Abstract
Rheumatic immune-related adverse events (irAEs) have long been underestimated. However, arthralgia or myalgia are common side effects of immune checkpoint inhibitors (ICPi) reported in up to 10-20% of patients in clinical trials. Although rheumatic irAEs are rarely life-threatening, patients' quality of life can be considerably restricted due to pain, stiffness and limited mobility. Rheumatic irAEs can resemble known rheumatic and musculoskeletal diseases (RMDs), but often do not fulfil the classification criteria of classical entities and standardised evidence-based guidelines for their management are so far lacking. Herein, we discuss specific characteristics of rheumatic irAEs and present a comprehensible diagnostic and therapeutic approach to the management of these side effects.
Rheumatic immune-related adverse events (irAEs) have long been underestimated owing to the fact that chronic musculoskeletal symptoms are frequent in the general population and even more in patients with cancer due to the pain and paraneoplastic symptoms caused by the disease and the frequently associated physical inactivity. Moreover, musculoskeletal pain is a common unspecific side effect of many drugs. Arthralgia or myalgia has been reported in up to 10–20% of patients treated with immune checkpoint inhibitors (ICPi) in clinical trials.1–3 A further aspect is that the Common Terminology Criteria for Adverse Events (CTCAE) grading system used by oncologist is not adapted to classify rheumatological side effects, and therefore, does not adequately reflect severity and complexity of these symptoms. In this regard, musculoskeletal events with substantial functional impact (eg, limiting instrumental activities of daily living) may be only a grade 2 event by the CTCAE system whereas they would be a grade 3 event in the Rheumatology Common Toxicity Criteria system.3 4 Although rheumatic irAEs can cause substantial pain, disability and high level of suffering, the current lack of awareness regarding these events in physicians results in under-reporting.1 Therefore, actively asking and searching for rheumatic irAEs in patient examination is of importance. As such, the routine integration of patient-reported outcome measurement represents also a further step in the assessment of these side effects.5
Rheumatic irAEs can resemble known rheumatic and musculoskeletal diseases (RMDs). However, they often do not fulfill the classification criteria of classical entities. For diagnostic and therapeutic management, rheumatic irAEs can be divided in several subtypes according to the leading signs and symptoms (table 1).1–4 6–9
Table 1.
Subtypes of rheumatic immune-related adverse events (irAE) in reference to known rheumatic and musculoskeletal diseases (RMDs) according to typical leading symptoms and possible laboratory findings
| Subtype | Leading signs and symptoms | Possible laboratory findings |
| Inflammatory arthritis | Painful joint swelling, morning stiffness, relief by movement. Symmetrical affected small joints can indicate rheumatoid arthritis-like phenotype. | CRP ↑, ESR ↑, *RF+, anti-CCP+, ANA+, HLA-B27+ |
| Polymyalgia rheumatica-like | Symmetrical polymyalgia of proximal limbs, morning stiffness, pain and difficulties when getting up from sitting/lying position and/or lifting arms. May be associated with large vessel vasculitis. | CRP ↑, ESR ↑ |
| Psoriatic arthritis-like | Asymmetrical (mono-/oligo) arthritis, dactylitis, tendinitis/tenosynovitis, enthesitis. | †CRP ↑, ESR ↑ |
| Sicca (Sjögren syndrome)-like | Dryness of eyes, mouth and genital area (mostly irreversible). Arthralgia/myalgia are possible. | †CRP ↑, ESR ↑ *ANA+, SSA/SSB+ |
| Polymyositis-like | Weakness of proximal limbs, stiffness and aching of muscles. Assess for presence of bulbar symptoms (dysphagia, dyspnoea, slurred speech, diplopia), myocarditis and interstitial lung disease. | CK ↑, †CRP ↑, †ESR ↑ *ANA+, myositis panel+ (eg, Jo1, Mi-2, SRP etc) |
| Scleroderma-like | Raynaud′s phenomenon, acral ulcers, sclerodactylitis, skin thickening, calcinosis, telangiectasia, possible lung involvement. | †CRP ↑, *ESR ↑ *ANA+, centromere pattern, Scl70+ |
| Vasculitis-like |
Large vessel vasculitis: headache, jaw/ extremities claudication, visual impairment/AION; Small vessel vasculitis: purpura/leukocytoclastic vasculitis (skin), nasal bloody discharge/ulcers (ENT), foamy urine/proteinuria, nephritic sediment, oedema, hypertension (kidney) dyspnoea/cough (lung) |
CRP ↑, ESR ↑ *ANCA+ |
| Sarcoid-like | Lymphadenopathy (bihilary), lung nodules, arthralgia/arthritis, red/painful eye (uveitis). Histology: non-caseating granulomas. | CRP ↑, ESR ↑ *ACE+, sCD25+ |
| Differential diagnosis: non-inflammatory musculoskeletal symptoms | Worsening by movement and in the course of the day, relief by resting and heat application. Bony formations along the joints (osteophytes) in osteoarthritis. Possible transition into activated osteoarthritis with signs of inflammation. | Normal values expected |
*When present, likelihood of an actual persisting rheumatic disease may increase
†Possible in rheumatic irAE, but not common in actual RMD.
AION, anterior ischaemic optic neuropathy; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies;Anti-CCP, anti-cyclic citrullinated peptide; CK, creatine kinase; CRP, C reactive protein; ENT, ear, nose and throat;ESR, blood sedimentation rate; HLA-B27, human leukocyte antigen B27; RF, rheumatoid factor;SRP, anti-signal recognition particle autoantibody; SSA/SSB, anti-Ro/SSA or anti-La/SSB autoantibodies; sCD25, soluble interleukin-2 receptor.
Currently, a reliable differentiation between a rheumatic irAE or the onset of a persisting RMD is not possible. Furthermore, in >50% of patients with pre-existing RMD, flares of the disease can occur during ICPi treatment.10 11 Additionally, important differential diagnosis, such as metastatic, paraneoplastic or infectious disease, should be considered.
When a rheumatic irAE is suspected, we recommend a timely rheumatological workup preferably before the start of glucocorticoid treatment. Abnormal immunological laboratory parameters, such as rheumatoid factor, anticitrullinated peptide antibodies and antinuclear antibodies, can point towards an actual RMD.1–4 6–9 Of note, rheumatic irAEs can present with considerably increased C reactive protein with levels >100 mg/L.6 Furthermore, imaging techniques should be used to search for evidence of inflammation: joint ultrasound is a fast, readily available and non-invasive method for detection of synovitis, tendinitis/tenosynovitis and enthesitis. Whereas MRI is validated and recommended for detection of several inflammatory manifestations of RMDs (eg, synovitis, tendinitis/tenosynovitis, enthesitis, myositis and vasculitis), positron emission tomography (PET) or PET-CT has mainly been validated and recommended for the detection of vasculitis. However, a few studies demonstrated a good correlation between fusion PET-CT and MRI for the detection of synovitis, and a good sensitivity and specificity for PET-CT also in context of ICPi-associated arthritis. Given that synovitis is detectable in MRI and PET, but often not addressed in the radiology report, routine tumour assessments should be also reviewed for this aspect when musculoskeletal symptoms occur.1–4 6–9 12 13
Additionally, histological confirmation of certain findings such as myositis, scleroderma or sarcoidosis is desirable for further therapeutic management. Other irAE may occur simultaneously with rheumatic irAE and should be looked for in the examination. However, an association with particular non-rheumatic irAEs has not been observed yet.2 8
Patients suffering from rheumatic irAEs have better tumour response and survival rates.1 2 8 However, the question whether the treatment of the irAE may actually counteract the antitumour immune response and survival benefit in these patients is currently the subject of controversial discussion and requires further research.1–4 6–8 10 11 14 Thus, treatment of patients with rheumatic irAE presents a compromise between best possible symptom reduction to allow ICPi continuation and the minimal possible immunosuppression to avoid potential interference with the antitumour response induced by ICPi. This therapeutic management contrasts the treatment in patients with RMDs, where the ‘treat-to-target’ strategy aims at achieving complete remission whenever possible.15
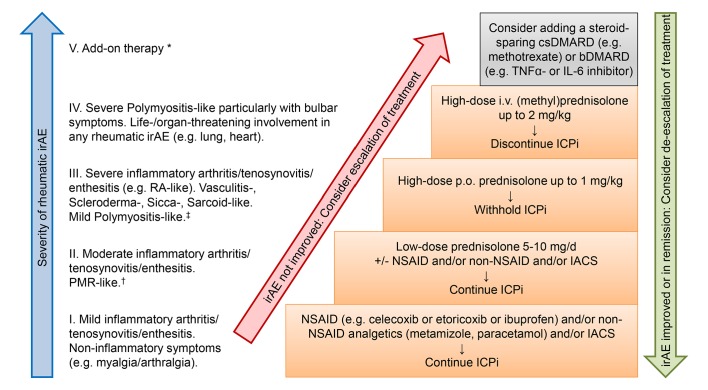
When a rheumatic irAE is diagnosed, we follow our therapeutic management algorithm given in figure 1. Similar algorithms were previously suggested for inflammatory arthritis,3 9 16 polymyalgia rheumatica-like and myositis syndrome.9 First, the choice of the appropriate therapy is determined by the severity of symptoms: rheumatic irAEs are mostly mild to moderate and mainly the therapy aims at pain relief and improving functionality in activities of daily life.1–4 6–9 Usually, the condition can be managed in outpatient setting. In rare cases rheumatic irAEs are life threatening and require inpatient treatment, with myositis with bulbar symptoms being the most severe example. Therefore, timely consultation of a rheumatologist is strongly recommended in severity grade III–V symptoms. However, it should already be considered in severity grade I–II symptoms, particularly when these do not sufficiently respond to the suggested symptomatic therapy.
Figure 1.
Suggested therapeutic management according to subtypes and severity of rheumatic immune-related adverse events (irAE). *Add-on therapy with DMARDs (disease-modifying antirheumatic drugs) can take up to 12 weeks until onset of therapeutic response. †Consultation of a rheumatologist should be considered. ‡Timely consultation of a rheumatologist is strongly recommended. bDMARDs, biological DMARDs; csDMARDs, conventional synthetic DMARDs; ICPi, immune checkpoint inhibitors; IACS, intra-articular corticosteroid injections; IL-6, Interleukin 6; NSAID, non-steroidal anti-inflammatory drug; PMR, polymyalgia rheumatica; RA, rheumatoid arthritis; TNFα, tumour necrosis factor α.
Second, the time until onset of response to a particular drug plays a major role in the choice of treatment. Glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs) and non-NSAID analgetics are the first-line therapy, as a response can be expected in several hours up to few days. Depending on the severity of irAE, we suggest to first use NSAID in mild to moderate symptoms and to escalate to glucocorticoids in case of an insufficient response (figure 1), however, their use may be limited by the comorbidities. Additionally, when only a few joints are involved, intra-articular corticosteroid injections can be considered. In contrast, depending on the substance, disease-modifying antirheumatic drugs (DMARDs) can take up to 12 weeks until onset of therapeutic response. We, therefore, regard them as second-line therapy to be added when response to glucocorticoids is insufficient or high doses are needed for control of signs and symptoms.
Although data from ICPi-treated patients with advanced non-small-cell lung cancer who received ≥10 mg of prednisone at baseline (for respiratory symptoms, fatigue and brain metastases) suggest that above the prednisolone dose of 10 mg/day the response to ICPi is impaired, the data are still scarce.17 Furthermore, it is not clear yet whether the use of glucocorticoid after initiation of ICPi is associated with poorer outcomes. However, given the known potential side effects of glucocorticoids, particularly in high-risk patients, the target of reaching a prednisone dose <10 mg within a few weeks seems to be a desirable approach.
It is common practice to withhold ICPi when high-dosed glucocorticoids are needed.1–4 6–9 ICPi re-exposition can be considered when symptoms of the rheumatic irAE are in remission or sufficiently controlled. However, permanent ICPi discontinuation should be strongly considered when life-threatening irAE occurred.1–4 6–9 Unfortunately, so far there are not enough data available on the recurrence rate of rheumatic irAE following ICPi restart.
Depending on the severity of the irAE, a timely re-evaluation of treatment response should be scheduled. If the symptoms have not improved sufficiently, we recommend escalation of the treatment to higher doses glucocorticoids and/or addition of DMARDs. Both conventional synthetic DMARDs (csDMARDs) and biological DMARDs (bDMARDs) are in use. There is not enough data available so far to recommend the use of a certain substance. Mostly, either methotrexate, sulfasalazine and/or hydroxychloroquine as csDMARDs or tumour necrosis factor (TNF) or interleukin six receptor-inhibitors as bDMARDs are used with varying success. Polymyositis-like disease may additionally be treated with intravenous immunoglobulins. Less experience is available on other bDMARDs and targeted synthetic DMARDs including the Janus kinase-inhibitors.
Of note, even though some substances have been available for more than a decade, negative effects on tumour response and recurrence cannot be ruled out completely. However, since carcinogenesis can be associated with chronic local inflammation, the use of DMARDs may also be protective in some cases, as has been shown for the reduced rate of colorectal cancer in patients with ulcerative colitis treated with TNF-inhibitors.18 Collectively, at this stage, we recommend to use DMARDs cautiously even in patients with cancer without ICPi-treatment.
At the present time, the management of rheumatic irAEs particularly in regard to the use of DMARDs differs greatly between centres. We, therefore, appreciate the efforts of a task force consisting of rheumatologists and oncologist under the aegis of the European League Against Rheumatism to standardise the management of rheumatic irAEs and refer to the recommendations that are to be expected in later 2019.
Footnotes
Contributors: KB wrote the manuscript including the design of the figure and table. H-ML, JL and KJ provided critical feedback and contributed to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: KB: Consultancy and/or speaker fees and/or travel reimbursements: Abbvie, BMS, Janssen, MSD, Mundipharma, Novartis, Pfizer, Roche, UCB. Scientific support: Abbvie, Novartis. H-ML: Consultancy and/or speaker fees and/or travel reimbursements: Abbvie, MSD, BMS, Pfizer, Celgene, Medac, GSK, Roche, Chugai, Novartis, UCB, Janssen-Cilag, Astra-Zeneca, Lilly. Scientific support and/or educational seminars and/or clinical studies: Abbvie, MSD, BMS, Pfizer, Celgene, Medac, GSK, Roche, Chugai, Novartis, UCB, Janssen-Cilag, Astra-Zeneca, Lilly, Baxter, SOBI, Biogen, Actelion, Bayer Vital, Shire, Octapharm, Sanofi, Hexal, Mundipharm, Thermo Fisher. JL: Consultancy and speaker fees: Abbvie, AstraZeneca, BMS, Celgene, Hospira, Janssen-Cilag, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB. Scientific support: Novartis, Pfizer. KJ: Consultancy and/or speaker fees: MSD, Merck, Amgen, Hexal, Riemser, Helsinn, Tesaro, Kreussler, Voluntis, Pfizer, Pomme-med.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Buder-Bakhaya K, Benesova K, Schulz C, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother 2018;67:175–82. 10.1007/s00262-017-2069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 3. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working Group. J Immunother Cancer 2017;5 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappelli LC, Gutierrez AK, Bingham CO, et al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017;69:1751–63. 10.1002/acr.23177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jordan K, Aapro M, Kaasa S, et al. European Society for medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 2018;29:36–43. 10.1093/annonc/mdx757 [DOI] [PubMed] [Google Scholar]
- 6. Leipe J, Christ LA, Arnoldi AP, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open 2018;4:e000714 10.1136/rmdopen-2018-000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calabrese C, Kirchner E, Kontzias K, et al. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017;3:e000412 10.1136/rmdopen-2016-000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liew DFL, Leung JLY, Liu B, et al. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheum Dis 2019;22:297–302. 10.1111/1756-185X.13444 [DOI] [PubMed] [Google Scholar]
- 9. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 11. Gutzmer R, Koop A, Meier F, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 2017;75:24–32. 10.1016/j.ejca.2016.12.038 [DOI] [PubMed] [Google Scholar]
- 12. Gholamrezanezhad A, Basques K, Batouli A, et al. Clinical Nononcologic applications of PET/CT and PET/MRI in musculoskeletal, orthopedic, and rheumatologic imaging. AJR Am J Roentgenol 2018;210:W245–W263. 10.2214/AJR.17.18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun J, Baraliakos X. [Magnetic resonance imaging in rheumatology]. Z Rheumatol 2016;75:582–5. 10.1007/s00393-016-0139-6 [DOI] [PubMed] [Google Scholar]
- 14. Bertrand F, Montfort A, Marcheteau E, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun 2017;8 10.1038/s41467-017-02358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoffer MA, Schoels MM, Smolen JS, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. 10.1136/annrheumdis-2015-207526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv264–6. 10.1093/annonc/mdy162 [DOI] [PubMed] [Google Scholar]
- 17. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed Death-Ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 18. Boal Carvalho P, Cotter J. Mucosal healing in ulcerative colitis: a comprehensive review. Drugs 2017;77:159–73. 10.1007/s40265-016-0676-y [DOI] [PubMed] [Google Scholar]