Abstract
Objective
This review was conducted to identify interventions effective in improving uptake and retention of HIV-positive mothers and their infants in prevention of mother to child transmission (PMTCT) services in low-income and middle-income countries (LMICs) in order to inform programme planning.
Methods
We conducted a systematic review of studies comparing usual care with any intervention to improve uptake and retention of HIV-positive pregnant or breastfeeding women and their children from birth to 2 years of age in PMTCT services in LMICs. Twenty-two electronic databases were searched from inception to 15 January 2018, for randomised, quasi-randomised and non-randomised controlled trials, and interrupted time series studies; reference lists of included articles were searched for relevant articles. Risk of bias was assessed using the Cochrane Effective Practice and Organisation of Care group criteria. Random-effects meta-analysis was conducted for studies reporting similar interventions and outcomes.
Results
We identified 29 837 articles, of which 18 studies were included in our review. Because of heterogeneity in interventions and outcome measures, only one meta-analysis of two studies and one outcome was conducted; we found a statistically significant increase in antiretroviral therapy (ART) use during pregnancy for integration of HIV and antenatal care relative to standard non-integrated care (pooled AOR=2.69; 95% CI 1.25 to 5.78, p=0.0113). The remaining studies assessing other patient, provider or health system interventions were synthesised narratively, with small effects seen across intervention categories for both maternal and infant PMTCT outcomes based predominately on evidence with moderate to high risk of bias.
Conclusions
Evidence on the effectiveness of interventions to improve uptake and retention of mothers and infants in PMTCT care is lacking. Our findings suggest that integration of HIV and antenatal care may improve ART use during pregnancy. Future studies to replicate promising approaches are needed. Improved reporting of key methodological criteria will facilitate interpretation of findings and improve the utility of evidence to PMTCT programme planners.
PROSPERO registration number
CRD42015020829.
Keywords: HIV, prevention of mother to child transmission, interventions, uptake, retention
Strengths and limitations of this review.
A comprehensive search was conducted, including grey literature sources and hand searching.
A broad range of intervention categories as well as both maternal and infant outcomes from across the spectrum of the prevention of mother to child transmission (PMTCT) cascade were included.
Our search was limited to studies conducted in low-income and middle-income countries (LMICs) in order to increase utility of findings to LMIC PMTCT programmers.
The multifaceted nature of the interventions and variability in outcomes reported limited our ability to combine studies statistically.
Due to the small number of studies included in the meta-analysis, publication bias could not be examined.
Introduction
In 2015, 150 000 new HIV infections and 110 000 HIV-related deaths occurred globally among children <15 years of age, with mother to child transmission the leading cause of new HIV infections among children.1 2 Despite effectiveness of prevention of mother to child transmission (PMTCT) of HIV regimens,3 4 uptake of and retention in PMTCT care remain below target in many low-income and middle-income countries (LMICs).4–6 While progress has been made in understanding barriers to uptake and retention of women and their infants in PMTCT services,7 evidence to provide guidance to LMIC implementers and policy makers seeking to optimise PMTCT services remains limited.
Eight systematic reviews have been conducted on strategies to optimise PMTCT. Two of these reviews evaluated the effectiveness of interventions, specifically male involvement8 and integration of services,9 to improve coverage of PMTCT services. These reviews were limited by the lack of studies to provide recommendations. A third review10 examined the effects of integration of antenatal care (ANC) with postnatal and other health services for a broad range of maternal health outcomes in LMICs; although some PMTCT studies and outcomes were included, this was not the focus of the review. A fourth systematic review evaluated interventions for improving initiation of antiretroviral therapy (ART) in pregnant women11 and found the evidence quality insufficient to support recommendations. A fifth systematic review12 assessed the impact of China’s PMTCT cascade in improving uptake and outcomes at various steps along the cascade; specific interventions implemented to operationalise the cascade were not reported. Three systematic reviews have been published since the initiation of the present review. One review evaluated non-pharmacological interventions to improve quality of care and maternal health outcomes in Sub-Saharan Africa.13 While a small number of included studies reported PMTCT outcomes, this was not a primary focus of the review. A second review focused on postpartum retention of women in PMTCT and ART care.14 This review focused on a limited portion of the PMTCT cascade. A third review15 focused on interventions to improve PMTCT service delivery and promote retention. This review included a range of study designs and studies conducted in both high-income and low-middle-income countries, and as such is of less value as a guide to decision making for PMTCT policy and programming in LMICs. Overall, review evidence to guide LMIC PMTCT programme planning remains limited by lack of high-quality studies; focus of past reviews on limited portions of the PMTCT cascade and/or focus on HIV care in general rather than PMTCT specifically; and inclusion of high-income country studies, where the context of PMTCT care is often substantially different from LMICs.
This review was developed in collaboration with knowledge users from the Malawi Ministry of Health’s HIV treatment and care technical working group. The objective of this current review was to identify what interventions at the patient, provider or health system level are effective compared with no intervention or usual care in improving uptake and retention of HIV-positive mothers and their infants in PMTCT services. Given the unique challenges facing PMTCT health services in LMICs, this review is targeted to provide guidance for PMTCT policy and programming in LMICs, and therefore included a broad range of intervention categories, as well as both maternal and infant outcomes from across the spectrum of the PMTCT cascade.
Methods
Protocol
A protocol was developed for this review based on the Cochrane Handbook for Systematic Reviews16 and the Cochrane Effective Practice and Organisation of Care (EPOC) group17 and registered with PROSPERO (available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015020829#.VXHCNUZBn5I). The complete protocol was previously published and the methods are presented briefly here.18 Our findings are reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for reporting systematic reviews.19
Patient and public involvement
No patients were involved in this study.
Eligibility criteria
We included studies reporting the effectiveness of interventions in improving uptake and/or retention of HIV-positive pregnant or breastfeeding women and their children from birth to 2 years of age or termination of breast feeding in PMTCT services. We included randomised, quasi-randomised and non-randomised controlled trials, and interrupted time series studies that compared usual care or no intervention with any type of intervention at the patient, provider or health system level. Although included in error in the PROSPERO registration for our review, controlled before-and-after studies were not included in the protocol manuscript or search. Studies were included if conducted in LMICs as defined by the EPOC filter20 and updated using the most recent World Bank Country and Lending group classification.21 Studies that included both high-income and low-income/middle-income countries were eligible for inclusion if LMICs results could be abstracted. No restriction was placed based on language of publication, publication status, study time frame or duration of follow-up.
Information sources and literature search
A search strategy was developed in consultation with an experienced information specialist (MA) and peer-reviewed by two additional information specialists (EC, JM) using the Peer Review of Electronic Search Strategies checklist.22 The following databases were searched from inception to 31 July 2015 and subsequently updated using the same search strategy for the period 31 July 2015–15 January 2018, using medical subject heading (MeSH) headings and text words related to HIV, pregnancy, breast feeding, mother to child transmission, interventions, treatment uptake and retention, and LMICs: MEDLINE, EMBASE, The WHO Global Health Library, CAB Abstracts, EBM Reviews, cumulative index to nursing and allied health literature (CINAHL), HealthSTAR, Web of Science, Scopus, PsycINFO, population information online (POPLINE), education resources information center (ERIC), national library of medicine (NLM) Gateway, latin american and caribean health sciences literature (LILACS), Google Scholar, database of abstracts and reviews of effectiveness (DARE), ProQuest Dissertation & Theses and Sociological Abstracts, OpenGrey, the Cochrane Library, WHO International Clinical Trials Registry, Controlled Clinical Trials, and ClinicalTrials.gov. Several databases planned for inclusion in our search were no longer available or not accessible by our group at the time of the search and were therefore not included: AIDS Education Global Information System, British Library Catalogue and the New York Academy of Grey Literature. In addition, we searched the reference lists of included articles and contacted several experts in the field to enquire about eligible unpublished or in-progress studies. See online supplementary file 1 for complete MEDLINE search strategy.
bmjopen-2018-024907supp002.pdf (103KB, pdf)
Study selection and data collection process
A screening checklist was developed and piloted by two authors (LMPR, MvL) independently on a sample of 50 citations prior to screening, with two rounds necessary to reach >90% agreement. Two authors (LMPR, MvL) then independently screened citations in two phases; first the titles, then abstracts were screened, and second the full-text articles were screened. Translation software was used to screen articles at the titles and abstracts level, with no non-English articles remaining at the full article review phase. A data abstraction form was created using the EPOC data collection form17 and a calibration exercise done by two authors to ensure consistency in screening and data extraction. A calibration exercise was conducted with completed data extraction forms compared and discussed for each of the first three articles to ensure consistency; data extraction was then completed for the remaining articles independently and in duplicate by two authors, and discrepancies resolved by consensus (LMPR, MvL). Information abstracted from each study included population, intervention, comparator, context, outcomes, study design, time frame and appropriateness of analysis (adjustment for design effect). The primary outcomes were percentage of HIV-positive women receiving or initiated on ART prophylaxis or treatment, percentage of infants born to HIV-positive mothers receiving or initiated on ART prophylaxis, and percentage of women and infants retained in PMTCT care/completing the ART regimen as defined by the PMTCT regimen used.18 Secondary outcomes included: percentage of infants completing postexposure HIV testing 4–6 weeks after birth and percentage of infants completing postexposure HIV testing 6 weeks following termination of breast feeding for all infants with known HIV exposure; percentage of HIV-exposed infants testing positive for HIV; adverse events; major or minor congenital malformations; small for gestational age; premature delivery; stillbirth; and infant death within the first 2 years of life.18
When necessary to clarify published data or to obtain unpublished data, we contacted the primary authors of studies meeting the inclusion criteria. The authors were contacted by email on two occasions and given 1 month to respond. Ten authors (11 reports) were contacted when data needed to calculate risk ratios were not available in the publication. Three responded and provided the requested data, six could not be reached, and one replied but was unwilling to share the additional data as they were submitting the manuscript for publication.
Methodological quality/risk of bias appraisal
Risk of bias was assessed for each study in duplicate by two authors (LMPR, MvL) using the Cochrane EPOC criteria for assessing risk of bias.17 Given the small number of studies included in the meta-analysis, risk of publication bias could not be examined using funnel plots. Selective reporting bias was assessed through review of trial registrations where available and categorised as unclear if not registered.
Data synthesis
Interventions were classified independently by two authors (LMPR, MvL) using the EPOC taxonomy for health system interventions and discrepancies resolved through discussion.23 Clinical heterogeneity was determined based on patient, intervention and outcome characteristics. Descriptive synthesis of study results was conducted for all studies and is reported narratively and in tabular form. Where appropriate, random-effects meta-analysis was conducted to estimate intervention effects using the Metafor Package in the statistical software R.24 Statistical heterogeneity was examined using the I2 statistic, with I2 ≥75% indicating significant heterogeneity.16
Results
Literature search
A total of 29 837 articles were identified through the database and hand search. After duplicates were removed 21 354 titles and abstracts were screened and 95 articles reviewed in full. Thirty-four articles representing 18 studies with 16 companion reports met the eligibility criteria (figure 1).
Figure 1.
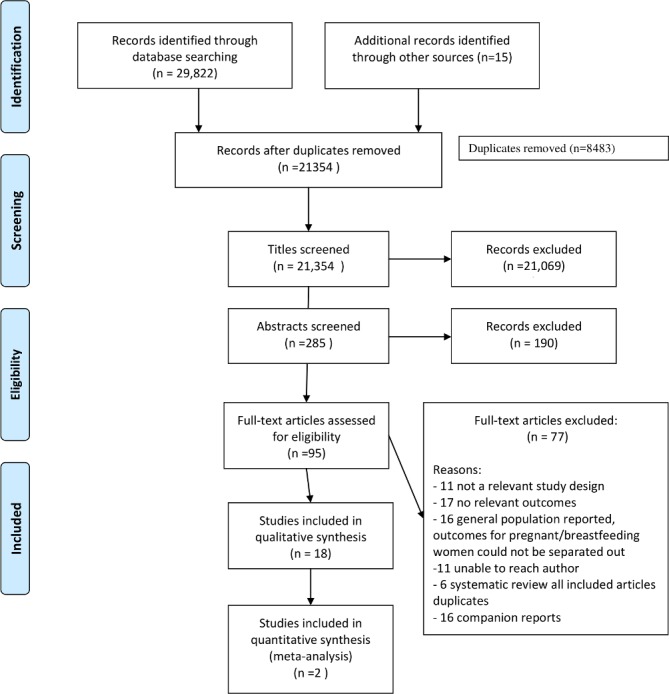
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of search results and screening.
Study characteristics
Study characteristics are outlined in table 1.
Table 1.
Characteristics of included studies
| Author(s), year |
Intervention level/type |
Study design |
Country (geographical location in country) |
Study population |
Intervention | Comparison | EPOC intervention classification |
Participants (n) | Participant characteristics |
Outcomes |
| Ezeanolue, 2015 | Patient | Mixed methods including small cluster RCT | Nigeria (Enugu State) | Self-identified pregnant women ≥18 years who attended any church site. | Monthly baby showers offered health education and onsite laboratory testing including HIV testing, and mama packs for essential items during pregnancy. | Usual care. |
|
40 churches, 3002 patients. |
|
|
| Reynolds, 2010 | Patient | Cluster RCT | Kenya (Coast, Rift Valley and Western provinces) | HIV-positive pregnant women ≥18 and at least 32 weeks’ gestation. | PMTCT providers trained to prepare and counsel women on how to store and administer take-home nevirapine infant dose. | Usual care. |
|
10 clusters, 160 patients. |
|
|
| Weiss, 2014 | Patient | RCT | South Africa (Gert Sibande and Nkangala districts) | HIV-positive pregnant women, 24–30 weeks’ gestation, and ≥18 years of age, recruited and asked to invite their male partner to enrol as a couple. | 4 successive weekly sessions employed a cognitive-behavioural approach and addressed HIV, safer sex, sexual negotiation and PMTCT issues. Sessions were closed, structured, of gender-concordant groups, led by trained gender-matched facilitators and conducted in ANCs. | Time-matched health education sessions. |
|
12 clusters, 478 couples. |
|
|
| Yotebieng, 2016 | Patient | RCT | Democratic Republic of Congo (Kinshasa) | Newly diagnosed HIV-positive women, ≤32 weeks’ gestation, registering for ANC. | Participants received small, escalating cash payments, starting at US$5 and increasing by US$1 each visit, if attended scheduled clinic appointments and completed recommended actions. Incentive reset to its original value if mother failed to complete any actions required at a specific visit. | Usual care. |
|
433 women. |
|
|
| Richter, 2014 | Patient/Provider | Cluster RCT | South Africa (KwaZulu-Natal) | HIV-positive women, ≥18 years of age and <34 weeks pregnant. | 8-session intervention conducted by peer mentors (4 antenatal, 4 postnatal) to support HIV-positive women through pregnancy and early motherhood. HIV-positive women recruited, trained and certified as peer mentors prior to implementation; inperson supervision was provided weekly. | Usual care. |
|
8 clusters, 1200 patients. |
|
|
| Kieffer, 2011 | Provider | Cluster RCT | Swaziland | All pregnant women presenting for delivery at participating maternity facilities. | 1-day training course provided to nurse- midwives to increase knowledge and skills in provision of PMTCT and to enhance confidence and counselling skills. | Usual care. |
|
6 clusters, 2444 patients. |
|
|
| Dryden-Peterson, 2015 | Provider/System | Stepped-wedge cluster RCT | Botswana (Gaborone) | ART-naïve, HIV-positive women registering at antenatal clinic before 26 weeks’ gestation. | 2-hour clinical staff education sessions on protocols for CD4 testing; open-source platform permitting automated SMS to monitor/deliver CD4 results between central labs and clinics; longitudinal support for tracing women eligible for ART initiation. | Usual care. |
|
19 clusters, 336 women. |
|
|
| Mwapasa, 2017 | Provider/System | 3-arm, cluster RCT | Malawi (Salima and Mangochi districts) | HIV-positive pregnant women initiated on option B+ regimen. | MIP: integration of HIV/ANC, routine tracing. MIP+SMS: integrated HIV/ANC care, SMS sent to community health worker to trace if appointment missed. |
Usual care: non-integrated care, routine tracing as for MIP. |
|
30 clusters, 1350 women. |
|
|
| Oyeledun, 2017 | Provider/System | Cluster RCT | Northern Nigeria (Benue and Kaduna states) | HIV-positive, women, gestational age ≤34 weeks, who were ART-naive and agreed to start lifelong ART. | QI teams established, visits by coaches and collaborative meetings. | Routine MOH support. |
|
32 clusters (6 later excluded), 532 women (21 withdrew, leaving 511 in total). |
|
|
| Phiri, 2017 | Provider/System | 3-arm, cluster RCT | Malawi (SE, SW and Central West Zones) | Pregnant and breastfeeding HIV-positive women and their infants. Up to three male sex partners could be enrolled per patient. | FBPS: women received SOC and met with ‘mentor mothers’, HIV-positive women who had recently completed PMTCT and were on ART. Mentor mothers provided one-on-one support at each clinic visit, led weekly clinic-based support groups and contacted women within 1 week of a missed appointment. CBPS: women received SOC and met with ‘expert mothers’, HIV-positive women who recently completed PMTCT and were on ART. Expert mothers conducted routine home visits to provide HIV education and clinic visit reminders, and led monthly community-based support group meetings. Expert mothers were responsible for contacting women in the community within 1 week of a missed clinic visit. |
SOC facilities provided routine HIV care according to Malawi MOH guidelines. According to national guidelines, women who fail to attend the clinic within 60 days of a missed appointment are supposed to be traced. However, this rarely occurs in the routine programme. |
|
21 clusters, 1269 women. |
|
|
| Tomlinson, 2014 | Provider/System | Cluster RCT | South Africa (Umlazi) | Pregnant women aged ≥17 and their newborns residing in the clusters during the recruitment period. | CHWs were trained to carry out structured home visits using motivational interviewing for breastfeeding counselling. Women were scheduled to receive seven home-based visits during pregnancy and postdelivery. Low birthweight neonates received two extra visits within the first week. | In control clusters, CHWs provided information and support on accessing social welfare grants and conducted three home-based visits: during pregnancy and postdelivery. |
|
30 clusters, 3957 women. |
|
|
| Aliyu, 2016 | System | Cluster RCT | Rural north-central Nigeria (Niger State) | HIV-positive women and their infants, presenting for ANC or delivery who met one of the following criteria: unknown HIV status at presentation; history of ART prophylaxis or treatment, but not receiving ARTs at presentation; or known HIV status but had never received treatment. | Integrated package of PMTCT services that included point-of-care CD4 cell count or percentage testing, transition of decentralised PMTCT tasks to trained midwives, integrated mother and infant care services, active influential family member (male partner) participation, and community involvement (male community peer champions providing outreach, education and linkage of male partners to key referral services). | SOC included health information, opt-out HIV testing, infant feeding counselling, referral for CD4 cell counts and treatment, ART prophylaxis, and early infant diagnosis. |
|
12 clusters, 369 patients. |
|
|
| Geelhoed, 2013 | System | Cluster RCT | Mozambique (Tete Province) | Public primary health facilities providing maternal child health and PMTCT services. Mothers and their children up to 5 years of age. |
Reorganised services to deliver integrated consultations and services for mothers and their children up to 5 years of age. | Usual care. |
|
6 clusters. | Not available. |
|
| Killam, 2010 | System | Stepped-wedge cluster RCT | Zambia (Lusaka) | ART-eligible pregnant women presenting at participating clinics. | Integration of ART care into ANC. Women already receiving ART at the general ART clinic encouraged to continue receiving their services in the general ART clinic. | Usual care. |
|
8 clusters, 31 536 patients. |
|
|
| Odeny, 2014 | System | RCT | Kenya (Nyanza region) | HIV-positive women attending antenatal or HIV care, ≥18 years of age, between 28 weeks’ gestation and delivery, enrolled in PMTCT, access to mobile phone. | Custom-built, automated software to send and receive text messages. Sent 14 text messages, up to 8 sent during pregnancy, and weekly for the first 6 weeks after delivery. | Usual care. |
|
388 patients. |
|
|
| Rotheram-Borus, 2014 | System | Cluster RCT | South Africa (Cape Town) | Pregnant women ≥18 years of age from Cape Town townships. | Antenatal and postnatal home visits by CHW in addition to standard clinic-based care. | Usual care. |
|
26 clusters (2 later removed), 1144 eligible women. |
|
|
| Rustagi, 2016 | System | Cluster RCT | Cote d’Ivoire, Kenya, Mozambique | Public and non-profit health facilities with PMTCT services. Pregnant women presenting for antenatal care. | A five-step, facility-level systems analysis and improvement intervention designed to maximise effectiveness of PMTCT service delivery by improving understanding of inefficiencies. | Usual care. |
|
36 clusters, 1876 patients. | Not available. |
|
| Turan, 2015 | System | Cluster RCT | Kenya (Nyanza Province) | Pregnant HIV-positive women ≥18, not enrolled in HIV care at baseline and their infants. | Integrated clinics provided PMTCT and HIV care and treatment services within existing ANC services, starting prenatally and continuing until a definitive paediatric HIV diagnosis was obtained or the child reached 18 months of age. | Non-integrated ANC clinics provided routine PMTCT services and referred HIV-positive pregnant women to a separate HIV clinic at the same facility. |
|
12 clusters, 1172 women. |
|
|
ANC, antenatal care; ART, antiretroviral therapy; AZT, zidovudine; C, control; CHW, community health worker; EPOC, Effective Practice and Organisation of Care; FBPS, facility-based peer support; HAART, highly active antiretroviral therapy; I, intervention; MIP, methods routine paper; MOH, ministry of health; NVP, nevirapine; PMTCT, prevention of mother to child transmission; RCT, randomised controlled trial; SE, south east; SMS, short message service; SOC, standard of care; SW, south west.
The studies included 14 cluster randomised controlled trials (RCTs) with parallel study design, 2 cluster RCTs with stepped-wedge design and 2 RCTs. The number of clusters ranged from 6 to 40, and participants across all study types ranged from 160 to 31 536. All included studies were conducted in Sub-Saharan Africa between 2005 and 2016. Half of included studies reported multifaceted interventions, including two or more EPOC category components (9/18), and as a result several were categorised at more than one intervention level: patient (4), provider (1), system (7), patient/provider (1) or provider/system (5). Interventions directed all or in part to the health system level were most common (12/18). Integration (5/18), role expansion or task shifting (5/18), outreach services (4/18), and use of information and communication technology (4/18) were the most common EPOC intervention categories employed alone or as part of a complex intervention.
Reporting of population characteristics varied widely across studies as did outcome definitions. Seven studies limited participation to pregnant women 17–18 years of age or older; the median age across the studies ranged from 23 to 29.7 years. Marital status was reported in 14 studies, and varied widely from 9% to 99% of women who were married or had a live-in partner. Maternal education level was reported in 12 studies; 5 studies reported the majority of women having no or primary education, 5 studies reported the majority of women having received secondary education, and 2 reported mean/median years of education (10.3 years, 10 years (range 8–12 years)). Maternal employment (6/18) and parity (2/18) status were reported in a minority of studies (table 1). No prespecified adverse events were reported in the identified studies.
Reported outcomes varied substantially across studies, with few studies within intervention categories reporting comparable outcomes. For example, five studies reported interventions employing integration alone (2) or in combination with other interventions (3), with only one PMTCT outcome in common among the two studies employing integration alone. The most commonly reported outcomes were maternal ART use during pregnancy and labour and delivery, infant prophylaxis at birth, and infant HIV testing at 6–8 weeks.
As a result of the multifaceted nature of the majority of interventions employed, and variability in PMTCT outcomes reported, the ability to combine results statistically was limited.
Methodological quality
Risk of bias was assessed using the Cochrane EPOC risk of bias criteria.17 Five of the 18 studies were appraised as low risk of bias on three or more (4 studies with three criteria, 1 study with four criteria) of the six main criteria. The most common issues encountered were unclear reporting of randomisation (8/18) and allocation concealment (11/18), and unclear reporting or high risk of bias due to lack of blinding of participants/personnel (18/18) and blinding of outcome assessment (16/18) (the complete risk of bias is included as an additional file; online supplementary table).
bmjopen-2018-024907supp001.pdf (103KB, pdf)
Meta-analysis of effect of integration of care on ART use during pregnancy
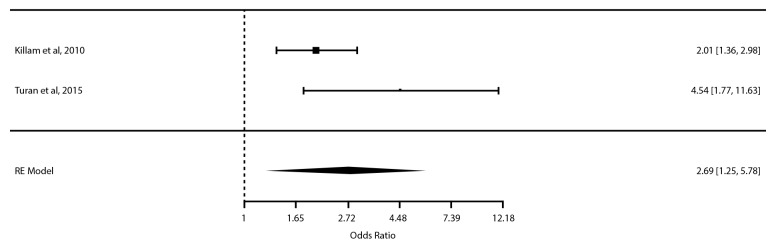
We expected variation in the implementation of integrated care of ART therapy into ANC in the two studies, conducted in clinics in Zambia and Kenya. We also expected some variation in standard of care (SOC) in the two settings, particularly with respect to eligibility and timing of ART initiation across the two studies, both of which experienced policy changes during the course of the study. We therefore used a random-effects meta-analysis to derive the combined effect estimate of integrated care based on theoretical grounds, although the I2 was not significant. Two studies assessing integration of HIV and ANC relative to usual non-integrated care were combined in a meta-analysis of 1887 patients25 26; there was increased use of ARTs during pregnancy with integration of HIV and ANC compared with standard non-integrated care (adjusted odds ratio (AOR)=2.69; 95% CI 1.25 to 5.78; p=0.0113, I2=59.26%) (figure 2) (see online supplementary file for fixed-effects meta-analysis diagram).
Figure 2.
Forest plot of meta-analysis of integration of HIV and antenatal care compared with usual (non-integrated care) effect on antiretroviral therapy use during pregnancy. RE, random effects.
Descriptive synthesis
Details of included studies (country, intervention, population characteristics, outcomes and so on) are outlined in table 1. Outcomes according to level(s) of intervention and according to PMTCT outcome are outlined in tables 2 and 3, respectively.
Table 2.
Results of included studies by level of intervention
| Author, year | Intervention level | EPOC intervention classification |
Intervention | Control | Outcomes Intervention group |
Outcomes Control group |
Risk ratio (95% CI) | Adjusted statistics where provided |
| Ezeanolue, 2015 | Patient | · Outreach services. | Monthly baby showers. | Usual care. |
|
|
|
|
| Reynolds, 2010 | Patient | · Self-management. · Educational outreach. |
Take-home infant nevirapine dose. | Usual care. |
|
|
|
|
| Weiss, 2014 | Patient | · Group (couple) vs individual care. | Couples HIV risk reduction and PMTCT education sessions. | Time-matched general education sessions. |
|
|
|
|
| Yotebieng, 2016 | Patient | · Conditional cash transfer. | Cash payments for clinic attendance and acceptance of recommended services. | Usual care. |
|
|
|
|
| Richter, 2014 | Patient/Provider | · Role expansion or task shifting. · Educational meetings. |
Peer mentor-led educational meetings. | Usual care. |
|
|
|
|
| Kieffer, 2011 | Provider | · Educational meetings. | 1-day PMTCT training for nurses and midwives. | No additional training. |
|
|
|
|
| Dryden-Peterson, 2015 | Provider/System | · The use of information and communication technology. · Educational meetings. |
Staff training in point-of-care CD4 testing and automated SMS results reporting to staff, support for patient tracing. | Usual care. |
|
|
|
|
| Mwapasa, 2017 | Provider/System | · Integration. · The use of information and communication technology. |
MIP: integration of antenatal and HIV care, routine patient tracing. MIP+SMS: integrated care and use of SMS enhanced tracing. |
Usual non-integrated care and patient tracing. |
|
|
|
|
| Oyeledun, 2017 | Provider/System | · Continuous quality improvement. | QI teams established, coaching and collaborative meetings. | Routine MOH support. |
|
|
|
|
| Phiri, 2017 | Provider/System | · Role expansion or task shifting Outreach services. · The use of information and communication technology. |
FBPS from mentor mothers. CBPS from mentor mothers. |
SOC. |
|
|
|
|
| Tomlinson, 2014 | Provider/System | · Role expansion or task shifting. · Outreach services. |
10 structured home visits from community health workers addressing PMTCT and newborn care. | 3 home visits from community health workers providing support in accessing social welfare grants. |
|
|
|
|
| Aliyu, 2016 | System |
|
Integrated package of PMTCT services, family/male partner participation, community champions. | Usual care. |
|
|
|
|
| Geelhoed, 2013 | System | · Integration. · Educational meetings |
Integrated maternal child health and HIV care. | Usual non-integrated care. |
|
|
|
|
| Killam, 2010 | System | · Integration. | Integration of antenatal and HIV care. | Usual non-integrated care. |
|
|
|
|
| Odeny, 2014 | System | · The use of information and communication technology. | SMS text messages during pregnancy and after delivery. | Usual care. |
|
|
|
|
| Rotheram-Borus, 2014 | System | · Role expansion or task shifting. · Outreach services. |
Antenatal and postnatal home visits from community health workers. | Usual care. |
|
|
|
|
| Rustagi, 2016 | System | · Continuous quality improvement. | Facility-level systems analysis and improvement intervention. | No intervention. |
|
|
|
|
| Turan, 2015 | System | · Integration. | Integrated HIV and antenatal care. | Usual, non-integrated care. |
|
|
|
|
AOR, adjusted odds ratio; ARD, adjusted risk difference; ARR, adjusted relative risk; ART, antiretroviral therapy; AZT, Zidovudine; CBPS, community-based peer support; EPOC, Effective Practice Organisation of Care; FBPS, facility-based peer support; HAART, highly active antiretroviral therapy; MIP, methods routine paper; MOH, ministry of health; NVP, nevirapine; PMTCT, prevention of mother to child transmission; QI, quality improvement; SMS, short message service; SOC, standard of care.
Table 3.
Results of the included studies by PMTCT outcome
| PMTCT outcome | Author, year | EPOC category(ies) | Intervention | Outcome Intervention group, n (%) |
Outcome Control group, n (%) |
Risk ratio (95% CI) |
| ART use in pregnancy | Turan, 2015 | Integration. | Integration of ANC and HIV services. | 138/173 (80) | 75/152 (49) | 1.61 (1.35 to 1.93)* |
| Killam, 2010 | Integration. | Integration of ANC and HIV services. | 278/846 (32.9) | 103/716 (14.4) | 2.28 (1.86 to 2.80)* | |
| Ezeanolue, 2015 | Outreach services. | Monthly church-based ‘baby showers’, including educational games, delivery supply packs, lab testing and contact point for follow-up. | 24/41 (65) | 12/32 (50) | 1.56 (0.93 to 2.62)* | |
| Phiri, 2017 | Role expansion or task shifting: outreach services: the use of information and communication technology. | Facility-based peer support from mentor mothers arm. Community-based peer support from mentor mothers arm. |
355/394 (90) 366/428 (86) |
361/447 (81) | 1.06 (1.00 to 1.12) 1.12 (1.06 to 1.18)* |
|
| Aliyu, 2016 | Role expansion/task shifting: Integration: packages of care. |
Integrated package of PMTCT services: point-of-care CD4 testing, decentralised PMTCT care, integrated mother/infant services, community champions. | 166/172 (97) | 77/197 (39) | 2.47 (2.07 to 2.95)* | |
| Dryden-Peterson, 2015 | The use of information and communication technology: educational meetings. | Staff training and support to antenatal clinics, SMS transmission of HIV results to clinic staff. | 56/154 (36.4) | 37/153 (24.2) | 1.50 (1.06 to 2.13) | |
| Oyeledun, 2017 | Continuous quality improvement. | A quality improvement initiative. | 261/264 (98.9) | 233/247 (94.3) | 1.05 (1.01 to 1.08) | |
| Rotheram-Borus, 2014 | Role expansion or task shifting: outreach services. | Antenatal and postnatal home community health worker home visits. | 169/179 (94.4) | 149/159 (93.7) | 1.01 (0.95 to 1.06) | |
| Rustagi, 2016 | Continuous quality improvement. | Facility-level system analysis and improvement intervention. | 575/839 (69) | 664/1037 (64) | 1.07 (1.00 to 1.14) | |
| Richter, 2014 | Role expansion or task shifting: educational meetings. | Peer-led educational meetings. | 340/377 (90.2) | 455/466 (95.5) | 0.92 (0.89 to 0.96)** | |
| ART in labour and delivery | Kieffer, 2011 | Educational meetings. | 1-day PMTCT knowledge and skills training for nurses and midwives. | 373/465 (80) | 325/472 (69) | 1.17 (1.08 to 1.26)* |
| Weiss, 2014 | Group (couple) vs individual care. | Couples-based HIV/PMTCT counselling. | 9/12 (75) | I6/12 (50) | 1.50 (0.78 to 2.88) | |
| Richter, 2014 | Role expansion or task shifting: educational meetings. | Peer-led education meetings. | 282/377 (74.8) 361/377 (95.8) |
334/466 (71.7) 456/466 (97.9) |
1.04 (0.96 to 1.13) 0.98 (0.95 to 1.00) |
|
| Geelhoed, 2013 | Integration: educational meetings. | Integration of maternal/child health and HIV services antepartum and post partum. | 112/121 (93) | 93/96 (97) | 0.96 (0.90 to 1.02) | |
| Rotheram-Borus, 2014 | Role expansion or task shifting: outreach services. | Antenatal and postnatal community health worker home visits. | 164/179 (91.6) 166/179 (92.7) |
147/159 (92.5) 142/159 (89.3) |
0.99 (0.93 to 1.06) 1.04 (0.97 to 1.11) |
|
| Turan, 2015 | Integration. | Integration of ANC and HIV services. | 28/173 (16) | 84/152 (55) | 0.29 (0.20 to 0.42)** | |
| ART in postpartum period | Turan, 2015 | Integration | Integration of ANC and HIV services. | 22/173 (13) | 57/152 (38) | 0.34 (0.22 to 0.53)** |
| ART across the PMTCT cascade | Yotebieng, 2016 | Conditional cash transfers. | Conditional cash transfers. | 146/216 (67.6) | 116/217 (53.5) | 1.26 (1.08 to 1.48)* |
| Turan, 2015 | Integration. | Integration of ANC and HIV services. | 37/176 (21.0) | 23/153 (15.0) | 1.40 (0.87 to 2.24) | |
| Infant prophylaxis at birth | Rotheram-Borus, 2014 | Role expansion or task shifting: outreach services. | Antenatal and postnatal home community health worker home visits. | 171/179 (95.5) | 141/159 (88.7) | 1.08 (1.01 to 1.14)* |
| Reynolds, 2010 | Self-management: educational outreach. | Take-home infant prophylaxis. | 80/85 (94) | 66/75 (88) | 1.07 (0.97 to 1.18) | |
| Richter, 2014 | Role expansion or task shifting: educational meetings. | Peer-led educational meetings. | 364/377 (96.6) 348/377 (92.3) |
451/466 (96.8) 374/466 (80) |
1.00 (0.97 to 1.02) 1.15 (1.09 to 1.21) |
|
| Oyeledun, 2017 | Continuous quality improvement. | Quality improvement intervention. | 138/209 (66) | 145/194 (74.7) | 0.88 (0.78 to 1.00) | |
| Geelhoed, 2013 | Integration: educational meetings. | Integration of maternal/child health and HIV services antepartum and post partum. | 117/126 (93) | 95/95 (100) | 0.93 (0.88 to 0.97) | |
| Turan, 2015 | Integration. | Integration of ANC and HIV services. | 50/173 (29) | 106/152 (70) | 0.41 (0.32 to 0.54)** | |
| Infant HIV testing at 6–10 weeks | Oyeledun, 2017 | Continuous quality improvement. | Quality improvement intervention. | 102/209 (48.8) | 49/194 (25.3) | 1.93 (1.46 to 2.55)* |
| Tomlinson, 2014 | Role expansion or task shifting: outreach services. | Increased training of and home visits by community health workers. | 420/571 (73.6) | 465/698 (66.6) | 1.10 (1.03 to 1.19)* | |
| Odeny, 2014 | The use of information and communication technology. | Antenatal and postnatal SMS texts to patients. | 172/187 (92.0) | 154/181 (85.1) | 1.08 (1.00 to 1.16)* | |
| Turan, 2015 | Integration. | Integration of ANC and HIV services. | 143/568 (25) | 106/594 (18) | 1.41 (1.13 to 1.76) | |
| Rotheram-Borus, 2014 | Role expansion or task shifting: outreach services. | Antenatal and postnatal home community health worker home visits. | 155/160 (96.9) | 132/140 (94.3) | 1.03 (0.98 to 1.08) | |
| Rustagi, 2016 | Continuous quality improvement. | Facility-level system analysis and quality improvement intervention. | 283/604.4 (47) | 270/710.6 (38) | 1.23 (1.09 to 1.40) | |
| Phiri, 2017 | Role expansion or task shifting: outreach services: the use of information and communication technology. | Facility-level peer mentor support arm. Community-based peer mentor support arm. |
200/289 (69) 95/286 (68) |
169/273 (62) | 1.12 (0.99 to 1.26) 1.23 (1.11 to 1.38)* |
|
| Infant HIV-positive at 6 weeks | Turan, 2015 | Integration. | Integration of ANC and HIV services. | I6/143 (4.2) | 7/106 (6.6) | 0.64 (0.22 to 1.84) |
| Weiss, 2014 | Group (couple) vs individual care. | Couples-based HIV/PMTCT counselling. | 1/30 (3.3) | 3/39 (7.7) | 0.43 (0.05 to 3.96) | |
| Yotebieng, 2016 | Conditional cash transfers. | Conditional cash transfers. | 5/169 (3.0) | 6/156 (3.9) | 0.77 (0.24 to 2.47) | |
| Phiri, 2017 | Role expansion or task shifting: outreach services: the use of information and communication technology. | Facility-level peer mentor support arm. Community-based peer mentor support arm. |
1/199 (1) 2/195 (2) |
2/169 (1) | 0.42 (0.04 to 4.64) 0.87 (0.12 to 6.09) |
|
| Retention in care at 6–8 weeks | Yotebieng, 2016 | Conditional cash transfers. | Conditional cash transfers. | 174/216 (80.6) | 157/217 (72.4) | 1.11 (1.00 to 1.23)* |
| Aliyu, 2016 | Role expansion/task shifting: Integration: packages of care. |
Integrated package of PMTCT services: point-of-care CD4 testing, decentralised PMTCT care, integrated mother/infant services, community champions. | 125/150 (83) | 15/170 (9) | 9.44 (5.60 to 15.4)* | |
| Ezeanolue, 2015 | Outreach services. | Monthly church-based ‘baby showers’ including educational games, delivery supply packs, lab testing and contact point for follow-up. | 33/41 (81) | 28/32 (88) | 0.92 (0.75 to 1.12) | |
| Retention in care at 12 months | Mwapasa, 2017 | Integration: the use of information and communication technology. | Integration of ANC and HIV care and routine patient tracing arm. Integration of ANC and HIV care and SMS enhanced patient tracing arm. |
M: 89/461 (19.3) M: 334/461 (72.4) I: 32/386 (8.3) I: 291/386 (75.4) M: 115/493 (23.3) M: 332/493 (67) I: 82/399 (20.1) I: 323/399 (80.9) |
M: 90/396 (22.7) M: 274/396 (69.1) I: 32/300 (10.7) I: 234/300 (78.0) |
M: 0.85 (0.65 to 1.10) M: 1.05 (0.96 to 1.14) I: 0.78 (0.49 to 1.24) I: 0.97 (0.89 to 1.05) M: 1.03 (0.81 to 1.31) M: 0.97 (0.89 to 1.06) I: 1.93 (1.32 to 2.82) I: 1.04 (0.96 to 1.12) |
| Phiri, 2017 | Role expansion or task shifting: outreach services: the use of information and communication technology. | Facility-level peer mentor support arm. Community-based peer mentor support arm. |
277/366 (78) 258/355 (74) |
261/361 (74) | 1.05 (0.96 to 1.14) 1.01 (0.92 to 1.10)* |
|
| Retention in care at 24 months | Phiri, 2017 | Role expansion or task shifting: outreach services: the use of information and communication technology. |
Facility-level peer mentor support arm. Community-based peer mentor support arm. |
223/428 (52) 298/428 (70) 211/394 (54) 292/394 (74) |
169/447 (38) 255/447 (57) |
1.38 (1.19 to 1.60) 1.22 (1.10 to 1.35)* 1.42 (1.22 to 1.65)* 1.30 (1.18 to 1.43)* |
*significant in favor of intervention.
**significant in favor of control.
ANC, antenatal care; ART, antiretroviral therapy; EPOC, Effective Practice Organisation of Care; I, infant; M, maternal; PMTCT, prevention of mother to child transmission; SMS, short message service.
Findings of the narrative synthesis are outlined below first as intervention types within intervention target categories (patient, provider, system) and then by PMTCT outcome.
Descriptive synthesis of findings according to intervention target level(s)
Findings according to level of intervention are outlined in table 2.
Patient-level interventions
Four studies evaluated interventions primarily targeted at the patient level.27–30 Risk of bias ranged from three to six of the six criteria rated as high or unclear. Ezeanolue et al 27 included 40 clusters and 3024 patients and evaluated a complex intervention that included monthly baby showers at participating churches where expectant mothers participated in educational games, received ‘mama packs’ containing supplies needed during delivery (sterile gloves, alcohol swabs, clean razor and so on) and laboratory testing, and were given a contact point for follow-up. Women in the intervention group were found to be significantly more likely to complete linkage to care and receive ARTs during pregnancy (relative risk (RR)=1.56 (95% CI 0.93 to 2.62); AOR=2.8 (95% CI 1.02 to 4.79)), but no difference was identified between groups in accessing care at 6–8 weeks post partum. Reynolds et al 28 included 10 clusters and 203 patients in a study that provided prepackaged syringes of infant nevirapine (NVP) doses to be given by mothers who delivered at home; no difference was found in the proportion of infants receiving NVP after delivery. Weiss et al 29 included 12 clusters and 239 couples and evaluated a couples-based PMTCT intervention compared with SOC. They found no statistically significant difference in PMTCT regimen adherence, defined as ART detected in mothers’ blood, ART detected in infants’ blood or in the rate of infant HIV infection. Yotebieng et al 30 included 433 patients and evaluated whether conditional cash transfers improved adherence, acceptance of and retention in PMTCT services to 6 weeks post partum. They found women in the intervention group were significantly more likely to be retained in care (RR=1.11 (95% CI 1.00 to 1.23)), and to have attended all clinic visits and to have accepted recommended PMTCT services (RR=1.26 (95% CI 1.08 to 1.48)). No difference was found in infant HIV-positive rates at 6 weeks.
Patient-level/provider-level interventions
One study by Richter et al 31 included 8 clusters and 1200 patients and reported an intervention directed at both patients and providers in which peer mentors were trained to provide inperson education sessions for patients. Risk of bias was rated as high or unclear on five of the six criteria.31 They found patients in the intervention group were significantly less likely to adhere to ARTs during pregnancy (zidovudine (AZT) or highly active antiretroviral therapy (HAART)) (RR=0.92 (95% CI 0.89 to 0.96); AOR=0.44 (95% CI 0.26 to 0.74)). No statistically significant effects were found on the remaining outcomes, including ART use during labour and delivery (NVP or HAART or AZT or HAART), infant NVP at birth, and infant ART postbirth/breast feeding. Although participants were reassessed at 6 and 12 months, we were unable to reach the authors for additional information on long-term outcomes.
Provider-level interventions
Kieffer et al 32 included 6 clusters and 2444 patients and evaluated the impact of a 1-day PMTCT knowledge and skills training course for nurses and midwives compared with standard training alone (no intervention); risk of bias was rated high or unclear on five of the six criteria. They found a statistically significant increase in the proportion of women with ART detected in cord blood as a marker of ART use during labour and delivery (RR=1.17 (95% CI 1.08 to 1.26)).
Provider-level/system-level interventions
Five studies reported interventions directed at both the provider and health system level.33–37 Risk of bias ranged from two to five of the six criteria rated as high or unclear. Dryden-Peterson et al 33 included 19 clusters and 366 patients and provided staff training, automated transmission of HIV test results to clinic staff via short message service (SMS), and ongoing support to antenatal clinics (ie, education for new staff, supporting SMS printers, monitoring and addressing clinic underperformance). There was a trend towards an increase in the proportion of mothers initiated on ARTs by 30 weeks’ gestation in the intervention group.
Mwapasa et al 34 conducted a three-arm cluster RCT with 30 clusters and 1350 patients to assess the impact of two different patient tracing methods routine paper (MIP) and SMS triggered tracing (MIP+SMS), combined with integrated care against SOC. They found no significant difference in maternal retention in care at 12 months in either intervention group relative to controls using study definitions or ministry of health definitions for retention. They found no statistically significant difference in infant retention in care at 12 months in either intervention group relative to controls using study definitions or ministry of health definitions for retention.
Oyeledun et al 35 compared a continuous quality improvement intervention including coaching visits and collaborative meetings with standard ministry of health support in 32 clusters and 511 patients. They found no significant difference in retention in care at 6 months, in initiation of ART prophylaxis in infants within 72 hours of birth or in the proportion of women initiated on ARTs within 2 weeks of enrolment. They found significantly improved rates of infant HIV testing at 6–10 weeks (RR=1.93 (95% CI 1.46 to 2.55); adjusted relative risk (ARR)=1.76 (95% CI 1.27 to 2.42)).
Phiri et al 36 conducted a three-arm cluster RCT with 21 clusters and 1269 women evaluating facility-based peer support (FBPS) and community-based peer support (CBPS) from expert mothers against SOC. They found non-significant improvement with FBPS and small statistically significant improvements with CBPS in uptake of ARTs (RR=1.12 (95% CI 1.06 to 1.18); adjusted risk difference (ARD)=0.09 (95% CI 0.01 to 0.18)), retention in care at 1 year (RR=1.01 (95% CI 0.92 to 1.10); ARD=0.08 (95% CI 0.04 to 0.20)) and retention in care at 2 years (RR=1.42 (95% CI 1.22 to 1.65); ARD=0.16 (95% CI 0.03 to 0.30)), relative to SOC. Retention in care at 2 years was significant for both FBPS (RR=1.22 (95% CI 1.10 to 1.35)) and CBPS (RR=1.30 (95% CI 1.18 to 1.43)) using ministry of health definitions for retention in care. Infant HIV testing at 6 weeks was significantly higher in the CBPS only (RR=1.23 (95% CI 1.11 to 1.38)). There was no difference in infant HIV-positive rates at 6 weeks in either intervention group.
Tomlinson et al 37 included 3957 patients in 30 clusters and evaluated the impact of increased training of community health workers and increased home visits by community health workers during delivery and postdelivery to provide PMTCT counselling and newborn care. They found a significantly increased proportion of infants receiving HIV testing at 6 weeks in the intervention group (RR=1.10 (95% CI 1.03 to 1.19); ARR=1.10 (95% CI 0.97 to 1.25)) and no difference in mother to child HIV transmission at 12 weeks.
System-level interventions
Seven studies reported interventions at the system level.24 25 38–42 Risk of bias ratings for system-level intervention studies ranged from two to five of the six criteria rated as high or unclear risk of bias. Aliyu et al 38 evaluated an integrated package of PMTCT services including point-of-care CD4 testing, decentralised care, integrated mother/infant services and community involvement through male champions, compared with SOC across 12 clusters and 369 patients. They found significant improvement in the proportion of eligible women started on ART for PMTCT (RR=2.47 (95% CI 2.07 to 2.95); ARR=3.3 (95% CI 1.4 to 7.8)), and in retention of mother–infant in care at 6 weeks (RR=9.44 (95% CI 5.60 to 15.4); ARR=9.1 (95% CI 5.2 to 15.9)) and 12 weeks’ post partum (RR=11.40 (95% CI 6.40 to 20.34); ARR=10.3 (95% CI 5.4 to 19.7)).
Geelhoed et al 39 included 6 clusters and 217 patients in the postintervention period and evaluated the impact of integration of HIV and maternal child health services during both antenatal and postnatal periods. They found no improvement in the proportion of women receiving ARTs during labour and delivery, proportion of infants receiving prophylaxis within 48 hours, and proportion of HIV-positive infants.
Killam et al 26 assessed the impact of integration of antenatal and HIV care relative to usual care (antenatal and HIV care separate) in 8 clusters and 31 536 patients. They found a statistically significant increase in the proportion of eligible women receiving ARTs during pregnancy (RR=2.28 (95% CI 1.86 to 2.80); AOR=2.01 (95% CI 1.37 to 2.95)).
Odeny et al 40 evaluated use of automated SMS to patients (n=388) during pregnancy and postdelivery. They found statistically significant improvements in maternal antenatal clinic attendance (RR=1.66 (95% CI 1.03 to 2.70)) and infant HIV testing by 8 weeks (RR=1.08 (95% CI 1.00 to 1.16)).
Rotheram-Borus et al 41 assessed the impact of home visits by community health workers in addition to clinic care in 24 clusters and 1144 patients. They found significant improvement in the proportion of infants receiving NVP within 24 hours of birth (RR=1.08 (95% CI 1.01 to 1.14); AOR 2.94 (95% CI 1.41 to 6.12)) and AZT dispensed for infant and used as prescribed in the intervention group (RR=1.08 (95% CI 1.01 to 1.14); AOR 2.95 (95% CI 1.12 to 7.73)). There was no significant difference in maternal AZT/HAART use prior to labour or during labour, maternal NVP/HAART use at onset of labour, and infant 6-week HIV testing relative to controls.
Rustagi et al 42 evaluated a systems analysis and improvement intervention across 36 clusters in 3 countries, including 1876 patients. They found no significant improvement in the proportion of pregnant women receiving ARTs (RR=1.07 (95% CI 1.00 to 1.14)) or infants tested for HIV by 6–8 weeks (RR=1.23 (95% CI 1.09 to 1.40)).
Turan et al 25 included 12 clusters and 1172 patients and examined the effects of integration of HIV and ANC compared with standard non-integrated care. Self-reported maternal ART use across the PMTCT spectrum, predelivery, during delivery and postdelivery, was not significantly different between groups, although it was significantly higher during pregnancy (RR=1.61 (95% CI 1.35 to 1.93); AOR=4.05 (95% CI 2.00 to 8.00)). ART use was significantly lower among intervention sites during labour and delivery (RR=0.29 (95% CI 0.20 to 0.42); AOR=0.16 (95% CI 0.04 to 0.68)) and postdelivery (RR=0.34 (95% CI 0.22 to 0.53); AOR=0.24 (95% CI 0.08 to 0.70)). Infant ART use after birth was significantly lower in intervention sites (RR=0.41 (95% CI 0.32 to 0.54); AOR=0.18 (95% CI 0.09 to 0.35)); although infant HIV testing was increased at 6 weeks and 9 months in intervention sites, the difference was not statistically significant. No difference was found for infant HIV infection rates at 6 weeks or 9 months.
Descriptive synthesis of findings according to PMTCT outcomes
Findings according to PMTCT outcome are outlined in table 3. The vast majority of studies reported short-term PMTCT outcomes with ART use during pregnancy (10/18) and labour and delivery (6/18), infant prophylaxis at birth (6/18), and infant HIV testing at 6–10 weeks (5/18). Overall, findings are often mixed and effect sizes small, with many of uncertain clinical significance.
Five studies found significant improvements in ART use during pregnancy, with RR ranging from 1.12 to 2.48.25–27 36 38 Effective interventions included integration of ANC and HIV services (RR=1.61 (95% CI 1.35 to 1.93); AOR=4.05 (95% CI 2.00 to 8.00)25 and RR=2.28 (95% CI 1.86 to 2.80); AOR=2.01 (95% CI 1.37 to 2.95))26; monthly baby showers at participating churches providing education through games, ‘mama packs’ containing delivery supplies, laboratory testing and a contact point for follow-up (RR 1.56 (95% CI 0.93 to 2.62); AOR=2.8 (95% CI 1.02 to 4.79))27; CBPS from mentor mothers (RR=1.12 (95% CI 1.06 to 1.18); ARD=0.09 (95% CI 0.01 to 0.18))36; and an integrated package of PMTCT services including point-of-care CD4 testing, decentralised PMTCT care, integrated mother/infant services and community champions (RR=2.47 (95% CI 2.07 to 2.95); ARR 3.3 (95% CI 1.4 to 7.8)).38 Four studies evaluating staff training and support to antenatal clinics and automated SMS transmission of HIV test results to clinic staff,33 a quality improvement initiative,35 community health worker antenatal and postnatal home visits,41 and facility-level systems analysis and improvement intervention42 found no significant difference in ART use during pregnancy. One study evaluating peer mentor-led educational meetings found ART adherence during pregnancy lower in the intervention group.31
Six studies reported ART use during labour and delivery, with four of six finding no significant effect,29 31 39 41 one finding a significant but small improvement (RR=1.17)32 and one finding significantly reduced ART use in the intervention group (RR=1.614).25 The one study that found a small significant effect employed a 1-day PMTCT knowledge and skills training course for nurses and midwives (RR=1.17 (95% CI 1.08 to 1.26)).32 Ineffective interventions included couples-based PMTCT intervention,29 peer mentor-led educational meetings,31 integration of maternal child health and HIV services,39 and community health worker antenatal and postpartum home visits.41 In contrast to the findings for ART use during pregnancy, ART use during labour and delivery was significantly lower with integration of ANC and HIV care (RR=0.29 (95% CI 0.20 to 0.42); AOR=0.16 (95% CI 0.04 to 0.68)).25
Only one study evaluated ART use in the postpartum period and found significantly reduced ART use during this period (RR=0.34 (95% CI 0.22 to 0.53); AOR=0.24 (95% CI 0.08 to 0.70)) with integration of ANC and HIV care.25 Two additional studies evaluated uptake across the cascade, with conditional cash transfer found to significantly improve uptake of PMTCT recommendations (RR=1.26 (95% CI 1.08 to 1.48))30 and no difference found for integration of ANC and HIV services.25
Six studies evaluated infant HIV prophylaxis at birth. One of six studies reported a small significant improvement in infant HIV prophylaxis at birth with community health worker home visits (RR=1.08 (95% CI 1.01 to 1.14); AOR 2.94 (95% CI 1.41 to 6.12)),41 one of six significantly reduced infant prophylaxis at birth with integration of ANC and HIV care (RR=0.41 (95% CI 0.32 to 0.54); AOR=0.18 (95% CI 0.09 to 0.35)),25 and four of six studies finding no significant difference with take-home NVP dosing,28 peer mentor-led educational meetings,31 a quality improvement intervention,35 and integration of maternal child health and HIV services during both the antenatal and postpartum periods.39
Seven studies reported infant HIV testing at 6–10 weeks. Three of seven found significantly improved rates of infant testing by 6–10 weeks of age, with RR ranging from 1.08 to 1.93,35 37 40 three of seven no difference,25 41 42 and one study finding a mixed effect of peer support.36 Improvements in infant HIV testing were found for a quality improvement intervention (RR=1.93 (95% CI 1.46 to 2.55); ARR=1.76 (95% CI 1.27 to 2.42)),35 increased training of and home visits from community health workers (RR=1.10 (95% CI 1.03 to 1.19); ARR=1.10 (95% CI 0.97 to 1.25)),37 and SMS texts to patients both antenatally and postdelivery (RR=1.08 (95% CI 1.00 to1.16)).40 One study found mixed effects of peer support on infant HIV testing, with CBPS found to significantly improve infant HIV testing at 6 weeks (RR=1.23 (95% CI 1.11 to 1.38)) and no difference found for FBPS.36 No difference was found for integration of ANC and HIV care,25 home visits from community health workers,41 or a facility-level system analysis and quality improvement intervention.42
Outcome definitions for retention in care and infant HIV-positive rates were highly variable, ranging from 6 weeks to 2 years for the former, and from 6 weeks to 1 year for the latter. As for other PMTCT outcomes noted above, relatively more short-term outcomes (6 weeks) were reported for retention and infant HIV-positive rates. Three studies evaluated maternal or maternal/infant retention in care at 6 weeks, with two studies evaluating conditional cash transfers30 and an integrated package of PMTCT services including point-of-care CD4 testing, decentralised care, integrated mother/infant services and community champions,38 finding significantly improved retention (RR=1.11 (95% CI 1.00 to 1.23) and RR=9.44 (95% CI 5.60 to 15.4); ARR=9.1 (95% CI 5.2 to 15.9)) at 6 weeks, and a third employing monthly baby showers finding no difference.27 Two studies examined retention in care at 1 year. One study evaluating integration of ANC and HIV care with and without SMS enhanced tracing in a three-arm trial and found no difference in maternal or infant retention at 1 year.34 A second study evaluated the effect of CBPS and FBPS on retention in care at 1 and 2 years, in a three-arm trial. They found non-significant improvement with FBPS and small statistically significant improvements with CBPS in retention in care at 1 year (RR=1.01 (95% CI 0.92 to 1.10)) and 2 years (RR=1.42 (95% CI 1.22 to 1.65)) using trial data.36 Retention in care at 2 years was significant for both FBPS (RR=1.22 (95% CI 1.10 to 1.35)) and CBPS (RR=1.30 (95% CI 1.18 to 1.43)) using ministry of health definitions for retention in care.
Four studies examined infant HIV-positive rates at 6–10 weeks post partum. Evaluated interventions included integration of ANC and HIV care,25 couples-based HIV/PMTCT counselling,29 conditional cash transfers30 and peer support.36 All found no difference.
Discussion
Eighteen studies were included in our review. Heterogeneity of interventions and outcome reported limited both comparison across studies and intervention categories, as well as opportunities for meta-analysis. The majority of studies were of moderate to high risk of bias, primarily due to limitations inherent to health systems research and unclear reporting of key methodological factors.
Based on our review findings, several interventions appear promising. In the single meta-analysis conducted with data from two studies,25 26 we found a significant increase in ART use during pregnancy with integration of HIV and ANC compared with standard non-integrated care. Consistent with the findings of our meta-analysis, a narrative review of three studies found small positive effects of integration of HIV and ANC, alone or as part of a complex intervention, on ART use during pregnancy. However, the effects of integration on PMTCT outcomes during labour and delivery and postdelivery were less clear, with no difference found for some studies34 39 and for some outcomes,25 and one study finding reduced ART use during labour and delivery and postdelivery.25 While the findings of Turan et al 25 occurred in the setting of resource challenges impacting implementation and relatively low numbers of adherence reports beyond the antenatal period, this was the case for both intervention and control groups. Therefore, as integrated care is now common practice, future work focusing on how integration of maternal child health and HIV care may be optimised alone or in combination with other interventions to optimise PMTCT outcomes beyond the antenatal period is needed.
Four studies evaluating different approaches to outreach services alone or in combination with other interventions found small positive effects on linkage to care, ART use during pregnancy and labour/delivery, and early infant HIV testing. Two studies found positive effects of role expansion or task shifting, in the form of peer mentorship support, on ART use during pregnancy and, when combined with outreach services, positive effects were seen on long-term retention in care and early infant HIV testing. Additional strategies found to have positive effects on PMTCT outcomes, each in a single study, included educational meetings, conditional cash transfers, continuous quality improvement, and use of information and communication technology.
An important finding of the present review is the high degree of variability in outcome definitions and relative lack of longer term outcome data. While in some instances variability of outcome definitions may be considered a strength where both self-report and biological markers of ART use are included, variability in timing of outcomes limits comparison across studies and opportunities for meta-analysis, and as a result limits the strength of conclusions and utility of the findings to PMTCT knowledge users. Although uptake and early retention in PMTCT services are clearly critical to reducing HIV transmission, longer term outcomes are equally important to understanding how retention in care can be optimised to reduce late HIV transmission. Utility of future work would be substantially improved through both standardisation of timing of PMTCT outcomes and through funding opportunities that would allow for evaluation of longer term outcomes.
In keeping with other systematic reviews focused on interventions aimed at improving PMTCT care and outcomes published to date,8 9 13–15 our review found the evidence base available to guide PMTCT programme planning remains limited. Similar to the systematic review by Tudor Car et al,9 which included a single study and found improved ART use in labour/delivery from integration of care, our single meta-analysis including two studies found a positive effect of integration on maternal ART use during pregnancy. Wekesah et al 13 included 73 studies, only 2 of which met the inclusion criteria for the present review, and they also found variable effects of non-drug interventions on both quality of care and maternal health outcomes. Geldsetzer et al 14 included 10 articles, with 2 overlapping studies included in our review, and focused on postpartum retention of women in PMTCT and ART care. This latter review, which included both high-income countries and LMICs and a broader range of study designs, focused on a limited portion of the PMTCT cascade. It found inconsistent effects of integration and weak evidence of phone interventions on retention in PMTCT care. Ambia and Mandala15 focused on interventions to improve PMTCT service delivery and promote retention. Their review was conducted over a similar time frame to the present review; however, it differs from the present review in its inclusion of high-income country studies, inclusion of a range of study designs and in its approach to categorisation of interventions. Thirty-four studies were included in their review, 11 of which were included in the present review. They found weak evidence for improvement of early infant HIV diagnosis from mobile-phone based interventions and for male involvement in reducing infant HIV transmission.
Given the focus of the present review on providing evidence-based guidance to PMTCT programme planners and implementers-based LMICs, our review differs from the reviews noted above in several ways. First, to optimise the quality of evidence, we limited our review to randomised and non-randomised controlled trials and interrupted times series studies. Second, to increase the applicability of findings to LMIC implementers, we limited our review to studies conducted in LMICs. Third, we included a broad range of intervention categories and included both maternal and infant outcomes from across the spectrum of the PMTCT cascade. Finally, in order to provide information of direct relevance to implementation planning, we categorised and analysed interventions at both the the level at which they are implemented (patient, provider, system) and using the EPOC intervention classification scheme, which groups interventions based on the intervention process/activities employed.
Limitations
While agreement on data extraction was not calculated, an initial calibration exercise was carried out to ensure consistency in data extraction. Following this, comparison of completed data extraction forms revealed few differences. Although no study was excluded for language, it is possible that use of translation software may have resulted in exclusion of an eligible study due to inaccurate translation. Additionally, while unlikely to have led to a significant difference in results, the updated search of the ERIC database was conducted in ProQuest rather than EBSCO as the latter was not accessible to the second information technologist.
The multifaceted nature of the majority of interventions evaluated and variability in PMTCT outcomes reported limited our ability to combine studies statistically and to separate effective/ineffective features of the interventions. In addition, efforts to contact authors for data necessary for risk ratio calculations were ineffective in several cases. Due to the small number of studies included in the meta-analysis, publication bias could not be examined. Additionally, although prespecified in our protocol, interpretation of findings, most commonly infant HIV infection rates, is limited by lack of power to assess secondary outcomes among the included studies. As 7 of the 18 studies limited participation to women 17–18 years of age or older, results may be less generalisable to younger mothers. Finally, although the EPOC search filter is designed to identify articles from all LMICs, only articles from Sub-Saharan Africa were included in the review. Results therefore may be less generalisable to LMICs outside Sub-Saharan Africa. In addition, this finding highlights limitations in the evidence to date and where funding should be targeted for future research based on knowledge users’ needs.
Future directions
Overall, evidence to date to guide PMTCT programming is limited. In particular, effects were generally small and often mixed across studies, and based on a small number of studies that were largely at moderate to high risk of bias. Further research is needed to improve both quantity and quality of data. First, replication of promising approaches is needed. Second, improved publication reporting to ensure key methodological factors are addressed and to provide detail on the likely impact of factors that cannot be modified through design. This transparency in reporting will enhance interpretation and utility of findings in informing PMTCT policy and programme decision making. For example, while the nature of designs for evaluating PMTCT interventions often makes blinding of participants impossible, description of the context and likely impact would aid interpretation. Additionally, use of blinded outcome assessment or objective outcomes such as laboratory confirmation of ART in blood samples will increase study impact. Third, given the inherent difficulties in evaluating complex interventions, increased use of designs to facilitate evaluation, for example, factorial designs of multiple-arm studies, would be of value. Fourth, efforts to include a variety of key outcomes across the PMTCT cascade and longer term outcomes in particular where feasible would allow for increased comparison across interventions.
Conclusions
The body of evidence synthesised in this review and in the literature to date on effectiveness of interventions to improve uptake and retention of mothers and infants in PMTCT care is limited by low-quality evidence. A single meta-analysis of two studies employing integration of antenatal and HIV care suggested a potential for improvement of ART use during pregnancy based on weak evidence. Overall findings are mixed and effect sizes small and of uncertain clinical significance. In order to improve the utility of evidence to programme planners, future studies should strive to include key outcomes across the range of the PMTCT cascade where feasible, reduce risk of bias where possible, and improve reporting of key methodological factors to allow for improved assessment of risk of bias and understanding of the likely impact of risk of bias where it cannot be addressed in design.
bmjopen-2018-024907supp003.pdf (176.8KB, pdf)
Supplementary Material
Acknowledgments
We thank Melanie Anderson for her assistance with developing the search strategy and conducting the initial search, Alissa Epworth for conducting the search update, and Elise Cogo and Jessie McGowan for peer reviewing the search strategy.
Footnotes
Contributors: LMPR and MvL conceived the study. LMPR and SES developed the search strategy. LMPR prepared and registered the protocol. LMPR and MvL completed all stages of article screening, data abstraction and risk of bias appraisal. LMPR prepared the initial evidence tables and manuscript. LMPR conducted the meta-analysis with support from BP. MCH, NER, SP, ML and FC provided content expertise and assisted with preparation of the protocol and manuscript. All authors provided critical revision of the manuscript.
Funding: LMPR was funded by a KT Canada Strategic Training Initiative in Health Research Fellowship award in 2014. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation and Quality of Care. NER was funded by the National Institute of Mental Health (Grant K99 MH104154-01A1) and the National Institute of Allergy and Infectious Diseases (P30 AI50410 and R01 AI131060-01).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
Patient consent for publication: Not required.
References
- 1. UNAIDS. Preventing mother-to-child transmission of HIV. 2016. http://www.unaids.org/en/resources/presscentre/featurestories/2016/october/20161024_EMotherToChildT (Accessed Jun 2018).
- 2. Avert. Prevention of mother-to-child transmission (PMTCT) of HIV. 2018. https://www.avert.org/professionals/hiv-programming/prevention/prevention-mother-child (Accessed Jun 2018).
- 3. World Health Organization. Mother-to-child transmission of HIV. 2018. http://www.who.int/hiv/topics/mtct/about/en/ (Accessed Jun 2018).
- 4. World Health Organization. Prevention of mother-to-child transmission (PMTCT): Situation and trends. 2018. http://www.who.int/gho/hiv/epidemic_response/PMTCT_text/en/ (Accessed Jun 2018).
- 5. World Health Organization. Global update on the health sector response to HIV, 2014. 2014. http://apps.who.int/iris/bitstream/10665/128196/1/WHO_HIV_2014.15_eng.pdf (Accessed Mar 2015).
- 6. World Health Organization. PMTCT strategic vision 2010–2015: Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. 2010. http://www.who.int/hiv/pub/mtct/strategic_vision/en/index.html (Accessed Mar 2015).
- 7. Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2013;16:18588 10.7448/IAS.16.1.18588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brusamento S, Ghanotakis E, Tudor Car L, et al. Male involvement for increasing the effectiveness of prevention of mother-to-child HIV transmission (PMTCT) programmes. Cochrane Database Syst Rev 2012;10:CD009468 10.1002/14651858.CD009468.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tudor Car L, van-Velthoven MH, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database Syst Rev 2011;24:CD008741 10.1002/14651858.CD008741.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Jongh TE, Gurol-Urganci I, Allen E, et al. Integration of antenatal care services with health programmes in low- and middle-income countries: systematic review. J Glob Health 2016;6:1–15. 10.7189/jogh.06.010403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Govindasamy D, Meghij J, Kebede Negussi E, et al. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings--a systematic review. J Int AIDS Soc 2014;17:19032 10.7448/IAS.17.1.19032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng H, Chow EP, Zhao Y, et al. Prevention of mother-to-child HIV transmission cascade in China: a systematic review and meta-analysis. Sex Transm Infect 2016;92:116–23. 10.1136/sextrans-2014-051877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wekesah FM, Mbada CE, Muula AS, et al. Effective non-drug interventions for improving outcomes and quality of maternal health care in sub-Saharan Africa: a systematic review. Syst Rev 2016;5:137 10.1186/s13643-016-0305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geldsetzer P, Yapa HM, Vaikath M, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc 2016;19:20679 10.7448/IAS.19.1.20679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc 2016;19:20309 10.7448/IAS.19.1.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. www.cochrane-handbook.org (Accessed Jan 2015).
- 17. Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2013. [Google Scholar]
- 18. Puchalski Ritchie LM, van Lettow M, Hosseinipour MC, et al. The effectiveness of interventions to improve uptake and retention of HIV-infected pregnant and breastfeeding women and their infants in prevention of mother-to-child transmission care programs in low- and middle-income countries: protocol for a systematic review and meta-analysis. Syst Rev 2015;4:144 10.1186/s13643-015-0136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cochrane Effective Practice and Organisation of Care (EPOC). LMIC filter. 2018. http://epoc.cochrane.org/lmic-filters (Accessed Mar 2015).
- 21. The World Bank. World Bank country and lending groups. 2018. http://data.worldbank.org/about/country-classifications/country-and-lending-groups (Accessed Jan 2015).
- 22. Sampson M, McGowan J, Cogo E, et al. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol 2009;62:944–52. 10.1016/j.jclinepi.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 23. EPOC Taxonomy. Cochrane effective practice and organisation of Care (EPOC). 2015. https://epoc.cochrane.org/epoc-taxonomy (Accessed Jan 2015).
- 24. Viechtbauer W. Metafor: meta-analysis package for R. 2017. https://cran.r-project.org/web/packages/metafor/index.html (Accessed Aug 2017).
- 25. Turan JM, Onono M, Steinfeld RL, et al. Implementation and operational research: effects of antenatal care and HIV treatment integration on elements of the PMTCT cascade: results from the SHAIP Cluster-Randomized Controlled Trial in Kenya. J Acquir Immune Defic Syndr 2015;69:e172–81. 10.1097/QAI.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS 2010;24:85–91. 10.1097/QAD.0b013e32833298be [DOI] [PubMed] [Google Scholar]
- 27. Ezeanolue EE, Obiefune MC, Ezeanolue CO, et al. Effect of a congregation-based intervention on uptake of HIV testing and linkage to care in pregnant women in Nigeria (Baby Shower): a cluster randomised trial. Lancet Glob Health 2015;3:e692–e700. 10.1016/S2214-109X(15)00195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds HW, Gachuno O, Kayita J, et al. Cluster randomised trial of the uptake of a take-home infant dose of nevirapine in Kenya. East Afr Med J 2010;87:284–93. [PubMed] [Google Scholar]
- 29. Weiss SM, Peltzer K, Villar-Loubet O, et al. Improving PMTCT uptake in rural South Africa. J Int Assoc Provid AIDS Care 2014;13:269–76. 10.1177/2325957413488203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yotebieng M, Thirumurthy H, Moracco KE, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV 2016;3:e85–e93. 10.1016/S2352-3018(15)00247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richter L, Rotheram-Borus MJ, Van Heerden A, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS Behav 2014;18:706–15. 10.1007/s10461-014-0694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kieffer MP, Nhlabatsi B, Mahdi M, et al. Improved detection of incident hiv infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. JAIDS Journal of Acquired Immune Deficiency Syndromes 2011;57:e85–91. 10.1097/QAI.0b013e31821acc6e [DOI] [PubMed] [Google Scholar]
- 33. Dryden-Peterson S, Bennett K, Hughes MD, et al. An augmented SMS intervention to improve access to antenatal CD4 testing and ART initiation in HIV-infected pregnant women: a cluster randomized trial. PLoS One 2015;10:e0117181 10.1371/journal.pone.0117181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mwapasa V, Joseph J, Tchereni T, et al. Impact of mother-infant pair clinics and Short-Text Messaging Service (SMS) reminders on retention of hiv-infected women and HIV-Exposed Infants in eMTCT Care in Malawi: A Cluster Randomized Trial. J Acquir Immune Defic Syndr 2017;75:S123–31. 10.1097/QAI.0000000000001340 [DOI] [PubMed] [Google Scholar]
- 35. Oyeledun B, Phillips A, Oronsaye F, et al. The effect of a continuous quality improvement intervention on retention-in-care at 6 months postpartum in a PMTCT program in Northern Nigeria: results of a cluster randomized controlled study. J Acquir Immune Defic Syndr 2017;75:S156–64. 10.1097/QAI.0000000000001363 [DOI] [PubMed] [Google Scholar]
- 36. Phiri S, Tweya H, van Lettow M, et al. Impact of facility- and community-based peer support models on maternal uptake and retention in Malawi’s Option B+ HIV prevention of mother-to-child transmission program: a 3-arm cluster randomized controlled trial (PURE Malawi). J Acquir Immune Defic Syndr 2017;75:S140–8. 10.1097/QAI.0000000000001357 [DOI] [PubMed] [Google Scholar]
- 37. Tomlinson M, Doherty T, Ijumba P, et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health 2014;19:256–66. 10.1111/tmi.12257 [DOI] [PubMed] [Google Scholar]
- 38. Aliyu MH, Blevins M, Audet CM, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV 2016;3:e202–11. 10.1016/S2352-3018(16)00018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geelhoed D, Lafort Y, Chissale É, et al. Integrated maternal and child health services in Mozambique: structural health system limitations overshadow its effect on follow-up of HIV-exposed infants. BMC Health Serv Res 2013;13:207 10.1186/1472-6963-13-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Odeny TA, Bukusi EA, Cohen CR, et al. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS 2014;28:2307–12. 10.1097/QAD.0000000000000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rotheram-Borus MJ, Tomlinson M, le Roux IM, et al. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLoS One 2014;9:e105934 10.1371/journal.pone.0105934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rustagi AS, Gimbel S, Nduati R, et al. Implementation and operational research: impact of a systems engineering intervention on PMTCT service delivery in côte d’Ivoire, Kenya, Mozambique: a cluster randomized trial. J Acquir Immune Defic Syndr 2016;72:e68–76. 10.1097/QAI.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024907supp002.pdf (103KB, pdf)
bmjopen-2018-024907supp001.pdf (103KB, pdf)
bmjopen-2018-024907supp003.pdf (176.8KB, pdf)