Abstract
Introduction
Survivors of critical illness often experience significant morbidities, including muscle weakness and impairments in physical functioning. This muscle weakness is associated with longer duration mechanical ventilation, greater hospital costs and increased postdischarge impairments in physical function, quality of life and survival. Compared with standard of care, the benefits of greater protein intake combined with structured exercise started early after the onset of critical illness remain uncertain. However, the combination of protein supplementation and exercise in other populations has demonstrated positive effects on strength and function. In the present study, we will evaluate the effects of a combination of early implementation of intravenous amino acid supplementation and in-bed cycle ergometry exercise versus a ‘usual care’ control group in patients with acute respiratory failure requiring mechanical ventilation in an intensive care unit (ICU).
Methods and analysis
In this multicentre, assessor-blinded, randomised controlled trial, we will randomise 142 patients in a 1:1 ratio to usual care (which commonly consists of minimal exercise and under-achievement of guideline-recommended caloric and protein intake goals) versus a combined intravenous amino acid supplementation and in-bed cycle ergometery exercise intervention. We hypothesise that this novel combined intervention will (1) improve physical functioning at hospital discharge; (2) reduce muscle wasting with improved amino acid metabolism and protein synthesis in-hospital and (3) improve patient-reported outcomes and healthcare resource utilisation at 6 months after enrolment. Key cointerventions will be standardised. In-hospital outcome assessments will be conducted at baseline, ICU discharge and hospital discharge. An intent-to-treat analysis will be used to analyse all data with additional per-protocol analyses.
Ethics and dissemination
The trial received ethics approval at each institution and enrolment has begun. These results will inform both clinical practice and future research in the area. We plan to disseminate trial results in peer-reviewed journals, at national and international conferences, and via nutritional and rehabilitation-focused electronic education and knowledge translation platforms.
Trial registration number
NCT03021902; Pre-results.
Keywords: Enteral Nutrition, Rehabilitation Medicine, Exercise, Parenteral Nutrition
Strengths and limitations of this study.
This is the first randomised controlled trial (RCT) evaluating the combination of exercise and protein supplementation started in the early phase of critical illness.
We have developed a rigorous framework to evaluate the effect of the study intervention on the patient’s functional recovery and outcomes.
As a phase II RCT, the study has a relatively small sample size recruited from four participating centres.
This study evaluates a combined intervention and will not be able to independently evaluate the effect of the nutrition versus exercise on study outcomes.
Introduction
Up to 20 million people worldwide receive life support in intensive care units (ICUs) each year.1 More than 750 000 Americans require mechanical ventilation annually,2 3 and 300 000 receive ventilation for >5 days.3–6 More critically ill patients are surviving hospitalisation due to recent medical advances.7 However, this survival comes at a cost. ICU survivors frequently experience significant post-ICU morbidities, commonly physical morbidities, including muscle weakness and impairments in physical functioning that can persist for years.8–14 Muscle weakness in the ICU is associated with delayed liberation from ventilation, extended ICU and hospital stays, worse long-term survival, physical functioning and quality of life (QOL).8 9 14–18 A recent review stated that the19 highest ranking research priority in the critical care nutrition/metabolism field was to evaluate the effect of protein dose with active and passive mobilisation in the acute phase of critical illness.
Recent randomised controlled trials (RCTs) have shown that providing increased total calories to ICU patients may not improve outcomes.20–28 However, only two of these RCTs delivered >50% of recommended protein, and these two delivered only ~33% of the protein dose targeted in our RCT.20 28 Moreover, observational studies report that optimising daily protein intake, rather than total caloric intake, decreases infections, mechanical ventilation duration, time to discharge and mortality.29–33 Although some studies report that increased protein intake is associated with greater muscle wasting,34 later ICU discharge35 and increased mortality,36 these studies did not adjust for a key confounding variables: the total time that protein was received or total caloric energy received.37 Moreover, a small RCT demonstrated that greater protein intake is associated with improved pulmonary function in ICU patients with chronic obstructive pulmonary disease (COPD),38 and attenuation of the muscle loss observed in the context of critical illness.39 40 A recent study found that infusing intravenous amino acids for 3 hours in ICU patients improved protein balance and stimulated an anabolic response.41 Many small trials have found that 2.0–2.8 g/kg/day of protein intake is safe and improves nitrogen balance.42–51 A recent RCT of IV amino acids of up to 2.0 g/kg/day in 474 ICU patients demonstrated safety, and although amino acid therapy did not preserve kidney function (the primary outcome), this study did not measure any performance-based measures and did not combine the amino acids intervention with exercise.52 A posthoc analysis from the trial examining the effects of baseline kidney function on mortality found those with normal kidney function had a reduction in 90-day mortality.53 Furthermore, those with baseline kidney dysfunction and/or those with a risk of progression of acute kidney injury (AKI) found no significant effect on 90-day mortality. Recent guidelines and systematic reviews recommend up to 2.0–2.5 g/kg/day and suggest that these doses are safe; thus, we propose to evaluate a similar dose.54 55
With increasing recognition of physical complications after critical illness, recent studies have evaluated exercise interventions started early after ICU admission. These studies have demonstrated safety and feasibility, and provide some evidence of reduced myofibrillar proteolysis, less muscle atrophy and consequently, improved strength and physical functioning, and decreased durations of mechanical ventilation and ICU stay.56–59 However, these studies are mainly single site, with modest sample sizes, and have grossly under-delivered protein to patients. Moreover, a number of recent trials of early exercise interventions have largely been negative.60–62 Hence, further evaluation of early ICU exercise interventions is needed, particularly in combination with amino acid supplementation, to reduce muscle weakness and physical complications after critical illness.
In various patient populations, combining protein and exercise has the greatest benefits compared with either nutrition or exercise alone. In older people, combined exercise and protein supplements improve protein synthesis and strength versus either intervention alone.63–67 In one study, combined exercise and supplementation in elderly patients increased muscle strength by 40% over exercise alone and by 130% over supplementation alone.67 In patients with obesity,68 HIV/AIDs69 and chronic obstructive pulmonary disease,70 as well as healthy volunteers undergoing bed rest,71 72 the combination of exercise and nutritional intervention (vs nutrition alone) yields the greatest benefit on muscle mass and strength. In a meta-analysis, protein supplementation and exercise (vs exercise alone) enhanced strength and muscle mass in non-critically ill adults.73 Moreover, a recent RCT of 93 ICU survivors, conducted in the out-patient setting, demonstrated that combined oral amino acid supplementation and exercise improved 6 min walk distance (6MWD, the primary outcome for our proposed trial) measured 3 months after enrolment, compared with either amino acids or exercise alone.74 We chose to use in-bed cycling because loss of lean body mass with bed rest is most pronounced in the legs.75 76 Our research finding also demonstrates greater weakness in legs versus arms in ICU patients.77 Moreover, leg strength is critical to ambulation, and thus key to functional independence and living at home.78–83 Although the generalisability of these findings to patients in the early phase of critical illness is unclear, these data provide biologic plausibility that this combined intervention may reduce physical impairments.84
Our multicentred, assessor-blinded, phase II RCT delivers a combination of intravenous amino acid supplementation and in-bed cycle ergometry exercise early after the onset of critical illness, versus usual care, in patients requiring mechanical ventilation in the ICU. The hypothesis is this novel RCT is that the combined intervention will (1) improve physical functioning at hospital discharge; (2) reduce muscle wasting with improved amino acid metabolism and protein synthesis in-hospital and (3) improve patient-reported outcomes and healthcare resource utilisation at 6 months after enrolment.
Methods and analysis
Study design
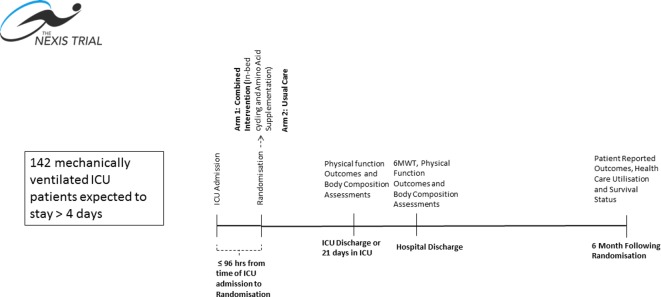
This is a phase II multicentre, assessor-blinded, RCT conducted at four academic medical centres in the USA: the University of Vermont Medical Center (UVMMC), Johns Hopkins University (JHU), Harborview Medical Center (HMC) and Wake Forest University Baptist Medical Center (WFUBMC). The data co-ordinating centre is the Clinical Evaluation Research Unit (CERU) at the Kingston General Hospital in Kingston Canada (see www.ceru.ca). See figure 1 for study design. This trial is funded by the National Institutes of Health (NIH) and the notice of award was received in April 2017 with the duration of the trial is expected to be 5 years.
Figure 1.
Study design and timeline. ICU, intensive care unit; 6MWT, 6 min walk time.
Eligibility criteria
Eligibility criteria are listed in table 1. We expect our intervention to be most effective when delivered early85; thus, we will limit enrolment to the first 96 hours after intubation. Patients hospitalised for a longer time prior to their ICU admission may be less responsive to our intervention.34 As such, we have excluded patients if they have spent greater than 5 days admitted to hospital in the 14 days leading up to the current ICU admission.
Table 1.
Inclusion and exclusion criteria for study entry
| Inclusion criteria | Exclusion criteria | Rationale for exclusion |
|
1. >96 continuous hours of mechanical ventilation before enrolment | Intervention most effective delivered early85 |
| 2. Expected death or withdrawal of life-sustaining treatments within this hospitalisation | Patients unlikely to receive benefit | |
| 3. No expectation for any nutritional intake within the subsequent 72 hours | Intervention intended to occur in addition to standard clinical nutritional intake | |
| 4. Severe chronic liver disease (MELD score ≥20) or acute hepatic failure | Amino acid supplementation may be harmful in patients with severe liver disease | |
| 5. Documented allergy to the amino acid intervention | Unable to receive proposed intervention | |
| 6. Metabolic disorders involving impaired nitrogen utilisation | Unable to receive amino acid infusion | |
| 7. Not ambulating independently prior to illness that leads to ICU admission (use of gait aid permitted) | Unable to perform outcome assessments | |
| 8. Pre-existing primary systemic neuromuscular disease (eg, Guillain Barre) | May not benefit from proposed intervention (ie, different mechanism of muscle weakness) | |
| 9. Neuromuscular blocker infusion (eligible once infusion discontinued if other inclusion criteria met) | Do not meet safety criteria for cycling intervention | |
| 10. Intracranial or spinal process affecting motor function | May not benefit from proposed intervention (ie, different mechanism of muscle weakness) | |
| 11. Pre-existing cognitive impairment or language barrier that prohibits outcomes assessment | Unable to perform outcome assessments | |
| 12. Patients in hospital >5 days prior to ICU admission | Muscle weakness likely already established | |
| 13. Lower extremity impairments that prevent cycling (eg, amputation, knee/hip injury) | Unable to receive proposed cycling intervention | |
| 14. Remaining intubated for airway protection only | Less likely to have muscle weakness and benefit from the interventions | |
| 15. Weight ≥150 kg | Exceeds maximum weight permitted for use of the cycle device | |
| 16. Physician declines patient enrolment | Not appropriate to conduct trial | |
| 17. Insufficient intravenous access | Need dedicated access for nutrition intervention for several hours a day | |
| 18. Pregnant | Unknown effects in fetus | |
| 19. Incarcerated | Vulnerable population |
ICU, intensive care unit.
Participant selection and recruitment
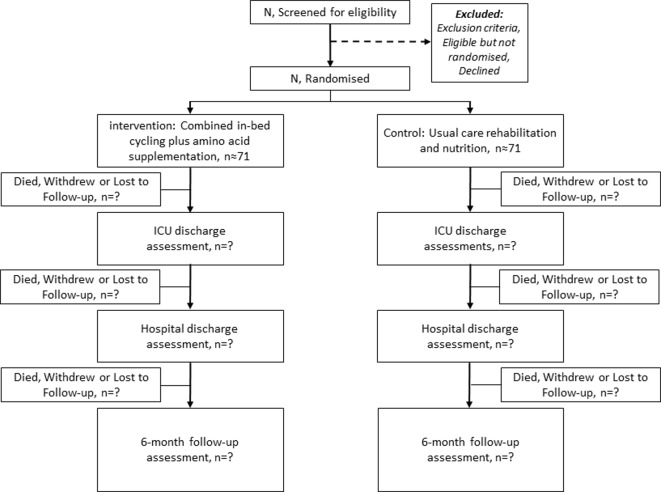
This combined intervention is targeted to critically ill patients with acute respiratory failure. Eligibility is determined by daily screening at the study site ICUs by trained members of the research team. Final approval of patient eligibility must be given by a trained site investigator. The planned flow of patients through the study is depicted in figure 2. All data will be captured in an electronic database to monitor recruitment at each study site.
Figure 2.
Consort diagram giving the flow of participants throughout the study. ICU, intensive care unit; N/n, number.
Consent
Once patients have been screened and confirmed by the site investigator or a subinvestigator, the participant or Legally Authorised Representative is approached for informed consent (online supplementary file). The research staff engages the Legally Authorized Representative (LAR) in a conversation to discuss the trial and ensure that they have understood the material. They are given ample time to review the materials and ask any relevant questions.
bmjopen-2018-027893supp001.pdf (543.5KB, pdf)
Randomisation
After consent, patients are randomised 1:1 to receive either (1) the combined intravenous amino acid and cycle ergometry exercise intervention or (2) usual care. Randomisation will be stratified by site and hospital length of stay prior to randomisation (<48 hours vs >48 hours). Randomisation will further be restricted by using permuted blocks of random size within strata. The randomisation list was commuter generated by the senior biostatistician at the data coordinating centre who is uninvolved with site enrolment and unaware of which site codes map to which sites. The randomisation is implemented using the data coordinating centre’s secure central web-based randomisation system which maintains concealment of future allocations and has been used successfully for several large international RCTs.
Blinding
Due to the nature of in-bed cycle ergometry, it is not possible to blind this study to patients, families or ICU clinicians. To minimise bias, blinded assessors are completing all outcome assessments and we are collecting data on key cointerventions to evaluate balance between groups in cointerventions. To assist with blinding outcome assessments, patients are prompted (using standardised language) not to disclose their perception of treatment allocation during the outcome assessments.86 Moreover, to evaluate effectiveness of blinding, outcome assessors document their ‘best guess’ regarding allocation (intervention vs control) at each assessment.86 87
Study intervention
Description of the amino acid intervention
The amino acid intervention is provided in addition to ‘usual care’ enteral and/or parenteral nutrition via an intravenous infusion (Clinisol 15% by Baxter) to target a total protein delivery of 2.0–2.5 g/kg/day. Consistent with a prior RCT demonstrating safety in the ICU,52 amino acids are delivered based on ideal body weight.88 Given a typical daily enteral/parenteral protein intake for ICU patients of 1.2 g/kg/day,89 the expectation is to administer approximately 1.2 g/kg/day of amino acid infusion (which yields 1.0 g/kg/day protein90) to reach a total of 2.2 g/kg/day total protein. Amino acids are infused via an indwelling central venous catheter, when available. The solution also can be diluted to a 7.5% solution and delivered via peripheral venous catheter if needed. Clinicians caring for patients in both groups are encouraged to maintain euvolemia with diuresis, when clinically appropriate. Intravenous amino acids beginas close to the time of randomisation as feasible. The target dose, delivered as a continuous infusion, will be started during or immediately after the exercise session. Usually, a participant will receive the amino acid infusion every day that the participant receives the cycling intervention. However, on a day when the cycling intervention is withheld (eg, safety reason), the amino acid infusion will still be given. Amino acid infusion will continue until ICU discharge or 21 days, whichever occurs earlier. If the patient is discharged from ICU, then readmitted, amino acid administration will continue until the 21st calendar day after randomisation.
Guidelines recommend that ICU patients with AKI receive standard protein intake as it appears to be utilised, improves nitrogen balance and does not lead to increased urea generation.55 Additionally, guidelines suggest that ICU patients receiving renal replacement therapy (RRT) should receive increased protein, up to a maximum of 2.5 g/kg/day.55 Therefore, patients with moderate to severe AKI or chronic kidney disease who are not yet receiving RRT will be allowed to participate in the study. However, because these patients are at risk of developing azotemia with increased amino acid intake, the urea will be monitored daily as part of standard of care. If urea is >100 mg/dL and there is no plan for RRT that day, the amino acid infusion can be decreased by half of current rate in discussion with the ICU clinical team. It is important to note that azotemia, alone, has not been shown to be harmful; nevertheless, this approach will be taken.54
Description of in-bed cycle ergometry exercise
The cycling intervention will be delivered by trained staff, and started as close to the time of randomisation as feasible (ie, within 24 hours of randomisation). All sites will use a MotoMed Letto II cycle ergometer. The cycling sessions will occur according to a detailed protocol beginning with a safety assessment and continue for the first at least 5 days per week. The safety guidelines for cycling and the cessation of a cycling session can be found in box 1. The intervention group will receive cycling sessions, for up to 45 min duration (as tolerated by patient),83 with vigorous verbal encouragement to promote active cycling. This goal of a 45 min cycling duration was chosen for several reasons: (1) to help prevent under-dosing of the exercise, with it being >2x the dose delivered in the prior positive RCT of cycle ergometry (20 min/ day in ICU),83 (2) our own experience suggests feasibility/tolerability of sessions >20 min in duration91 and (3) even longer cycling sessions may not be beneficial, with a recent small RCT reporting no difference in muscle loss between cycling 1 hour versus 2 hours per day.56 The implementation of the cycling intervention is protocolised to provide graduated resistance during each session and between daily sessions. Cycling will continue through ICU discharge or 21 calendar days after randomisation, whichever occurs earlier (same as amino acids). The intervention specifically occurs during the ICU stay (and not post-ICU) since this represents the portion of hospitalisation in which patients are most exposed to bed rest/immobility (ie, due to ventilation and severity of illness). If the patient is discharged from ICU, then readmitted, the cycling intervention will continue until the 21st calendar day after randomisation. Proper implementation of the cycling will be overseen locally by site investigators and research staff. The data coordinating centre will run periodic data reports to review the implementation of the combined intervention. The protocol that is used in this RCT is adapted from an existing protocol that was developed and extensively used at one of the study sites.91
Box 1. Cycling safety guidelines.
-
Criteria to not commence cycling session
(Cycling should not occur if any of the following conditions are present for greater than 15 min within the 2 hours prior to cycling.)
Heart rate <50 or >140 bpm, or new arrhythmia.
New onset of chest pain of potential cardiac origin.
Presence of femoral extracorporeal membrane oxygenation (ECMO) or intra-aortic balloon pump (IABP).
Mean arterial pressure <65 mm Hg or below target or >120 mm Hg or above target.
-
A single vasopressor as outlined below:
Dopamine >12.5 mcg/kg/min.
Phenylephrine >2 mcg/kg/min.
Norepinephrine >1 mcg/kg/min.
-
≥2 vasopressors at same time, as outlined below:
Vasopressin at ≥0.04 units/min.
Dopamine >10.0 mcg/kg/min.
Phenylephrine >1.6 mcg/kg/min.
Norepinephrine >0.8 mcg/kg/min.
Participant is pale/sweaty and requests not to start due to feeling unwell.
FiO2 >0.8.
PEEP >15 cm H2O.
SpO2 falls >10% of resting level or <85% for more than 60 s, or below target level for more than 60 s in participants with abnormal baseline SpO2.
Recent receipt of neuromuscular blocker medication.
Clinical team’s opinion that participant should not receive cycling despite the absence of above criteria.
-
Criteria to terminate cycling session
Heart rate <50 or >140 bpm or new arrhythmia.
New onset of chest pain of potential cardiac origin.
Presence of femoral ECMO or IABP.
Mean arterial pressure <65 mm Hg or below target or >120 mm Hg or above target.
-
A single vasopressor as outlined below:
Dopamine >12.5 mcg/kg/min.
Phenylephrine >2 mcg/kg/min.
Norepinephrine >1 mcg/kg/min.
-
≥2 vasopressors at same time, as outlined below:
Vasopressin at ≥0.04 units/min.
Dopamine >10.0 mcg/kg/min.
Phenylephrine >1.6 mcg/kg/min.
Norepinephrine >0.8 mcg/kg/min.
Participant is pale/sweaty and requests to stop due to feeling unwell.
FiO2>0.8.
PEEP >15 cm H2O.
SpO2 falls>10% of resting level or <85% for more than 60 s, or below target level for more than 60 s in participants with abnormal baseline SpO2.
Fatigue.
Patient declines to continue.
Clinical team’s opinion that participant should not stop cycling despite the absence of above criteria.
Standard ICU cointerventions for all patients
Important aspects of routine care will be standardised for both intervention and usual care group patients based on pre-existing protocols at all study sites, including (1) turning by nurse every 2 hours while bedbound; (2) frequency, duration and intervention type of all physical therapy and occupational therapy sessions in the ICU; (3) dietician consultation and prescription of a standard enteral nutrition formula (at approximately 20–25 kcal/kg/day and 1.2 g/kg/day) within 48 hours after intubation; (4) blood glucose control with insulin; (5) electrolyte replacement protocols; (6) daily sedation interruption or minimisation and (7) daily spontaneous breathing trials as part of ventilator weaning protocols.
On-study data collection
Demographic, laboratory, physiological, nutritional and rehabilitation data will be collected at study enrolment, then daily in the ICU as outlined in tables 2 and 3. The database will be under password protection in an institutional computer located at the CERU and only the research staff will have access.
Table 2.
Patient variables—collected at enrolment
| Patient variables—collected at enrolment | Collection method |
| Age/sex/ethnicity/race demographic data | Chart review |
| Body mass index | Chart review |
| Comorbidities: Charlson* and functional indices** | Chart review |
| Baseline function: Functional Status Score for ICU77 | Proxy interview (see Outcomes below) |
| Baseline function: Katz Activities of Daily Living Scale154 and Lawton IADL | Proxy interview (see Outcomes below) |
| Baseline function: SF-36 Physical Function domain and walk impairment question | Proxy interview |
| Clinical Frailty Scale | Proxy interview |
| ICU admission diagnosis (eg, sepsis, renal failure) | Chart review |
| Severity of illness: APACHE II† | Chart review |
| Patient location immediately prior to ICU and to hospital admission | Chart review |
*Charlson Index: a score for in- patients derived from 19 comorbidities; an increased score reflecting increased 1 -year mortality. 155
**Functional Index: an 18-diagnosis scale for ICU patients predicting 1 - year SF-36 Physical Function score; increased score reflecting worse function. 156–158
†APACHE II: a severity of illness index using age, medical conditions and acute physiology, with higher scores reflecting increased short-term mortality. 159
IADL, instrumental activities of daily living; ICU, intensive care unit.
Table 3.
ICU-related variables—collected at enrolment and DAILY during ICU stay*
| ICU variables—collected at enrolment and daily during ICU stay | Collection method |
| Sedation medications and dose, with sedation status—RASS score160 and CAM-ICU | Chart review |
| Neuromuscular blocker, corticosteroid drug use and dose | Chart review |
| Insulin dose and blood glucose level | Chart review |
| SOFA§ organ failure score (including vasopressor data) | Chart review |
| Creatinine, creatine phosphokinase, blood urea nitrogen | Chart review |
| Nutrition received (calories/protein, type and route of feeding) | Chart review |
| Mobility/rehabilitation received | Chart review |
| Compliance with proposed intervention regimen | CRF review |
§SOFA: a validated composite score of 6 organ systems used to assess the severity of ICU organ dysfunction.161
CRF, case report form; RASS, Richmond Agitation-Sedation Scale.
Primary outcome
In developing our evaluation framework, we followed the recommendations of a recent expert consensus statement.92 The primary aim of this study is to measure in-patient muscle strength and physical functioning. This will be evaluated using one primary and multiple secondary outcomes. The timing of these are provided in table 4. The primary outcome will be the walking distance achieved during a 6 min walk test (6MWT) measured at hospital discharge. Implementation of the test will be based on the 2014 ATS standards, with adaptation, as needed, for the in-patient setting and ICU survivor population.93 The test will only be performed once, rather than twice as recommended due to the feasibility of asking critically ill patients to perform the test multiple times. The 6MWT is a reliable, valid, responsive measure of physical function94 for survivors of acute respiratory failure. Hospital discharge was chosen as the endpoint to assess the primary outcome, in congruence with other cycling studies.60 95 96
Table 4.
Primary and secondary outcomes—all performed by blinded assessors*
| Instrument | Assessment timing | |
| Primary outcome | ||
| Physical functioning | 6 min walk distance (6MWD) | Hospital discharge |
| Secondary outcomes | ||
| Overall strength-upper and lower extremity | MRC sum-score | Hospital discharge |
| Quadriceps force-lower extremity strength | Hand-held dynamometry | Hospital discharge |
| Distal strength-hand grip strength | Hand grip dynamometry | ICU and hospital discharge |
| Overall Physical Functional status | SPPB and FSS-ICU | ICU and hospital discharge |
| Physical functioning (ADL) | Katz ADL | Hospital discharge |
| Mortality | Chart review | ICU and hospital discharge |
| Length of ventilation, ICU and hospital stay | Chart review | ICU and hospital discharge |
| ICU readmission and reintubation | Chart review | Hospital discharge |
| Hospital-acquired infections | Chart review | Hospital discharge |
| Discharge location (eg, home vs rehab) | Chart review | Hospital discharge |
| Body composition | Ultrasound of rectus femoris, vastus intermedius, tibialis anterior | Enrolment, ICU and hospital discharge |
| Body composition (when clinically available) | Chest CT scan (above the aortic arch) | Only when clinically available |
| Body composition (when clinically available) | Abdominal CT scan at third lumbar vertebra | Only when clinically available |
| Health-related quality of life | SF-36 and EQ-5D-5L | Telephone survey at 6 months |
| Physical functioning | Katz ADL; Lawton IADL | Telephone survey at 6 months |
| Physical functioning | Return to baseline work/activity | Telephone survey at 6 months |
| Physical functioning | Living location | Telephone survey at 6 months |
| Mental and cognitive functioning | MoCA-BLIND, HADS and IES-R | Telephone survey at 6 months |
| Healthcare resource utilisation | Admission to ICU, hospital, rehabilitation and nursing facility | Telephone survey at 6 months |
ADL, activities of daily living; IADL, instrumental activities of daily living; FSS-ICU, Functional Status Score for ICU; ICU, intensive care unit; MRC, Medical Research Council; SPPB, Short Physical Performance Battery.
Secondary outcomes
Secondary strength-related outcome measures will include the following.
Overall strength using Medical Research Council (MRC) sum-score evaluated via standardised ‘manual muscle testing’ with each of 12 muscle groups assessed using a 6-point MRC scale97 and summed to a total score (range: 0–60).8 9 14 77 85 98–100
Quadriceps force, via hand-held dynamometry83 101 for of both lower extremities. Each will be scored by averaging the results of three trials.102 103
Distal strength measured via isometric hand grip strength via a hydraulic hand dynamometer performed bilaterally as per American Society of Hand Therapist guidelines104 and evaluated using normal values.105
Secondary Physical Functioning outcomes will include the following.
Short Physical Performance Battery which measures balance, walking speed and rising from a chair78 80 106–110
Functional Status Score for ICU, which is a 5-item, 35-point assessment of bed mobility, transfers and ambulation, designed for ICU patients77 86 111 112 and was designed and validated specifically in ICU patients evaluated 8-point Functional Independence Measure response scale used throughout rehabilitation assessments113–116 and is responsive to change during recovery for ICU patients.77 86 112 117 118
Body composition
Body composition will be assessed using additional secondary outcomes. Ultrasound (US) will be used to measure rectus femoris and vastus intermedius cross-sectional area and thickness using a published protocol.34 71 119–123 Changes in muscle echodensity will be measured using quantitative greyscale analysis.34 123–125 We will do US at baseline (shortly after randomisation), ICU discharge and hospital discharge. Chest CT and abdominal CT scans will be obtained when clinically available, with chest CT used to measure pectoralis muscle area as it correlates with clinical outcomes in patients with COPD, lung cancer and critical illness,126–128 and abdominal CT scan used to measure abdominal and visceral adipose tissue at the level of the third lumbar vertebra. We will obtain all CT data, when performed for clinical purposes at any point during hospital stay (including during admission process prerandomisation), for comparative analyses between participants in the intervention and control groups. Due to expense, radiation exposure, and required transport out of the ICU for CT, we will not obtain specific research CT scans.
Hospital Acquired Infections
An additional secondary outcome is hospital-acquired infections. Data suggest that increased protein intake reduces infections.20 30 33 Recording culture results and antibiotics administered, along with pertinent clinical data will enable adjudication of infectious complications using pre-existing methodology.129 130
Patient-reported outcomes
Finally, outcomes after hospital discharge will be assessed via 6-month phone-based follow-up. Health-related QOL will be measured using SF-36 version 2 (SF-36 v2) and EQ-5D-5L. The SF-36 is valid and reliable across a variety of patient groups, including ICU survivors.131 132 The EQ-5D-5L is included, in addition to SF-36 v2, because it is suitable for patients with inattention and fatigue,133 134 recommended for use in ICU survivors.135 136 Physical functional status will be measured using Katz activities of daily living (ADL)137 and Lawton’s Instrumental ADL (IADL)138 scales, as well as return to baseline work/activity and living location. Mental and cognitive function will be measured, in addition, using the HADS, IES-R and MoCA-BLIND screening questionnaires as part the recommended Core Outcome Measurement Set for evaluating postdischarge outcomes in acute respiratory failure survivors.136 Healthcare resource utilisation will be assessed through a structured interview regarding admissions to hospital, skilled nursing and rehabilitation facilities as done in prior research.11 139–142 In order to improve retention, a call will be made to participants at 3 months to update contact information and act as a reminder of upcoming follow-up assessments to be completed at the 6-month time point.
Adverse events
Patients will be monitored daily, their medical records examined and their care providers queried for adverse events that are serious and unexpected in nature. Unexpected SAEs will be recorded from the time of randomisation until ICU discharge or day 21, whichever comes first. These unexpected SAEs which are inconsistent with underlying pathophysiology or progression of underlying disease will be documented in study source documents and reported unexpected SAEs that are related or possibly related to participation in the study will be reported to the participating site IRBs, DSMB and NHLBI on an expedited basis. Deidentified reporting will occur within 7 calendar days of receipt of the initial report for fatal and life-threatening events. All other events (ie, non-fatal and non-life-threatening) will be reported within 15 calendar days of receipt of the initial report. In addition, we will capture events that may not be serious but may be related to the amino acid infusion (uremia>100 mg/dL, peripheral phlebitis) or cycle ergometry exercise (times when the safety criteria thresholds for blood pressure, heart rate are reached and cycling stopped).
Statistical analysis
Power calculation
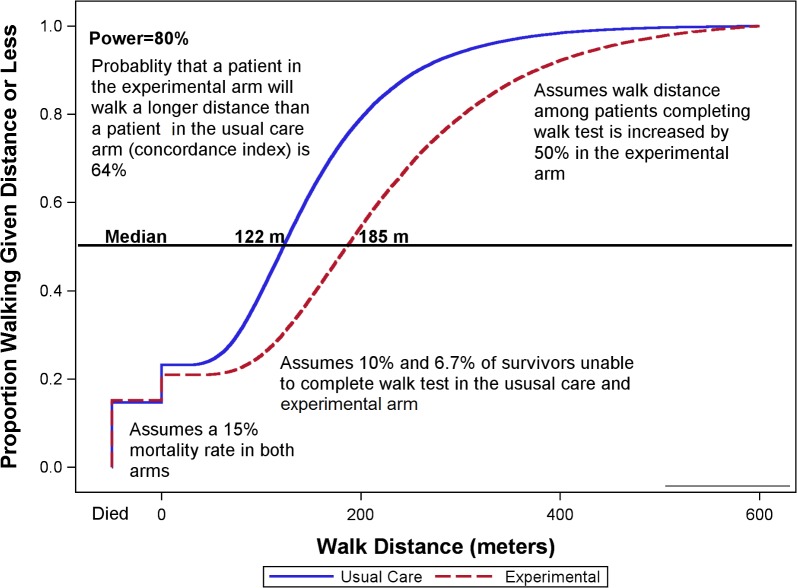
Sample size was determined based on the primary outcome, 6MWT at hospital discharge. We plan to enrol 64 evaluable patients per arm (total n=128 evaluable patients) which achieves >80% power using the Wilcoxon Rank-sum test at a two-sided alpha=0.05 to detect differences in 6MWD distribution between the two groups with discrimination (ie, ROC AUC or concordance index)>64%. Figure 3 depicts a sample scenario yielding a concordance index of 64% and 80% power.
Figure 3.
Sample empirical distribution function of walk distance.
Using a traditional t-test approach, this sample size would have 80% power with α=0.05 (two sided) to detect a 50-m difference in 6MWD (with SD=100 based on our prior RCT data in ICU patients (NCT01206166)). We expect <10% non-participation rate for the primary outcome (ie, patients capable of 6MWD but declining testing); thus, we will enrol a total of 142 patients.
Primary analysis
All analyses will follow the intention-to-treat principle. A two-tailed p-value<0.05 will be considered statistically significant. The 6MWD will be compared between the two groups using the rank-based Mann-Whitney U test.143 This approach allows inclusion of all randomised patients, per the intent-to-treat principle, by assigning decedents a lower value than all survivors (eg, −1) and assigning patients incapable of performing the 6MWD test a value of 0, including the rare patients discharged while still receiving mechanical ventilation.143 144 In addition to this rank-based test, we will describe the differences in 6MWD between the two groups graphically as shown in figure 3. Time from randomisation to 6MWD testing will be described by arm, and a sensitivity analysis will adjust for the time to testing before comparing the adjusted ranked 6MWD between arms. A per-protocol analysis will also be performed by excluding patients randomised to the intervention arm who do not receive at least 3 days of the combined intervention and patients in the usual care group who stay less than 3 days in the ICU.
Secondary analysis
Secondary continuous outcomes will be analysed similar to the primary outcome. Categorical secondary outcomes will include death and unable-to-perform as potential categories and will be analysed using Fisher’s exact tests. As secondary outcomes will be considered hypothesis-generating, we will not formally correct for multiple comparisons but will consider the number of secondary comparisons when interpreting our results.
The number of missing values will be described for all outcomes. Outcomes will not be considered missing due to death since the proposed statistical methods include decedents in the between arm comparisons. Patient characteristics will be compared between those with versus without missing outcomes.145–147 If >5% of outcome data are missing, multiple imputation will be used,148 and for the primary analysis a ‘missing not at random’ sensitivity analysis will be performed using the tipping point approach of the pattern mixture model with multiple imputation, as per the SAS MI procedure.149 150
Patient and public involvement
This research question was the highest priority topic arising from an expert panel on research priorities for intensive care nutrition and metabolism.19 The outcomes and associated measurement instruments being utilised have been informed by a robust international Delphi consensus process with a panel that included almost 25% patient/family representatives,151 152 and is also informed by many foundational studies (leading up to the Delphi) that included patient/family input, as summarised in a recent publication.153 The intervention that formed the foundation for this RCT was extensively tested with critically ill patients for its feasibility, including any need to stop the intervention due to patient request, agitation, pain or physiological issues.91 We did not include patients and members of public in the design process, recruitment or conducting of the study. The results will be published in peer-reviewed journals and reference to these works will be posted on our websites (eg, www.criticalcarenutrition.com), which are in the public space.
Ethics
The study sponsor is the NIH/NHBLI. The University of Vermont is the lead site. The NIH/NHBLI will take no part in design, conduct of the study, collection, management, analysis, interpretation of the data, preparation, review and approval of the manuscript. We have constituted a data monitoring committee to provide a third-party assessment of interim analyses and review of the scientific literature as it evolves over the duration of the trial.
Discussion
We are conducting the first randomised trial of combined exercise and nutrition applied early in the context of critically illness. We will test whether this intervention improves the functional recovery and QOL of survivors of critical illness. If proven effective, this combined intervention has potential to improve care of ICU patients and have an important public health impact on the growing number of ICU survivors. After completion of this phase II RCT, a decision regarding progression to a phase III RCT will be based on the study findings of feasibility, safety and benefit, in addition to funding considerations. The limitations of work include a lack of blinding of the study interventions, which is impossible to do. Accordingly, we have blinded outcome assessors and standardised key cointerventions, and will report on their use in each group. We are utilising novel rank-based statistical analyses to account for patients who die before assessment of the primary outcome, as well as patients who are unable to walk at hospital discharge. The small sample size and the limited number of sites limits the generalisability of our findings. Nevertheless, our results will surely inform both clinical practice and future research in this area.
Supplementary Material
Footnotes
Contributors: DKH, RS, DN, DCF, CH, AD, ND and MM designed the study and the protocol submission. DKH and GJC wrote the manuscript. RS, DN, DCF, CH, AD, ND and MM revised the manuscript and approved the final version.
Funding: This work was supported by the NIH grant number R01HL132887, along with an unrestricted research grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices.
Competing interests: For purposes of conducting this NIH/NHLBI-funded clinical trial, Dr Heyland reports a grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. For purposes of conducting this NIH/NHLBI-funded clinical trial, Dr Needham reports a grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. For purposes of conducting this NIH/NHLBI-funded clinical trial, Dr Stapleton reports a grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. Dr Mourtzakis, John Clarke and Andrew Day have nothing to disclose. Dr Files reports grants from National Institute of Health during the conduct of the study. Dr Files also reports a grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. Dr Hough reports grants from NIH during the conduct of the study. Dr Hough also reports a grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Devices. Nicolaas E Deutz declares no conflicts of interests, but discloses that he is a coinventor of several patents, owned by others, has served on scientific advisory boards for Novartis and Baxter and has been a consultant for Abbott Nutrition, Ajinomoto, OCERA and VitaNext. Texas A&M CTRAL receives funding from NIH, NSF, Abbott Nutrition, ICAAS, ESPEN fellowship, George Abramson Donation, Yani Mizubuti Donation and Internal Grants.
Ethics approval: The trial received approval of the ethics committee at each institution. It will be conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the approved protocol. We plan to disseminate the results in peer-reviewed journals, at national and international conferences and via content specific web-based knowledge translation platforms (see and.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Adhikari NK, Fowler RA, Bhagwanjee S, et al. . Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339–46. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn JM, Goss CH, Heagerty PJ, et al. . Hospital volume and the outcomes of mechanical ventilation. N Engl J Med Overseas Ed 2006;355:41–50. 10.1056/NEJMsa053993 [DOI] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Luippold RS, Sulsky S, et al. . Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med 2008;36:724–30. 10.1097/CCM.0B013E31816536F7 [DOI] [PubMed] [Google Scholar]
- 4. Cox CE, Martinu T, Sathy SJ, et al. . Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med 2009;37:2888–94. 10.1097/CCM.0b013e3181ab86ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacIntyre NR, Epstein SK, Carson S, et al. . Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest 2005;128:3937–54. 10.1378/chest.128.6.3937 [DOI] [PubMed] [Google Scholar]
- 6. Frutos-Vivar F, Esteban A, Apezteguía C, et al. . Outcome of mechanically ventilated patients who require a tracheostomy. Crit Care Med 2005;33:290–8. 10.1097/01.CCM.0000150026.85210.13 [DOI] [PubMed] [Google Scholar]
- 7. Spragg RG, Bernard GR, Checkley W, et al. . Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med 2010;181 10.1164/rccm.201001-0024WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali NA, O’Brien JM, Hoffmann SP, et al. . Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261–8. 10.1164/rccm.200712-1829OC [DOI] [PubMed] [Google Scholar]
- 9. De Jonghe B, Sharshar T, Lefaucheur JP, et al. . Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859 10.1001/jama.288.22.2859 [DOI] [PubMed] [Google Scholar]
- 10. Herridge MS, Cheung AM, Tansey CM, et al. . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med Overseas Ed 2003;348:683–93. 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 11. Cheung AM, Tansey CM, Tomlinson G, et al. . Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538–44. 10.1164/rccm.200505-693OC [DOI] [PubMed] [Google Scholar]
- 12. Herridge MS, Tansey CM, Matté A, et al. . Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 13. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. . Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 2012;185 10.1164/rccm.201103-0503OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan E, Dowdy DW, Colantuoni E, et al. . Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42:849–59. 10.1097/CCM.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens RD, Marshall SA, Cornblath DR, et al. . A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009;37:S299–308. 10.1097/CCM.0b013e3181b6ef67 [DOI] [PubMed] [Google Scholar]
- 16. Sharshar T, Bastuji-Garin S, Stevens RD, et al. . Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 2009;37:3047–53. 10.1097/CCM.0b013e3181b027e9 [DOI] [PubMed] [Google Scholar]
- 17. Hermans G, Van Mechelen H, Clerckx B, et al. . Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2014;190:410–20. 10.1164/rccm.201312-2257OC [DOI] [PubMed] [Google Scholar]
- 18. Dinglas VD, Aronson Friedman L, Colantuoni E, et al. . Muscle Weakness and 5-Year Survival in Acute Respiratory Distress Syndrome Survivors. Crit Care Med 2017;45:446–53. 10.1097/CCM.0000000000002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arabi YM, Casaer MP, Chapman M, et al. . The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 2017;43:1239–56. 10.1007/s00134-017-4711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heidegger CP, Berger MM, Graf S, et al. . Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381:385–93. 10.1016/S0140-6736(12)61351-8 [DOI] [PubMed] [Google Scholar]
- 21. Doig GS, Simpson F, Finfer S, et al. . Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 2008;300:2731–41. 10.1001/jama.2008.826 [DOI] [PubMed] [Google Scholar]
- 22. Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med Overseas Ed 2014;370:1227–36. 10.1056/NEJMra1304623 [DOI] [PubMed] [Google Scholar]
- 23. Rice TW, Wheeler AP, Thompson BT, et al. . Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307 10.1001/jama.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casaer MP, Mesotten D, Hermans G, et al. . Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506–17. 10.1056/NEJMoa1102662 [DOI] [PubMed] [Google Scholar]
- 25. Arabi YM, Tamim HM, Dhar GS, et al. . Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011;93:569–77. 10.3945/ajcn.110.005074 [DOI] [PubMed] [Google Scholar]
- 26. Doig GS, Simpson F, Sweetman EA, et al. . Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130 10.1001/jama.2013.5124 [DOI] [PubMed] [Google Scholar]
- 27. Casaer MP. Muscle weakness and nutrition therapy in ICU. Curr Opin Clin Nutr Metab Care 2015;18:162–8. 10.1097/MCO.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 28. Arabi YM, Aldawood AS, Haddad SH, et al. . Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med Overseas Ed 2015;372:2398–408. 10.1056/NEJMoa1502826 [DOI] [PubMed] [Google Scholar]
- 29. Alberda C, Gramlich L, Jones N, et al. . The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009;35:1728–37. 10.1007/s00134-009-1567-4 [DOI] [PubMed] [Google Scholar]
- 30. Heyland DK, Stephens KE, Day AG, et al. . The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr 2011;30:148–55. 10.1016/j.clnu.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 31. Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake!. Crit Care Med 2011;39:2619–26. 10.1097/CCM.0b013e318226641d [DOI] [PubMed] [Google Scholar]
- 32. Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, et al. . Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr 2012;31:462–8. 10.1016/j.clnu.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 33. Nicolo M, Heyland DK, Chittams J, et al. . Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. JPEN J Parenter Enteral Nutr 2016;40 10.1177/0148607115583675 [DOI] [PubMed] [Google Scholar]
- 34. Puthucheary ZA, Rawal J, McPhail M, et al. . Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 35. Casaer MP, Wilmer A, Hermans G, et al. . Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 2013;187:247–55. 10.1164/rccm.201206-0999OC [DOI] [PubMed] [Google Scholar]
- 36. Braunschweig CL, Freels S, Sheean PM, et al. . Role of timing and dose of energy received in patients with acute lung injury on mortality in the Intensive Nutrition in Acute Lung Injury Trial (INTACT): a post hoc analysis. Am J Clin Nutr 2017;105:411–6. 10.3945/ajcn.116.140764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heyland D, Earthman C, Compher C. Acute muscle wasting among critically ill patients. JAMA 2014;311:621–2. 10.1001/jama.2013.285420 [DOI] [PubMed] [Google Scholar]
- 38. Hsieh LC, Chien SL, Huang MS, et al. . Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr 2006;15:544–50. [PubMed] [Google Scholar]
- 39. Fetterplace K, Deane AM, Tierney A, et al. . Targeted full energy and protein delivery in critically ill patients: a pilot Randomized Controlled Trial (FEED Trial). JPEN J Parenter Enteral Nutr 2018;42:1252–62. 10.1002/jpen.1166 [DOI] [PubMed] [Google Scholar]
- 40. Ferrie S, Allman-Farinelli M, Daley M, et al. . Protein Requirements in the Critically Ill. Journal of Parenteral and Enteral Nutrition 2016;40:795–805. 10.1177/0148607115618449 [DOI] [PubMed] [Google Scholar]
- 41. Liebau F, Sundström M, van Loon LJ, et al. . Short-term amino acid infusion improves protein balance in critically ill patients. Crit Care 2015;19:106 10.1186/s13054-015-0844-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Twyman D, Young AB, Ott L, et al. . High protein enteral feedings: a means of achieving positive nitrogen balance in head injured patients. JPEN J Parenter Enteral Nutr 1985;9:679–84. 10.1177/0148607185009006679 [DOI] [PubMed] [Google Scholar]
- 43. Scheinkestel CD, Kar L, Marshall K, et al. . Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition 2003;19(11-12):909–16. 10.1016/S0899-9007(03)00175-8 [DOI] [PubMed] [Google Scholar]
- 44. Wolfe RR, Goodenough RD, Burke JF, et al. . Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg 1983;197:163–71. 10.1097/00000658-198302000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 1987;205:288–94. 10.1097/00000658-198703000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greig PD, Elwyn DH, Askanazi J, et al. . Parenteral nutrition in septic patients: effect of increasing nitrogen intake. Am J Clin Nutr 1987;46:1040–7. 10.1093/ajcn/46.6.1040 [DOI] [PubMed] [Google Scholar]
- 47. Müller TF, Müller A, Bachem MG, et al. . Immediate metabolic effects of different nutritional regimens in critically ill medical patients. Intensive Care Med 1995;21:561–6. 10.1007/BF01700160 [DOI] [PubMed] [Google Scholar]
- 48. Scheinkestel CD, Adams F, Mahony L, et al. . Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition 2003;19:733–40. 10.1016/S0899-9007(03)00107-2 [DOI] [PubMed] [Google Scholar]
- 49. Singer P. High-dose amino acid infusion preserves diuresis and improves nitrogen balance in non-oliguric acute renal failure. Wien Klin Wochenschr 2007;119(7-8):218–22. 10.1007/s00508-007-0794-3 [DOI] [PubMed] [Google Scholar]
- 50. Verbruggen SC, Coss-Bu J, Wu M, et al. . Current recommended parenteral protein intakes do not support protein synthesis in critically ill septic, insulin-resistant adolescents with tight glucose control. Crit Care Med 2011;39:2518–25. 10.1097/CCM.0b013e3182257410 [DOI] [PubMed] [Google Scholar]
- 51. Iapichino G, Radrizzani D, Scherini A, et al. . Essential and non-essential amino acid requirement in injured patients receiving total parenteral nutrition. Intensive Care Med 1988;14:399–405. 10.1007/BF00262896 [DOI] [PubMed] [Google Scholar]
- 52. Doig GS, Simpson F, Bellomo R, et al. . Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 2015;41:1197–208. 10.1007/s00134-015-3827-9 [DOI] [PubMed] [Google Scholar]
- 53. Zhu R, Allingstrup MJ, Perner A, et al. . The effect of IV Amino acid supplementation on mortality in ICU Patients may be dependent on kidney function: post hoc subgroup analyses of a multicenter randomized trial. Crit Care Med 2018;46:1293–301. 10.1097/CCM.0000000000003221 [DOI] [PubMed] [Google Scholar]
- 54. Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012;96:591–600. 10.3945/ajcn.111.032078 [DOI] [PubMed] [Google Scholar]
- 55. McClave SA, Martindale RG, Vanek VW, et al. . Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nut 2016;40:159–211. 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]
- 56. Preiser J-C, De Prato C, Harvengt A, et al. . Passive Cycling limits myofibrillar protein catabolism in unconscious patients: a pilot study. J Nov Physiother 2014;4:1–6. [Google Scholar]
- 57. Tipping CJ, Harrold M, Holland A, et al. . The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med 2017;43:171–83. 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]
- 58. Devlin JW, Skrobik Y, Gélinas C, et al. . Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825–73. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 59. Hickmann CE, Castanares-Zapatero D, Deldicque L, et al. . Impact of very early physical therapy during septic shock on skeletal muscle: a randomized controlled trial. Crit Care Med 2018;46:1436–43. 10.1097/CCM.0000000000003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fossat G, Baudin F, Courtes L, et al. . Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: a randomized clinical trial. JAMA 2018;320:368–78. 10.1001/jama.2018.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morris PE, Berry MJ, Files DC, et al. . Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016;315:2694–702. 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moss M, Nordon-Craft A, Malone D, et al. . A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016;193:1101–10. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Symons TB, Sheffield-Moore M, Mamerow MM, et al. . The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging 2011;15:376–81. 10.1007/s12603-010-0319-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–9. 10.1097/MCO.0b013e328333aa66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tieland M, Dirks ML, van der Zwaluw N, et al. . Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–9. 10.1016/j.jamda.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 66. Bonnefoy M, Cornu C, Normand S, et al. . The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br J Nutr 2003;89:731–8. 10.1079/BJN2003836 [DOI] [PubMed] [Google Scholar]
- 67. Fiatarone MA, O’Neill EF, Ryan ND, et al. . Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–75. 10.1056/NEJM199406233302501 [DOI] [PubMed] [Google Scholar]
- 68. Villareal DT, Chode S, Parimi N, et al. . Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med Overseas Ed 2011;364:1218–29. 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Botros D, Somarriba G, Neri D, et al. . Interventions to address chronic disease and HIV: strategies to promote exercise and nutrition among HIV-infected individuals. Curr HIV/AIDS Rep 2012;9:351–63. 10.1007/s11904-012-0135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Payne C, Larkin PJ, McIlfatrick S, et al. . Exercise and nutrition interventions in advanced lung cancer: a systematic review. Curr Oncol 2013;20:321 10.3747/co.20.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arbeille P, Kerbeci P, Capri A, et al. . Quantification of muscle volume by echography: comparison with MRI data on subjects in long-term bed rest. Ultrasound Med Biol 2009;35:1092–7. 10.1016/j.ultrasmedbio.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 72. Trappe TA, Burd NA, Louis ES, et al. . Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol 2007;191:147–59. 10.1111/j.1748-1716.2007.01728.x [DOI] [PubMed] [Google Scholar]
- 73. Cermak NM, Res PT, de Groot LC, et al. . Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 74. Jones C, Eddleston J, McCairn A, et al. . Improving rehabilitation after critical illness through outpatient physiotherapy classes and essential amino acid supplement: A randomized controlled trial. J Crit Care 2015;30:901–7. 10.1016/j.jcrc.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 75. Ferrando AA, Stuart CA, Brunder DG, et al. . Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat Space Environ Med 1995;66:976–81. [PubMed] [Google Scholar]
- 76. LeBlanc AD, Schneider VS, Evans HJ, et al. . Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 1992;73:2172–8. 10.1152/jappl.1992.73.5.2172 [DOI] [PubMed] [Google Scholar]
- 77. Zanni JM, Korupolu R, Fan E, et al. . Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care 2010;25:254–62. 10.1016/j.jcrc.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 78. Guralnik JM, Ferrucci L, Pieper CF, et al. . Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31. 10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 79. Studenski S, Perera S, Wallace D, et al. . Physical performance measures in the clinical setting. J Am Geriatr Soc 2003;51:314–22. 10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 80. Guralnik JM, Ferrucci L, Simonsick EM, et al. . Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–62. 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities. A clinical report. Phys Ther 1988;68:1371–3. 10.1093/ptj/68.9.1371 [DOI] [PubMed] [Google Scholar]
- 82. Langlois JA, Keyl PM, Guralnik JM, et al. . Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health 1997;87:393–7. 10.2105/AJPH.87.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Burtin C, Clerckx B, Robbeets C, et al. . Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009;37:2499–505. 10.1097/CCM.0b013e3181a38937 [DOI] [PubMed] [Google Scholar]
- 84. Puthucheary Z, Harridge S, Hart N. Skeletal muscle dysfunction in critical care: wasting, weakness, and rehabilitation strategies. Crit Care Med 2010;38:S676–82. 10.1097/CCM.0b013e3181f2458d [DOI] [PubMed] [Google Scholar]
- 85. Schweickert WD, Pohlman MC, Pohlman AS, et al. . Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–82. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kho ME, Truong AD, Zanni JM, et al. . Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care 2015;30:32–9. 10.1016/j.jcrc.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Villamar MF, Contreras VS, Kuntz RE, et al. . The reporting of blinding in physical medicine and rehabilitation randomized controlled trials: a systematic review. J Rehabil Med 2013;45:6–13. 10.2340/16501977-1071 [DOI] [PubMed] [Google Scholar]
- 88. Hamwi GJ, Therapy: changing dietary concepts. New York, NY: American Diabetes Association, 1964:73–8. [Google Scholar]
- 89. Heyland DK, Dhaliwal R, Wang M, et al. . The prevalence of iatrogenic underfeeding in the nutritionally ’at-risk' critically ill patient: Results of an international, multicenter, prospective study. Clin Nutr 2015;34 10.1016/j.clnu.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 90. Hoffer LJ. How much protein do parenteral amino acid mixtures provide? Am J Clin Nutr 2011;94:1396–8. 10.3945/ajcn.111.023390 [DOI] [PubMed] [Google Scholar]
- 91. Kimawi I, Lamberjack B, Nelliot A, et al. . Safety and feasibility of a protocolized approach to in-bed cycling exercise in the intensive care unit: quality improvement project. Phys Ther 2017;97:593–602. 10.1093/ptj/pzx034 [DOI] [PubMed] [Google Scholar]
- 92. Heyland DK, Stapleton RD, Mourtzakis M, et al. . Combining nutrition and exercise to optimize survival and recovery from critical illness: Conceptual and methodological issues. Clin Nutr 2016;35:1196–206. 10.1016/j.clnu.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 93. Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 94. Chan KS, Pfoh ER, Denehy L, et al. . Construct validity and minimal important difference of 6-minute walk distance in survivors of acute respiratory failure. Chest 2015;147:1316–26. 10.1378/chest.14-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kho ME, Molloy AJ, Clarke FJ, et al. . Multicentre pilot randomised clinical trial of early in-bed cycle ergometry with ventilated patients. BMJ Open Respir Res 2019;6:e000383 10.1136/bmjresp-2018-000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kho ME, Molloy AJ, Clarke FJ, et al. . TryCYCLE: a prospective study of the safety and feasibility of early in-bed cycling in mechanically ventilated patients. PLoS One 2016;11:e0167561 10.1371/journal.pone.0167561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Editorial Committee for the Guarantors of Brain. Aids to the examination of the peripheral nervous system. London: Ballière Tindall 1986. [Google Scholar]
- 98. Routsi C, Gerovasili V, Vasileiadis I, et al. . Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 2010;14:R74 10.1186/cc8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zanotti E, Felicetti G, Maini M, et al. . Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003;124:292–6. 10.1378/chest.124.1.292 [DOI] [PubMed] [Google Scholar]
- 100. Fan E, Ciesla ND, Truong AD, et al. . Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med 2010;36:1038–43. 10.1007/s00134-010-1796-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bohannon RW, Andrews AW. Interrater reliability of hand-held dynamometry. Phys Ther 1987;67:931–3. 10.1093/ptj/67.6.931 [DOI] [PubMed] [Google Scholar]
- 102. Baldwin CE, Paratz JD, Bersten AD. Muscle strength assessment in critically ill patients with handheld dynamometry: an investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care 2013;28:77–86. 10.1016/j.jcrc.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 103. Vanpee G, Segers J, Van Mechelen H, et al. . The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med 2011;39:1929–34. 10.1097/CCM.0b013e31821f050b [DOI] [PubMed] [Google Scholar]
- 104. Massy-Westropp N, Rankin W, Ahern M, et al. . Measuring grip strength in normal adults: reference ranges and a comparison of electronic and hydraulic instruments. J Hand Surg Am 2004;29:514–9. 10.1016/j.jhsa.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 105. Mathiowetz V, Kashman N, Volland G, et al. . Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985;66:69–74. [PubMed] [Google Scholar]
- 106. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 107. Simonsick EM, Maffeo CE, Rogers SK, et al. . Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci 1997;52:M264–74. 10.1093/gerona/52A.5.M264 [DOI] [PubMed] [Google Scholar]
- 108. Corti MC, Guralnik JM, Salive ME, et al. . Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 1994;272:1036–42. 10.1001/jama.1994.03520130074036 [DOI] [PubMed] [Google Scholar]
- 109. Melzer D, Lan TY, Guralnik JM. The predictive validity for mortality of the index of mobility-related limitation--results from the EPESE study. Age Ageing 2003;32:619–25. 10.1093/ageing/afg107 [DOI] [PubMed] [Google Scholar]
- 110. Ostir GV, Markides KS, Black SA, et al. . Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci 1998;53:M491–5. 10.1093/gerona/53A.6.M491 [DOI] [PubMed] [Google Scholar]
- 111. Tipping CJ, Young PJ, Romero L, et al. . A systematic review of measurements of physical function in critically ill adults. Crit Care Resusc 2012;14:302–11. [PubMed] [Google Scholar]
- 112. Thrush A, Rozek M, Dekerlegand JL. The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long-term acute care hospital: a prospective cohort study. Phys Ther 2012;92:1536–45. 10.2522/ptj.20110412 [DOI] [PubMed] [Google Scholar]
- 113. Ottenbacher KJ, Hsu Y, Granger CV, et al. . The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996;77:1226–32. 10.1016/S0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- 114. The inpatient rehabilitation facility–patient assessment instrument (IRF-PAI) training manual. 2012. http://www.cms.gov/.
- 115. Heinemann AW, Kirk P, Hastie BA, et al. . Relationships between disability measures and nursing effort during medical rehabilitation for patients with traumatic brain and spinal cord injury. Arch Phys Med Rehabil 1997;78:143–9. 10.1016/S0003-9993(97)90255-0 [DOI] [PubMed] [Google Scholar]
- 116. Hamilton BB, Laughlin JA, Fiedler RC, et al. . Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 1994;26:115–9. [PubMed] [Google Scholar]
- 117. Huang M, Chan KS, Zanni JM, et al. . Functional Status Score for the ICU: an international clinimetric analysis of validity, responsiveness, and minimal important difference. Crit Care Med 2016;44:e1155–e64. 10.1097/CCM.0000000000001949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Parry SM, Huang M, Needham DM. Evaluating physical functioning in critical care: considerations for clinical practice and research. Crit Care 2017;21:249 10.1186/s13054-017-1827-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Seymour JM, Ward K, Sidhu PS, et al. . Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 2009;64:418–23. 10.1136/thx.2008.103986 [DOI] [PubMed] [Google Scholar]
- 120. Marquis K, Debigaré R, Lacasse Y, et al. . Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809–13. 10.1164/rccm.2107031 [DOI] [PubMed] [Google Scholar]
- 121. Shrikrishna D, Patel M, Tanner RJ, et al. . Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012;40:1115–22. 10.1183/09031936.00170111 [DOI] [PubMed] [Google Scholar]
- 122. Tillquist M, Kutsogiannis DJ, Wischmeyer PE, et al. . Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr 2014;38:886–90. 10.1177/0148607113501327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cartwright MS, Kwayisi G, Griffin LP, et al. . Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve 2013;47:255–9. 10.1002/mus.23525 [DOI] [PubMed] [Google Scholar]
- 124. Rooyackers O, Wernerman J. Imaging opens possibilities both to target and to evaluate nutrition in critical illness. Crit Care 2014;18:144 10.1186/cc13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Connolly B, MacBean V, Crowley C, et al. . Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: a systematic review. Crit Care Med 2015;43:897-905 10.1097/CCM.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 126. Kinsey CM, San José Estépar R, van der Velden J, et al. . Lower pectoralis muscle area is associated with a worse overall survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2017;26:38–43. 10.1158/1055-9965.EPI-15-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McDonald ML, Diaz AA, Ross JC, et al. . Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014;11:326–34. 10.1513/AnnalsATS.201307-229OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Khan M, Itty R, Chieng H, et al. . ICU admission skeletal muscle mass, in-hospital outcomes and 6-months mortality: a prospective study. Abstract #320 in session A104, presented at the American Thoracic Society International Conference in Washington, DC on 5/21/17. 2017.
- 129. Heyland D, Muscedere J, Wischmeyer PE, et al. . A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97. 10.1056/NEJMoa1212722 [DOI] [PubMed] [Google Scholar]
- 130. Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005;33:1538–48. 10.1097/01.CCM.0000168253.91200.83 [DOI] [PubMed] [Google Scholar]
- 131. Chrispin PS, Scotton H, Rogers J, et al. . Short Form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia 1997;52:15–23. 10.1111/j.1365-2044.1997.015-az014.x [DOI] [PubMed] [Google Scholar]
- 132. Heyland DK, Hopman W, Coo H, et al. . Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med 2000;28:3599–605. 10.1097/00003246-200011000-00006 [DOI] [PubMed] [Google Scholar]
- 133. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 134. Dowdy DW, Eid MP, Sedrakyan A, et al. . Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med 2005;31:611–20. 10.1007/s00134-005-2592-6 [DOI] [PubMed] [Google Scholar]
- 135. Angus DC, Carlet J. 2002 Brussels Roundtable Participants. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med 2003;29:368–77. 10.1007/s00134-002-1624-8 [DOI] [PubMed] [Google Scholar]
- 136. Connolly B, Hough CL. Coloring by Number? Core outcome measures and the canvas of intensive care unit survivorship. Am J Respir Crit Care Med 2017;196:1087–9. 10.1164/rccm.201706-1239ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Katz IR. On the inseparability of mental and physical health in aged persons: lessons from depression and medical comorbidity. Am J Geriatr Psychiatry 1996;4:1–16. 10.1097/00019442-199624410-00001 [DOI] [PubMed] [Google Scholar]
- 138. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 139. Needham DM, Dinglas VD, Bienvenu OJ, et al. . One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 2013;346:f1532 10.1136/bmj.f1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Needham DM, Dennison CR, Dowdy DW, et al. . Study protocol: the improving care of acute lung injury patients (ICAP) study. Crit Care 2006;10:R9 10.1186/cc3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Clermont G, Kong L, Weissfeld LA, et al. . The effect of pulmonary artery catheter use on costs and long-term outcomes of acute lung injury. PLoS One 2011;6:e22512 10.1371/journal.pone.0022512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ruhl AP, Lord RK, Panek JA, et al. . Health care resource use and costs of two-year survivors of acute lung injury. An observational cohort study. Ann Am Thorac Soc 2015;12:392–401. 10.1513/AnnalsATS.201409-422OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials 1999;20:408–22. 10.1016/S0197-2456(99)00022-7 [DOI] [PubMed] [Google Scholar]
- 144. Colantuoni E, Scharfstein DO, Wang C, et al. . Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ 2018;360:j5748 10.1136/bmj.j5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Diehr P, Johnson LL. Accounting for missing data in end-of-life research. J Palliat Med 2005;8(Suppl 1):s-50–7. 10.1089/jpm.2005.8.s-50 [DOI] [PubMed] [Google Scholar]
- 146. Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley & Sons, 1987. [Google Scholar]
- 147. Shafer J. Missing data in longitudinal studies: a review. Nashville: National Institute on Drug Abuse, 2005. [Google Scholar]
- 148. Carpenter J, Kenward M. Multiple imputation and its application. Hoboken, New Jersey: John Wiley & Sons, 2012. [Google Scholar]
- 149. National Research Council. The prevention and treatment of missing data in clinical trials. Panel on handling missing data in clinical trials. Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press, 2010. [Google Scholar]
- 150. Yuan Y. Sensitivity analysis in multiple imputation for missing data. Paper presented at: proceedings of the SAS Global Forum 2014 Conference. 2014. http://support.sas.com/resources/papers/proceedings14/SAS270-2014.pdf.
- 151. Turnbull AE, Sepulveda KA, Dinglas VD, et al. . Core domains for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Crit Care Med 2017;45:1001–10. 10.1097/CCM.0000000000002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Needham DM, Sepulveda KA, Dinglas VD, et al. . Core outcome measures for clinical research in acute respiratory failure survivors. An international modified delphi consensus study. Am J Respir Crit Care Med 2017;196:1122–30. 10.1164/rccm.201702-0372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dinglas VD, Faraone LN, Needham DM. Understanding patient-important outcomes after critical illness: a synthesis of recent qualitative, empirical, and consensus-related studies. Curr Opin Crit Care 2018;24:401–9. 10.1097/MCC.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Katz S, Ford AB, Moskowitz RW, et al. . Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 155. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 156. Fan E, Gifford JM, Chandolu S, et al. . The functional comorbidity index had high inter-rater reliability in patients with acute lung injury. BMC Anesthesiol 2012;12:21 10.1186/1471-2253-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Groll DL, To T, Bombardier C, et al. . The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595–602. 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 158. Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 2005;33:1549–56. 10.1097/01.CCM.0000168609.98847.50 [DOI] [PubMed] [Google Scholar]
- 159. Knaus WA, Draper EA, Wagner DP, et al. . APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 160. Ely EW, Truman B, Shintani A, et al. . Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003;289:2983–94. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 161. Vincent JL, Moreno R, Takala J, et al. . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027893supp001.pdf (543.5KB, pdf)