Abstract
Background
Amlodipine (AML) is the initial therapy most commonly prescribed for patients with hypertension in China. However, AML monotherapy is often less effective in achieving blood pressure (BP) control than other agents.
Objective
We performed a clinical study to evaluate efficacy and safety of a combination therapy with AML, olmesartan (OLM), or an OLM/hydrochlorothiazide (HCTZ) compound for Chinese patients with mild-to-moderate hypertension.
Methods
In the clinical trial, patients were initially treated with OLM 20 mg/d combined with AML 5 mg/d. Then OLM was uptitrated to 40 mg/d or changed to an OLM/HCTZ (20/12.5 mg/d) compound if the patients did not reach the target of seated diastolic BP <90 mm Hg (<80 mm Hg in patients with diabetes) after 8 weeks.
Results
The overall response rate of the combination therapy was 59.2% (95% CI, 54.23%–63.97%) at Week 2 and gradually increased to 97.1% (95% CI, 94.93%–98.47%) at the end of the study (Week 16).
Conclusions
The combination therapy with OLM or OLM/HCTZ was well tolerated. The total incidence of adverse events was 42.9% (n = 176). Most of the adverse events were mild in severity (39.5%; n = 162) and not associated with the drugs (33.2%). In conclusion, combination therapy with AML, OLM, or OLM/HCTZ can significantly lower BP safely and achieve a high BP control rate in patients with mild-to-moderate hypertension in China. ClinicalTrial.org identifier: ChiCTR-ONC-12001963.
Key words: amlodipine, antihypertensive, combination therapy, hydrochlorothiazide, hypertension, olmesartan
Introduction
Hypertension is a major global public-health problem leading to high risk of cardiovascular and kidney diseases. It has been estimated that by 2025 the number of individuals with hypertension will be 1.56 billion.1 In China, the prevalence of diagnosed hypertension is approximately 27.86%,2 but the blood pressure (BP) control rate is quite low (6.1%),3 especially in patients treated with monotherapy.4
Calcium-channel blockers (CCBs)—which dilate arteries by reducing calcium flux into cells, effectively lowering BP—are commonly used as an initial treatment for hypertension, particularly in China. Amlodipine besilate (AML) is the most frequently prescribed antihypertensive CCB in China.5 However, several clinical studies demonstrated that BP cannot be adequately controlled by monotherapy with AML 5 mg/d.6, 7, 8 In the 2013 European Society of Hypertension/European Society of Cardiology Guidelines it was emphasized that monotherapy with any drug at any dose (even maximum dose) can only effectively lower BP in limited populations of patients with hypertension and that most patients require combination therapy with at least 2 antihypertensive agents to reach BP control.9 Moreover, combining antihypertensive agents that lower BP via different mechanisms may minimize the likelihood of dose–dependent adverse effect.
Indeed, coadministration of an angiotensin II antagonist and a CCB is considered an effective and well-tolerated therapeutic option for hypertension treatment.10 Olmesartan medoxomil (OLM), an angiotensin receptor blocker (ARB), selectively and competitively inhibits the type 1 angiotensin II receptor without affecting other receptors regulating the cardiovascular system11 and has been shown to lower BP with a high degree of efficacy.12, 13, 14 Volpe et al15 demonstrated that more than 70% of patients treated actively with the combination therapy of OLM/AML 20/5 mg achieved their BP goal by Week 24. However, it is still unclear how a combination therapy with AML, OLM, or OLM/hydrochlorothiazide (HCTZ) can contribute to reaching BP goals in Chinese patients with hypertension compared with inadequate BP control on initial AML 5 mg/d. Therefore, our study was designed to demonstrate the high BP lowering efficacy of combination therapy with AML, OLM, or OLM/HCTZ in patients with hypertension.
Materials and Methods
Subjects
Patients enrolled in the study were required to be outpatients aged 18 to 75 years either newly diagnosed or with a history of primary mild-to-moderate hypertension, previously treated with AML 5 mg/d for more than 4 weeks without any kind of other antihypertension drugs without achieving BP control (defined as seated diastolic BP [SeDBP] <90 mm Hg [<80 mm Hg for patients with diabetes], or mean SeDBP <90 mm Hg [<100 mm Hg for patients with diabetes] and mean seated systolic BP [SeSBP] <180 mm Hg [<170 mm Hg for patients with diabetes]). They must also have been willing and able to use the drug in accordance with the study protocol.
The main exclusion criteria were patients with suspected or known secondary hypertension, SeSBP/SeDBP ≥180/110 mm Hg (≥170/100 mm Hg for patients with diabetes), diagnosis of insulin-dependent diabetes, diagnosed with uncontrolled noninsulin-dependent diabetes as indicated by fasting plasma glucose >200 mg/dL (11.1 mmol/L), diagnosis of diabetic peripheral neuropathy or autonomic neuropathy, those with serious cardiovascular diseases or clinically significant hepatic impairment, severe renal impairment or other conditions that would not allow for the safe completion of the protocol, or use of beta-receptor blockers for medical needs. Also excluded were patients with a history of drug dependency, allergy to any of the study drugs or supplements, pregnant or lactating women, or women of childbearing age who were unwilling to or could not take effective contraception.
The protocol was approved by an appropriate local ethics committee, and all patients provided written informed consent before their enrollment.
Study design
This study was a prospective, open-label, and multicenter study implemented in 19 sites in China. The study schedule and treatment regimen of study drugs are shown in Figure 1. The included patients were administered OLM 20 mg/d combined with AML 5 mg/d for 8 weeks. If SeDBP was not adequately controlled to <90 mm Hg (<80 mm Hg for patients with diabetes), the OLM combination therapy was changed to 1 of the 2 following regimens at a physician’s discretion according to the patient’s condition at Week 8 or Week 12: double dose of OLM (add to 40 mg) with AML 5 mg once a day, or AML 5 mg/d plus OLM/HCTZ (20/12.5 mg) once a day. Patients were discontinued from the study and received appropriate treatment if they had SeSBP and/or SeDBP ≥180/110 mm Hg (≥170/100 mm Hg for patients with diabetes) at any time during the study’s duration.
Figure 1.
Study design. AML = amlodipine; Follow-up = 16-week combination study; HCTZ = hydrochlorothiazide; OLM = olmesartan medoxomil.
End points of the study
The primary end point was the proportion of patients with a clinical response at Week 16, defined as reached BP goal (SeSBP/SeDBP <140/90 mm Hg for patients without diabetes and <130/80 mm Hg for patients with diabetes), the mean change of SeDBP from baseline was >10 mm Hg, or the mean change of SeSBP from baseline was >20 mm Hg.
Secondary outcome variables were the proportion of patients who achieved their BP goal defined after Week 4, Week 8, Week 12, and Week 16 of the treatment and the mean change of SeSBP and SeDBP from the baseline at Week 2, Week 4, Week 8, Week 10, Week 12, and Week 16.
Safety assessments included adverse events (AEs), adverse drug reactions (ADRs), serious adverse events, and abnormal laboratory parameters.
Statistical analysis
We estimated the sample size in regard to precision of responder rate of all treatment groups at Week 16. When the expected goal rate is 80.0%, using the large sample normal approximation, the sample size should be >385 to satisfy the criteria of within 0.04 of 2-sided 95% CI for a single proportion. Four hundred patients were needed (considering withdraw). The full analysis set consisted of all patients randomized to treatment who received at least 1 dose of the assigned treatment based on the principle of intention to treat. The per-protocol analysis set excluded patients who did not meet our inclusion and exclusion criteria and those who were lost at follow-up, withdrew early from the trial, had major deviations from the planned time schedule, failed to complete the trial medication, had low compliance, or did not attend the final visit. The safety data set (SS) was used to evaluate safety based on the safety index of the patients who received the test drugs at least once.
Quantitative data were analyzed using ANOVA and qualitative data were assessed using the Fisher exact test or χ2 test. Wilcoxon signed-rank tests were utilized for grading AEs. The statistical significance was set at 2-tailed P < 0.05. The analysis was performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Patients’ disposition and baseline characteristics
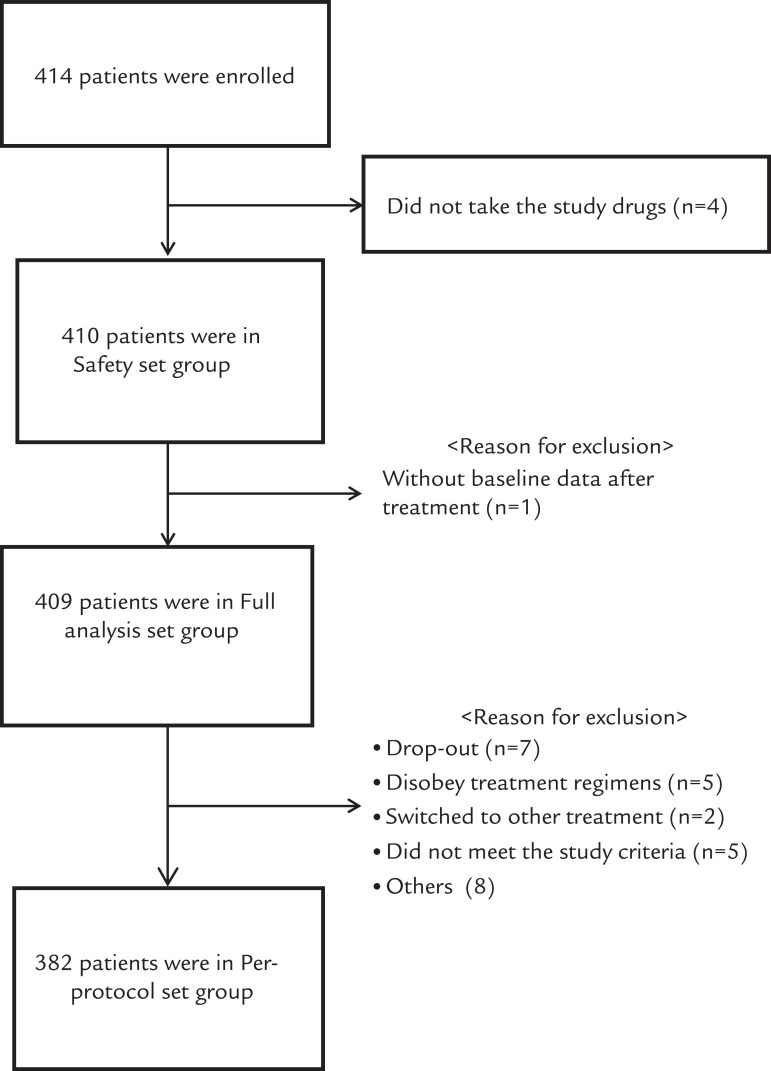
Four hundred nineteen patients were screened at 19 sites. A total of 414 eligible patients were enrolled. The percentage of patients in the full analysis set (n = 409), per-protocol analysis set (n = 382), and SS (n = 410) were 98.8%, 92.3%, and 99.0%, respectively (Figure 2).
Figure 2.
Patient flow. Others included dropouts and urine protein ≥ ++ (n = 2); switched to other treatment, dropped out, and compliance did not reach 80%–120% (n = 2); switched to other treatment, dropped out, did not meet the study criteria, and compliance did not reach 80%–120% (n = 1); switched to other treatment and dropped out (n = 1); and did not meet the study criteria and dropped out (n = 2).
Demographic and clinical characteristics of patients are shown in Table I. The mean age was 57.2 (9.44) years. Men made up 54.5% of the patients. The mean SeSBP/SeDBP at screening was 149.3 (10.11) mm Hg/95.9(5.23) mm Hg and the mean duration of hypertension was 110.3 (98.65) months. The mean treatment time of AML 5 mg/d was 23.9 (37.70) months. The major concomitant diseases included hyperlipidemia (n = 72; 17.9%), coronary heart disease (n = 14; 3.4%), and diabetes mellitus (n = 54; 13.2%).
Table I.
Baseline demographic characteristics (full analysis set n = 409).
| Characteristic | Result |
|---|---|
| Score | |
| Body mass index | 25.7 |
| Mean (SD) | |
| Age | 57.2 (9.44) |
| BMI | 25.7 |
| Hypertension history (mo) | 110.3 (98.65) |
| Previous treatment duration of amlodipine 5 mg/d (mo) | 23.9 (37.70) |
| Seated systolic blood pressure (mm Hg) | 149.3 (10.11) |
| Seated diastolic blood pressure (mm Hg) | 95.9 (5.23) |
| Heart rate | 73.2 (8.24) |
| n (%) | |
| Male | 223 (54.5) |
| Major concomitant disease | |
| Hyperlipidemia | 72 (17.6) |
| Coronary heart disease | 14 (3.4) |
| Diabetes mellitus | 54 (13.2) |
| Major concomitant medication | |
| Yes | 159 (38.9) |
| No | 250 (61.1) |
BMI = body mass index
BP response rate
The mean proportion of patients who responded at Week 2 was 59.2% (95% CI, 54.23%–63.97%). This response rate was gradually increased at Week 4, 8, 10, and 12. At the end of treatment (Week 16), the mean overall response rate was 97.1% (95% CI, 94.93%–98.47%) (Figure 3).
Figure 3.
The mean blood pressure response rate of patients. The histogram illustrates a stratified analysis for different dosing regimens (full analysis set). AML = amlodipine; HCTZ = hydrochlorothiazide; OLM = olmesartan medoxomil.
The number of patients in each dosing regimen group is shown in Table II. Most of the patients (n = 333) were treated with OLM/AML 20/5 mg/d by the end of treatment, including the patients shown in Table II (n = 331); 2 patients withdrew due to lack of efficacy (failure) at Week 8. Another 62 patients required a double dose of OLM (OLM/AML 40/5 mg/d) or OLM/HCTZ (OLM/AML/HCTZ 20/5/12.5 mg/d) up to Week 16 to control BP, as shown in Table II (n = 34 and n = 27) and 1 patient had to withdraw due to failure at Week 8. According to our stratified analysis for dosing regimen, the response rate in each regimen group was 99.4% for OLM/AML 20/5 mg/d, 91.2% for AML/OLM 40/5 mg/d, and 92.9% for OLM/AML/HCTZ 20/5/12.5 mg/d at Week 16 (Figure 3).
Table II.
Adjustment of therapy during the 16-week follow-up.⁎
| Week of follow-up | AML 5+ OLM 20 | AML 5+ OLM 40 | AML 5+ OLM 20/HCTZ 12.5 | Total |
|---|---|---|---|---|
| n | ||||
| 0 | 409 | 0 | 0 | 409 |
| 8 | 344 | 30 | 22 | 406 |
| 10 | 344 | 30 | 22 | 406 |
| 12 | 331 | 34 | 27 | 407 |
| 16 | 331 | 34 | 27 | 407 |
AML 5 = amlodipine 5 mg/d; HCTZ 12.5 = hydrochlorothiazide 12.5 mg/d; OLM 20 = olmesartan medoxomil 20 mg/d; OLM 40 = olmesartan medoxomil 40 mg/d.
The dosing regimen was altered at Week 8 or Week 12 once based on different patients’ medical conditions. All data were collected from the full analysis set group.
Mean change of SeSBP and SeDBP
After 16 weeks of the combination therapy all dosing regimens resulted in a significant decrease in mean SeSBP (Figure 4A) and SeDBP (Figure 4B). The changes in mean SeSBP/SeDBP from baseline at each treatment period were –13.3 (10.97)/–10.7 (7.64) mm Hg at Week 2, –17.1 (11.23)/–13.6 (7.18) mm Hg at Week 4, –19.2 (12.01)/–15.8 (7.68) mm Hg at Week 8, –21.5 (11.26)/–16.9 (7.25) mm Hg at Week 10, –23.0 (11.10)/–18.52 (6.93) mm Hg at Week 12, and –24.1 (10.98)/–19.1 (6.81) mm Hg at Week 16 (P < 0.001 from baseline), respectively.
Figure 4.
Change from baseline in (A) seated systolic blood pressure (SBP) and (B) seated diastolic blood pressure (DBP) for overall patients (full analysis set [FAS] n = 409)
Achievement rate of BP goal
A high ratio (88.0%) of patients achieved the BP goal of SeSBP and SeDBP at Week 16 (Figure 5A). In the further analysis for individual SeSBP or SeDBP goal ratio, 90% of patients reached the SeSBP goal (Figure 5B), and 92.9% of patients reached the SeDBP goal (Figure 5C) after 16 weeks of treatment.
Figure 5.
Blood pressure goal rate for overall patients (full analysis set [FAS] n= 409). Panels indicate the proportion of overall patients who achieved the blood pressure target for (A) seated systolic blood pressure (SBP)/seated diastolic blood pressure (DBP), (B) SBP alone, and (C) DBP alone.
Safety assessments
Of 410 patients in the SS, 110 experienced at least 1 AE each. The total number of AEs was 176 (42.9%) during 16 weeks of treatment. Among patients experiencing AEs, the incidence of mild, moderate, and severe AEs was 39.5% (n = 162), 3.2% (n = 13), and 0.2% (n = 1), respectively. The frequent AEs (≥1%) were hyperuricemia (n = 30; 7.3%), hyperlipidemia (n = 23; 6.8%), dizziness (n = 12; 2.9%), hepatic dysfunction (n = 8; 2.0%), and headache (n = 4; 1.0%).
A total of 40 AEs (22.7%) in 24 patients were defined as ADRs. The major ADRs were dizziness (n = 8; 2.0%), hyperuricemia (n = 5; 1.2%), hepatic dysfunction (n = 3; 0.7%), headache (n = 3; 0.7%), and fatigue (n = 3; 0.7%) (Table III). ADR numbers for each study drug were 16 (3.9%) for OLM, 18 (4.4%) for AML, and 7 (25.0%) for OLM/HCTZ. Dizziness was the most frequent ADR for OLM (n = 7; 1.7%) and AML (n = 8; 2.0%) and hyperuricemia for OLM/HCTZ (n = 5; 17.9%) (Table IV). All ADRs were resolved without sequelae at the end of treatment.
Table III.
Summary of adverse events (AEs) and adverse drug reactions (ADRs).
| AE or ADR | Safety data set (n = 410) |
|---|---|
| n (%) | |
| All AEs | 176 (42.9) |
| Severity of AE | |
| Mild | 162 (39.5) |
| Moderate | 13 (3.2) |
| Severe | 1 (0.2) |
| ≥1% of AEs | |
| Hyperuricemia | 30 (7.3) |
| Hyperlipidemia | 23 (6.8) |
| Dizziness | 12 (2.9) |
| Hepatic dysfunction | 8 (2.0) |
| Headache | 4 (1.0) |
| All ADRs | 40 (9.8) |
| ≥0.7% of ADRs | |
| Dizziness | 8 (2.0) |
| Hyperuricemia | 5 (1.2) |
| Hepatic dysfunction | 3 (0.7) |
| Headache | 3 (0.7) |
| Fatigue | 3 (0.7) |
Table IV.
Summary of adverse events (AEs) and adverse drug reactions (ADRs) based on study drug. Total number of AEs = 176.
| Event or reaction | Result |
|---|---|
| n (%) | |
| Causality of all AEs with study drugs | |
| Not related | 136 (77.3) |
| Related | 40 (22.7) |
| Study drugs related with AEs | |
| OLM | 16 (3.9)⁎ |
| AML | 18 (4.4)† |
| OLM/HCTZ | 7 (25.0)‡ |
| Major AEs related with OLM | |
| Dizziness | 7 (1.7) |
| Major AEs related with AML | |
| Dizziness | 8 (2.0) |
| Major AEs related with OLM/HCTZ | |
| Hyperuricemia | 5 (17.9) |
AML = amlodipine; HCTZ = hydrochlorothiazide; OLM = olmesartan medoxomil.
Patients who received OLM = 410.
Patients who received AML = 410.
Patients who received OLM/HCTZ = 28.
There was 1 severe adverse effect (bone fracture of the lower leg) that occurred in this trial. It was not related to the study drugs.
Discussion
In a majority of clinical trials exploring OLM and AML combination therapy, the patients are not from Asia and there are limited numbers of Chinese cohorts. To our best knowledge, our study is first multicenter, open-label real-world study that demonstrates the efficacy and safety of OLM/AML and/or HCTZ in lowering BP in Chinese patients with hypertension who experienced poor control with AML 5 mg/d monotherapy. Most patients treated with combination therapy—approximately 97.1% at the end of the study—responded quickly and achieved a high response rate. Consistent with the effect on the primary outcomes, OLM/AML and/or HCTZ combination therapy during the 16 weeks also produced significant lowering in SeSBP/SeDBP. In our study, most of the patients (n = 333; 81.2%) received the basic dose of OLM/AML 20/5 mg/d and showed relatively high response (99.4%) of BP lowering effect. Most recently, a similar study conducted by Zhu et al16 demonstrated that OLM/AML 20/5 mg/d was superior to OLM 40 mg or AML 5 mg monotherapy in lowering BP in Chinese patients with mild-to-moderate hypertension and inadequate BP control on monotherapy. The response rate of OLM/AML 20/5 mg/d was superior to that of AML 5 mg monotherapy (84.5% vs 66.7%). Therefore, our results further confirm that dual combination therapy with the basic dosage of OLM/AML 20/5 mg/d is sufficient to produce significant BP lowering in patients with mild-to-moderate hypertension who failed to respond to AML 5 mg/d monotherapy.
More importantly, in the present study, a titration strategy was applied for the patients who still had uncontrolled BP with OLM/AML 20/5 mg/d at Week 8 or Week 12. Based on their BP status, the physicians switched to prescribing the highest dose of OLM combination (40 mg) or OLM/HCTZ compound (20/12.5 mg) for them. For patients overall, the primary end point of overall SeSBP/SeDBP response at the end of the treatment was as high as 97.1%, which is much higher than expected. Additionally, the mean changes of SeSBP/SeDBP from baseline were statistically significant during each time point (P < 0.001).
One possible explanation for these beneficial results is that the combination of different antihypertensive drugs may address the multifactorial nature of hypertension as a disease with many pathways. ARBs and CCBs have different pharmacologic pathways for lowering BP. In our study, some patients were not sensitive to CCBs (eg, AML monotherapy), which means their renin-angiotensin system may exert more important roles in BP control. Hence, these patients may be more sensitive to ARB (eg, OLM) combination treatment. Several global and Chinese guidelines3, 9, 17, 18 have recommended >2 combination therapies with different categories of antihypertensive drugs. The dose regimen administered in our study is more suitable for Chinese patients than that of experts’ recommendations. Another possible reason is that a double dose of OLM or OLM/HCTZ 20/12.5 mg/d was administered for patients whose BP was still uncontrolled with OLM/AML 20/5 mg/d at Week 8 or Week 12. This adding-dose strategy was entirely based on different patients’ medical conditions, which might contribute to the greater BP lowering and higher BP control rate achieved at the end of the study. Thus, our study provided more evidence to support the conclusion that combination therapy with an ARB and a CCB is more effective than monotherapy. Furthermore, compared with several other ARBs, OLM is more effective than losartan, candesartan, or valsartan monotherapy over 24 hours, the daytime, night-time, and end-of-dosing interval periods and was at least as efficacious as irbesartan.19, 20, 21 OLM is globally known as 1 of the ARBs associated with antihypertensive effect. AML is 1 of most commonly used drugs in each antihypertensive drug category in China. Moreover, the OLM/HCTZ combination provided substantial reductions in SBP/DBP that were greater than monotherapy with either agent alone.22 Hence, combination therapy with these drugs is a valuable tool for Chinese physicians.
The design of our study was not the same as that of previous studies. But, the result of Blood Pressure Control in All Subgroups with Hypertension study (BP-CAUSH) is relatively comparable.23 The baseline BP level of BP-CAUSH study patients was 154/92 mm Hg, which was similar to our study (149/96 mm Hg). In our study, most patients were suitable for therapy with OLM/AML 20/5 mg/d (84.3% of total patients), whereas in the BP-CAUSH study the regimen likely selected was forced titration to triple combination with OLM/AML/HCTZ 20/5/25 mg/d and 20/5/12.5 mg/d (49.7% and 69.9% of total patients, respectively). In addition, the patients in the BP-CAUSH study presented with risk factors such as hyperglycemia, metabolic syndrome, and higher body mass index. Generally, the result of the BP goal ratio in our study is higher than in BP-CAUSH, although our dosage of the OLM combination was lower and mainly a dual combination.
The response rate of patients with diabetes at Week 16 was 88.9%. However, the BP goal ratio in patients with diabetes in our study was 38.9%, which was quite a bit lower than that in the previous study—55%—reported by Ram et al.24 The main reason was that a higher dose of OLM combination (OLM/AML/HCTZ 40/10/12.5 and 40/10/25 mg/d) and a longer treatment period (18 weeks) were employed in the study by Ram et al24 than in our study. Indeed, patients with hypertension and diabetes require a higher dosage of OLM combination (dual or triple therapy) according to their individual conditions. Moreover, the number of patients with diabetes in our study was quite small (n = 54), so our efficacy results for patients with diabetes should only be used as a reference. Further clinical study of patients with hypertension and diabetes in China is needed.
Combination therapy with OLM/AML was safe and well tolerated in patients with hypertension, and no new safety issues were observed. Dizziness was the only OLM- and ALM-related AE that occurred in >1% of cases. This may be due to overlowering of BP. In addition, the reported OLM/HCTZ-related AE was mainly hyperuricemia (17.9%), which is a common metabolic side effect of taking thiazide diuretics.25 Although the exact mechanism of HCTZ-induced hyperuricemia remains unclear, it is possible that HCTZ may increase urine acid through diverse mechanisms, including impairment of urine acid secretion secondary to volume depletion.26 Several previous studies found that AML is often associated with a relatively high rate of peripheral edema.27, 28 Conversely, in this study, the incidence of peripheral edema (0.5% in AEs and 0.2% in ADRs) was extremely low with combination therapy.
Several limitations of this study should be noted. The open-label, single-arm design of the study may possibly have results with treatment bias due to lack of blinding. Also, the sample of patients with diabetes was relatively small (n = 54). Because of low statistical power, these results need to be evaluated with caution when extrapolating the results to similar populations seen in clinical practice. In addition, the long-term efficacy and safety of OLM/AML or AML/OLM/HCTZ has been reported previously,29, 30 and the total treatment period of 16 weeks in our study is comparably short. It would be interesting to carry out double-blind and placebo-controlled studies to evaluate long-term efficacy of large populations of patients with diabetes and hypertension in the future.
Conclusions
This study confirmed the fact that combination therapy with OLM/AML or AML/OLM/HCTZ can effectively control BP and is a well-tolerated option for patients with hypertension who have not adequately responded to AML monotherapy. More importantly, the majority of patients with diabetes and hypertension whose BP was not controlled by antihypertensive monotherapy also achieved BP control with an OLM/AML-based combination therapy.
Acknowledgment
All authors contributed to literature search, figure creation, study design, data collection, data interpretation, and writing of the manuscript and approved the final version.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- 1.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X.L., Chen J., Cui Y.L. Current status of primary hypertension in China: an epidemiological study of 12 provinces, 1 autonomous region and 1 municipality. Chinese Medical Journal. 2006;86(17):1148–1152. [PubMed] [Google Scholar]
- 3.Liu L.S. 2010 Chinese guidelines for the management of hypertension. Chinese Journal of Cardiology. 2011;39(7):579–615. [PubMed] [Google Scholar]
- 4.Xiao Y.L. Antihypertensive drugs for patients with hypertension: combined therapy and individual treatment. Guide of China Medicine. 2009;7(14):74–75. [Google Scholar]
- 5.Han W.H., Chen H.Q. Analysis of application of antihypertensive drugs in a hospital from 2009 to 2011. Practical Preventive Medicine. 2012;19(6):9350936. [Google Scholar]
- 6.Flack J.M., Calhoun D.A., Satlin L. Efficacy and safety of initial combination therapy with amlodipine/valsartan compared with amlodipine monotherapy in black patients with stage 2 hypertension: the EX-STAND study. Journal of Human Hypertension. 2009;23:479–489. doi: 10.1038/jhh.2008.153. [DOI] [PubMed] [Google Scholar]
- 7.Drummond W., Munger M.A., Essop M.R. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. The Journal of Clinical Hypertension. 2009;9(10):742–750. doi: 10.1111/j.1524-6175.2007.06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrader J., Salvetti A., Calvo C. The combination of amlodipine/valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. The International Journal of Clinical Practices. 2009;63(2):217–222. doi: 10.1111/j.1742-1241.2008.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G., Fagard R., Narkiewicz K. 2013 ESH/ESC guidelines for the management of arterial hypertension. Journal of Hypertension. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 10.Chrysant S.G., Oparil S., Melino M., Karki S., Lee J., Heyrman R. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens (Greenwich) 2009;11(9):475–482. doi: 10.1111/j.1751-7176.2009.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner G.T., Jarvis B. Olmesartan Medoxomil. Drugs. 2002;62(9):1345–1353. doi: 10.2165/00003495-200262090-00005. [DOI] [PubMed] [Google Scholar]
- 12.Brunner H.R., Laeis P. Clinical efficacy of olmesartan medoxomil. J Hypertens Suppl. 2003;21(2):S43–S46. doi: 10.1097/00004872-200305002-00008. [DOI] [PubMed] [Google Scholar]
- 13.Giles T.D., Oparil S., Silfani T., Wang A., Walker F. Comparison of increasing doses of olmesartan medcxoniil. Losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens (Greenwich) 2007;9(3):187–195. doi: 10.1111/j.1524-6175.2007.06395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith D., Dubiel R., Jones M. Use of 24-hour ambulatory blood pressure monitoring lo assess antihypertensive efficacy: a comparison of olmesartan medoxomil. Losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5(1):41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Volpe M., Brommer P., Haag U., Miele C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29(1):11–25. doi: 10.2165/0044011-200929010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J.R., Zhang S.Y., Gao P.J. Efficacy and safety of olmesartan medoxomil/amlodipine fixed-dose combination for hypertensive patients uncontrolled with monotherapy. Arch Pharm Res. 2014 July:25. doi: 10.1007/s12272-014-0446-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Mancia G., De Backer G., Dominiczak A. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 18.Dahlof B., Sever P.S., Poulter N.R. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial- Blood Pressure Lowering Arm (ASCOT-BPLA): a mulicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 19.Brunner H.R., Stumpe K.O., Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Invest. 2003;23(7):419–430. doi: 10.2165/00044011-200323070-00001. [DOI] [PubMed] [Google Scholar]
- 20.Oparil S., Williams D., Chrysant S.G., Marbury T.C., Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich) 2001;3(5):283–291. doi: 10.1111/j.1524-6175.2001.01136.x. 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith D.H., Dubiel R., Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5(1):41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kostis J.B., Silfani T. The combination of olmesartan medoxomil plus hydrochlorothiazide in subjects with stage 2 hypertension: Results of a randomized, double-blind, factorial-design study. Am J Hypertens. 2004;17(S1):114A. [Google Scholar]
- 23.Weir M.R., Hsueh W.A., Nesbitt S.D. A titrate-to-goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed-dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide. J Clin Hypertens (Greenwich) 2011;13(6):404–412. doi: 10.1111/j.1751-7176.2011.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram C.V., Sachson R., Littlejohn T., Qian C., Shojaee A., Stoakes K.A., Neutel J.M. Management of hypertension in patients with diabetes using an amlodipine-, olmesartan medoxomil-, and hydrochlorothiazide-based titration regimen. Am J Cardiol. 2011;107(9):1346–1352. doi: 10.1016/j.amjcard.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Elliott W.J., Weber R.R., Murphy M.B. A double-blind, randomized, placebo-controlled comparison of the metabolic effects of low-dose hydrochlorothiazide and indapamide. J Clin Pharmacol. 1991;31(8):751–757. doi: 10.1002/j.1552-4604.1991.tb03772.x. [DOI] [PubMed] [Google Scholar]
- 26.Vandell A.G., McDonough W., Gong Y. Hydrochlorothiazide-induced hyperuricaemia in the pharmacogenomic evaluation of antihypertensive responses study. Journal of Internal Medicine. 2014 Feb 12 doi: 10.1111/joim.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonetti G., Maganai B., Pessina A., Rappelli A., Trimarco B., Zanchett A. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. American Journal of Hypertension. 2002;15(11):932–940. doi: 10.1016/s0895-7061(02)03000-5. [DOI] [PubMed] [Google Scholar]
- 28.White W., Duprez D., St, Hillarie R., Krause S., Roniker B., Kuse-Hamilton J., Weber M. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41(5):1021–1026. doi: 10.1161/01.HYP.0000067463.13172.EA. [DOI] [PubMed] [Google Scholar]
- 29.Volpe M., Miele C., Haag U. Efficacy and safety of a stepped-care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate-to-severe hypertension: an open-label, long-term study. Clinical Drug Investigation. 2009;29:381–391. doi: 10.2165/00044011-200929060-00002. [DOI] [PubMed] [Google Scholar]
- 30.Oparil S., MelinOLMee J. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: The TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–1269. doi: 10.1016/j.clinthera.2010.07.008. [DOI] [PubMed] [Google Scholar]