Abstract
It may be possible to classify patients with Aβ positive (+) mild cognitive impairment (MCI) into fast and slow decliners according to their biomarker status. In this study, we aimed to develop a risk prediction model to predict fast decline in the Aβ+ MCI population using multimodal biomarkers. We included 186 Aβ+ MCI patients who underwent florbetapir PET, brain MRI, cerebrospinal fluid (CSF) analyses, and FDG PET at baseline. We defined conversion to dementia within 3 years (= fast decline) as the outcome. The associations of potential covariates (MCI stage, APOE4 genotype, corrected hippocampal volume (HV), FDG PET SUVR, AV45 PET SUVR, CSF Aβ, total tau (t-tau), and phosphorylated tau (p-tau)) with the outcome were tested and nomograms were constructed using logistic regression models in the training dataset (n=124, n of fast decliners=52). The model was internally validated with the testing dataset (n=62, n of fast decliners=22). The multivariable analysis (including CSF t-tau) showed that MCI stage (late MCI vs. early MCI; OR 15.88, 95% CI 4.59, 54.88), APOE4 (OR 5.65, 95% CI 1.52, 20.98), corrected HV*1000 (OR 0.22, 95% CI 0.09, 0.57), FDG SUVR*10 (OR 0.43, 95% CI 0.27, 0.71), and loge CSF t-tau (OR 6.20, 95% CI 1.48, 25.96) were associated with being fast decliners. In the second model including CSF p-tau instead of t-tau, the above associations remained the same, with a significant association between loge CSF p-tau (OR 4.53, 95% CI 1.26, 16.31) and fast decline. The constructed nomograms showed excellent predictive performance (90%) on validation with the testing dataset. Among Aβ+ MCI patients, our findings suggested that multimodal AD biomarkers are significantly associated with being classified as fast decliners. A nomogram incorporating these biomarkers might be useful in early treatment decisions or stratified enrollment of this population into clinical trials.
Keywords: Amyloid, Mild cognitive impairment, Alzheimer's disease, Multimodal biomarkers, Nomogram, Conversion to dementia
Highlights
-
•
About 40% of Aβ+ MCI patients progressed to dementia within 3 years (fast decliner).
-
•
Neurodegeneration markers were predictive of fast decline in Aβ+ MCI patients.
-
•
Presence of APOE4 and late MCI stage were predictive of fast decline in Aβ+ MCI patients.
-
•
The fast decliner prediction model shows an excellent predictive performance.
1. Introduction
Mild cognitive impairment (MCI) is considered a transitional state between normal aging and Alzheimer's disease (AD) (Morris et al., 2001; Petersen et al., 2001). However, rates of clinical deterioration of MCI patients are variable, as some patients quickly progress to AD dementia while others remain stable or even revert to normal cognition (Busse et al., 2006; Larrieu et al., 2002; Petersen, 2004). This may be attributable to heterogeneous underlying pathologies of this population (DeCarli, 2003). Rapid development of molecular imaging has enabled the detection of amyloid-β (Aβ), a hallmark of AD pathology, using positron emission tomography (PET) in living patients, both in the MCI as well as the dementia stages. Previous studies have shown that 40–60% of MCI patients are Aβ positive (Aβ +) on PET; these patients are classified as MCI due to AD, with evidence of 40% to 80% risk of conversion to AD dementia within 3 years, a level that is 4 to 9 fold higher than their Aβ negative counterparts (Doraiswamy et al., 2014; Okello et al., 2009; Wolk et al., 2009).
Although Aβ is an AD-specific pathology, Aβ burden is not linearly correlated with symptom severity. AD biomarker modeling shows that tau neurofibrillary tangles (NFT), hypometabolism and brain atrophy, as more downstream biomarkers, are more closely associated with clinical symptoms. In fact, a recent AV-1451 PET study (Maass et al., 2017), which investigated NFT burden in the brain, reported that Aβ+ MCI patients exhibit in-vivo Braak stages ranging from I/II to V/VI. Also, previous studies have shown that higher CSF p-tau levels (Buerger et al., 2002; Ewers et al., 2007), hippocampal atrophy (Jack Jr. et al., 1999), and hypometabolism measured by [F18] fluorodeoxyglucose (FDG) PET are able to significantly predict conversion to AD in MCI patients (Drzezga et al., 2003) as well. Therefore, it would be reasonable to expect that Aβ+ MCI patients could be classified into fast and slow decliners according to their downstream biomarker status. However, most previous studies have included all MCI patients regardless of Aβ status. As abnormal neurodegeneration markers such as hypometabolism and hippocampal atrophy can be observed even in non-AD conditions, these multimodal biomarkers might not be able to specifically reflect the prognosis of Aβ+ MCI patients. Also, it is not known whether neocortical Aβ burden and presence of APOE4 genotype are associated with disease progression in specifically Aβ+ MCI patients. Therefore, it is necessary to demonstrate the importance of these multimodal biomarkers as predictors for clinical outcomes specifically in the Aβ+ MCI population.
The predictive power of these multimodal biomarkers is particularly important, because while drugs targeting Aβ have been developed and actively applied in clinical trials in AD dementia, most have ended in failure. In this regard, many recent clinical trials have focused on Aβ+ population, not yet demented, as a target group. However, it is still not clear how long of a delay exists from the beginning of Aβ deposition to dementia development, although a previous study showed that this delay may be decades long (Villemagne et al., 2013). Therefore, narrowing the range of best candidates even among Aβ+ MCI patients is clinically essential. Specifically, identifying the Aβ+ MCI patients subjects likely to progress most rapidly is critical to early treatment decisions as well as to stratified enrollment in clinical trials.
In this study, we aimed to develop a model to predict the risk of fast decliners in the Aβ+ MCI population using multimodal biomarkers. To promote the application of this prediction model to the clinical setting, we used a nomogram method, which is graph-based, simple, and easy to quickly interpret. We hypothesized that not only a combination of neurodegeneration markers such as reduced hippocampal volume on MRI, hypometabolism measured by FDG PET and increased CSF tau levels, but also Aβ burden or APOE4 would be associated with disease progression, because it is possible that Aβ burden and APOE4 might contribute to disease progression through a pathway independent of tau or neurodegeneration. We also expected that a nomogram featuring these markers could intuitively predict the possibility of Aβ+ MCI patients being fast decliners at an individual level.
2. Methods
2.1. Study participants
We used the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner (Weiner et al., 2010). Our study population primarily consisted of subjects from ADNI Go and ADNI-2. The major goal of ADNI has been to reveal the progression of MCI and AD using MRI, positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment. Full inclusion/exclusion criteria are described in detail at http://adni.loni.usc.edu/methods/documents/. Briefly, all subjects were between the ages of 55 and 90 years, had completed at least 6 years of education, were fluent in Spanish or English, and were free of any other significant neurologic diseases. MCI participants had a subjective memory complaint with a Clinical Dementia Rating (CDR) score of 0.5 (Petersen et al., 2010). The stage of MCI (early and late) patients are determined using the Wechsler Memory Scale (WMS) Logical Memory II; Early MCI (EMCI) subjects must have education adjusted scores between approximately 0.5 and 1.5 SD below the mean of Cognitively Normal (on delayed recall of one paragraph from WMS Logical Memory II). All subjects gave written informed consent prior to participation.
In this study, we included MCI patients who underwent 3.0T MRI scanning and 18F-AV45 (florbetapir) PET at baseline. As of 24th January 2018, a total of 463 patients met this qualification, and their baseline diagnoses were EMCI (n = 305) and late MCI (LMCI, n = 158). Among these, we included in the present study 254 patients with Aβ positivity on AV45 PET, which we defined as standardized uptake value ratios (SUVR) above a cutoff value of 1.11 (Landau et al., 2013; Landau et al., 2012) (145 EMCI and 109 LMCI).
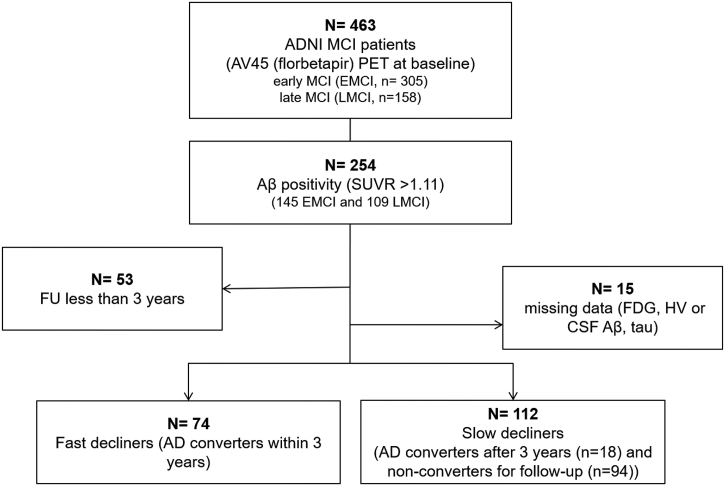
Patients were followed up at 6- to 12-month intervals with clinical diagnostic assessments. Conversion to AD was established at individual recruitment sites (Landau et al., 2011b), and we defined fast decliners as patients who converted to AD within three years of follow-up after baseline PET and MRI scans. A total of 53 patients who were followed up for less than three years with no AD conversion were excluded, and 15 additional patients were excluded from analyses because of missing data at baseline (11 for missing CSF data, three for missing hippocampal volume, and one for missing FDG SUVR). Thus, 74 fast and 112 slow decliners (patients who converted after 3 years (n = 18) or did not convert during the 3-year follow-up (n = 104)) were included in analyses (Fig. 1).
Fig. 1.
Flowchart showing inclusion and exclusion of participants included within the study.
Abbreviations: N, number; MCI, mild cognitive impairment; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; FDG, Fluorodeoxyglucose; HV, hippocampal volume; CSF, cerebrospinal fluid; AD, Alzheimer's dementia.
2.2. Clinical data collection
Basic demographics and clinical data were extracted from the ADNIMERGE dataset from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/) in January 2018. Extracted clinical data included presence of APOE4 genotype, hippocampal volume (HV), total intracranial volume (ICV), FDG SUVR (Average FDG SUVR of bilateral angular, inferior temporal, and posterior cingulate regions — AD signature regions — relative to pons/vermis reference region)(Landau et al., 2011; Landau et al., 2010), AV45 SUVR (Average AV45 SUVR of frontal, anterior cingulate, precuneus, and parietal cortex relative to the cerebellum), and AD biomarkers (Aβ(1–42), total tau (t-tau) and phosphorylated tau (p-tau)) from CSF drawn at baseline. The detailed protocols for image processing and CSF analyses have been described in previous studies (Bittner et al., 2016; Hsu et al., 2002; Landau et al., 2011b) and in the ADNI methods section at http://adni.loni.usc.edu/.
2.3. Statistical analysis
For model construction, we randomly divided the dataset into the training and the testing datasets, with a ratio of 2:1. Demographic data and biomarker characteristics of fast and slow decliners (in the total, the training and the testing dataset) were summarized with frequency and proportion or median and interquartile range.
In the training dataset, biomarkers used as potential predictors were age, MCI stage (early and late stage), APOE4, CSF Aβ (1–42) (=CSF Aβ), t-tau and p-tau, FDG SUVR, AV45 SUVR, and corrected HV (HV in mm3/ICV in mm3). Variables which had p value <0.1 for differences between groups from univariable analyses were included in multivariable analysis and the model of the significant variables from multivariable analysis was re-estimated as the final model. AV45 SUVR, CSF t-tau and p-tau were natural log transformed (loge) due to skewed distribution, and FDG SUVR, AV45 SUVR and corrected HV variables were multiplied by 10, 10 and 1000 respectively because of relatively small scale. We assessed multicollinearity using the variance inflation factor (VIF) and found that CSF t-tau and p-tau levels showed VIF > 4. Therefore, they were considered correlated, which led us to make two separate multivariable logistic regression analyses including each variable. Association of biomarker with fast decliner in multivariable logistic model was presented with OR (Odds Ratio) and 95% CI (Confidence Interval) of OR.
Nomograms were formulated based on the results of multivariable analysis using R 3.4.3(http://www.r-project.org) with rms packages. Detailed methods about nomogram construction have been described previously (Jang et al., 2017). To validate the predictive accuracy of a prediction model developed using the training dataset, we quantified nomogram performance by discrimination and calibration. In discrimination step, predictive performance was determined with a concordance index (C-index, the area under the receiver operating characteristic curve), which quantifies the level of concordance between predicted probabilities and the actual chance of having the event of interest. Internal validation of performance was estimated with a bootstrapping method (1000 replications) and a 10-fold cross validation of the training dataset, and the testing dataset. Calibration was graphically assessed with the relationship between the actual observed probabilities and predicted probabilities (calibration curve).
Finally, we tried to show that final prediction models we constructed had best model fitness and predictive performance compared with others. Therefore, we compared the fitness and predictive performance of different models with various combinations of biomarkers using the likelihood ratio test and 95% CI for AUC, respectively. We made several models by adding, one by one, independent variables (in the order of easiness of access in clinic). Model 1 has MCI stage as an only independent variable, because MCI stage is easily obtainable information from neuropsychological test. Model 2 has APOE4 genotyping as an additional variable, and model 3 and 4 have HV/ICV and FDG PET SUVR as an additional variable, respectively. Model 5 has MCI stage, APOE4 genotyping, HV/ICV, and FDG SUVR as independent variables, while Model 6 and 7 have additionally CSF t-tau and p-tau levels as an independent variable, respectively. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R 3.4.3 (Vienna, Austria; http://www.R-project.org/). Statistical significance was defined as two-tailed p < 0.05.
3. Results
3.1. Differences in clinical characteristics of fast and slow decliners
The demographic characteristics of the study subjects in the total (N = 186, 74 fast and 112 slow decliners), the training dataset (N = 124), and the testing dataset (N = 62) are shown in Table 1.
Table 1.
Demographics and biomarkers between slow and fast decliners of Aβ + MCI.
| Total (N = 186) |
Training dataset (N = 124) |
Testing dataset (N = 62) |
||||
|---|---|---|---|---|---|---|
| Slow decliners (N = 112) | Fast decliners (N = 74) | Slow decliners (N = 72) | Fast decliners (N = 52) | Slow decliners (N = 40) | Fast decliners (N = 22) | |
| Demographicsa | ||||||
| Age, years | 72.0 (67.8,77.4) | 74.3 (69.5,77.8) | 70.7 (67.3,77.3) | 73.6 (69.5,77.3) | 72.7 (69.4,77.45) | 74.45 (70,79.3) |
| Sex, % female | 52 (46.4) | 31 (41.9) | 30 (41.7) | 20 (38.5) | 22 (55) | 11 (50) |
| Education, years | 16 (13.5,18) | 16 (15,18) | 16 (13.5,18) | 16 (15.5,18) | 16 (13.5,18) | 16 (14,18) |
| MCI stage, % late MCI | 28 (25) | 54 (73) | 11 (15.3) | 37 (71.2) | 17 (42.5) | 17 (77.27) |
| MMSE | 28.5 (27, 29) | 27 (26, 28) | 28.5 (27,30) | 27.5 (26,29) | 28.5 (27.5,29) | 27 (26,28) |
| Biomarkersa | ||||||
| APOE 4+ carriers | 64 (57.1) | 60 (81.8) | 42 (58.3) | 42 (80.8) | 22 (55) | 18 (81.8) |
| CSF Aβ(1–42), pg/mLb | 740.8 (625.3956.6) | 697.7 (563.2806.0) | 738.1 (613.7959) | 699.8 (569,808.3) | 740.7 (635.4953.3) | 658.1 (520.3799.3) |
| CSF t-tau, pg/mLb | 284.3 (227.9347.5) | 372.2 (283.7514.2) | 286.9 (233.7374.3) | 361.6 (288.2497.3) | 279.9 (211.2327.3) | 391.4 (283.7542.7) |
| CSF p-tau, pg/mLb | 27.6 (21.0,35.6) | 36.3 (28.2,51.9) | 28.2 (21.5,36.4) | 35.2 (28.8,51.1) | 26.9 (19.5,31.9) | 38.7 (28.0,56.9) |
| HV mm3 | 7217.3 (6421.5, 7916.5) | 6325.6 (5715, 6996) | 7271.4 (6498,7948) | 6400.4 (5797.5, 7063.5) | 7119.9 (6300, 7841.5) | 6148.8 (5549, 6702) |
| HV/ICVb | 0.0047 (0.0042,0.0053) | 0.0041 (0.0038,0.0045) | 0.0048 (0.0042,0.0054) | 0.0041 (0.0039,0.0045) | 0.0046 (0.0042,0.0051) | 0.0043 (0.0038,0.0046) |
| AV45 PET SUVRb | 1.31 (1.20,1.45) | 1.45 (1.31,1.55) | 1.3 (1.2,1.5) | 1.4 (1.3,1.6) | 1.3 (1.2,1.4) | 1.5 (1.3,1.5) |
| FDG SUVRb | 1.30 (1.23,1.36) | 1.14 (1.07,1.22) | 1.3 (1.2,1.4) | 1.2 (1.1,1.2) | 1.3 (1.2,1.4) | 1.1 (1.1,1.2) |
MCI, mild cognitive impairment; APOE, Apolipoprotein E; CSF, cerebrospinal fluid; Aβ(1–42), Amyloid-β 1–42; t-tau, total tau; p-tau, phosphorylated tau; HV, hippocampal volume; ICV, total intracranial volume; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; FDG, Fluorodeoxyglucose.
Values are median (interquartile range) or number (percentage).
Obtained from ADNI dataset.
Table 2 shows the result of univariable logistic analysis in the training dataset. There were no significant differences in age (p = 0.475), sex (p = 0.720), and education years (p = 0.196) between fast and slow decliners. There was a higher frequency of LMCI (37 (71.2%) vs. 11 (15.3%), p < 0.001) and APOE4 carriers among fast decliners (42/52 (80.8%) vs. 42/72 (58.3%) p = 0.008) than among slow decliners.
Table 2.
Univariable analysis for the association of biomarker predictors with fast decliners of Aβ + MCI in the training dataset.
| Predictors | Slow decliners (N = 72) | Fast decliners (N = 52) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 70.7 (67.3,77.3) | 73.6 (69.5,77.3) | 1.02 (0.97,1.08) | 0.475 |
| Sex, % female | 30 (41.7) | 20 (38.5) | 0.88 (0.42,1.81) | 0.720 |
| Education, years | 16 (13.5,18) | 16 (15.5,18) | 1.10 (0.95,1.27) | 0.196 |
| MCI stage, % late MCI | 11 (15.3) | 37 (71.2) | 13.68 (5.68,32.93) | <0.001 |
| Biomarkers | ||||
| APOE 4+ carriers | 42 (58.3) | 42 (80.8) | 3 (1.30,6.91) | 0.010 |
| CSF Aβ (1–42), pg/mL | 738.1 (613.7959) | 699.8 (569,808.3) | 0.998 (0.996,1) | 0.018 |
| CSF t-tau, pg/mL | 286.9 (233.7374.3) | 361.6 (288.2497.3) | 6.33 (2.13,18.82) | <0.001 |
| CSF p-tau, pg/mL | 28.2 (21.5,36.4) | 35.2 (28.8,51.1) | 5.23 (1.95,14) | 0.001 |
| HV/ICV | 0.0048 (0.0042,0.0054) | 0.0041 (0.0039,0.0045) | 0.26 (0.14,0.48) | <0.001 |
| AV45 PET SUVR | 1.3 (1.2,1.5) | 1.4 (1.3,1.6) | 1.75 (1.26,2.43) | <0.001 |
| FDG SUVR | 1.3 (1.2,1.4) | 1.2 (1.1,1.2) | 0.37 (0.25,0.56) | <0.001 |
OR, Odds ratio; CI, confidence interval; MCI, mild cognitive impairment; FDG, Fluorodeoxyglucose; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; CSF, cerebrospinal fluid; t-tau, total tau; p-tau, phosphorylated tau; HV, hippocampal volume; ICV, total intracranial volume; APOE, Apolipoprotein E.
AD biomarker characteristics were significantly different between fast and slow decliners in the training dataset. Compared to slow decliners, fast decliners showed a lower level of CSF Aβ (699.8 (569, 808.3) vs. 738.1 (613.7, 959), p = 0.018), and higher levels of CSF t-tau (361.6 (288.2, 497.3) vs. 286.9 (233.7, 374.3), p < 0.001) and p-tau (35.2 (28.8, 51.1) vs. 28.2 (21.5, 36.4), p = 0.001). Corrected HV (0.0041 (0.0039, 0.0045) vs. 0.0048 (0.0042, 0.0054), p < 0.001) and FDG PET SUVR (1.2 (1.1, 1.2) vs. 1.3 (1.2, 1.4), p < 0.001) were significantly lower and AV45 SUVR (1.4 (1.3, 1.6), 1.3 (1.2, 1.5), p < 0.001) was higher in fast decliners than in slow decliners. (Table 2).
3.2. Predictors for classification as fast decliners in multivariable logistic regression analysis
In the training dataset, the first multivariable analysis (Model 1 including CSF t-tau) showed that MCI stage (LMCI vs. EMCI; OR 15.88, 95% CI 4.59, 54.88), APOE4 (OR 5.65, 95% CI 1.52, 20.98), corrected HV*1000 (OR 0.22, 95% CI 0.09, 0.57), FDG SUVR*10 (OR 0.44, 95% CI 0.27, 0.71), and loge CSF t-tau (OR 6.20, 95% CI 1.48, 25.96) were associated with being fast decliners. The second multivariable analysis (Model 2 including CSF p-tau) showed that MCI stage (LMCI vs. EMCI; OR 15.98, 95% CI 4.69, 54.44), APOE4 (OR 5.71, 95% CI1.56, 20.88), corrected HV*1000 (OR 0.23, 95% CI 0.09,0.57), FDG SUVR*10 (OR 0.45, 95% CI 0.28,0.72), and loge CSF p-tau (OR 4.53, 95% CI 1.26, 16.31) were associated with being fast decliners (Table 3).
Table 3.
Final model for the association of biomarker predictors with fast decliners of Aβ + MCI in the training dataset: Multivariable analysis.
| Predictors | Model 1 (including CSF t-tau) |
Model 2 (including CSF p-tau) |
||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | 95% CI | p-value | β | OR | 95% CI | p-value | |
| MCI stage (LMCI vs EMCI (ref)) | 2.77 | 15.88 | 4.59,54.88 | <0.001 | 2.77 | 15.98 | 4.69,54.44 | <0.001 |
| Presence of APOE4 | 1.73 | 5.65 | 1.52,20.98 | 0.010 | 1.74 | 5.71 | 1.56,20.88 | 0.009 |
| HV/ICV*1000 | −1.50 | 0.22 | 0.09,0.57 | 0.002 | −1.46 | 0.23 | 0.09,0.57 | 0.002 |
| FDG SUVR *10 | −0.83 | 0.44 | 0.27,0.71 | 0.001 | −0.80 | 0.45 | 0.28,0.72 | 0.001 |
| Loge CSF t-tau (pg/mL) | 1.82 | 6.20 | 1.48,25.96 | 0.013 | N/A | |||
| Loge CSF p-tau (pg/mL) | N/A | 1.51 | 4.53 | 1.26,16.31 | 0.021 | |||
OR, Odds ratio; MCI, mild cognitive impairment; LMCI, late MCI; EMCI, early MCI; CI, confidence interval; FDG, Fluorodeoxyglucose; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; CSF, cerebrospinal fluid; t-tau, total tau; p-tau, phosphorylated tau; HV, hippocampal volume; ICV, total intracranial volume; APOE, Apolipoprotein E.
3.3. Nomograms as prediction models
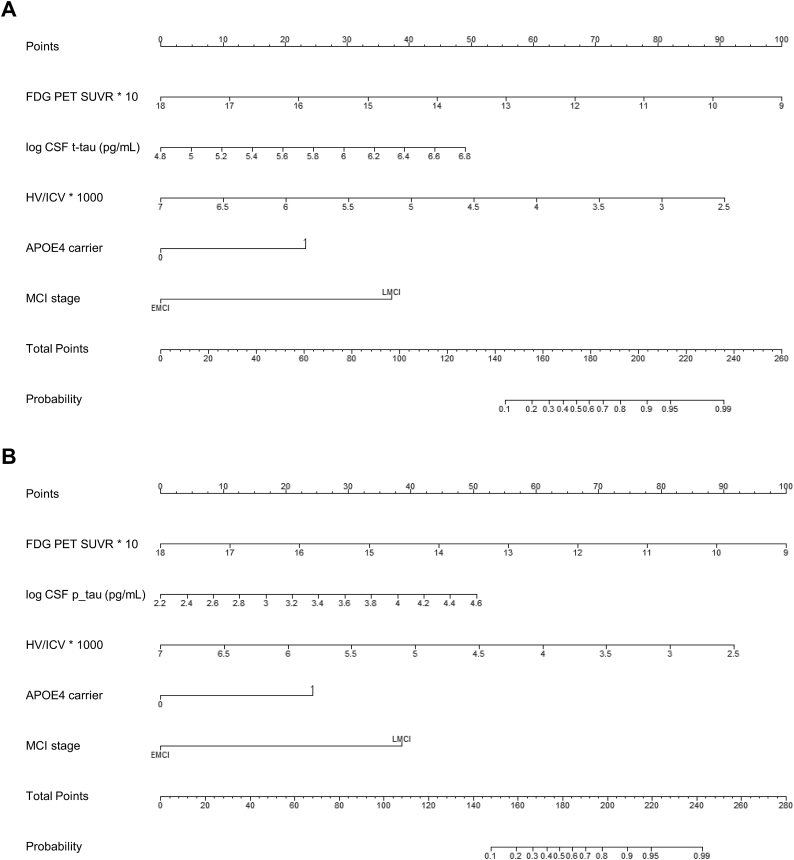
We finally constructed nomograms using the multivariable analyses results (Fig. 2). A specific point was matched to each variable based on the beta coefficients from regression analyses described above. The total points made from the sum of each point indicate the overall risk score. This can be applied to predict the risk of being classified a fast decliner as shown in Fig. 3, which shows how to interpret the nomogram using the exemplary cases of the low and high risk biomarker profiles.
Fig. 2.
Nomograms for predicting fast decliners including (A) CSF t-tau (B) CSF p-tau.
Abbreviations: FDG, Fluorodeoxyglucose; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; CSF, cerebrospinal fluid; t-tau, total tau; p-tau, phosphorylated tau; HV, hippocampal volume; ICV, intracranial volume; APOE, Apolipoprotein E.
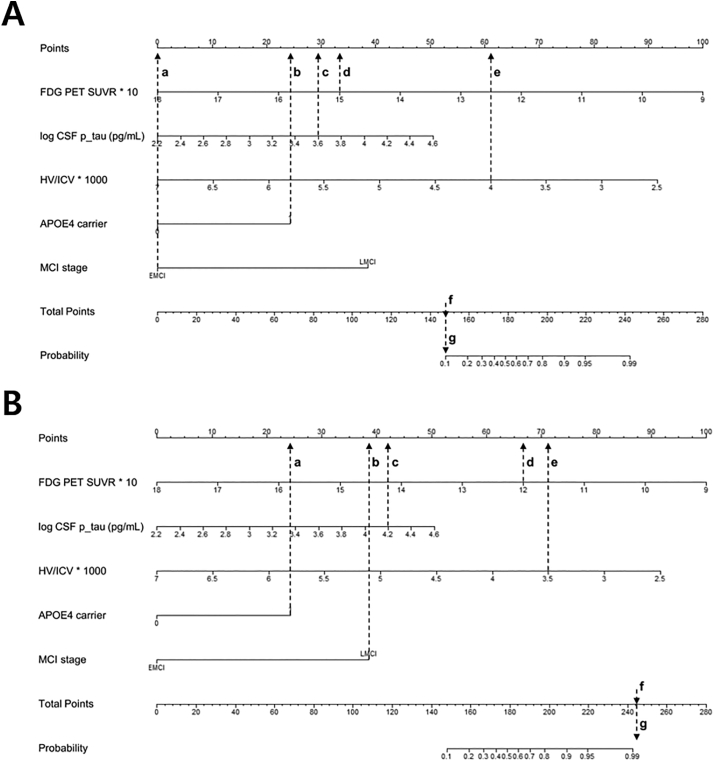
Fig. 3.
Nomogram interpretation.
The first exemplary case (A) is EMCI (a) and an APOE4 carrier (b) and has loge CSF p-tau of 3.6 (c), FDG PET SUVR of 1.5 (d), and HV/ICV of 0.005 (e). Therefore, the point was matched to each variable. The total points (f) made from the sum of each point (a,0 + b,24 + c,29 + d,33 + e,61) indicate the overall risk score (f = 147). This subsequently is matched to a probability of <10%(g), which demonstrates that this case has a low risk profile to predict fast decline. Likewise, the second exemplary case (B) is an APOE4 carrier (a) and LMCI (b) and loge CSF p-tau of 4.2 (c), FDG PET SUVR of 1.2 (d), and HV/ICV of 0.0035 (e). The total points (f) made from the sum of each point (a,24.5 + b,38 + c,42.5 + d,67 + e,71) indicate the overall risk score (f = 243), which subsequently is matched to a probability of 99% (g), very high risk for fast decline.
The prediction performance of Model 1 was 0.929 in the training dataset and 0.912/ 0.910 by bootstrap sampling/10-fold cross validation and 0.901 in the testing dataset. The prediction performance of Model 2 was 0.929 in the training dataset and 0.913/0.899 in bootstrap sampling/10-fold cross validation and 0.907 in the testing dataset. The nonparametric calibration curves showed that the bias corrected calibration plots (which were generated from internal validation based on 1000 bootstrap resamples) showed virtually no departure from ideal lines, which means the nomograms are well calibrated (Supplementary Fig. 1).
3.4. Comparison with prediction models including different combinations of biomarkers
The prediction models with different combinations of biomarkers are shown in Table 4. We evaluated the model fitness using Akaike information criterion (AIC) and R-square. Given that lower AIC and higher R-square indicate the better model regarding fitness, model 6 and 7 fitted the data the best as shown in the Table 4 and it was confirmed with likelihood ratio test (p < 0.003). We evaluated the predictive performance using area under curve (AUC), Brier Score (the mean squared error between predictions and outcome) and error rate. Given that higher AUC and lower error rate/Brier score indicate better predictive performance, the model 1, 2 showed relatively low predictive performance, while the model 4,5,6,7,8,9 showed relatively high predictive performance. Regarding 95% CI for AUC, the model 4,5,6,7,8, and 9 showed the higher AUC than model 1, 2 (p < 0.05).
Table 4.
Models with different combinations of biomarkers using the total dataset.
| Model | AIC | R-Square | AUC | 95% CI for AUC |
Brier Score | Error Rate | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Model 1 | MCI stage | 211.115 | 0.206 | 0.74 | 0.186 | 0.805 | 0.186 | 0.258 |
| Model 2 | MCI stage + APOE4 | 204.882 | 0.24 | 0.786 | 0.18 | 0.848 | 0.18 | 0.274 |
| Model 3 | MCI stage + APOE4 + HV/ICV | 185.003 | 0.325 | 0.847 | 0.157 | 0.902 | 0.157 | 0.226 |
| Model 4 | MCI stage + APOE4 + FDG | 156.007 | 0.422 | 0.898 | 0.123 | 0.944 | 0.123 | 0.172 |
| Model 5 | MCI stage + APOE4 + HV/ICV + FDG | 150.648 | 0.445 | 0.906 | 0.119 | 0.949 | 0.119 | 0.183 |
| Model 6 | MCI stage + APOE4 + HV/ICV + FDG + CSF t-tau | 143.731 | 0.471 | 0.919 | 0.111 | 0.958 | 0.111 | 0.172 |
| Model 7 | MCI stage + APOE4 + HV/ICV + FDG + CSF p tau | 143.768 | 0.471 | 0.919 | 0.111 | 0.958 | 0.111 | 0.167 |
| Model 8 | APOE4 + HV/ICV + FDG + CSF t-tau | 159.603 | 0.417 | 0.893 | 0.127 | 0.94 | 0.127 | 0.161 |
| Model 9 | APOE4 + HV/ICV + FDG + CSF p tau | 160.462 | 0.415 | 0.892 | 0.127 | 0.939 | 0.127 | 0.151 |
AIC, Akaike information criterion; AUC, Area under ROC; CI, confidence interval; FDG, Fluorodeoxyglucose; PET, Positron emission tomography; SUVR, Standardized uptake value ratio; CSF, cerebrospinal fluid; t-tau, total tau; p-tau, phosphorylated tau; HV, hippocampal volume; ICV, total intracranial volume; APOE, Apolipoprotein E.
4. Discussion
We divided Aβ+ MCI patients into two groups—fast and slow decliners—according to whether they progressed to AD within three years of follow-up. We then compared clinical and biomarker characteristics between these groups and developed predictive models for fast decliners group status using multivariable analysis with multimodal biomarkers as potential predictors. Our major findings were as follows: 1) about 40% of Aβ+ MCI patients progressed to dementia within three years, and were classified as fast decliners; 2) advanced MCI stage (LMCI), higher CSF t-tau or p-tau, lower HV, hypometabolism in AD signature regions (=lower FDG SUVR), and the presence of APOE4 were significantly predictive of fast decliners group status among Aβ+ MCI patients; 3) the predictive model for being classified as fast decliners that we developed using these biomarkers showed an excellent predictive performance (90%) on validation with the testing dataset.
Our first major finding was that about 40% of Aβ+ MCI patients progressed to dementia within three years. In the present study, the conversion rate among Aβ+ MCI patients seems to be lower than previously reported (Okello et al., 2009; van Rossum et al., 2012; Ye et al., 2018), as prior studies have shown that 50–80% of Aβ+ MCI patients convert to dementia in 3 years. In this study, about half the study subjects (104 of 186) were diagnosed with EMCI at the time of AV45 imaging, which might have contributed to a low dementia conversion rate; a previous study demonstrated that EMCI patients take longer to progress to dementia compared with LMCI (Jessen et al., 2014). This is also consistent with our study finding that LMCI has a 15-fold higher risk of being fast decliners. Therefore, this finding suggests that even Aβ+ MCI patients have varied clinical courses. Additionally, we investigated the dementia conversion rate in Aβ- MCI patients using the same ADNI dataset, although we did not include it in the present study. The result was that, among 158 patients who were followed up for 3 years, 152 patients (96.2%) were slow decliners while only six patients (3.8%) were fast decliners, from which we concluded that Aβ- MCI patients have a much lower chance of dementia conversion in three years than Aβ+ patients.
Our second major finding was that higher CSF t-tau or p-tau, lower HV, hypometabolism in AD signature regions, and the presence of APOE4 were significantly predictive of Aβ+ MCI patients being classified as fast decliners. Considering that higher CSF t-tau or p-tau, hippocampal atrophy and hypometabolism are characterized as neurodegeneration markers, our finding is consistent with previous studies showing that Aβ+ MCI patients have worse prognosis when they have additional abnormal neurodegeneration markers (Bittner et al., 2016; Knopman et al., 2013). In particular, given that CSF p-tau correlates with pathologic neurofibrillary tangle burden (Buerger et al., 2006; Clark et al., 2003), which itself is well correlated with disease progression in AD, it is reasonable to expect that elevated CSF p-tau could predict the risk of Aβ+ MCI patients being classified as fast decliners. This is because, while all participants have elevated brain Aβ, they might exhibit variable Braak stages from I/II to V/IV (Maass et al., 2017), and more detailed information regarding p-tau could more specifically inform risk of future decline. Furthermore, our results show that nonspecific AD neurodegeneration markers, such as hippocampal atrophy or hypometabolism (representing synaptic dysfunction and neuronal loss), are also strongly associated with clinical deterioration once patients have elevated Aβ deposition on PET.
As expected, CSF Aβ levels and AV45 PET SUVR were not associated with fast decliners in the multivariable analyses. The previous study suggested that a combination of CSF Aβ and CSF tau had better predictive accuracy for AD conversion in MCI patients than CSF tau alone (van Rossum et al., 2010). However, in this article, MCI consists of both Aβ+ and Aβ- subjects. As Aβ positivity increases the risk of dementia conversion in MCI population, combination of CSF Aβ and tau measures must be a better predictor in that case. On the contrary, we included only Aβ+ MCI patients at the first place, CSF Aβ were not found to be associated with being fast decliners in multivariable analysis. Likewise, considering that the amyloid cascade hypothesis states that Aβ accumulation leads to a cascade of events including tau hyperphosphorylation and neuronal degeneration as downstream processes, Aβ load on AV45 PET scans would not be related with being classified as fast decliners after adjusting for CSF tau, neurodegeneration markers, and cognition (as MCI stage). In this study, we also found that APOE4 carriage was a significant predictor for fast decliners group status among Aβ+ MCI patients, which suggests that the effect of APOE4 on disease progression in the Aβ+ MCI population may be as important as its effect on Aβ deposition in normal individuals (Risacher et al., 2015). This might be because APOE4 affects cognition in Aβ independent as well as Aβ dependent mechanisms, by inducing tau hyperphosphorylation, and undergoing neuron-specific proteolysis subsequently leading to mitochondrial energy disruptions and cell death (Mahley et al., 2006).
Our final major finding was that the predictive model using multimodal AD biomarkers showed an excellent predictive performance (90%) on validation with the testing dataset for predicting who would be fast decliners among the Aβ+ MCI population. Although clinical information such as MCI stage was found to be an important predictive variable, we demonstrated that the model with MCI stage alone performed worse than the model with MCI stage and additional multimodal biomarkers, in terms of predictive performance and model fitness. Therefore, we could insist that implementing these multimodal biomarkers in clinical practice ensure more accurate prediction for disease progression in Aβ+ MCI population. Importantly, the nomograms we constructed in this study are easy to apply to clinical data. In the present study, we used the ADNI dataset; ADNI is a large cohort of well-characterized subjects, and clinical and imaging data are based on standardized protocols and analyses. Therefore, as shown in Fig. 3, we could easily apply our nomograms to ADNI datasets to predict the probability of being fast declines. In terms of classification of patients, we expect that this prediction model may provide additional risk stratification information, which is helpful to optimize the selection of patients who may benefit from Aβ+ targeting therapies in clinical trials.
We were able to conduct this study because of the availability of various clinical data through ADNI. However, there are several limitations in this study. First, the definition of fast or slow decliners was based on the diagnosis with respect to conversion to AD, not on the trajectories of objective neuropsychological test results. However, it is meaningful to predict which patients are closest to clinical deterioration in a clinical setting. Second, we used an a priori established SUVR cutoff for Aβ positivity, despite the clinical utility of visual assessment. However, a quantitative SUVR cutoff is more sensitive compared to visual assessment to predict at-risk patients (Schreiber et al., 2015), and we could easily obtain SUVR values from the ADNIMERGE dataset, which enables agreement with many other ADNI studies in terms of the study methods.
5. Conclusions
In conclusion, we found advanced MCI stage, neurodegeneration markers such as high level of CSF tau, hippocampal atrophy, and hypometabolism in AD signature regions, and presence of APOE4 to be independently associated with early disease progression in Aβ + MCI patients. The constructed nomograms including these multimodal AD biomarkers could help clinicians to predict fast decliners among Aβ + MCI patients with excellent predictive performance.
Disclosures
All authors have no conflicts of interest to disclose.
Declaration of Competing Interest
The authors declare no financial or other conflicts of interests.
Acknowledgements
SWS receives funding from the Brain Research Program through the National Research Foundation (NRF) of Korea (2016M3C7A1913844), the Korea government (MSIP) through the NRF of Korea grant (2017R1A2B2005081), the Brain Research Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2018M3C7A1056512), the Research of Korea Centers for Disease Control and Prevention (2018-ER6203-01). J-KS receives funding from the Brain Research Program through the NRF funded by the Ministry of Science & ICT (No. 2017M3C7A1048092). HJK receives a support by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI18C1629).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101941.
Contributor Information
Joon-Kyung Seong, Email: jkseong@korea.ac.kr.
Sang Won Seo, Email: sw72.seo@samsung.com.
Appendix A. Supplementary data
Supplementary material
References
- Bittner T., Zetterberg H., Teunissen C.E., Ostlund R.E., Jr., Militello M., Andreasson U., Hubeek I., Gibson D., Chu D.C., Eichenlaub U., Heiss P., Kobold U., Leinenbach A., Madin K., Manuilova E., Rabe C., Blennow K. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–526. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Buerger K., Teipel S.J., Zinkowski R., Blennow K., Arai H., Engel R., Hofmann-Kiefer K., McCulloch C., Ptok U., Heun R., Andreasen N., DeBernardis J., Kerkman D., Moeller H., Davies P., Hampel H. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- Buerger K., Ewers M., Pirttila T., Zinkowski R., Alafuzoff I., Teipel S.J., DeBernardis J., Kerkman D., McCulloch C., Soininen H., Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Busse A., Hensel A., Guhne U., Angermeyer M.C., Riedel-Heller S.G. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- Clark C.M., Xie S., Chittams J., Ewbank D., Peskind E., Galasko D., Morris J.C., McKeel D.W., Jr., Farlow M., Weitlauf S.L., Quinn J., Kaye J., Knopman D., Arai H., Doody R.S., DeCarli C., Leight S., Lee V.M., Trojanowski J.Q. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- De Carli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- Doraiswamy P.M., Sperling R.A., Johnson K., Reiman E.M., Wong T.Z., Sabbagh M.N., Sadowsky C.H., Fleisher A.S., Carpenter A., Joshi A.D., Lu M., Grundman M., Mintun M.A., Skovronsky D.M., Pontecorvo M.J., Group, A.A.S Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol. Psychiatry. 2014;19:1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A., Lautenschlager N., Siebner H., Riemenschneider M., Willoch F., Minoshima S., Schwaiger M., Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- Ewers M., Buerger K., Teipel S.J., Scheltens P., Schroder J., Zinkowski R.P., Bouwman F.H., Schonknecht P., Schoonenboom N.S., Andreasen N., Wallin A., DeBernardis J.F., Kerkman D.J., Heindl B., Blennow K., Hampel H. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y., Schuff N., Du A.T., Mark K., Zhu X., Hardin D., Weiner M.W. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J. Magn. Reson. Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Petersen R.C., Xu Y.C., O'Brien P.C., Smith G.E., Ivnik R.J., Boeve B.F., Waring S.C., Tangalos E.G., Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H., Ye B.S., Woo S., Kim S.W., Chin J., Choi S.H., Jeong J.H., Yoon S.J., Yoon B., Park K.W., Hong Y.J., Kim H.J., Lockhart S.N., Na D.L., Seo S.W. Prediction model of conversion to dementia risk in subjects with amnestic mild cognitive impairment: a longitudinal, multi-center clinic-based study. J. Alzheimers Dis. 2017;60:1579–1587. doi: 10.3233/JAD-170507. [DOI] [PubMed] [Google Scholar]
- Jessen F., Wolfsgruber S., Wiese B., Bickel H., Mosch E., Kaduszkiewicz H., Pentzek M., Riedel-Heller S.G., Luck T., Fuchs A., Weyerer S., Werle J., van den Bussche H., Scherer M., Maier W., Wagner M., German Study on Aging Cognition, Dementia in Primary Care Patients AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10:76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Knopman D.S., Jack C.R., Jr., Wiste H.J., Weigand S.D., Vemuri P., Lowe V.J., Kantarci K., Gunter J.L., Senjem M.L., Mielke M.M., Roberts R.O., Boeve B.F., Petersen R.C. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann. Neurol. 2013;73:472–480. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Harvey D., Madison C.M., Reiman E.M., Foster N.L., Aisen P.S., Petersen R.C., Shaw L.M., Trojanowski J.Q., Jack C.R., Jr., Weiner M.W., Jagust W.J., Alzheimer’s disease neuroimaging, I Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L., Weiner M.W., Jagust W.J. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L., Weiner M.W., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S., Weiner M.W., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Lu M., Joshi A.D., Pontecorvo M., Mintun M.A., Trojanowski J.Q., Shaw L.M., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann. Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu S., Letenneur L., Orgogozo J.M., Fabrigoule C., Amieva H., Le Carret N., Barberger-Gateau P., Dartigues J.F. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Maass A., Landau S., Baker S.L., Horng A., Lockhart S.N., La Joie R., Rabinovici G.D., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017;157:448–463. doi: 10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R.W., Weisgraber K.H., Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C., Storandt M., Miller J.P., McKeel D.W., Price J.L., Rubin E.H., Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Okello A., Koivunen J., Edison P., Archer H.A., Turkheimer F.E., Nagren K., Bullock R., Walker Z., Kennedy A., Fox N.C., Rossor M.N., Rinne J.O., Brooks D.J. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V., Ritchie K., Rossor M., Thal L., Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J., Jack C.R., Jr., Jagust W.J., Shaw L.M., Toga A.W., Trojanowski J.Q., Weiner M.W. Alzheimer’s disease neuroimaging initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher S.L., Kim S., Nho K., Foroud T., Shen L., Petersen R.C., Jack C.R., Jr., Beckett L.A., Aisen P.S., Koeppe R.A., Jagust W.J., Shaw L.M., Trojanowski J.Q., Weiner M.W., Saykin A.J., Alzheimer’s Disease Neuroimaging Initiative APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11:1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Landau S.M., Fero A., Schreiber F., Jagust W.J., Alzheimer’s Disease Neuroimaging Initiative Comparison of visual and quantitative florbetapir F 18 positron emission tomography analysis in predicting mild cognitive impairment outcomes. JAMA Neurol. 2015;72:1183–1190. doi: 10.1001/jamaneurol.2015.1633. [DOI] [PubMed] [Google Scholar]
- van Rossum I.A., Vos S., Handels R., Visser P.J. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J. Alzheimers Dis. 2010;20:881–891. doi: 10.3233/JAD-2010-091606. [DOI] [PubMed] [Google Scholar]
- van Rossum I.A., Vos S.J., Burns L., Knol D.L., Scheltens P., Soininen H., Wahlund L.O., Hampel H., Tsolaki M., Minthon L., L'Italien G., van der Flier W.M., Teunissen C.E., Blennow K., Barkhof F., Rueckert D., Wolz R., Verhey F., Visser P.J. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology. 2012;79:1809–1816. doi: 10.1212/WNL.0b013e3182704056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., Ames D., Rowe C.C., Masters C.L., Australian Imaging Biomarkers, Lifestyle Research Group Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Weiner M.W., Aisen P.S., Jack C.R., Jr., Jagust W.J., Trojanowski J.Q., Shaw L., Saykin A.J., Morris J.C., Cairns N., Beckett L.A., Toga A., Green R., Walter S., Soares H., Snyder P., Siemers E., Potter W., Cole P.E., Schmidt M., Alzheimer’s Disease Neuroimaging Initiative The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6(202–211) doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk D.A., Price J.C., Saxton J.A., Snitz B.E., James J.A., Lopez O.L., Aizenstein H.J., Cohen A.D., Weissfeld L.A., Mathis C.A., Klunk W.E., De-Kosky S.T. Amyloid imaging in mild cognitive impairment subtypes. Ann. Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B.S., Kim H.J., Kim Y.J., Jung N.Y., Lee J.S., Lee J., Jang Y.K., Yang J.J., Lee J.M., Vogel J.W., Na D.L., Seo S.W. Longitudinal outcomes of amyloid positive versus negative amnestic mild cognitive impairments: a three-year longitudinal study. Sci. Rep. 2018;8:5557. doi: 10.1038/s41598-018-23676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material