Abstract
Background
Polymorphic alleles of the vitamin D (vitD)-binding protein (VDBP) gene are associated with discriminatory differences in circulating concentrations of 25-hydroxyvitamin D (25-D), the indicator of vitD status (sufficiency defined by the Endocrine Society as ≥75 nmol/L). Within a diverse group of children, we hypothesized that reaching recommended daily allowance (RDA) of vitD intake would have differential impact on vitD status depending on VDBP variability.
Methods
VDBP alleles (Gc1S, Gc1F, Gc2) in 123 children (1–4 annual visits/child; ages 1–8 years) were compared for relationships with serum 25-D concentrations and daily vitD intake.
Results
In African-American children, reaching the vitD RDA was associated with significantly higher mean serum 25-D concentrations for the 20% carrying the VDBP 1S allele than for the large majority without this allele (77 vs. 61 nmol/L 25-D; p = 0.038). Children with the Gc1S/1S homozygous genotype (30% Caucasians, 24% Hispanics, 2% African-Americans) who met RDA had 51% (39 nmol/L) greater mean serum 25-D than those below RDA (p < 0.0001).
Conclusions
VDBP genetic variability was a significant factor affecting childhood vitD status when following RDA guidelines. This study may inform public health policy of uniformity in recommended childhood vitD dosage, especially regarding racially/ethnically associated disparities.
Introduction
Vitamin D (vitD)-binding protein (VDBP), originally called Gc globulin, is one of the most abundant serum proteins and transports 85–90% of circulating vitD metabolites.1,2 VDBP is also the product of one of the most polymorphic genes in the human population.3,4 For the most common VDBP alleles, significant frequency differences can be demonstrated within even small groups of racially diverse people.5–7 VDBP variants may directly impact interpretation of clinical data, as the isoforms have different binding affinities for vitD metabolites and are associated with discriminatory serum concentrations of these metabolites, including 25-hydroxyvitamin D (25-D; 25OHD; calcidiol), the principal circulating form accepted as the indicator of “vitD status.”8–10 Taken together with racial/ethnic-associated differences in vitD deficiency rates, as well as other health disparities, VDBP genetic variations may have important implications that have yet to be fully addressed in public health policy.
Among VDBP polymorphic alleles, three that result in distinct protein phenotypes have been most studied in association with racial/ethnic variations and potential effects on vitD metabolite measurements. Using traditional nomenclature directly referring to the protein phenotypes, the alleles are: (a) Gc1F (rs7041-T/rs4588-C); (b) Gc1S (rs7041-G/rs4588-C, single-nucleotide polymorphism (SNP) conferring D416E amino acid change from Gc1F); and (c) Gc2 (rs7041-T/rs4588-A, SNP conferring T420K amino acid change from Gc1F).7 Due to the amino acid substitutions, 1S and 2 VDBP phenotypes are also distinguished from the 1F isoform by significant post-translational glycosylation differences. As Gc1S and Gc2 polymorphisms are in linkage disequilibrium (i.e., both do not occur on the same chromosome), three allelic haplotypes and six genotypes are found in humans.11 Thus, one or two isoforms of VDBP circulate, depending if an individual has a homozygous (1F/1F, 1S/1S, 2/2) or heterozygous (1F/2, 1F/1S, 1S/2) genotype. The VDBP 1F allele, likely the primordial/wild-type form, predominates in individuals of African ancestry, while 1S and 2 alleles are more frequently associated with European or Asian ancestry.5–7
Though synthesized endogenously, vitD deficiencies are common due to the fact that de novo biosynthesis of parent-compound cholecalciferol (vitD3) involves a non-enzymatic, photolysis reaction requiring skin exposure to ultraviolet B radiation, the efficiency of which is affected by factors such as geography, skin tone, body mass index (BMI), and human behavior.12,13 Thus, because natural food sources are uncommon, vitD (D3 or D2, ergocalciferol) is commonly taken as a dietary supplement. Currently, the recommended daily allowance (RDA) of vitD for all children over 12 months old is 600 IU (15 μg), raised from 400 IU (10 μg) by the Institute of Medicine (IOM) in 2010.14
Most health policy agencies, including the IOM, World Health Organization, and Endocrine Society, agree that maintenance of serum 25-D >50 nmol/L (20 ng/mL) prevents most bone development and calcium homeostasis disorders associated with severe vitD deficiency.13,15 This 25-D concentration can generally be achieved by daily vitD intake of 400–600 IU (10–15 μg). However, as understanding of the extent of vitD functioning in the human body has greatly expanded in recent years, including knowledge of numerous intracrine/paracrine roles, the Endocrine Society now recommends that serum 25-D ≥75 nmol/L (30 ng/mL) be considered vitD sufficient, above the range typically found in many Americans, especially those in Black or Hispanic populations.13,16,17
Since vitD status and VDBP polymorphisms are both associated with racial/ethnic differences, this study was designed to test the hypothesis that VDBP genetic variability in children affect correlations between vitD intake (diet plus supplementation) and the clinical measurement of vitD status (serum 25-D concentration). Specifically, within a diverse group of children (participants in follow-up studies to two completed clinical trials involving their pregnant mothers), we wanted to determine if vitD intake in the range of the current RDA resulted in serum vitD concentrations of ≥75 nmol/L 25-D, regardless of VDBP allelic variations (Gc1F, 1S or 2). The results clearly showed that VDBP isoforms significantly impacted the association of vitD intake to vitD status. Particularly, the 75% of African-American children in the study with the Gc1F/1F genotype did not reach this threshold of mean 25-D concentration with RDA, a result significantly different from the minority of African-Americans who carried the Gc1S VDBP allele. These data may challenge the current uniformity of vitD-related public health policy in children.
Methods
Study design
Participants were 123 children born to mothers previously enrolled in two completed pregnancy vitD supplementation trials (Carol L. Wagner, Stuart A. Hamilton, Bruce W. Hollis, P.I.s).18–20 This was part of a prescribed follow-up study conducted at the Medical University of South Carolina (MUSC). The subjects themselves were not part of a vitD supplementation protocol and not subject to any exclusion criteria. Parents gave their written informed consent for their child’s participation (MUSC Institutional Review Board protocol HR# 19641), completed sociodemographic questionnaires, and chose the racial/ethnic group to which their child belonged. All subjects were identified as African-American, Hispanic, or non-Hispanic Caucasian.
Participating children returned for 1 to 4 annual clinical visits, totaling 333 available data collection points. Eighty-fivepercent of children had their first visit at ages 3–5 years. For 333 total visits, represented ages, rounded off to year, were: 1 (1%), 2 (4%), 3 (16%), 4 (22%), 5 (26%), 6 (17%), 7 (11%), and 8 (3%) years old (subsequent visits were scheduled as close to 12 months later as possible). Participants were 48% male and 52% female.
At each annual visit, blood was collected for measurement of total serum 25-D concentration. Blood cells from one visit per subject were used for VDBP genotyping. Other data obtained from most visits included weight, skin colorimetry (IMS SmartProbe, Milford, CT, USA), and detailed dietary information.
VDBP genotyping
Three VDBP alleles were analyzed: Gc1F (rs7041-T/rs4588-C), Gc1S (rs7041-G/rs4588-C), and Gc2 (rs7041-T/rs4588-A).7 DNA was extracted from blood using the GeneJet Whole Blood Genomic DNA Purification kit (Thermo Fisher, Waltham, MA, USA). Genotypes were then determined by restriction-fragment length polymorphism (RFLP) analyses, as previously described.21 Briefly, DNA was amplified by PCR using a Realplex Mastercycler2 (Eppendorf, Hauppauge, NY, USA) and oligonucleotide primers: forward, 5′-TATGATCTCGAAGAGGCATG-3′; reverse, 5′-AATCACAGTAAAGAGGAGGT-3′ (synthesized by Integrated DNA Technologies, Coralville, IA, USA; GenBank L10641.1). PCR amplicons were then treated with either HaeIII or StyI restriction enzymes that cleave at Gc1S or Gc2 polymorphic sites, respectively. Resulting fragments were separated on 4–12% TBE polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and visualized by GelRed stain (Biotium, Fremont, CA, USA) to assign one of six possible genotypes based on fragment sizes. RFLP analyses were repeated for any samples with unclear results; also, random samples were repeated for quality control. PCR reagents and enzymes were from New England Biolabs (Ipswich, MA, USA).
Measurement of circulating 25-D
A direct radioimmunoassay developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN, USA) was used to measure total serum 25-D (D2 and D3) at each annual follow-up visit.22 A total of 333 serum samples (one to four samples per VDBP-genotyped subject) were available. Based on Endocrine Society recommendations, vitD deficiency was defined as serum 25-D <50 nmol/L (20 ng/mL), insufficiency as ≥50 to <75 nmol/L (≥20 to <30 ng/mL), and sufficiency as ≥75 nmol/L (≥30 ng/mL).15
Dietary information
At the time of each annual serum collection, parents identified type and frequency of any dietary supplements children were currently taking so that supplemental vitD intake could be calculated. Because most manufacturers of children’s vitamins raised vitD content from 400 to 600 IU (10–15 μg) while this multi-year study was ongoing (following revised IOM recommendations), determination of supplemental intake was problematic for subjects at some visits and a value of 500 IU (12.5 μg) was used.14 At most visits, parents also completed the Block Kids 2–7 Food Frequency Questionnaire (FFQ) to ascertain the child’s usual eating pattern over the previous 6 months, with specific calculation of vitD intake (NutritionQuest, Berkeley, CA, USA).23 Completed FFQ forms were sent to the NutritionQuest processing center, and these data later reviewed for accuracy by a registered dietitian in the MUSC Clinical and Translational Research Center. Cholecalciferol (D3) and ergocalciferol (D2) intakes were not considered separately. All children in this study were receiving <1000 IU/day (25 μg) total vitD from diet and supplementation at the time of their annual visits.
For normalization between subjects, daily vitD intake was divided by weight (IU/kg). Based on average weights of subjects at the time of serum collection (19.6 ± 5.4 kg), daily vitD intake of either < or >25 IU/kg (625 ng/kg) was chosen to statistically compare sufficient numbers of children with low or high daily intake, respectively. For most children, this 25 IU/kg threshold corresponded to 400–600 IU/day, within the range of the RDA.
Data analyses and statistics
Comparisons of circulating 25-D concentrations to VDBP variants or race/ethnicity were made using Student’s t tests or analysis of variance, as appropriate. If numbers were sufficient for statistical power, 25-D values were compared in numerous ways: (a) mean of all subjects’ samples per grouping; (b) if subject had more than one annual serum sample, 25-D values associated with vitD intake above or below RDA were averaged before using in group mean calculations; (c) the 25-D value of each subject’s sample taken at the time of highest or lowest daily vitD intake; or (d) for individual subjects who provided different serum samples associated with both intake groups (above and below vitD RDA), paired analyses was performed using 25-D values from same subjects (values averaged if subject had more than one such sample per intake group). Linear relationships between vitD intake and circulating 25-D concentrations were determined by calculating Pearson’s correlation coefficient (r). Multiple regression analyses were used to determine correlations of 25-D with both vitD intake and sun exposure. For comparison, no groups with n ≤ 5 were used for calculations. P values <0.05 were considered significant.
Results
Subjects and samples available for this study
VDBP genotyping was performed for three well-characterized alleles (Gc1F, 1S and 2) using blood from 123 children of diverse racial/ethnic backgrounds born to mothers previously enrolled in two completed pregnancy vitD supplementation trials. The participating children in this follow-up study were not part of a vitD supplementation protocol themselves. The children returned for one to four annual clinical visits, for a total of 333 data collection points that were eligible for subsequent analyses; that is, samples for which VDBP genotype, 25-D measurements, and detailed dietary/supplement information were available (Table 1). The majority of these serum samples came from subjects 3–6 years old. To compare children of significantly different sizes over the study period, total daily vitD intake (from estimated dietary and supplemental contribution) was normalized either by body weight (as IU/kg) or calculated BMI. Though both methods obtained similar results, normalization by weight was slightly more linearly correlated to observed effects on 25-D concentration; thus, those values are reported herein.
Table 1.
VDBP genetic variability in subject population by race/ethnicity and samples available for this study
| A. Number of individual subjects (% of total individuals within group) | |||||
|---|---|---|---|---|---|
| B. Number of annually collected serum samples (% of represented genotypes within group) | |||||
| C. Samples collected when subjects were meeting the vitD RDA (normalized to >25 IU/kg) | |||||
| VDBP genotype or haplotype | All subjects | African-American | Caucasian | Hispanic | |
| 1F/1F | A. | 33 (27%) | 30 (75%) | 0 (0%) | 3 (6%) |
| B. | 84 (25%) | 75 (74%) | – | 9 (6%) | |
| C. | 32 | 30 | – | 2 | |
| 1S/1F | A. | 25 (20%) | 6 (15%) | 6 (18%) | 13 (26%) |
| B. | 72 (22%) | 17 (17%) | 15 (17.6%) | 40 (27%) | |
| C. | 24 | 7 | 11 | 6 | |
| 1S/1S | A. | 23 (19%) | 1 (2.5%) | 10 (30.5%) | 12 (24%) |
| B. | 60 (18%) | 2 (2%) | 23 (27%) | 35 (24%) | |
| C. | 18 | 0 | 14 | 4 | |
| 1S/2 | A. | 24 (19.5%) | 1 (2.5%) | 10 (30.5%) | 13 (26%) |
| B. | 68 (20%) | 1 (1%) | 27 (31.8%) | 40 (27%) | |
| C. | 25 | 0 | 13 | 12 | |
| 1F/2 | A. | 13 (10.5%) | 2 (5%) | 3 (9%) | 8 (16%) |
| B. | 36 (11%) | 6 (6%) | 10 (11.8%) | 20 (14%) | |
| C. | 14 | 3 | 4 | 7 | |
| 2/2 | A. | 5 (4%) | 0 (0%) | 4 (12%) | 1 (2%) |
| B. | 13 (4%) | – | 10 (11.8%) | 3 (2%) | |
| C. | 1 | – | 1 | 0 | |
| All genotypes | A. | 123 subjects | 40 | 33 | 50 |
| B. | 333 | 101 | 85 | 147 | |
| C. | 114 (34%) | 40 (40%) | 43 (51%) | 31 (21%) | |
| 1F allelea (rs7041-T/rs4588-C) | A. | 71 (58%) | 38 (95%) | 9 (27%) | 24 (48%) |
| B. | 192 (58%) | 98 (97%) | 25 (29%) | 69 (47%) | |
| C. | 70 | 40 | 15 | 15 | |
| 1S alleleb (rs7041-G/rs4588-C) | A. | 72 (59%) | 8 (20%) | 26 (79%) | 38 (76%) |
| B. | 200 (60%) | 20 (20%) | 65 (76%) | 115 (78%) | |
| C. | 67 | 7 | 38 | 22 | |
| 2 allelec (rs7041-T/rs4588-A) | A. | 42 (34%) | 3 (8%) | 17 (52%) | 22 (44%) |
| B. | 117 (35%) | 7 (7%) | 47 (55%) | 63 (43%) | |
| C. | 40 | 3 | 18 | 19 | |
a1F/1F, 1S/1F, and 1F/2 genotypes
b1S/1F, 1S/1S, and 1S/2 genotypes
c1S/2, 1F/2, and 2/2 genotypes
Factors affecting study design and approach
The use of all available samples from the participating subjects was necessary for the statistical power to uncover some differences among the six VDBP genotypes due to numerous variables in this study population:
Frequencies of the three VDBP alleles were dramatically different among the subjects in the study (Table 1). For example, 75% of the African-American children, but no non-Hispanic Caucasians, had the homozygous 1F/1F genotype. Conversely, nearly one-third of Caucasians, but only a single African-American child, had the homozygous 1S/1S genotype. Overall, only 20% of African-Americans in the study carried the 1S allele, most with the 1S/1F genotype. Comparing percentages in lines a and b of Table 1, the 333 available samples were extremely representative of the allelic frequencies within the population of individual subjects.
Total daily vitD intake between children also differed between racial/ethnic groups, with Hispanic children having much lower average daily intake than African-Americans or Caucasians (Table 1, line c). These differences in intake were largely attributed to the varying percentages of children taking supplements containing vitD at the time of the clinical visit: Caucasians (71%), African-Americans (47%), and Hispanics (25%). Consequently, only 21% of Hispanics in this study were receiving >25 IU/kg (>625 ng/kg) vitD daily (similar to 400–600 IU total vitD for most subjects), within the range of the RDA. All children had total daily vitD intakes of <1000 IU at the time of their annual clinical visits.
At the time of serum collection, a SmartProbe skin colorimeter was used as the primary estimate of sun exposure (i.e., by measuring the differences in skin pigmentation at 450 nm between areas normally exposed or covered by clothing), but other information was not available (e.g., hours of outside activity, sunscreen usage, etc.), nor were all clinic visits during the same time of year. These additional data are considered important for accurate estimates, especially for darker-skinned individuals. For children with low vitD intakes (below the RDA), significant linear correlations were calculated between sun exposure and circulating 25-D; however, this relationship was obscured in children vitD intakes above the RDA (data not shown). As the focus of this study was the relationship to vitD status between different VDBP genotypes and vitD RDA (which is based solely on dietary/supplemental intake of vitD, not endogenous biosynthesis), these estimates of sun exposure were not considered further.
Taken together, some relationships of VDBP genotypes to a child’s vitD status and daily intake were more apparent if the subjects were analyzed separately by racial/ethnic groups, and that is the approach followed to clarify some of the data reported herein. However, due to the low percentage of Hispanic children meeting the vitD RDA at the time of serum collection, this group could not be analyzed individually.
Effects of vitD intake on children’s circulating 25-D concentrations
Without considering VDBP genotype, the children in this study differed significantly in vitD status, as measured by mean serum 25-D concentration (Fig. 1). As a group, Caucasians had much higher average 25-D concentrations than did African-Americans or Hispanics and met the ≥75 nmol/L threshold recommended by the Endocrine Society, even with low daily vitD intake.
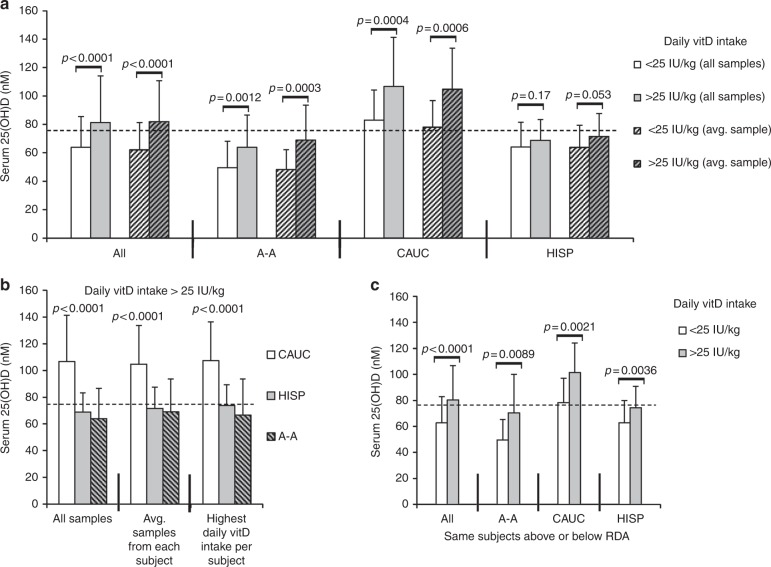
Fig. 1.
Effects of vitD intake on serum 25-D concentrations in children (all VDBP isoforms). a Mean 25-D (nmol/L) compared between Caucasian, Hispanic, and African-American children regularly reaching the vitD RDA (normalized as >25 IU/kg; representing 400–600 IU daily intake), or below the RDA at the time of annual serum collection. Statistics calculated using all available serum samples or the average of 25-D values for each subject’s samples. b Direct comparisons of serum 25-D in different groups of children meeting or exceeding vitD RDA; all samples, average of each subject’s samples, or single sample from each subject at the time of highest daily vitD intake (80 total subjects). c Comparisons of mean 25-D within the same children who were both below and above the vitD RDA at different annual serum collections (55 total subjects). P values <0.05 considered significant. Dashed line in each graph represents threshold serum concentration of ≥75 nmol/L 25-D. vitD, Vitamin D, VDBP, vitamin D binding protein; 25-D, 25-hydroxyvitamin D, RDA recommended daily allowance
If all subjects were considered together, the RDA of vitD intake (normalized to ≥25 IU/kg) did raise the children’s mean 25-D levels above 75 nmol/L (Fig. 1a), from an average of 64L to 81 nmol/L 25-D in children regularly receiving below or above the vitD RDA, respectively. However, as groups, African-American and Hispanic children on average showed minor effects of higher amounts of daily vitD intake on measured vitD status and had <75 nmol/L mean circulating 25-D, even with reaching the RDA (all subjects in this study had daily vitD intake of < 000 IU) (Fig. 1a, b). As illustrated in Fig. 1, these relationships between vitD intake and vitD status were consistent in this population regardless of the method of sample analyses used, including paired analyses within the same children comparing 25-D values from different annual samples collected when those subjects were either below or above regular RDA intake (Fig. 1c).
No statistically significant sex-associated differences were seen in these results when daily vitD intakes were normalized (data not shown).
Relationships between VDBP genotype, vitD intake, and circulating 25-D concentration
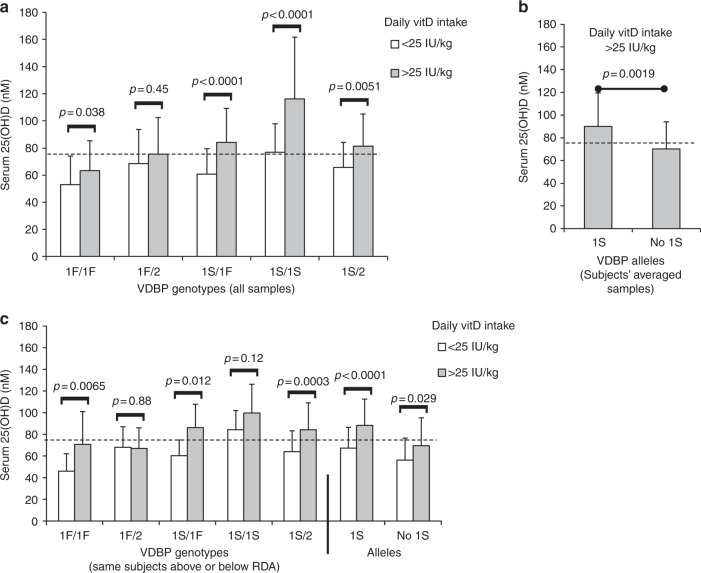
As shown in Fig. 2, children who carried the VDBP 1S allele had the highest serum 25-D concentrations. Specifically, homozygous individuals (1S/1S) demonstrated the most significant differences in vitD status between groups with high or low daily vitD intake, with a mean 25-D 39 nmol/L (51%) higher in children meeting the RDA. On average, 1S/1S children were also most likely to reach 75 nmol/L 25-D, even with low vitD intake.
Fig. 2.
The VDBP 1S allele was associated with the highest circulating 25-D concentrations in children reaching the vitD RDA. a Comparisons of mean 25-D between children of different VDBP genotypes who regularly reached vitD RDA (normalized as >25 IU/kg) or not at the time of annual serum collection (308 total samples). No group of n ≤ 5 compared (affected only 2/2 genotype). b Mean 25-D in children reaching the vitD RDA compared between subjects who carried the 1S allele and those who did not (47 and 33, respectively). If an individual child had more than one such sample, 25-D values were averaged. c Association of VDBP genotypes to mean 25-D within the same children who were both below and above vitD RDA at different annual serum collections (55 total subjects). Also shown are children grouped by carriers of the 1S allele per se. Dashed line in each graph represents threshold serum concentration of ≥75 nmol/L 25-D. vitD, Vitamin D, VDBP, vitamin D binding protein; 25-D, 25-hydroxyvitamin D, RDA recommended daily allowance
Conversely, the VDBP 1F allele was associated with lowest vitD status, and mean 25-D values of homozygous 1F/1F individuals meeting the RDA were <75 nmol/L, regardless of the method of sample analysis used. Serum 25-D concentrations in carriers of the VDBP 2 allele appeared to be intermediate between those of 1F and 1S (as homozygous VDBP 2/2 subjects were only 5% of the study population, data from these children could not considered separately in all statistical calculations). For the sample groups large enough to statistically compare, no significant sex-associated differences were seen in these relationships between VDBP genotype and 25-D concentration (data not shown).
The VDBP 1S/1S genotype is associated with the highest serum 25-D concentrations in non-Hispanic Caucasian children
As shown above, the majority of Caucasian children in this study had circulating 25-D >75 nmol/L at the time of their annual clinical visits (Fig. 1), and these children were most likely to be meeting the vitD RDA intake (Table 1). Thus, statistical comparisons of all individual VDBP genotypes in subjects with low vitD intake were not possible. However, within this non-Hispanic Caucasian population, VDBP 1S/1S individuals consistently had the highest circulating 25-D concentrations and the largest mean difference (40 nmol/L) between those either below or above vitD RDA intake (Fig. 3). Also, the linear correlations between vitD intake and 25-D were strongest between 1S/1S individuals (r = 0.55; p = 0.011), than with other genotypes (r = 0.35, p = 0.0068) (data not shown).
Fig. 3.
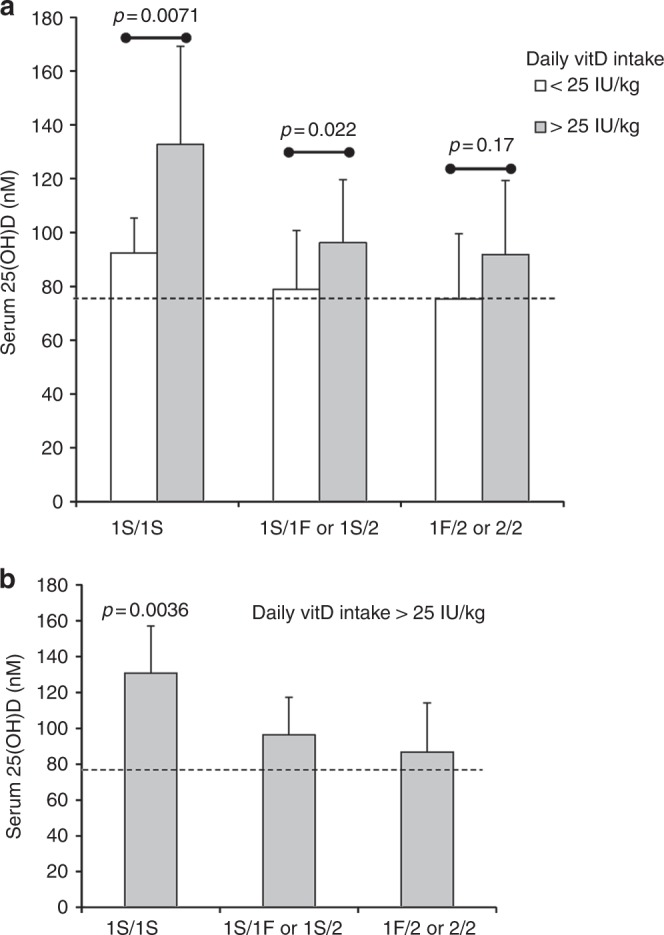
The VDBP 1S/1S genotype is associated with the highest circulating 25-D in non-Hispanic Caucasian children. a Comparisons of mean 25-D in Caucasian children of different VDBP genotype who reached vitD RDA or not at the time of annual serum collection. The three groups shown met sufficient statistical power (81 serum samples). b Caucasian children meeting the RDA at the time of sample collection (27 subjects). If an individual child had more than one such serum sample, 25-D values were averaged. Dashed line in each graph represents threshold serum concentration of ≥75 nmol/L 25-D. vitD, Vitamin D, VDBP, vitamin D binding protein; 25-D, 25-hydroxyvitamin D, RDA recommended daily allowance
The VDBP 1S allele is associated with higher circulating 25-D concentrations in African-American children
Seventy five percent of African-American children in this study were homozygous for the VDBP 1F allele. By comparison, only three Hispanic and no Caucasian children in this study had this 1F/1F genotype. Twenty percent of the remaining African-American children carried the VDBP 1S allele (1S/1F, 1S/2 or 1S/1S genotypes). At the time of their clinical visits, 40% of African-American children in this study were regularly meeting the vitD RDA (Table 1).
For African-American children with the majority VDBP 1F/1F genotype, meeting the RDA was not associated with mean serum 25-D ≥75 nmol/L, regardless of the method of sample analysis (Fig. 4). Even in the subset of 1F/1F children obtaining substantially above RDA (between 600 and 1000 IU daily), mean serum 25-D did not reach 75 nmol/L (data not shown).
Fig. 4.
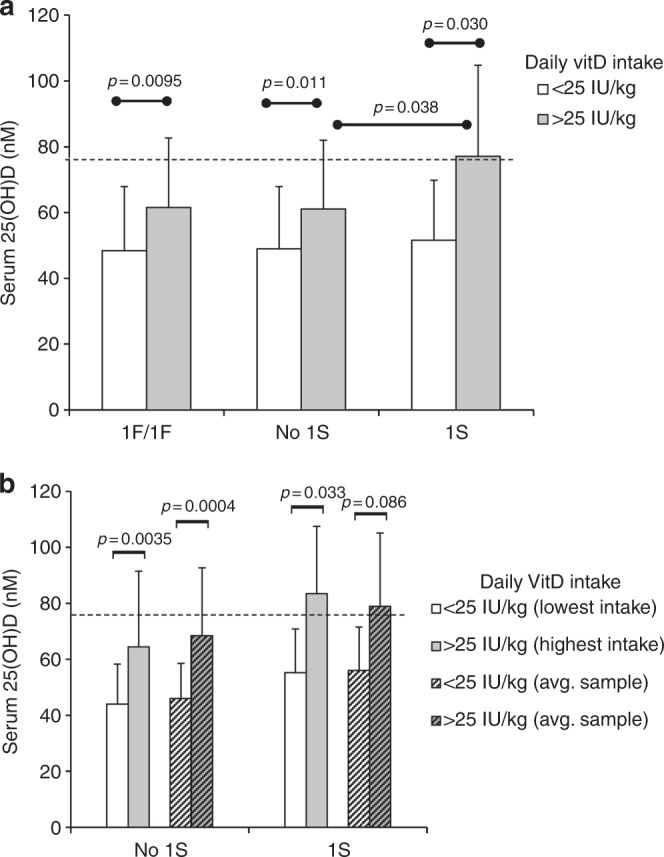
The VDBP 1S allele is associated with mean serum 25-D >75 nmol/L in African-American children who meet or exceed RDA. a Comparisons of mean 25-D in three groups of African-American children (93 serum samples; 1S group: genotypes 1S/1F, 1S/2, and 1S/1S; no 1S group: 1F/1F and 1F/2) who reached vitD RDA or not at the time of annual serum collection. b Comparisons of 25-D in serum samples collected at the time of lowest or highest daily vitD intake from African-American children either carrying the 1S allele or not (12 and 43 subjects, respectively); also, averaged 25-D values of all serum samples per subject either below or above RDA. Dashed line in each graph represents threshold serum concentration of ≥75 nmol/L 25-D
Conversely, the presence of the VDBP 1S isoform was associated with mean 25-D above the 75 nmol/L threshold in African-American children reaching the RDA (77–83 nmol/L mean serum 25-D, depending on the type of sample analysis used) (Fig. 4a, b). Though only 20% of these children carried the 1S allele (i.e., 1S/1F, 1S/2 and 1S/1S genotypes), mean 25-D concentration was still significantly higher when meeting the RDA (p value = 0.038) than in those children who did not carry the 1S allele (i.e., 1F/1F and 1F/2 genotypes) (Fig. 4a). Also, linear correlations between vitD intake and 25-D were stronger for those children who carried the 1S allele (r = 0.41; p = 0.04), compared to those who did not (r = 0.24; p = 0.03) (data not shown).
Discussion
This study analyzed the association of vitD intake to circulating 25-D concentration (i.e., vitD status) according to VDBP genetic diversity in a group of 123 children. These subjects returned for up to four annual follow-up clinical visits after their mothers completed clinical studies involving vitD supplementation during pregnancy.18–20 The results reported herein clearly demonstrate that the VDBP 1S allele is associated with the highest vitD status in children who regularly receive the RDA of vitD intake (currently set 600 IU by the IOM for children older than 12 months). The Endocrine Society currently defines “vitD sufficiency” as serum 25-D ≥75 nmol/L (≥30 ng/mL).15 Most strikingly, in those subjects who reached the RDA, the VDBP 1S allele was associated with mean circulating 25-D above this concentration even in in African-American children, a population that regularly falls below this threshold of vitD sufficiency. However, only 20% of African-American children in this study carried the 1S allele (most with the 1S/1F genotype), which is consistent with the frequency seen other studies.4,6,10,24
Conversely, children homozygous for the VDBP 1F allele, including 75% of the African-Americans in this study, were much more likely to be vitD “insufficient” by standard definition (i.e., circulating 25-D ≥50 to <75 nmol/L), even when reaching the RDA. Thus, though the data here suggest further investigation through a controlled clinical study, in this population of African-American children, only the cohort that carried the VDBP 1S allele reached mean vitD sufficiency with RDA intake as currently defined. Though previous studies have also found a general association with the VDBP 1S allele and higher circulating 25-D concentrations,10,21,25–29 to our knowledge, this is the first time that the vitD intake within the range of the RDA has been so conclusively linked to differential effects on childhood vitD status due to VDBP genetic variability.
Probably originating as humans radiated out of Africa, the Gc1F, 1S, and 2 polymorphic alleles of VDBP have likely been maintained for millennia, and their frequency variations are well known to be associated with racial ancestry.5–7 Speculation as to the reasons for maintenance of this genetic diversity has varied widely from an association with skin tone, geographic differences in diet or sun exposure, and immunological pressure from pathogens.6,30,31 In this study of children from the southeastern United States, the frequencies of these VDBP alleles among racial/ethnic groups were very similar to those found in previous publications.4,6,10,24 Over 100 other VDBP polymorphisms have been found in the human population, but their frequencies and relationships to vitD status are much less established.3,4
Though beyond the scope of this study, there is an on-going debate concerning the meaning of vitD status as determined by circulating 25-D, and if estimated calculations of free (unbound to protein) or “bioavailable” (free plus fraction bound to albumin, rather than to VDBP) metabolite would be better assessments of vitD sufficiency.32–35 Determination of vitD metabolite bioavailability is further complicated by the polymorphic forms of VDBP, which may have different steady-state circulating concentrations (e.g., resulting from protein synthesis and/or turnover rates) and 25-D-binding affinities.9,26 Taken together, the known associations of VDBP polymorphisms with quantitative differences in circulating 25-D concentrations among individuals could have important implications for vitD-related physiology.11 This would be especially true concerning the intracrine and paracrine functions of vitD, such as in immune cells, which likely compete with circulating VDBP for binding 25-D (these cells, like many others, can synthesize the active metabolite, 1,25(OH)2D, from 25-D for intracellular use).36–38 For example, in vitro assays clearly demonstrate that increasing VDBP concentrations can abrogate monocyte/macrophage vitD signaling, an effect that can be discriminated by using different VDBP allelic forms.38
The meanings of vitD “sufficiency” or “deficiency” are still controversial. If referring solely to circulating 25-D concentrations associated with the endocrine role of vitD, then serum calcium and PTH concentration, bone density, and growth are likely good clinical correlates of deficiency. Even most current studies addressing the relationship of free or bioavailable vitD metabolites to VDBP genetic diversity are solely focused on this endocrine role of vitD (e.g., are African-Americans truly vitD deficient with low circulating 25-D if they have high bone density?). However, a large body of research over the past few decades has now elucidated hundreds of other genes and physiologic processes affected by vitD, many of which are associated with subtle, homeostatic regulation that could potentially influence life-long health, and are much more difficult to correlate to simple clinical/pathological measurements. This accumulated research is what primarily led the Endocrine Society to distinguish between deficiency, insufficiency, and sufficiency in circulating 25-D. Although the official recommendation of sufficiency in that report was for circulating 25-D of ≥75 nmol/L, even a range of 100–150 nmol/L (40–60 ng/ml) was considered safe and would likely guarantee full sufficiency in most children and adults.15
Though future research may indeed change interpretations of vitD sufficiency, the primary objective of our study was to determine if potential relationships existed between VDBP polymorphisms and the currently accepted clinical determination of vitD sufficiency in children as defined by the Endocrine Society, and if these data would impact the current uniformity of vitD RDA as determined by the IOM. There were several limitations to this study, most associated with the moderate sample size (123 subjects, 333 data collection points) and the fact that the children were not part of a vitD supplementation protocol. Most of these limitations were incorporated into the study design as discussed in detail in Results section. Since this population of children were not part of a clinical trial of vitD supplementation themselves, most factors normally controlled in such a study (e.g., equivalent vitD intake cohorts) were highly variable. In fact, this prevented the separate analysis of Hispanic children here, since the large majority of the subjects in this group fell far below the RDA of vitD intake. Though beyond the scope of this report, many other measurements needed to further elucidate the physiological effects associated with VDBP diversity were also unavailable, including circulating PTH, Ca2+, 1,25-dihydroxyvitamin D, and free or bioavailable 25-D. Thus, interpretations here were limited to the primary data available: serum 25-D concentration and detailed dietary information. Again, however, these are the only two measurements currently accepted as indicators of vitD status/sufficiency and RDA of vitD intake, respectively.
In summary, these results clearly indicate that VDBP genotypic variability is a significant factor in determining vitD status associated with recommended vitD intake in children. Furthermore, these data suggest additional studies addressing recommended standards as they relate specifically to African-American children, the majority of whom lack the 1S allele associated with higher circulating 25-D. Along with recent research into the significance of vitD metabolite bioavailability, these data may challenge public health policy related to current uniformity in recommended vitD daily allowance, especially with regard to racially/ethnically associated disparities in children.
Acknowledgements
We would like to thank Reneé Washington and Dr. Kaleena Dezsi for assistance in completing these studies. This study was supported by Thrasher Research Fund and NIH/NCRR UL1 RR029882, UL1 TR0000062.
Author contributions
Conception/design/data acquisition and analyses: All authors. Drafting/revision: D.A.N., J.E.B., C.L.W. Final approval: All authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cooke NE, David EV. Serum vitamin D-binding protein is a third member of the albumin and alpha fetoprotein gene family. J. Clin. Invest. 1985;76:2420–2424. doi: 10.1172/JCI112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol. Metab. 2000;11:320–327. doi: 10.1016/S1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 3.Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox. Sang. 1988;54:215–225. doi: 10.1159/000461809. [DOI] [PubMed] [Google Scholar]
- 4.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr. Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 6.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum. Genet. 1986;72:281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 7.Braun A, Bichlmaier R, Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): allelic differences of the common genetic GC types. Hum. Genet. 1992;89:401–406. doi: 10.1007/BF00194311. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum. Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 10.Lauridsen AL, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif. Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 11.Malik S, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit. Rev. Clin. Lab. Sci. 2013;50:1–22. doi: 10.3109/10408363.2012.750262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Food and Nutrition Board. Dietary Reference Intakes for Vitamin D and Calcium. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (National Academy Press, Washington, 2010).
- 15.Holick MF, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 16.Wright NC, et al. Defining physiologically “normal” vitamin D in African Americans. Osteoporos. Int. 2012;23:2283–2291. doi: 10.1007/s00198-011-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004;158:531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011;26:2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner CL, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am. J. Obstet. Gynecol. 2013;208:137 e131–113. doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner CL, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J. Steroid Biochem. Mol. Biol. 2013;136:313–320. doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schellenberg D, et al. Vitamin D binding protein variants and the risk of COPD. Am. J. Respir. Crit. Care Med. 1998;157:957–961. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 22.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin. Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 23.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J. Am. Diet. Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 24.Speeckaert MM, Speeckaert R, van Geel N, Delanghe JR. Vitamin D binding protein: a multifunctional protein of clinical importance. Adv. Clin. Chem. 2014;63:1–57. doi: 10.1016/B978-0-12-800094-6.00001-7. [DOI] [PubMed] [Google Scholar]
- 25.Janssens W, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 26.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin. Chem. 2001;47:753–756. [PubMed] [Google Scholar]
- 27.Braithwaite VS, et al. Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children. Bone. 2015;74:166–170. doi: 10.1016/j.bone.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu L, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin. Biochem. 2009;42:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Yuen AW, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med. Hypotheses. 2010;74:39–44. doi: 10.1016/j.mehy.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Kappelman J, et al. First Homo erectus from Turkey and implications for migrations into temperate Eurasia. Am. J. Phys. Anthropol. 2008;135:110–116. doi: 10.1002/ajpa.20739. [DOI] [PubMed] [Google Scholar]
- 32.Bikle, D., Bouillon, R., Thadhani R. & Schoenmakers I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol.173, 105–116 (2017). [DOI] [PMC free article] [PubMed]
- 33.Powe CE, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun RF, et al. Vitamin D and DBP: the free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014;144:132–137. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shieh A, et al. Effects of cholecalciferol vs calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J. Clin. Endocrinol. Metab. 2017;102:1133–1140. doi: 10.1210/jc.2016-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffery LE, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J. Immunol. 2012;189:5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 38.Chun RF, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J. Clin. Endocrinol. Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]