Abstract
All mammals begin life in social groups, but for some species, social relationships persist and develop throughout the course of an individual’s life. Research in multiple rodent species provides evidence of relatively conserved circuitry underlying social behaviors and processes such as social recognition and memory, social reward, and social approach/avoidance. Species exhibiting different complex social behaviors and social systems (such as social monogamy or familiarity preferences) can be characterized in part by when and how they display specific social behaviors. Prairie and meadow voles are closely related species that exhibit similarly selective peer preferences but different mating systems, aiding direct comparison of the mechanisms underlying affiliative behavior. This chapter draws on research in voles as well as other rodents to explore the mechanisms involved in individual social behavior processes, as well as specific complex social patterns. Contrasts between vole species exemplify how the laboratory study of diverse species improves our understanding of the mechanisms underlying social behavior. We identify several additional rodent species whose interesting social structures and available ecological and behavioral field data make them good candidates for study. New techniques and integration across laboratory and field settings will provide exciting opportunities for future mechanistic work in non-model species.
Keywords: social behavior, group living, sociality, neuroendocrinology, neural circuits, model species, prairie vole, meadow vole
1. Introduction
All mammals exhibit some degree of social behavior, but the extent to which they are social varies widely across species. Social behavior is associated with costs, including increased risk of disease transmission and competition for resources. Nonetheless, benefits from increased predator detection, defense, and, in some species cooperative breeding, can lead to the evolution of sociality (Clutton-Brock & Lukas, 2012; Lukas & Clutton-Brock, 2012a, 2012b, 2013; reviewed in Lee, 1994). Social relationships are best studied in species that display specific traits of interest. For example, social monogamy is rare among rodents (<5%) but has been studied in prairie voles and California mice. Group living has been investigated in meadow voles, naked mole rats, social tuco-tucos, striped mice, and other colonial rodents (reviewed in Anacker & Beery, 2013; Beery, Kamal, Sobrero, & Hayes, 2016; Beery, 2018). In this chapter we discuss mechanisms underlying behaviors supporting life in social groups, and how they may vary between species. We focus on prairie and meadow voles, two closely related species that provide an ideal opportunity to investigate diversity of mechanism and social system.
Despite the advantages of examining species that exhibit particular characteristics of interest, mammalian research is dominated by studies of mice and rats, which in 2009 made up approximately 90% of mammalian physiology studies (Beery & Zucker, 2011 supplementary material). Mice and rats have provided important insights into social behavior—intense focus on a small number of model organisms allows for great depth of study, and the development of of cutting-edge technologies for these species makes them well-situated for mechanistic work. However, both species are gregarious and do not exhibit selective affiliation, making them inappropriate models for studies of adult social bonds and social preferences (Beery, Christensen, Lee, & Blandino, 2018; Schweinfurth et al., 2017). Furthermore, laboratory rodents are often highly inbred and far removed from the ecological contexts in which the social traits of interest evolved (but see Chalfin et al., 2014), making it difficult to determine links between behavior and natural history.
It is also possible that the mechanisms that are relevant for one species may not apply to another species with a different social organization. Even among species with similar social organization, the mechanisms that support sociality may be different (e.g. socially monogamous rodents and socially monogamous non-human primates and humans likely exhibit some key differences in mechanism). By examining the shared and unique basis of behaviors across species, we can hope to effectively determine how and when we can translate research across species, and potentially to humans. Laboratory studies of diverse species, for whom detailed studies of their field ecology and behavior are available, will improve our understanding of the species-specificity and generality of mechanisms supporting different social behaviors (Johnson & Young, 2018; Taborsky et al., 2015).
Although ultimate/evolutionary explanations are not the focus of this chapter, they may inform our understanding of proximate mechanisms of social behavior by suggesting hypotheses concerning whether mechanisms are likely to vary between ecological, phylogenetic, and behavioral contexts. For example, because reward pathways play an essential role in assessing salience of external stimuli such as a potential mate or high-calorie foods, and thereby in motivating appetitive behaviors, we would expect them to be highly conserved across vertebrate taxa (O’Connell & Hofmann, 2011b). Thus, we approach the question of what circuits underlie social behavior with evolution in mind, by considering how species with varying social structures derive different benefits from life in groups. We focus on rodents throughout, as they are the most widely used animal model for many fields including neurobiology, and include discussion of both field and laboratory experiments. We describe the neural circuitry underlying specific social behaviors and processes before synthesizing them into complex social behaviors and social systems. We summarize relevant work in rats and mice in service of a more in-depth discussion of work in voles, as these classic models have significantly informed work in voles. We then highlight some of the most socially distinctive rodents in the wild in order to illustrate potential candidates for future study, as well as describe the natural behaviors of already well-studied animals. The chapter concludes with remarks about new techniques that may help advance comparative work, as well as future directions in the study of neural mechanisms of social behavior.
2. Circuits underlying social behaviors and processes
Complex social behaviors, such as prairie vole pair bonding and rat maternal behavior, rely on specific social behaviors and processes. Here, we discuss social recognition and memory, social reward, and social approach/avoidance. These processes are often interrelated (Figure 1); for example, social recognition is a form of short-term social memory. Social memory is necessary to form long-lasting social relationships, as individuals must recognize and remember their partner. In order for social recognition and social memory to lead to the formation of a social relationship, an individual must exhibit decreased fear and anxiety toward a prospective partner, thereby allowing for social approach rather than avoidance. Social reward may mediate motivation to approach, and may also reinforce social preferences. Although aggression is often thought to directly oppose sociality (and will not be discussed here), aggression can also play an important role in mediating social preferences. For example, if a relationship has been formed that is highly selective and/or motivating, an individual may exhibit aggression toward unfamiliar individuals.
Figure 1.
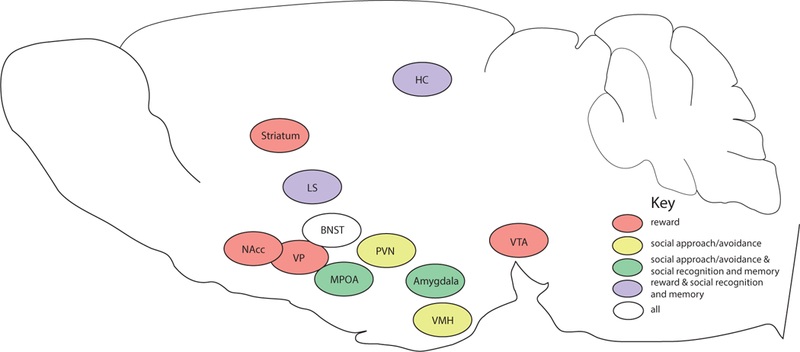
Key brain regions associated with social behavior. Abbreviations: BNST (bed nucleus of the stria terminalis), HC (hippocampus), LS (lateral septum), MPOA (medial preoptic area), NAcc (nucleus accumbens), PVN (paraventricular nucleus of the hypothalamus), VMH (ventromedial nucleus of the hypothalamus), VP (ventral pallidum), VTA (ventral tegmental area). Brain regions explicitly discussed in the text are depicted, as well as: BNST (Bielsky & Young, 2004; Lebow & Chen, 2016; O’Connell & Hofmann, 2011b; Walker, Toufexis, & Davis, 2003), hippocampus (Broadbent, Squire, & Clark, 2004; Brown & Aggleton, 2001; Hölscher, Jacob, & Mallot, 2003; Kogan, Frankland, & Silva, 2000; O’Connell & Hofmann, 2011b), ventral pallidum (Smith, Tindell, Aldridge, & Berridge, 2009), VMH (Colpaert, 1975; Grossman, 1972). The sagittal section was adapted from the mouse brain atlas of Paxinos and Franklin (2012).
2.1. Social recognition and memory
Social recognition and social memory are closely related and are important for life in some social groups, as animals may need to recognize and remember specific individuals in order to assess how to behave toward these individuals (e.g. in cases where strangers need to be quickly identified, or in cases where familiarity is a proxy for kinship recognition and degree of relatedness determines how an individual behaves toward a conspecific). The importance of social recognition and social memory to social structure will be discussed in Section 3.
Social recognition in rodents is measured via behavioral tests such as the habituation/dishabituation test, which measures time spent investigating a conspecific stimulus animal after repeated exposure, followed by a novel animal (reviewed in Ferguson, Young, & Insel, 2002; Gheusi, Bluthé, Goodall, & Dantzer, 1994). Behavioral tests such as these are based on the tendency of rats and mice to investigate unfamiliar individuals more than they would familiar individuals.
Many laboratory studies on social recognition have demonstrated the importance of two neuropeptides: oxytocin and vasopressin. Male vasopressin 1a receptor (V1aR) knockout mice showed impaired social recognition (Bielsky, Hu, Szegda, Westphal, & Young, 2004), and reexpression of V1aR in the lateral septum restored the behavior (Bielsky, Hu, Ren, Terwilliger, & Young, 2005). Similarly, greater V1aR expression and higher vasopressin activity in the lateral septum is associated with better social recognition (Everts & Koolhaas, 1999; Landgraf et al., 1995, 2003) and social investigation in male rats and prairie voles (Ophir, Zheng, Eans, & Phelps, 2009). Male mice with a mutant form of the oxytocin gene did not exhibit social recognition (Ferguson et al., 2000), and oxytocin infusion in the medial amygdala rescued the behavior (Ferguson, Aldag, Insel, & Young, 2001). Studies have mostly focused on males, but evidence suggests that oxytocin and potentially vasopressin are also important for social recognition in females (Clipperton-Allen et al., 2012, but see Bluthe, Shoenen, & Dantzer, 1990). Furthermore, evidence suggests an important interplay with sex steroid hormones (Bluthe, Schoenen, & Dantzer, 1990).
Unsurprisingly, social recognition is mediated through more than just neuropeptide action, involving important connections to and from other systems. Dopaminergic, noradrenergic, and glutamatergic systems have all been implicated in social recognition, as well as muscarinic acetylcholine receptor activation, with neuropeptides likely serving as neuromodulators of neurotransmitter release (Bielsky & Young, 2004; Dluzen, Muraoka, Engelmann, Ebner, & Landgraf, 2000; Ferguson et al., 2002; Winslow & Insel, 2004). The vomeronasal system has also been found to play an essential role in social recognition, with the medial amygdala, and oxytocin in the medial amygdala, mediating relevant information processing in a sex-specific manner (Bergan, Ben-Shaul, & Dulac, 2014; Li & Dulac, 2018; Li et al., 2017; Yao, Bergan, Lanjuin, & Dulac, 2017). Due to the complexity and overlap of the neural circuits underlying all social behaviors and processes (Figure 1), those relevant to social recognition may be difficult to isolate.
Beyond social recognition, some animals maintain long-term social bonds that require social memory. In this chapter, social memory will refer to the set of behaviors routinely cited in the literature as distinct from short-term social recognition, including kin recognition, pair-bond maintenance, selective pregnancy termination, territoriality, and maintenance of stable dominance hierarchies (Gheusi et al., 1994; Winslow & Insel, 2004). For example, pair-bond maintenance in prairie voles persists even after long-term separation (1–4 weeks) from a partner; at reunion, prairie voles show a preference for a partner (DeVries & Carter, 1999; Sun, Smith, Lei, Liu, & Wang, 2014). The dopamine and opioid systems play important roles in mediating the longevity of these mate bonds (Aragona et al. 2006; Resendez, Kuhnmuench, Krzywosinski, & Aragona, 2012). Social memory is also necessary for the maintenance of territoriality and dominance hierarchies, and the agonistic interactions between individuals that are involved in this maintenance are often modulated by social stress (reviewed in Tamashiro, Nguyen, & Sakai, 2005; van der Kooij & Sandi, 2012). Rats and mice in particular have been studied for their ability to spontaneously form stable dominance hierarchies. For example, glucocorticoids (Timmer & Sandi, 2010) and oxytocin receptor density (OTR) in the medial amygdala and lateral septum (Timmer, Cordero, Sevelinges, & Sandi, 2011) play important roles in the formation of dominance hierarchy-related social memory in male rats. Similarly, higher brain gene expression of gonadotropin-releasing hormone (GnRH) in the medial preoptic area (MPOA) of the hypothalamus is associated with opportunity for social ascent in male mice (Williamson, Romeo, & Curley, 2017). Higher corticotropin releasing factor (CRF) mRNA in the medial and central nuclei of the amygdala and the MPOA, and glucocorticoid receptor mRNA in the hippocampus, are associated with higher dominance status (So, Franks, Lim, & Curley, 2015).
2.2. Social reward
In some species, social reward plays an important role in stable social groups by motivating initial social contact and later reinforcing this social contact. The neurobiology of reward has been studied extensively in the context of sexual behavior and appetitive behaviors such as drug addiction (reviewed in Beloate & Coolen, 2017; Young, Gobrogge, & Wang, 2011). Reward has also been well studied in maternal behavior and social play in rodents, especially rats and mice (Trezza, Campolongo, & Vanderschuren, 2011). These studies have focused on dopamine, opioid, and serotonin neurotransmission as targets for manipulation. Furthermore, mounting evidence suggests that reward mechanisms associated with social behavior—specifically, social behavior for which an individual is highly motivated, including parental behavior and pair bonding—are very similar to those associated with sexual behavior and drug addiction. Some have even suggested these kinds of social attachments are themselves addictive (Insel, 2003; Young, Gobrogge, & Wang, 2011). From a fitness perspective, it is not difficult to understand why it would be beneficial for sexual behavior, parental behavior, and pair bonding to be highly rewarding and therefore highly motivating. Drugs of abuse co-opt conserved reward mechanisms to manipulate behavior.
Investigators seeking to illuminate the reward mechanisms associated with social behavior have likewise designed experiments that target the mesolimbic dopamine reward pathway. For example, activation of dopamine D2-type receptors—and concurrent interaction with OTR—is necessary for opposite-sex pair-bond formation in female prairie voles (Liu & Wang, 2003; Wang et al., 1999). Specifically, nucleus accumbens dopamine is critical for pair bonding in both male and female prairie voles (Aragona et al., 2006; Aragona, Liu, Curtis, Stephan, & Wang, 2003; Gingrich, Liu, Cascio, Wang, & Insel, 2000; Liu & Wang, 2003). D1-like activation in the rostral shell of the nucleus accumbens prevented pair-bond formation in male prairie voles, whereas D2-like activation facilitated it (Aragona et al., 2006). Furthermore, upregulation of D1-type receptors in the nucleus accumbens is associated with pair-bond maintenance. These dopamine-manipulated prairie voles show differences in partner preference but not in the number of mating bouts, distinguishing between sexual and social behavior. Consistent with this finding, prairie and meadow voles show similar increases in extracellular dopamine in the striatum after mating (Curtis, Stowe, & Wang, 2003). Thus, the necessity of dopamine in prairie vole pair-bond formation is specific to the partner preference, and not to any effects on mating.
It is likely that the mesolimbic dopamine reward pathway’s role in reinforcing certain social behaviors is conserved across vertebrates (Bruce & Braford, 2009; O’Connell & Hofmann, 2011a). It has also been suggested that significant overlap exists between the reward system and the social behavior network, and that these circuits were present even in early vertebrates (O’Connell & Hofmann, 2011b). However, despite the importance of dopamine in prairie vole pair bonding, blocking it does not impair peer affiliation in female meadow voles (Beery & Zucker, 2010) or prairie voles (Lee et al., unpublished data). Thus, dopamine appears to be more important for selective relationships with mates than with peers.
2.3. Social approach/avoidance
Many animals find novel stimuli, including novel social stimuli, fear- and anxiety-inducing. This fear and anxiety must be countered to shift from an initial avoidance response to social approach and thereby social behavior. For example, nulliparous rats are fearful of pups, avoiding them and sometimes acting aggressively toward them, sometimes to the point of infanticide (Fleming & Anderson, 1987). The onset of maternal behavior involves changes in approach/avoidance, whereby a maternal rat has greater tendency to approach pup stimuli than avoid it (Rosenblatt & Mayer, 1995). The MPOA has been pinpointed as a significant region for this shift, depressing antagonistic neural systems related to avoidance behaviors and activating appetitive neural systems related to approach behaviors (Numan, 2007). Furthermore, maternal memory may involve maternal experience-induced synaptic plasticity within relevant neural circuits, such that pup stimuli can more effectively activate these maternal circuits (Numan & Stolzenberg, 2009). These authors suggest such synaptic plasticity may include downregulation of the female rat’s withdrawal/avoidance system toward pups, so that pup stimuli are less likely to activate avoidance behavior. This approach-avoidance model applies to non-maternal social behavior as well (reviewed in O’Connell & Hofmann, 2011a).
In female meadow voles, a similar shift from avoidance to approach behaviors occurs with seasonal changes in day length. In the summer—or under long day-length conditions in a laboratory setting—meadow voles are aggressive and territorial (Madison, 1980; Madison & McShea, 1987; McShea & Madison, 1984). In the winter—under short day-length conditions—they become more tolerant of conspecifics and live in communal groups. Day-length dependent changes in social behavior in the laboratory are concomitant with changes in brain and peripheral hormone circulation that may facilitate this behavioral shift. For example, both OTR and CRF receptors (1 and 2) vary with day length and with individual huddling in female meadow voles (Beery & Zucker, 2010; Beery, Vahaba, & Grunberg, 2014). Glucocorticoid secretion also varies seasonally, and stress and glucocorticoid exposure alter the formation of both same- and opposite-sex partner preferences in voles (Anacker, Reitz, Goodwin, & Beery, 2016; DeVries, DeVries, Taymans, & Carter, 1996; Pyter, Weil, & Nelson, 2005).
Social approach can be measured in multiple ways: latency to approach a conspecific, amount of social contact with a conspecific, and social preference (preference for a social stimulus over a non-social stimulus). Social approach is not equivalent to affiliative behavior; it may refer only to the tolerance of an individual for a conspecific, or the amount an individual investigates and interacts with a conspecific. Sociability tests in rats and mice have found that oxytocin facilitates social approach and prevents social avoidance in these animals (reviewed in Lukas & Neumann, 2013). Although vasopressin—specifically, AVPR1a antagonist—did not have a clear effect on social approach (Lukas et al., 2011), AVPR1b antagonist reduced social avoidance in mice after social defeat (Litvin, Murakami, & Pfaff, 2011).
Social experience modulates the balance between approach and avoidance. For example, social contact itself may decrease fear and anxiety (reviewed in Hostinar, Sullivan, & Gunnar, 2014). Rats, prairie voles, guinea pigs, mice, California mice, and Siberian hamsters all show social buffering, whereby animals exhibit modulated stress responses with social interaction (reviewed in Beery & Kaufer, 2015). Unsurprisingly, oxytocin and opioids have been implicated in social buffering (reviewed in Kikusui et al., 2006). For example, social buffering in prairie voles is oxytocin-dependent (Burkett et al., 2016)—specifically, it involves oxytocin in the paraventricular nucleus (PVN) of the hypothalamus (Smith & Wang, 2014). Conversely, social isolation in highly social animals may cause fear and anxiety. Disruption of both peer and mate relationships in prairie voles has been used as a model for affective disorders such as depression and anxiety. Social isolation induces behavioral, cardiac, autonomic, and neuroendocrine changes relevant to anxiety and depression in male and female prairie voles (Grippo, Gerena, et al., 2007; Grippo, Cushing, & Carter, 2007; Grippo, Lamb, Carter, & Porges, 2007; Grippo, Wu, Hassan, & Carter, 2008). Pair-bond disruption in prairie voles causes depression-related behaviors and an increase in adrenocorticotropic hormone (ACTH) and corticosterone (McNeal et al., 2014). Changes in CRF are also associated with passive stress-coping after pair-bond disruption (Bosch, Nair, Ahern, Neumann, & Young, 2009), and isolation in juvenile prairie voles (Ruscio, Sweeny, Hazelton, Suppatkul, & Carter, 2007). These studies illustrate the intimate relationship between social approach, social avoidance, and the initiation or loss of a social bond.
3. Studying rodent sociality across social structures
The nature of sociality varies widely across rodents, such that group-living species may differ in group size, composition (e.g. kin, non-relatives, subadults, adult peers, mates), and the role of specific, selective relationships. Many laboratory studies focus on the mechanisms supporting individual social behaviors and processes. In moving from specific behaviors to social structure, rodent groups with inter- and intraspecific variation in sociality are particularly useful for comparison across different social structures. For example, the African mole-rat family is the only mammalian clade to contain eusocial species. Naked mole-rats are the most highly specialized of these species, studied in the lab for mechanisms underlying their social behavior, with a focus on effects of sex and social status (Hathaway, Faykoo-Martinez, Peragine, Mooney, & Holmes, 2016; Mooney, Coen, Holmes, & Beery, 2015; Mooney, Douglas, & Holmes, 2014; Rosen, Vries, Goldman, Goldman, & Forger, 2007), as well as for comparison with solitary Cape mole-rats (Coen, Kalamatianos, Oosthuizen, Poorun, Faulkes, & Bennett, 2015; Kalamatianos et al., 2010). Similarly, South American tuco-tucos are members of a genus consisting of social and non-social species, studied in the lab for oxytocin and vasopressin receptor binding differences (Beery, Lacey, & Francis, 2008). Examination of additional species with diverse social behaviors will aid our understanding of the specificity and generality of mechanisms underlying different aspects of life in groups (see Table 1 for examples of some promising species). The majority of research on mechanisms supporting peer sociality is in voles, which provide an excellent opportunity to examine inter- and intra-specific variation in social behavior.
Table 1.
Natural (field) behavior of a small sampling of well-studied rodents that exhibit diverse and interesting social behaviors.
| Genus/species | Group size/composition | Mating system/social relationships | Home range/dispersal | Other features | References |
|---|---|---|---|---|---|
| Naked mole-rats | Dominant breeding female and up to a few breeding males Closely related conspecifics that help care for young, forage, maintain nest/tunnels, defend against predators Specific caste/body size associated with each worker role Live in large colonies of 200–300 individuals, with a mean group size of ~80 Litters of up to 28 offspring |
Eusociality Monogamy/polyandry Highly inbred Non-breeding adults reproductively suppressed Reproductive suppression reversible and maintained by aggression/threats from dominant female |
Successful dispersal infrequent Subordinate adults usually remain in natal nest |
Arguably most social of all rodents Related species with varying degrees of sociality |
(Faulkes & Bennett, 2007; Jarvis, 1981; Lacey & Sherman, 1991; Nowak, 1999; Sherman et al., 1991) |
| Black-tailed prairie dogs | Live in large colonies (towns) made up of coteries (family groups) Coteries generally consist of 1–2 adult males, 1–6 adult females, and offspring One colony may contain 15–26 coteries |
Polygyny Little or no multiple mating by females, even if other males available Communal nesting with close female relatives Mothers nurse communally after juveniles from different litters begin to share burrows |
Delay in dispersal and reproduction compared to more solitary prairie dog species Coterie territory boundaries well-defined and defended Females generally remain in natal coterie Males disperse to other coteries within the same colony Males and females sometimes disperse outside of colony |
Known for alarm calls, specific to species and approach of predators | (Foltz & Hoogland, 1981; Hoogland, 1982, 1983, 1985, 2007, 2013; Michener & Murie, 1983; Nowak, 1999) |
| Beavers | Pair-bonded male and female breeding pair and their offspring Older family members may live with family group |
Social and sexual monogamy Bi-parental care Long-lasting pair bonds Degree of social interaction inversely related to age |
Territory defense Territory marking (scent mounds) Older family members may be juveniles delaying dispersal |
Construct lodges and dams from mud and sticks | (Busher, 2007; Godin, 1977; Jenkins & Busher, 1979; Nowak, 1999; Wilsson 1971) |
| Belding’s ground squirrels | Female and her offspring | Non-defense polygyny Multiple paternity of litters common (promiscuity rather than true polygyny) Dominance hierarchies determine male reproductive success No mate guarding (clear first male advantage) Mothers and daughters cooperate to defend against infanticide Antipredator warning calls—females display nepotistic behavior |
All males disperse from natal nest Mothers and daughters set up territories near each other |
Most comprehensive data on rodent sociality exists for ground-dwelling squirrels (which include prairie dogs, marmots, chipmunks, and ground squirrels) Kin/social recognition important Long social memory |
(Hanken & Sherman, 1981; Hare & Murie, 2007; Mateo, 2010; Nowak, 1999; Sherman, 1981, 1985; Sherman & Morton, 1984) |
| Prairie voles | Male-female pair and their offspring Shift toward communal groups in fall/winter, but less pronounced change than in meadow voles |
Social but not sexual monogamy Bi-parental care Mate-guarding by males Alloparental care by older siblings Exhibits specific preferences for known peers and mates |
Male-female pairs have overlapping home ranges that they defend | Most well studied of vole species, and extensively studied in the lab | (Carter & Getz, 1993; Getz, McGuire, Pizzuto, Hofmann, & Frase, 1993; Getz, 1962, 1972; Getz & Carter, 1996; Ophir, Phelps, Sorin, & Wolff, 2007; Solomon & Jacquot, 2002; Thomas & Birney, 1979) |
| Meadow voles | Breeding female dyads in spring Solitary in summer Maternal family group, and some adult and subadult males in fall Mixed-lineage social group in winter |
Promiscuous Intolerant toward conspecifics during breeding season Nests in groups and shares territories during non-breeding season Exhibits specific preferences for known peers |
Females defend territories during breeding season Home ranges overlap increasingly through fall/winter Males disperse from natal nest |
Seasonal sociality | (Boonstra, Xia, & Pavone, 1993; Dark, Zucker, & Wade, 1983; Ferkin, 1988; Madison, 1980; Madison, FitzGerald, & McShea, 1984; Madison & McShea, 1987; McShea & Madison, 1984; Turner, Iverson, & Severson, 1983) |
| Mice (Lab) | Large hierarchical group Territorial groups (demes) founded by one male and multiple females |
Social hierarchy Polygyny Interactions between males relatively rare and usually aggressive |
Less complex burrows than rats (often contain a single cavity occupied by a single male) Males territorial Highly variable home ranges (and population densities) based on food availability and other related factors |
One of the most well studied lab animals Many genetic techniques exist for mice that do not exist for most (if not all) other species Spontaneously form stable dominance hierarchies in the lab Environmental richness an important factor in determining features of their group living |
(Bronson, 1979; Lidicker, 1976; Lloyd, 1975; MacKintosh, 1973; Nowak, 1999; Poole & Morgan, 1973, 1976; Schmid-Holmes, Drickamer, Robinson, & Gillie, 2001; Zegeren, 1979) |
| Rats (Lab) | Large hierarchical group (colony) | Social hierarchy, but less absolute than in mice Polygynandry, with all males mating but dominant male mating the most |
Less territorial than mice Highly variable home ranges (and population densities) based on food availability, etc. |
One of the most well studied lab animals Ideal for studying mechanisms of mothering due to well-defined suite of maternal behaviors Also studied for their gregariousness and pro-sociality Environmental richness an important factor in determining features of their group living |
(Berdoy & Drickamer, 2007; Calhoun, 1948, 1962; MacDonald, Mathews, & Berdoy, 1999; McClintock & Anisko, 1982; McClintock, Anisko, & Adler, 1982; Nowak, 1999) |
| California mice | Small, semi-permanent family groups | Social and sexual monogamy Bi-parental care Fathers display all parental behaviors beside nursing as much as mothers |
Mated pairs have largely overlapping home ranges | Studied for their paternal care and winner effect | (Bedford & Hoekstra, 2015; Gubernick & Alberts, 1987; Gubernick & Nordby, 1993; Nowak, 1999; Ribble, 1992; Ribble & Salvioni, 1990) |
| Social tuco-tuco | Social species in a clade with many solitary and a few other social species for comparisons Variation in group size and composition Up to 6 closely related adult females, at most one immigrant adult male, and dependent young |
All females reproduce Communal nesting Male and females contribute to care of young |
2/3 of females remain in natal burrows All males disperse after becoming adults and after each breeding season |
Allonursing observed in lab Cooperative behaviors: digging, alarm calling |
(Lacey, 2004; Lacey, Braude, & Wieczorek, 1997, 1998; Lacey & Wieczorek, 2004; Tammone, Lacey, & Relva, 2013) |
| Degus | Colonial 2–5 females and dependent young Number of adult males unclear (single or multiple) |
All females reproduce Communal nesting Male(s) and females contribute to care of young |
High turnover rate of group members No sex bias in dispersal |
Allonursing observed in lab Cooperative behaviors: digging, alarm calling |
(Ardiles et al., 2013; Colonnello, Iacobucci, Fuchs, Newberry, & Panksepp, 2011; Ebensperger, Chesh, et al., 2011; Ebensperger, Ramírez-Estrada, et al., 2011; Ebensperger, Hurtado, Soto-Gamboa, Lacey, & Chang, 2004; Fulk, 1976) |
| Striped mice | Facultative sociality Social populations: Up to 30 adult individuals (2–4 breeding females, 1 breeding male, adult offspring) |
Social populations: Communal breeding, philopatric young Solitary populations: Males and females only interact to mate |
Social populations: Territorial social groups, males mostly patrol borders Solitary populations: Females inhabit exclusively female home ranges and disperse as juveniles; males inhabit home ranges that overlap with many females |
Social flexibility allows for intraspecific comparison between social and solitary states Reproductive competition in group-living individuals may lead to dispersal and solitary-living High population density may lead to group-living Fitness of each tactic dependent on environment Captive animals show bi-parental care |
(Schoepf & Schradin, 2012; Schradin, 2005, 2006; Schradin et al., 2012; Schradin, König, & Pillay, 2010) |
3.1. Synthesizing social processes to understand selective relationships in voles
Prairie voles and meadow voles are closely related but behaviorally disparate species that have been well studied in both the field and lab. By identifying which social behavior processes are important in specific contexts, such as prairie vole pair bonds and meadow vole peer relationships, we can predict important mechanistic differences in prairie and meadow vole social behavior.
Socially monogamous prairie voles are an increasingly popular rodent model of social behavior, and research on this species has informed our understanding of oxytocin and its importance in selective social bonds. Oxytocin, vasopressin, and dopamine are important molecules in the formation and maintenance of pair bonds in both males and females (Aragona et al., 2003; Cho, DeVries, Williams, & Carter, 1999; Wang et al., 1999). Specifically, oxytocin has been found to be particularly important for females (Insel & Hulihan, 1995; Williams, Carter, & Insel, 1992; Williams, Insel, Harbaugh, & Carter, 1994), and vasopressin for males (Donaldson, Spiegel, & Young, 2010; Lim & Young, 2004; Liu, Curtis, & Wang, 2001; Winslow, Hastings, Carter, Harbaugh, & Insel, 1993). Prairie voles have also been studied for their bi-parental behavior (Thomas & Birney, 1979) and selective same-sex social bonds (Beery et al., 2018; DeVries, Johnson, & Carter, 1997; Lee, Goodwin, Freitas, & Beery, in review).
Sexually promiscuous meadow voles have been studied as contrasts to socially monogamous prairie voles, with some studies assessing whether specific manipulations cause meadow voles to behave more like prairie voles. For example, meadow vole males were successfully manipulated to show more partner preference for an opposite-sex partner using viral vector V1aR gene transfer into the ventral forebrain (Lim et al., 2004). However, a similar experiment using viral vector OXTR gene transfer into the nucleus accumbens of females failed to enhance partner preference for opposite-sex partners (Ross et al., 2009). Meadow voles have also been studied for their ability to form selective same-sex relationships (e.g. Beery, Loo, & Zucker, 2008; Ondrasek et al., 2015; Parker & Lee, 2003). Perhaps unsurprisingly, oxytocin mediates prairie and meadow vole social behavior in different ways. While prairie vole mate bonds rely on oxytocin, oxytocin is not necessary for meadow vole peer relationships, although oxytocin can both enhance and eliminate peer partner preferences, acting in different brain regions (Anacker, Christensen, LaFlamme, Grunberg, & Beery, 2016; Beery & Zucker, 2010). This and other differences in mechanism may be predicted by identifying the social behavior processes relevant to specific complex social behaviors and social systems. For example, the relative importance of social recognition, reward, and social approach shift according to context. Pair bonding, parental care, and group living all provide variations on context that lead to variations in mechanism.
In reproductive pair bonds, prairie voles strongly exhibit all social behaviors and processes discussed previously. Social recognition, as well as social investigation and memory, are essential to pair-bond formation (Young, Young, & Hammock, 2005). Social recognition and decreased fear and anxiety are necessary for voles to approach potential mates and form a pair bond. Paired voles show partner preference even after prolonged separation, indicating long-term social memory. Following pair-bond formation, both sexes display increased aggression toward conspecifics of either sex (Carter & Getz, 1993). Aggression, especially inter-male aggression, allows prairie voles to maintain the exclusivity of their pair bond by mate guarding and defending their territory, and is also important for parental care via defense of young.
Meadow vole peer relationships share several behavioral elements in common with reproductive pair-bonds: they are also selective, enduring, and depend on long term social memory. In contrast to prairie vole pair bonds, meadow vole peer relationships do not appear to be dependent on dopamine signaling (Beery & Zucker, 2010) and do not result in aggression toward extra-pair conspecifics. It is possible that meadow vole peer relationships involve increased tolerance toward all conspecifics rather than increased affiliation toward individuals, and that these bonds are neither as highly motivating nor as highly selective as those of prairie voles. Ongoing work is investigating the role of reward in prairie vole peer relationships, to elucidate whether the mechanisms of prairie vole pair-bond formation and maintenance are specific to the species (prairie vole) or specific to the behavior (pair bonding). Prairie voles can be socially conditioned to show place preference for mates but not long-term cagemates (Goodwin et al., 2018), and preliminary data suggests that prairie voles exhibit greater lever pressing for mates compared to peers (Lopez et al., Beery Lab, unpublished data). It seems likely that prairie vole mate bonds and meadow vole peer relationships are so different because the former are reproductive in nature and the latter are not. Indeed, preliminary data suggests that prairie vole peer relationships, like meadow vole peer relationships, do not rely on the dopaminergic reward pathway (Lee et al., Beery Lab, unpublished data).
This example of two closely related species exhibiting different social behaviors and underlying mechanisms highlights the advantage of the comparative perspective. Understanding affiliation will require study of both peer and mate relationships in multiple species.
4. Sociality in free-living rodents
Rodents are the most diverse, numerous, and widespread of all mammals (Wolff & Sherman, 2007). Sociality is widespread in rodents, with at least 70 documented social species in 39 genera (Lacey & Sherman, 2007). Social rodents are found in a vast range of environments and exhibit distinct social behaviors, even within the same genus. These features make them particularly valuable models for comparative work. Rodents are also favorite lab subjects, due to their generally small size, fast life history, ease of attainability, and availability. Thus, rodents are ideal subjects for understanding social behavior mechanisms in the context of ecological relevance and phylogenetic relatedness. Table 1 summarizes the social behaviors of some of the most socially distinctive rodents in the wild. For example, beavers, prairie voles, and California mice all provide an opportunity to study social monogamy, a rare behavior among mammals. Naked mole-rats and social tuco-tucos are examples of opportunities to study sociality in direct comparison to closely-related non-social species. Although some of these social rodents have been used in laboratory studies of affiliative behavior, the mechanisms of social behavior for many more remain to be elucidated.
The most studied rodents in the lab—rats and lab mice—are not often studied in their natural environments. Special strains of rats and mice have been bred to study specific behaviors or physiological characteristics, and these specific strains do not occur naturally in the wild. While some rodent researchers use wild-caught animals or animals bred from wild-caught as their subjects, or outbreed with wild-caught animals to maintain genetic diversity, these studies are not the norm (but see: research on wild rats, Ibe, Onyeanusi, Hambolu, & Ayo, 2010; Ruan & Zhang, 2016). However, inbred strains of mice and rats have allowed for the development of genetic tools in these animals before any others (e.g. knock-out mice have been used in research for many years). Furthermore, a variety of behavioral tests have been standardized in laboratory rodents, including tests of anxiety-like and depressive-like behavior (open field test, light-dark box test, elevated plus maze, forced swim test), spatial memory (Morris water maze), aggression (resident-intruder test), and affiliation (partner preference test). Although these laboratory techniques have allowed for incredible depth in mechanistic research, breadth is also necessary to inform translatability of mechanistic findings. Thus, much may be gained by increasing efforts to research the neurobiology of species with field data, and to research species with neurobiology data in the field.
5. New techniques and future directions
The studies discussed above use a variety of neural techniques including lesions and stimulation of different brain regions, pharmacological manipulations, and neurochemical measurement. Additional new methods promise to both enhance the specificity of control of particular circuits, and to bring genetic manipulations and measurements to species beyond the laboratory mouse. This will help increase the diversity of laboratory models.
5.1. Genetic techniques
Until recently, lab mice dominated genetic manipulation experiments, with transgenic mice employed extensively to study the roles of specific genes, receptors, and other players. Since the birth of genetic technology, the genomes of many more species have been sequenced, and transgenic prairie voles (Donaldson, Yang, Chan, & Young, 2009), zebra finches (Agate, Scott, Haripal, Lois, & Nottebohm, 2009), sticklebacks (Kingsley et al., 2004), and marmosets (Kishi, Sato, Sasaki, & Okano, 2014; Miller et al., 2016) now exist. CRISPR/Cas9 is one of many new genetic manipulation techniques that is fine-scale, reversible, and can be performed in vivo (in mice, Swiech et al., 2015). It has recently been successfully applied to rhesus macaques (Kang et al., 2015; reviewed in Luo, Li, & Su, 2016; Niu et al., 2014; Wan et al., 2015). This technique is also being developed in prairie and meadow voles, as well as many other species. Zinc finger nucleases (ZFN) and transcription activator-like effector nucleases (TALENs) can also now be used to make precise, targeted genome modifications in model species that include zebrafish, rats, and mice (Gaj, Gersbach, & Barbas, 2013).
However, most organisms still lack fully sequenced genomes. Transcriptome analysis provides a high-throughput, low-cost means of genomic sequencing by which refined differences or changes in gene expression are identified. Because this next-generation sequencing (e.g. RNA-seq) can be used in animals with few to no genomic resources, it is useful for studying non-model systems (Blumstein et al., 2010; Ekblom & Galindo, 2011; Ockendon et al., 2016; Taborsky et al., 2015). De novo transcriptome assembly allows for the identification of novel transcripts and does not require a reference genome. In fact, many new techniques, including optogenetics and chemogenetics, can be applied in both traditional and nontraditional systems and are equally effective in each. Optogenetics and chemogenetics, like CRISPR/Cas9, can activate and suppress neural action at a very fine scale; they are also reversible and can be performed in vivo.
Despite the recent advances in technology that can be applied to non-model species, tools currently available primarily in mice provide a level of specificity and refinement that cannot yet be rivaled. Combined with techniques that can be used in both model and non-model species, such as optogenetics and chemogenetics, these methods have much to contribute to understanding the mechanisms underlying social behavior (e.g. Beloate, Omrani, Adan, Webb, & Coolen, 2016; Burgos-Robles et al., 2017; McHenry et al., 2017).
As new techniques become available for a larger number of species, they should be adopted when possible. We are only now emerging from a bottleneck created by the beginnings of genetic technology, which had been a “genetic revolution” that consisted of sequences and tools for only a few species (Brenowitz & Zakon, 2015). Now that genetic techniques are available for more than just a few species, more comparative, mechanistic work will be made possible.
5.2. New conceptual directions
The idea that no single neurochemical or brain region controls a behavior is now well accepted. For social behaviors, a complex, interconnected circuit is thought to play an important role in diverse social behaviors: the so-called social behavior network (Newman, 1999) or social decision-making network (O’Connell & Hofmann, 2012). A growing movement has recently called for an integrative and comparative approach, arguing for the importance of both an ultimate and proximate perspective, both field and lab work, and the study of non-model organisms across a wide range of taxa (Blumstein et al., 2010; Hofmann et al., 2014; Rubenstein & Hofmann, 2015; Taborsky et al., 2015). Field work alone does not uncover mechanism, and lab work alone does not consider the context of an animal’s behavior—its functional significance in a specific ecological environment, and its evolutionary history. Furthermore, there is such incredible diversity in behavior and the mechanisms that underlie it, even within a single genus, that both basic biological processes and the human brain and disease can only hope to be understood by comparative, wide-ranging research.
While the availability of new technology has proven essential to dissecting functional circuits, it has sometimes promoted a focus on technique rather than question. Many researchers argue neuroscientists have forgotten brains belong to behaving animals—that “neuroscience needs behavior” (Krakauer, Ghazanfar, Gomez-Marin, MacIver, & Poeppel, 2017) and that “nothing in neuroscience makes sense except in light of behavior” (Hofmann, Renn, & Rubenstein, 2016). Researchers may find themselves inundated with vast amounts of genetic data, but it is important to return to context and life history (Kültz et al., 2013).
Ultimately, we must not seek to understand a specific behavior in a specific species alone, but to understand the social brain, how it functions, and how it evolved.
Acknowledgements
The authors were funded by award R15MH113085 from the National Institute of Mental Health of the National Institutes of Health.
References
- Agate RJ, Scott BB, Haripal B, Lois C, & Nottebohm F (2009). Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proceedings of the National Academy of Sciences, 106(42), 17963–17967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, & Beery AK (2013). Life in groups: the roles of oxytocin in mammalian sociality. Frontiers in Behavioral Neuroscience, 7 10.3389/fnbeh.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, & Beery AK (2016). Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology, 68, 156–162. 10.1016/j.psyneuen.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, & Beery AK (2016). Stress impairs new but not established relationships in seasonally social voles. Hormones and Behavior 79, 52–57. doi: 10.1016/j.yhbeh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, & Wang Z (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. The Journal of Neuroscience, 23(8), 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, & Wang Z (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience, 9(1), 133–139. 10.1038/nn1613 [DOI] [PubMed] [Google Scholar]
- Ardiles A, Ewer J, Acosta ML, Kirkwood A, Martinez A, Ebensperger L, … Palacios AG (2013). Octodon degus (Molina 1782): A model in comparative biology and biomedicine. Cold Spring Harbor Protocols, 2013(4), 312–318. 10.1101/pdb.emo071357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford NL, & Hoekstra HE (2015). Peromyscus mice as a model for studying natural variation. eLife, 4, e06813 10.7554/eLife.06813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, & Blandino KL (2018). Specificity in sociality: Mice and prairie voles exhibit different patterns of peer affiliation. Frontiers in Behavioral Neuroscience, 12. doi: 10.3389/fnbeh.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Kamal Y, Sobrero R, & Hayes LD (2016). Comparative neurobiology and genetics of mammalian social behavior. In Sociobiology of caviomorph rodents: An integrated view Hoboken, NJ: Wiley Blackwell. [Google Scholar]
- Beery AK, & Kaufer D (2015). Stress, social behavior, and resilience: Insights from rodents. Neurobiology of Stress, 1, 116–127. 10.1016/j.ynstr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, & Francis DD (2008). Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). The Journal of Comparative Neurology, 507(6), 1847–1859. 10.1002/cne.21638 [DOI] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, & Zucker I (2008). Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Hormones and Behavior, 54(1), 153–159. 10.1016/j.yhbeh.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, & Grunberg DM (2014). Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Hormones and Behavior 66, 779–786. doi: 10.1016/j.yhbeh.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Beery AK, & Zucker I (2010). Oxytocin and same-sex social behavior in female meadow voles. Neuroscience, 169(2), 665–673. 10.1016/j.neuroscience.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Beery AK, & Zucker I (2011). Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews, 35(3), 565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloate LN, & Coolen LM (2017). Influences of social reward experience on behavioral responses to drugs of abuse: Review of shared and divergent neural plasticity mechanisms for sexual reward and drugs of abuse. Neuroscience & Biobehavioral Reviews, 83, 356–372. 10.1016/j.neubiorev.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Beloate LN, Omrani A, Adan RA, Webb IC, & Coolen LM (2016). Ventral tegmental area dopamine cell activation during male rat sexual behavior regulates neuroplasticity and d-amphetamine cross-sensitization following sex abstinence. Journal of Neuroscience, 36(38), 9949–9961. 10.1523/JNEUROSCI.0937-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M, & Drickamer LC (2007). Comparative social organization and life history of Rattus and Mus. In Rodent societies: An ecological & evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Bergan JF, Ben-Shaul Y, & Dulac C (2014). Sex-specific processing of social cues in the medial amygdala. eLife, 3 10.7554/eLife.02743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Ren X, Terwilliger EF, & Young LJ (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron, 47(4), 503–513. 10.1016/j.neuron.2005.06.031 [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Szegda KL, Westphal H, & Young LJ (2004). Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology, 29(3), 483–493. 10.1038/sj.npp.1300360 [DOI] [PubMed] [Google Scholar]
- Bielsky IF, & Young LJ (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides, 25(9), 1565–1574. 10.1016/j.peptides.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Ebensperger LA, Hayes LD, Vásquez RA, Ahern TH, Burger JR, … Young LJ (2010). Toward an integrative understanding of social behavior: New models and new opportunities. Frontiers in Behavioral Neuroscience, 4 10.3389/fnbeh.2010.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe R-M, & Dantzer R (1990). Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Research, 535(2), 301–304. [DOI] [PubMed] [Google Scholar]
- Bluthe R-M, Schoenen J, & Dantzer R (1990). Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Research, 519(1–2), 150–157. 10.1016/0006-8993(90)90073-K [DOI] [PubMed] [Google Scholar]
- Boonstra R, Xia X, & Pavone L (1993). Mating system of the meadow vole, Microtus pennsylvanicus. Behavioral Ecology, 4(1), 83–89. 10.1093/beheco/4.1.83 [DOI] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, & Young LJ (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 34(6), 1406–1415. 10.1038/npp.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, & Zakon HH (2015). Emerging from the bottleneck: Benefits of the comparative approach to modern neuroscience. Trends in Neurosciences, 38(5), 273–278. 10.1016/j.tins.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, & Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences, 101(40), 14515–14520. 10.1073/pnas.0406344101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH (1979). The reproductive ecology of the house mouse. The Quarterly Review of Biology, 54(3), 265–299. [DOI] [PubMed] [Google Scholar]
- Brown MW, & Aggleton JP (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2(1), 51–61. 10.1038/35049064 [DOI] [PubMed] [Google Scholar]
- Bruce LL, & Braford MR (2009). Evolution of the limbic system. In Encyclopedia of Neuroscience, vol 4, ed. Squire LR Oxford: Academic Press. [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, … Tye KM (2017). Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nature Neuroscience, 20(6), 824–835. 10.1038/nn.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal F. B. M. de, & Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science, 351(6271), 375–378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busher P (2007). Social organization and monogamy in the beaver. In Rodent societies: An ecological & evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Calhoun JB (1948). Mortality and movement of brown rats (Rattus norvegicus) in artificially supersaturated populations. The Journal of Wildlife Management, 12(2), 167–172. 10.2307/3796412 [DOI] [Google Scholar]
- Calhoun JB (1962). The ecology and sociology of the Norway rat (US Public Health Service Publication no. 1008) Washington, DC: US Government Printing Office. [Google Scholar]
- Carter CS, & Getz LL (1993). Monogamy and the prairie vole. Scientific American, 268(6), 100–106. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, … De Maeyer E (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science, 268(5218), 1763–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfin L, Dayan M, Levy DR, Austad SN, Miller RA, Iraqi FA, … Kimchi T (2014). Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nature Communications, 5, 4569 10.1038/ncomms5569 [DOI] [PubMed] [Google Scholar]
- Cho MM, Courtney A, Williams JR, & Carter CS (1999). The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 113(5), 1071–1079. 10.1037/0735-7044.113.5.1071 [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Lee AW, Reyes A, Devidze N, Phan A, Pfaff DW, & Choleris E (2012). Oxytocin, vasopressin and estrogen receptor gene expression in relation to social recognition in female mice. Physiology & Behavior, 105(4), 915–924. 10.1016/j.physbeh.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, & Lukas D (2012). The evolution of social philopatry and dispersal in female mammals. Molecular Ecology, 21(3), 472–492. 10.1111/j.1365-294X.2011.05232.x [DOI] [PubMed] [Google Scholar]
- Coen CW, Kalamatianos T, Oosthuizen MK, Poorun R, Faulkes CG, & Bennett NC (2015). Sociality and the telencephalic distribution of corticotrophin-releasing factor, urocortin 3, and binding sites for CRF type 1 and type 2 receptors: A comparative study of eusocial naked mole-rats and solitary Cape mole-rats. Journal of Comparative Neurology, 523(16), 2344–2371. doi: 10.1002/cne.23796. [DOI] [PubMed] [Google Scholar]
- Colonnello V, Iacobucci P, Fuchs T, Newberry RC, & Panksepp J (2011). Octodon degus. A useful animal model for social-affective neuroscience research: Basic description of separation distress, social attachments and play. Neuroscience & Biobehavioral Reviews, 35(9), 1854–1863. 10.1016/j.neubiorev.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Colpaert FC (1975). The ventromedial hypothalamus and the control of avoidance behavior and aggression: Fear hypothesis versus response-suppression theory of limbic system function. Behavioral Biology, 15(1), 27–44. 10.1016/S0091-6773(75)92053-2 [DOI] [PubMed] [Google Scholar]
- Curtis J, Stowe J, & Wang Z (2003). Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience, 118(4), 1165–1173. 10.1016/S0306-4522(03)00032-0 [DOI] [PubMed] [Google Scholar]
- Dark J, Zucker I, & Wade GN (1983). Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 245(3), R334–R338. [DOI] [PubMed] [Google Scholar]
- DeVries AC, & Carter CS (1999). Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Canadian Journal of Zoology, 77(6), 885–889. 10.1139/z99-054 [DOI] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, & Carter CS (1996). The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences 93, 11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, & Carter CS (1997). Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Canadian Journal of Zoology, 75(2), 295–301. 10.1139/z97-037 [DOI] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Ebner K, & Landgraf R (2000). Oxytocin induces preservation of social recognition in male rats by activating α-adrenoceptors of the olfactory bulb. European Journal of Neuroscience, 12(2), 760–766. 10.1046/j.1460-9568.2000.00952.x [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Spiegel L, & Young LJ (2010). Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behavioral Neuroscience, 124(1), 159–163. 10.1037/a0018094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Yang S-H, Chan AWS, & Young LJ (2009). Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biology of Reproduction, 81(6), 1189–1195. 10.1095/biolreprod.109.077529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensperger LA, Chesh AS, Castro RA, Tolhuysen LO, Quirici V, Burger JR, … Hayes LD (2011). Burrow limitations and group living in the communally rearing rodent, Octodon degus. Journal of Mammalogy, 92(1), 21–30. 10.1644/09-MAMM-S-383.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensperger LA, Hurtado MJ, Soto-Gamboa M, Lacey EA, & Chang AT (2004). Communal nesting and kinship in degus (Octodon degus). Naturwissenschaften, 91(8), 391–395. 10.1007/s00114-004-0545-5 [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, Ramírez-Estrada J, León C, Castro RA, Tolhuysen LO, Sobrero R, … Hayes LD (2011). Sociality, glucocorticoids and direct fitness in the communally rearing rodent, Octodon degus. Hormones and Behavior, 60(4), 346–352. 10.1016/j.yhbeh.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Ekblom R, & Galindo J (2011). Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity, 107(1), 1–15. 10.1038/hdy.2010.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts HG, & Koolhaas J (1999). Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behavioural Brain Research, 99(1), 7–16. 10.1016/S0166-4328(98)00004-7 [DOI] [PubMed] [Google Scholar]
- Faulkes CG, & Bennett NC (2007). African mole-rats: Social and ecological diversity. In Rodent societies: An ecological & evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience, 21(20), 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, & Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nature Genetics, 25(3), 284–8. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, & Insel TR (2002). The neuroendocrine basis of social recognition. Frontiers in Neuroendocrinology, 23(2), 200–224. 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferkin MH (1988). The effect of familiarity on social interactions in meadow voles, Microtus pennsylvanicus: A laboratory and field study. Animal Behaviour, 36(6), 1816–1822. 10.1016/S0003-3472(88)80121–0 [DOI] [Google Scholar]
- Fleming AS, & Anderson V (1987). Affect and nurturance: Mechanisms mediating maternal behavior in two female mammals. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 11(2–3), 121–127. 10.1016/0278-5846(87)90049-2 [DOI] [PubMed] [Google Scholar]
- Foltz DW, & Hoogland JL (1981). Analysis of the mating system in the black-tailed prairie dog (Cynomys ludovicianus) by likelihood of paternity. Journal of Mammalogy, 62(4), 706–712. 10.2307/1380592 [DOI] [Google Scholar]
- Fulk GW (1976). Notes on the activity, reproduction, and social behavior of Octodon degus. Journal of Mammalogy, 57(3), 495–505. 10.2307/1379298 [DOI] [Google Scholar]
- Gaj T, Gersbach CA, & Barbas CF (2013). ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology, 31(7), 397–405. 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL (1962). Aggressive behavior of the meadow and prairie voles. Journal of Mammalogy, 43(3), 351–358. 10.2307/1376942 [DOI] [Google Scholar]
- Getz LL (1972). Social structure and aggressive behavior in a population of Microtus pennsylvanicus. Journal of Mammalogy, 53(2), 310–317. 10.2307/1379167 [DOI] [Google Scholar]
- Getz LL, & Carter CS (1996). Prairie-vole partnerships. American Scientist, 84(1), 56–62. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, & Frase B (1993). Social organization of the prairie vole (Microtus ochrogaster). Journal of Mammalogy, 74(1), 44–58. 10.2307/1381904 [DOI] [Google Scholar]
- Gheusi G, Bluthé R-M, Goodall G, & Dantzer R (1994). Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behavioural Processes, 33(1–2), 59–87. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, & Insel TR (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 114(1), 173–183. 10.1037//0735-7044.114.1.173 [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, & Carter CS (2007). Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine, 69(2), 149–157. 10.1097/PSY.0b013e31802f054b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, & Carter CS (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology, 32(8–10), 966–980. 10.1016/j.psyneuen.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, & Porges SW (2007). Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biological Psychiatry, 62(10), 1162–1170. 10.1016/j.biopsych.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, & Carter CS (2008). Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety, 25(6), E17–E26. 10.1002/da.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin AJ (1977). Wild mammals of New England Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Grossman SP (1972). Aggression, avoidance, and reaction to novel environments in female rats with ventromedial hypothalamic lesions. Journal of Comparative and Physiological Psychology, 78(2), 274–283. 10.1037/h0032284 [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, & Alberts JR (1987). The biparental care system of the California mouse, Peromyscus californicus. Journal of Comparative Psychology, 101(2), 169–177. 10.1037//0735-7036.101.2.169 [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, & Nordby JC (1993). Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behavioral Ecology and Sociobiology, 32(3), 211–219. [Google Scholar]
- Hanken J, & Sherman PW (1981). Multiple paternity in Belding’s ground squirrel litters. Science, 212(4492), 351–353. 10.1126/science.7209536 [DOI] [PubMed] [Google Scholar]
- Hare JF, & Murie JO (2007). Ecology, kinship, and ground squirrel sociality: Insights from comparative analyses. In Rodent societies: An ecological & evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Hathaway GA, Faykoo-Martinez M, Peragine DE, Mooney SJ, & Holmes MM (2016). Subcaste differences in neural activation suggest a prosocial role for oxytocin in eusocial naked mole-rats. Hormones and Behavior, 79, 1–7. 10.1016/j.yhbeh.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, … Rubenstein DR (2014). An evolutionary framework for studying mechanisms of social behavior. Trends in Ecology & Evolution, 29(10), 581–589. 10.1016/j.tree.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Renn SCP, & Rubenstein DR (2016). Introduction to symposium: New frontiers in the integrative study of animal behavior: Nothing in neuroscience makes sense except in light of behavior. Integrative and Comparative Biology, 56(6), 1192–1196. 10.1093/icb/icw127 [DOI] [PubMed] [Google Scholar]
- Hölscher C, Jacob W, & Mallot HA (2003). Reward modulates neuronal activity in the hippocampus of the rat. Behavioural Brain Research, 142(1), 181–191. 10.1016/S0166-4328(02)00422-9 [DOI] [PubMed] [Google Scholar]
- Hoogland JL (2007). Alarm calling, multiple mating, and infanticide among Black-tailed, Gunnison’s, and Utah prairie dogs. In Rodent societies: An ecological & evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Hoogland JL (1982). Prairie dogs avoid extreme inbreeding. Science, 215(4540), 1639–1641. 10.1126/science.215.4540.1639 [DOI] [PubMed] [Google Scholar]
- Hoogland JL (1983). Nepotism and alarm calling in the black-tailed prairie dog (Cynomys ludovicianus). Animal Behaviour, 31(2), 472–479. [Google Scholar]
- Hoogland JL (1985). Infanticide in prairie dogs: Lactating females kill offspring of close kin. Science, 230(4729), 1037–1040. 10.1126/science.230.4729.1037 [DOI] [PubMed] [Google Scholar]
- Hoogland JL (2013). Prairie dogs disperse when all close kin have disappeared. Science, 339(6124), 1205–1207. 10.1126/science.1231689 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014) Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibe CS, Onyeanusi BI, Hambolu JO, & Ayo JO (2010). Sexual dimorphism in the whole brain and brainstem morphometry in the African giant pouched rat (Cricetomys gambianus, Waterhouse 1840). Folia Morphologica, 69(2), 69–74. [PubMed] [Google Scholar]
- Insel TR (2003). Is social attachment an addictive disorder? Physiology & Behavior, 79(3), 351–357. 10.1016/S0031-9384(03)00148-3 [DOI] [PubMed] [Google Scholar]
- Insel TR, & Hulihan TJ (1995). A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience, 109(4), 782–789. 10.1037/0735-7044.109.4.782 [DOI] [PubMed] [Google Scholar]
- Jarvis JUM (1981). Eusociality in a mammal: Cooperative breeding in naked mole-rat colonies. Science, 212(4494), 571–573. [DOI] [PubMed] [Google Scholar]
- Jenkins SH, & Busher PE (1979). Castor canadensis. Mammalian species, (120), 1–8.
- Johnson ZV and Young LJ (2018), Evolutionary diversity as a catalyst for biological discovery. Integrative Zoology. Accepted Author Manuscript doi: 10.1111/1749-4877.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamatianos T, Faulkes CG, Oosthuizen MK, Poorun R, Bennett NC, & Coen CW (2010). Telencephalic binding sites for oxytocin and social organization: A comparative study of eusocial naked mole-rats and solitary cape mole-rats. Journal of Comparative Neurology, 518(10), 1792–1813. 10.1002/cne.22302 [DOI] [PubMed] [Google Scholar]
- Kang Y, Zheng B, Shen B, Chen Y, Wang L, Wang J, … Huang X (2015). CRISPR/Cas9-mediated Dax1 knockout in the monkey recapitulates human AHC-HH. Human Molecular Genetics, 24(25), 7255–7264. 10.1093/hmg/ddv425 [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions: Biological Sciences, 361(1476), 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Zhu B, Osoegawa K, De Jong PJ, Schein J, Marra M, … Myers R (2004). New genomic tools for molecular studies of evolutionary change in threespine sticklebacks. Behaviour, 141(11), 1331–1344. [Google Scholar]
- Kishi N, Sato K, Sasaki E, & Okano H (2014). Common marmoset as a new model animal for neuroscience research and genome editing technology. Development, Growth & Differentiation, 56(1), 53–62. 10.1111/dgd.12109 [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, & Silva AJ (2000). Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus, 10(1), 47–56. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, & Poeppel D (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron; Cambridge, 93(3), 480–490. [DOI] [PubMed] [Google Scholar]
- Kültz D, Clayton DF, Robinson GE, Albertson C, Carey HV, Cummings ME, … Todgham AE (2013). New frontiers for organismal biology. BioScience, 63(6), 464–471. 10.1525/bio.2013.63.6.8 [DOI] [Google Scholar]
- Lacey EA (2004). Sociality reduces individual direct fitness in a communally breeding rodent, the colonial tuco-tuco (Ctenomys sociabilis). Behavioral Ecology and Sociobiology, 56(5), 449–457. [Google Scholar]
- Lacey EA, Braude SH, & Wieczorek JR (1997). Burrow sharing by colonial tuco-tucos (Ctenomys sociabilis). Journal of Mammalogy, 78(2), 556–562. [Google Scholar]
- Lacey EA, Braude SH, & Wieczorek JR (1998). Solitary burrow use by adult Patagonian tuco-tucos (Ctenomys haigi). Journal of Mammalogy, 79(3), 986–991. [Google Scholar]
- Lacey EA, & Sherman PW (1991). Social organization of naked mole-rat colonies: evidence for divisions of labor. The biology of the naked mole-rat, 275, 336. [Google Scholar]
- Lacey EA, & Sherman PW (2007). The ecology of sociality in rodents. In Rodent societies: An ecological and evolutionary perspective, ed. Wolff JO, & Sherman PW Chicago, IL: The University of Chicago Press. [Google Scholar]
- Lacey EA, & Wieczorek JR (2004). Kinship in colonial tuco-tucos: Evidence from group composition and population structure. Behavioral Ecology, 15(6), 988–996. 10.1093/beheco/arh104 [DOI] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, … Young LJ (2003). Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. European Journal of Neuroscience, 18(2), 403–411. 10.1046/j.1460-9568.2003.02750.x [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, & Engelmann M (1995). V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. Journal of Neuroscience, 15(6), 4250–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC (1994). Social structure and evolution. In Behaviour and Evolution, ed. Slater PJB, & Halliday TR Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lidicker WZ (1976). Social behaviour and density regulation in house mice living in large enclosures. Journal of Animal Ecology, 45(3), 677–697. 10.2307/3575 [DOI] [Google Scholar]
- Li Y, & Dulac C (2018). Neural coding of sex-specific social information in the mouse brain. Current Opinion in Neurobiology, 53, 120–130. 10.1016/j.conb.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, … Dulac C (2017). Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell, 171(5), 1176–1190.e17. 10.1016/j.cell.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M, & Young L (2004). Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience, 125(1), 35–45. 10.1016/j.neuroscience.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, & Young LJ (2004). Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature, 429(6993), 754–757. 10.1038/nature02539 [DOI] [PubMed] [Google Scholar]
- Litvin Y, Murakami G, & Pfaff DW (2011). Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiology & Behavior, 103(3–4), 393–403. 10.1016/j.physbeh.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, & Wang Z (2001). Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 115(4), 910–919. 10.1037//0735-7044.115.4.910 [DOI] [PubMed] [Google Scholar]
- Liu Y, & Wang Z (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience, 121(3), 537–544. 10.1016/S0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Lloyd JA (1975). Social structure and reproduction in two freely growing populations of house mice (Mus musculus L.). Animal Behaviour, 23, 413–424. 10.1016/0003-3472(75)90089-5 [DOI] [Google Scholar]
- Lukas D, & Clutton-Brock T (2012a). Cooperative breeding and monogamy in mammalian societies. Proceedings of the Royal Society B: Biological Sciences, 279(1736), 2151–2156. 10.1098/rspb.2011.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, & Clutton-Brock T (2012b). Life histories and the evolution of cooperative breeding in mammals. Proceedings of the Royal Society B: Biological Sciences, 279(1744), 4065–4070. 10.1098/rspb.2012.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, & Clutton-Brock TH (2013). The evolution of social monogamy in mammals. Science, 341(6145), 526–530. 10.1126/science.1238677 [DOI] [PubMed] [Google Scholar]
- Lukas M, & Neumann ID (2013). Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behavioural Brain Research, 251, 85–94. 10.1016/j.bbr.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, & Neumann ID (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology, 36(11), 2159–2168. 10.1038/npp.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Li M, & Su B (2016). Application of the genome editing tool CRISPR/Cas9 in non-human primates. Zoological Research, 37(4), 241–219. 10.13918/j.issn.2095-8137.2016.4.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald DW, Mathews F, & Berdoy M (1999). The behaviour and ecology of Rattus norvegicus: From opportunism to kamikaze tendencies Singleton, G; Hinds, L.; Leirs, H, 49–80. [Google Scholar]
- MacKintosh JH (1973). Factors affecting the recognition of territory boundaries by mice (Mus musculus). Animal Behaviour, 21(3), 464–470. 10.1016/S0003-3472(73)80006-5 [DOI] [Google Scholar]
- Madison DM (1980). Space use and social structure in meadow voles, Microtus pennsylvanicus. Behavioral Ecology and Sociobiology, 7(1), 65–71. [Google Scholar]
- Madison DM, FitzGerald RW, & McShea WJ (1984). Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): Possible consequences for population cycling. Behavioral Ecology and Sociobiology, 15(1), 9–17. [Google Scholar]
- Madison DM, & McShea WJ (1987). Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: A microtine model. American Zoologist, 27(3), 899–908. 10.1093/icb/27.3.899 [DOI] [Google Scholar]
- Mateo JM (2010). Self-referent phenotype matching and long-term maintenance of kin recognition. Animal Behaviour, 80(5), 929–935. 10.1016/j.anbehav.2010.08.019 [DOI] [Google Scholar]
- McClintock MK, & Anisko JJ (1982). Group mating among Norway rats I. Sex differences in the pattern and neuroendocrine consequences of copulation. Animal Behaviour, 30(2), 398–409. 10.1016/S0003-3472(82)80051-1 [DOI] [Google Scholar]
- McClintock MK, Anisko JJ, & Adler NT (1982). Group mating among Norway rats II. The social dynamics of copulation: Competition, cooperation, and mate choice. Animal Behaviour, 30(2), 410–425. 10.1016/S0003-3472(82)80052-3 [DOI] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, … Stuber GD (2017). Hormonal gain control of a medial preoptic area social reward circuit. Nature Neuroscience, 20(3), 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, … Grippo AJ (2014). Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience : Basic & Clinical, 180, 9–16. 10.1016/j.autneu.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea WJ, & Madison DM (1984). Communal nesting between reproductively active females in a spring population of Microtus pennsylvanicus. Canadian Journal of Zoology, 62(3), 344–346. [Google Scholar]
- Michener GR, & Murie JO (1983). Black-tailed prairie dog coteries: Are they cooperatively breeding units? The American Naturalist, 121(2), 266–274. [Google Scholar]
- Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, & Wang X (2016). Marmosets: A neuroscientific model of human social behavior. Neuron, 90(2), 219–233. 10.1016/j.neuron.2016.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SJ, Coen CW, Holmes MM, & Beery AK (2015). Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience, 303, 261–269. 10.1016/j.neuroscience.2015.06.043 [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Douglas NR, & Holmes MM (2014). Peripheral administration of oxytocin increases social affiliation in the naked mole-rat (Heterocephalus glaber). Hormones and Behavior, 65(4), 380–385. 10.1016/j.yhbeh.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Nelson RJ (1997). The use of genetic “knockout” mice in behavioral endocrinology research. Hormones and Behavior, 31(3), 188–196. [DOI] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Annals of the New York Academy of Sciences, 877(1), 242–257. 10.1111/j.1749-6632.1999.tb09271.x [DOI] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, … Sha J (2014). Generation of gene-modified Cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell, 156(4), 836–843. 10.1016/j.cell.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Nowak RM (1999). Walker’s Mammals of the World (6th ed.). Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Numan M (2007). Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology, 49(1), 12–21. 10.1002/dev.20198 [DOI] [PubMed] [Google Scholar]
- Numan M, & Stolzenberg DS (2009). Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology, 30(1), 46–64. 10.1016/j.yfrne.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Ockendon NF, O’Connell LA, Bush SJ, Monzón-Sandoval J, Barnes H, Székely T, … Urrutia AO (2016). Optimization of next-generation sequencing transcriptome annotation for species lacking sequenced genomes. Molecular Ecology Resources, 16(2), 446–458. 10.1111/1755-0998.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011a). Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Frontiers in Neuroendocrinology, 32(3), 320–335. 10.1016/j.yfrne.2010.12.004 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011b). The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology, 519(18), 3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science, 336(6085), 1154–1157. 10.1126/science.1218889 [DOI] [PubMed] [Google Scholar]
- Ondrasek NR, Wade A, Burkhard T, Hsu K, Nguyen T, Post J, & Zucker I (2015). Environmental modulation of same-sex affiliative behavior in female meadow voles (Microtus pennsylvanicus). Physiology & Behavior, 140, 118–126. 10.1016/j.physbeh.2014.12.021 [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, & Wolff JO (2007). Morphological, genetic, and behavioral comparisons of two prairie vole populations in the field and laboratory. Journal of Mammalogy, 88(4), 989–999. 10.1644/06-MAMM-A-250R.1 [DOI] [Google Scholar]
- Ophir AG, Zheng D-J, Eans S, & Phelps SM (2009). Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 123(5), 979. [DOI] [PubMed] [Google Scholar]
- Parker KJ, & Lee TM (2003). Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. Journal of Comparative Psychology, 117(3), 283–289. 10.1037/0735-7036.117.3.283 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Franklin KB (2012). The mouse brain in stereotaxic coordinates Elsevier Academic Press. [Google Scholar]
- Poole TB, & Morgan HDR (1973). Differences in aggressive behaviour between male mice (Mus musculus L.) in colonies of different sizes. Animal Behaviour, 21(4), 788–795. 10.1016/S0003-3472(73)80105-8 [DOI] [PubMed] [Google Scholar]
- Poole TB, & Morgan HDR (1976). Social and territorial behaviour of laboratory mice (Mus musculus L.) in small complex areas. Animal Behaviour, 24(2), 476–480. 10.1016/S0003-3472(76)80056-5 [DOI] [Google Scholar]
- Pyter LM, Weil ZM, & Nelson RJ (2005). Latitude affects photoperiod-induced changes in immune response in meadow voles (Microtus pennsylvanicus ). Canadian Journal of Zoology 83, 1271–1278. doi: 10.1139/z05-121. [DOI] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, & Aragona BJ (2012). κ-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. Journal of Neuroscience, 32(20), 6771–6784. 10.1523/JNEUROSCI.5779-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble DO (1992). Dispersal in a monogamous rodent, Peromyscus californicus. Ecology, 73(3), 859–866. [Google Scholar]
- Ribble DO, & Salvioni M (1990). Social organization and nest co-occupancy in Peromyscus californicus, a monogamous rodent. Behavioral Ecology and Sociobiology, 26(1), 9–15. 10.1007/BF00174020 [DOI] [Google Scholar]
- Rosen GJ, Vries GJD, Goldman SL, Goldman BD, & Forger NG (2007). Distribution of vasopressin in the brain of the eusocial naked mole-rat. Journal of Comparative Neurology, 500(6), 1093–1105. 10.1002/cne.21215 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, & Mayer AD (1995). An analysis of approach/withdrawal processes in the initiation of maternal behavior in the laboratory rat. In Research in developmental and comparative psychology, Vol. 1 Behavioral development: Concepts of approach/withdrawal and integrative levels (pp. 177–230), ed. Hood KE, Greenberg G, & Tobach E. New York, NY: Garland Publishing. [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, & Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience, 29:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C, & Zhang Z (2016). Laboratory domestication changed the expression patterns of oxytocin and vasopressin in brains of rats and mice. Anatomical Science International, 91(4), 358–370. 10.1007/s12565-015-0311-0 [DOI] [PubMed] [Google Scholar]
- Rubenstein DR, & Hofmann HA (2015). Editorial overview: The integrative study of animal behavior. Current Opinion in Behavioral Sciences, 6, v–viii. 10.1016/j.cobeha.2015.11.009 [DOI] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, & Carter CS (2007). Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior, 51(1), 54–61. 10.1016/j.yhbeh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Schmid-Holmes S, Drickamer LC, Robinson AS, & Gillie LL (2001). Burrows and burrow-cleaning behavior of house mice (Mus musculus domesticus). The American Midland Naturalist; Notre Dame, 146(1), 53–62. [Google Scholar]
- Schoepf I, & Schradin C (2012). Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). Journal of Animal Ecology, 81(3), 649–656. 10.1111/j.1365-2656.2011.01939.x [DOI] [PubMed] [Google Scholar]
- Schradin C (2005). When to live alone and when to live in groups: Ecological determinants of sociality in the African striped mouse (Rhabdomys pumilio, Sparrman, 1784). Belgian Journal of Zoology, 135(Suppl. 1), 77–82. [Google Scholar]
- Schradin C (2006). Whole-day follows of striped mice (Rhabdomys pumilio), a diurnal murid rodent. Journal of Ethology, 24(1), 37–43. 10.1007/s10164-005-0158-2 [DOI] [Google Scholar]
- Schradin C, König B, & Pillay N (2010). Reproductive competition favours solitary living while ecological constraints impose group-living in African striped mice. Journal of Animal Ecology, 79(3), 515–521. 10.1111/j.1365-2656.2009.01651.x [DOI] [PubMed] [Google Scholar]
- Schradin C, Lindholm AK, Johannesen J, Schoepf I, Yuen C-H, König B, & Pillay N (2012). Social flexibility and social evolution in mammals: A case study of the African striped mouse (Rhabdomys pumilio). Molecular Ecology, 21(3), 541–553. 10.1111/j.1365-294X.2011.05256.x [DOI] [PubMed] [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, et al. (2017). Do female Norway rats form social bonds? Behavioral Ecology and Sociobiology 71, 98. [Google Scholar]
- Sherman PW (1981). Kinship, demography, and Belding’s ground squirrel nepotism. Behavioral Ecology and Sociobiology, 8(4), 251–259. [Google Scholar]
- Sherman PW (1985). Alarm calls of Belding’s ground squirrels to aerial predators: Nepotism or self-preservation? Behavioral Ecology and Sociobiology, 17(4), 313–323. [Google Scholar]
- Sherman PW, Jarvis JUM, & Alexander RD, (Eds.). (1991). The biology of the naked mole-rat Princeton, NJ: Princeton University Press. [Google Scholar]
- Sherman PW, & Morton ML (1984). Demography of Belding’s ground squirrels. Ecology, 65(5), 1617–1628. 10.2307/1939140 [DOI] [Google Scholar]
- Smith AS, & Wang Z (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biological Psychiatry, 76(4), 281–288. 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, & Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behavioural Brain Research, 196(2), 155–167. 10.1016/j.bbr.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So N, Franks B, Lim S, & Curley JP (2015). A social network approach reveals associations between mouse social dominance and brain gene expression. PLOS ONE, 10(7), e0134509 10.1371/journal.pone.0134509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, & Jacquot JJ (2002). Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Canadian Journal of Zoology, 80(5), 951–955. 10.1139/z02-053 [DOI] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, & Wang Z (2014). Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural Brain Research, 265, 22–31. 10.1016/j.bbr.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, … Zhang F (2015). In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nature Biotechnology, 33(1), 102–106. 10.1038/nbt.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky M, Hofmann HA, Beery AK, Blumstein DT, Hayes LD, Lacey EA, … Rubenstein DR (2015). Taxon matters: Promoting integrative studies of social behavior. Trends in Neurosciences, 38(4), 189–191. 10.1016/j.tins.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, & Sakai RR (2005). Social stress: From rodents to primates. Frontiers in Neuroendocrinology, 26(1), 27–40. 10.1016/j.yfrne.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Tammone MN, Lacey EA, & Relva MA (2012). Habitat use by colonial tuco-tucos (Ctenomys sociabilis): Specialization, variation, and sociality. Journal of Mammalogy, 93(6), 1409–1419. 10.1644/11-MAMM-A-266.1 [DOI] [Google Scholar]
- Thomas JA, & Birney EC (1979). Parental care and mating system of the prairie vole, Microtus ochrogaster. Behavioral Ecology and Sociobiology, 5(2), 171–186. [Google Scholar]
- Timmer M, Cordero MI, Sevelinges Y, & Sandi C (2011). Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology, 36(11), 2349–2356. 10.1038/npp.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M, & Sandi C (2010). A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrinology, 35(10), 1543–1552. 10.1016/j.psyneuen.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, & Vanderschuren LJMJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience, 1(4), 444–458. 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BN, Iverson SL, & Severson KL (1983). Seasonal changes in open-field behavior in wild male meadow voles (Microtus pennsylvanicus). Behavioral and Neural Biology, 39(1), 60–77. 10.1016/S0163-1047(83)90637-4 [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, & Sandi C (2012). Social memories in rodents: Methods, mechanisms and modulation by stress. Neuroscience & Biobehavioral Reviews, 36(7), 1763–1772. 10.1016/j.neubiorev.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, & Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463(1), 199–216. 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Wan H, Feng C, Teng F, Yang S, Hu B, Niu Y, … Zhou Q. (2015). One-step generation of p53 gene biallelic mutant Cynomolgus monkey via the CRISPR/Cas system. Cell Research, 25(2), 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, & Insel TR (1999). Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behavioral Neuroscience, 113(3), 602. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, & Insel T (1992). Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Annals of the New York Academy of Sciences, 652(1), 487–489. 10.1111/j.1749-6632.1992.tb34393.x [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, & Carter CS (1994). Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). Journal of Neuroendocrinology, 6(3), 247–250. 10.1111/j.1365-2826.1994.tb00579.x [DOI] [PubMed] [Google Scholar]
- Williamson CM, Romeo RD, & Curley JP (2017). Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Hormones and Behavior, 87, 80–88. 10.1016/j.yhbeh.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Wilsson L (1971). Observations and experiments on the ethology of the European beaver (Castor fiber L.). Viltrevy, 8(3), 115–266. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, & Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature; London, 365(6446), 545–8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, & Insel TR (2004). Neuroendocrine basis of social recognition. Current Opinion in Neurobiology, 14(2), 248–253. 10.1016/j.conb.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Wolff JO, & Sherman PW, (Eds.). (2007). Rodent societies: An ecological and evolutionary perspective Chicago, IL: The University of Chicago Press. [Google Scholar]
- Yao S, Bergan J, Lanjuin A, & Dulac C (2017). Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife, 6, e31373 10.7554/eLife.31373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, & Wang Z (2011). The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neuroscience and Biobehavioral Reviews, 35(3), 498–515. 10.1016/j.neubiorev.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, & Hammock EAD (2005). Anatomy and neurochemistry of the pair bond. The Journal of Comparative Neurology, 493(1), 51–57. 10.1002/cne.20771 [DOI] [PubMed] [Google Scholar]
- Zegeren KV (1979). Variation in aggressiveness and the regulation of numbers in house mouse populations. Netherlands Journal of Zoology, 30(4), 635–770. 10.1163/002829679X00241 [DOI] [Google Scholar]


