Methanogenic hydrocarbon degradation is the major process for oil degradation in subsurface oil reservoirs and is blamed for the formation of heavy oil and oil sands. Addition of n-alkanes to fumarate yielding alkyl-substituted succinates is a well-characterized anaerobic activation mechanism for hydrocarbons and is the most common activation mechanism in the anaerobic biodegradation of n-alkanes with chain lengths less than C16. However, the activation mechanism involved in the methanogenic biodegradation of n-alkanes longer than C16 is still uncertain. In this study, we analyzed a methanogenic enrichment culture amended with a mixture of C16 to C20 n-alkanes. These n-alkanes can be activated via fumarate addition by mixed cultures containing Smithella and Desulfatibacillum species under methanogenic conditions. These observations provide a fundamental understanding of long-n-alkane metabolism under methanogenic conditions and have important applications for the remediation of oil-contaminated sites and for energy recovery from oil reservoirs.
KEYWORDS: (1-methylalkyl)succinate, biodegradation, fumarate addition, hydrocarbon, long n-alkanes, methanogenesis
ABSTRACT
Methanogenic degradation of n-alkanes is prevalent in n-alkane-impacted anoxic oil reservoirs and oil-polluted sites. However, little is known about the initial activation mechanism of the substrate, especially n-alkanes with a chain length above C16. Here, a methanogenic C16 to C20 n-alkane-degrading enrichment culture was established from production water of a low-temperature oil reservoir. At the end of the incubation (364 days), C16 to C20 (1-methylalkyl)succinates were detected in the n-alkane-amended enrichment culture, suggesting that fumarate addition had occurred in the degradation process. This evidence is supported further by the positive amplification of the assA gene encoding the alpha subunit of alkylsuccinate synthase. A phylogenetic analysis shows these assA amplicons to be affiliated with Smithella and Desulfatibacillum clades. Together with the high abundance of these clades in the bacterial community, these two species are postulated to be the key players in the degradation of C16 to C20 n-alkanes in the present study. Our results provide evidence that long n-alkanes are activated via a fumarate addition mechanism under methanogenic conditions.
IMPORTANCE Methanogenic hydrocarbon degradation is the major process for oil degradation in subsurface oil reservoirs and is blamed for the formation of heavy oil and oil sands. Addition of n-alkanes to fumarate yielding alkyl-substituted succinates is a well-characterized anaerobic activation mechanism for hydrocarbons and is the most common activation mechanism in the anaerobic biodegradation of n-alkanes with chain lengths less than C16. However, the activation mechanism involved in the methanogenic biodegradation of n-alkanes longer than C16 is still uncertain. In this study, we analyzed a methanogenic enrichment culture amended with a mixture of C16 to C20 n-alkanes. These n-alkanes can be activated via fumarate addition by mixed cultures containing Smithella and Desulfatibacillum species under methanogenic conditions. These observations provide a fundamental understanding of long-n-alkane metabolism under methanogenic conditions and have important applications for the remediation of oil-contaminated sites and for energy recovery from oil reservoirs.
INTRODUCTION
Methanogenic biodegradation of petroleum hydrocarbons in subsurface reservoirs contributes to the occurrence of heavy oil and deterioration of oil quality (1, 2). Alternatively, it has been speculated that residual oil hydrocarbons can be converted to methane for energy recovery by subsurface microorganisms (3, 4). n-Alkanes as the major components of crude oils have been reported to be biodegraded under methanogenic conditions (5, 6). Different activation mechanisms for the anaerobic degradation of n-alkanes, including fumarate addition, hydroxylation/carboxylation, intrahydroxylation, and other mechanisms, have been proposed (7).
Fumarate addition is the most well-characterized activation mechanism of n-alkane degradation under anaerobic conditions. It is catalyzed by alkylsuccinate synthase to add a subterminal carbon of an n-alkane to the double bond of fumarate (7). This has been demonstrated under sulfate- and nitrate-reducing conditions with pure isolates or mixed cultures (8). Dodecylsuccinic acid and (1-methylpentyl)succinate were detected in a sulfate-reducing enrichment culture amended with n-dodecane (9) and in a pure culture of nitrate-reducing strain HxN1 growing on n-hexane (10), respectively. The functional assA genes (encoding the alpha subunit of alkylsuccinate synthase) have been detected in methanogenic alkane-degrading enrichment cultures (11, 12) and in oil-containing zones (13, 14). Recently, Berdugo-Clavijo et al. detected C5 and C6 (1-methylalkyl)succinates in a methanogenic crude oil-degrading enrichment culture derived from oil production water (15). Using the same oil field production water as a source of microorganisms, Toth et al. further detected C1 to C9 (1-methylalkyl)succinates in methanogenic enrichment cultures utilizing crude oil (16). C15 and C16 (1-methylalkyl)succinates were identified in methanogenic enrichment cultures derived from oily sludge-contaminated sediments of an oil field incubated with pentadecane and hexadecane, respectively (17). Additionally, C1 to C8 alkylsuccinates have been detected in production fluids from oil reservoirs (14, 18–20). However, alkylsuccinates derived from n-alkanes longer than C16 have not been detected under methanogenic conditions.
While the mechanism of fumarate addition is used by most anaerobic n-alkane-degrading bacteria, there are several strains known to activate n-alkanes by other mechanisms, including hydroxylation at the subterminal carbon atom, followed by carboxylation at C-3. This was observed for the sulfate-reducing strain Hxd3 (21). Previous studies showed that this strain carries genes encoding an ethylbenzene dehydrogenase-like enzyme rather than genes coding for the alkylsuccinate synthase enzyme (22). A second alternative mechanism for the activation of n-alkanes is the reduction of NO species or the dismutation of oxygenated nitrogen species (NO3– or NO2–) to generate oxygen, which may participate in the hydroxylation with a monooxygenase in the nitrate-reducing strain HdN1 (23). Recently, anaerobic biodegradation of n-alkanes was also observed in archaea. The sulfate-reducing archaeon Archaeoglobus fulgidus strain VC-16 carrying genes encoding an enzyme closely related to alkylsuccinate synthase may activate n-alkanes via the fumarate addition mechanism (24). The archaeal genus “Candidatus Syntrophoarchaeum” can activate n-butane via alkyl-coenzyme M formation in an anaerobic thermophilic enrichment culture (25).
Although the metabolite profiles of methanogenic biodegradation of long n-alkanes have been determined previously (11, 12, 26), the detection of putative initial intermediates is still limited. Even though a significant increase of assA gene expression has been observed in an octacosane-degrading culture, the corresponding fumarate addition product of octacosane has still not been detected due to the extremely low concentration and high turnover of these metabolites in the syntrophic culture (26). Interestingly, Oberding and Gieg (26) detected several dicarboxylic acids and speculated that both ends of the long n-alkane can be activated simultaneously by fumarate addition, leading ultimately to the generation of a carboxylic acid group at each end of the molecule. Their study indicated that a new activation mechanism may be involved in methanogenic long-n-alkane degradation due to the extreme length and nonreactivity of the n-alkane. Here, we report the formation of long-chain (1-methylalkyl)succinates (C16 to C20) in a methanogenic C16 to C20 n-alkane-degrading enrichment culture and show that fumarate addition occurs in methanogenic long-n-alkane degradation.
RESULTS
Methane production in methanogenic enrichment cultures.
Methane production was detected after the initial 85 days of incubation and ultimately reached about 57 μmol at the end of the incubation (364 days) in the n-alkane-amended enrichment cultures (Fig. 1). Methane was not produced in the control cultures (Fig. 1). This suggests that the net methane detected in the n-alkane-amended enrichment cultures was generated mainly from n-alkanes. Volatile fatty acid analysis showed the presence of formate, acetate, propionate, and butyrate in both cultures, but levels were higher in the amended enrichment cultures than in the control cultures (see Table S1 in the supplemental material). Residual n-alkane analysis showed that a total of 0.25 mmol of n-alkanes has been consumed in the n-alkane-amended enrichment cultures (Table S2).
FIG 1.

Accumulative methane production in the enrichment cultures amended with a total of 0.90 mmol C16 to C20 n-alkanes (0.18 mmol each) (squares) and the control cultures without n-alkane (circles). Error bars represent 1 standard error for duplicate cultures.
(1-Methylalkyl)succinate in methanogenic enrichment cultures.
At the end of the incubation, a range of long-chain (1-methylalkyl)succinates (C16 to C20) was identified in the n-alkane-amended enrichment cultures (Fig. 2). The mass spectra of these compounds are shown in Fig. 3. No (1-methylalkyl)succinate was detected in the control cultures. Consistent with previous studies, the putative downstream metabolites of the fumarate addition mechanism, such as 2-methyl and 4-methyl fatty acids (27), were not detectable in both enrichment cultures (with and without alkanes) (12, 17). None of the putative metabolites involved in the hydroxylation/carboxylation mechanism for anaerobic n-alkane degradation, such as 2-ethylalkanoic acid and 2-acetylalkanoic acid, were detectable (28). Also, no dicarboxylic acids were detected in this study.
FIG 2.
Partial GC-MS selected ion chromatogram (m/z 128 and m/z 174) of an ethylated extract of the methanogenic enrichment cultures amended with C16 to C20 n-alkanes. The presence of five (1-methylalkyl)succinate diethyl esters were identified as (1-methylpentadecyl)succinate diethyl ester (C16), (1-methylhexadecyl)succinate diethyl ester (C17), (1-methylheptadecyl)succinate diethyl ester (C18), (1-methyloctadecyl)succinate diethyl ester (C19) and (1-methylnonadecyl)succinate diethyl ester (C20). These (1-methylalkyl)succinates had been generated by fumarate addition to the n-alkanes hexadecane (C16), heptadecane (C17), octadecane (C18), nonadecane (C19), and eicosane (C20), respectively.
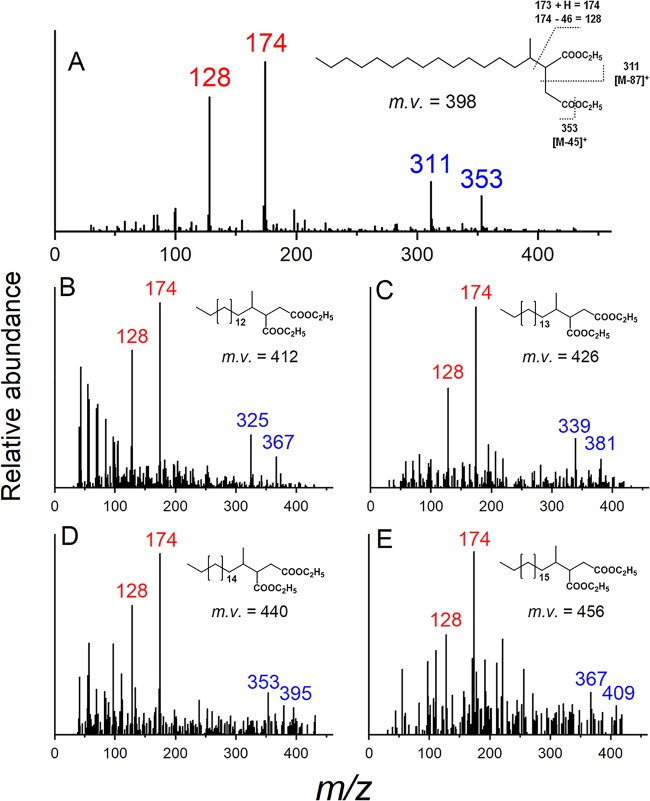
FIG 3.
Mass spectra of (1-methylalkyl)succinate diethyl esters detected in the methanogenic enrichment cultures amended with C16 to C20 n-alkanes. These alkylsuccinates were tentatively identified as (1-methylpentadecyl)succinate diethyl ester (A), (1-methylhexadecyl)succinate diethyl ester (B), (1-methylheptadecyl)succinate diethyl ester (C), (1-methyloctadecyl)succinate diethyl ester (D), and (1-methylnonadecyl)succinate diethyl ester (E).
Diversity and phylogenetic analysis of the assA gene.
Six assA operational taxonomic units (OTUs) amplified from two primers were obtained from the n-alkane-amended enrichment cultures. The phylogenetic analysis indicated that four assA OTUs (assA OTU 1 to 4, comprising 84.5% of the total sequences) are related to assA gene sequences of Smithella species (Fig. 4). The assA OTU 5 was closely (92% similarity) related to assA sequences of Desulfosarcina alkanivorans strain PL12 (BAU09525) (Fig. 4) and accounted for 8.7% of the total sequences. D. alkanivorans strain PL12 is capable of utilizing n-hexane and n-decane as the substrates under sulfate-reducing conditions (29). The assA OTU 6 comprised 6.8% of the total sequences and was most closely related (85% similarity) to assA sequences of Desulfatibacillum alkenivorans strain AK-01 (ABH11460) (Fig. 4). No amplification products were obtained in the control cultures.
FIG 4.
Neighbor-joining tree of AssA amino acid sequences recovered from methanogenic enrichment cultures amended with C16 to C20 n-alkanes (in blue). No assA gene was obtained in the control cultures without the addition of n-alkanes. Bootstrap values (no less than 50%) of 1,000 resamplings are shown near nodes. The alpha subunit of the benzylsuccinate synthase (BssA) sequence of Aromatoleum aromaticum EBN1 was used as an outgroup.
Bacterial community composition.
A total of 88 bacterial 16S rRNA gene sequences (26 OTUs) were obtained from the inoculum. Most notably, a bacterial OTU closely related to Syntrophus sp. clone B2 originating from a methanogenic hexadecane-degrading enrichment culture was detected (Fig. S1) (5).
Bacterial OTUs affiliated with the genus Smithella (family Syntrophaceae) represented less than 0.1% of the total bacterial reads in the control cultures but were enriched in the n-alkane-amended enrichment cultures, where they accounted for 24.4% of the total bacterial reads (Fig. 5A). Desulfatibacillum (family Desulfobacteraceae) produced 5.3% of the total bacterial reads in the n-alkane-amended enrichment cultures but was not found in the control cultures (Fig. 5A). Desulfatibacillum was comprised by a single OTU, which showed 97% similarity to Desulfatibacillum alkenivorans AK-01 (CP001322). Other significant bacterial taxa detected included Anaerolineaceae, Desulfovibrio (family Desulfovibrionaceae), Porphyromonadaceae, and Synergistaceae (Fig. 5A).
FIG 5.
Microbial community composition recovered from Illumina sequencing of 16S rRNA genes in methanogenic enrichment cultures amended with C16 to C20 n-alkanes (C16-C20) and the control cultures not amended with n-alkane (control). (A) Bacterial community composition. Only taxa abundant (at the family level) in more than 5% of the total bacterial reads in at least one sample are shown. (B) Archaeal community composition. Only taxa abundant (at the genus level) in more than 1% of the total archaeal reads in at least one sample are shown.
Archaeal community composition.
A total of 110 archaeal 16S rRNA gene sequences (7 OTUs) were obtained from the inoculum. The archaeal community was composed mainly of hydrogenotrophic methanogens (Methanocalculus and Methanoculleus) and acetoclastic methanogens (Methanosaeta) (Fig. S2).
For the n-alkane-amended enrichment cultures, members of Methanocalculus and Methanosaeta were dominant and accounted for 83.4% and 10.2% of the total archaeal sequences, respectively (Fig. 5B). In contrast, Methanothermobacter comprised 98.5% of the total archaeal reads in the control cultures (Fig. 5B). These results are consistent with the phylogenies of mcrA genes. In the n-alkane-amended cultures, 97.0% and 3.0% of mcrA gene clones belonged to the genera Methanocalculus and Methanosaeta, respectively (Fig. S3). In the control cultures, 80.6% of the clones clustered with the genus Methanothermobacter (Fig. S3).
DISCUSSION
In the present study, a methanogenic enrichment culture derived from oil field production water utilizing C16 to C20 n-alkanes was successfully enriched. A previous study had identified assA genes in the production water from the same oil field, suggesting that alkanes may be activated via a fumarate addition mechanism in this anoxic habitat (14). However, no fumarate addition products from n-alkanes had been detected in the same production water (sample X1 described by Bian et al. [14]). The only metabolites detected in the production water were naphthoate, laurate, myristate, and palmitate (14). At the end of our incubation, C16 to C20 (1-methylalkyl)succinates were identified, providing evidence that C16 to C20 n-alkanes are activated via fumarate addition under methanogenic conditions. This result is confirmed further by the detection of assA genes.
Smithella was the most abundant bacterium in n-alkane-amended cultures at the end of the incubation. This bacterium is generally considered an n-alkane degrader under methanogenic conditions. First, Zengler et al. reported that Syntrophus (later known as Smithella) was related to hexadecane degradation in a methanogenic enrichment incubated with anoxic ditch sediment (5). Later, Gray et al. demonstrated that the growth of Smithella positively correlated with the degradation of alkanes in methanogenic cultures with the addition of crude oil (30). Siddique et al. showed that Smithella was dominant in methanogenic cultures amended with an n-alkane mixture (C14, C16, and C18) (31). Recently, genomic analysis indicated that Smithella contains assA genes. Thus, Smithella can catalyze the fumarate addition reaction for anaerobic n-alkane degradation (32–34). This observation was demonstrated further by the detection of methyl pentadecyl succinic acid and methyl tetradecyl succinic acid in methanogenic cultures (dominated by Smithella) amended with hexadecane and pentadecane, respectively (17). Smithella was also found to participate in n-octacosane biodegradation under methanogenic conditions, where the methanogenic biodegradation of n-octacosane might be achieved via fumarate addition (26, 35). Considering the primary abundance of Smithella and the detection of Smithella-related assA genes allows the conclusion that this genus most likely plays a key role in the biodegradation of C16 to C20 n-alkanes in the present study.
In the present study, Desulfatibacillum was comprised by a single OTU closely related to Desulfatibacillum alkenivorans AK-01. D. alkenivorans AK-01 is capable of utilizing C13 to C18 n-alkanes as a source of carbon and energy under sulfate-reducing conditions (36). It has been reported that D. alkenivorans AK-01 can degrade hexadecane in cocultures with hydrogenotrophic methanogens (37). Microorganisms related to D. alkenivorans AK-01 have also been detected in a methanogenic hexadecane-degrading consortium enriched from a Shengli oil field (38). In addition, an assA OTU closely related to sequences of strain AK-01 was also found in the present study, indicating that this bacterium can activate n-alkanes via fumarate addition. Hence, we postulate that Desulfatibacillum species may degrade n-alkanes in cooperation with methanogens (such as Methanocalculus) and activate n-alkanes via a fumarate addition mechanism.
In summary, a methanogenic consortium capable of degrading C16 to C20 n-alkanes has been enriched from production water of a low-temperature petroleum reservoir. The biochemical degradation pathways involved in the anaerobic conversion of n-alkanes to methane are proposed in the present study (Fig. 6). Metabolites and functional gene analysis prove that the methanogenic biodegradation of C16 to C20 n-alkanes is started via fumarate addition. Smithella and Desulfatibacillum have been identified as the key degraders. Our results add evidence that fumarate addition is a key mechanism in the methanogenic biodegradation of n-alkanes.
FIG 6.
Proposed biochemical degradation pathways in the methanogenic biodegradation of C16 to C20 n-alkanes. The metabolites and functional genes detected in the methanogenic enrichment cultures are represented in red, while metabolites not detected are represented in black.
MATERIALS AND METHODS
Enrichment cultures.
The oil production water was collected from block 6 in Xinjiang Kelamayi oil field, Xinjiang Autonomous Region, China. Its physicochemical characteristics have been described previously (39). The temperature of the oil reservoir is approximately 21°C. The production water, after sampling and transportation back to the laboratory, was stored anaerobically in a large serum bottle before use as the inoculum. All enrichment cultures were set up in duplicate, containing 48 ml of basal medium (40) and 2 ml of inoculum. n-Alkane-amended enrichment cultures contained 0.90 mmol of n-alkanes (0.18 mmol of each n-alkane: n-hexadecane [C16; ≥99%], n-heptadecane [C17; ≥99%], n-octadecane [C18; ≥99%], n-nonadecane [C19; ≥99%], and n-eicosane [C20; ≥99%]; Sigma-Aldrich, Milwaukee, WI, USA). The control cultures received inoculation but no n-alkanes. All enrichment cultures were stationary and at room temperature (∼21°C) in the dark.
Analysis of headspace gas, volatile fatty acids and residual n-alkanes.
Methane in the serum bottles was measured periodically by sampling the headspace and analysis by gas chromatography (GC model 9890B; Shanghai Ling-Hua Instruments, Inc.) with a flame ionization detector. Two hundred microliters of the headspace gas was withdrawn with a syringe for GC analysis. The program of the GC analysis and the standard calibration curve have been described previously (41). The concentration of volatile fatty acids was measured using an ion chromatograph (model ICS-1100; Dionex, USA). The mobile phase consisted of KOH in a gradient elution mode. Residual n-alkanes in the serum bottles were extracted with n-hexane and then quantified with the addition of a surrogate standard (cetyl chloride). The dried extract was then analyzed by GC-mass spectrometry (GC-MS) (40).
Detection of putative metabolites.
After genomic DNA extraction from a 10-ml culture aliquot for microbial community analysis, the aliquot was transferred to a 250-ml round-bottom flask and then saponified (reflux at 100°C for 8 h) with 50 ml of 1 M KOH (1:1 methanol-deionized water). After cooling to room temperature, the content was acidified to pH <2 with HCl, followed by the addition of 10 ml of ethyl acetate to extract organic acids (repeated three times). The organic phase was collected and pooled into a 100-ml round-bottom flask and processed by rotary evaporation to remove the solvent. The flask, equipped with a water separator, was transferred to an oil bath and then refluxed with 10 ml of ethanol, 10 ml of cyclohexane, and 0.2 g of NaHSO4 at 80°C for 8 h. At the end of the reaction, ethanol and cyclohexane were removed and 10 ml of deionized water was added. Also, 10 ml of ethyl acetate was added three times to extract esters and the organic phase was collected. The extract was concentrated to about 200 μl and dried over anhydrous Na2SO4 prior to GC-MS analysis.
An Agilent 7890A GC, fitted with an Agilent capillary column (HP-5MS, 30 m by 0.25 mm by 0.25 μm) and a mass detector (MSD 5975C), was used for GC-MS analysis. The injector temperature was 280°C. The oven temperature was held initially at 60°C for 2 min and then increased at 20°C/min to 280°C for 30 min. The MS detector was run in the scan mode from 30 to 1,000 mass units. Diagnostic ion fragments of diethyl (1-methylalkyl)succinates are m/z 128, m/z 174, [M-45]+, and [M-87]+ (42).
DNA extraction.
DNA was collected from 10 ml culture broth of each culture (the inoculum, n-alkane-amended enrichment cultures, and control cultures), followed by centrifugation at 12,000 × g for 10 min at 4°C. The biomass pellets were collected for DNA extraction by using an AxyPrep bacterial genomic DNA maxiprep kit (Axygen Biosciences, USA) in accordance with the manufacturer’s instructions.
16S rRNA gene sequencing.
16S rRNA genes of the inoculum were amplified using primers 8F/805R (43) for bacteria and primers 340F/1000R (44) for archaea, and PCR analyses were performed according to a previous study (45), which was followed by purification and cloning. The positive clones were sent for Sanger sequencing (45). Sequences for rRNA genes were deposited in GenBank (see below).
Illumina sequencing of 16S rRNA genes was conducted for microbial community analysis of n-alkane-amended cultures and control cultures. The extracted DNA was amplified using touchdown PCR by primer sets 515F (5′-GTGCCAGCMGCCGCGG-3′)/907R (5′-CCGTCAATTCMTTTRAGTTT-3′) (46) for bacteria and 344F (5′-ACGGGGYGCAGCAGGCGCGA-3′)/915R (5′-GTGCTCCCCCGCCAATTCCT-3′) (47) for archaea. After PCR amplification, the final PCR products were sequenced using Illumina next-generation sequencing in accordance with previously described methods (41). The valid sequences were clustered into OTUs at a 97% species cutoff and classified against SILVA release 128 sequences.
assA and mcrA gene sequencing.
The assA genes were amplified using primer set assA2F and assA2R (12) and primer set ass/bss F and ass/bss R (13). For primers assA2F and assA2R, the PCR program was performed as follows: initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 60 s, and a final elongation step at 72°C for 10 min. For primers ass/bss F and ass/bss R, PCR conditions were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 45 s, 55°C for 60 s, 72°C for 2 min, and a final elongation step at 72°C for 10 min. The assA genes were amplified successfully by both primer sets. The primer set MLF/MLR (48) was used for the amplification of mcrA genes. PCR conditions were as follows: 95°C for 5 min, followed by 38 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 60 s, and a final elongation step at 72°C for 10 min. After purification and cloning, Sanger sequencing was conducted for assA and mcrA genes. Protein sequences were translated from the valid nucleotide sequences.
Phylogenetic analysis of 16S rRNA, assA, and mcrA genes.
Nucleotide sequences of 16S rRNA genes (the inoculum) and protein sequences of assA and mcrA genes were classified into operational taxonomic units (OTUs) at 97% similarity. Representative sequences were compared to the GenBank database using BLAST to match the closest sequences. Phylogenetic analyses were conducted using MEGA6.0 software (49) with the neighbor-joining method and 1,000 bootstrap replicates.
Data availability.
Sequences for 16S rRNA genes were deposited under GenBank accession numbers MH202660 to MH202747 and MH202773 to MH202882. Raw data of Illumina sequencing were deposited in the NCBI BioSample database under accession numbers SAMN08904494, SAMN08904495, SAMN08904499, and SAMN08904500. Protein sequences translated from the valid nucleotide sequences for assA and mcrA genes were deposited in the NCBI database under accession numbers MH192586 to MH192646, MH827880 to MH827921, and MH192810 to MH192905.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 41530318 and 41373070), the NSFC/RGC Joint Research Fund (no. 41161160560), the Shanghai Fundamental Research Program (no. 15JC1401400), the Fundamental Research Funds for the Central Universities (no. 222201817017, 50321101917017, and 22221818014), and the Research Program of State Key Laboratory of Bioreactor Engineering.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00985-19.
REFERENCES
- 1.Head IM, Jones DM, Larter SR. 2003. Biological activity in the deep subsurface and the origin of heavy oil. Nature 426:344–352. doi: 10.1038/nature02134. [DOI] [PubMed] [Google Scholar]
- 2.Jones DM, Head IM, Gray ND, Adams JJ, Rowan AK, Aitken CM, Bennett B, Huang H, Brown A, Bowler BF, Oldenburg T, Erdmann M, Larter SR. 2008. Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451:176–180. doi: 10.1038/nature06484. [DOI] [PubMed] [Google Scholar]
- 3.Gieg LM, Duncan KE, Suflita JM. 2008. Bioenergy production via microbial conversion of residual oil to natural gas. Appl Environ Microbiol 74:3022–3029. doi: 10.1128/AEM.00119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head IM, Gray ND, Larter SR. 2014. Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front Microbiol 5:566. doi: 10.3389/fmicb.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zengler K, Richnow HH, Rosselló-Mora R, Michaelis W, Widdel F. 1999. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401:266–269. doi: 10.1038/45777. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez N, Richnow HH, Vogt C, Treude T, Krüger M. 2016. Methanogenic hydrocarbon degradation: evidence from field and laboratory studies. J Mol Microbiol Biotechnol 26:227–242. doi: 10.1159/000441679. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan AV. 2013. Enzymes involved in the anaerobic oxidation of n-alkanes: from methane to long-chain paraffins. Front Microbiol 4:89. doi: 10.3389/fmicb.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbadinga SM, Wang LY, Zhou L, Liu JF, Gu JD, Mu BZ. 2011. Microbial communities involved in anaerobic degradation of alkanes. Int Biodeterior Biodegradation 65:1–13. doi: 10.1016/j.ibiod.2010.11.009. [DOI] [Google Scholar]
- 9.Kropp KG, Davidova IA, Suflita JM. 2000. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl Environ Microbiol 66:5393–5398. doi: 10.1128/AEM.66.12.5393-5398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabus R, Wilkes H, Behrends A, Armstroff A, Fischer T, Pierik AJ, Widdel F. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J Bacteriol 183:1707–1715. doi: 10.1128/JB.183.5.1707-1715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Li KP, Mbadinga SM, Yang SZ, Gu JD, Mu BZ. 2012. Analyses of n-alkanes degrading community dynamics of a high-temperature methanogenic consortium enriched from production water of a petroleum reservoir by a combination of molecular techniques. Ecotoxicology 21:1680–1691. doi: 10.1007/s10646-012-0949-5. [DOI] [PubMed] [Google Scholar]
- 12.Aitken CM, Jones DM, Maguire MJ, Gray ND, Sherry A, Bowler BFJ, Ditchfield AK, Larter SR, Head IM. 2013. Evidence that crude oil alkane activation proceeds by different mechanisms under sulfate-reducing and methanogenic conditions. Geochim Cosmochim Acta 109:162–174. doi: 10.1016/j.gca.2013.01.031. [DOI] [Google Scholar]
- 13.Callaghan AV, Davidova IA, Savage-Ashlock K, Parisi VA, Gieg LM, Suflita JM, Kukor JJ, Wawrik B. 2010. Diversity of benzyl- and alkylsuccinate synthase genes in hydrocarbon-impacted environments and enrichment cultures. Environ Sci Technol 44:7287–7294. doi: 10.1021/es1002023. [DOI] [PubMed] [Google Scholar]
- 14.Bian XY, Mbadinga SM, Liu YF, Yang SZ, Liu JF, Ye RQ, Gu JD, Mu BZ. 2015. Insights into the anaerobic biodegradation pathway of n-alkanes in oil reservoirs by detection of signature metabolites. Sci Rep 5:9801. doi: 10.1038/srep09801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdugo-Clavijo C, Gieg LM. 2014. Conversion of crude oil to methane by a microbial consortium enriched from oil reservoir production waters. Front Microbiol 5:197. doi: 10.3389/fmicb.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toth CRA, Gieg LM. 2017. Time course-dependent methanogenic crude oil biodegradation: dynamics of fumarate addition metabolites, biodegradative genes, and microbial community composition. Front Microbiol 8:2610. doi: 10.3389/fmicb.2017.02610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin QS, Feng DS, Liu PF, He Q, Li X, Liu AM, Zhang H, Hu GQ, Cheng L. 2017. Metagenomic characterization of Candidatus Smithella cisternae strain M82_1, a syntrophic alkane-degrading bacteria, enriched from the Shengli oil field. Microbes Environ 32:234–243. doi: 10.1264/jsme2.ME17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan KE, Gieg LM, Parisi VA, Tanner RS, Tringe SG, Bristow J, Suflita JM. 2009. Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ Sci Technol 43:7977–7984. doi: 10.1021/es9013932. [DOI] [PubMed] [Google Scholar]
- 19.Gieg LM, Davidova IA, Duncan KE, Suflita JM. 2010. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ Microbiol 12:3074–3086. doi: 10.1111/j.1462-2920.2010.02282.x. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Gieg LM. 2013. In situ detection of anaerobic alkane metabolites in subsurface environments. Front Microbiol 4:140. doi: 10.3389/fmicb.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heider J, Szaleniec M, Sünwoldt K, Boll M. 2016. Ethylbenzene dehydrogenase and related molybdenum enzymes involved in oxygen-independent alkyl chain hydroxylation. J Mol Microbiol Biotechnol 26:45–62. doi: 10.1159/000441357. [DOI] [PubMed] [Google Scholar]
- 22.Heider J, Schühle K. 2013. Anaerobic biodegradation of hydrocarbons including methane, p 605–634. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes: prokaryotic physiology and biochemistry. Springer, Berlin, Heidelberg. [Google Scholar]
- 23.Zedelius J, Rabus R, Grundmann O, Werner I, Brodkorb D, Schreiber F, Ehrenreich P, Behrends A, Wilkes H, Kube M, Reinhardt R, Widdel F. 2011. Alkane degradation under anoxic conditions by a nitrate-reducing bacterium with possible involvement of the electron acceptor in substrate activation. Environ Microbiol Rep 3:125–135. doi: 10.1111/j.1758-2229.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khelifi N, Amin Ali O, Roche P, Grossi V, Brochier-Armanet C, Valette O, Ollivier B, Dolla A, Hirschler-Réa A. 2014. Anaerobic oxidation of long-chain n-alkanes by the hyperthermophilic sulfate-reducing archaeon, Archaeoglobus fulgidus. ISME J 8:2153–2166. doi: 10.1038/ismej.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laso-Pérez R, Wegener G, Knittel K, Widdel F, Harding KJ, Krukenberg V, Meier DV, Richter M, Tegetmeyer HE, Riedel D, Richnow HH, Adrian L, Reemtsma T, Lechtenfeld OJ, Musat F. 2016. Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 539:396–401. doi: 10.1038/nature20152. [DOI] [PubMed] [Google Scholar]
- 26.Oberding LK, Gieg LM. 2018. Methanogenic paraffin biodegradation: alkylsuccinate synthase gene quantification and dicarboxylic acid production. Appl Environ Microbiol 84:e01773-17. doi: 10.1128/AEM.01773-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkes H, Rabus R, Fischer T, Armstroff A, Behrends A, Widdel F. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch Microbiol 177:235–243. doi: 10.1007/s00203-001-0381-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Bian XY, Zhou L, Mbadinga SM, Yang SZ, Liu JF, Gu JD, Mu BZ. 2016. Synthesis and characterization of anaerobic degradation biomarkers of n-alkanes via hydroxylation/carboxylation pathways. Eur J Mass Spectrom (Chichester) 22:31–37. doi: 10.1255/ejms.1402. [DOI] [PubMed] [Google Scholar]
- 29.Higashioka Y, Kojima H, Nakagawa T, Sato S, Fukui M. 2009. A novel n-alkane-degrading bacterium as a minor member of p-xylene-degrading sulfate-reducing consortium. Biodegradation 20:383–390. doi: 10.1007/s10532-008-9229-8. [DOI] [PubMed] [Google Scholar]
- 30.Gray ND, Sherry A, Grant RJ, Rowan AK, Hubert CR, Callbeck CM, Aitken CM, Jones DM, Adams JJ, Larter SR, Head IM. 2011. The quantitative significance of Syntrophaceae and syntrophic partnerships in methanogenic degradation of crude oil alkanes. Environ Microbiol 13:2957–2975. doi: 10.1111/j.1462-2920.2011.02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddique T, Penner T, Semple K, Foght JM. 2011. Anaerobic biodegradation of longer-chain n-alkanes coupled to methane production in oil sands tailings. Environ Sci Technol 45:5892–5899. doi: 10.1021/es200649t. [DOI] [PubMed] [Google Scholar]
- 32.Embree M, Nagarajan H, Movahedi N, Chitsaz H, Zengler K. 2014. Single-cell genome and metatranscriptome sequencing reveal metabolic interactions of an alkane-degrading methanogenic community. ISME J 8:757–767. doi: 10.1038/ismej.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan B, Nesbø C, Foght J. 2014. Re-analysis of omics data indicates Smithella may degrade alkanes by addition to fumarate under methanogenic conditions. ISME J 8:2353–2356. doi: 10.1038/ismej.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan B, Dong X, Sensen CW, Foght J. 2013. Metagenomic analysis of an anaerobic alkane-degrading microbial culture: potential hydrocarbon-activating pathways and inferred roles of community members. Genome 56:599–611. doi: 10.1139/gen-2013-0069. [DOI] [PubMed] [Google Scholar]
- 35.Wawrik B, Marks CR, Davidova IA, McInerney MJ, Pruitt S, Duncan KE, Suflita JM, Callaghan AV. 2016. Methanogenic paraffin degradation proceeds via alkane addition to fumarate by 'Smithella' spp. mediated by a syntrophic coupling with hydrogenotrophic methanogens. Environ Microbiol 18:2604–2619. doi: 10.1111/1462-2920.13374. [DOI] [PubMed] [Google Scholar]
- 36.So CM, Young LY. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl Environ Microbiol 65:2969–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callaghan AV, Morris BE, Pereira IA, McInerney MJ, Austin RN, Groves JT, Kukor JJ, Suflita JM, Young LY, Zylstra GJ, Wawrik B. 2012. The genome sequence of Desulfatibacillum alkenivorans AK-01: a blueprint for anaerobic alkane oxidation. Environ Microbiol 14:101–113. doi: 10.1111/j.1462-2920.2011.02516.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng L, Rui J, Li Q, Zhang H, Lu Y. 2013. Enrichment and dynamics of novel syntrophs in a methanogenic hexadecane-degrading culture from a Chinese oilfield. FEMS Microbiol Ecol 83:757–766. doi: 10.1111/1574-6941.12031. [DOI] [PubMed] [Google Scholar]
- 39.Wang LY, Duan RY, Liu JF, Yang SZ, Gu JD, Mu BZ. 2012. Molecular analysis of the microbial community structures in water-flooding petroleum reservoirs with different temperatures. Biogeosciences 9:4645–4659. doi: 10.5194/bg-9-4645-2012. [DOI] [Google Scholar]
- 40.Wang LY, Gao CX, Mbadinga SM, Zhou L, Liu JF, Gu JD, Mu BZ. 2011. Characterization of an alkane-degrading methanogenic enrichment culture from production water of an oil reservoir after 274 days of incubation. Int Biodeterior Biodegradation 65:444–450. doi: 10.1016/j.ibiod.2010.12.010. [DOI] [Google Scholar]
- 41.Ma L, Zhou L, Mbadinga SM, Gu JD, Mu BZ. 2018. Accelerated CO2 reduction to methane for energy by zero valent iron in oil reservoir production waters. Energy 147:663–671. doi: 10.1016/j.energy.2018.01.087. [DOI] [Google Scholar]
- 42.Bian XY, Mbadinga SM, Yang SZ, Gu JD, Ye RQ, Mu BZ. 2014. Synthesis of anaerobic degradation biomarkers alkyl-, aryl- and cycloalkylsuccinic acids and their mass spectral characteristics. Eur J Mass Spectrom (Chichester) 20:287–297. doi: 10.1255/ejms.1280. [DOI] [PubMed] [Google Scholar]
- 43.Savage KN, Krumholz LR, Gieg LM, Parisi VA, Suflita JM, Allen J, Philp RP, Elshahed MS. 2010. Biodegradation of low-molecular-weight alkanes under mesophilic, sulfate-reducing conditions: metabolic intermediates and community patterns. FEMS Microbiol Ecol 72:485–495. doi: 10.1111/j.1574-6941.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 44.Gantner S, Andersson AF, Alonso-Sáez L, Bertilsson S. 2011. Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J Microbiol Methods 84:12–18. doi: 10.1016/j.mimet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Liang B, Wang LY, Zhou Z, Mbadinga SM, Zhou L, Liu JF, Yang SZ, Gu JD, Mu BZ. 2016. High frequency of Thermodesulfovibrio spp. and Anaerolineaceae in association with Methanoculleus spp. in a long-term incubation of n-alkanes-degrading methanogenic enrichment culture. Front Microbiol 7:1431. doi: 10.3389/fmicb.2016.01431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong J, Liu Y, Lin X, Zhang H, Zeng J, Hou J, Yang Y, Yao T, Knight R, Chu H. 2012. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ Microbiol 14:2457–2466. doi: 10.1111/j.1462-2920.2012.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casamayor EO, Massana R, Benlloch S, Ovreas L, Diez B, Goddard VJ, Gasol JM, Joint I, Rodriguez-Valera F, Pedros-Alio C. 2002. Changes in archaeal, bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ Microbiol 4:338–348. doi: 10.1046/j.1462-2920.2002.00297.x. [DOI] [PubMed] [Google Scholar]
- 48.Luton PE, Wayne JM, Sharp RJ, Riley PW. 2002. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148:3521–3530. doi: 10.1099/00221287-148-11-3521. [DOI] [PubMed] [Google Scholar]
- 49.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences for 16S rRNA genes were deposited under GenBank accession numbers MH202660 to MH202747 and MH202773 to MH202882. Raw data of Illumina sequencing were deposited in the NCBI BioSample database under accession numbers SAMN08904494, SAMN08904495, SAMN08904499, and SAMN08904500. Protein sequences translated from the valid nucleotide sequences for assA and mcrA genes were deposited in the NCBI database under accession numbers MH192586 to MH192646, MH827880 to MH827921, and MH192810 to MH192905.