Abstract
Background:
The identification of an early biomarker for autism spectrum disorder (ASD) would improve the determination of risk, leading to earlier diagnosis and, potentially to earlier intervention and improved outcomes.
Methods:
Data were generated from the Early Markers for Autism (EMA) study, a population-based case-control study of prenatal and neonatal biomarkers of ASD. Newborn bloodspots of children with ASD (N=370), developmental delay (DD, N=140), and general population (GP, N=378) controls were analyzed for 42 different immune markers using a Luminex multiplex platform. Comparisons of immune marker concentrations between groups were examined using logistic regression and Partial Least Squares Discriminant Analysis.
Results:
Children with ASD had significantly increased neonatal levels of IL-6 and IL-8 compared to GP controls. An increase in IL-8 was especially significant in the ASD group with early onset compared to the GP group with an adjusted odds ratio of 1.97 (95%CI 1.39–2.83 p=0.00014). In addition, children with ASD had significantly elevated levels of Eotaxin-1, IFN-γ, and IL-12p70 relative to children with developmental delay (DD). We observed no significant differences in levels of immune markers between the DD and GP groups.
Conclusion:
Elevated levels of some inflammatory markers in newborn bloodspots indicated a higher degree of immune activation at birth in children who were subsequently diagnosed with ASD. The data from this exploratory study suggest that with further expansion, the development of neonatal bloodspot testing for cytokine/chemokine levels might lead to the identification of biomarkers that provide an accurate assessment of ASD risk at birth.
Keywords: Cytokine, Chemokine, neonatal, bloodspot, autism, developmental delay
Introduction:
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by social and communication deficits and repetitive behaviors. ASD is currently estimated to occur in 1–2% of children in the United States (1-4). While a number of genetic risk factors have been identified for ASD, recent evidence has shown that little more than half of the risk for developing ASD can be attributed to genetic mutations, and over 40% of risk is likely due to unknown environmental factors (5, 6). There is evidence that a subset of children with ASD display alterations in immune function including differences in immune cell numbers/function, immunoglobulin levels, and cytokine/chemokine levels relative to typically developing controls (7). While activation of the immune system is classically associated with defense against invading pathogens, there is now evidence that the immune system plays a significant role in neurodevelopment (8) and in the regulation of neural plasticity throughout life (9, 10).
Cytokines and chemokines are cell-signaling molecules used by the immune system to orchestrate the appropriate response to physiological challenges. Most are highly pleiotropic, serving as immune mediators, growth factors, and chemotactic signals for cellular migration during development (7, 11, 12). Differences in circulating cytokine/chemokine levels are amongst the most commonly reported immune abnormalities in individuals already diagnosed with ASD (13, 14). The numerous studies on immune dysregulation in ASD suggest that cytokine/chemokine profiles at birth may be useful biomarkers for predicting risk of ASD (7, 15).
A reliable ASD diagnosis typically not given until at least two years of life (16-18). The most effective treatment currently available is behavioral intervention, and its success depends upon initiating treatment as early as possible (19). Neonatal bloodspots are potentially useful for biomarker discovery. Several large statewide and national programs conducting universal newborn screening for genetic and metabolic disorders collect and store newborn bloodspots. These archives provide researchers the ability to retrospectively analyze newborn samples for children subsequently diagnosed with various developmental outcomes. Several studies, including our own group, have examined newborn bloodspots for cytokine/chemokine differences as potential biomarkers for ASD (20, 21). Inconsistent results between these studies can likely be attributed to small study sample sizes, differences in outcome definition and covariate inclusion, as well as assay sensitivity. In our previous study by Zerbo et al. (20), a significant number of samples had signal levels that fell below the threshold of detection, limiting the interpretability of the results. However, a high sensitivity assay has since been developed recently with an expanded set of cytokines and chemokines for analysis.
The current study was designed to examine whether newborn screening bloodspots cytokine/chemokine levels could provide early markers for ASD risk in a larger cohort of individuals than previously analyzed, utilizing a recently expanded high-sensitivity immunoassay. Moreover, we aimed to examine whether different behavioral subsets within the ASD group are associated with unique cytokine/chemokine profiles in newborn bloodspots.
Methods
Study Population:
Previous publications have described the study population in detail (20, 22). In brief, our samples were obtained from a large population based, nested case-control study, the Early Markers for Autism (EMA) study, which was designed to investigate archived biological samples for markers of exposure and susceptibility to ASD. All study subjects were born between March 2000 and July 2003 in the same 3 counties in Southern California to women who participated in California’s prenatal screening program and for whom both a prenatal maternal blood sample and a newborn bloodspot were available for analysis. The sociodemographic characteristics of the screened women, which represented 70% of all pregnant women, were similar to the characteristics of all women in study counties and birth years. Two behavioral groups - ASD and Developmental Delay without ASD (DD) were initially identified from the California Department of Development Services (DDS) system of 21 regional centers (RC), which coordinates services for persons with ASD and other developmental disabilities. A general population (GP) control group was randomly sampled from the birth certificate files after excluding all past or current DDS/RC clients and frequency matched to ASD cases by sex, birth month, and birth year. This study was approved by the institutional review boards of the California Health and Human Services Agency and Kaiser Permanente of Northern California.
Diagnostic Validation:
For children identified from DDS/RC as ASD or DD, medical record abstractors compiled detailed diagnostic and clinical data from the RC records according to a protocol developed by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (23). All children were between 4.5 and 9 years old at time of record review and abstraction. A developmental pediatrician subsequently performed an expert clinical review of abstracted data to confirm the initial DDS/RC diagnosis. A final study classification of ASD was given if Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria were met. Final classification of DD was based on standardized cognitive and adaptive test scores found in RC records, with composite scores of <70 categorized DD, while all scores ≥70 or some scores <70 and others ≥70 was categorized no DD. Additionally, those classified as ASD were further categorized as “Early Onset” (EO: no statement of loss of social and/or language skills) or “Regressive” (Reg: clear loss of previously acquired language and/or social skills); and also classified according to presence (YesID) or absence (NoID) of intellectual disability. Intellectual disability was defined as developmental/cognitive score and adaptive composite score <70, as for DD. The final study population consisted of 378 GP children, 140 children with DD, and 370 children with ASD that were further broken down into EO/Reg and YesID/NoID subgroups as reported in Table 1.
Table 1:
Classification of Study Subjects
| Behavioral Classification | Number of subjects |
|---|---|
| General Population (GP) | 378 |
| Developmental Delay (DD) | 140 |
| Autism Spectrum Disorder (ASD) | 370 |
| ASD subgroup classifications | |
| Onset type | |
| Early onset | 269 |
| Regressive | 92 |
| Unknown | 9 |
| Intellectual disability | |
| Yes | 163 |
| No | 180 |
| Unknown | 27 |
Specimen Collection:
Capillary blood was collected at birth by heel stick method and spotted onto standardized filter paper for routine newborn screening of various endocrine, metabolic, and genetic disorders. After collection, specimens were transported without temperature control by courier to a regional screening laboratory for testing. Any bloodspots remaining were then catalogued and stored at −20°C by the California Department of Public Health (CDPH). All bloodspots included in this study were collected within 72 hours of birth.
Blood Spot Elution:
Dried bloodspot samples were received as three 3mm punches per subject in a single well of 96-well plates and stored at −80°C until elution. For elution, each sample received 200μl of elution buffer (0.5% BSA in 50ml PBS with 1 tablet of Roche Complete Protease Inhibitor Cocktail; Roche Applied Science, Indianapolis, IN) and was placed on a plate shaker overnight at 4°C. The eluates were then isolated from the filter paper spots and a small 4μl aliquot used for bicinchoninic acid assay (BCA) (Thermo Scientific, Rockford, IL) determination of total protein to normalize cytokine/chemokine levels against blood sample quantity variation.
Immune Marker Measurement:
Immediately following overnight elution, neonatal levels of peripheral blood immune markers were determined using commercially available Luminex multiplex magnetic bead assays. We combined a Bio-Plex Pro Human Chemokine kit (Bio-Rad, Hercules, CA) containing a mix of 40 different immune markers (Supplementary Table 1) with two individual single-plex beads of the same company, interleukin (IL)-12p70 and IL-13. The assay was run according to the manufacturer’s directions. Briefly, 50 μL of bloodspot eluate was incubated with fluorescently-labeled capture antibody-coated beads in a 96-well plate on a plate shaker for one hour at room temperature. After incubation, the sample-bead mix was removed, washed, and biotinylated detection antibodies added for 1 hour at room temperature with shaking. The reaction mixture was detected by the addition of streptavidin-phycoerythrin and incubated on a plate shaker at room temperature for 30 minutes. Following a repeat of the washing step, beads were resuspended in sheath fluid for 5 minutes on the plate shaker. Plates were read on a Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed using Bio-Plex Manager software (Bio-Rad Laboratories) with a five-parameter model used to calculate final concentrations and values (expressed in pg/mL). Reference samples were run on each plate to determine assay consistency, and all samples were run blinded to child developmental outcome.
Statistical Analysis:
Socio-demographic factors were compared across groups using Chi-square tests for categorical variables and ANOVA for continuous variables. All immune markers were normalized for sampling variation in blood collection by dividing total protein content in the eluate as determined by BCA assay. Those that fell below the minimum level of detection (MLD) were assigned a value of MLD/2. Data were then natural log transformed to reduce variance and outlier influence. The range for each analyte and median for each behavioral group, as well as the percentage of samples that fell below the MLD, are reported in Supplementary Table 1. Immune markers that were highly skewed or had more than 40% non-detects were divided into quartiles (supplemental Table 2) and dichotomized as follows: <10% vs. 11–100%; >90% vs. 0–89%. Our previous study on neonatal blood spots indicated that the most prominent risk effects were found in these strata (20). A single cytokine (GM-CSF) was detected in less than 50% of samples and was therefore divided into detect vs non-detect groups for analysis. The majority of analytes were not categorized into quartiles or dichotomized and were analyzed continuously. Odds ratio (OR) represents a 1 SD change from the comparison group.
We performed unadjusted and adjusted logistic regression analyses with the child’s diagnostic group as the outcome of interest and each immune marker as the predictor. We made comparisons between the three main behavioral classifications, ASD vs GP, ASD vs DD, and DD vs GP. In addition, the ASD group was further broken down into those with and without intellectual disability (ASD-YesID and ASD-NoID) and those with Early Onset ASD (ASD-EO) or Regression ASD (ASD-Reg). Comparisons were then made between each of these subgroups vs. GP, and vs. DD.
We ran a separate model for each immune marker and included covariates of interest in the adjusted model that were chosen a priori including: birth type (C-section vs. vaginal), gender, gestational age at birth, birth weight, postnatal age at bloodspot collection, maternal and paternal age, maternal and paternal education, birth season, maternal birth place, child’s birth year, maternal and paternal race, and Bio-Plex plate number. All analyses were run using SPSS version 22 (IBM, New York, NY).
Partial Least Squares Discriminant Analysis (PLS-DA) was performed to examine whether different combinations of multiple cytokines could be used to differentiate between child developmental outcomes (All permutations performed noted in Table 4). Initially, linear regression analysis was performed on each transformed immune marker individually using the co-variates stated above to generate residuals for use in the PLS-DA. Eotaxin-2, ENA-78, GM-CSF, Eotaxin-1, IFN-γ, IL-4, MCP-4, and IL-13 all violated assumptions of linearity in the linear regression model and were therefore excluded from the PLS-DA. The PLS-DA was computed using the web-based Metaboanalyst software in accordance with the protocol by Xia and Wishart (24). Analysis was performed using Leave-One-Out Cross-Validation (LOOCV) and prediction accuracy performance measure for determining the number of latent variables. The permutation statistic was performed using prediction accuracy during training with 2000 permutations.
Table 4:
Permutation statistics for all group interactions by PLS-DA.
| Groups | p-value | |
|---|---|---|
| ASD | vs GP | 0.380 |
| DD | vs GP | 0.990 |
| ASD-NoID | vs GP | 0.470 |
| ASD-YesID | vs GP | 0.847 |
| ASD-EO | vs GP | 0.710 |
| ASD-Reg | vs GP | 0.950 |
| ASD | vs DD | 0.410 |
| ASD-NoID | vs DD | 0.470 |
| ASD-YesID | vs DD | 0.847 |
| ASD-EO | vs DD | 0.390 |
| ASD-Reg | vs DD | 0.780 |
| ASD-YesID | vs ASD-NoID | 0.183 |
| ASD-Reg | vs ASD-EO | 0.423 |
Results:
Population Characteristics:
There were no differences between the GP, DD, and ASD groups with respect to maternal weight, method of delivery (C-section vs. vaginal), gestational age, age at blood collection, or year of birth (Table 2). ASD and GP groups were sex-matched by design to reflect the male bias in ASD, but the DD group was not, resulting in a skew towards females in that group. Birth weight was significantly lower in the DD group than in the ASD and GP groups. With respect to parental demographics, the ASD group was more likely to have parents who were more highly educated, and non-Hispanic when compared to the GP group. In contrast, children in the DD group were more likely to be born in winter, have parents who were younger, less educated, of Hispanic heritage, and mothers who were heavier and born in Mexico when compared to the GP group.
Table 2:
Characteristics of the Early Markers for Autism Study Population
| GP (n=378) |
DD (n=140) |
ASD (n=370) |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ASD vs GP |
ASD vs DD |
DD vs GP |
|
| BirthType | |||||||||
| C-Section | 104 | 27.5% | 40 | 28.6% | 104 | 28.1% | 0.856 | 0.280 | 0.150 |
| Vaginal | 274 | 72.5% | 100 | 71.4% | 266 | 71.9% | |||
| Gender | |||||||||
| Male | 311 | 82.3% | 79 | 56.4% | 303 | 81.9% | 0.891 | 0.000 | 0.000 |
| Female | 67 | 17.7% | 61 | 43.6% | 67 | 18.1% | |||
| Maternal Education | |||||||||
| Less HS Grad | 93 | 24.6% | 57 | 40.7% | 55 | 14.9% | 0.000 | 0.000 | 0.000 |
| HS Grad | 102 | 27.0% | 34 | 24.3% | 75 | 20.3% | |||
| College | 128 | 33.9% | 40 | 28.6% | 168 | 45.4% | |||
| Post-Grad | 55 | 14.6% | 9 | 6.4% | 72 | 19.5% | |||
| Paternal Education | |||||||||
| Less HS Grad | 82 | 21.7% | 52 | 37.1% | 54 | 14.6% | 0.002 | 0.000 | 0.000 |
| HS Grad | 98 | 25.9% | 42 | 30.0% | 71 | 19.2% | |||
| College | 136 | 36.0% | 39 | 27.9% | 166 | 44.9% | |||
| Post-Grad | 62 | 16.4% | 7 | 5.0% | 79 | 21.4% | |||
| Birth Season | |||||||||
| Winter | 70 | 18.5% | 36 | 25.7% | 69 | 18.6% | 0.993 | 0.151 | 0.036 |
| Spring | 119 | 31.5% | 43 | 30.7% | 115 | 31.1% | |||
| Summer | 105 | 27.8% | 32 | 22.9% | 106 | 28.6% | |||
| Fall | 84 | 22.2% | 29 | 20.7% | 80 | 21.6% | |||
| Maternal Birth Place | |||||||||
| US | 186 | 49.2% | 64 | 45.7% | 183 | 49.5% | 0.11 | 0.000 | 0.000 |
| Mexico | 108 | 28.6% | 59 | 42.1% | 85 | 23.0% | |||
| Other | 84 | 22.2% | 17 | 12.1% | 102 | 27.6% | |||
| Birth Year | |||||||||
| 2000 | 73 | 19.3% | 33 | 23.6% | 65 | 17.6% | 0.883 | 0.235 | 0.154 |
| 2001 | 98 | 25.9% | 37 | 26.4% | 92 | 24.9% | |||
| 2002 | 154 | 40.7% | 53 | 37.9% | 158 | 42.7% | |||
| 2003 | 53 | 14.0% | 17 | 12.1% | 55 | 14.9% | |||
| Maternal Ethnicity | |||||||||
| Hispanic | 171 | 45.2% | 96 | 68.6% | 140 | 37.8% | 0.04 | 0.000 | 0.000 |
| Non-Hispanic | 207 | 54.8% | 44 | 31.4% | 230 | 62.2% | |||
| Paternal Ethnicity | |||||||||
| Hispanic | 163 | 43.1% | 96 | 68.6% | 141 | 38.1% | 0.163 | 0.000 | 0.000 |
| Non-Hispanic | 215 | 56.9% | 44 | 31.4% | 229 | 61.9% | |||
| P-value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | ASD vs GP |
ASD vs DD |
DD vs GP |
|
| Gestational Age (Days) |
277.484 | 12.8808 | 275.786 | 14.9444 | 275.535 | 13.6808 | 0.508 | 1.000 | 0.921 |
| Age (hours) at blood spot collection |
31.947 | 11.7752 | 32.957 | 13.9794 | 31.624 | 11.885 | 1.000 | 0.952 | 0.990 |
| Birth Weight (grams) | 3455.352 | 480.4057 | 3283.157 | 543.831 | 3491.03 | 499.4389 | 0.979 | 0.001 | 0.013 |
| Maternal Age (years) | 28.751 | 5.4439 | 26.871 | 6.3221 | 29.905 | 5.6256 | 0.093 | 0.000 | 0.017 |
| Paternal Age | 31.608 | 6.0102 | 30.364 | 7.2452 | 32.762 | 6.0196 | 0.172 | 0.002 | 0.456 |
| Maternal Weight (lbs) | 149.89 | 33.83 | 156.94 | 36.96 | 151.51 | 34.45 | 0.12 | 0.053 | 0.0093 |
ASD: Autism spectrum disorder; DD: Developmental delay; GP: General population. p-values determined by Chi-square and ANOVA
Immune Markers and Diagnosis:
Only three analytes including ENA-78, Eotaxin-2, and GM-CSF had greater than 10% of samples with undetectable levels (Supplementary Table 1) and were thus analyzed as dichotomous variables. Results of all logistic regression analyses for all study group comparisons are reported in Supplementary Table 2. Table 3 displays analytes with statistically significant findings for any study group comparisons.
Table 3:
Significant Adjusted Odds Ratios (95% CI) for Immune Markers in the EMA Study
| ASD vs GP |
ASD- NoID vs GP |
ASD- YesID vs GP |
ASD- EO vs GP |
ASD- Reg vs GP |
ASD vs DD |
ASD- NoID vs DD |
ASD- YesID vs DD |
ASD-EO vs DD |
ASD- Reg vs DD |
ASD- YesID vs ASD- NoID |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-8 | b1.7 (1.2 – 2.4) | a1.6 (1.1 – 2.4) | b1.8 (1.2 – 2.8) | c1.9 (1.3 – 2.8) | |||||||
| IL-6 | a1.4 (1.0 – 2.1) | ||||||||||
| IL-12p70 | a2.2 (1.1 – 4.7) | b2.9 (1.4 – 6.1) | b4.0 (1.6 – 9.5) | a2.4 (1.0 – 5.7) | b3.2 (1.4 – 7.0) | ||||||
| IFN-g Q2 | b2.3 (1.2 – 4.3) | b2.6 (1.3 – 5.2) | a2.2 (1.1 – 4.2) | a2.7 (1.2 – 6.3) | |||||||
| EOTAXIN-1 Q4 | a1.8 (1.1 – 3.1) | b2.2 (1.2 – 4.1) | b2.9 (1.4 – 5.8) | a2.0 (1.0 – 3.8) | b3.0 (1.3 – 6.8) | ||||||
| EOTAXIN-1 Q3 | a2.1 (1.1 – 3.9) | a2.1 (1.0 – 4.3) | a2.1 (1.1 – 4.1) | a2.1 (1.1 – 3.9) | |||||||
| EOTAXIN-1 Q2 | a1.8 (1.0 – 3.1) | a2.3 (1.1 – 4.5) | a0.5 (0.2 – 0.8) | ||||||||
| 6CKINE | a2.1 (1.0 – 4.1) | a1.9 (1.0 – 3.8) | |||||||||
| IL-13 Q4 | a2.1 (1.1 – 4.1) | ||||||||||
| TNF-a >10% | a0.4 (0.1 – 0.9) | ||||||||||
| IL-4 ≥90% | a2.2 (1.2 – 4.2) | ||||||||||
| GCP-2 | a2.2 (1.0 – 4.6) | a3.1 (1.2 – 8.0) | |||||||||
| CTACK | a0.5 (0.2 – 0.9) | ||||||||||
| BCA-1 | a2.8 (1.1 – 7.5) | ||||||||||
| EOTAXIN-3 | a2.4 (1.1 – 5.6) | ||||||||||
| IL-10 | a2.7 (1.1 – 7.0) | ||||||||||
| MIP-1a | 2.3 (1.0 – 5.4) | ||||||||||
| MCP-4 >10% | a0.3 (0.1 – 0.8) |
Significant Adjusted Odds Ratios representing 1 SD change and 95% CI from Supplementary Table 2. For Quartile analyses Q1 was used as the reference for Q2, Q3, and Q4. Above 90% odds ratios (≥90%) use <90% as the reference. Above 10% odds ratios (>10%) use ≤10% as the reference.
0.05> p ≥0.01
0.01> p ≥0.001
p< 0.001
ASD versus GP Controls:
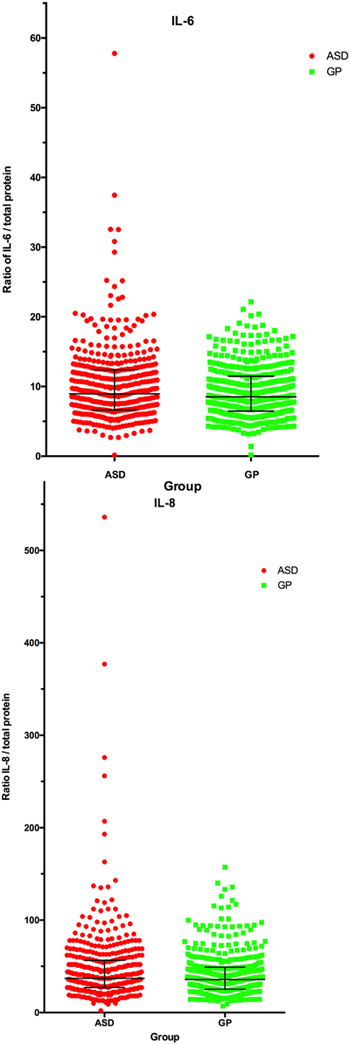
Compared to GP controls, there was increased risk of ASD associated with higher levels of the inflammatory chemokine IL-8 and cytokine IL-6 (Table 3)(Figure 2).
Figure 2:
Representative scatterplot of data from ASD and GP subject for IL-6 (A) and IL-8 (B). Data are presented as a ratio of the cytokine concentration/total protein concentration of the bloodspot eluate. This was done to normalize differences based on total protein content of the eluate.
ASD with and without Intellectual Disability versus GP Controls:
We further divided the ASD group into subgroups based on intellectual disability to determine if cytokines contributed differently to risk of phenotypically distinct populations within the spectrum. The inflammatory chemokine IL-8 was the only chemokine for which higher levels were associated with significantly increased odds of both ASD-NoID and ASD-YesID compared to GP controls. Higher levels of inflammatory cytokine IL-12p70, and chemokines Eotaxin-1 (Q2 and Q4 vs. Q1), and GCP-2 (granulocyte chemotactic protein 2) were associated with increased odds for the ASD-NoID group only, and the highest levels of IL-4 (≥90% vs. <90%) were uniquely associated with ASD-YesID.
ASD with Regression or Early Onset vs GP Controls:
We further examined if there were individual cytokines that contributed to risk of developing either Early Onset or Regressive ASD when compared to GP controls. We found an increased risk associated with elevated IL-8 in the ASD-EO group relative to GP controls (Table 3). The ASD-Reg group was unique as a sub-phenotype within ASD, with an increase in the chemokines BCA-1 (B cell-attracting chemokine 1) and Eotaxin-3, and the regulatory cytokine IL-10 in association with elevated risk relative to the GP controls.
ASD versus DD controls:
When compared to DD controls, increased odds of developing ASD were associated with significantly higher levels of the inflammatory cytokines IL-12p70, IFN-γ (Q2 vs Q1), and the chemokine Eotaxin-1 (Q3 and Q4 vs Q1), but lower levels of TNF-α (≤10% vs >10%) (Table 3).
ASD with and without Intellectual Disability versus DD Controls:
Both the ASD-NoID and ASD-YesID groups demonstrated increased odds corresponding to higher levels of IL-12p70 and Eotaxin-1 (Q3 vs. Q1) relative to DD controls (Table 3). The odds of ASD-NoID were elevated with higher levels of Eotaxin-1 (Q4 and Q2 vs. Q1), 6CKINE (T cell chemoattractant with angiogenic properties), IL-13 (Q4 vs. Q1), and GCP-2 when compared to DD controls. The odds of ASD-YesID were elevated with higher levels of IFN-γ (Q2 vs Q1) and Eotaxin-1 (≥90% vs <90%) when compared to DD controls (Table 3).
ASD with Regression or Early Onset vs DD Controls:
Similar to the ASD group as a whole, we found increased odds of ASD-EO associated with higher levels of IL-12p70, IFN-γ (Q2 vs Q1), Eotaxin-1 (Q4 and Q3 vs Q1), and 6CKINE relative to the DD group (Table 3). The risk of developing ASD-Reg relative to DD is also increased with higher levels of IFN-γ and Eotaxin-1 (Q4 vs. Q1), but unlike ASD-EO, decreased risk with higher levels of MCP-4 (chemoattractant for monocytes and T cells) (>10% vs. <10%) (Table 3).
DD versus GP:
We observed no significant differences in any of the immune marker levels measured for the DD group relative to the GP controls (Supplementary Table 2).
ASD with Intellectual Disability versus ASD without Intellectual Disability:
When considering the ASD-YesID phenotype relative to the ASD-NoID phenotype within the ASD group we found that elevated levels of Eotaxin-1 and CTACK (cutaneous T-cell-attracting chemokine) were associated with lower odds of ASD-YesID compared to ASD-NoID (Table 3).
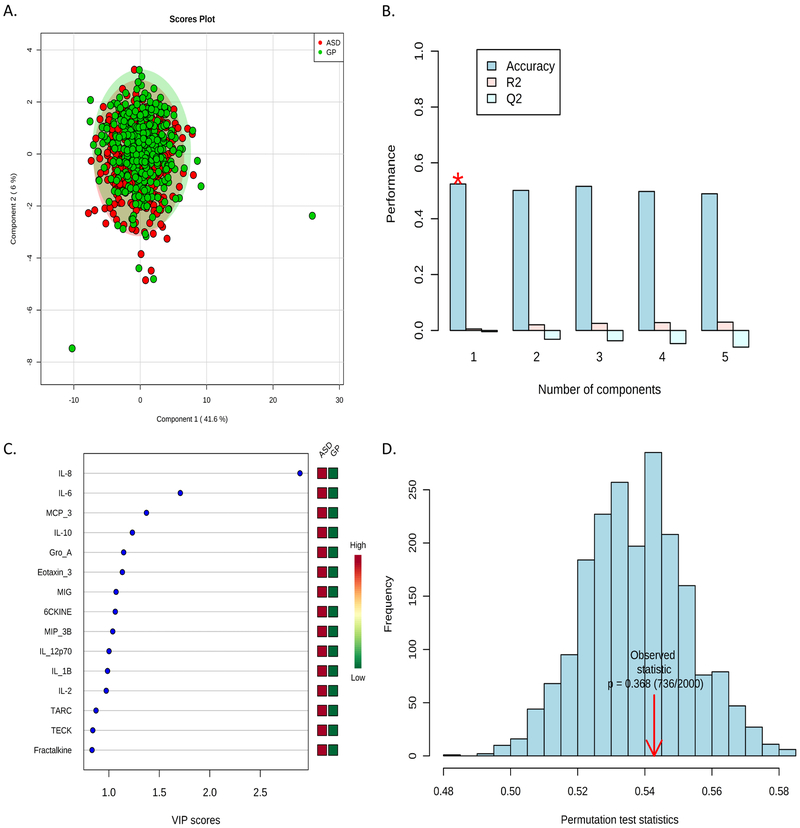
PLS-DA Multivariate Analysis:
Individual immune marker data, independent of diagnosis, were subjected to a linear regression analysis with the same covariates as the logistic regression model above. Residuals generated through this analysis were used as adjusted data points for inclusion in the PLS-DA model. Figure 1 is a representative analysis comparing the GP and ASD groups, showing the individual subjects plotted in component space, the cross-validation method, permutation statistic, and the top 15 Variable Importance in Projection (VIP) scores. The VIP scores are a weighted sum of squares of the PLS loadings that takes into account the amount of Y-variance explained by each component. In Figure 1, when comparing ASD vs GP we find IL-8 and IL-6 as the most important variables, replicating our findings in the logistic regression analysis, with all other group comparisons summarized in Table 4. Using the available immune marker data, we were unable to differentiate between behavioral groups when plotted in component space. Likewise, a significant number of randomizations outperformed the predictive power of our behavioral group labels in the permutation test statistic. These results suggest that while there are individual cytokine and chemokine levels that are significantly different between ASD and GP controls, there were no obvious combinations of cytokine and/or chemokines that could differentiate across case status.
Figure 1:
Representative Multivariate Analysis between ASD and GP groups on Linear Regression Residuals Using PLS-DA to determine combined cytokine profiles. A.) PLS-DA 2D score plot. B.) Bar plots showing the three performance measures using different numbers of components. The red ‘*’ indicates the measure used for this analysis. C.) The top 15 immune markers ranked by VIP score. D.) The results of permutation tests summarized in a histogram with the observed statistic highlighted by red arrow.
Discussion:
The goal of this study was to examine cytokine and chemokine levels in neonatal bloodspots as potential early markers for ASD risk. The high sensitivity assays used herein proved to be an efficient detection method as only three analytes remained at undetectable levels in greater than 10% of the samples, delivering the most extensive analysis of newborn cytokines and chemokines to date. Most notably, we found an increased risk of ASD associated with select markers of immune activation when compared to GP and DD controls, but surprisingly no such associations for the DD group compared to GP controls.
In previous work using a less sensitive assay, Zerbo et al. found increased levels of MCP-1 and decreased levels of RANTES in the newborn bloodspots of ASD cases compared to general population controls (20). In addition, they also found decreased neonatal levels of both MIP-1a and RANTES in DD cases compared to GP controls. In the present study, MCP-1 levels were found to be comparable between all groups, whereas levels of MIP-1a were slightly higher in the ASD-Reg group compared with the GP controls. Unfortunately, RANTES was unavailable for inclusion in the high sensitivity kit used in the present study so no direct comparisons between studies can be made for this chemokine.
In comparison to previous studies on neonatal bloodspots from non-EMA sample populations, this study finds an increased risk of ASD with intellectual disability associated with elevated levels of IL-4 compared to GP controls and IFN-γ when compared to DD controls, which is in direct contradiction to the findings of Abdallah et al. (25) where they noted decreased levels of IFN-γ, IL-4 and IL-10. However, these data are in agreement with a study by Krakowiak et al. (21) which demonstrated an increase in IL-4 in individuals with ASD associated with increased odds of what is referred to as severe ASD (Autism Diagnostic Observation Schedule comparison score ≥7). We find a similar tendency in this study with an increase in IL-4 uniquely associated with greater odds of ASD-YesID, what some would consider a more impaired form of ASD. The inconsistencies in findings between this study and previous studies can likely be attributed to advancements in the Luminex methodology and reagents. In addition, the current study had significantly more samples per group compared to the three previous studies on neonatal blood spots, providing more statistical power to the analysis.
Our findings of increased risk of ASD associated with elevated neonatal levels of the inflammatory cytokines/chemokins IL-8 (ASD compared to GP controls), IL-12p70 and Eotaxin-1(ASD without intellectual disability compared to GP controls), and IFN-γ (ASD (total and YesID) relative to DD controls) are of particular interest. The chemokines IL-8 and Eotaxin-1 are upregulated in response to local innate immune activation thereby recruiting neutrophils and eosinophils that phagocytize debris and aid in tissue remodeling, respectively. The results for IL-8 suggest early differences in immune function between those children with early onset ASD, and those with the regressive form of the disorder. Increased IL-8 in neonatal serum is also associated with other neurological disorders including Cerebral Palsy (26, 27) and seizures induced by hypoxic-ischemic encephalopathy (28). Likewise, children experiencing a traumatic brain injury have dramatically increased IL-8 in the CSF and measurable levels in serum that correlate with unfavorable outcomes (29). Given these numerous associations between IL-8 and damage to the CNS, our data may implicate IL-8 as an indicator of early neuro-immune dysfunction in children with ASD, especially those that experience an early onset of symptoms.
IL-12p70 is an innate immune cytokine that initiates inflammation and drives the development of IFN-γ producing T-cells thereby perpetuating a pro-inflammatory environment. While very little is known about the exact mechanism by which cytokines may influence neurodevelopment and ASD related behavioral outcomes, several other studies have found the presence of pro-inflammatory cytokines to be associated with ASD (7, 12, 30). Previously, circulating levels of IL-6, IL-8, IL-12, and Eotaxin-1 were elevated in children with ASD post-diagnosis between the ages of 2–5 years (31, 32). These related findings suggest that blood levels of these inflammatory cytokines could be a persistent phenotype of the ASD population. Studies are underway to explore this further.
We also performed a multivariate analysis on select immune markers to assess whether combinations of immune markers would be able to predict behavioral outcome. The PLS-DA method was unable to clearly distinguish between behavioral groups. Thus, our results suggest that there is no obvious neonatal cytokine or chemokine combined profile that predicts child developmental outcome.
It would be of further interest to explore the relationship between altered cytokine/chemokine levels in newborn blood spots and the maternal cytokine profile during gestation. A preliminary analysis using the maternal samples linked to our neonatal specimens was limited by the lack of measurement of the same analyte in both maternal and neonatal samples; the 15 cytokines/chemokines that did overlap had no correlation (33). While the initial exploratory analyses were not conclusive, we will continue to address the relationship between the maternal and neonatal cytokine profile in ongoing studies.
Limitations of this study, and the EMA population in general, include the method by which behavioral groups were classified. Subjects from the EMA study did not undergo standardized clinical evaluation by the research team, but rather diagnosis was dependent on expert review of abstracted medical records as part of diagnostic eligibility for developmental services from regional centers. Since we did not have deep phenotypic data on the children in this study population, we examined associations by standard subgroups (onset type, ID status) and did report some differences across subgroup. However, a more refined examination of ASD phenotype in relation to neonatal biological markers is clearly important and could be addressed in other study populations with prospective data collection and longitudinal follow-up of children. Our analyses were limited by the information routinely collected on birth certificates which did not include many potentially relevant environmental factors. However, there is no evidence that these factors are associated with neonatal cytokine/chemokine levels, and as such, they are unlikely to confound the associations we observed. While no consistent IQ test was performed on all children, ID status was determined in a consistent manner based on the cognitive assessment that was performed. In addition, the environmental conditions of bloodspot samples were not controlled during transportation, which might have led to degradation of sample integrity. However, this limitation applies equally to all bloodspots, so while actual levels measured in the samples may have been subjected to this degradation, the relative levels of cytokines and chemokines between individual samples should not be affected. Finally, as newborn bloodspots are taken at a single time point, we are limited in that these data reflect a snapshot of the infant circulating cytokines and chemokines at birth. Future studies that evaluate the child’s immune function over time would be of great interest.
Despite these limitations, our study was strengthened by the use of a highly sensitive assay for immune markers, which resulted in an increase in the number of cytokines and chemokines detected compared to previous studies. Further, our study utilized a significantly larger sample size than previous studies, allowing for the examination of different behavioral subgroups within the ASD population. The findings from this exploratory study require replication and extension in future studies to examine the involvement of immune molecules in the development of ASD in early life.
Supplementary Material
Key Resource Table
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | Roche Complete Protease Inhibitor Cocktail | Roche Applied Science, Indianapolis, IN | Millipore Sigma #11697498001 | |
| Commercial Assay Or Kit | Pierce bicinchoninic acid protein assay (BCA) | Thermo Scientific, Rockford, IL) | #23225 | |
| Bio-Plex Pro Human Chemokine kit | Bio-Rad, Hercules, CA | #171ak99mr2 | ||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | Bio-Plex Manager software | Bio-Rad, Hercules, CA | Comes with the BioPlex200 reader | |
| Transfected Construct | ||||
| Other |
Acknowledgments:
This study was supported by grants 3R01ES016669 from National Institute of Environmental Health Sciences; 5R01MH072565 from the National Institute of Mental Health, and the NICHD funded IDDRC 054 (U54HD079125). Banked specimens were provided by Project Baby’s Breath (M Kharrazi and G DeLorenze, Co-Principal Investigators) under the direction of the California Genetic Disease Screening Program. The views expressed are those of the authors and do not necessarily represent those of the California Department of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. (2009): Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 124:1395–1403. [DOI] [PubMed] [Google Scholar]
- 2.Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention (2012): Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 61:1–19. [PubMed] [Google Scholar]
- 3.Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC (2013): Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. National health statistics reports.1–11, 11 p following 11. [PubMed] [Google Scholar]
- 4.Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. (2011): Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 168:904–912. [DOI] [PubMed] [Google Scholar]
- 5.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. (2014): Most genetic risk for autism resides with common variation. Nat Genet. 46:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014): The familial risk of autism. Jama. 311:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mead J, Ashwood P (2015): Evidence supporting an altered immune response in ASD. Immunol Lett. 163:49–55. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013): Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 33:4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. (2011): The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 477:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziv Y, Schwartz M (2008): Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 22:167–176. [DOI] [PubMed] [Google Scholar]
- 11.Kuban KC, O’Shea TM, Allred EN, Fichorova RN, Heeren T, Paneth N, et al. (2015): The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr Neurol. 52:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu N, Li X, Zhong Y (2015): Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm. 2015:531518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goines PE, Ashwood P (2013): Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 36:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer A, Van de Water J (2017): The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology. 42:284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao EY (2013): Immune dysregulation in autism spectrum disorder. International review of neurobiology. 113:269–302. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- 17.Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. (2000): The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30:205–223. [PubMed] [Google Scholar]
- 18.Le Couteur A, Haden G, Hammal D, McConachie H (2008): Diagnosing autism spectrum disorders in pre-school children using two standardised assessment instruments: the ADI-R and the ADOS. J Autism Dev Disord. 38:362–372. [DOI] [PubMed] [Google Scholar]
- 19.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, et al. (2012): Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 51:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. (2014): Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflammation. 11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, et al. (2017): Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol Psychiatry. 81:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, et al. (2008): Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Res. 1:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C (2003): Prevalence of autism in a US metropolitan area. JAMA. 289:49–55. [DOI] [PubMed] [Google Scholar]
- 24.Xia J, Wishart DS (2011): Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 6:743–760. [DOI] [PubMed] [Google Scholar]
- 25.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. (2012): Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 252:75–82. [DOI] [PubMed] [Google Scholar]
- 26.Carlo WA, McDonald SA, Tyson JE, Stoll BJ, Ehrenkranz RA, Shankaran S, et al. (2011): Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J Pediatr. 159:919–925 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, et al. (2003): Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 53:600–607. [DOI] [PubMed] [Google Scholar]
- 28.Youn YA, Kim SJ, Sung IK, Chung SY, Kim YH, Lee IG (2012): Serial examination of serum IL-8, IL-10 and IL-1Ra levels is significant in neonatal seizures induced by hypoxic-ischaemic encephalopathy. Scand J Immunol. 76:286–293. [DOI] [PubMed] [Google Scholar]
- 29.Lo TY, Jones PA, Minns RA (2010): Combining coma score and serum biomarker levels to predict unfavorable outcome following childhood brain trauma. J Neurotrauma. 27:2139–2145. [DOI] [PubMed] [Google Scholar]
- 30.Onore C, Careaga M, Ashwood P (2012): The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity. 26:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 25:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 232:196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traglia M, Croen LA, Jones KL, Heuer LS, Yolken R, Kharrazi M, et al. (2018): Cross-genetic determination of maternal and neonatal immune mediators during pregnancy. Genome Med. 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.