Abstract
Objective:
To systematically review and critically evaluate studies reporting alcohol exposure during pregnancy and miscarriage.
Methods:
We searched PubMed, EMBASE, PsycINFO, and ProQuest Theses for publications from January 1970 to January 2019. We identified studies about alcohol exposure during pregnancy and miscarriage. Information about study population, alcohol exposure assessment, outcome definition, covariates, and measures of association were collected. We assessed study quality using an adapted Newcastle-Ottawa Scale. Data were abstracted by two investigators independently. We conducted a random-effects meta-analysis to calculate the association between alcohol exposure and miscarriage risk and performed subgroup analyses to determine robustness of results to study differences. For studies reporting dose-specific effects, a pooled dose-response association was estimated using generalized least squares regression with and without restricted cubic spline terms for number of drinks consumed per week.
Results:
Of 2,164 articles identified, 24 were eligible for inclusion. Meta-analysis of data from 231,808 pregnant women finds those exposed to alcohol during pregnancy have a greater risk of miscarriage compared to those who abstained (odds ratio [OR] 1.19, 95% confidence intervals [CI] 1.12, 1.28). Estimates did not vary by study design, study country, or method of alcohol ascertainment. For alcohol use of five or fewer drinks per week, each additional drink per week was associated with a six percent increase in miscarriage risk (OR 1.06, 95% CI 1.01, 1.10). Common study limitations reflect challenges inherent to this research, including difficulty recruiting participants early enough in pregnancy to observe miscarriage and collecting and quantifying information about alcohol consumption during pregnancy that accurately reflects use.
Conclusions:
This review provides evidence that alcohol consumption during pregnancy is associated with a dose-mediated increase in miscarriage risk. Future studies evaluating change in alcohol use in pregnancy are needed to provide insight into how alcohol consumption prior to pregnancy recognition impacts risk.
Keywords: alcohol, drinking, miscarriage, pregnancy, spontaneous abortion
INTRODUCTION
Miscarriage occurs in up to one in six recognized pregnancies (Avalos et al., 2012; Goldhaber and Fireman, 1991; Wilcox et al., 1988), is costly to the healthcare system, and can be emotionally devastating regardless of whether pregnancy was planned (Lok and Neugebauer, 2007; Nikcevic et al., 1998). Though miscarriage is common, few modifiable determinants of pregnancy loss are known. In the United States, 10% of pregnant women and more than 50% of nonpregnant women endorse using alcohol within the past 30 days (Tan et al., 2015). Similarly, studies in other developed countries indicate alcohol use occurs in approximately half of women at pregnancy onset and is prevalent to a lesser extent after recognition (O’Keeffe et al., 2015; Tough et al., 2006). The large number of women exposed to alcohol in pregnancy makes it imperative that we understand the relationship between alcohol use and miscarriage.
While alcohol exposure in pregnancy has been repeatedly linked to adverse outcomes, estimates of alcohol’s effect on miscarriage range from protective to increasing risk 3.8-fold. A previous systematic review provides a qualitative summary of the literature about low-to-moderate alcohol consumption in pregnancy and finds five of eight studies suggest use increases miscarriage risk (Henderson et al., 2007). Our review extends previous work by incorporating all studies of alcohol use in pregnancy and providing a meta-analysis of the association.
In this review, we aimed to systematically review the literature and calculate a summary estimate for the association between alcohol exposure during pregnancy and miscarriage. Research about alcohol use and miscarriage faces methodologic challenges including recruiting participants early enough in pregnancy to observe loss, accurately measuring alcohol consumption, and quantifying exposure in a way that is reflective of use (Bailey and Sokol, 2011). Therefore, our secondary objective was to assess the quality of past studies and identify opportunities for future research.
METHODS AND MATERIALS
The literature search, study selection, coding plan, and meta-analysis adhere to the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) statement and the MOOSE guidelines for reporting systematic reviews and meta-analysis of observation studies (Liberati et al., 2009; Stroup et al., 2000).
Search strategy and study selection
Studies were identified through searches of electronic databases (PubMed, EMBASE, PsycINFO, ProQuest, and ClinicalTrials.gov) in January 2019 using the following terms: ‘spontaneous abortion’ or ‘miscarriage’ or ‘pregnancy loss’ or ‘abortion’ and ‘alcohol’ or ‘ethanol’ (See Appendix S1 for full search strategy). To ensure capture of all relevant studies, investigators conducted backward and forward citation searches of included studies. Only studies published after January 1, 1970 and available in English were included.
Original studies evaluating the association between alcohol exposure during pregnancy and miscarriage risk were eligible. Exposure was defined as alcohol use during pregnancy and outcome was miscarriage. Studies that only evaluated pre-conception alcohol use were excluded. Studies of induced abortions were excluded. Because gestational age threshold for miscarriage varied between studies, we did not exclude based on miscarriage definition, but instead performed sensitivity analyses conditioned on definition.
Titles and abstracts were screened by A.C.S. and one other author (C.L.Y., L.L, or S.Z.). If a study was not excluded by both reviewers at the abstract screening stage, we conducted a full text review. A full text review and eligibility decision was made independently by both A.C.S. and S.Z. Discrepancies were adjudicated by S.H.J., who was masked to prior decisions.
Data extraction
A.C.S. and S.Z. conducted data extraction using standardized forms in REDCap hosted at Vanderbilt University (Harris et al., 2009). Differences were resolved by S.H.J. Data abstraction elements included study design, study years, country, counts of study participants by exposure status and pregnancy outcome, recruitment setting, exposure window, reference group definition, exposure definition and operationalization, miscarriage definition, outcome comparator, crude and adjusted effect estimates and confidence intervals for the association, and factors included in adjusted models. If a dose-response analysis was performed, crude and adjusted effect estimates were collected for all dose categories. We contacted study authors for missing values (seven of eleven authors provided additional information).
To assess study quality, we used an adapted Newcastle-Ottawa scale (Table 1), which scores participant recruitment, exposure assessment, outcome assessment, and statistical modeling (Wells et al., 2013). Two reviewers (A.C.S. and S.Z.) collected information about participant inclusion (comparing methods for recruitment of exposed and unexposed in cohort studies and case and control identification for case-control studies), loss to follow-up/non-participation rates, average gestational age at recruitment, timing of alcohol exposure assessment (before or after pregnancy outcome), exposure assessment method (self-administered questionnaire or interviewer-conducted survey), assessment of alcohol consumption change during pregnancy, alcohol exposure operationalization, statistical modeling, and covariates included in the adjusted analysis.
Table 1.
Adapted Newcastle-Ottawa Scale Quality Domains
| Recruitment |
| Equitable recruitment of exposed and unexposed (cohort studies) |
| Equitable recruitment of cases and controls (case-control studies) |
| Recruitment allows for selection of participants representative of general population |
| Minimal loss to follow-up (< 20% loss or < 5% non-participation rate) |
| More than 80% of participants recruited prior to 10 weeks’ gestation |
| Outcome Ascertainment |
| Appropriate comparator group (pregnancies surviving past 20 weeks’ gestation) |
| Exposure Ascertainment |
| Exposure assessed prior to pregnancy outcome to minimize risk of bias (cohort studies) |
| Exposure assessed through self-administered questionnaires to minimize reporting bias |
| Study queried change in consumption during pregnancy |
| Statistical Modeling |
| Alcohol modeled as a time-varying exposure |
| Adjusted for maternal age +/− other confounders |
| Use of time-to event analysis |
Data synthesis
We quantified the association between alcohol exposure and miscarriage risk using random-effects meta-analysis. We evaluated alcohol use as both a dichotomous (exposed versus unexposed) and a continuous variable (number of drinks per week). Random-effects models were used to account for dispersion of true effect across study contexts. Analyses included adjusted data when available. When effect estimates were not reported, odds ratios were calculated using counts provided in the text. Heterogeneity was assessed using the I2 statistics, which estimates the proportion of heterogeneity attributable to true between-study differences. We evaluated publication bias using a funnel plot and Egger regression.
For studies reporting dose-specific effects, we used random-effects meta-analysis to estimate the association between amount of alcohol consumed and miscarriage. We converted alcohol exposure categories to average number of drinks per week. We used the midpoint of each study-specific exposure category and, for open-ended categories, we divided the interval of the next highest category by two and added that value to the lower boundary of the highest category (e.g., if categories were 0, 1–4, 5–8, and ≥9, doses used in the model would be 0, 2.5, 6.5, and 10.5). We used generalized least squares regression models to perform a random-effects meta-analysis estimating a log-linear trends between alcohol dose and miscarriage risk. This method accounts for non-independence between effect estimates using the same reference category (i.e., effect estimates for multiple doses in a single study) (Greenland and Longnecker, 1992). We evaluated the possibility of a non-linear relationship between alcohol dose and miscarriage risk using restricted cubic splines (Orsini et al., 2006). We used three knots since the inclusion of four or more did not improve model fit by the likelihood ratio test and knot placement was determined by Harrell’s recommended percentiles (Harrell, 2001). We analyzed studies reporting dose-effects in terms of hazard ratios (HR) separately as to not combine estimates that incorporate survival data with those that do not.
For both methods of operationalizing alcohol exposure, we performed a series of subgroup analyses to investigate robustness of findings to study differences. We evaluated whether findings varied when we restricted the analysis to cohort studies, case-control studies, studies that only included first trimester miscarriages, studies that included all miscarriages (i.e., excluding the studies that only included first trimester miscarriages), studies presenting adjusted results, studies that recruited 80% of more of the cohort prior to ten weeks gestation, studies with equitable recruitment between study groups (cases and controls for case-control studies and exposed versus non-exposed for cohort studies), or studies that assessed alcohol use prior to pregnancy outcome.
Analyses were performed in Stata (Version 14.2, StataCorp, College Station, TX). We used the “metan” package to estimate aggregate odds ratios (ORs) and 95% confidence intervals (CIs) and the “glst” package to estimate the dose-response effect.
RESULTS
Study selection and study characteristics
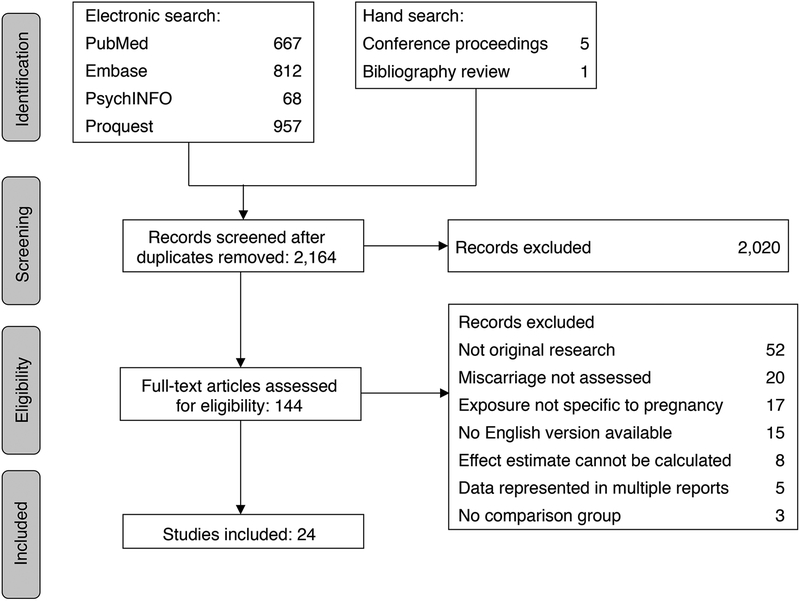
We identified 2,136 unique articles. Twenty-four studies were eligible for analysis including 231,808 pregnant women (Figure 1) (Armstrong et al., 1992; Avalos et al., 2014; Borges et al., 1997; Boyles et al., 2000; Buck Louis et al., 2016; Cavallo et al., 1995; Chiodo et al., 2012; Conde-Ferraez et al., 2013; Davis et al., 1982; Dlugosz et al., 1996; Feodor Nilsson et al., 2014; Halmesmaki et al., 1989; Han et al., 2012; Harlap and Shiono, 1980; Kesmodel et al., 2002; Kline et al., 1980; Long et al., 1994; Maconochie et al., 2007; Parazzini et al., 1994; Paszkowski et al., 2016; Rasch, 2003; Windham et al., 1992; Windham et al., 1997; Xu et al., 2014). If data from the same study sample was present in multiple reports (Avalos et al., 2009; Andersen et al., 2012; Kline et al., 1981; Strandberg-Larsen et al., 2008; Zhang and Bracken, 1996), the report with the most complete information was used. Fourteen were cohort studies and ten were case-control (Table 2). The United States contributed the largest proportion of studies (38%), followed by Denmark (13%) and the United Kingdom (13%). Included studies were published between 1980 and 2016 and sample size ranged from 161 to 89,339 participants.
Figure 1.
Flow diagram of studies identified for the systematic review.
Table 2.
Characteristics of studies in systematic review
| Author, Year |
Study Design | Country | Study Years | n (SAB/no SAB) |
Recruited population | Exposure Ascertainment | Miscarriage Definitiona | Comparator |
|---|---|---|---|---|---|---|---|---|
| Armstrong, 1992 | Cohort | Canada | 1982–1984 | 47,146 (10,191/36,955) |
Women delivering or receiving care for SAB across 11 hospitals | In-person interview for first trimester exposure in index and prior pregnancies | <28 | Births |
| Avalos, 2014 | Cohort | USA | 1996–1998 | 1,061 (172/889) |
KPNC members with record of a positive pregnancy test prior to 10 weeks’ gestation | In-person interview prior to 15 weeks’ gestation | ≤20 | Pregnancies surviving past 20 weeks |
| Borges, 1997 | Cohort | Mexico | 1988 | 4,634 (197/4,437) |
Women with a prior pregnancy randomly surveyed in urban areas of Mexico | In-person interview for alcohol consumption in most recent pregnancy | — | Pregnancies not ending in SAB |
| Boyles, 2000 | Case-Control | USA | 1995–1997 | 970 (400/570) |
Women presenting to the emergency department before 22 weeks’ gestation | In-person interview during emergency department visit | ≤22 | Pregnancies surviving past 22 weeks |
| Buck Louis, 2016 | Cohort | USA | 2005–2009 | 344 (98/246) |
Couples discontinuing contraception with the intention of becoming pregnant | Daily lifestyle journals pre-conception through seven weeks post-conception | ≤22 | Pregnancies surviving past 22 weeks |
| Cavallo, 1995 | Cohort | Italy | — | 527 (55/472) |
Women at first blood test during pregnancy | In-person interview during hospital visit | — | Live births |
| Chiodo, 2012 | Cohort | USA | 1999–2001 | 302 (23/279) |
Women initiating prenatal care before 28 weeks’ gestation at urban clinics | In-person interview repeated at each prenatal visit | ≤20 | Pregnancies surviving past 20 weeks |
| Conde-Ferraez, 2013 | Case-Control | Mexico | 2008–2009 | 281 (143/138) |
Women receiving curettage for SAB (cases) or delivering at term (controls) | In-person interview during hospitalization | ≤20 | Live, term births |
| Davis, 1982 | Cohort | UK | 1980 | 973 (22/951) |
Women at booking prenatal visit at study hospital | Self-administered questionnaire at booking visit | — | Stillbirths and live births |
| Dlugosz, 1996 | Cohort | USA | 1988–1992 | 2,839 (135/2,704) |
Women initiating prenatal care before 16 weeks’ gestation | At-home interview before 17 weeks’ gestation about exposure in first month | <28 | Live births |
| Feodor Nilsson, 2014 | Cohort | Denmark | 1996–2002 | 89,339 (3,018/86,321) | DNBC women initiating prenatal care before 22 weeks’ gestation | CATI targeted for 12 weeks’ gestation | ≤22 | Pregnancies surviving past 22 weeks |
| Halmesmaki, 1989 | Case-Control | Finland | — | 161 (80/81) |
Women presenting to hospital for SAB (cases) or prenatal ultrasound (controls, gestational age-matched) | In-person interview at hospitalization | — | Live, term births |
| Han, 2012 | Cohort | South Korea | — | 3,507 (254/3,253) |
Women participating in the Korean Motherisk Program | Self-administered questionnaire repeated at each prenatal visit | — | Pregnancies surviving past SAB cutoff |
| Harlap, 1980 | Cohort | USA | 1974–1977 | 32,019 (1,503/30,516) | KPNC members initiating prenatal care before 28 weeks’ gestation | Self-administered questionnaire during prenatal care | <28 | Pregnancies surviving past 28 weeks |
| Kesmodel, 2002 | Cohort | Denmark | 1989–1996 | 24,663 (321/24,342) |
Women initiating prenatal care before 8 weeks’ gestation at participating hospital | Self-administered mailed questionnaire (median GA 14.7 weeks) | ≤28 | Pregnancies surviving past 28 weeks |
| Kline, 1980 | Case-Control | USA | 1974–1978 | 1,248 (616/632) |
Women presenting to hospital for SAB (cases) or for non-SAB pregnancy outcome (controls, age- and hospital-matched) | Interview at pregnancy outcome | — | Pregnancies surviving past 28 weeks |
| Long, 1994 | Case-Control | UK | — | 3,443 (95/3,348) |
Consecutive women presenting with SAB or singleton live births past 28 weeks’ gestation (controls) | Interview at admission for SAB (cases) or at first prenatal clinic visit (controls) | <13 | Live births occurring past 28 weeks |
| Maconochie, 2007 | Case-Control | UK | 1980–2001 | 6,458 (569/5,889) |
Women responding to a postal survey indicating their most recent pregnancy ended in first trimester SAB (cases) or survived past 13 weeks (controls) | Self-administered postal survey in 2001 (pregnancies since 1980 included) | <13 | Pregnancies surviving past 13 weeks |
| Parazzini, 1994 | Case-Control | Italy | 1990–1993 | 1,276 (462/814) |
Women presenting to hospital for SAB (cases) or delivery (controls, hospital-matched) | In-person interview during hospitalization for pregnancy outcome | <13 | Live, term births (normal weight and Apgar score) |
| Paszkowski, 2016 | Cohort | Poland | 2001–2004 | 242 (105/137) |
Women hospitalized for threatened abortion | Self-administered questionnaire | — | Live, term births |
| Rasch, 2003 | Case-Control | Denmark | 1994–1996 | 1,454 (320/1,134) |
Women hospitalized for a D&C for SAB (cases) or women initiating prenatal care and between 6–16 weeks of gestation (controls) | Self-administered questionnaire during hospitalization (cases) or during first prenatal visit (controls) | 6–16 | Pregnancies surviving past 16 weeks |
| Windham, 1992 | Case-Control | USA | 1986–1987 | 1,919 (623/1,296) |
Women presenting to hospital for SAB (cases) or delivery (controls, hospital- and LMP-matched) | CATI after pregnancy outcome | <20 | Live births |
| Windham, 1997 | Cohort | USA | 1990–1991 | 5,142 (500/4,642) | KPNC member initiating prenatal care before 12 weeks’ gestation | Telephone interviews within two weeks of scheduling first prenatal visit | ≤20 | Pregnancies surviving past 20 weeks |
| Xu, 2014 | Case-Control | China | 2009–2012 | 1,860 (620/1,240) |
Women presenting to hospital for SAB (cases) or attending prenatal care past 13 weeks’ gestation (controls, age-matched) | In-person interview within week of loss (cases) or during gestation (controls) | <13 | Pregnancies surviving to 13 weeks |
Abbreviations: KPNC, Kaiser Permanente Northern California; DNBC, Danish National Birth Cohort; D&C, dilation and curettage; LMP, last menstrual period; CATI, computer-assisted telephone interview; — represents missing data.
Reported as gestational age range used to define miscarriage
Twelve of the twenty studies reporting an effect estimate found some level of alcohol exposure was associated with an increased risk of miscarriage (Table S1). Studies varied in methods for assessing alcohol use in pregnancy and measuring risk. Participants in thirteen studies were asked to report the average number of drinks they consumed in a typical week or day, while six studies classified alcohol as a dichotomous exposure. Other studies collected more granular information about alcohol use whether that be daily use reported in a self-administered questionnaire (Buck Louis et al., 2016), daily use in the past two weeks reported at each prenatal visit (Chiodo et al., 2012), or total number and type of drinks consumed since last menstrual period (Avalos et al., 2014).
Risk of bias
Included studies scored between two and eight out of nine on the New Castle Ottawa Scale (higher scores reflecting better study quality; Figure S1). Some deducted quality domains may have been met, but were not counted if the publication lacked sufficient information for scoring. Twelve out of twenty-four studies assessed alcohol exposure after pregnancy outcome. Fifteen out of twenty-four collected information about alcohol exposure through interviews while the remainder used self-administered questionnaires. Out of the fourteen cohorts, six recruited the majority of participants in the first trimester or pre-conception. In eight out of ten case-control studies, cases were recruited when receiving emergency care and controls were recruited at birth. Neither visual inspection of the funnel plot nor Egger’s regression were suggestive of publication bias (Figure S2; Egger’s regression p-value 0.96).
Synthesis of results
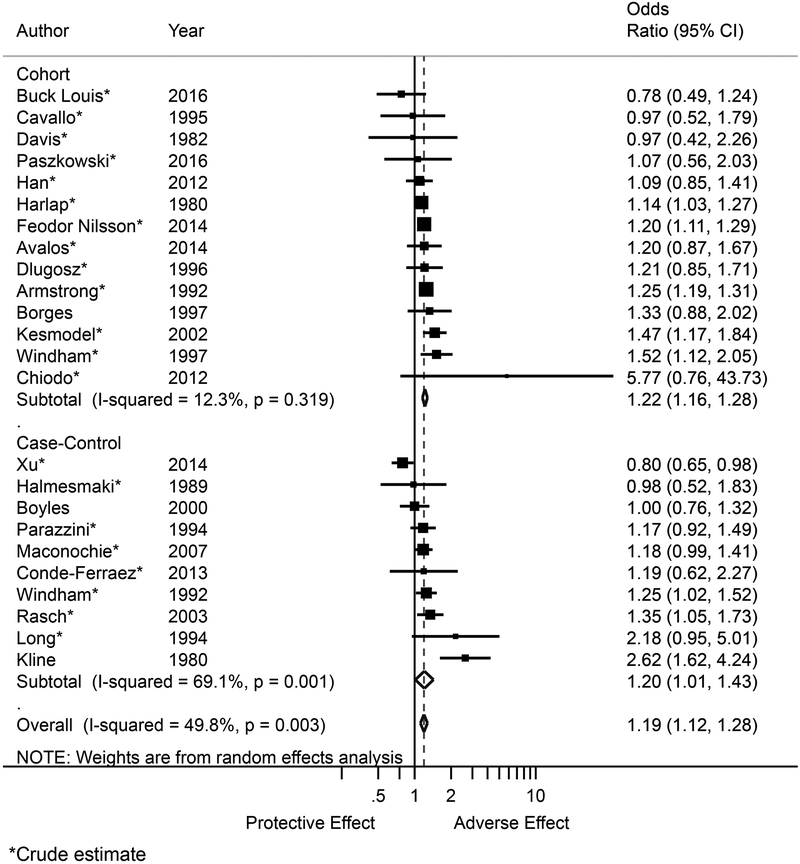
In our meta-analysis of the association between alcohol use and miscarriage, exposed pregnancies where 19% more likely to end in miscarriage (OR 1.19, 95% CI 1.12, 1.28; Figure 2). There was significantly less between-study heterogeneity among cohort studies compared to case control studies (I2: 12.3%, 95% CI 0.0%, 34.7% [low heterogeneity] versus 69.1%, 95% CI 56.8%, 77.9% [ moderately high heterogeneity]). Pooled estimates among cohort and case-control studies were similar (OR 1.22, 95% CI 1.16, 1.28 versus OR 1.20, 95% CI 1.01, 1.43; Table 3). Only three studies reported an adjusted risk estimate for the effect of alcohol operationalized as a dichotomous exposure (exposed/unexposed) (Borges et al., 1997; Boyles et al., 2000; Kline et al., 1980).
Figure 2.
Forest plot for the association between alcohol exposure during pregnancy and risk of miscarriage with subgroup estimates by study design. Size of point estimate markers indicates weight in meta-analysis. Abbreviations: OR, odds ratio; CI, confidence interval.
Table 3.
Association between alcohol use during pregnancy and miscarriage, subgroup analyses
| Analysis | Number of Studies | OR | 95% CI | τ2 |
|---|---|---|---|---|
| All eligible studies | 24 | 1.19 | 1.12, 1.28 | 0.004 |
| Cohort studies | 14 | 1.22 | 1.16, 1.28 | 0.001 |
| Case-control studies | 10 | 1.20 | 1.01, 1.43 | 0.045 |
| Studies only including first trimester miscarriages | 5 | 1.09 | 0.89, 1.33 | 0.033 |
| Studies including all miscarriages | 18 | 1.23 | 1.15, 1.31 | <0.001 |
| Studies with adjusted estimates | 3 | 1.48 | 0.86, 2.53 | 0.185 |
| Studies with majority of participants recruited in the first trimester | 8 | 1.17 | 1.03, 1.33 | 0.009 |
| Studies with equitable recruitment between study groups | 14 | 1.19 | 1.12, 1.27 | 0.001 |
| Studies that assess alcohol use before pregnancy outcome | 11 | 1.20 | 1.11, 1.30 | 0.004 |
Abbreviation: OR, odds ratio; CI, confidence interval.
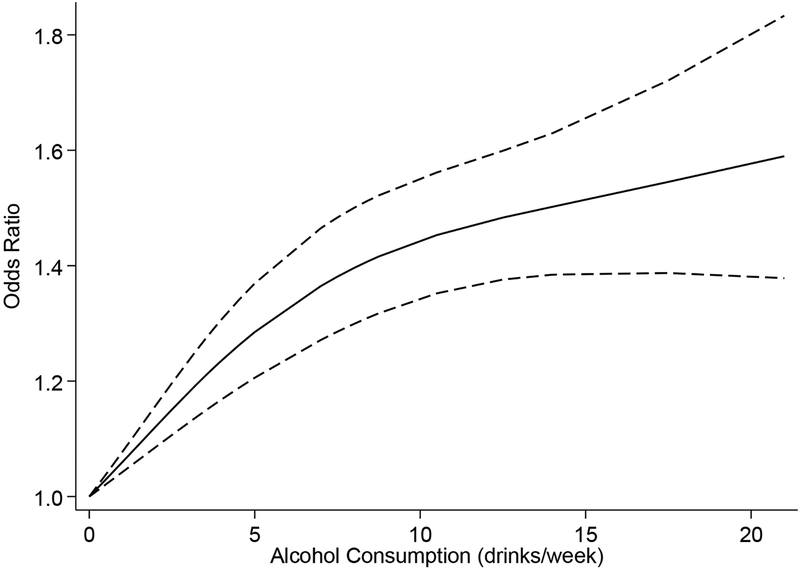
Seventeen studies reported dose-specific effects of alcohol on miscarriage risk. We pooled studies using survival and non-survival estimates separately so only like measures were combined. In the random effects meta-analysis of the twelve studies using non-survival data, there was a dose-dependent relationship between alcohol use and miscarriage (Figure 3 [spline model], Table S2). For alcohol use in pregnancy of five or fewer drinks per week, each additional drink per week was associated with a 6% increase in risk (OR 1.06, 95% CI 1.01, 1.10 [log-linear model]). Estimates were similar when comparing results from cohort and case-control studies and when restricting analysis to studies that fulfilled key risk of bias domains (Table 4). The pooled effect was lower among studies restricted to only first trimester miscarriages when compared to studies that included all miscarriages (OR 1.02, 95% CI 1.00, 1.04 versus OR 1.07, 95% CI 1.012, 1.13). When aggregating the five studies reporting dose-specific effects using survival data, each additional drink per week in pregnancy associated with a 13% increase in miscarriage hazard (HR 1.13, 95% CI 1.04, 1.22). Subgroup analyses by miscarriage definition could not be carried out for survival data estimates due to the limited number of studies.
Figure 3.
Dose-response trend for average number of alcoholic drinks per week during pregnancy and miscarriage risk, spline model. Dashed lines represent the 95% confidence interval, knots selected using Harrell’s recommended percentiles located at 0, 3.5, and 14 drinks per week.
Table 4.
Risk of miscarriage for each additional drink per week in pregnancy from studies not using survival data,a linear model, subgroup analyses
| Analysis | Number of Studies | ORb | 95% CI | τ2 |
|---|---|---|---|---|
| All eligible studiesc | 12 | 1.06 | 1.01, 1.10 | 0.004 |
| Cohort studies | 6 | 1.03 | 1.02, 1.03 | <0.001 |
| Case-control studies | 6 | 1.09 | 0.96, 1.23 | 0.023 |
| Studies only including first trimester miscarriages | 4 | 1.02 | 1.00, 1.04 | <0.001 |
| Studies including all miscarriages | 8 | 1.07 | 1.02, 1.13 | 0.005 |
| Studies with adjusted estimates | 9 | 1.05 | 1.00, 1.11 | 0.005 |
| Studies with majority of participants recruited in the first trimester | 2 | 1.05 | 1.01, 1.10 | <0.001 |
| Studies with equitable recruitment between study groups | 6 | 1.03 | 1.01, 1.04 | <0.001 |
| Studies that assess alcohol use before pregnancy outcome | 5 | 1.03 | 1.01, 1.04 | <0.001 |
Abbreviations: OR, odds ratio; CI, confidence interval
Estimates from survival data evaluated separately
Log-linear estimate valid for alcohol use of five or fewer drinks per week
DISCUSSION
Main findings
In this systematic review of alcohol use during pregnancy and miscarriage, we found exposure is associated with a dose-dependent increase in risk. The most common limitations observed in this literature included imperfect capture of pregnancies ending in miscarriage and oversimplified methods for classifying alcohol use during pregnancy. Public health entities recommend complete abstinence for women who are or could become pregnant (Green et al., 2016; U.S. Depeartment of Helath and Human Services, 2005), yet 8 to 20% of women drink alcohol during pregnancy and more than half are exposed in early gestation (McCormack, 2017; Popova, 2017; Subtances Abuse and Mental Health Services Administration, 2013; Tan, 2015; Tough et al., 2006). Despite the stated limitations, this review of twenty-four studies affirms previous guidance that no amount of alcohol exposure is known to be safe and provides specific information about incremental risk for each additional drink per week consumed.
We aimed to capture literature with data about the relationship between alcohol and miscarriage in this review. A past systematic review described significantly increased risk among women with low-to-moderate alcohol use in five of eight identified studies (Henderson et al., 2007). The present review includes an additional sixteen studies and alcohol use was significantly associated with miscarriage in more than half of reports, though individual effects varied in magnitude. The aggregate risk estimate was attenuated compared with a meta-analysis of three studies (OR 1.35 versus 1.19; total N 3,156 versus 231,808) (Makarechian et al., 1998). Unlike this prior meta-analysis, we required included studies to evaluate miscarriage as an outcome independent of stillbirth and we estimated the dose-response risk-relationship.
Considerations
Since most miscarriages occur in early pregnancy (Avalos et al., 2012), enrolling women soon after pregnancy detection is critical for capturing a representative sample of miscarriages. Six of the fourteen cohort studies in this review either did not recruit most participants within the first trimester or did not report average gestational age at enrollment. This limits the generalizability of findings for very early losses. Recruitment was also limited in case-control studies. Eight of the ten depended upon hospital-based recruitment of miscarriages, which may lead to selection bias since up to 75% of women opt for expectant management of miscarriage and never receive emergency or inpatient care (Luise et al., 2002). Finally, we are unable to comment on the relationship between alcohol and the estimated one in five pregnancies to end prior to detection (Wilcox et al., 1988) since the studies in this meta-analysis only included recognized pregnancies.
Exposure to alcohol was collected through maternal self-report in all studies. Alcohol use during pregnancy is stigmatized and desirability bias, or the tendency to respond in a way viewed favorably by others, may impact reporting (Bailey and Sokol, 2011). Degree of social desirability bias depends on method of data collection and sense of anonymity, with bias being stronger for in-person interviews than self-administered questionnaires (Bowling, 2005; Ernhart et al., 1988). Eight of the included studies assessed alcohol exposure through self-administered questionnaires while others used in-person or telephone interviews. Data collection regarding alcohol use in early pregnancy is logistically difficult and often takes place after miscarriage occurs even in cohort studies, making recall bias a common vulnerability (Bailey and Sokol, 2011; Feldman et al., 1989). Generally, women who experience an adverse pregnancy outcomes are more likely to report exposure (Rockenbauer et al., 2001), but the stigma attached to alcohol use in pregnancy makes the direction of reporting bias difficult to anticipate and may vary from woman to woman (Del Boca and Darkes, 2003). While self-reported is currently the best method for measuring alcohol use, it is important to interpret findings in light of these limitations.
Alcohol use is generally classified as number of drinks consumed per week. This convention does not capture number of drinking episodes per week, episodic dose, or binge drinking. A prior review of moderate alcohol use and binge drinking and pregnancy health found few studies reported on miscarriage risk and those that did reported inconsistent effects (Meyer-Leu et al., 2011). Further investigation of how these factors influence risk of miscarriage is warranted. Methods for determining amount of alcohol consumed did not uniformly account for alcohol content by liquor type and drink size. Both pregnant women and women in the general population tend to overestimate the size of a standard drink (Kaskutas and Graves, 2001; Kerr et al., 2005). On average, alcohol content of a drink as judged by women in the general population is 43% more than a standard drink (Kerr et al., 2005). As a result, dose categories used in the dose-response analysis likely approximate true exposure to varying degrees. Imprecision in alcohol dose assignment would diminish the ability to precisely estimate a dose-response relationship. Additionally, three of the seventeen studies with information about dose-specific effects were not adjusted for potential confounders. Nonetheless, the subgroup analysis of studies with adjusted estimates did not differ from the estimate including all dose-specific effects (OR 1.05 versus 1.06).
Since only two studies reported miscarriage risk by alcohol type, we could not provide a pooled estimate for how this characteristic relates to risk. One study indicated women who drank only spirits during pregnancy had a greater than two-fold risk of miscarriage compared to abstainers, while drinking only wine, only beer, or a combination of alcohol types was not associated with increased miscarriage risk (Avalos et al., 2014). The other study did not detect an association between number of glasses of wine or total alcoholic beverages per week and miscarriage risk (Parazzini et al., 1994).
Timing of alcohol exposure during pregnancy likely plays a critical role in determining risk of miscarriage (Hertz-Picciotto et al., 1996), but there is no consensus on how to leverage this information when measuring risk. More than half of women consume alcohol during pregnancy, but most quit or sharply decrease their consumption upon pregnancy recognition (Day et al., 1993; McCormack et al., 2017; Pryor et al., 2017). While half of the studies in this review assessed whether a change from pre-pregnancy alcohol use occurred, this information was seldomly incorporated into measures of association. Most commonly, alcohol use was classified as consumption after pregnancy recognition, while some studies calculated an across-pregnancy average. These approaches are limited since the first neglects the effect of early alcohol exposure and the second disregards that most use occurs in early gestation and then rapidly tapers after pregnancy detection. One study evaluated risk by week of exposure and demonstrated that consuming three or more beverages in weeks eight through ten of pregnancy conferred the most risk (Windham et al., 1997). Kline and colleagues measured the effect of duration of alcohol use in pregnancy and found that each additional day of exposure increased relative risk of miscarriage by three percent (1981). Five studies included in this review described risk associated with pre-pregnancy alcohol use in a separate analysis, with discordant results. Two additional studies found that peri-conceptional use was not associated with miscarriage (Gaskins et al., 2016) or only associated with risk at very high levels of exposure (greater than ten drinks per week) (Henriksen et al., 2004). Since “pre-pregnancy” alcohol use may persist into early gestation to varying extents, evaluating these behaviors separately likely fails to tell the whole story. Future studies investigating alcohol use before and after a change in consumption occurs and timing of that change could provide more specific information about the ramifications of timing of pregnancy awareness and alcohol use cessation.
CONCLUSION
This review provides evidence that alcohol use during pregnancy increases risk of miscarriage and the relationship is dose-dependent. These findings align with public health guidance that no amount of alcohol during pregnancy is known to be safe. Our results also suggest incremental decreases in alcohol exposure dose may translate to risk reduction. Information about how pattern of alcohol use in early pregnancy influences risk is scarce. Most women reduce or quit consuming alcohol after pregnancy detection and risk likely depends on when in gestation alcohol use occurs. Future studies that prioritize recruitment of participants early in gestation and use more sophisticated methods for incorporating information about pattern of exposure into measures of risk would provide needed insight into how timing of alcohol use in pregnancy relates to miscarriage.
Supplementary Material
Acknowledgements:
The authors have no conflict of interest to declare. Supported by CTSA award (UL1TR000445) from the National Center for Advancing Translational Sciences. Stipend support for ACS was provided in part by the National Institute of General Medical Studies award for the Vanderbilt Medical-Scientist Training Program (T32GM07347) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F30HD094345).
REFERENCES
- Andersen AM, Andersen PK, Olsen J, Gronbaek M, Strandberg-Larsen K (2012) Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol 41:405–413. [DOI] [PubMed] [Google Scholar]
- Armstrong BG, McDonald AD, Sloan M (1992) Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health 82:85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos LA, Kaskutas LA, Block G, Li DK (2009) Do multivitamin supplements modify the relationship between prenatal alcohol intake and miscarriage? Am J Obstet Gynecol 201:563.e561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos LA, Galindo C, Li DK (2012) A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A 94:417–423. [DOI] [PubMed] [Google Scholar]
- Avalos LA, Roberts SC, Kaskutas LA, Block G, Li DK (2014) Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst Use Misuse 49:1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Sokol RJ (2011) Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health 34:86–91. [PMC free article] [PubMed] [Google Scholar]
- Borges G, Tapia-Conyer R, Lopez-Cervantes M, Medina-Mora ME, Pelcastre B, Marina FF (1997) Alcohol consumption and pregnancy in the Mexican National Addiction Survey. Cad Saude Publica 13:205–211. [DOI] [PubMed] [Google Scholar]
- Bowling A (2005) Mode of questionnaire administration can have serious effects on data quality. J Public Health 27:281–291. [DOI] [PubMed] [Google Scholar]
- Boyles SH, Ness RB, Grisso JA, Markovic N, Bromberger J, Cifelli D (2000) Life event stress and the association with spontaneous abortion in gravid women at an urban emergency department. Health Psychol 19:510–514. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sapra KJ, Schisterman EF, Lynch CD, Maisog JM, Grantz KL, Sundaram R (2016) Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: The LIFE Study. Fertil Steril 106:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo F, Russo R, Zotti C, Camerlengo A, Ruggenini AM (1995) Moderate alcohol consumption and spontaneous abortion. Alcohol Alcohol 30:195–201. [PubMed] [Google Scholar]
- Chiodo LM, Bailey BA, Sokol RJ, Janisse J, Delaney-Black V, Hannigan JH (2012) Recognized spontaneous abortion in mid-pregnancy and patterns of pregnancy alcohol use. Alcohol 46:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Ferraez L, Chan May Ade A, Carrillo-Martinez JR, Ayora-Talavera G, Gonzalez-Losa Mdel R (2013) Human papillomavirus infection and spontaneous abortion: A case-control study performed in Mexico. Eur J Obstet Gynecol Reprod Biol 170:468–473. [DOI] [PubMed] [Google Scholar]
- Davis PJM, Partridge JW, Storrs CN (1982) Alcohol consumption in pregnancy: How much is safe? Arch Dis Child 57:940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Cottreau CM, Richardson GA (1993) The epidemiology of alcohol, marijuana, and cocaine use among women of childbearing age and pregnant-women. Clin Obstet Gynecol 36:232–245. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J (2003) The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction 98 Suppl 2:1–12. [DOI] [PubMed] [Google Scholar]
- Dlugosz L, Belanger K, Hellenbrand K, Holford TR, Leaderer B, Bracken MB (1996) Maternal caffeine consumption and spontaneous abortion: A prospective cohort study. Epidemiology 7:250–255. [DOI] [PubMed] [Google Scholar]
- Ernhart CB, Morrow-Tlucak M, Sokol R, Martier S (1988) Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res 12:506–511. [DOI] [PubMed] [Google Scholar]
- Feldman Y, Koren G, Mattice D, Shear H, Pellegrini E, MacLeod SM (1989) Determinants of recall and recall bias in studying drug and chemical exposure in pregnancy. Teratology 40:37–45. [DOI] [PubMed] [Google Scholar]
- Feodor Nilsson S, Andersen PK, Strandberg-Larsen K, Andersen AM (2014) Risk factors for miscarriage from a prevention perspective: A nationwide follow-up study. BJOG 121:1375–1384. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Rich-Edwards JW, Williams PL, Toth TL, Missmer SA, Chavarro JE (2016) Prepregnancy low to moderate alcohol intake is not associated with risk of spontaneous abortion or stillbirth. J Nutr 146:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber MK, Fireman BH (1991) The fetal life table revisited: Spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology 2:33–39. [PubMed] [Google Scholar]
- Green PP, McKnight-Eily LR, Tan CH, Mejia R, Denny CH (2016) Vital signs: Alcohol-exposed pregnancies – United States, 2011–2013. Morb Mortal Wkly Rep:91–97. [DOI] [PubMed] [Google Scholar]
- Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- Halmesmaki E, Valimaki M, Roine R, Ylikahri R, Ylikorkala O (1989) Maternal and paternal alcohol consumption and miscarriage. Br J Obstet Gynaecol 96:188–191. [DOI] [PubMed] [Google Scholar]
- Han JY, Choi JS, Ahn HK, Kim MH, Chung JH, Ryu HM, Kim MY, Yang JH, Nava-Ocampo AA (2012) Foetal and neonatal outcomes in women reporting ingestion of low or very low alcohol intake during pregnancy. J Matern-Fetal Neo M 25:2186–2189. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH (1980) Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet 2:173–176. [DOI] [PubMed] [Google Scholar]
- Harrell FE (2001). Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, USA: Springer. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Gray R, Brocklehurst P (2007) Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG 114:243–252. [DOI] [PubMed] [Google Scholar]
- Henriksen TB, Hjollund NH, Jensen TK, Bonde JP, Andersson AM, Kolstad H, Ernst E, Giwercman A, Skakkebaek NE, Olsen J (2004) Alcohol consumption at the time of conception and spontaneous abortion. Am J Epidemiol 160:661–667. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Pastore LM, Beaumont JJ (1996) Timing and patterns of exposures during pregnancy and their implications for study methods. Am J Epidemiol 143:597–607. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2001) Pre-pregnancy drinking: How drink size affects risk assessment. Addiction 96:1199–1209. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Tujague J, Brown SE (2005) A drink is a drink? Variation in the amount of alcohol contained in beer, wine and spirits drinks in a us methodological sample. Alcohol Clin Exp Res 29:2015–2021. [DOI] [PubMed] [Google Scholar]
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ (2002) Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol Alcohol 37:87–92. [DOI] [PubMed] [Google Scholar]
- Kline J, Shrout P, Stein Z, Susser M, Warburton D (1980) Drinking during pregnancy and spontaneous abortion. Lancet 2:176–180. [DOI] [PubMed] [Google Scholar]
- Kline J, Levin B, Stein Z, Susser M, Warburton D (1981) Epidemioloigc detection of low dose effects on the developing fetus. Enviorn Health Perspect 42: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok IH, Neugebauer R (2007) Psychological morbidity following miscarriage. Best Pract Res Clin Obstet Gynaecol 21:229–247. [DOI] [PubMed] [Google Scholar]
- Long MG, Waterson EJ, MacRae KD, Murray-Lyon IM (1994) Alcohol consumption and the risk of first trimester miscarriage. J Obstet Gynaecol 14:69–70. [Google Scholar]
- Luise C, Jermy K, Collons WP, Bourne TH (2002) Expectant management of incomplete, spontaneous first-trimester miscarriage: Outcome according to initial ultrasound criteria and value of follow-up visits. Ultrasound Obstet Gynecol 19:580–582. [DOI] [PubMed] [Google Scholar]
- Maconochie N, Doyle P, Prior S, Simmons R (2007) Risk factors for first trimester miscarriage: Results from a UK-population-based case-control study. BJOG 114:170–186. [DOI] [PubMed] [Google Scholar]
- Makarechian N, Argo K, Kevlin J, Trepaniere E, Koren G, Einarson TR (1998) Association between moderate alcohol consumption during pregnancy and spontaneous abortion, stillbirth, and premature birth: A meta-analysis. Can J Clin Pharmacol 5:171–176. [Google Scholar]
- McCormack C, Hutchinson D, Burns L, Wilson J, Elliott E, Allsop S, Najman J, Jacobs S, Rossen L, Olsson C, Mattick R (2017) Prenatal alcohol consumption between conception and recognition of pregnancy. Alcohol Clin Exp Res 41:369–378. [DOI] [PubMed] [Google Scholar]
- Meyer-Leu Y, Lemola S, Daeppen JB, Deriaz O, Gerber S (2011). Association of moderate alcohol use and binge drinking during pregnancy with neonatal health. Alcohol Clin Exp Res 35:1669–1677. [DOI] [PubMed] [Google Scholar]
- Nikcevic AV, Tunkel SA, Nicolaides KH (1998) Psychological outcomes following missed abortions and provision of follow-up care. Ultrasound Obstet Gynecol 11:123–128. [DOI] [PubMed] [Google Scholar]
- O’Keeffe LM, Kearney PM, McCarthy FP, Khashan AS, Greene RA, North RA, Poston L, McCowan LME, Baker PN, Dekker GA, Walker JJ, Taylor R, Kenny LC (2015) Prevalence and predictors of alcohol use during pregnancy: Findings from international multicentre cohort studies. BMJ Open 5:e006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6:40–57. [Google Scholar]
- Parazzini F, Tozzi L, Chatenoud L, Restelli S, Luchini L, La Vecchia C (1994) Alcohol and risk of spontaneous abortion. Hum Reprod 9:1950–1953. [DOI] [PubMed] [Google Scholar]
- Paszkowski M, Czuczwar P, Wozniak S, Paszkowska M, Szkodziak P, Patyra K, Paszkowski T (2016) Selected non-somatic risk factors for pregnancy loss in patients with abnormal early pregnancy. Ann Agric Environ Med 23:153–156. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G, Rehm J (2017) Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. JAMA 5:e290–e299. [DOI] [PubMed] [Google Scholar]
- Pryor J, Patrick SW, Sundermann AC, Wu P, Hartmann KE (2017) Pregnancy intention and maternal alcohol consumption. Obstet Gynecol 129:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch V (2003) Cigarette, alcohol, and caffeine consumption: Risk factors for spontaneous abortion. Acta Obstet Gynecol Scand 82:182–188. [DOI] [PubMed] [Google Scholar]
- Rockenbauer M, Olsen J, Czeizel AE, Pedersen L, Sørensen HT, Group TE (2001) Recall bias in a case-control surveillance system on the use of medicine during pregnancy. Epidemiology 12:461–466. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2005) U.S. Surgeon General Releases Advisory on Alcohol Use in Pregnancy U.S. Department of Health and Human Services, Washington, DC: Available at: http://come-over.to/FAS/SurGenAdvisory.htm. Accessed May 15, 2016. [Google Scholar]
- Strandberg-Larsen K, Nielsen NR, Gronbaek M, Andersen PK, Olsen J, Andersen AM (2008) Binge drinking in pregnancy and risk of fetal death. Obstet Gynecol 111:602–609. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2013) Results from the 2012 national survey on drug use and health: Summary of national findings Substance Abuse and Mental Health Services Administration, Rockville, MD: Available at: https://www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf. Accessed May 15, 2016. [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D (2015) Alcohol use and binge drinking among women of childbearing age: United States, 2011–2013. Morb Mortal Wkly Rep 64:1042–1046. [DOI] [PubMed] [Google Scholar]
- Tough S, Tofflemire K, Clarke M, Newburn-Cook C (2006) Do women change their drinking behaviors while trying to conceive? An opportunity for preconception counseling. Clin Med Res 4:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-anlyses. Available at: Ottawa, Canada: Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 5, 2016. [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC (1988) Incidence of early loss of pregnancy New Engl J Med 319:189–194. [DOI] [PubMed] [Google Scholar]
- Windham GC, Fenster L, Swan SH (1992) Moderate maternal and paternal alcohol consumption and the risk of spontaneous abortion. Epidemiology 3:364–370. [DOI] [PubMed] [Google Scholar]
- Windham GC, Von Behren J, Fenster L, Schaefer C, Swan SH (1997) Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology 8:509–514. [DOI] [PubMed] [Google Scholar]
- Xu G, Wu Y, Yang L, Yuan L, Guo H, Zhang F, Guan Y, Yao W (2014) Risk factors for early miscarriage among Chinese: A hospital-based case-control study. Fertil Steril 101:1663–1670. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bracken MB (1996) Tree-based, two-stage risk factor analysis for spontaneous abortion. Am J Epidemiol 144:989–996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.