Abstract
Objective
This post hoc, pooled, subgroup analysis of two randomised studies evaluated baseline characteristics that may influence the efficacy and safety of naldemedine in patients with opioid-induced constipation (OIC) and cancer.
Methods
Data for patients who received 0.2 mg naldemedine or placebo were pooled from randomised, placebo-controlled, phase IIb and phase III studies. Proportions of spontaneous bowel movement (SBM) responders and patients with diarrhoea were assessed for each treatment group. For the patient subgroups with or without possible blood–brain barrier (BBB) disruptions, changes in Numerical Rating Scale (NRS) and Clinical Opioid Withdrawal Scale (COWS) scores were assessed.
Results
A total of 307 patients were included in this analysis (naldemedine: n=155; placebo: n=152). The pooled proportion of SBM responders was 73.5% with naldemedine versus 35.5% with placebo. There was a significant increase in the proportion of SBM responders with naldemedine versus placebo (38.0% (95% CI 27.6% to 48.4%); p<0.0001). Greater proportions of SBM responders and patients who experienced diarrhoea were observed with naldemedine versus placebo in all subgroups. Changes from baseline in NRS and COWS scores were similar with naldemedine or placebo in patients with or without brain metastases.
Conclusions
Although not powered to detect statistically significant differences in treatment effect among subgroups, this study demonstrated that naldemedine appeared to benefit patients with OIC and cancer, irrespective of baseline characteristics, and did not seem to affect analgesia or withdrawal–even in patients with potential BBB disruptions. Baseline characteristics did not appear to affect the incidence of diarrhoea in patients who received naldemedine.
Trial registration numbers
JapicCTI-111510 and JapicCTI-132340.
Keywords: opioid-induced constipation, cancer pain, naldemedine, spontaneous bowel movement, subgroup analysis
Key questions.
What is already known about this subject?
Opioid-induced constipation (OIC) is one of the most common adverse events associated with the use of opioid analgesics and has a significant negative impact on patients’ health-related quality of life.
Naldemedine is a peripherally acting μ-opioid receptor antagonist that is approved to treat OIC in adults with chronic non-cancer pain (in the USA and Japan) or in patients with cancer (in Japan).
In two randomised, placebo-controlled studies (phase IIb and phase III), treatment with naldemedine resulted in significantly greater proportions of spontaneous bowel movement (SBM) responders and greater changes from baseline in SBM frequency per week compared with placebo.
What does this study add?
This pooled, post hoc, subgroup analysis of two randomised, placebo-controlled studies of naldemedine evaluated baseline characteristics that may influence the efficacy and safety of naldemedine in patients with OIC and cancer.
Naldemedine, at a dose of 0.2 mg, appeared to benefit patients with OIC and cancer irrespective of baseline characteristics.
Naldemedine, at a dose of 0.2 mg, did not appear to affect analgesia or withdrawal, as measured by Numerical Rating Scale and Clinical Opioid Withdrawal Scale, even in patients with potential blood–brain barrier disruptions.
How might this impact on clinical practice?
This study provides additional safety and efficacy data for naldemedine in the treatment of OIC, with the potential to contribute to the relief of iatrogenic suffering in patient receiving opioid therapy.
Introduction
Opioid agonists, such as morphine and oxycodone, effectively modulate pain by binding μ-opioid receptors in the central and peripheral nervous systems and are typically the first choice for physicians in the treatment of cancer pain.1 2 Opioids, however, can bind and activate enteric μ-opioid receptors, leading to opioid-induced constipation (OIC).3 OIC is one of the most common adverse events (AEs) associated with the use of opioid analgesics and has a significantly negative impact on patients’ health-related quality of life.3–5
Laxatives are commonly used as a first-line treatment for OIC; however, they do not treat the underlying cause of the condition, are ineffective in >50% of patients and can have varying efficacy depending on the class of opioid used.3 6 7 As an example, lubiprostone—a prescription-strength laxative approved by the US Food and Drug Administration (FDA) for the treatment of OIC8 9—provides significant improvement in responder rates for patients receiving phenanthrene (eg, morphine, oxycodone or hydromorphone) or phenylpiperidine opioids (eg, fentanyl or remifentanil), but not diphenylheptane opioids (methadone or propoxyphene).10 Another class of therapeutics, known as ‘peripherally acting μ-opioid receptor antagonists’ (PAMORAs), target opioid receptors in the gastrointestinal tract, with minimal penetration across the blood–brain barrier (BBB), thereby reducing OIC without affecting analgesia.3 6 9
Naldemedine is a PAMORA that is approved to treat OIC in adults with chronic non-cancer pain (in the USA and Japan) or in patients with cancer (in Japan).11 12 In a systematic review and meta-analysis of randomised controlled clinical trials of pharmacological therapies for the treatment of OIC, naldemedine demonstrated greater efficacy for the treatment of OIC (defined as ≥3 bowel movements (BMs) per week and an increase of ≥1 BM per week over baseline) versus other therapies.13
In two randomised, placebo-controlled studies (phase IIb and phase III), treatment with naldemedine resulted in significantly greater proportions of spontaneous bowel movement (SBM) responders and greater changes from baseline in SBM frequency per week compared with placebo.14 15 In both studies, the safety profiles of naldemedine were similar and the most common treatment-emergent AE was diarrhoea.14 15 Although naldemedine did not increase opioid withdrawal in either study, as measured by the Clinical Opioid Withdrawal Scale (COWS) scoring method, patients with a disrupted BBB are thought to be at increased risk for opioid withdrawal or reduced analgesia.11 14–16
The efficacy and safety of PAMORAs, much like the efficacy and safety of laxatives, may be affected by patient characteristics and/or the types of opioids used to treat patients. For example, in clinical trials, treatment with the FDA-approved PAMORA naloxegol resulted in an increased incidence of gastrointestinal AEs in patients receiving methadone compared with other opioids.17 The specific factors that may influence the efficacy and safety of naldemedine have not been well studied. Here, we present the results of a pooled, post hoc, subgroup analysis of two randomised, placebo-controlled studies of naldemedine14 15 to evaluate factors that may influence the efficacy and safety of naldemedine in patients with OIC and cancer.
Methods
Study design
This pooled analysis comprises data from two randomised, double-blind, placebo-controlled studies (phase IIb (JapicCTI-11151015) and phase III (JapicCTI-13234014)). In both studies, patients with cancer and OIC were enrolled and received once-daily oral naldemedine (0.1, 0.2 or 0.4 mg (phase IIb); 0.2 mg (phase III)) or placebo for 14 days. The study designs for the phase IIb and phase III studies have been previously published.14 15 Briefly, eligible patients were aged ≥18 years (phase IIb) or ≥20 years (phase III) and had been given a diagnosis of cancer that did not directly affect gastrointestinal function, had OIC (defined as experiencing, during the 2 weeks prior to randomisation, ≤5 SBMs and straining, incomplete evacuation and/or hard stools in ≥25% of all BMs), had an Eastern Cooperative Oncology Group performance status ≤2 and had received opioids for ≥2 weeks prior to screening.
Both studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice and the Declaration of Helsinki and were approved by corresponding institutional review boards.14 15 Before the start of each study, all patients provided written informed consent.
Outcome measures
In the phase IIb study, the primary endpoint was the change from baseline in SBM frequency per week.15 Secondary endpoints included the proportion of SBM responders (defined as subjects with ≥3 SBMs per week and an increase of ≥1 SBM per week from baseline), change from baseline in SBM frequency without straining per week and change from baseline in SBMs with a feeling of complete evacuation (complete spontanteous bowel movements (CSBMs)) per week.15 In the phase III study, the primary endpoint was the proportion of SBM responders.14 Secondary endpoints included the change from baseline in SBM frequency per week, change from baseline in SBM frequency without straining per week and change from baseline in CSBMs per week.14 In both studies, safety-related endpoints were evaluated by recording AEs with severity grades according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, Numerical Rating Scale (NRS) scores of pain intensity and assessments of opioid withdrawal by the COWS scoring method.14 15 Primary and secondary endpoints and safety outcomes have been previously reported separately.14 15
Subgroup analysis
In this post hoc subgroup analysis, patients who received 0.2 mg naldemedine or placebo in the phase IIb or III studies were pooled and analysed according to the overall population and by subgroup. The differences in the proportions of SBM responders and the proportions of patients with diarrhoea were assessed. Specific subgroups included age (<65 years, ≥65 years or ≥75 years), body mass index (<18.5 kg/m2, ≥18.5 kg/m2 to <25 kg/m2 or ≥25 kg/m2), sex (male or female), prior opioid type (oxycodone, morphine or fentanyl), average oral morphine equivalent total daily dose (TDD) of opioid at baseline (<60 mg, ≥60 mg to <120 mg or ≥120 mg), prior opioid administration route (oral or transdermal), the use of prior regular laxative (yes or no), the prior regular laxative type used (magnesium oxide, sennoside A+B or other), prior anticancer therapy (yes or no) and possible BBB disruption (defined as patients with the presence of brain metastasis; yes or no). Additionally, changes from baseline in NRS and COWS scores for patients with a possible disruption to the BBB were assessed for each treatment group.
Statistical methods
Efficacy analyses were conducted in the full analysis set (defined as all patients who were randomly assigned and received study treatment (0.2 mg naldemedine or placebo) and for whom any efficacy data were obtained). Safety analyses were conducted in the safety analysis set (defined as all patients who were randomly assigned and received study treatment). Both efficacy and safety analyses were conducted for the overall population and according to the subgroups listed. Data from the phase IIb study for patients who received 0.1 mg or 0.4 mg naldemedine were not included in either analysis set. The differences in the proportions of SBM responders with naldemedine versus placebo and their 95% CIs were calculated using the method by Koch et al 18 and are shown as a forest plot for the overall pooled population and by subgroup. The proportions of patients with diarrhoea based on CTCAE were calculated similarly. The CTCAE terminology was chosen to define diarrhoea to eliminate any potential effects on the reported incidence of diarrhoea from the primary phase III and phase IIb studies (ie, patient-reported BMs with a score of 7 on the Bristol Stool Form Scale in the phase IIb study versus patient-reported diarrhoea in the phase III study). All statistical analyses were conducted by using SAS software (V.9.2).
Changes from baseline in NRS and COWS scores at each timepoint were analysed for the subgroup of patients with or without a possible BBB disruption by mixed-effects model repeated measures with baseline and study as the covariates, and treatment group, time and time-by-treatment group interactions as fixed effects. The covariance matrix for the time factor was assumed to be unstructured. Plots of least squares means of changes and their 95% CIs over time were presented by treatment group for the pooled studies within each subgroup.
Results
Patients
A total of 307 patients were included in this pooled, post hoc, subgroup analysis (naldemedine: n=155; placebo: n=152). The baseline characteristics and patient demographics were relatively well balanced between treatment groups and across both studies (table 1). Overall, the most frequent primary tumour location was in the lung, present in 40.6% of patients who received naldemedine and 49.3% of patients who received placebo. Patient dispositions for the primary studies have been previously published.14 15
Table 1.
Patient demographics and baseline characteristics
| Parameter | Phase IIb15 | Phase III14 | Pooled | |||
| Naldemedine (n=58) | Placebo (n=56) |
Naldemedine (n=97) | Placebo (n=96) |
Naldemedine (n=155) | Placebo (n=152) |
|
| Mean age, years (SD) | 63.4 (10.4) | 64.2 (9.6) | 63.8 (9.4) | 64.6 (11.8) | 63.7 (9.7) | 64.4 (11.0) |
| Age category, years, n (% ) | ||||||
| <40 | 3 (5.2) | 1 (1.8) | 1 (1.0) | 3 (3.1) | 4 (2.6) | 4 (2.6) |
| ≥40–<65 | 26 (44.8) | 25 (44.6) | 49 (50.5) | 42 (43.8) | 75 (48.4) | 67 (44.1) |
| ≥65 | 29 (50.0) | 30 (53.6) | 47 (48.5) | 51 (53.1) | 76 (49.0) | 81 (53.3) |
| ≥75 | 4 (6.9) | 5 (8.9) | 9 (9.3) | 26 (27.1) | 13 (8.4) | 31 (20.4) |
| Sex, n (%) | ||||||
| Male | 34 (58.6) | 34 (60.7) | 59 (60.8) | 60 (62.5) | 93 (60.0) | 94 (61.8) |
| Female | 24 (41.4) | 22 (39.3) | 38 (39.2) | 36 (37.5) | 62 (40.0) | 58 (38.2) |
| Mean BMI, kg/m2 (SD) | 21.8 (2.96) | 21.5 (3.66) | 21.5 (3.59) | 20.8 (3.63) | 21.6 (3.36) | 21.1 (3.64) |
| I category, kg/m2, n (% ) | ||||||
| <18.5 | 9 (15.5) | 9 (16.1) | 17 (17.5) | 26 (27.1) | 26 (16.8) | 35 (23.0) |
| ≥18.5–<25.0 | 39 (67.2) | 40 (71.4) | 67 (69.1) | 59 (61.5) | 106 (68.4) | 99 (65.1) |
| ≥25.0–<30.0 | 10 (17.2) | 5 (8.9) | 11 (11.3) | 10 (10.4) | 21 (13.5) | 15 (9.9) |
| ≥30.0 | 0 | 2 (3.6) | 2 (2.1) | 1 (1.0) | 2 (1.3) | 3 (2.0) |
| Primary tumour location, n (% ) | ||||||
| Lung | 21 (36.2) | 30 (53.6) | 42 (43.3) | 45 (46.9) | 63 (40.6) | 75 (49.3) |
| Breast | 13 (22.4) | 13 (23.2) | 22 (22.7) | 17 (17.7) | 35 (22.6) | 30 (19.7) |
| Large intestine | 3 (5.2) | 0 | 3 (3.1) | 3 (3.1) | 6 (3.9) | 3 (2.0) |
| Other | 21 (36.2) | 13 (23.2) | 30 (30.9) | 31 (32.3) | 51 (32.9) | 44 (28.9) |
| Race, n (% ) | ||||||
| Asian | 58 (100.0) | 56 (100.0) | 97 (100.0) | 96 (100.0) | 155 (100.0) | 152 (100.0) |
| Prior use, n (% ) | ||||||
| Anticancer drugs | 36 (62.1) | 34 (60.7) | 72 (74.2) | 62 (64.6) | 108 (69.7) | 96 (63.2) |
| Routine laxatives | 58 (100.0) | 56 (100.0) | 72 (74.2) | 74 (77.1) | 130 (83.9) | 130 (85.5) |
| Rescue laxatives | 56 (96.6) | 54 (96.4) | 93 (95.9) | 89 (92.7) | 149 (96.1) | 143 (94.1) |
|
Mean (SD) daily dose of
opioids,* mg |
82.3 (87.2) | 85.5 (98.5) | 57.3 (46.4) | 69.5 (99.5) | 66.7 (65.6) | 75.4 (99.1) |
| Total daily dose of opioids,* n (% ) | ||||||
| <60 mg | 31 (53.4) | 28 (50.0) | 50 (51.5) | 57 (59.4) | 81 (52.3) | 85 (55.9) |
| ≥60–<120 mg | 13 (22.4) | 15 (26.8) | 34 (35.1) | 25 (26.0) | 47 (30.3) | 40 (26.3) |
| ≥120 mg | 14 (24.1) | 13 (23.2) | 13 (13.4) | 14 (14.6) | 27 (17.4) | 27 (17.8) |
| Prior opioid type used, n (% ) | ||||||
| Oxycodone | 41 (70.7) | 34 (60.7) | 67 (69.1) | 68 (70.8) | 108 (69.7) | 102 (67.1) |
| Morphine | 8 (13.8) | 9 (16.1) | 7 (7.2) | 8 (8.3) | 15 (9.7) | 17 (11.2) |
| Fentanyl | 7 (12.1) | 14 (25.0) | 22 (22.7) | 22 (22.9) | 29 (18.7) | 36 (23.7) |
| Possible BBB disruption,† n (%) | 13 (22.4) | 10 (17.9) | 9 (9.3) | 9 (9.4) | 22 (14.2) | 19 (12.5) |
Parts of this table were data used from previously published reports by the authors. Adapted with permission from: Katakami N, et al. J Clin Oncol 2017;35:3859–66; and Katakami N, et al. J Clin Oncol 2017;35:1921–8.
*Oral morphine equivalent.
†Defined as the presence of brain metastasis.
BBB, blood–brain barrier; BMI, body mass index; SD, standard deviation.
Efficacy
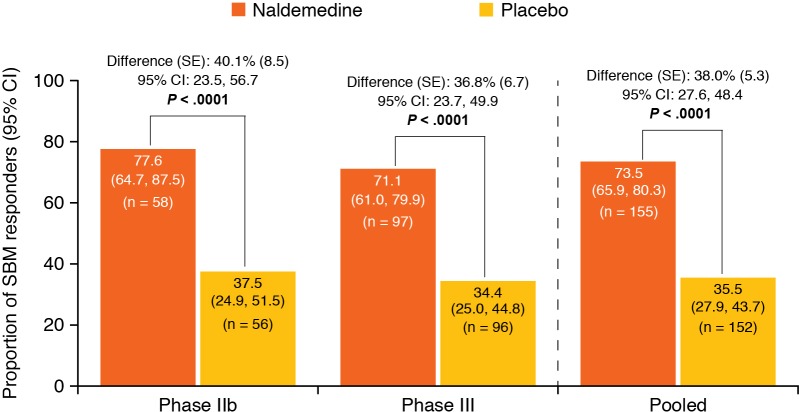
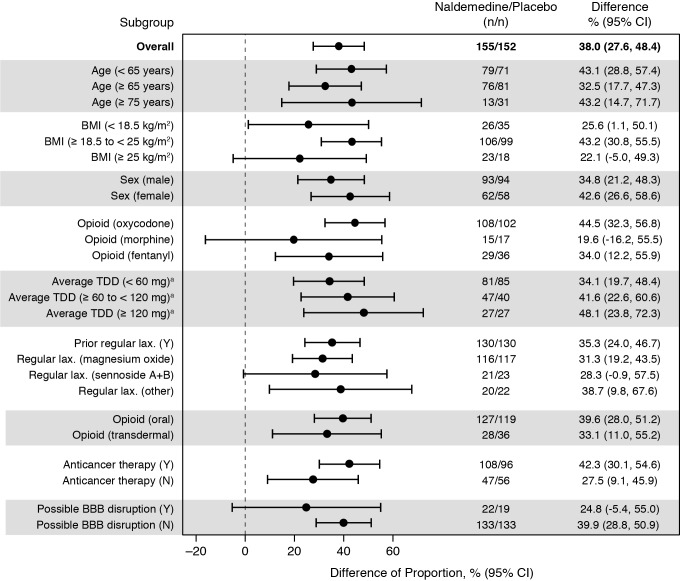
The pooled proportion of SBM responders was 73.5% (114/155) for patients who received naldemedine and 35.5% (54/152) for patients who received placebo (figure 1), and there was a significant difference in the proportions of SBM responders between treatment groups (38.0% (95% CI 27.6% to 48.4%); p<0.0001). In all subgroups evaluated, the point estimates for the differences in the proportions of responders between treatment groups were greater than zero (figure 2). Specifically, in the opioid subgroups, the differences in the proportions of responders with naldemedine vs placebo were 44.5% (95% CI 32.3% to 56.8%) for the patients who received oxycodone, 19.6% (95% CI −16.2% to 55.5%) for the patients who received morphine and 34.0% (95% CI 12.2% to 55.9%) for the patients who received fentanyl. The differences in the proportions of responders who received naldemedine versus placebo decreased numerically as the average oral morphine equivalent TDD of opioids decreased. For patients who received an oral morphine equivalent TDD of ≥120 mg, the difference was 48.1% (95% CI 23.8% to 72.3%), whereas for patients who received an oral morphine equivalent TDD of ≥60–≤120 mg, the difference was 41.6% (95% CI 22.6% to 60.6%), and for patients who received an oral morphine equivalent TDD of <60 mg, the difference was 34.1% (95% CI 19.7% to 48.4%). The difference in the proportions of responders who received naldemedine vs placebo was greater in the subgroup of patients with anticancer treatment (42.3% (95% CI 30.1% to 54.6%)) than in the subgroup of patients without anticancer treatment (27.5% (95% CI 9.1% to 45.9%)).
Figure 1.
Proportions of SBM responders and differences of proportions of SBM responders during the 2-week treatment period (full analysis set). SBM, spontaneous bowel movement; SE; standard error. Adapted with permission from: Katakami N, et al. J Clin Oncol 2017;35:3859–66; and Katakami N, et al. J Clin Oncol 2017;35:1921–8.
Figure 2.
Differences in the proportions of SBM responders by subgroups (full analysis set). aOral morphine equivalent. BBB, blood–brain barrier; BMI, body mass index; LAX; laxative; N, no; SBM, spontaneous bowel movement; TDD, total daily dose at baseline; Y, yes.
Safety
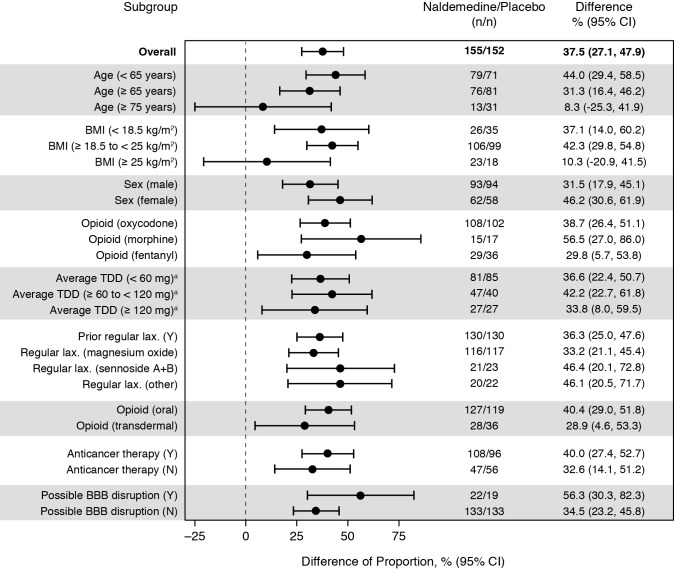
Overall, a higher proportion of patients who received naldemedine compared with placebo had diarrhoea (difference in proportions: 37.5% (95% CI 27.1% to 47.9%)) (figure 3). In all subgroups evaluated, the point estimates for the differences in the proportions of patients who experienced diarrhoea between treatment groups were greater than zero. The difference in the proportions of patients with diarrhoea who received naldemedine versus those who received placebo was larger in the subgroup of patients with a possible disruption in BBB (difference of proportions: 56.3% (95% CI 30.3% to 82.3%)) than in the subgroup of patients without a disruption in the BBB (difference of proportions: 34.5% (95% CI 23.2% to 45.8%)).
Figure 3.
Differences in the proportions of patients with diarrhoea based on Common Terminology Criteria for Adverse Events by subgroups (safety analysis set). aOral morphine equivalent. BBB, blood–brain barrier; BMI, body mass index; LAX, laxative; N, no; TDD, total daily dose at baseline; Y, yes.
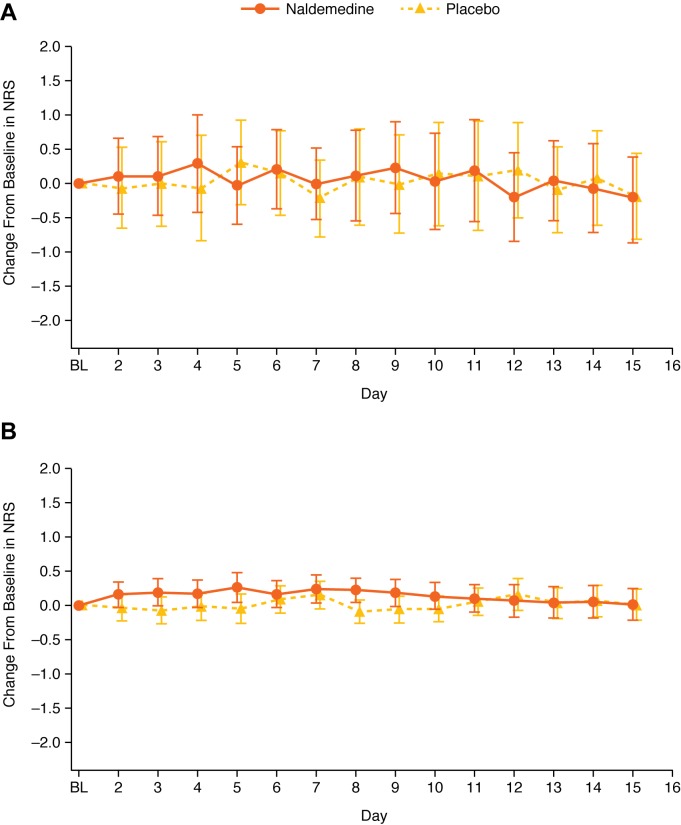
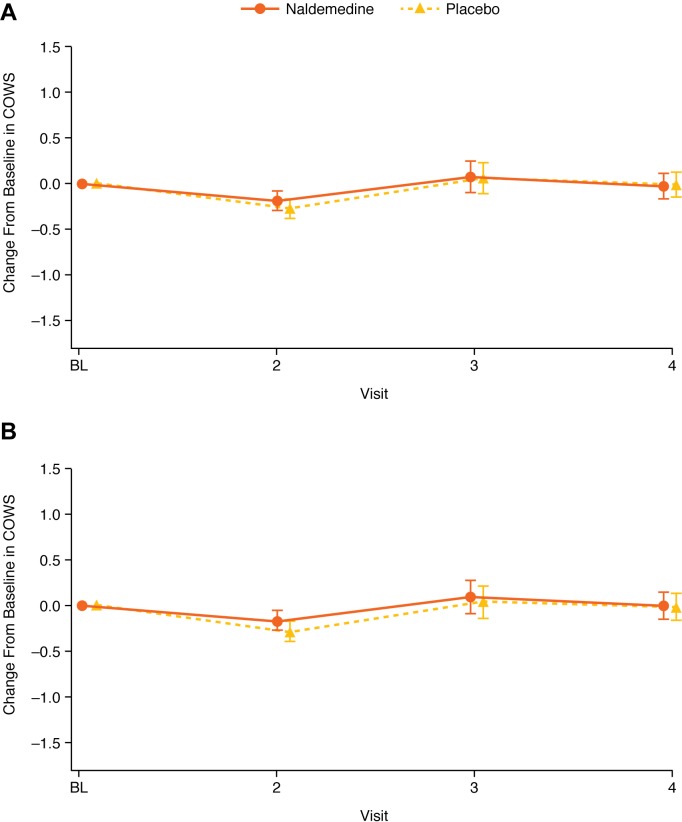
Further analyses in the subgroup of patients with a possible BBB disruption were conducted to evaluate the potential effect of naldemedine on pain and opioid withdrawal. Changes from baseline in NRS scores and COWS scores were similar between treatment groups, irrespective of potential BBB disruption (figures 4 and 5). In patients with a possible BBB disruption, the maximum absolute difference in the change from baseline between treatment groups was 0.39 for NRS scores and 0.27 for COWS scores. In patients without a disruption in BBB, the maximum absolute difference in the change from baseline between treatment groups was 0.32 for NRS scores and 0.12 for COWS scores.
Figure 4.
Change from baseline in NRS scores in patients with possible disruption (A) or without possible disruption (B) to BBB (LS mean; error bars are 95% CI) (safety analysis set). BBB, blood–brain barrier; BL, baseline; LS, least squares; NRS, Numerical Rating Scale.
Figure 5.
Change from baseline in COWS scores in patients with possible disruption (A) or without possible disruption (B) to BBB (LS mean; error bars are 95% CI) (safety analysis set). BBB, blood–brain barrier; BL, baseline; COWS, Clinical Opiate Withdrawal Scale; LS, least squares.
Discussion
In this pooled, post hoc, subgroup analysis of two randomised clinical studies of naldemedine, the proportion of SBM responders and the incidence of diarrhoea in the various subgroups evaluated were generally consistent with that observed in the overall pooled patient population. These data suggest that there were no obvious baseline characteristics that affected the efficacy or safety of 0.2 mg naldemedine in patients with OIC and cancer.
Several randomised studies have concluded that minimal amounts of naldemedine cross the BBB; however, patients with a disrupted BBB are thought to be at increased risk for opioid withdrawal or reduced analgesia.14 19 20 In this analysis, the differences in the proportions of patients with diarrhoea who received naldemedine versus those who received placebo were relatively large for patients with or without a potential BBB disruption. Although diarrhoea can be a symptom of opioid withdrawal,21 no other notable symptoms of withdrawal were observed in this analysis. Specifically, treatment with either 0.2 mg naldemedine or placebo resulted in similar changes from baseline in both NRS and COWS scores in patients with or without brain metastases (a proxy for possible BBB disruption). Moreover, in this patient subgroup, the maximum absolute differences from baseline in NRS and COWS scores were small. These results suggest that naldemedine may demonstrate efficacy without reducing the analgesic effects of opioids, even in patients with BBB disruption. However, data for this particular subgroup should be interpreted with caution because of the small number of patients (n=41) and the wide 95% CIs observed, and because not all brain metastases are associated with a disrupted BBB.22 Further evaluations of the efficacy and safety of naldemedine in a larger population of patients with a known disruption of the BBB are needed to better understand the potential effects of naldemedine in this patient subgroup.
This pooled post hoc analysis was not powered to detect statistically significant differences between subgroups. Many of the subgroups were particularly small (n<40 in either treatment group), including those with patients: aged ≥75 years, with BMI <18.5 or ≥25 kg/m2, who received morphine or fentanyl, who received an average oral morphine equivalent TDD of opioid ≥120 mg, who received sennoside A+B or laxatives other than sennoside A+B or magnesium oxide, who received transdermal opioids, and patients with a possible BBB disruption.
Overall, in these two clinical studies, naldemedine at a dose of 0.2 mg appeared to benefit patients with OIC and cancer regardless of baseline characteristics, and baseline characteristics did not appear to affect the incidence of diarrhoea in patients who received naldemedine.
Acknowledgments
All authors had complete access to the data that support this publication. The authors would like to thank all patients who participated in the primary studies.
Footnotes
Contributors: IO, HI, TY, YT, HS and ES contributed to the conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript and agree to be accountable for all aspects of the work, which includes ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by Shionogi & Co, Ltd, Osaka, Japan. Medical writing support was provided by Jeffrey Bratz, PhD, of Oxford PharmaGenesis Inc, Newtown, Pennsylvania, USA, and was funded by Shionogi & Co, Ltd, Osaka, Japan.
Competing interests: IO has received lecture fees and non-financial support from Shionogi & Co, Ltd. HI has received non-financial support from Shionogi & Co, Ltd. TY, YT, HS and MO are employees of Shionogi & Co, Ltd. ES has received lecture fees and non-financial support from Shionogi & Co., Ltd.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Rosenblum A, Marsch LA, Joseph H, et al. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol 2008;16:405–16. 10.1037/a0013628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider G, Voltz R, Gaertner J. Cancer pain management and bone metastases: an update for the clinician. Breast Care 2012;7:113–20. 10.1159/000338579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95. 10.1111/nmo.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007;61:1181–7. 10.1111/j.1742-1241.2007.01415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and wellness survey. J Opioid Manag 2009;5:137–44. 10.5055/jom.2009.0014 [DOI] [PubMed] [Google Scholar]
- 6. Brenner DM, Chey WD. An evidence-based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl 2014;2:38–46. 10.1038/ajgsup.2014.8 [DOI] [Google Scholar]
- 7. Emmanuel A, Johnson M, McSkimming P, et al. Laxatives do not improve symptoms of opioid-induced constipation: results of a patient survey. Pain Med 2017;18:1932–40. 10.1093/pm/pnw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Center for Drug Evaluation and Research Amitiza (lubiprostone) [package insert]. Rockville, MD: Sucampo Pharma Americas, LLC and Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2018. [Google Scholar]
- 9. Nee J, Zakari M, Sugarman MA, et al. Efficacy of treatments for opioid-induced constipation: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:1569–84. 10.1016/j.cgh.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 10. Webster LR, Brewer RP, Lichtlen P, et al. Efficacy of lubiprostone for the treatment of opioid-induced constipation, analyzed by opioid class. Pain Med 2018;19:1195–205. 10.1093/pm/pnx212 [DOI] [PubMed] [Google Scholar]
- 11. Shionogi Symproic (naldemedine) [package insert]. Florham Park, NJ: Shionogi Inc, 2018. [Google Scholar]
- 12. Pharmaceuticals and Medical Devices Agency Report on the deliberation results: Symproic tablets 0.2 Mg, 2017. Available: https://www.pmda.go.jp/files/000222647.pdf [Accessed 30 Nov 2018].
- 13. Luthra P, Burr NE, Brenner DM, et al. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and network meta-analysis. Gut 2018. 10.1136/gutjnl-2018-316001. [Epub ahead of print: 05 May 2018]. [DOI] [PubMed] [Google Scholar]
- 14. Katakami N, Harada T, Murata T, et al. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J Clin Oncol 2017;35:3859–66. 10.1200/JCO.2017.73.0853 [DOI] [PubMed] [Google Scholar]
- 15. Katakami N, Oda K, Tauchi K, et al. Phase IIB, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol 2017;35:1921–8. 10.1200/JCO.2016.70.8453 [DOI] [PubMed] [Google Scholar]
- 16. Kaufman MB. Pharmaceutical approval update. P T 2017;42:502–4. [PMC free article] [PubMed] [Google Scholar]
- 17. Movantik (naloxegol) tablets [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2018. [Google Scholar]
- 18. Koch GG, Carr GJ, Amara IA, et al. Categorical data analysis : Berry DA, Statistical Methodology in the Pharmaceutical Sciences. Boca Raton, FL: CRC Press, Taylor & Francis Group, 1989. [Google Scholar]
- 19. Hale M, Wild J, Reddy J, et al. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol 2017;2:555–64. 10.1016/S2468-1253(17)30105-X [DOI] [PubMed] [Google Scholar]
- 20. Webster LR, Nalamachu S, Morlion B, et al. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain 2018;159:987–94. 10.1097/j.pain.0000000000001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol 2014;33:561–7. 10.1177/0960327112450901 [DOI] [PubMed] [Google Scholar]
- 22. Yonemori K, Tsuta K, Ono M, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010;116:302–8. 10.1002/cncr.24735 [DOI] [PubMed] [Google Scholar]