells with R72 form of mutant p53 have increased migration, invasion and mTOR/p70S6K signaling and poorer survival in breast cancer patients (41). It is likely that this polymorphism alone is not sufficient to induce tumorigenesis, however, it may endorse tumor growth in conjunction with alterations of other tumor suppressor genes.
Supplementary Material
Acknowledgments
We acknowledge Jalpa Patel, Navin Chintala and Kelly Hartley for technical assistance, the Developmental Corporation of Abilene for support, and the use of tissues procured by the NDRI supported by NIH 2 U42 OD011158. This work was supported by the National Institute of Health (R01CA190209 to M.M.M.), the Cancer Prevention and Research Institute of Texas (RP120168 to M.K.), the U.S. Department of Defense (TS140010 to M.K.), Laura W. Bush Institute for Women’s Health (seed grants to M.M.M. and M.K.).
Financial support: This work was supported by the National Institute of Health (R01CA190209 to M.M.M.), the Cancer Prevention and Research Institute of Texas (RP120168 to M.K.), the U.S. Department of Defense (TS140010 to M.K.), Laura W. Bush Institute for Women’s Health (seed grants to M.M.M. and M.K.).
Footnotes
Conflict of interest statement: The authors declare no conflict of interest
References
- 1.Giannikou K, Malinowska IA, Pugh TJ, Yan R, Tseng YY, Oh C, et al. Whole Exome Sequencing Identifies TSC1/TSC2 Biallelic Loss as the Primary and Sufficient Driver Event for Renal Angiomyolipoma Development. PLoS Genet. 2016;12(8):e1006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henske EP, and McCormack FX. Lymphangioleiomyomatosis - a wolf in sheep’s clothing. J Clin Invest. 2012;122(11):3807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battelli C, and Cho DC. mTOR inhibitors in renal cell carcinoma. Therapy. 2011;8(4):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Kowalski ML, Carson RP, Bridges LR, and Ess KC. Heterozygous inactivation of tsc2 enhances tumorigenesis in p53 mutant zebrafish. Disease models & mechanisms. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, et al. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 2007;26(23):4812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habib SL, Yadav A, Mahimainathan L, and Valente AJ. Regulation of PI 3-K, PTEN, p53, and mTOR in Malignant and Benign Tumors Deficient in Tuberin. Genes Cancer. 2011;2(11):1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong LC, Westlake G, Snow JP, Cawthon B, Armour E, Bowman AB, et al. Heterozygous loss of TSC2 alters p53 signaling and human stem cell reprogramming. Hum Mol Genet. 2017;26(23):4629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzam GA, Frank AK, Hollstein M, and Murphy ME. Tissue-specific apoptotic effects of the p53 codon 72 polymorphism in a mouse model. Cell Cycle. 2011;10(9):1352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank AK, Leu JI, Zhou Y, Devarajan K, Nedelko T, Klein-Szanto A, et al. The codon 72 polymorphism of p53 regulates interaction with NF-{kappa}B and transactivation of genes involved in immunity and inflammation. Mol Cell Biol. 2011;31(6):1201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pim D, and Banks L. p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer. 2004;108(2):196–9. [DOI] [PubMed] [Google Scholar]
- 11.Whibley C, Pharoah PD, and Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9(2):95–107. [DOI] [PubMed] [Google Scholar]
- 12.Kung CP, Khaku S, Jennis M, Zhou Y, and Murphy ME. Identification of TRIML2, a novel p53 target, that enhances p53 SUMOylation and regulates the transactivation of proapoptotic genes. Mol Cancer Res. 2015;13(2):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung CP, Leu JI, Basu S, Khaku S, Anokye-Danso F, Liu Q, et al. The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction. Cell Rep. 2016;14(10):2413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kung CP, Liu Q, and Murphy ME. The codon 72 polymorphism of p53 influences cell fate following nutrient deprivation. Cancer Biol Ther. 2017;18(7):484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YJ, Bailey JM, Rovira M, and Leach SD. Sphere-forming assays for assessment of benign and malignant pancreatic stem cells. Methods Mol Biol. 2013;980:281–90. [DOI] [PubMed] [Google Scholar]
- 17.Tyburczy ME, Dies KA, Glass J, Camposano S, Chekaluk Y, Thorner AR, et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genet. 2015;11(11):e1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JH, Patel B, Bonala S, Manne S, Zhou Y, Vadrevu SK, et al. Notch transactivates Rheb to maintain the multipotency of TSC-null cells. Nat Commun. 2017;8(1):1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SD, Stallcup W, Lefkove B, Govindarajan B, Au KS, Northrup H, et al. Expression of the neural stem cell markers NG2 and L1 in human angiomyolipoma: are angiomyolipomas neoplasms of stem cells? Mol Med. 2007;13(3–4):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner N, Wagner KD, Scholz H, Kirschner KM, and Schedl A. Intermediate filament protein nestin is expressed in developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R779–87. [DOI] [PubMed] [Google Scholar]
- 21.Ishizaki M, Ishiwata T, Adachi A, Tamura N, Ghazizadeh M, Kitamura H, et al. Expression of nestin in rat and human glomerular podocytes. J Submicrosc Cytol Pathol. 2006;38(2–3):193–200. [PubMed] [Google Scholar]
- 22.Yang P, Cornejo KM, Sadow PM, Cheng L, Wang MS, Xiao Y, et al. Renal Cell Carcinoma in Tuberous Sclerosis Complex. American Journal of Surgical Pathology. 2014;38(7):895–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W, Cheville JC, Sadow PM, Gopalan A, Fine SW, Al-Ahmadie HA, et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol. 2013;26(10):1355–64. [DOI] [PubMed] [Google Scholar]
- 24.Karbowniczek M, Zitserman D, Khabibullin D, Hartman T, Yu J, Morrison T, et al. The evolutionarily conserved TSC/Rheb pathway activates Notch in tuberous sclerosis complex and Drosophila external sensory organ development. The Journal of clinical investigation. 2010;120(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21(5):562–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo JL, Yang Q, Tong WM, Hergenhahn M, Wang ZQ, and Hollstein M. Knock-in mice with a chimeric human/murine p53 gene develop normally and show wild-type p53 responses to DNA damaging agents: a new biomedical research tool. Oncogene. 2001;20(3):320–8. [DOI] [PubMed] [Google Scholar]
- 27.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–19. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Zou Y, Nowotschin S, Kim SY, Li QV, Soh CL, et al. The p53 Family Coordinates Wnt and Nodal Inputs in Mesendodermal Differentiation of Embryonic Stem Cells. Cell Stem Cell. 2017;20(1):70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallier L, Reynolds D, and Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275(2):403–21. [DOI] [PubMed] [Google Scholar]
- 30.Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, and Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113(3):301–14. [DOI] [PubMed] [Google Scholar]
- 31.Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003;17(10):1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Jiang T, Li Q, Wang J, Yang D, Li X, et al. Nodal activates smad and extracellular signal-regulated kinases 1/2 pathways promoting renal cell carcinoma proliferation. Mol Med Rep. 2015;12(1):587–94. [DOI] [PubMed] [Google Scholar]
- 33.Duan W, Li R, Ma J, Lei J, Xu Q, Jiang Z, et al. Overexpression of Nodal induces a metastatic phenotype in pancreatic cancer cells via the Smad2/3 pathway. Oncotarget. 2015;6(3):1490–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9(8):587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, and Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol. 2007;27(10):3732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laws AM, and Osborne BA. p53 regulates thymic Notch1 activation. Eur J Immunol. 2004;34(3):726–34. [DOI] [PubMed] [Google Scholar]
- 37.Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y, et al. The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer. 2017;16(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karbowniczek M, Yu J, and Henske EP. Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am J Pathol. 2003;162(2):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, and Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313(1):107–17. [DOI] [PubMed] [Google Scholar]
- 40.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13(3):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu S, Gnanapradeepan K, Barnoud T, Kung CP, Tavecchio M, Scott J, et al. Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1alpha. Genes Dev. 2018;32(3–4):230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunaratna RT, Santos A, Luo L, Nagi C, Lambertz I, Spier M, et al. Dynamic role of the codon 72 p53 single-nucleotide polymorphism in mammary tumorigenesis in a humanized mouse model. Oncogene. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noon AP, Vlatkovic N, Polanski R, Maguire M, Shawki H, Parsons K, et al. p53 and MDM2 in renal cell carcinoma: biomarkers for disease progression and future therapeutic targets? Cancer. 2010;116(4):780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.