Abstract
This review article is an overview of the session at the European Society for Medical Oncology (ESMO) Asia 2018 Congress entitled: 'Cancer medicines in Asia and Asia-Pacific: What is available, and is it effective enough?'. The article provides an overview of the session speakers’ views on the impact that the lack of accessibility and availability of medicines has on patient outcomes in the treatment of breast cancer, colorectal cancer and lung cancer, responsible for more than one-third of cancer deaths in the Asian region. It also lists the various global policy initiatives that ESMO supports to promote the best cancer care in the Asian and Asia-Pacific region. The review presents extrapolated data from the ‘ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe’, which reveals several disparities among Asian countries, across the different income levels. In low- and middle-income countries, some barriers to the accessibility of anticancer medicines include the lack of government reimbursement, budget allocation for healthcare and quality-assured generic and biosimilar medicines, as well as shortages and patent rights. Throughout the article, the session presenters provide their views on strategies that can be considered to overcome these barriers.
Keywords: cancer medicines, availability of cancer medicines in Asia and Asia-Pacific, reimbursement of medicines, ESMO-MCBS, WHO Model List of Essential Medicines
Introduction
Cancer is the leading cause of death and disability worldwide. According to the 2018 Global Cancer Observatory (GCO), produced by the International Agency for Research on Cancer, there will be an estimated 18.1 million new cancer cases and 9.6 million cancer deaths in 2018, of which 3.5 million of the new cases and 1.9 of the cancer deaths are estimated in less-developed countries.1
Globally, Asia will see nearly half of the new cases and more than half of the cancer deaths in 2018, partly because the region has almost 60% of the world’s population and partly because the region has a higher frequency of certain cancer types, such as liver and stomach cancer, that are associated with poorer prognosis and higher mortality rates. In addition, restricted prevention strategies are in place in some Asian countries, including limited hepatitis B vaccination along with limited access to timely diagnosis and treatment.1
According to estimates of the GCO, of the 29.5 million new cancer cases expected by 2040, 30%–50% are avoidable with cancer prevention, which means nearly 15 million lives could be saved. Indeed, the implementation of cancer prevention strategies would result in the reduction of global cancer mortality, supporting the fulfilment of the United Nations (UN) 2030 Sustainable Development Goal 3.4 to reduce cancer deaths by one-third by 2030. Seventy percent of the lives saved would be in low- and middle-income countries.2
Of the 24 million new cancer cases in 2030, more than 20% of all malignant tumours and 25% of cancer diagnosis in low- and middle-income countries can be attributed to tobacco and human papilloma virus, respectively.2
The estimated total annual economic cost of cancer is significant and is increasing. In 2010, it was approximately US$ 1.16 trillion, the equivalent of more than 2% of the total global gross domestic product (GDP).3 Even this impressively high figure does not include the additional substantial longer-term costs to families and caregivers.3
Investing strategically in cancer care and control has a high return on investment. A reasonable estimate shows that the world could have saved between US$ 100 billion and US$ 200 billion in 2010 by investing in prevention, early detection and effective treatment of cancer.3
Cancer management requires a comprehensive framework and multidisciplinary care. The main drivers of cost across the cancer continuum of care are the cancer workforce, medical devices and technology, cancer medicines, infrastructure utilisation costs, and capital investment. For this reason, it is imperative to invest and prioritise cancer financial resources within a coherent, comprehensive and consistent national cancer control plan with a valuable evaluation and monitoring framework, including a strategy for the procurement and reimbursement of cancer medicines.4 Indeed, where financial investments for cancer care are not prioritised, a general decrease in cancer-related patient outcomes is observed. This often results in catastrophic health expenditures due to out-of-pocket expenses for cancer patients and their families.5 When ranking all countries by cancer mortality-to-incidence ratio (MIR), an indicator of health system performance, there is a clear correlation between health expenditure and MIR. In Asia, for instance, wealthy countries register lower values of MIR (i.e. Australia, MIR=0.38) than resource-limited areas (ie, Mongolia, Tajikistan or Kyrgyzstan, MIR>0.7).6 As a consequence of the lack of adequate health financing in poor-resources countries, cancer health services require substantial out-of-pocket costs by patients, widening the gap between affordable cancer care and increased cancer mortality.
Access to newer, targeted agents is often limited because of the unavailability of molecular companion diagnostic tests. In the Asian population, a high level of pathogenetic mutations of epidermal growth factor receptor (EGFR) in advanced non-small-cell lung carcinoma (NSCLC) patients is observed (up to 50% of cases), potentially warranting EGFR-targeting therapy7 with tyrosine-kinase inhibitors, often available as generics.8 However, the lack of molecular testing often represents an obstacle for appropriate use of these therapies. In fact, in 2016, a global survey-based study reported that in 49 countries, only a minority (40%) have access to sustained availability of EGFR testing, with uneven availability in Southeast Asian countries, where the testing is available only in selected subnational laboratories.9 Interestingly, the affordability of the companion diagnostic test for EGFR seemed to be correlated to the Human Development Index, which ranks countries on levels of life expectancy, education, and per capita income indicators. Those countries with lower per capita income faced higher out-of-pocket expenses related to the cost of the diagnostic test.9
The affordability and accessibility of effective and quality cancer medicines remains a serious challenge for the sustainability of health systems, including the issues of medicine shortages and out-of-pocket expenses for patients.
The aim of this review article is to provide an overview of the authors’ views of the impact that the lack of accessibility and availability of medicines has on patient outcomes in the treatment of breast, colorectal and lung cancer (BCL), responsible for more than one-third of cancer deaths, in the World Health Organization (WHO) Regions of Southeast Asia, Western Pacific and the countries on the Asian continent in the WHO Eastern Mediterranean Region.1 The review presents extrapolated data from the ‘ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe’. The analysis also highlights the gaps between the most effective treatments for cancer, included in the ESMO Clinical Practice Guidelines,10 and the medicines available and accessible in several Asian countries.
Access to cancer medicines: a global policy issue
Access to quality and affordable medicines is a major issue in public health policy. Both the WHO and the United Nations have adopted resolutions, programmes and tools to ensure adequate supplies and access to safe, affordable, and effective quality medicines. The main recent initiatives and resolutions on access to medicines are described in table 1.
Table 1.
Recent WHO and United Nations initiatives and resolutions on access to medicines
| When | What | Why | Objective |
| Since 1977 | WHO Model List of Essential Medicines (EML)51–53 | To provide an internationally recognisable set of selected, effective, safe and affordable medicines to help countries choose how to treat their priority health needs. | Serves as guidance for national authorities to develop their own national EMLs and to prioritise the reimbursement and sustainable supply of those medicines. The WHO EML Cancer Medicines Working Group recommended using the ESMO-Magnitude of Clinical Benefit Scale as a screening tool to identify candidate medicines potentially suitable for inclusion in the WHO EML. |
| 1998 | WHO Action Programme on Essential Drugs54 | To conceptualise the importance of providing EMLs. | Shows that the availability of medicines increases the credibility of health services, motivates health workers and positions non-governmental organisations as key stakeholders for technical support and country-level dialogue with governments. |
| 2014 | WHO Resolution on Access to Essential Medicines55 | To encourage WHO Member States to implement effective national medicines policies. | Ensures equity of access to affordable, safe, effective and quality-assured essential medicines, the use of scientific evidence for the selection of the medicines on national EMLs, and adherence to evidence-based clinical practice guidelines for the use of essential medicines in clinical practice. |
| 2015 | United Nations 2030 Sustainable Development Goals (SDGs)56 57 |
SDG 3 is related to health, with the objective of ensuring healthy lives and promoting well-being for all at all ages. | Sets targets UN Member States should achieve by the year 2030. The targets should address the public health issue of NCDs, which include cancer. Target 3.4’s goal is by the year 2030 to reduce by one-third premature mortality from NCDs through prevention and treatment, and to promote mental health and well-being. Target 3.8’s goal is to achieve universal health coverage, including financial risk protection, access to quality essential healthcare services, and access to safe, effective, quality and affordable essential medicines and vaccines for all. |
| 2017 | World Health Assembly Cancer Resolution 70.1258 | To specifically address cancer within the NCD family, and to complement other WHO resolutions on cancer and NCDs to ensure a comprehensive approach. | Addresses cancer prevention and control within an integrated approach. The resolution calls for the development and implementation of comprehensive national cancer control plans that cover the entire spectrum of cancer care from research to survivorship. |
| 2017 | WHO Prequalification of two biosimilars—trastuzumab and rituximab59 | To assess the safety and efficacy of biosimilars globally. The WHO prequalification process is the only global medicines quality assurance programme. | Supports national and global efforts to increase access to, and the affordability of, selected biotherapeutic products and their corresponding similar biotherapeutic products. This is a crucial development for biosimilars in oncology, providing a rigorous assessment with a tracked and transparent methodology, to inform the regulatory agencies about the prioritisation and selection of cancer medicines and the alternative quality and valuable molecules available that should also be considered. |
EMLs, essential medicines lists; NCDs, noncommunicable diseases; SDGs, Sustainable Development Goals.
Global commitments by national governments on access to medicines have increased in the last year, along with the publication of many reports. During the 2018 World Health Assembly, the WHO presented a report on ‘Addressing the global shortage of, and access to, medicines and vaccines’.11 Based on this report, the WHO was asked to develop a ‘Roadmap for access to medicines and vaccines 2019–2023’.12 In December 2018, the WHO published a technical report on 'Pricing of cancer medicines and its impacts'.13
Medicine shortages also present a major challenge related to access to medicines and can lead to less effective or more toxic treatments for cancer patients.14 Therefore, in May 2017, ESMO, together with The Economist Intelligence Unit, issued a report on how to prevent and manage shortages of essential medicines in Europe, with a list of six recommendations that can also provide guidance globally.14 One of those recommendations is that countries should establish a national strategic plan for medicine shortages, underpinned by national legislation and funding.14 Further pragmatic strategies were developed by an ESMO Leadership Generation Program paper in 2018 entitled ‘Global cancer control: responding to the growing burden, rising costs and inequalities in access’.15 One strategy is that countries at a minimum ensure that the cancer medicines on the WHO Model List of Essential Medicines are available and affordable for everyone.15
At the UN level, the 2018 UN Political Declaration on the prevention and control of noncommunicable diseases (NCDs)16 was adopted by all UN Member States in September 2018 and underlined the commitment by countries to strengthen and reorient health systems towards the achievement of universal health coverage, including access to essential diagnostics, medicines, vaccines, technologies and palliative care.
The commitment to ensure access to medicines is of particular importance at the global level in the WHO Southeast Asia Region, where countries adopted the 2018 Delhi Declaration17 to make essential medicines, vaccines, diagnostics and medical devices affordable and accessible to all, both within the region and beyond. The Declaration calls for the reduction of out-of-pocket cancer medicine payments by patients, and for strengthening national policies, regulation and supply chain management to improve access to medicines and vaccines.
These global resolutions, initiatives and reports underline the importance of the topic of access to medicines, and the repeated commitment by countries to adequately address it. However, the challenge remains today. To help shed light on this issue, ESMO collected first-of-its-kind data from its members and from oncology pharmacists around the world about their daily experience with access to cancer medicines. For the purpose of this review, data were extrapolated from the results of the ‘ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe’18 for countries in Asia, and are discussed in the sections below.
Medicines with the highest ESMO-Magnitude of Clinical Benefit Scale scores, and what is available in Asia and Asia-Pacific
The ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) 19 20 represents a critical step in addressing the public policy issue of the value of cancer care for the appropriate use of limited resources to deliver effective and affordable cancer care. It is a standardised, generic, validated approach to stratify and score the magnitude of clinical benefit that can be anticipated from anticancer therapies. Since 2016, new cancer medicines or indications approved by the European Medicines Agency have been scored and presented either in the ESMO Clinical Practice Guidelines where relevant, or as an ESMO e-Update. Medicines which obtain the highest scores, A and B (curative setting) or 5 and 4 (noncurative setting), are suggested as the highest priority for rapid endorsement by national bodies across Europe.
The availability and accessibility of cancer medicines is essential for medical oncologists to be able to treat patients with anticancer therapies and according to the evidence-based ESMO Clinical Practice Guidelines. However, challenges have risen within countries regarding the implementation of evidence-based clinical practice guidelines. The utilisation of guidelines by clinicians and the adaptability of guidelines to a country’s health system capacity requires a robust mechanism that can track the level of guideline adherence and aggregate data on patient treatment decisions.21 Evidence-based clinical guidelines may represent an indicator for the general status of healthcare, the type of interventions recommended and prioritised, and may help to identify gaps or insufficiencies in cancer care in the most critical steps of immediate patient care.
The ‘ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe’18 aimed to evaluate the following: (1) the availability on national formulary of licensed antineoplastic medicines across the globe; (2) patient out-of-pocket costs for the medications; (3) the actual availability of the medication for a patient with a valid prescription; (4) information relating to possible factors adversely impacting the availability of antineoplastic agents and (5) the impact of the country’s level of economic development on these parameters.
The study was led by the ESMO Global Policy Committee, and conducted in collaboration with the ESMO Executive Board, ESMO National Representatives and other ESMO committees, including those covering European Policy, Education, Practising Oncologists, as well as the ESMO Faculty. There were three external collaborating partners, the Union for International Cancer Control, the Institute of Cancer Policy of King’s College London and the European Society of Oncology Pharmacy. The WHO was a supporting partner in this project as part of an ongoing 3-year work plan between WHO and ESMO, who enjoys ‘official relations status’ with WHO since 2013.22 Implementation and data analysis of the survey results were performed by independent researchers from the collaborating partner organisations.
Study results on the availability of cancer medicines in Asia and Asia-Pacific
In the Asian cohort, surveys were submitted by 38 individual reporters from 18 countries (tables 2 and 3).
Table 2.
2015 ESMO International Consortium study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Number of field reporters for each Asian country that responded to the survey18
| Number of field reporters per country | Country |
| 5 | India, Japan |
| 3 | Myanmar, Korea (South), Singapore, Thailand |
| 2 | Malaysia, Nepal, Vietnam, Philippines |
| 1 | Afghanistan, Bangladesh, Cambodia, China, Indonesia, Iran, Kazakhstan, Pakistan |
Table 3.
2015 ESMO International Consortium study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Country demographics18
| Total countries | Surveyed countries | Per cent of countries surveyed in region (%) | Total population (billions) | Surveyed population (billions) | Per cent of surveyed population (%) | |
| Sub-Saharan Africa | 51 | 11 | 21.5 | 0.795 | 0.262 | 32.9 |
| Asia and India | 29 | 18 | 62.1 | 3.703 | 3.601 | 97.2 |
| North America | 5 | 2 | 40.0 | 0.332 | 0.332 | 100.0 |
| Oceania | 21 | 2 | 9.5 | 0.033 | 0.024 | 72.7 |
| Middle East | 16 | 12 | 80.0 | 0.195 | 0.153 | 78.4 |
| North Africa | 6 | 4 | 66.7 | 0.161 | 0.155 | 96.2 |
| Latin America and Caribbean | 45 | 14 | 31.1 | 0.562 | 0.502 | 89.3 |
| Total | 173 | 63 | 36.4 | 5.781 | 5.029 | 86.9 |
The country data are stratified according to the level of each country’s economic development based on Word Bank criteria23 classifying them as high-income country (HIC), upper middle-income country (UMIC), lower middle-income country (LMIC) and low-income country (LIC) and are presented alphabetically. For clarity of presentation, and to highlight findings of inequity and impact, the study focused on medications included in the WHO’s Model List of Essential Medicines,24 and recently approved medications by the US Food and Drug Administration and/or the European Medicines Agency, that are not on the WHO list but have an ESMO-MCBS score greater than two (2), corresponding to a moderate to high level of clinical benefit.19 20
By definition, medications included in the WHO’s Model List of Essential Medicine24 are highly effective and clinically beneficial. They are included in the evidence-based ESMO Clinical Practice Guidelines10 because they have a high level of benefit for patient outcomes, either alone or in combination with expensive innovative medicines, and many are used in the curative setting.
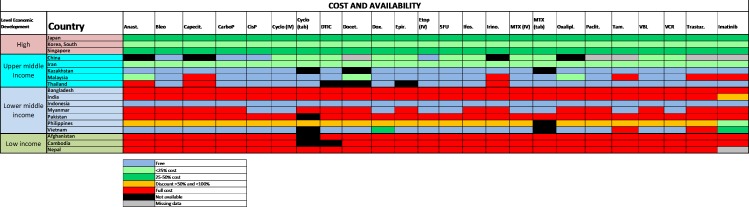
In HICs and in UMICs, most of these medicines are on formulary and are available to patients at a subsidised cost (figure 1). Overall in LMICs, and in LICs, reports of formulary deficiencies are greater, and the survey showed that in many countries, patients incur full out-of-pocket cost even for generic, inexpensive anticancer medications that are on the WHO Model List of Essential Medicines.18 These observations are most pertinent in Bangladesh, India, Myanmar, Pakistan, Afghanistan, Cambodia and Nepal.
Figure 1.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data for medications on the WHO Model List of Essential Medicines: Formulary availability and out-of-pocket costs.18 Anast, anastrozole; Bleo, bleomycin; Capecit, capecitabine; CarboP, carboplatin; CisP, cisplatin; Cyclo, cyclophosphamide; DTIC, dacarbazine; Docet, docetaxel; Epir, eprirubicin; Etop, etoposide; Ifos, ifosfamide; Irino, irinotecan; MTX, methotrexate; Oxalipl, oxaliplatin; Paclit, paclitaxel; Tam, tamoxifen; Trastuz, trastuzumab; VBL, vinblastine; VCR, vincristine.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
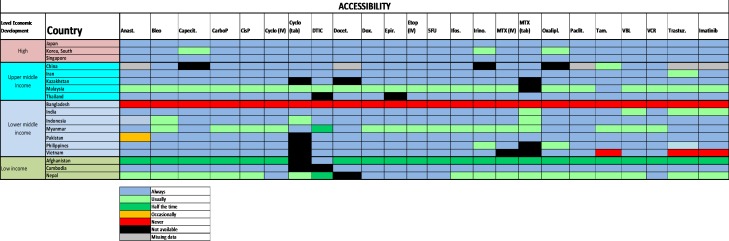
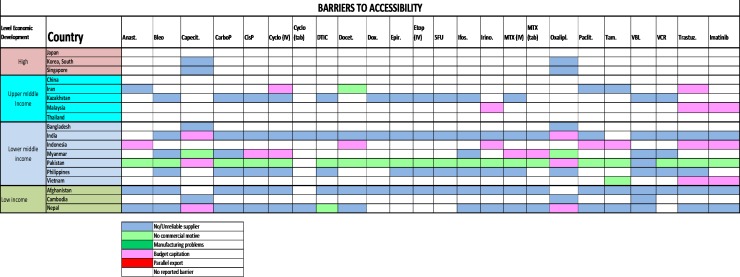
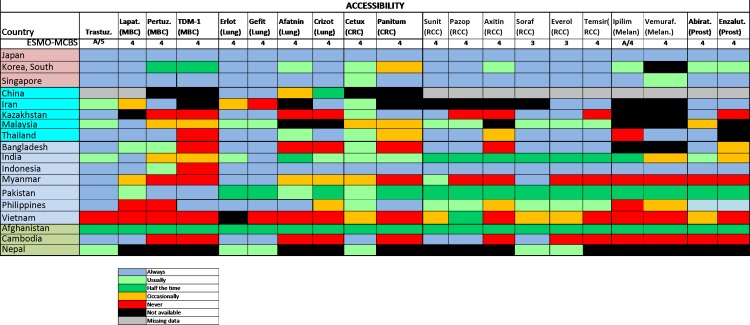
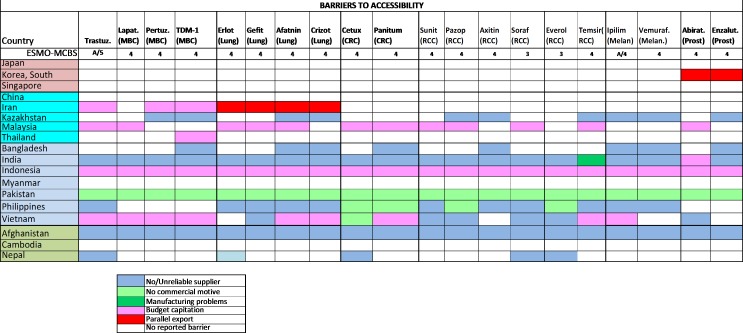
Problems with the accessibility of medicines were reported to be substantially more prevalent in middle-income countries and LICs (figure 2): most severely in Bangladesh, but also in Malaysia, Myanmar, Afghanistan and Nepal. The dominant reported barrier to accessibility was a lack of reliable supply, especially because of lack of commercial interest or budgetary restraint (figure 3). The ESMO-Economist Intelligence Unit Report on how to prevent and manage shortages of cancer medicines recommends addressing lack of supply related to commercial interest by providing incentives for suppliers to enter and remain in nation markets.14
Figure 2.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data of medications on the WHO Model List of Essential Medicines: Actual availability (accessibility with a valid prescription).18 Anast, anastrozole; Bleo, bleomycin; Capecit, capecitabine; CarboP, carboplatin; CisP, cisplatin; Cyclo, cyclophosphamide; DTIC, dacarbazine; Docet, docetaxel; Epir, eprirubicin; Etop, etoposid; Ifos, ifosfamide; Irino, irinotecan; MTX, methotrexate; Oxalipl, oxaliplatin; Paclit, paclitaxel; Tam, tamoxifen; Trastuz, trastuzumab; VBL, vinblastine; VCR, vincristine.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
Figure 3.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data on medications on the WHO Model List of Essential Medicines: Dominant Barrier to Accessibility.18 Anast, anastrozole; Bleo, bleomycin; Capecit, capecitabine; CarboP, carboplatin; CisP, cisplatin; Cyclo, cyclophosphamide; DTIC, dacarbazine; Docet, docetaxel; Epir, eprirubicin; Etop, etoposide; Ifos, ifosfamide; Irino, irinotecan; MTX, methotrexate; Oxalipl, oxaliplatin; Paclit, paclitaxel; Tam, tamoxifen; Trastuz, trastuzumab; VBL, vinblastine; VCR, vincristine.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
Approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2)
Data gathered in the ESMO study regarding medications with relatively recent marketing approval, by either the US Food and Drug Administration or the European Medicines Agency, and not included in the 2015 WHO Model List of Essential Medicines, were cross-referenced with scores derived from the ESMO-Magnitude of Clinical Benefit Scale using the revised version 1.1 of the scale.20
At the time of the ESMO survey in 2015, there were 19 medications from seven disease groups that were not on the WHO Model List of Essential Medicines and had an ESMO-MCBS score greater than two (2): lapatinib, pertuzumab and trastuzumab emtansine (TDM-1; breast cancer); erlotinib, gefitinib, afatinib (EGFR-mutated NSCLC), crizotinib (ALK/ROS1 rearranged NSCLC); cetuximab and panitumumab (RAS/RAF wild-type colorectal cancer); sunitinib, pazopanib, axitinib, sorafenib, everolimus and temsirolimus (renal cell cancer); ipilimumab and vemurafenib (cutaneous melanoma) and abiraterone and enzalutamide (castration-resistant prostate cancer).
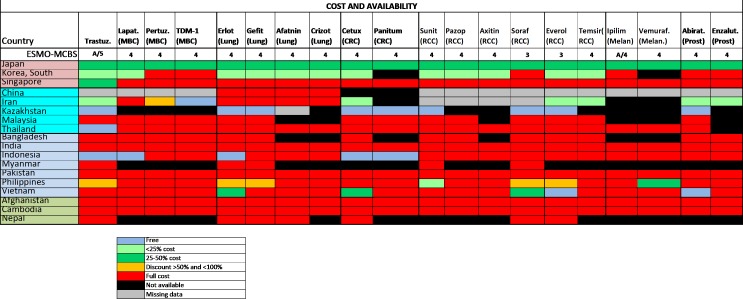
In most middle-income countries and LIC, the survey shows that these medications were very infrequently available at reduced cost to patients (figure 4) and many of them were not available at all due to accessibility issues or non-approval by the national regulatory agencies (figure 5). High out-of-pocket costs were less frequent in HICs where the medications were almost always on formulary (figure 4). The overall accessibility of these agents was less than for those medications included in the WHO Model List of Essential Medicines, and problems of accessibility were greater in LMIC and LIC (figure 5). Even in HICs, limited accessibility was sporadically reported (figure 5).
Figure 4.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data of recently approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2): Formulary availability and out-of-pocket costs.18 Abirat, abiraterone; Aftatin, atafinib; Axitin, axitinib; Cetux, cetuximab; CRC, colorectal cancer; Enzalut, enzalutamide; Erlot, eroltinib; Everol, everolimus; Gefit, gefitinib; Ipilim, ipilimumab; Lapat, lapatinib; MBC, metastatic breast cancer; Melan, melanoma; Panitum, panitumumab; Pazop, pazopanib; Pertuz, pertuzumab; Prost, prostate; RCC, renal cell cancer; Soraf, sorafenib; Suni, sunitinib; Vemuraf, vemurafenib.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
Figure 5.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data of recently approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2): Actual availability (accessibility with a valid prescription).18 Aftatin, atafinib; Axitin, axitinib; Cetux, cetuximab; CRC, colorectal cancer; Erlot, eroltinib; Everol, everolimus; Gefit, gefitinib; Ipilim, ipilimumab; Lapat, lapatinib; MBC, metastatic breast cancer; Melan, melanoma; Panitum, panitumumab; Pazop, pazopanib; Pertuz, pertuzumab; Prost, prostate; RCC, renal cell cancer; Soraf, sorafenib; Suni, sunitinib; Vemuraf, vemurafenib.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
The dominant reported barrier in HIC and UMIC was budgetary constraints, while in LMIC and LIC, lack of supplier or commercial motivation was increasingly dominant. Parallel export, whereby shortages are caused by the export of relatively inexpensive medications for foreign use, was infrequently reported as a major cause of lack of accessibility (figure 6).
Figure 6.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: (1) Asian data of recently approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2): Dominant barrier to accessibility.18 Abirat, abiraterone; Aftatin, atafinib; Axitin, axitinib; Cetux, cetuximab; CRC, colorectal cancer; Erlot, eroltinib; Enzalut, enzalutamide; Everol, everolimus; Gefit, gefitinib; Ipilim, ipilimumab; Lapat, lapatinib; MBC, metastatic breast cancer; Melan, melanoma; Panitum, panitumumab; Pazop, pazopanib; Pertuz, pertuzumab; Prost, prostate; RCC, renal cell cancer; Soraf, sorafenib; Suni, sunitinib; Vemuraf, vemurafenib.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017
What is the impact of the availability of cancer medicines on breast, colorectal and lung cancer patient care?
The data from the ESMO International Consortium Study reveal several disparities among Asian countries, across the different income levels. The ESMO study revealed the lack of availability in some countries of essential medicines on the WHO Model List of Essential Medicines including oxaliplatin, and capecitabine—which are the backbone for the treatment of colorectal cancer both in the curative and advanced setting. In one of the countries, aromatase inhibitors were unavailable, possibly affecting the outcome of breast cancer patients. Importantly, the use of aromatase inhibitors represents a crucial strategy for the treatment of hormone receptor-positive metastatic breast cancer patients, both in premenopausal and postmenopausal women, resulting in longer disease control.25 26
In 17% of the surveyed Asian countries (n=3/18), pertuzumab was not available and had a serious impact on HER2-overexpressing advanced breast cancer patients, with an estimated loss of 15.7 months of median survival, according to clinical trials data, along with less control over breast cancer-related symptoms as a measure of quality of life.27 In the same countries, TDM1 was also not available, contributing to an adjunctive loss of 4-6 months in the median overall survival28 when used as second-line treatment after trastuzumab-based therapy failed.
In five countries, no targeted approach for ALK-rearranged NSCLC was detectable. According to clinical trials data, frontline use of crizotinib in the metastatic setting provides a greater tumour control rate, with effective tumour shrinkage, resulting in significant reduction in lung cancer symptoms and improvement in quality of life (physical, social, emotional and professional) when compared with platinum-based chemotherapy doublets, along with a longer progression-free survival (PFS).29
A trend for out-of-pocket full cost in LMICs, and free-of-charge medicines in HICs, can be observed for the WHO essential medicines required for the treatment of breast cancer (anastrozole, cyclophosphamide, docetaxel or paclitaxel, doxorubicin or epirubicine, tamoxifen, trastuzumab, capecitabine, platinum compounds, vinorelbine, methotrexate, 5-fluorouracil), colorectal cancer (capecitabine or 5-fluorouracil, irinotecan, oxaliplatin) and lung cancer (cisplatin or carboplatin, docetaxel or paclitaxel, vinorelbine). In all the LICs (n=3), and more than half of LMICs (n=4/7), all of these medicines were concurrently available only at full cost; in UMICs and HICs, these medicines were variably reimbursed or free-of-charge for patients.
The newer molecules (lapatinib, pertuzumab, T-DM1, erlotinib, gefitinib, afatinib, crizotinib, cetuximab, panitumumab) not included in the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2), for the treatment of breast, colorectal and lung cancers, were all contemporarily accessible with partial reimbursement only in one HIC, and otherwise generally provided only at full cost to patients in UMICs, LMICs and LICs. For example, EGFR-targeting agents for the frontline treatment of EGFR-mutated NSCLC were available for free, or partially reimbursed, in one-third of the Asian countries surveyed (n=6), but never in LICs.
The Asian population has a high lung cancer burden responsible for nearly 20% of cancer mortality, and it has the highest global rates of EGFR-mutated types.1 7 For that reason, the availability of quality and effective EGFR-targeting therapy (TKI) is of relevant public health interest. Where effective treatment exists, but is only available at full price, the provision of the optimal evidence-based treatment is often unaffordable or only available to patients and their families at very high costs, resulting in catastrophic personal health expenditures.30 The unavailability of TKI in frontline treatment of advanced EGFR-mutated NSCLC patients may result in poorer prognosis, with a loss of PFS between 1.7 and 8.5 months,31–34 and with compelling likelihood that the lack of access to medicines has a negative impact on patient outcomes, including duration of survival and quality of life.
Financial toxicity of cancer treatment in LMICs
Achieving the UN 2030 Sustainable Development Goal 3.8 on Universal Health Coverage requires that everyone, everywhere can access needed healthcare without experiencing financial ruin as a result of that care. In 2017, more than 800 million people across the globe experienced out-of-pocket expenses for health that were greater than 10% of their total income, and of these nearly 180 million people had expenditures higher than 25% of their total income. As a result, 100 million people every year fall into poverty as a consequence of these catastrophic healthcare expenditures.35
These figures are calculated based on out-of-pocket payments for all access to health services in the previous year, not cancer alone. However, they reveal the picture of the financial risk associated with accessing healthcare services, particularly in instances where financial protection systems, such as prepooled publicly funded financing, are not available.
Current literature from low- and middle-income settings paints a bleak picture of the financial hardships associated with accessing cancer care. In Haiti, where 74% of people earn less than $2 per day, the median out-of-pocket expenses associated with cancer treatment were $717.36 Studies across multiple sites in Asia indicate that patients in all countries, even those with universal health coverage schemes, face the potential for financial catastrophe as a result of cancer care. A minimum 25% of cancer patients in Thailand, and as high as 76% of cancer patients in India, report high out-of-pocket expenses, and the need to sell assets and borrow funds to pay for cancer treatment.37
Due to the high costs involved in cancer care, in settings where financial resources are limited, cancer care is simply not included in publicly funded health benefit packages,38 leading to massive economic losses due to this inaction,39 especially in low- and middle-income settings where the treatment gaps and survival rate differentials are greatest.38
The investment needs for cancer control in low- and middle-income countries are not fully quantified. The WHO investment case estimated that an additional $1.1 trillion is needed over the next 5 years for low- and middle-income countries to scale-up universal health coverage, leading to almost 25 million lives saved, and a return on investment of $1.4 for every $1 invested. While this may sound low, in the 50 countries currently spending less than $100 USD per capita on health, this underspending can represent significant additional expenditure.40
Given the high initial capital investment needs, responding to the cancer burden is complicated for many countries. Despite rhetoric about the need for increased donor financing for NCDs, the reality is that cancer services in low- and middle-income countries are largely funded through domestic resources and will continue to be so into the future.41 However, it is an area where true catalytic investments to strengthen health systems for cancer responses, for example by building radiotherapy units, could have magnified benefits.42
Strategies to improve accessibility and availability of cancer medicines in low- and middle-income countries
Cancer treatment is seriously affected by the availability and affordability of medicines. In low- and middle-income countries, the decision to use anticancer agents is based mainly on the economic status of each country.43 Major barriers to the accessibility of anticancer medicines are lack of government reimbursement, budget allocation for healthcare, and quality-assured generic and biosimilar medicines, as well as shortages and patent rights.
Possible strategies to improve the ‘availability’ of cancer medicines include:
Shortening the time for the approval and registration of cancer medicines in low- and middle-income countries.
Improving the availability of valuable high-cost medicines if they are on the national list of essential medicines and included in the national clinical practice guidelines.
Increasing the budget allocation for effective high-cost anticancer medicines for specific indications.
Possible strategies to improve the ‘affordability’ of cancer medicines are primarily based on the cost of the medicine and include:
Price negotiation (usually by the government), including value-based pricing.
Availability of quality-assured generic and biosimilar medicines.44
Patient assistance programmes either from pharmaceutical companies or from nonprofit organisations.
Compulsory licensing.
With 225 new cancer medicines expected to be marketed by 2020, an increase in spending for oncology medicinal products is anticipated to surpass 1 trillion Euros.44 The clinical value of a biosimilar is equal to the originator medicine, and it is estimated that the use of biosimilars can create a potential savings for cancer medicine expenditures in the USA and the European Union of 100 billion Euros by 2020.45 The lower cost of biosimilars represents an opportunity to reshape the market and introduce competition to the originator medicine, thereby impacting the financial burden of cancer.46
In addition to these strategies, several countries issue their own guidelines on price allocation according to the country’s GDP per capita. Price negotiations can have a great impact on affordability, and sometimes lead to pharmaceutical companies producing a second brand of the same medicine.47
Health technology assessment (HTA) should be used to assess medicine costs according to cost-effectiveness analysis and budget impact. The results obtained can provide useful decision-making data to the government, which can encourage them to reimburse important medications. The ESMO-Magnitude of Clinical Benefit Scale19 20 is a useful tool that can assist HTA bodies to prioritise cancer medicines according to their clinical benefit.
In 2001, the World Trade Organization Members adopted the Doha Declaration,48 which includes the Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities. The TRIPS flexibilities include the right to suspend patent privileges, to grant voluntary or compulsory licenses, to practice parallel imports, and to allow production and importation of low-price generic medicines. The Doha Declaration strengthens the right of World Trade Organization Members to make full use of these tools when necessary to protect public health and improve access to affordable medicines for developing countries.49
Tiered pricing is the concept of selling medicines and vaccines in developing countries at prices systematically lower than in developed countries and has also been proposed to help the least developed countries.50
The WHO supports these pricing policies.
In addition to pricing and reimbursement, an increase in the number of researchers in low- and middle-income countries will improve access to cancer medicines through new clinical trials and medicine development, along with a better understanding of the inter-ethnical variability in response to treatments and safety.
Conclusions
In summary, there is no single solution. Policies need to be evidence-based and adapted at the country level where global and national commitments are integrated harmoniously. The conceptual framework for the availability, accessibility and affordability of cancer medicines has been outlined at the highest political levels, with all countries agreeing to achieve the UN 2030 Sustainable Development Goals, and the 2017 WHO Cancer Resolution, which call for improved access to cancer medicines, palliative care, vaccines and medical devices. While these strategies and resolutions are not legally binding, they represent an official government commitment that should be ethically and morally binding.
On the national level, national cancer control plans can shape the policy commitments by the government and the Ministry of Health because they contain regulatory information on how cancer medicines will be approved, procured, distributed and reimbursed. The ESMO-MCBS is an important tool to help governments review and adapt their national medicine list, making sure it contains the most cost-effective medicines that provide the greatest value to patients. Laws and regulations should prevent patient discrimination based on the unaffordability of care.
Good policies require good evidence-based data. In the absence of good country data, policy recommendations risk to be general and not country-based. For example, the data on EGFR-mutated NSCLC epidemiology in the Asian population,6 as discussed above, requires cancer policy to be based on a country’s cancer burden. Good country data also determines the country’s national essential medicines list, as well as reimbursement decisions for expensive targeted medicines. A clear histology and molecular profiling of a country’s cancer epidemiology provides clearer strategies for medicine prioritisation and procurement. Basing cancer prevention and control policies on country-specific data is the principle of tailored oncology and can provide the conceptual framework for ‘precision’ policy-making. Oncology providers are key stakeholders in global discussions about cancer management and access to medicines, and they can support global oncology programmes with their knowledge and expertise.
Acknowledgments
We thank the ESMO Public Policy Steering Committee for their feedback on the content of this review. We also acknowledge the contribution of ESMO public policy staff member Martina Galotti, for her editorial support, editing and referencing of the content.
Footnotes
Contributors: The project was developed under the guidance and coordination of Alexandru Eniu. All of the authors contributed to the writing of this manuscript and approved the final submitted version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The authors alone are responsible for the views expressed in this paper, and they do not necessarily represent the decisions, policy or views of WHO, ESMO or the authors’ institutions or affiliations.
Competing interests: AE has received research support from AstraZeneca, Pfizer, Celltrion, Novartis, none in relation to this publication.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. . Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. WHO Cancer, 2018. Available: http://www.who.int/news-room/fact-sheets/detail/cancer [Accessed 17 Sep 2018].
- 3. Stewart BW, Wild CP, World cancer report 2014. Lyon: International Agency for Research on Cancer, 2014. [Google Scholar]
- 4. Best A, Hiatt RA, Cameron R, et al. . The evolution of cancer control research: an international perspective from Canada and the United States. Cancer Epidemiol Biomarkers Prev 2003;12:705–12. [PubMed] [Google Scholar]
- 5. Chahoud J, Semaan A, Rieber A, et al. . Wealth, health expenditure, and cancer: a national perspective. J Natl Compr Canc Netw 2016;14:972–8. 10.6004/jnccn.2016.0104 [DOI] [PubMed] [Google Scholar]
- 6. Batouli A, Jahanshahi P, Gross CP, et al. . The global cancer divide: relationships between national healthcare resources and cancer outcomes in high-income vs. middle- and low-income countries. J Epidemiol Glob Health 2014;4:115–24. 10.1016/j.jegh.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han B, Tjulandin S, Hagiwara K, et al. . EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: the IGNITE study. Lung Cancer 2017;113:37–44. 10.1016/j.lungcan.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 8. Hill A, Gotham D, Fortunak J, et al. . Target prices for mass production of tyrosine kinase inhibitors for global cancer treatment. BMJ Open 2016;6 10.1136/bmjopen-2015-009586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carbonnaux M, Souquet P-J, Meert A-P, et al. . Inequalities in lung cancer: a world of EGFR. Eur Respir J 2016;47:1502–9. 10.1183/13993003.01157-2015 [DOI] [PubMed] [Google Scholar]
- 10. ESMO ESMO Clinical Practice Guidelines. Available: https://www.esmo.org/Guidelines [Accessed 21 Sep 2018].
- 11. WHO WHA71.12 - Addressing the global shortage of, and access to, medicines and vaccines, 2018. Available: http://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_12-en.pdf [Accessed 17 Sep 2018].
- 12. WHO Roadmap for access 2019‐2023,Comprehensive support for access to medicines and vaccines. Zero draft V2, 2018. Available: http://www.who.int/medicines/access_use/Roadmap_English.pdf?ua=1 [Accessed 17 Sep 2018].
- 13. WHO Technical Report: Pricing of cancer medicines and its impacts, 2018. Available: https://apps.who.int/iris/bitstream/handle/10665/277190/9789241515115-eng.pdf?ua=1 [Accessed 30 Jan 2019].
- 14. The Economist Intelligence Unit, ESMO Cancer medicines shortages in Europe: Policy recommendations to prevent and manage shortages, 2017. Available: http://www.eiu.com/graphics/marketing/pdf/ESMO-Cancer-medicines-shortages.pdf [Accessed 17 Sep 2018].
- 15. Prager GW, Braga S, Bystricky B, et al. . Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018;3:e000285 10.1136/esmoopen-2017-000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. UN Political Declaration on NCDS, 2018. Available: https://www.un.org/pga/73/2018/09/18/ncds-political-declaration-silence-procedure/ [Accessed 17 Sep 2018].
- 17. WHO SEARO Delhi Declaration, improving access to essential medical products in the South-East Asia region and beyond, 2018. Available: http://apps.who.int/iris/bitstream/handle/10665/274331/Delhi-Declaration.pdf?sequence=5&isAllowed=y [Accessed 19 Sep 2018].
- 18. Cherny NI, Sullivan R, Torode J, et al. . ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe. Ann Oncol 2017;28:2633–47. 10.1093/annonc/mdx521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cherny NI, Sullivan R, Dafni U, et al. . A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for medical oncology magnitude of clinical benefit scale (ESMO-MCBS). Ann Oncol 2015;26:1547–73. 10.1093/annonc/mdv249 [DOI] [PubMed] [Google Scholar]
- 20. Cherny NI, Dafni U, Bogaerts J, et al. . ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol 2017;28:2340–66. 10.1093/annonc/mdx310 [DOI] [PubMed] [Google Scholar]
- 21. Rauh S, Arnold D, Braga S, et al. . Challenge of implementing clinical practice guidelines. Getting ESMO's guidelines even closer to the bedside: introducing the ESMO Practising Oncologists' checklists and knowledge and practice questions. ESMO Open 2018;3:e000385 10.1136/esmoopen-2018-000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ullrich A, Ciardiello F, Bricalli G, et al. . ESMO and WHO: 14 years of working in partnership on cancer control. ESMO Open 2016;1:e000012 10.1136/esmoopen-2015-000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The World Bank Countries and economies. Available: https://data.worldbank.org/country [Accessed 19 Sep 2018].
- 24. WHO The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2015. WHO technical report series. Geneva: WHO, 2015. [Google Scholar]
- 25. Early Breast Cancer Trialists' Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet 1998;351:1451–67. 10.1016/S0140-6736(97)11423-4 [DOI] [PubMed] [Google Scholar]
- 26. Thürlimann B, Keshaviah A, Coates AS, et al. . A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747–57. 10.1056/NEJMoa052258 [DOI] [PubMed] [Google Scholar]
- 27. Swain SM, Baselga J, Kim S-B, et al. . Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34. 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diéras V, Miles D, Verma S, et al. . Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42. 10.1016/S1470-2045(17)30312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon BJ, Mok T, Kim D-W, et al. . First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med 2014;371:2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 30. Chino F, Peppercorn JM, Rushing C, et al. . Going for broke: a longitudinal study of patient-reported financial sacrifice in cancer care. J Oncol Pract 2018;14:e533–46. 10.1200/JOP.18.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scagliotti GV, Parikh P, von Pawel J, et al. . Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 32. Yang JJ, Zhou Q, Yan HH, et al. . A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568–74. 10.1038/bjc.2016.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han J-Y, Park K, Kim S-W, et al. . First-SIGNAL: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 34. Zhou C, Wu Y-L, Chen G, et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 35. WHO and The World Bank Tracking Universal Health Coverage: 2017 Global Monitoring Report, 2017. Available: http://apps.who.int/iris/bitstream/handle/10665/259817/9789241513555-eng.pdf;jsessionid=FDBD617A25822ABDC9E613914AB3C24F?sequence=1 [Accessed 19 Sep 2018].
- 36. O'Neill KM, Mandigo M, Pyda J, et al. . Out-of-pocket expenses incurred by patients obtaining free breast cancer care in Haiti: a pilot study. Surgery 2015;158:747–55. 10.1016/j.surg.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 37. ACTION Study Group Policy and priorities for national cancer control planning in low- and middle-income countries: lessons from the association of Southeast Asian Nations (ASEAN) Costs in Oncology prospective cohort study. Eur J Cancer 2017;74:26–37. 10.1016/j.ejca.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 38. Farmer P, Frenk J, Knaul FM, et al. . Expansion of cancer care and control in countries of low and middle income: a call to action. The Lancet 2010;376:1186–93. 10.1016/S0140-6736(10)61152-X [DOI] [PubMed] [Google Scholar]
- 39. Sullivan R, Alatise OI, Anderson BO, et al. . Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015;16:1193–224. 10.1016/S1470-2045(15)00223-5 [DOI] [PubMed] [Google Scholar]
- 40. WHO New Perspectives on Global Health Spending for Universal Health Coverage, 2018. Available: http://apps.who.int/iris/bitstream/handle/10665/259632/WHO-HIS-HGF-HFWorkingPaper-17.10-eng.pdf?sequence=1 [Accessed 19 Sep 2018].
- 41. Knaul F, Horton S, Yerramilli P, et al. . Chapter 17. Financing Cancer Care in Low-Resource Settings In: Cancer: disease control priorities. 3 3rd edn, 2015. [PubMed] [Google Scholar]
- 42. Atun R, Jaffray DA, Barton MB, et al. . Expanding global access to radiotherapy. Lancet Oncol 2015;16:1153–86. 10.1016/S1470-2045(15)00222-3 [DOI] [PubMed] [Google Scholar]
- 43. Sruamsiri R, Ross-Degnan D, Lu CY, et al. . Policies and programs to facilitate access to targeted cancer therapies in Thailand. PLoS One 2015;10 10.1371/journal.pone.0119945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabernero J, Vyas M, Giuliani R, et al. . Biosimilars: a position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open 2016;1:e000142 10.1136/esmoopen-2016-000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. IMS Institute for Health Informatics Delivering on the potential of biosimilar medicines. Parsippany: IMS Institute for Health Informatics, 2016. [Google Scholar]
- 46. Wolff-Holz E, Garcia Burgos J, Giuliani R, et al. . Preparing for the incoming wave of biosimilars in oncology. ESMO Open 2018;3:e000420 10.1136/esmoopen-2018-000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subramanian R, Baqri R. Branding: when one is not enough. PharmExce. com, 2016. Available: http://www.pharmexec.com/branding-when-one-not-enough [Accessed 25 Sep 2018].
- 48. WHO The Doha Declaration on the TRIPS agreement and public health. Available: https://www.who.int/medicines/areas/policy/doha_declaration/en/ [Accessed 10 Sep 2018].
- 49. Nicol D, Owoeye O. Using TRIPS flexibilities to facilitate access to medicines. Bull World Health Organ 2013;91:533–9. 10.2471/BLT.12.115865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moon S, Jambert E, Childs M, et al. . A win-win solution?: a critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health 2011;7 10.1186/1744-8603-7-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. WHO WHO Model List of Essential Medicines, 2017. Available: http://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1 [Accessed 19 Sep 2018].
- 52. Eniu A, Torode J, Magrini N, et al. . Back to the 'essence' of medical treatment in oncology: the 2015 WHO Model List of Essential Medicines. ESMO Open 2016;1:e000030 10.1136/esmoopen-2015-000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. WHO WHO EML Cancer Medicines Working Group (CMWG): Report of the meeting 22-23 March 2018. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 54. WHO, Action Programme on Essential Drugs and Vaccines, London School of Hygiene and Tropical Medicine & Koninklijk Instituut voor de Tropen . An evaluation of WHO's action programme on essential drugs, 1989. Available: http://www.who.int/iris/handle/10665/62965 [Accessed 19 Sep 2018].
- 55. WHO WHA67.22 - Access to Essential Medicines. WHA Resolution; Sixty-seventh World Health Assembly, 2014. Available: http://apps.who.int/medicinedocs/documents/s21453en/s21453en.pdf [Accessed 17 Sep 2018].
- 56. UN Sustainable Development Goals. Available: https://sustainabledevelopment.un.org/?menu=1300 [Accessed 17 Sep 2018].
- 57. UN Sustainable Development Goal 3. Available: https://sustainabledevelopment.un.org/sdg3 [Accessed 17 Sep 2018].
- 58. WHO WHA70.12 - Cancer Prevention and Control in the Context of an Integrated Approach. WHA Resolution; Seventieth World Health Assembly, 2017. Available: http://apps.who.int/medicinedocs/documents/s23233en/s23233en.pdf [Accessed 17 Sep 2018].
- 59. WHO Pilot procedure for prequalification of biotherapeutic products and similar biotherapeutic products, 2018. Available: http://www.who.int/medicines/regulation/biotherapeutic_products/en/ [Accessed 17 Sep 2018].