Abstract
Although frequently discussed in terms of sex dimorphism, the neurobiology of sexual orientation and identity is unknown. We report multimodal magnetic resonance imaging data, including cortical thickness (Cth), subcortical volumes, and resting state functional magnetic resonance imaging, from 27 transgender women (TrW), 40 transgender men (TrM), and 80 heterosexual (40 men) and 60 homosexual cisgender controls (30 men). These data show that whereas homosexuality is linked to cerebral sex dimorphism, gender dysphoria primarily involves cerebral networks mediating self–body perception. Among the homosexual cisgender controls, weaker sex dimorphism was found in white matter connections and a partly reversed sex dimorphism in Cth. Similar patterns were detected in transgender persons compared with heterosexual cisgender controls, but the significant clusters disappeared when adding homosexual controls, and correcting for sexual orientation. Instead, both TrW and TrM displayed singular features, showing greater Cth as well as weaker structural and functional connections in the anterior cingulate-precuneus and right occipito-parietal cortex, regions known to process own body perception in the context of self.
Keywords: gender dysphoria, gender identity, MRI, own body, sex differences, sexual orientation
Introduction
Sexual orientation and gender identity are fundamental facets of human experience. Sexual orientation signifies the sex of the object of one’s sexual attraction, whereas gender identity denotes a complex interrelationship among an individual’s genital sex, one’s internal sense of self, and one’s outward presentations and behaviors (gender expression). Despite their existential role, our knowledge about the neurobiological underpinnings of sexual orientation and gender identity is very limited. The present study seeks to identify their possible cerebral signatures by comparing data from multimodal magnetic resonance imaging (MRI) measurements among groups comprised of transgender males—individuals with male gender identity, but assigned female at birth (TrM) and transgender females—individuals with female gender identity, but assigned male at birth, (TrW), homosexual and heterosexual cisgender controls. According to recent nomenclature, “cisgender” refers to, as opposed to transgender, individuals who identify as their sex assigned at birth [American Psychiatric Association (2013), Diagnostic and statistical manual of mental disorders: DSM-5, 5th edns.] Sexual orientation among transgender persons is usually, and also in the present study, operatively defined in relation to the sex assigned at birth.
The distinction between sexual orientation and gender identity is particularly important to maintain when trying to understand the biological underpinnings of gender dysphoria (GD) and its most common form, transgenderism. One reason is that the prevalence of homosexuality and bisexuality is greater among populations with GD [about 50% compared with <10% among cisgender populations (Drummond et al. 2008; Rowniak and Chesla 2013)]. This aspect is usually neglected. If it is not taken into consideration, any difference detected between GD populations and heterosexual cisgender controls is potentially biased and could be a mixed effect of homosexuality and GD. Furthermore, it has been reported that the majority of boys expressing gender variant behavior in childhood, i.e., cross-gender (play) behavior, turn out to be cisgender homosexual men (Zucker and Bradley 1995). Because boys with GD, as opposed to homosexual men, often receive hormonal treatment, it is important to sharpen the diagnostic tools and find possible biomarkers of GD irrespective of sexual orientation. A further reason to distinguish between possible neurobiology of sexual orientation and gender identity is the notion by Blanchard, who based on his clinical observations proposed that homosexual and non-homosexual subjects with GD have different aetiologies and developmental patterns, and differences in brain structure (Blanchard 1989a, 1989b, 2008). This finding has since been supported as well as contradicted, and further studies based on quantitative experimental data are needed in order to properly evaluate its relevance.
Differentiating GD phenotypes with regard to sexual orientation was recently reemphasized by Kreukels and Guillamon (2016) in their review of brain imaging studies of GD. In this review, they discuss the impact of age at onset of GD in regard to etiology, and they also propose a cortical development theory (primarily in the early onset GD) where the brain phenotype of TrM would present a mixture of feminine, masculine, and defeminized traits, whereas that of TrW would present a mixture of masculine, feminine, and demasculinized traits.
Both the etiology of GD and the factors underlying the development of a homosexual, bisexual, or heterosexual orientation are believed to be linked to prenatal and early post-natal sex hormone exposure (Swaab and Hofman 1995; Swaab et al. 1995; Balthazart 2011) and to a less prominent sexual differentiation of the brain (Hines 2011; Kreukels and Guillamon 2016; Swaab and Garcia-Falgueras 2009). However, brain imaging studies employed to test this notion, while being largely consistent in showing structural and functional differences among cisgender male and female controls (Filipek et al. 1994; Goldstein et al. 2001; Luders et al. 2006; Savic and Arver 2011, 2014; Bramen et al. 2012; Lentini et al. 2013), seem rather inconsistent with respect to the findings among subjects with GD. This especially applies to TrM, for which some studies have reported a cerebral pattern congruent with that of cisgender females (Zubiaurre-Elorza et al. 2013; Hoekzema et al. 2015), while others have found partly similar structural and functional neural characteristics as in cisgender males (Simon et al. 2013; Burke et al. 2017), and still others have identified a pattern different from both cisgender male and cisgender female control groups (Junger et al. 2014; Kranz et al. 2014; Manzouri et al. 2017). Corresponding studies of TrW seem more consistent, showing a “female” pattern both with respect to fractional anisotropy (FA), reflecting white matter connections, and cortical thickness (Rametti, Carrillo, Gomez-Gil, Junque, Segovia et al. 2011; Zubiaurre-Elorza et al. 2014). Some findings, however, diverge from this pattern, showing, for example, values in between those of male and female controls regarding white matter integrity (Hahn et al. 2015) and a thicker mesial frontal lobe cortex compared to cisgender male controls though male and female cisgender controls did not differ in this region (Luders et al. 2012). In sum, while it seems that cerebral morphology and function differ between cisgender and transgender subjects, there has not been consistent evidence for systematical patterns, possibly because most of the studies compare heterosexual control groups with mixed homo, hetero, and bisexual transsexual groups. Another reason for the lack of clarity is that most reports are from investigations based on single methods measuring either cerebral anatomy or function and do not take a multifaceted viewpoint on the possible neurobiology of GD. Furthermore, the majority of the available brain imaging studies do not address the principal features of GD—a strong perception of incongruence between one’s sense of self and one’s body, a discomfort with one’s own body, and a feeling of estrangement towards one’s physical sex (Cohen-Kettenis and Pfafflin 2010).
Own body perception is believed to be molded by a reciprocal interaction between sensory perceptions of one’s physical appearance, based on self-observation and the reactions of others (Cash 2002), and one’s own body image representation in the brain (Vocks et al. 2010). We recently published a series of studies suggesting that cerebral networks involved in own body perception in the context of self, [including the pregenual anterior cingulate cortex (pACC), temporo-parietal junction, and fusiform body area] are different in individuals with GD compared with cisgender persons (Savic and Arver 2011, 2014; Feusner et al. 2016, 2017; Manzouri et al. 2017; Feusner et al. 2017). We also put forward a hypothesis that the discomfort with their own bodies reported by individuals with GD is linked to the nodes of the default mode network (DMN). The DMN is an intrinsic connectivity network that previous studies have shown to be involved in mind-wandering and self-referential thinking (Mason et al. 2007; Northoff and Panksepp 2008; Christoff et al. 2009). The DMN has been found to overlap with areas involved in own body perception in the context of self but has received little attention in studies of GD. One theoretical possibility is that, for individuals with GD, the typical physical traits of their sex assigned at birth are not incorporated into their own body image representation in the brain—and that this is associated with specific functional and structural cerebral signatures. Congruent with this notion, a recent MEG and MRI study of TrM (pre-treatment) showed that the response in the somatosensory cortex corresponding to the breast-thorax area of the homunculus was reduced compared with sex-matched controls (Case et al. 2017).
Contrary to the common notion, the neurobiology of GD may not necessarily be coupled to sex dimorphism. Sexual orientation, on the other hand, may well be. Indeed, studies of homosexual and heterosexual persons utilizing various measures—including performance on visuospatial and word fluency! tests (Rahman et al. 2011), anthropomorphic measures such as the right-hand digit2/digit4 ratio (Robinson and Manning 2000), functional connectivity of the amygdala, perception of putative pheromones (Savic et al. 2005; Berglund et al. 2008), and the thickness of the cuneus cortex (Abe et al. 2014)—all point to different, even sex-reversed features among homosexual study groups compared with heterosexual groups. Thus, the neuronal underpinnings of gender identity and sexual orientation may be different, despite the reported co-occurrence of homosexuality and GD.
In the present study, we sought to identify possible singular as well as common cerebral features among homosexual and GD persons in comparison with both homosexual and heterosexual cisgender controls. Rather than using a single metric, we employed both functional and anatomical MRI, which enabled the possible detection of coordinated characteristics in one group as compared with the other. We expected to find different structural and connectivity features among transgender groups compared with cisgender controls “primarily” within the circuits mediating own body perception in the context of self, irrespective of sex assigned at birth. Differences in sex dimorphic networks, on the other hand, were expected in relation to homosexual subjects.
Materials and Methods
The demographical data are presented in Table 1. Forty TrM (mean age 24, sd 6 years), 27 TrW (mean age 25, sd 5), 40 heterosexual cisgender males (mean age 30, sd 6 years), and 40 heterosexual cisgender females (mean age 29, sd 5) participated in the study, together with 30 homosexual cisgender men (HoM; mean age 31, sd 6 years) and 30 homosexual cisgender women (HoW; mean age 28, sd 5 years). The majority of TrW and TrM reported an early (age 3–4 years) awareness of their transgender identity, with only ~20% reporting an early post-pubertal onset. None of the transgender persons had received hormone treatment or sex confirmative surgery. The transgender persons were recruited by the Gender Team of the Center for Andrology and Sexual Medicine at Karolinska University Hospital (Stockholm, Sweden), a center specializing in the evaluation and treatment of individuals with GD. Between January 2011 and June 2016, all of the adults aged 18–45 who sought gender confirming medical interventions at the center, and were diagnosed with GD, more specifically transsexualism (F64.0), based on the ICD-10 diagnostic criteria, were consecutively approached to enter the study. Exclusion criteria consisted of previous or current hormonal treatment at the time of the first scanning session, having any known chromosomal or hormonal disorder, any current psychiatric disorder (as confirmed by the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 2010)) any neurological or other major medical disorder, and use of any medications with psychotropic effects (antipsychotic or antiepileptic agents, lithium, benzodiazepines, or opioid analgesics). We specifically excluded participants with autism spectrum disorder (ASD; diagnosed before being referred to the team) and participants who showed clinical signs of ASD when being assessed by the team. The control group consisted of individuals without GD, a neurological or psychiatric condition or family history of one, a substance abuse problem, and who were not taking ongoing medication.
Table 1.
Demographic data
| Unit | HeM | HeW | HoM | HoW | TrW | TrM | F-value (5, 201) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 40 | N = 40 | N = 30 | N = 30 | N = 27 | N = 40 | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Age | Year | 29.5 | 6.2 | 29.1 | 5.2 | 31.4 | 6.1 | 27.9 | 6.1 | 24.8 | 5.4 | 24.1 | 5.6 | 8.1 | 0.000 |
| Education | Year | 16.0 | 2.7 | 16.8 | 2.8 | 15.6 | 2.6 | 16.0 | 2.9 | 14.0 | 2.0 | 13.2 | 2.5 | 10.1 | 0.000 |
| Kinsey score | 0.3 | 0.5 | 0.5 | 0.9 | 5.6 | 05 | 5.5 | 0.5 | 3.6 | 2.2 | 4.5 | 1.7 | 146.4 | 0.000 | |
Note: F-values from group comparisons (one-way ANOVA). For the distribution of Kinsey scores, please see Materials and Methods.
Sexual orientation was assessed using the self-report Kinsey scale (Kegel 1953), a seven-point scale ranging from 0 (heterosexual, i.e., sexually attracted to the opposite sex) to 6 (homosexual, i.e., sexually attracted to the same sex). Sex refers here to the sex assigned at birth. The approach is described in detail in previous studies (Berglund et al. 2006; Savic and Lindström 2008). By design, the groups differed in terms of sexual orientation. The group average Kinsey scale scores are presented in Table 1. The transgender groups were heterogeneous with regard to sexual orientation. Among the 40 TrM subjects, 24 scored as gynephilic (scores 4–6), 10 as bisexual (score 3), and 6 as androphilic (scores 0–2). Among the 27 TrW subjects, 16 scored as androphilic (scores 4–6), 10 as gynephilic (scores 0–2), and 1 as bisexual (score 3).
The study was approved by the ethical committee of the Karolinska Institute (application number: Dnr 2011/281–31/4) and each participant provided signed consent before entering the study.
The serum hormone levels of transgender persons were assessed through routine clinical checkups, and we used the assessment closest in time to the MR sessions for the purpose of this study. No blood samples were collected among controls.
Data acquisition
Magnetic resonance imaging data was acquired on a 3-Tesla MRI medical scanner (Discovery 3 T GE-MR750, General Electric, Milwaukee, WI) equipped with a 32-channel /or 8-channel phased array receiving coil. 3D T1-weighted spoiled gradient (SPGR) images were acquired with 1 mm3 isotropic voxel size (TE = 3.1 ms, TR = 7.9 ms, TI = 450 ms, FoV = 24 cm, 176 axial slices, flip angle of 12 deg.). Resting state functional MRI was performed for 8 min with subjects’ eyes closed, with a gradient echo pulse sequence using a voxel size of 2.25 × 2.25 × 3 mm (TE = 30 ms, TR = 2500 ms, FoV = 28.8 cm, 45 bottom up interleaved axial slices, 3 mm thickness, flip angle of 90 deg.). In addition, multi-slice DTI was performed using an echo planar imaging sequence with 1 × 1 mm in-plane resolution, [TE = 83 ms, TR 8000 ms, FoV = 24 cm, 60 interleaved axial slices, thickness = 2.9 mm, 55 diffusion gradient directions (b = 1000), six B0, flip angle of 90 deg]. Finally, clinical sagittal FLAIR images were taken (TE/TR = 126.3/6000, TI = 1863, ETL = 140, ARC acceler. R = 2 × 2 slice, phase, FoV = 24 cm, 512 × 512, slice thickness = 1.2 mm). Images acquired with the 8-channel coil were used for the FreeSurfer analyses, see below because these T1 images had better demarcations between white and gray matter in the occipital cortex than T1 images acquired with the 32-channel coil. Images acquired with the 32-channel coil, were used for the functional magnetic resonance imaging (fMRI) and DTI analyses.
Cortical thickness and subcortical volume analyses
Cortical reconstruction and volumetric segmentation were performed using the FreeSurfer image analysis suite, version 5.1 (Fischl and Dale 2000; www.surfer.nmr.mgh.harvard.edu) to derive measures of cortical thickness, subcortical volumes, and total intracranial volume (TIV). The T1-weighted images were processed using the FreeSurfer software (version 5.1), which included several steps (skull stripping, Talairach transforms, atlas registration, spherical surface maps, and parcellations). The resulting images were visually inspected for accuracy for all participants and were manually edited when needed (primarily to improve skull stripping). Subcortical segmentations generated with FreeSurfer (Fischl et al. 2002; 2004) were used to calculate the volumes of five subcortical brain structures: the amygdala, hippocampus, caudate nucleus, putamen, and thalamus. These structures were selected because they usually show sex differences in regard to volume among cisgender sexual persons (Lentini et al. 2013; Savic and Arver 2014, 2011). In addition, the putamen is found to be involved in own body perception (Petkova et al. 2011), allowing us to test our primary hypotheses also with respect to subcortical volumes.
Fractional anisotropy
Diffusion images were analyzed and (motion) artifacts and eddy current distortions were corrected for using DTIPrep (Oguz et al. 2014). Using DTIfit, which is part of the FMRIB’s Diffusion Toolbox implemented in FSL v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), images were realigned to one of the non-weighted images using affine registration, non-brain tissue removal was done using BET (part of FMRIB Software Library ), and a tensor model was fitted to the diffusion data, defining the eigenvalues of the tensor for each voxel and calculating individual FA maps. Voxel-wise statistical analyses were performed using tract-based spatial statistics (TBSS). All of the subject’s FA maps were registered to the FMRIB58_FA template, and then transformed to MNI152 space. The normalized individual FA maps were averaged to create a group-wise mean FA white matter skeleton. A threshold of 0.3 was applied to reduce partial volume effects. The FA skeleton for subsequent voxel-wise statistical analyses was generated using the Randomize Tool (part of FSL). Whole-brain voxel-wise analyses were performed. Using Randomize, permutation-based non-parametric testing (5000 permutations) was carried out, applying the Threshold-Free Cluster Enhancement option. A cluster-forming threshold of P = 0.05 and a minimal cluster size of 100 voxels were applied. Results were considered significant at PFWE < 0.05 (family-wise error corrected). Anatomical locations were identified using the JHU White Matter Tractography atlas and JHU ICBM-DTI-81 white matter labels (Hua et al. 2008; Mori et al. 2008).
Resting state functional MRI
The analysis of resting state functional connectivity, using model-free independent component analysis (ICA; MELODIC), focused on possible group differences in relation to the DMN. The analyses were carried out in FSL v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), as described in a previous study from our group (Feusner et al. 2017).
Spatial preprocessing of the functional images was performed using SPM8 (Welcome Department of Cognitive Neurology) according to the standardized procedure including fieldmap correction using B0 images during the warping procedure (applying VDM’ option in the FieldMap Toolbox).
Movement correction was conducted with 18 movement regressors (six linear, their squares and cubes, SPM8 software) and through the use of ICA-AROMA, which automatically identifies and subsequently removes data-driven derived components that represent motion-related artifacts (Pruim et al. 2015). The spatial parameters were then applied to the slice-timed and realigned functional volumes that were finally resampled to 2 × 2 × 2 mm voxels and smoothed with a 6-mm full-width at half-maximum kernel.
The data were, then analyzed in FSL software v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), using a high-pass filter at 100 s before running individual ICAs (Beckmann and Smith 2004), (Multivariate Exploratory Linear Decomposition into Independent Components, Version 3.14), with automatic determination of dimensionality. The resulting component maps were then manually classified into components of interest and nuisance components in accordance with the criteria proposed in (Kelly et al. 2010). The nuisance components were subsequently regressed out of the original dataset using fsl_regfilt.
Group concat-ICA was performed on the entire cleaned dataset, resulting in 22 components. These components are usually used to run dual regression analysis, and the resulting general linear model (GLM) parameter estimate images are fed into FSL’s Randomize tool for non-parametric permutation inference in order to test the separate hypotheses about differences in connectivity among groups (Feusner et al. 2016). In light of our a priori hypothesis, only the DMN component was further examined for group comparisons, and the other 21 components were not used in the analysis for the present publication. The statistical design included using mean DVARS (Root Mean Square intensity difference of volume N to volume N + 1; Power et al. 2012) as a nuisance covariate. DVARS represent an index of the effects of motion.
Statistical analyses, general overview
The statistical analyses were used to test our two principal hypotheses: (1) that cerebral sex dimorphism would be less pronounced or even reversed in the homosexual and transgender study groups compared with heterosexual cisgender controls, and (2) that cerebral midline networks processing own body perception in the context of self would be different in the transgender groups than among heterosexual and homosexual cisgender control groups. The analyses were, therefore, focused on sexually dimorphic structures, and on networks involved in own body perception in the context of self. Evaluation of subcortical volumes and of rs-fMRI within the DMN as well as of FA values in specific white matter tracts were, thus, hypothesis based. The analysis of Cth was explorative, an approach that was regarded as relevant, considering that male–female differences in Cth are not regionally delimited according to available literature, and that FA values have been observed to be generally rather than regionally higher among men. Explorative analysis was, also employed for group comparisons of white matter integrity (in addition to the analysis of specific tracts), as some previous studies have shown both widespread and more limited differences between males and females.
Statistical analyses, separate methods
Cortical thickness
Group differences in Cth were calculated with Qdec statistical tool within the FreeSurfer software, using different slope different onset option, and using age and education as the nuisance covariates (thus, assuming group differences, and effects of age and education, two factors that differed between the groups, see Table 1, demographics). We employed a 10 mm filter, and a threshold of P < 0.05 with Monte Carlo correction.
Subcortical structural volumes
We first tested whether there were any group differences within the same sex for total intracranial volume (ICV) (two separate one-way ANOVAs, P < 0.025). Then, we examined whether there were group differences in subcortical volumes, using individual structural volume/ICV ratios as input values. Possible difference between cisgender homosexual groups and cisgender heterosexual groups was tested using multivariate (10 subcortical regions) GLM analysis (P < 0.05) (SPSS, version 24). Sex and sexual orientation were between factors and region the within-factor. Next, we investigated whether there was any effect of gender identity on relative structural volumes (Volume/ICV), using the same method [including all cisgender men (HeM and HoM), all cisgender women (HeW and HoW), TrW, and TrM]. We considered an overall 2 × 2 × 2 (sex, sexual orientation, and gender identity) ANOVA or GLM not to be suitable, as the factor of sexual orientation was not binary (homo or heterosexual) for the transgender groups.
Finally, we tested whether there were any group differences within each sex as assigned at birth, thus separately comparing HoM, HeM, and TrW as well as HoW, HeW, and TrM, again using region (structural volume) as the dependent variable and group as the fixed factor (univariate GLM (P < 0.025) due to two separate comparisons).
FA values
Group differences in FA values were first examined using the explorative, TBSS statistics. All results, if not specified otherwise, were considered significant at PFWE < 0.05 (family-wise error corrected), and a minimal cluster size of k > 100 voxels. We first used a 2 × 2 (sex by sexual orientation) ANOVA, followed by a separate 2 × 2 (sex by gender identity) ANOVA. Finally, two separate one-way ANOVAs, followed by post hoc comparisons (Scheffe’s post hoc test, P < 0.05) were conducted to specifically test the influences of gender identity and sexual orientation within the groups comprised of persons with the same sex assigned at birth; specifically, the comparisons were between TrW, HoM, and HeM and between TrM, HoW, and HeW, and included age as the covariate of no interest.
Rs-fMRI
Group differences were tested with dual regression analysis, and the resulting GLM parameter estimate images were fed into FSL’s randomize tool for non-parametric permutation inference in order to test the separate hypotheses about differences in connectivity within the DMN among groups.
The results are reported at a threshold of FWE corrected P < 0.01 (using mask as described by Feusner et al. (2017)). This more conservative threshold was selected due to the multiple comparisons involved.
Results
Demographics
Demographic data are presented in Table 1. As the six groups differed in age and years of education (two separate one-way ANOVAS, P < 0.05), these categories were used as covariates of no interest in all further comparisons. Post hoc Scheffe tests showed no differences between the two transgender groups, nor between the homosexual and heterosexual cisgender populations. The cisgender groups were at the two extremes of the sexual orientation scale, whereas the transgender groups were heterogeneous in this respect.
Analyses of cortical thickness
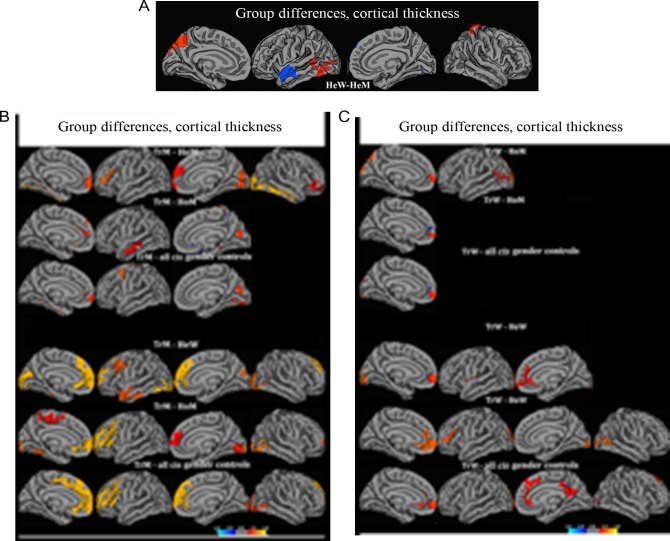
We replicated previous findings of significant sex differences between the two heterosexual cisgender control groups, as HeW were observed to have thicker parietal, occipital, and sensory motor cortices, while HeM displayed a thicker left superior temporal cortex (Table 2a, Fig. 1a). TrM showed no significant difference from HeM in the parietal Cth, and the anterior portion of their left superior temporal cortex was thicker than in HeW but not HeM. TrM thus displayed a “male pattern” (Table 2b). However, when including homosexual subjects in the control group and comparing TrM with all cisgender controls, and using Kinsey scores as the covariate, the cluster indicating thicker left superior temporal cortex as compared with HeW was no longer present (Fig. 1b); the parietal cortex, however, was still not thicker than in HeM. For TrW, on the other hand, the initial analysis revealed a thicker left parietal cortex in comparison to HeM but not HeW, and the Cth of their left superior temporal cortex did not differ significantly from that of HeW or HeM (Fig. 1c, Table 2b). This partly “female pattern” among TrW, however, was no longer present after re-running the comparisons including the homosexual cisgender controls and using Kinsey scores as the covariate. Thus, the significant “female-typical” cluster in the parietal cortex vis á vis male controls was no longer present when adding HoM, whereas Cth in the left superior temporal gyrus continued not to differ from that of both male and female cisgender controls (Fig. 1c, Table 2a). Hence, the features suggesting “sex-reversed” characteristics (in relation to the sex assigned at birth) among both transgender populations seemed to be attributed to a higher proportion of homosexual and bisexual persons among these groups. The non-differing values (ns in relation to cisgender men as well as cisgender women) concerning the left temporal cortex in TrW and the right parietal cortex among TrM remained, even when correcting for sexual orientation, whereas the “sex-reversed” patterns were no longer present.
Table 2a.
Clusters showing significant group difference in cortical thickness
| Cluster | HeW–HeM (positive −log10(P) values) | TrW–HeW (positive −log10(P) values) | TrW–HeM (positive −log10(P) values) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HeM–HeW (negative −log10(P) values) | HeW–TrW (negative −log10(P) values) | HeM–TrW (negative −log10(P) values) | |||||||
| Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | |
| L Superior temporal cortex | −2.7 | 15.4 | −48 −2 −20 | ||||||
| L rostral middle frontal cortex | 4.6 | 23.7 | −33 46 15 | 2.9 | 1.2 | −12 42 15 | |||
| L post-central + superior parietal cortex | 4.4 | 19.9 | −41 −44 20 | ||||||
| L lateral occipital cortex | 2.3 | 28.1 | −29 −84 2 | 3.8 | 23.1 | −18 −96 −8 | 2.7 | 12.4 | −29 −88 16 |
| L insular cortex | 3.7 | 15.2 | −37 −24 0 | ||||||
| L isthmus cingulate cortex | 2.9 | 13.4 | −7 −45 30 | ||||||
| L parietal cortex | 2.8 | 17.5 | −51 −43 9 | ||||||
| R occipito-temporal cortex | 3.0 | 14.6 | −4 −71 16 | ||||||
| R post-central gyrus | |||||||||
| R superior frontal cortex | 3.5 | 17.5 | 15 56 13 | ||||||
Note: Statistical threshold is P < 0.05, corrected for multiple comparisons (according to Monte Carlo permutations). Demeaned age and demeaned education were used as nuisance covariates. These data were generated by contrasts when only using heterosexual cis-sexual controls and are presented to allow comparisons with our previous publications with similar study groups. The filter was 10 mm. The Talairach’s coordinates indicate location of maximum difference; the “Region” column describes the coverage of the respective cluster.
R = right; L = left.
Figure 1.
Group differences in cortical thickness. Contrasts calculated at P < 0.05, FWE corrected for multiple comparisons (Monte Carlo permutation). The projection of cerebral hemispheres (MR images of the FreeSurfer atlas) is standardized. Scale is logarithmic and shows –log10 (P), with cool colors indicating negative contrast, and warm colors positive contrast. (A) Comparison between cisgender heterosexual men and women. (B) Comparisons between transgender men and controls. (C) Comparisons between transgender women and controls. The figures illustrate how significant clusters depend on whether the cisgender control groups are comprised of homosexual or heterosexual persons, or a mix of both. HoM, homosexual men; HeW, heterosexual women; HoM, homosexual men; HeM, heterosexual men. TrM, transgender men; TrW, transgender women.
Table 2b.
Clusters showing significant group difference in cortical thickness
| Cluster | TrM–TrW (positive −log10(P) values) | TrM–HeW (positive −log10(P) values) | TrM–HeM (positive −log10(P) values) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TrW–TrM (negative −log10(P) values) | HeW–TrM (negative −log10(P) values) | HeM–TrM (negative −log10(P) values) | |||||||
| Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | Maximum vertex-wise −log10(P) | Cluster size (cm2) | Talairach coordinates | |
| L superior temporal cortex | 1.8 | 10.5 | −45 5 −25 | ||||||
| L precentral cortex | 3.9 | 14.3 | −38 0 32 | ||||||
| L rostral anterior cingulate cortex | 3.4 | 23.5 | −6 33 −5 | ||||||
| L lateral occipital + cuneus cortexa | 2.7 | 19.1 | −19 −97 −5 | 3.6 | 15.9 | −28 −82 6 | |||
| L entorhinal cortex | 4.3 | 21.5 | −29 −12 −26 | ||||||
| R lateral occipital cortex | 2.6 | 11.5 | 25 −91 15 | ||||||
| R superior frontal cortex | 3.2 | 10.4 | 10 43 19 | 4.3 | 16.4 | 12 20 27 | |||
| R precalcarine cortex | 3.3 | 12.6 | 4 −71 16 | ||||||
| R lingual cortex | 5.3 | 24.1 | 12 −72 2 | ||||||
Note: Statistical threshold is P < 0.05, corrected for multiple comparisons (according to Monte Carlo permutations). Demeaned age and demeaned education were used as nuisance covariates. These data were generated by contrasts when only using heterosexual cis-sexual controls and are presented to allow comparisons with our previous publications with similar study groups. The filter was 10 mm. The Talairach’s coordinates indicate location of maximum difference; the “Region” column describes the coverage of the respective cluster.
aCovers parts of the right temporal cortex.
These findings motivated separate investigations of the corresponding data from the homosexual groups. We found that the Cth value for the left superior temporal cortex among HoW was in-between that of HeM and HeW. For HoM, the Cth value for the left parietal cortex was similar to that of HeW and larger than among HeM (Fig. 2), whereas their Cth value for the left superior temporal cortex was similar to that of HeM. The data for the homosexual groups are described in detail in a separate paper (Manzouri and Savic 2018), and also in Fig. 2 and Table 3. Notably, the differences between HoM and HeM were pronounced, while no significant clusters were detected when comparing HoW and HeW. HoM showed significantly greater Cth in the medial prefrontal and precuneus regions than all three other cisgender groups.
Figure 2.
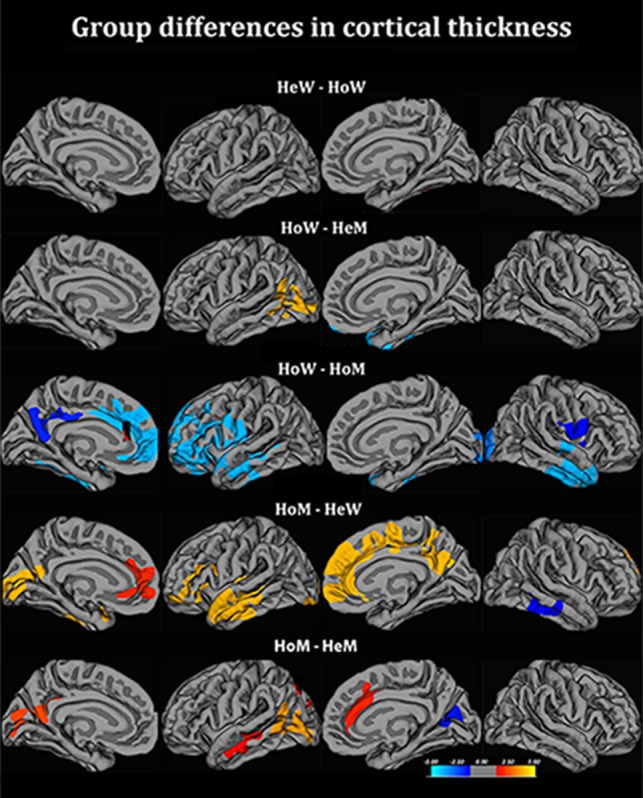
Group differences in cortical thickness between homosexual and heterosexual cisgender controls. Contrasts calculated at P < 0.05, FWE corrected for multiple comparisons (Monte Carlo permutation). The projection of cerebral hemispheres (MR images of the FreeSurfer atlas) is standardized. Scale is logarithmic and shows –log10(P), with cool colors indicating negative contrast, and warm colors positive contrast. HoM = homosexual men; HeW = heterosexual women; HoW = homosexual women; HeM = heterosexual men.
Table 3.
Differences in cortical thickness between homosexual and heterosexual cisgender controls
| Region | HoM–HeM | HoW–HoM | HoW–HeW | HoW–HeM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max, log10(P) | Size (cm2) | Coordinates | Max, log10(P) | Size (cm2) | Coordinates | Max, log10(P) | Size (cm2) | Coordinate | Max, log10(P) | Size (cm2) | Coordinates | |
| L middle temporal and parietal cortex | ||||||||||||
| L superior frontal cortex | ||||||||||||
| L pericalcarine cortex | ||||||||||||
| L precuneus | 3.6 | 14.3 | −6 −58 18a | −4.2 | 12.7 | −6 −66 36 | ||||||
| L lateral occipital and parietal cortex | 4.6 | 14.0 | −42 −81 2 | |||||||||
| L pars triangularis + prefrontal cortex | −5.3 | 178.4 | −41 30 −2b | |||||||||
| R cuneus | −3.4 | 11.6 | 4 −73 16 | |||||||||
| R rostral anterior cingulate cortex | 3.5 | 12.5 | 11 35 12 | |||||||||
| R inferior temporal cortex | ||||||||||||
| R insular, pre-post-central cortex | −6.0 | 13.2 | 56 3 6 | |||||||||
| R Superior frontal cortex | −5.5 | 35 | 8 51 15 | |||||||||
| R lateral occipital cortex | −2.9 | 17.1 | 32 −78 7 | |||||||||
Note: Negative values denote reverse contrast. The ‘Region’ column describes the coverage of the cluster. R = right; L = left. Clusters are calculated at P 0.05 corrected, using a 10-mm filter.
aIncludes left inferior and superior parietal lobes.
bLarge cluster including superior frontal and anterior cingulate cortex, left superior temporal gyrus.
Another prominent observation, concerning both transgender groups, was that the thickness of the pregenual anterior cingulate, superior frontal, and occipito-temporal cortices was significantly greater in TrM as well as in TrW in relation to both male and female cisgender controls. Furthermore, this difference between transgender subjects and cisgender controls remained when including the homosexual controls and accounting for Kinsey scores (Fig. 1b,c). Thus, these clusters were independent of sexual orientation. Notably, a direct comparison between the two transgender groups revealed no differences in the Cth of the medial prefrontal frontal lobes, confirming that the detected frontal lobe thickening was a feature shared by both transgender populations. The only difference between TrM and TrW was that the left lateral occipital cortex and the right lingual cortex (including the extrastriatal body area (EBA)) was thicker in TrM (−log10(P) = 2.7, size 19.0 cc, coordinate 10 −95 −5), and thus followed the pattern of the sex assigned at birth. In the parietal cortex, Cth was sub-significantly greater in TrM than in TrW, which, again, follows the pattern of the sex assigned at birth, although less prominently than among heterosexual controls. It is also worth mentioning that a direct comparison between all of the transgender subjects and all of the cisgender controls revealed thicker cortex among transgender persons, bilaterally in the prefrontal fronto-polar cortex, the right occipito-temporal cortex, and in the left temporo-parietal junction and the cuneus (Supplementary, Fig. 1). There were no regions in which a thinner cortex was found among transgender subjects as compared with controls.
In summary, transgender subjects showed a pattern of Cth in sexually dimorphic areas that was more in accordance with the sex they identify with. However, this pattern was primarily generated by the data from homosexual transgender subjects. To the contrary, the singular features shared by both transgender groups, the thickening of the mesial prefrontal cortex, the temporo-parietal cortex (right > left) and cuneus, were not associated with sexual orientation. To further test the possible effects of sexual orientation among transgender populations, we sub-classified the groups according to sexual orientation (Kinsey score 0–4 was defined as non-homosexual, 5–6 as homosexual), In these post hoc analyses of mean Cth, two interesting features emerged: the mesial prefrontal cortex was thicker in TrW and TrM than in controls, irrespective of sexual orientation. In the left superior temporal cortex, which is usually thicker in males, and in the parietal cortex, which is usually thicker in females, the non-homosexual transgender participants tended to follow the pattern for their sex assigned at birth to a greater extent than homosexual transgender subjects (Supplementary Fig. 2a,b).
In short, with regard to Cth, the pattern of sexual dimorphism was less pronounced among the homosexual populations, both cisgender and transgender. Furthermore, among the transgender groups (both TrM and TrW, irrespective of sexual orientation), singular characteristics were detected, however, not in the typically sexually dimorphic areas but within the DMN along the cerebral midline.
Subcortical structures and total intracranial volume
The ICV did not differ between the study groups of the same sex assigned at birth (females: F = 0.1(2.117), P = 0.896; males: F = 0.3(2.94), P = 0.719); see Table 4. Thus, with regard to ICV, data from TrM and TrW were in line with their sex assigned at birth, with higher values among TrW than TrM (Table 4). The sex (male, female) by sexual orientation (heterosexual, homosexual) GLM showed a significant main effect of sex but not sexual orientation [Wilk’s lambda for the corrected model = 0.006, F(10, 124) = 2208.8, P < 0.00, with F(10, 124) = 4.78, P < 0.00 for sex, and F(10, 124) = 0.311, P = 0.087 for sexual orientation]. There was no interaction between sex and sexual orientation F(10, 124) = 1.6, P < 0.099. Likewise, the sex (male and female) by gender identity (cisgender, transgender) GLM showed an effect of sex but not gender identity [Wilk’s lambda for the corrected model = 0.107, F(10, 127) = 0.125, P = 0.00; for effect of sex F(10, 127) = 4.46, P < 0.00 and sexual orientation F(10, 127) = 0.422, P = 0.934]; there was no interaction between sex and gender identity [F(10, 127 = 1.0, P = 0.099]. As shown in Table 4, and reported earlier, the relative volumes of the hippocampus, caudate, and thalamus were greater among the females assigned at birth, whereas the relative putamen volumes were greater among the males assigned at birth (Table 4). We also tested whether there was any effect of gender identity or sexual orientation for each sex as assigned at birth, thus separately comparing HoM, HeM, and TrW and HoW, HeW, and TrM. To avoid 0-effect due to opposite dimensionalities linked to sex dimorphism, we compared the caudate, thalamus, and hippocampus (with multivariate GLM) separately from the putamen (with univariate GLM), using region as the dependent variable and group as the fixed factor (P < 0.025 due to two comparisons—male and female sexes). There were no significant group differences between groups with the male sex [for the caudate, thalamus, and hippocampus, Wilks’ lambda was 0.805, F(12, 212) = 1.975, P = 0.028; for the putamen, Wilks’ lambda was 0.945, F(4, 220) = 1.569, P = 0.184)]. Nor did we find significant differences between groups with the female sex [for the caudate, thalamus, and hippocampus, Wilks’ lambda was 0.868, F(6, 92) = 1.124, P = 0.343; for the putamen, Wilks’ lambda was 0.891, F(2, 96) = 2.28, P = 0.065]. Thus, HoW and HoM as well as TrW and TrM followed the pattern of their sex assigned at birth.
Table 4.
Structural volumes
| Structural volumes (cm3) | HeM | HeW | TrM | TrW | HoM | HoW | P and F (3, 36), sex × sexual orientation | P and F (3,143), sex × gender identity |
|---|---|---|---|---|---|---|---|---|
| N = 40 | N = 40 | N = 40 | N = 27 | N = 30 | N = 30 | |||
| L caudate volume | 4.2 ± 0.5 | 3.9 ± 0.4 | 3.8 ± 0.4 | 4.0 ± 0.5 | 4.2 ± 0.6 | 3.9 ± 0.5 |
|
|
| R caudate volume | 4.2 ± 0.6 | 4.0 ± 0.4 | 3.9 ± 0.4 | 4.1 ± 0.5 | 4.2 ± 0.6 | 3.9 ± 0.4 |
|
|
| L putamen volume | 5.5 ± 0.6 | 4.9 ± 0.6 | 5.0 ± 0.4 | 5.6 ± 0.7 | 5.3 ± 0.5 | 5.1 ± 0.7 |
|
|
| R putamen volume | 5.3 ± 0.6 | 4.8 ± 0.6 | 4.9 ± 0.5 | 5.5 ± 0.7 | 5.2 ± 0.6 | 4.9 ± 0.7 |
|
|
| L hippocampus volume | 4.3 ± 0.4 | 4.0 ± 0.3 | 4.0 ± 0.4 | 4.4 ± 0.5 | 4.2 ± 0.4 | 4.2 ± 0.4 |
|
|
| R hippocampus volume | 4.4 ± 0.4 | 4.1 ± 0.3 | 4.1 ± 0.4 | 4.6 ± 0.4 | 4.3 ± 0.6 | 4.3 ± 0.5 |
|
|
| L amygdala | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.3 | 1.8 ± 0.2 |
|
|
| R amygdala | 2.0 ± 0.3 | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.3 | 2.1 ± 0.4 | 1.9 ± 0.3 |
|
|
| L thalamus volume | 7.5 ± 0.6 | 6.8 ± 0.5 | 6.8 ± 0.7 | 7.5 ± 0.6 | 7.1 ± 0.7 | 6.9 ± 0.5 |
|
|
| R thalamus volume | 7.5 ± 0.7 | 6.7 ± 0.6 | 6.8 ± 0.6 | 7.3 ± 0.6 | 7.1 ± 0.7 | 6.8 ± 0.5 |
|
|
| ICV volume | 1632.1 ± 130.6 | 1427.8 ± 118 | 1403.1 ± 224.2 | 1618.4 ± 118.1 | 1618.4 ± 123.1 | 1420.1 ± 131.1 |
|
|
Note: ICV, total intracranial volume; The P- and F-values indicate results from multivariate (10 subcortical regions) general linear model (GLM) analysis (P < 0.05), based on calculations of ratios between the respective structural volume and the ICV. Left column—P- and F-values from GLM analysis using sex and sexual orientation as between factors and region the within-factor; Right column—P- and F-values from GLM analysis using sex and gender identity as between factors and region as the within-factor.
As there were no significant effects of sexual orientation, no Kinsey corrected post hoc comparisons were carried out.
Structural connectivity indexed by fractional anisotropy values
FA voxel-wise statistics, using explorative whole-brain analysis
The results from a sex (male and female) by sexual orientation (heterosexual and homosexual) ANOVA including the four cisgender groups showed a main effect of sex (Fig. 3a and Supplementary Table 1). Notably, there was no effect of sexual orientation. Post hoc two-group t-test (P < 0.05) comparisons with respect to effects of sex revealed highly significant sex differences among heterosexual groups, HeM>HeW in almost all major tracts, even when reducing the amount of data to match the group size of homosexual subjects (N = 30 in each group; see Supplementary Table 1). In contrast, the homosexual groups barely differed from each other, with only a small cluster located in the left corticospinal tract (CST), which showed a significantly higher FA among HoM than HoW (Fig. 3a). Comparisons across groups showed significantly higher FA values among HeM compared with HoW, but the differences were less extensive than in relation to HeW. Likewise, but less pervasively, HoM had significantly higher FA values than HeW (Supplementary Fig. 3). Thus, the lack of sex difference among the homosexual groups was not due to one particular homosexual group. There were no tracts whose FA values were higher among cisgender women than cisgender men, irrespective of sexual orientation.
Figure 3.
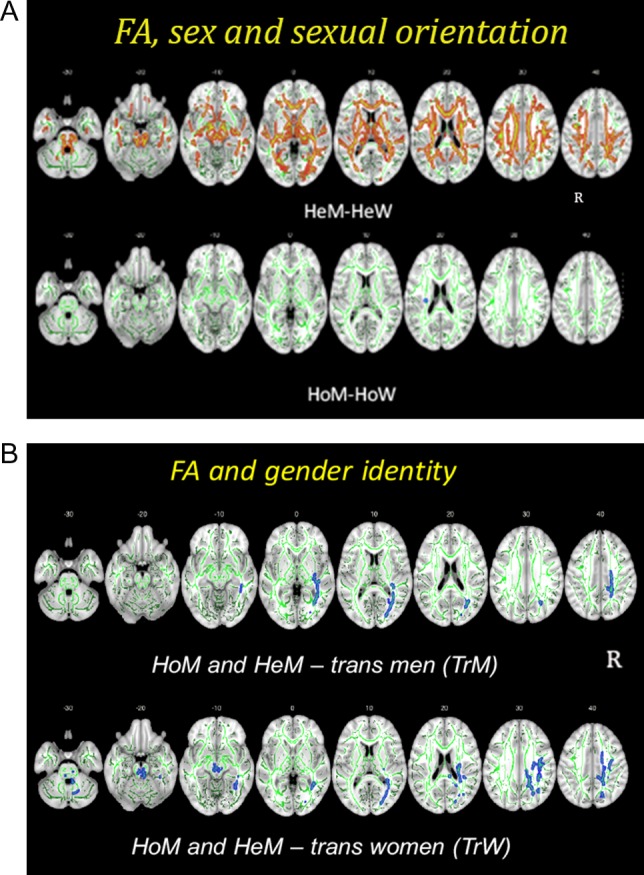
Group differences in fractional anisotropy (FA). The differences are illustrated in red–yellow and blue and superimposed on the group skeleton (green). Clusters calculated at P < 0.05, FWE corrected for multiple comparisons. (A) HeM–HeW (upper row, indicated in red–yellow) and HoM–HoW (lower row, indicated in blue). z-Coordinates of the MNI atlas are indicated. R = right side; L = left side. (B) Group differences between cisgender men (HoM and HeM) and transgender men (TrM, upper row), cisgender men (HoM and HeM) and transgender women (TrW, lower row). Whole-brain voxel-wise group differences in FA (P < 0.05, FWE corrected) between homosexual and heterosexual cisgender men (HoM/HeM) and transgender women (TrW, upper row), and transgender men (TrM, lower row). Significant clusters are projected on the mean FA skeleton (green) using the “tbss fill” procedure in FSL; slice labels indicate MNI z-coordinates; L = left, R = right. Separate comparisons between cisgender males, TrW, and TrM were allowed as the overall TBSS analysis showed a significant effect of sex. The method allows explorative visualization, which is not possible with the tract extraction model, and enabled us to observe the effect of gender identity in the right IFOF.
We found no significant (PFWE ≤ 0.05) interaction and no main effect of gender identity. Again, there was a main effect of sex, with higher FA for the right inferior fronto-occipital tract (IFOF) (k = 5802, F = 30.8), left thalamic radiation (k = 1435, F = 42.3), and right splenium of the corpus calosum, (CC) (k = 958, F = 31.3) among participants assigned as male at birth than among those assigned as female at birth (see Supplementary Table 3).
FA tract-wise statistics, and correcting for sexual orientation
Given previous data using tract-wise statistics showing “in-between” values for transgender populations (Rametti, Carrillo, Gomez-Gil, Junque, Segovia et al. 2011; Rametti, Carrillo, Gomez-Gil, Junque, Zubiarre-Elorza 2011), we sought to investigate whether similar results would be reproduced when carrying out tract-wise, and thus more selective, comparisons rather than explorative TBSS analyses, also taking into account sexual orientation. The data was therefore re-analyzed, extracting the mean FA values for those tracts previously found to be sex dimorphic (Menzler et al. 2011; Rametti, Carrillo, Gomez-Gil, Junque, Segovia et al. 2011) and comparing them across groups with and without adding Kinsey scores as the covariate [SPSS Statistics 21 (SPSS Inc., Chicago, IL)].
A two (sex) by two (gender identity) multivariate ANOVA, including Kinsey scores in addition to age as covariates of no interest, revealed a significant interaction effect of sex and gender identity for the right IFOF (P = 0.046). “Furthermore, there was a main effect of gender identity bilaterally for the IFOF (left: P = 0.035; right: P = 0.009)”. The main effect of sex, on the other hand, was found for the bilateral CST (left: P = 0.004, right: P = 0.027), superior longitudinal tract (SLF) (left: P = 0.008, right: P = 0.005), and left ILF (P = 0.039).
Group differences were then investigated separately within each group with the same sex assigned at birth, and Kinsey scores were utilized as the covariate of no interest in the one-way ANOVA.
Among at birth assigned males, a significant group difference was found for the bilateral IFOF (left: P = 0.022; right: P < 0.001), right SLF (P = 0.017), left CST (P = 0.039), forceps minor (P = 0.046), and left ILF (P = 0.048), with lower mean FA for all of these tracts among TrW compared with HeM, although only in the right IFOF in relation to HoM (see Fig. 3b). TrW also had significantly lower mean FA in relation to HoW, but, again, only in the bilateral IFOF (left: P = 0.020; right: P = 0.032) and the left ILF (P = 0.030). When comparing TrW to HeW, however, the mean FA was significantly higher among TrW for the bilateral CST (left: P = 0.017; right: P = 0.010) and the left SLF (P = 0.041). “Thus, in respect to FA values, TrW showed a sex-typical pattern regarding the CST and SLF, but a sex-atypical pattern regarding the IFOF and left ILF”.
In contrast, and similar to the results for whole-brain comparisons, the one-way ANOVA including the three female (sex assigned at birth) groups revealed no significant differences between TrM and HeW or HoW for any tract. A separate comparison between HeM and TrM, however, showed widespread clusters, whereas the contrast HoM–TrW revealed clusters only in the right IFOF (Fig. 3b), again, suggesting that the difference compared with cisgender controls, when taking into account sexual orientation, was confined to the right IFOF.
“Comparisons between TrW and TrM showed higher FA in TrM for the left ILF (P = 0.031), a finding that is in line with their gender identity for this particular tract. No differences were found for any of the other tracts”.
In summary, the findings from the DTI data followed the patterns of a generally less pronounced sexual differentiation among homosexual populations, “whereas the signature of transgender groups was confined to the IFOF”. The group comparisons of mean FA in specific tracts, after also accounting for the mixed sexual orientation among the transgender groups, confirmed a special role for the right IFOF in GD, as differences were found between TrW and all cisgender men (HoM and HeM), as well as between TrM and all cisgender men (Fig. 3b).
Post hoc comparison separating homosexual and non-homosexual transgender subjects
Given that tract-wise FA analyses showed effects of sexual orientation as well as GD, the character of a possible interaction between these two factors was explored by conducting tract-wise mean FA analyses that differentiated between homosexual and non-homosexual transgender subgroups.
Comparison between the four male assigned at birth groups (14 homosexual TrW, 10 non-homosexual TrW, 27 HoM, 37 HeM) (one-way ANOVA, Scheffe’s post hoc tests, P < 0.05) again only showed a significantly different mean FA for the IFOF (left: P = 0.023; right: P = 0.001). More specifically, for the “left IFOF, HeM showed significantly higher mean FA than non-homosexual TrW” (P = 0.029) and sub-significantly higher FA than homosexual TrW (P = 0.096); Notably, and concurring with the notion that IFOF has a singular phenotype in GD, HoM had a significantly higher mean FA for the “left IFOF” as compared with both non-homosexual (P = 0.005) and homosexual TrW (P = 0.043). For the right IFOF, both cisgender male groups had significantly higher mean FA than homosexual TrW (comparison with HeM, P = 0.005; with HoM, P = 0.017) and non-homosexual TrW groups (comparison with HeM, P = 0.001; with HoM, P = 0.005).
Again, there were no significant tract-wise differences in FA between the four female assigned at birth groups (24 homosexual TrM, 13 non-homosexual TrM, 27 HoW, 40 HeW). However, with regard to IFOF, both homosexual and non-homosexual TrM had similar mean FA values to the cisgender males, thus showing values in line with those of the gender they identified with, independent of sexual orientation. In contrast, for the fornix, CST, left SLF, and forceps minor, transgender persons followed the pattern of their sex assigned at birth, a pattern that was qualitatively but not significantly more pronounced among the heterosexual than homosexual transgender subjects. With the small number of subjects in the groups, the differences could not qualify as significant.
Group comparisons of functional connectivity within the DMN
Results from group comparisons of functional connectivity within the DMN are shown in Fig. 4 and Table 5. As for the other metrics, we first compared heterosexual cisgender male and female controls. No significant differences were found at PFWE < 0.01 corrected; however, when lowering the threshold to P < 0.05 FWE corrected, a cluster appeared with higher connectivity among HeW in the precuneus/PCC. We then investigated whether there were any group differences with respect to sexual orientation. As recently reported (Manzouri and Savic 2018), in both HoW and HoM the functional connectivity in the precuneus cortex and the pACC was significantly less pronounced as compared with both male and female heterosexual cisgender controls (Table 5). As among the heterosexual groups, there was no sex difference at PFWE corrected <0.01, but when lowering the threshold to PFWE corrected <0.05, a cluster appeared in the precuneus revealing less pronounced connections in HeM compared with HeW.
Figure 4.
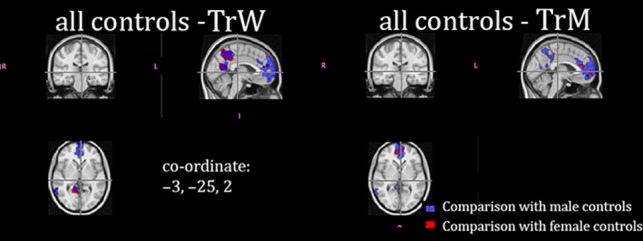
Group differences in rs-fMRI and in the default mode network (DMN). Significant group differences in resting state functional connections within the default mode network. Differences between all of the male cisgender control groups (both homosexual and heterosexual) are indicated in blue. Differences between all of the female cisgender control groups (both homosexual and heterosexual) are indicated in red. Clusters calculated at P < 0.01, FWE corrected. The MNI coordinate for the crosshair is indicated.
Table 5.
Group differences in resting state connectivity within the DMN
| A. Differences between cis and transgender persons | ||||
|---|---|---|---|---|
| Region | All cisgender men—TrW | All cisgender women—TrW | ||
| Cluster size (cc) | MNI coordinates | Cluster size (cc) | MNI coordinates | |
| ACC | 17.1 | −6 51 −18 | 13.5 | −9 51 13 |
| Precuneus (PCC) | 1.8 | 4 −52 16 | 1.5 | −1 −58 18 |
| Right Angular Gyrus, Middle Temporal Gyrus | 5.3 | 64 −51 1 | 5.8 | 43 −49 12 |
| Lateral Occipital Cortex, Middle Temporal Gyrus | 2.8 | −36 −63 13 | 3.3 | −45 −63 9 |
| L Middle Frontal Gyrus, Superior Frontal Gyrus | 2.3 | −28 33 36 | 1.7 | −27 33 39 |
| R Middle Frontal Gyrus, Superior Frontal Gyrus | 2.7 | 24 27 32 | 1.4 | 27 25 40 |
| Region | All cisgender men—TrM | All cisgender women—TrM | ||
|---|---|---|---|---|
| Cluster size (cc) | MNI coordinates | Cluster size (cc) | MNI coordinates | |
| ACC, frontal pole | 17.1 | −6 52 −13 | 10.2 | −7 61 −10 |
| Precuneus | 1.0 | 6 −57 54 | 1.8 | 0 −61 54 |
| B. Group differences between homosexual and heterosexual cisgender controls | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | HeW–HoW | HeM–HoM | HeW–HoM | HeM–HoW | ||||
| Cluster size (cm3) | MNI coordinates | Cluster size (cm3) | MNI coordinates | Cluster size (cm3) | MNI coordinates | Cluster size (cm3) | MNI coordinates | |
| ACC | 7.2 | −1 50 17 | 9.9 | 15 51 3 | 1.0 | −15 56 17 | 0.5 | −15 56 17 |
| Precuneus | 2.9 | −6 −50 3.0 | 0.6 | 11 −48 6 | ||||
| C. Sex differences among cisgender controls | ||||||
|---|---|---|---|---|---|---|
| All cisgender women–all cisgender men | All cisgender men–all cisgender women | |||||
| Cluster size (cc) | MNI coordinates | Cluster size (cc) | MNI coordinates | |||
Cuneus, supracalcarine cortex, precuneus
|
0.3 | 16 −66 21 | ||||
| D. Group differences between the two transgender groups | ||
|---|---|---|
| Region | TrW–TrM | |
| Cluster size (cc) | MNI coordinates | |
| Lingual Gyrus, precuneus | 3.5 | 10 −57 4 |
| Precuneus, PCC | 1.8 | 1 −55 34 |
Note: Significant clusters calculated at PFWE corrected <0.01, and using Kinsey scores as covariate.
Note: Significant clusters calculated at PFWE corrected <0.05.
Note: Clusters calculated at PFWE <0.01.
Note: ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; HeW, heterosexual women; HeM, heterosexual men; HoW, homosexual women; HoM, homosexual men.
Significant clusters calculated at PFWE corrected <0.05
As for the Cth and FA measures, the most striking effects were detected with regard to gender identity. Both TrW and TrM displayed less pronounced functional connectivity within the pACC of the DMN as well as in the posterior cingulate and precuneus portion of the DMN. Notably, this was found in relation to both male and female cisgender controls, and irrespective of whether the Kinsey scores were used as the nuisance variable (Table 5a,b). Thus, in both of these nodes of the DMN, functional connectivity was significantly more prominent in HoM and HoW than in TrM and in TrW (Table 5). As for comparisons with heterosexual controls, these differences were more widespread for TrW than TrM (Table 5).
Discussion
We investigated possible cerebral underpinnings to sexual orientation and gender identity. Specifically, we tested the hypothesis that GD is associated with variant cerebral anatomy and function within the circuits mediating self–own body perception, irrespective of birth sex assignment. A further hypothesis was that a different or even sex-reversed cerebral dimorphism would be found in cisgender homosexual subjects in comparison to cisgender heterosexual subjects. This is contrary to the more traditional hypothesis (which we also tested) that sex dimorphism would be “sex-reversed” primarily among transgender subjects (compared with cisgender heterosexual controls).
The generated data produced five major findings:
Among persons with GD, the FA values for the right IFOF were significantly lower than among cisgender controls. This difference remained when accounting for sexual orientation, and was most prominent among TrW.
Among persons with GD (both TrW and TrM), Cth was significantly greater compared with controls bilaterally in the mesial prefrontal cortex, in the cuneus-precuneus, and in the left lateral occipito-temporal cortex (including the right EBA). These cortical areas are interconnected via the IFOF, for which FA was found to be lower than in controls, suggesting that findings 1 and 2 are coordinated.
-
Among both transgender groups, the functional connections within the pACC and precuneus of the DMN were less pronounced than in both male and female cisgender controls.
These three findings were detected in relation to both homosexual and heterosexual controls. Thus, irrespective of the MR metric, group differences were detected in regard to the neuronal circuits along the cerebral midline, circuits known to be involved in the processing of own-body perception in the context of self. Together, these findings seem to characterize GD and accord with the behavioral hallmark of GD—the incongruence between perception of own body and self.
Among the homosexual groups, we found less pronounced sex differences than among cisgender heterosexual controls. This was true for Cth as well as FA and was not restricted to a particular area of the brain. A lower degree of sexual differentiation with regard to FA values as well as Cth, was detected among both cisgender and transgender homosexuals.
The findings among homosexual cisgender controls “were more pronounced among men”.
Several previous neuroimaging studies have suggested that, in comparison to heterosexual cisgender controls, sexual differentiation of the brain is less pronounced in transgender, and homosexual cisgender populations but none have compared transgender groups with both homosexual and heterosexual cisgender controls in the same setting. In this respect, the present study is unique, and the findings provide a new perspective on the neurobiology of GD as well as sexual orientation. While Cth and FA values showed signs of a less pronounced sexual dimorphism among both TrW and TrM, this pattern did not remain when taking into account sexual orientation and comparing each of the transgender groups with “all” cisgender controls. There were, however, other differences in FA and Cth values, which were independent of sexual orientation, and seem to be singular for GD. These observations suggest that GD is associated with specific neuroanatomical and connectivity traits along the cerebral midline. A less pronounced and even reversed sexual dimorphism, on the other hand, was found to be associated with homosexuality, including transgender homosexual persons. Only subtle “undifferentiated” (not different from cisgender men or cisgender women) findings in major sexually dimorphic regions and only in regard to Cth were detected among transgender groups when correcting for sexual orientation. These findings may potentially explain some inconsistencies in earlier reports (see introduction). For example, the impression from some previous studies that TrW would have a sex-reversed pattern of regional Cth, while TrM would express more sex-typical features may be biased by the fact that the participants with GD were homosexual and the controls were heterosexual in these studies (e.g., Zubiaurre-Elorza et al. 2014) and that differences between HoM and HeM are more pronounced than those between HoW and HeW, at least for Cth. Support for this difference can be found in the present observation that the frontal and lateral occipital-parietal Cth was greater among HoM but not HoW in comparison to heterosexual groups of the respective sexes, implying that it might also be greater in homosexual TrW but not homosexual TrM. This is based on the notion that androphilic TrW, having been assigned male sex at birth, can be regarded as HoM before corrective treatment, whereas gynephilic TrM can be regarded as HoW.
A possible sexual orientation bias should also be considered for findings of “in-between” FA values among TrM and TrW. Rametti and colleagues found such a pattern when comparing homosexual GD populations with heterosexual male and female controls (Rametti, Carrillo, Gomez-Gil, Junque, Segovia et al. 2011; Rametti, Carrillo, Gomez-Gil, Junque, Zubiarre-Elorza 2011). Kranz and colleagues, when investigating 23 TrM (19 homosexual), found no FA differences compared with control groups of mixed, albeit predominantly heterosexual, orientation, but did find significantly lower mean diffusivity compared with the female controls, indicating a “male-like” pattern (Kranz et al. 2014). The same research group subsequently used a graph theory approach with same study groups (Hahn et al. 2015), finding decreased intra-hemispheric connectivity ratios among TrM (less of a “male” pattern) and increased interhemispheric connectivity ratios among TrW (more of a “female” pattern). Together, these data indicated a less pronounced sexual differentiation among subjects with GD and were interpreted to reflect a unique “in-between” pattern among GD.
With regard to the third metric used in the present study, the rs-fMRI, the data are sparse. Ute Habel’s group, however, investigated several different networks in a TrW population of mixed sexual orientation, and found that “untreated” TrW had a “weaker” functional connectivity not only in the inferior temporal lobe in relation to male (presumably heterosexual) controls but also in the right calcarine gyrus and the left dorsolateral prefrontal cortex in relation to TrW who had received cross-sex hormonal treatment (Clemens et al. 2017). These data concur with the present findings of weaker functional connectivity among TrW and TrM.
In contrast to GD, previous imaging studies regarding homosexuality are rather consistent, showing a sex-reversed cerebral activation with pheromone like compounds (Savic et al. 2005), a sex-atypical pattern of resting state amygdala connectivity and hemispheric asymmetry (Savic and Lindstrom 2008), and, at least in homosexual men, sex-atypical structural homogeneity and resting state connectivity in the cuneus and pACC (Hu et al. 2013; 2014). Interestingly, in these studies, and also in several investigations of neuropsychological test performance, the difference in relation to heterosexual controls of the same sex was more pronounced among homosexual men than homosexual women (Beek et al. 2017). On the basis of these data and the present results, one could argue that sex dimorphism is associated with homosexual orientation rather than with GD, and that the hallmark of GD is linked to the own body-self processing networks. Signs of a different sex dimorphism among GD seem to reflect the increased prevalence of homosexuality and bisexuality among GD, a possibility needing further evaluation, optimally by direct comparisons between large groups of homosexual and heterosexual GD persons of the same sex assigned at birth. Such a comparison would require large groups of homosexual and heterosexual transgender persons and is also motivated by the present observation of subtle, yet detectable, signs of defeminization of the right parietal Cth among heterosexual TrM and a demasculinization of the left superior temporal Cth among heterosexual TrW. These very preliminary observations are in agreement with the notion of co-occurrence of transgenderism and homosexuality,
Own body image among persons with GD and its tentative underlying mechanisms
The emergence of a masculine or feminine identity is held to be strongly mediated by the early development of a male or female body-self-perception. Research data suggest that our brains have an imprinted own-body image, This image is reinforced by a multisensory integration of external and internal stimuli related to own body, as this body image and its congruence with the perception of self finally become permanent (Tsakiris 2010). Such a scenario requires neuronal processes involving somato-perception, somato-representation, and the link between neuronal networks mediating physical and the psychological self (Hodzic et al. 2009; Longo et al. 2010; Burke et al. 2017). The body model of identity integrity would involve neuronal processes in the right fronto-parietal cortex and possibly also in the insular cortex, which seem altered in persons with GD (Giummarra et al. 2011; Manzouri et al. 2017; Burke et al. 2017). One logical assumption is that the incongruence experienced by persons with GD between their own body image and perception of self could be due to developmental changes in the cortical anatomy of regions processing self–own body perception, which subsequently lead to a weakening of the structural and functional connections in these networks (as have been captured by rs-fMRI and DTI studies of GD persons). An alternative and not mutually exclusive possibility is that incongruent gender identification might have led to an aversion to sex-specific body parts, which over time might modify the cerebral processing of own body sex in the context of self. An additional factor could be rumination about own body-self incongruence. Given that Cth, white matter integrity (indexed by FA values), and resting state functional connectivity can undergo plastic changes, it is difficult to draw definite conclusions as to whether the observed characteristics among persons with GD were innate or acquired. Distinguishing this would require longitudinal studies, optimally from childhood onwards, which was beyond the scope of the present research.
Nonetheless, there are reasons to believe that at least some of our observations reflect underlying factors rather than the effects of GD. One reason is that other conditions involving body rumination, such as anorexia nervosa (AN) and body dysmorphic disorder (BDD), are not associated with similar fronto-occipital changes. Although BDD, like GD, has an early onset and is also related to the congruity between body and identity, persons with BDD have been found to display thinner rather than thicker frontal and temporal cortices compared with controls (Buchanan et al. 2013). Among subjects with AN, compared with controls, their cortex has been found to be “thinner” bilaterally in the superior parietal gyrus and in the right inferior parietal and superior frontal gyri, and their FA values found to be lower for the left corona radiate, the posterior thalamic radiation, and the left superior longitudinal fasciculus, but not in the IOF tract, as in the present study (Via et al. 2014; Fuglset et al. 2016).
Also, providing support for the underlying nature of our findings is the fact that frontal and parietal cortical thickening in TrM seems to persist after testosterone treatment, (at least 6 months after testosterone institution; Burke et al. 2017), and could, thus, be stable, and presumably inherent to the GD condition. Further support is provided by the finding that the increased prevalence of homosexuality among GD populations was associated with less pronounced sexual differentiation. Although the process underlying sexual attraction might be more complicated for persons with body-self incongruence, this by itself is unlikely to lead to a less pronounced sexual differentiation of the brain. In addition to having different cerebral signatures, homosexuality and GD might also share certain neuroanatomical and functional features (e.g., weaker pACC and precuneus/PCC connections, and less pronounced sex dimorphism with regard to Cth in the temporal and parietal cortex; see Supplementary Fig. 2), possibly underpinning both phenomena. They could be part of the defeminization and demasculinization processes as suggested by the cortical development theory for GD (Guillamon et al. 2016). In this context, it is worth considering Blanchard’s theory about homosexuality and transsexuality. This theory has been adopted by several researchers and holds that: (1) homosexual TrW are female-like with respect to several sexually dimorphic behaviors, while non-homosexual TrW are not (Blanchard 1989; 1989); (2) the brain of homosexual TrW are more female-typical in respect to sexually dimorphic structures, while non-homosexual TrW do not differ from HeM with regard to sexually dimorphic structures (Blanchard 2008); and (3) homosexual TrW represent an extreme form of male homosexuality. Our findings largely support the first two tenets, but deviate from the third, as we detected qualitatively singular features among TrW, independent of sexual orientation, which was not present among homosexual men. Blanchard does not differentiate between homosexual and heterosexual TrM. Interestingly, we found that differences between HoW and HeW regarding some sex dimorphic measures were less pronounced than those between HoM and HeM. As already discussed, this can have implications for the observed degree of sex variant dimorphism among TrM and TrW, further emphasizing the need for specific comparisons between homosexual and non-homosexual transsexual study groups.
Less pronounced sex dimorphism and its tentative mechanisms
Data from all three of the metrics employed in the present study suggested that both sexual orientation and gender identity are linked to the networks around the cerebral midline. Sex dimorphism was found to be less pronounced among the two homosexual control groups, and seemingly also among homosexual transgender persons, although our group sizes were not large enough to specifically compare homosexual and heterosexual transgender persons of the same sex.
Cth denotes neuronal size and number, dendritic density, and connections in the cortical columns (Rakic 1988). It is moderated by testosterone, which usually contributes to a thickening of the left superior temporal cortex, thinning of the parietal cortex, and, according to some studies, thickening of the cuneus (Zubiaurre-Elorza et al. 2014). Among male controls, testosterone levels are found to be inversely correlated to the thickness of the parietal and superior frontal lobe cortices (Nopoulos et al. 2000; Bramen et al. 2012; Nguyen et al. 2013; Savic and Arver 2014) and positively correlated to the thickness of the left superior temporal and occipital cortices (Herting et al. 2015). In addition, it has been shown that the possession of an allele conferring more efficient function of the androgen receptor (AR) is associated with a relatively thinner Cth specifically in the inferior parietal cortex in males and the inferior frontal cortex in females (Raznahan et al. 2010). It is, therefore, feasible to hypothesize that the greater Cth of the parietal and frontal cortices, as well as thinner Cth of the left cuneus, found among our HoM subjects could reflect regional hypoandrogenization (Fernandez et al. 2003; Rasgon et al. 2005). Cth is also moderated by estrogen, which in women contributes to thickening of the inferior parietal cortex and thinning of the temporal cortex, and in men to thinning of the frontal cortex (Peper et al. 2009; Witte et al. 2010; Koolschijn et al. 2014; Herting et al. 2015). As opposed to Cth, FA values are believed to reflect axonal packing and caliber and, perhaps, also the degree of myelinization (Lebel et al. 2008). With regard to sex hormones, FA is primarily influenced by testosterone and is typically higher in men (Huster et al. 2009; Menzler et al. 2011; Rametti, Carrillo, Gomez-Gil, Junque, Zubiarre-Elorza 2011; Rametti, Carrillo, Gomez-Gil, Junque, Segovia et al. 2011; Inano et al. 2013; Kanaan et al. 2014).
In sum, it is plausible that both the Cth and FA values among homosexual men and women (both cisgender and transgender) are being affected by sex hormonal factors, possibly in tandem with gene expression produced by some epigenetic process rendering hypoandrogenization among homosexual men and perhaps combined hyper-androgenization and hypo-estrogenization among homosexual women.
Why are differences from heterosexual cisgender controls more pronounced among HoM and TrW than HoW and TrM?
For cisgender homosexual controls, the differences found regarding cerebral sex dimorphism were more pronounced in HoM than HoW. This accords with previous neuropsychological findings showing that HoM perform similarly to HeW on certain verbal and mental rotation tasks, while HoW appear to perform in a more sex-typical manner at least on verbal tasks (Rahman et al. 2004). One tentative explanation is that behavioral differences over the course of a lifetime could account for the morphometric differences between HoW and HoM. However, all of our participants described having an early life (puberty) awareness of their sexual orientation that did not change over time. Moreover, there was no significant difference in the Kinsey scores of HoW and HoM (Table 1). An alternative hypothesis is that the coding of cerebral DMN circuits for sexual response to body morphology of “the same sex” could be stronger in HoM, possibly due to more pronounced aberrations in functional connections and in structural anatomy along the cerebral midline and the EBA, areas known to mediate body perception (Hodzic et al. 2009). Notably, when lowering the threshold to PFWE < 0.05, the weaker connection in the precuneus became more pronounced among HoM than HoW. This neurobiological explanatory model accords with the general view that female sexual orientation is more fluid and dynamic than male sexual orientation (Diamond 2000; Farr et al. 2014). It is also congruent with certain previous, yet anecdotal, studies suggesting that the genetic influence is significantly higher in male compared with female homosexuals (Hu et al. 1995). The present observations of differences between HoW and HoM should, however, be interpreted with caution, and any conclusions regarding underpinnings should await further studies.
Methodological considerations
The group sizes in the present study were sufficient for the results to be regarded as reliable (Liem et al. 2015) and were, to the best of our knowledge, larger than in any previous publications using brain imaging data among persons with GD. Due to patient flow during the time of recruitment, the group sizes differed between TrM and TrW. This was, however, taken into account in the statistical analyses. We chose to compare transgender populations with homosexual men and women rather than homosexual and bisexual persons as this was the first study to compare homosexuality and transgenderism, and given the limited group size, it was advisable to investigate the homosexual subjects who scored at the high end of the Kinsey scale first.
Unlike many previous studies, we combined anatomical and functional MRI data, which enabled an examination of the possible coordinated mechanisms and provided a stronger foundation for the hypothesis about the underlying neurobiology. Although there is no reason to suspect that sex hormone levels in HoW would be different from those in HeW, it would, admittedly, have been more optimal if we had collected data on these levels to verify this. However, given that we only included subjects who did not display any of the likely factors that would have predisposed them to changes in sex hormone levels, we did not foresee that collecting sex hormone data could be worthwhile. Furthermore, sex hormone levels were available via medical charts (with subject’s permission) for two-thirds of the HoW group, and these were within the normal range. As for the GD population, all of the participants were investigated prior to undergoing hormone replacement therapy, and their testosterone and estrogen values were within the normal range (125–1680 μmol /l−1 for estrogen and 1.2–3.5 μmol /l−1 for testosterone).
Age at onset of GD in our populations was mixed, although predominantly early. As stated in the introduction, it is believed that there are two types of GD: early (from 2 to 3 years old) and late onset (post-puberty); early onset GD has been reported for androphilic TrW and gynephilic TrW and late onset for gynephilic TrW and androphilic TrM (Guillamon et al. 2016). This could open us up to the criticism that we investigated mixed phenotypes. To include a further subgrouping would require much larger study groups—an undertaking that is hopefully feasible in the near future. It is, nevertheless, important to emphasize that despite the inhomogeneity, we clearly detected singular features among our GD populations, independent of their gender and sexual orientation. Thus, by taking advantage of the inhomogeneity of this population, we were able to identify commonalities regarding the involvement of cerebral networks, which, in addition, process the common behavioral hallmark of GD. Some of the present observations, however, also align with Blanchard’s subtyping, and with cortical development theory, such as our observation of less pronounced sex dimorphism among homosexual transgender subjects as compared with heterosexual transgender subjects.
It should be noted that the observed differences in FA and Cth between homosexual and heterosexual cisgender groups and cisgender and transgender groups cannot be attributed to brain size, as the ICV differed between the groups in accordance with their sex assigned at birth. Another potential issue worth commenting on is that although FA is a rather unspecific measure (axial and radial diffusivity (AD, RD) can provide more specific information), we still chose to limit our DTI analysis to FA because it regarded as a more stable and sensitive metric (Winston 2012), and our primary goal was to assess possible group differences rather than to analyze white matter microstructure. Such an effort would require use of advanced multi-compartment models such as CHARMED, AxCaliber or NODDI, and was beyond the scope of the present report. Another rationale was that non-FA diffusion measures are generally used to further characterize FA, but variations as a function of sex/gender have primarily been reported for FA. RD and AD will be elaborated in a separate study.
Finally, it is worth noting that we did not detect the expected sex difference in regard to amygdala volumes (Savic and Arver 2011; Lentini et al. 2013). This can be attributed to the small size of the amygdala and that the FreeSurfer software is not optimal for amygdala segmentation, something to consider in future studies of sexual dimorphism in relation to GD (Schoemaker et al. 2016).
Conclusion
Homosexual orientation was found to be associated with less pronounced cerebral sex dimorphism, a finding that appeared more prominent among men than women. Although a less pronounced cerebral sex dimorphism was detected in transgender persons compared with heterosexual cisgender controls, this seems primarily due to the higher proportion of homosexual persons in the GD groups, and does not seem to be the signature of GD. We suggest that GD is, instead, specifically linked to cerebral networks mediating self–body perception, possibly due to certain developmental and acquired changes.
Supplementary Material
Footnotes
We would like to thank Dr Cecilija Dhejne for her excellent clinical evaluations and recruitment of transgender persons. Conflict of Interest: None declared.
Funding
Swedish Research Council (I.S., DNA 2007—3107, and DNA: 2015—02716); FORTE (I.S.); AFA (I.S.).
Author's contribution
IS wrote the manuscript, and supervised and designed the study; AM conducted the experiments and analyzed the data.
References
- Abe C, Johansson E, Allzen E, Savic I. 2014. Sexual orientation related differences in cortical thickness in male individuals. PLoS One. 9(12):e114721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. 2011. Minireview: hormones and human sexual orientation. Endocrinology. 152(8):2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 23(2):137–152. [DOI] [PubMed] [Google Scholar]
- Beek TF, Cohen-Kettenis PT, Bouman WP, de Vries AL, Steensma TD, Witcomb GL, Arcelus J, Richards C, De Cuypere G, Kreukels BP. 2017. Gender Incongruence of childhood: clinical utility and stakeholder agreement with the World Health Organization’s proposed ICD-11 criteria. PLoS One. 12(1):e0168522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund H, Lindstrom P, Dhejne-Helmy C, Savic I. 2008. Male-to-female transsexuals show sex-atypical hypothalamus activation when smelling odorous steroids. Cereb Cortex. 18(8):1900–1908. [DOI] [PubMed] [Google Scholar]
- Berglund H, Lindstrom P, Savic I. 2006. Brain response to putative pheromones in lesbian women. Proc Natl Acad Sci U S A. 103(21):8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R. 1989. a. The classification and labeling of nonhomosexual gender dysphorias. Arch Sex Behav. 18(4):315–334. [DOI] [PubMed] [Google Scholar]
- Blanchard R. 1989. b. The concept of autogynephilia and the typology of male gender dysphoria. J Nerv Ment Dis. 177(10):616–623. [DOI] [PubMed] [Google Scholar]
- Blanchard R. 2008. Deconstructing the feminine essence narrative. Arch Sex Behav. 37(3):434–438; discussion 505-410. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER. 2012. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 7(3):e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BG, Rossell SL, Maller JJ, Toh WL, Brennan S, Castle DJ. 2013. Brain connectivity in body dysmorphic disorder compared with controls: a diffusion tensor imaging study. Psychol Med. 43(12):2513–2521. [DOI] [PubMed] [Google Scholar]
- Burke SM, Manzouri AH, Dhejne C, Bergstrom K, Arver S, Feusner JD, Savic-Berglund I. 2017. Testosterone effects on the brain in transgender men. Cereb Cortex. 1–15. doi:10.1093/cercor/bhx054. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Brang D, Landazuri R, Viswanathan P, Ramachandran VS. 2017. Altered white matter and sensory response to bodily sensation in female-to-male transgender individuals. Arch Sex Behav. 46(5):1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TF. 2002. The situational inventory of body-image dysphoria: psychometric evidence and development of a short form. Int J Eat Disord. 32(3):362–366. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 106(21):8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens B, Junger J, Pauly K, Neulen J, Neuschaefer-Rube C, Frolich D, Mingoia G, Derntl B, Habel U. 2017. Male-to-female gender dysphoria: Gender-specific differences in resting-state networks. Brain Behav. 7(5):e00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kettenis PT, Pfafflin F. 2010. The DSM diagnostic criteria for gender identity disorder in adolescents and adults. Arch Sex Behav. 39(2):499–513. [DOI] [PubMed] [Google Scholar]
- Diamond LM. 2000. Sexual identity, attractions, and behavior among young sexual-minority women over a 2-year period. Dev Psychol. 36(2):241–250. [DOI] [PubMed] [Google Scholar]
- Drummond KD, Bradley SJ, Peterson-Badali M, Zucker KJ. 2008. A follow-up study of girls with gender identity disorder. Dev Psychol. 44(1):34–45. [DOI] [PubMed] [Google Scholar]
- Farr RH, Diamond LM, Boker SM. 2014. Female same-sex sexuality from a dynamical systems perspective: sexual desire, motivation, and behavior. Arch Sex Behav. 43(8):1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Weis S, Stoffel-Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Greiff A, Ruhlmann J, et al. 2003. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci. 23(9):3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Dervisic J, Kosidou K, Dhejne C, Bookheimer S, Savic I. 2016. Female-to-male transsexual individuals demonstrate different own body identification. Arch Sex Behav. 45(3):525–536. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Lidstrom A, Moody TD, Dhejne C, Bookheimer SY, Savic I. 2017. Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging Behav. 11(4):964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr.. 1994. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 4(4):344–360. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33(3):341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. 2004. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 23(Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- Fuglset TS, Endestad T, Hilland E, Bang L, Tamnes CK, Landro NI, Ro O. 2016. Brain volumes and regional cortical thickness in young females with anorexia nervosa. BMC Psychiatry. 16(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giummarra MJ, Bradshaw JL, Nicholls ME, Hilti LM, Brugger P. 2011. Body integrity identity disorder: deranged body processing, right fronto-parietal dysfunction, and phenomenological experience of body incongruity. Neuropsychol Rev. 21(4):320–333. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr., Faraone SV, Tsuang MT. 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 11(6):490–497. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Junque C, Gomez-Gil E. 2016. A review of the status of brain structure research in transsexualism. Arch Sex Behav. 45(7):1615–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Küblböck M, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Winkler D, Kasper S, et al. 2015. Structural connectivity networks of transgender people. Cereb Cortex. 25(10):3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. 2015. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 10(3):e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. 2011. Gender development and the human brain. Annu Rev Neurosci. 34:69–88. [DOI] [PubMed] [Google Scholar]
- Hodzic A, Kaas A, Muckli L, Stirn A, Singer W. 2009. Distinct cortical networks for the detection and identification of human body. NeuroImage. 45(4):1264–1271. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Schagen SE, Kreukels BP, Veltman DJ, Cohen-Kettenis PT, Delemarre-van de Waal H, Bakker J. 2015. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology. 55:59–71. [DOI] [PubMed] [Google Scholar]
- Hu S, Pattatucci AM, Patterson C, Li L, Fulker DW, Cherny SS, Kruglyak L, Hamer DH. 1995. Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nat Genet. 11(3):248–256. [DOI] [PubMed] [Google Scholar]
- Hu S, Xu D, Peterson BS, Wang Q, He X, Hu J, Xu X, Wei N, Long D, Huang M, et al. 2013. Association of cerebral networks in resting state with sexual preference of homosexual men: a study of regional homogeneity and functional connectivity. PLoS One. 8(3):e59426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Xu D, Peterson BS, Wang Q, Lai J, Hu J, Wei N, Zhang M, Xu Y. 2014. Differing default mode network activities in men with homosexual or heterosexual preferences. J Sex Med. 11(10):2474–2484. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 39(1):336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. 2009. Hemispheric and gender related differences in the midcingulum bundle: a DTI study. Hum Brain Mapp. 30(2):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Yoshioka N, Mori H, Kunimatsu A, Abe O, Ohtomo K. 2013. Effects of age and gender on neuroanatomical volumes. J Magn Reson Imaging. 37(5):1072–1076. [DOI] [PubMed] [Google Scholar]
- Junger J, Habel U, Brohr S, Neulen J, Neuschaefer-Rube C, Birkholz P, Kohler C, Schneider F, Derntl B, Pauly K. 2014. More than just two sexes: the neural correlates of voice gender perception in gender dysphoria. PLoS One. 9(11):e111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Chaddock C, Allin M, Picchioni MM, Daly E, Shergill SS, McGuire PK. 2014. Gender influence on white matter microstructure: a tract-based spatial statistics analysis. PLoS One. 9(3):e91109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel AK. 1953. The Kinsey report. J Am Med Assoc. 153(14):1303–1304. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. 2010. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 189(2):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, Peper JS, Crone EA. 2014. The influence of sex steroids on structural brain maturation in adolescence. PLoS One. 9(1):e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Kublbock M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C, et al. 2014. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci. 34(46):15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukels BP, Guillamon A. 2016. Neuroimaging studies in people with gender incongruence. Int Rev Psychiatry. 28(1):120–128. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 40(3):1044–1055. [DOI] [PubMed] [Google Scholar]
- Lentini E, Kasahara M, Arver S, Savic I. 2013. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb Cortex. 23(10):2322–2336. [DOI] [PubMed] [Google Scholar]
- Liem F, Merillat S, Bezzola L, Hirsiger S, Philipp M, Madhyastha T, Jancke L. 2015. Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. NeuroImage. 108:95–109. [DOI] [PubMed] [Google Scholar]
- Longo MR, Azanon E, Haggard P. 2010. More than skin deep: body representation beyond primary somatosensory cortex. Neuropsychologia. 48(3):655–668. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, Jancke L, Toga AW. 2006. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 27(4):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Sanchez FJ, Tosun D, Shattuck DW, Gaser C, Vilain E, Toga AW. 2012. Increased cortical thickness in male-to-female transsexualism. J Behav Brain Sci. 2(3):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri A, Kosidou K, Savic I. 2017. Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cereb Cortex. 27(2):998–1010. [DOI] [PubMed] [Google Scholar]
- Manzouri A, Savic I. 2018. Cerebral sex dimorphism and sexual orientation. Hum Brain Mapp. 39(3):1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. 2007. Wandering minds: the default network and stimulus-independent thought. Science. 315(5810):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S. 2011. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. NeuroImage. 54(4):2557–2562. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, et al. 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 40(2):570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S, G. Brain Development Cooperative . 2013. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 23(6):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. 2000. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 98(1):1–13. [DOI] [PubMed] [Google Scholar]
- Northoff G, Panksepp J. 2008. The trans-species concept of self and the subcortical-cortical midline system. Trends Cogn Sci. 12(7):259–264. [DOI] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z. 2014. DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2009. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 34(3):332–342. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Bjornsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH. 2011. From part- to whole-body ownership in the multisensory brain. Curr Biol. 21(13):1118–1122. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. 2015. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 112:267–277. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Newland C, Smyth BM. 2011. Sexual orientation and spatial position effects on selective forms of object location memory. Brain Cogn. 75(3):217–224. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, Abrahams S. 2004. Developmental instability is associated with neurocognitive performance in heterosexual and homosexual men, but not in women. Behav Neurosci. 118(1):243–247. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1988. Specification of cerebral cortical areas. Science. 241(4862):170–176. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gomez-Gil E, Junque C, Segovia S, Gomez A, Guillamon A. 2011. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res. 45(2):199–204. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gomez-Gil E, Junque C, Zubiarre-Elorza L, Segovia S, Gomez A, Guillamon A. (2011). “The microstructure of white matter in male to female transsexuals before cross-sex hormonal treatment. A DTI study.” J Psychiatr Res 45(7): 949–954. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Magnusson C, Johansson AL, Pedersen NL, Elman S, Gatz M. 2005. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology. 30(6):558–567. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. 2010. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 107(39):16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ, Manning JT. 2000. The ratio of 2nd to 4th digit length and male homosexuality. Evol Hum Behav. 21(5):333–345. [DOI] [PubMed] [Google Scholar]
- Rowniak S, Chesla C. 2013. Coming out for a third time: transmen, sexual orientation, and identity. Arch Sex Behav. 42(3):449–461. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2011. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex. 21(11):2525–2533. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2014. Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb Cortex. 24(12):3246–3257. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Lindstrom P. 2005. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci U S A. 102(20):7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Lindstrom P. 2008. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc Natl Acad Sci U S A. 105(27):9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, Gauthier S, Pruessner JC. 2016. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage. 129:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B. 2010. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry. 71(3):313–326. [DOI] [PubMed] [Google Scholar]
- Simon L, Kozak LR, Simon V, Czobor P, Unoka Z, Szabo A, Csukly G. 2013. Regional grey matter structure differences between transsexuals and healthy controls—a voxel based morphometry study. PLoS One. 8(12):e83947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Garcia-Falgueras A. 2009. Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Funct Neurol. 24(1):17–28. [PubMed] [Google Scholar]
- Swaab DF, Hofman MA. 1995. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trends Neurosci. 18(6):264–270. [PubMed] [Google Scholar]
- Swaab DF, Slob AK, Houtsmuller EJ, Brand T, Zhou JN. 1995. Increased number of vasopressin neurons in the suprachiasmatic nucleus (SCN) of ‘bisexual’ adult male rats following perinatal treatment with the aromatase blocker ATD. Brain Res Dev Brain Res. 85(2):273–279. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. 2010. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 48(3):703–712. [DOI] [PubMed] [Google Scholar]
- Via E, Zalesky A, Sanchez I, Forcano L, Harrison BJ, Pujol J, Fernandez-Aranda F, Menchon JM, Soriano-Mas C, Cardoner N, et al. 2014. Disruption of brain white matter microstructure in women with anorexia nervosa. J Psychiatry Neurosci. 39(6):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S, Busch M, Schulte D, Gronermeyer D, Herpertz S, Suchan B. 2010. Effects of body image therapy on the activation of the extrastriate body area in anorexia nervosa: an fMRI study. Psychiatry Res. 183(2):114–118. [DOI] [PubMed] [Google Scholar]
- Winston GP. 2012. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg. 2(4):254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. 2010. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage. 49(2):1205–1212. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gomez-Gil E, Guillamon A. 2014. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med. 11(5):1248–1261. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gomez-Gil E, Segovia S, Carrillo B, Rametti G, Guillamon A. 2013. Cortical thickness in untreated transsexuals. Cereb Cortex. 23(12):2855–2862. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Bradley SJ. 1995. Gender identity disorder and psychosexual problems in children and adolescents. New York: Guilford Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.