Abstract
Evolution generates a remarkable breadth of living forms, but many traits evolve repeatedly, by still poorly understood mechanisms. A classic example of repeated evolution is the loss of pelvic hindfins in stickleback fish (Gasterosteus aculeatus). Repeated pelvic loss maps to recurrent deletions of a pelvic enhancer of the Pitx1 gene. Here, we identify molecular features contributing to these recurrent deletions. Pitx1 enhancer sequences form alternative DNA structures in vitro, and increase double-strand breaks and deletions in vivo. Enhancer mutability depends on DNA replication direction and is caused by (TG)-dinucleotide repeats. Modeling shows that elevated mutation rates can influence evolution under demographic conditions relevant for sticklebacks and humans. DNA fragility may thus help explain why the same loci are often used repeatedly during parallel adaptive evolution.
ONE SENTENCE SUMMARY:
DNA fragility and high mutation rates influence the genomic pathways of adaptive evolution.
MAIN TEXT
Many phenotypic traits evolve repeatedly in organisms adapting to similar environments, and studying these cases can reveal ecological and genetic factors shaping parallel evolution (1, 2). For example, loss of pelvic appendages has evolved repeatedly in mammals, amphibians, reptiles, and fishes. Marine stickleback fish (Gasterosteus aculeatus) develop a robust pelvic apparatus, whereas many freshwater populations have lost pelvic structures (3). Pelvic reduction is associated with particular ecological conditions, is likely adaptive, and maps to recurrent and independent deletions of a pelvic enhancer (Pel) upstream of the homeodomain transcription factor gene (Pitx1) that also show repeatable molecular signatures of positive selection (4–7). This unusual spectrum of regulatory deletions contrasts with the accumulation of single nucleotide changes in other studies (8, 9), hinting that special DNA features may shape adaptive variation at the Pitx1 locus (6).
Pel enhancer sequences show high predicted helical twist flexibility (6), a DNA feature associated with delayed replication and fragile site instability (10). To examine whether Pel forms alternative DNA structures in vitro, we used 2-dimensional electrophoresis to analyze distributions of plasmid topoisomers (11) (Fig. 1A). A control stickleback genomic region showed smooth curves characteristic of B-DNA (Fig. 1B). In contrast, Pel sequences from marine populations showed mobility shifts characteristic of alternative DNA structure formation (Fig. 1B). Structural transitions started at a negative superhelical density of −σ = 0.043 and changed apparent linking numbers by 10–16 helical turns, similar to shifts produced by Z-DNA (left-handed DNA, starting −σ = 0.046) occupying ~105–170 bp (12, 13). Pel sequences from pelvic-reduced populations did not show unusual electrophoretic transitions (Fig. 1B), suggesting that natural mutations remove sequences forming alternative DNA structures.
Figure 1. Marine but not freshwater Pel alleles form alternative structures in vitro.
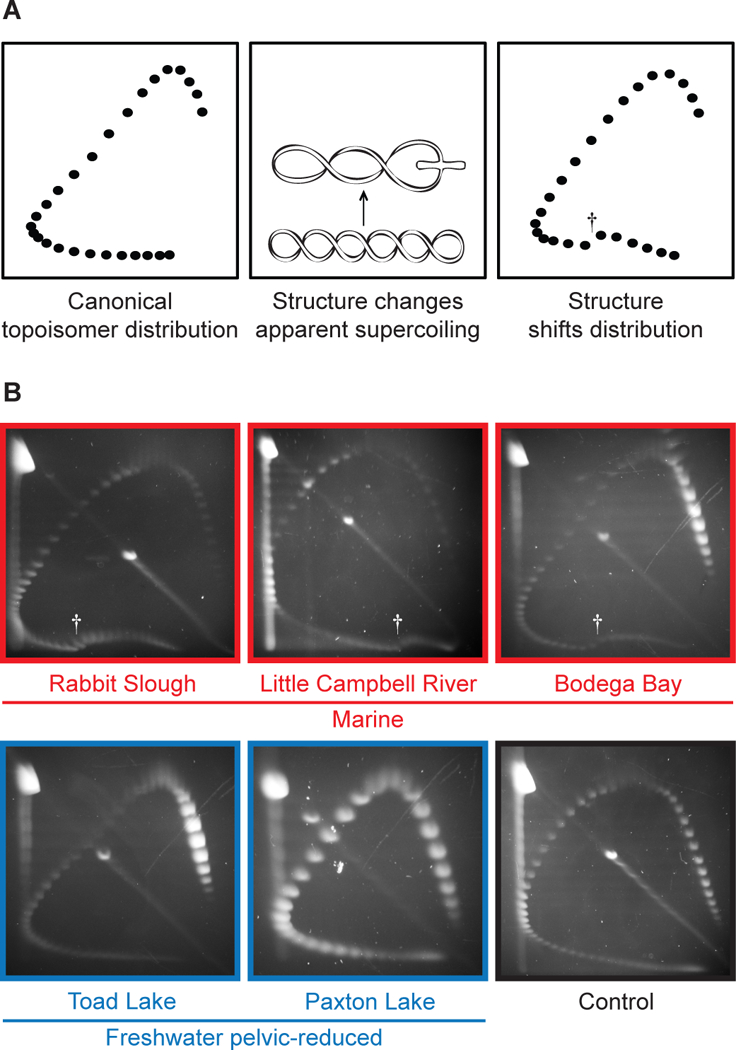
(A) Two-dimensional electrophoresis of circular DNA topoisomers. A distribution of plasmid topoisomers is separated on an agarose gel; each topological class forms one spot. Canonical B-DNA forms a smooth distribution. Alternative structures cause mobility shifts. Distribution shifts at the linking number inducing alternative structure. Dagger, mobility shift. (B) Pel from marine and freshwater pelvic-reduced populations. Control, AtpA1.
To test the effect of Pel sequences on chromosome stability in vivo, we measured the rate of DNA double-strand breaks in yeast artificial chromosomes (Fig. 2A). Constructs without added test regions broke at background rates of 3.37 breaks per 106 divisions (Fig. 2B), consistent with previous reports (14). Chromosomes containing marine Pel broke ~25–50 times more frequently (Fig. 2B), a rate even higher than previously analyzed human fragile sites (14). Pel from freshwater pelvic-reduced populations (but not freshwater pelvic-complete populations (fig. S1)) broke at rates similar to the control (Fig. 2B), suggesting that natural Pel mutations remove breakage-prone regions.
Figure 2. Marine but not freshwater Pel alleles break at high rates in yeast, in an orientation-dependent fashion.
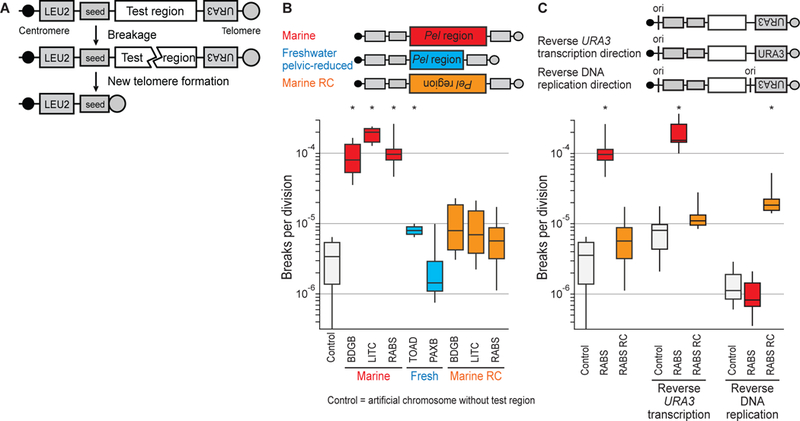
(A) Test DNA is inserted in a yeast artificial chromosome between two selectable markers (LEU2 and URA3) and downstream of a telomere seed site. Breakage results in loss of URA3. (B) Box-and-whisker plot of Pel breakage rates. Whisker ends indicate maximum and minimum of 6 fluctuation assays (10 cultures each). RC, reverse complement. *p < 0.01 (table S5). Population names, table S6. (C) Reversing replication direction through the test region, but not URA3 transcription direction, reverses orientation of fragility. ori, DNA replication origin.
Reverse complements of marine Pel broke ~10–20 times less frequently than identical sequences in the forward orientation (Fig. 2B). RNA transcription can influence fragile site breakage (15), but reversing transcription orientation of the nearby URA3 marker did not significantly affect Pel fragility (Fig. 2C). In contrast, adding a replication origin on the opposite side of Pel did switch fragility, making the forward sequence stable, and the reverse complement fragile (Fig. 2C). Thus, Pel fragility is markedly dependent on DNA replication direction.
Pel contains abundant runs of alternating pyrimidine-purine repeats (Fig. 3A, file S1), which can adopt alternative structures like Z-DNA, previously associated with deletions in bacteria, mice, and humans (16, 17). Three stretches of ~15, ~20, and ~50 (TG)-dinucleotide repeats in marine Pel total ~170 bp (consistent with linking number changes seen in topoisomer assays above). TG-repeats alone induced mobility shifts in topoisomer assays (Fig. 3B) (18) and elevated chromosome breakage in yeast, with longer repeats stimulating more breaks (Fig. 3C). In contrast, both long and short versions of the reverse complement sequence (CA-repeats) were stable (Fig. 3C), recapitulating the orientation dependence of Pel fragility.
Figure 3. (TG)-dinucleotide repeats recapitulate structure formation, high breakage rate, orientation-dependence, and deletion spectrum.
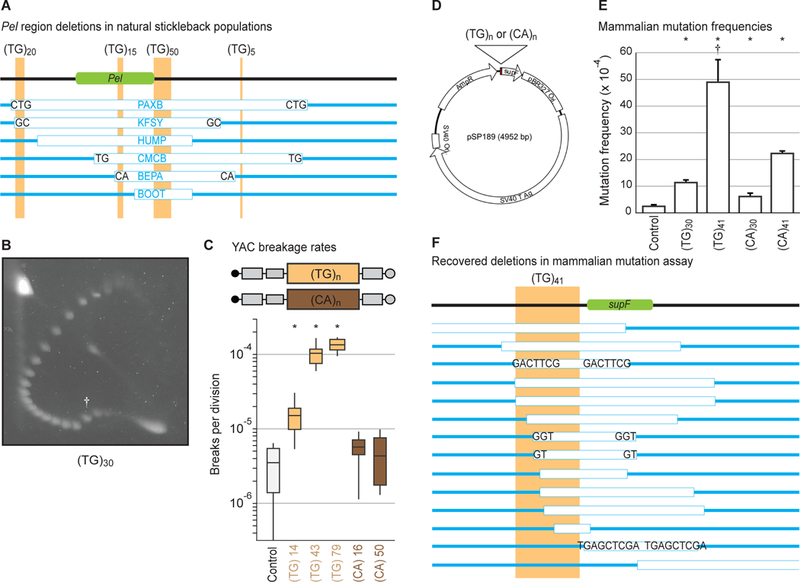
(A) To-scale maps of Pel in different freshwater pelvic-reduced populations (table S6). Green, Pel sequence driving pelvis expression (6). Light-brown, TG-repeats. White boxes, DNA deletions in indicated populations. Blue, DNA remaining. Letters, microhomologies at deletion junctions. (B) Two-dimensional gel for (TG)30. Dagger, mobility shift. (C) Yeast artificial chromosome breakage rates for TG- or CA-repeats of varying lengths. *p < 0.01 (table S5). (D) Reporter shuttle plasmid schematic. (E) Mammalian mutation frequencies. Error bars indicate SEM of 4–5 independent experiments. *p < 0.05 (Student’s t-test). Dagger, deletions dominate mutation spectrum (fig. S2A). (F) To-scale map of (TG)41-induced deletions in mammalian cells.
We also tested the effect of TG- and CA-repeats in mammalian COS-7 cells (Fig. 3D) (19). Dinucleotide repeats elevated mutation frequencies, with TG-repeats being more mutagenic than CA-repeats of comparable lengths, and longer repeats being more mutagenic than shorter repeats (Fig. 3E), consistent with results from yeast assays. Mutations stimulated by the most mutagenic sequence, (TG)41, were predominantly >100 bp deletions that removed part or all of the repeat and adjacent reporter gene (Fig. 3F, fig. S2A). Approximately 70% of deletion junctions contained microhomologies and insertions (Fig. 3F, fig. S2A–B), consistent with error-prone microhomology-mediated end-joining repair, and similar to junctions seen in stickleback pelvic-reduction alleles (6) (Fig. 3A). Ligation-mediated PCR suggested that breaks initiated near the dinucleotide repeats (fig. S2C). Taken together, our results indicate that TG-repeats form alternative DNA structures in vitro and can recapitulate the high mutation rates, orientation-dependence, and propensity to stimulate breaks and deletions of the full Pel region.
To determine the orientation of Pel sequences relative to DNA replication in sticklebacks (Fig. 4A, fig. S3), we sequenced S- and G-phase cells from developing embryos and calculated S:G read-depth ratios to determine replication timing (20). Pel is located in a timing transition region (Fig. 4B, fig. S4), consistent with unidirectional replication. The replication direction through Pel matches the fragile orientation (Fig. 4C), suggesting that Pel would form a TG-repeat-associated fragile site in vivo. Experimental CRISPR targeting confirmed that initiation of breaks in Pel were sufficient to trigger local DNA deletions and macroscopic loss of pelvic structures in genetic crosses (fig. S5).
Figure 4. Pel is located in the breakage-prone orientation in sticklebacks, generating a fragile site likely to contribute to parallel evolution in natural populations.
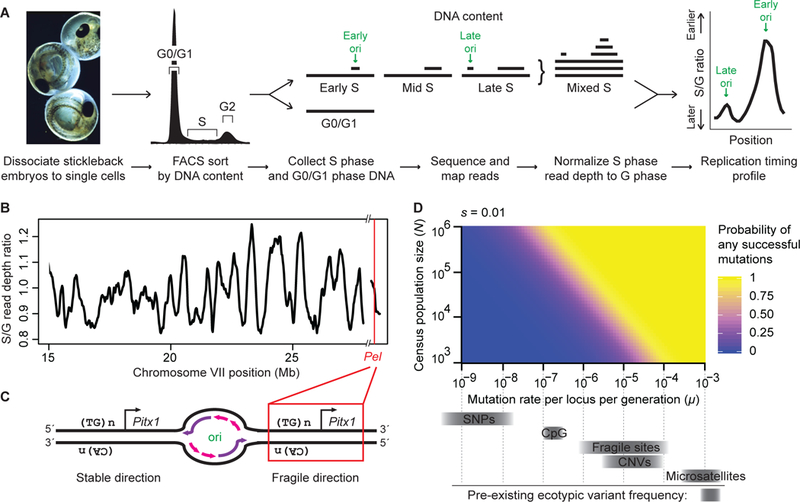
(A) Workflow for profiling genome-wide replication timing. (B) Stickleback chromosome VII replication timing. Red line, Pel locus, which is subtelomeric. Hash marks, reference genome assembly gap. (C) Diagrams of stable and fragile replication orientations. ori, origin bubble. Purple, newly synthesized leading strand. Pink, newly synthesized lagging strand. (D) Probability of at least one de novo mutation arising at a particular locus in 10,000 generations and eventually fixing, as a function of typical stickleback population sizes (N) and mutation rates (μ, grey bars) for single nucleotides (SNPs), copy number variants (CNVs), and fragile sites. De novo point mutations are unlikely to occur and fix in small vertebrate populations, even when conferring a selective advantage (s=0.01, modeled here). In contrast, mutations occurring at fragile sites are likely to arise and contribute to repeated evolution when conferring a selective advantage. For additional parameters, including neutrality (s=0), see fig. S6 and fig. S7.
Could elevated mutation rates contribute to reuse of Pel deletions in parallel evolution? Population genetic modeling indicates that new mutations occurring at the low rates of typical single nucleotide changes (~10−9) would rarely arise at a particular locus in postglacial stickleback populations, while mutations occurring at elevated rates (~10−5 for fragile sites) would arise often. When new mutations do occur, their subsequent fate is controlled by drift and selection (21). Neutral or small-effect point mutations will usually be lost or rise to fixation slowly, while deletions may cause larger phenotypic effects and can sweep if environmental conditions favor pelvic reduction (Fig. 4D, fig. S6, fig. S7). The combined effects on both the “arrival of the fittest” and the “survival of the fittest” may explain why recurrent Pel deletions are the predominant mechanism for evolving stickleback pelvic reduction. For other traits, ancient standing variants provide an alternative way to overcome the demographic constraints of waiting for de novo mutations in small populations, and can also lead to reuse of similar alleles in different populations (22, 23).
The demographic parameters typical of sticklebacks apply to many vertebrates evolving with small population sizes or facing rapid environmental changes. For example, migration of modern humans out of Africa occurred with relatively small populations adapting to new environments in 3,000 generations or less (24). Interestingly, nearly half of currently known mutations underlying adaptive traits in modern humans also appear to be produced by mechanisms with elevated mutation rates (table S1).
High mutation rates have been described at contingency loci in bacteria and other systems (25–30). Our studies add an important new example of DNA fragility contributing to repeated morphological evolution in vertebrates. Our data also highlight several mechanisms that could alter local mutation rates, including expansion/contraction of TG-repeats, changes in sequence orientation, or changes in DNA replication. Natural variation in such parameters may affect the evolvability of different loci and the particular genetic paths likely to be taken when ecological conditions favor a given phenotype. The sequence features associated with DNA fragility in the Pel region are also found in thousands of other positions in stickleback and human genomes (fig. S8). Notably, TG-repeats are enriched in other loci that have undergone recurrent ecotypic deletions during marine-freshwater stickleback evolution (31) (table S2, fig. S9), and near DNA breakage sites in humans (fig. S10). As causative changes are identified for more phenotypic traits, it will be interesting to see the extent to which DNA fragility has influenced the genes and mutations that underlie evolutionary change in nature.
Supplementary Material
ACKNOWLEDGEMENTS
We thank V. Tien, J. Le, M. Yau, M. Thakur, A. Muralidharan, M. Whitlock, B. Belotserkovskii, R. Driscoll, K. Cimprich, J. Wang, S. Quake, and A. Casper for experimental assistance or advice; R. Daugherty, J. Rollins, B. Lohman, R. Mollenhauer, M. Reyes, and F. von Hippel for help with fieldwork; C. Freudenreich for yeast strains; Z. Weng and B. Carter for help with high-throughput sequencing and cell sorting.
Funding: NIH grants 5P50HG2568 (D.M.K.), CA093729 (K.M.V.), 2T32GM007790 (J.I.W); NSF grant DEB0919184 (M.A.B.); NSF and Stanford CEHG Graduate Fellowships (K.T.X.), NIH Predoctoral Fellowship (ACT); HHMI investigator (D.M.K.).
Footnotes
Competing interests: None.
Data and materials availability: GEO accession GSE121537.
REFERENCES AND NOTES
- 1.Schluter D, Clifford EA, Nemethy M, McKinnon JS. Am Nat 163, 809–822 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Stern DL, Orgogozo V. Science 323, 746–751 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell MA. Biol J Linn Soc 31, 347–382 (1987). [Google Scholar]
- 4.Reimchen TE. Can J Zool 58, 1232–1244 (1980). [Google Scholar]
- 5.Bell MA, Orti G, Walker JA, Koenings JP. Evolution 47, 906–914 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Chan YF et al. Science 327, 302–305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karhunen M, Merila J, Leinonen T, Cano JM, Ovaskainen O. Mol Ecol Resour 13, 746–754 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Prud’homme B et al. Nature 440, 1050–1053 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Stern DL, Frankel N. Philos T R Soc B 368, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thys RG, Lehman CE, Pierce LC, Wang YH. Curr Genomics 16, 60–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowater R, Aboul-Ela F, Lilley DM. Methods Enzymol 212, 105–120 (1992). [DOI] [PubMed] [Google Scholar]
- 12.Nordheim A, Rich A. Proc Natl Acad Sci USA 80, 1821–1825 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich A, Nordheim A, Wang AH. AnnuRevBiochem 53, 791–846 (1984). [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Freudenreich CH. Mol Cell 27, 367–379 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmrich A, Ballarino M, Tora L. Mol Cell 44, 966–977 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Christensen LA, Vasquez KM. Proc Natl Acad Sci USA 103, 2677–2682 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Carbajal S, Vijg J, DiGiovanni J, Vasquez KM. J Natl Cancer Inst 100, 1815–1817 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamada H, Petrino MG, Kakunaga T, Seidman M, Stollar BD. Mol Cell Biol 4, 2610–2621 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Vasquez KM. Proc Natl Acad Sci USA 101, 13448–13453 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhind N, Gilbert DM. Cold Spring Harb Perspect Biol 5, a010132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura M. Genetics 47, 713–719 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colosimo PF et al. Science 307, 1928–1933 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Barrett RD, Schluter D. Trends Ecol Evol 23, 38–44 (2008). [DOI] [PubMed] [Google Scholar]
- 24.1000_Genomes_Project_Consortium. Nature 526, 68–74 (2015).26432245 [Google Scholar]
- 25.Moxon R, Bayliss C, Hood D. Annu Rev Genet 40, 307–333 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Stoltzfus A, Yampolsky LY. J Hered 100, 637–647 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Du X et al. Nucleic Acids Res 42, 12367–12379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galen SC et al. Proc Natl Acad Sci USA 112, 13958–13963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacolla A, Tainer JA, Vasquez KM, Cooper DN. Nucleic Acids Res 44, 5673–5688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hargreaves AD et al. Proc Natl Acad Sci USA 114, 7677–7682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe CB et al. Genome Res 28, 256–265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


