Abstract
Neurological adverse events from immune checkpoint inhibition are increasingly recognised, especially with combination anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) and anti-programmed death receptor 1 (anti-PD-1) therapies. Their presenting symptoms and signs are often subacute and highly variable, reflecting the numerous components of the nervous system. Given the risk of substantial morbidity and mortality, it is important to inform patients of symptoms that may be of concern, and to assess any suspected toxicity promptly. As with other immune-related adverse events, the cornerstone of management is administration of corticosteroids. Specialist neurology input is vital in this group of patients to guide appropriate investigations and tailor treatment strategies.
Keywords: immune-related adverse events, neurological, neurotoxicity, immune checkpoint inhibitor
INTRODUCTION
The spectrum of neurological toxicities from immune checkpoint inhibitors (ICIs) is incredibly diverse. While immune-related adverse events (irAEs) such as colitis tend to manifest only a few symptoms, or others may be picked up incidentally on blood tests in the case of hepatitis and thyroiditis, there are numerous components to the nervous system and these are intertwined with most other systems in the body. Neurological irAEs may be less common than others but the potential for long-term morbidity and mortality are substantial.1 It is important that clinicians be familiar with presenting symptoms and an approach to management to optimise outcomes for patients.
INFORMING THE PATIENT
When a patient is consented for an ICI regimen, our practice is always to discuss the potential for neurological toxicity to occur. This is especially relevant in the adjuvant setting where for some individuals with a lower risk of disease recurrence the possibility of neurological toxicity may be a deciding factor against treatment. We also alert patients to the fact that neurological irAEs can manifest in subtle ways. Those with pre-existing neuroinflammatory disorders require additional counselling as disease exacerbations may occur. Patients have contact details for members of our clinical team, including specialist nurses, and early reporting of symptoms is encouraged.
ONSET and SYMPTOMS
The highest incidence of neurological irAEs is reported with combination of ipilimumab and nivolumab (ipi +nivo) at around 14%, whereas anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) and anti-programmed death receptor 1 (anti-PD-1) monotherapy incidence is reported as 1% and 3%, respectively.2–4 There is no typical pattern to their timing of onset and symptoms may even occur after cessation of therapy.5 As a general rule, irAEs due to combination ipi +nivo tend to manifest within the first 3 months of treatment, whereas the range is broader with single agent anti-PD-1 antibodies such as nivolumab and pembrolizumab and onset may occur even 12 months after treatment initiation.5 A subacute pattern of symptom onset is characteristic of an inflammatory aetiology.
Symptoms may mimic those associated with recognised neuro-inflammatory conditions—for example, myositis, myasthenia gravis, acute inflammatory demyelinating polyradiculoneuropathy (AIDP) or Guillain Barre syndrome (GBS), chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), transverse myelitis or autoimmune encephalitis. Even when the clinical syndrome is recognisable, the investigative findings are often subtle or atypical. Multifocal inflammation can produce a confusing presentation. Meningitis, encephalitis, central nervous system (CNS) demyelination, optic neuritis, transverse myelitis, mononeuritis including phrenic nerve palsy, bilateral Bell’s palsy and Lambert-Eaton Syndrome have all been reported.4–7 Table 1 lists the signs and symptoms associated with various neurological irAEs.
Table 1.
Presenting signs and symptoms of neurological immune-related adverse events
| Neurological syndrome | Signs/symptoms |
| Myositis |
|
| Myasthenia gravis |
|
| AIDP (nadir <6 weeks, monophasic course)/CIDP (nadir >6 week, fluctuating course) |
|
| Aseptic meningitis |
|
| Encephalitis |
|
| Transverse myelitis |
|
 Denotes ‘red flag’ symptoms.
Denotes ‘red flag’ symptoms.
AIDP, acute inflammatory demyelinating polyradiculoneuropathy; CASPR2, contactin-associated protein 2; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; CSF, cerebrospinal fluid; EMG, electromyography; GCS, Glasgow coma scale; NMDAR, N-methyl-D-aspartate receptor.
ASSESSMENT AND WORK-UP
A pragmatic ‘mild’, ‘moderate’ and ‘severe’ categorisation is most useful to triage management, although common terminology criteria for adverse events (CTCAE)8 can be used as a framework.
In most cases, a prompt work-up is warranted. Exclusion of structural tumour-related pathology such as spinal cord compression or intra-cerebral metastases is a priority. An urgent lumbar puncture is essential in any febrile or immunocompromised patient to exclude possible bacterial meningitis.
A thorough neurological history and examination is informative. For example, gradually progressive limb girdle weakness with discomfort is seen in myositis. Diurnal fatiguability is pathognomonic for neuromuscular junction pathology (such as myasthenia gravis). Ascending sensory symptoms in a length-dependent pattern (tips of the toes, progressing proximally and involving hands when at the level of the knees) indicate a peripheral neuropathy while sensory involvement to the trunk but not involving the upper limbs points to a cord pathology. Changes in cognition or level of alertness are suggestive of encephalitis. Blood tests should include full blood count, biochemistry, liver function, erythrocyte sedimentation rate, Vitamin B12, folate, methylmalonic acid and homocysteine, HIV serology, thyroid function tests, haemoglobin A1c and consideration of a vasculitis screen (including hepatitis B and C serology).
The nature of the presenting symptoms should then direct other investigations. For example, in a myositis presentation creatine kinase levels, a myositis antibody panel and electromyography (EMG) are first-line investigations. In a myasthenic presentation, acetylcholine receptor and muscle-specific kinase (especially in bulbar-predominant presentations) antibodies and single-fibre EMG, repetitive nerve stimulations alongside routine nerve conduction studies (NCS) and EMG should be requested. In suspected peripheral neuropathies, NCS should be arranged; a demyelinating pattern is characteristic of AIDP and CIDP, while patchy axonal changes are seen in vasculitis. A nerve biopsy is recommended in suspected vasculitic neuropathy which typically presents as a painful, patchy motor and sensory neuropathy and may have multisystem involvement. Appropriate MRI is important for any CNS symptoms and should include T1, T2 and short-TI inversion recovery sequences with or without gadolinium. Although a lumbar puncture may not be indicated acutely, cerebrospinal fluid is informative even after treatment is commenced to look for lymphocytosis, oligoclonal bands and to exclude subtle leptomeningeal disease.
A large proportion of cases of myositis have concurrent cardiac involvement.9 10 Autonomic dysfunction may also be a feature of AIDP/GBS-like presentations. In these cases, rhythm monitoring, ECG, serum troponin and brain natriuretic peptide levels and an echocardiogram should be performed. Monitoring for occult neuromuscular respiratory involvement in myasthenia gravis with forced vital capacities (erect and supine) and arterial blood gases is important.
We advocate early involvement of a neurologist in the assessment of any patient with a suspected irAE. A multidisciplinary team is also vital in the management of patients.
Non-neurological irAEs may also present with neurological symptoms, for example headache as the hallmark of hypophysitis. Where this is suspected as a differential diagnosis, upfront steroids should be given to mitigate any risk of an adrenal crisis. Inflammatory arthropathy can present with carpal tunnel syndrome, which may be the predominant complaint.
MANAGEMENT
In cases where symptoms are suggestive of a potential neurological toxicity, we have a low threshold to withhold ICI therapy, even if symptoms are of ‘Grade 1’ (mild) severity.
In patients with overt, progressive symptoms with a functional impact (whether moderate or severe), corticosteroid therapy should be initiated promptly and inpatient admission considered. If there is any respiratory muscle involvement, patients should be admitted to centres where ventilation can be easily facilitated.
Generally, the choice of corticosteroid therapy is between prednisolone 0.5–1 mg/kg or 1–2 mg/kg of methylprednisolone. This should also be the first-line management in cases that resemble a GBS-like syndrome—despite a lack of benefit in post-infectious cases—as responses have been observed and the underlying pathogenesis may be different.6 In the event that symptoms fail to improve with corticosteroids, treatment options to consider include plasmapheresis and intravenous immunoglobulin (suggested bolus dose 2 g/kg over 5 days). Pyridostigmine may provide some additional benefit in myasthenia-like syndromes. Steroid injections are often helpful for carpal tunnel syndrome. The use of additional immunomodulators has been described in anecdotal reports, including infliximab, natalizumab, mycophenolate and cyclosporine.4 11
Steroids should be continued at the starting dose until objective improvement occurs in symptoms and/or functional state and then weaned over at least 4 weeks.
Management and, if possible, prevention of iatrogenic toxicity is important in patients receiving high dose corticosteroids. This may include Pneumocystic carinii pneumonia prophylaxis, blood pressure and blood glucose monitoring, gastric protection with a proton pump inhibitor, as well as consideration of bone density imaging where steroids are prolonged. Proximal weakness consequent to prolonged steroids can impair functional improvement and insomnia and mood changes are common.
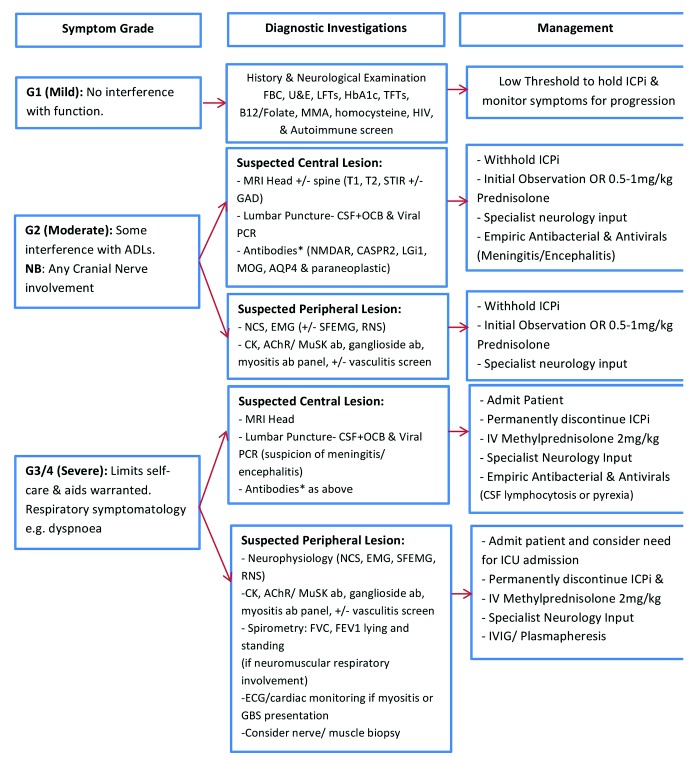
We have summarised our approach to investigation and management in figure 1. Other published guidelines also provide useful information for clinicians.12
Figure 1.
Approach to the investigation and management of neurological immune-related adverse events. AChR, acetylcholine receptor; ADLs, activities of daily living; AQP4, aquaporin-4; CASPR2, contactin-associated protein 2; CK, creatine kinase; CSF, cerebrospinal fluid; EMG, electromyogram; FBC, full blood count; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GAD, gadolinium; GBS, Guillain Barre syndrome; HbA1c, glycated haemoglobin; ICI, immune checkpoint inhibitor; IVIG, intravenous immunoglobulin; LGi1, leucine-rich glioma inactivated 1; LFTs, liver function tests; MMA, methymalonic acid; MOG, myelin oligodendrocyte glycoprotein; MuSK, muscle- specific kinase; NCS, nerve conduction studies; NMDAR, N-Methyl-D-aspartate receptor; OCB, oligoclonal bands; RNS, repetitive nerve stimulation; SF EMG, single-fibre electromyogram; STIR, short-TI inversion recovery; TFT, thyroid function tests; T1, T1-weighted image; T2, T2-weighted image; U&E, urea & electrolytes *According to presentation—discuss with neurologist.
OUTCOMES
Around a third of patients who develop immune-related neurotoxicity are left with residual impairment.4 When we reviewed the survival outcomes of this group of patients at our institution,4 we noted that both progression free and overall survival were longer than patients who did not develop neurological toxicity and a higher than expected response rate has also been reported in the meta-analysis by Cuzubbo et al.2 As such, advocating for intensive care management when required in patients with metastatic disease is justified, particularly where disease control is established.
TREATING PATIENTS WITH PRE-EXISTING NEUROLOGICAL SYNDROMES
There is a paucity of data on the outcomes of patients with pre-existing autoimmune neurological conditions who are treated with ICIs. One report of 14 patients with multiple sclerosis (MS) treated with ICIs noted two deaths due to relapsed disease.13 Another case report describes a radiologically isolated syndrome converted to definite MS.14 A series by Menzies et al included five patients with pre-existing neurological disorders, none of whom experienced a flare with anti-PD-1 therapy despite flares being documented in 38% of patients with non-neurological autoimmune conditions.15 Pre-existing neurological conditions should not be a contraindication to treatment; however, the potential risks for each patient need to be evaluated.5
FURTHER THERAPY
In patients who develop immune-related neurological toxicity prompting cessation of the ICI and need further treatment alternative therapies such as targeted therapy or chemotherapy are preferred. If patients develop irAEs after anti-CTLA4 monotherapy, treatment with an anti-PD-1 antibody is generally safe.15 A decision regarding the risks of further treatment must also be balanced against the risk of uncontrolled metastatic disease. An informed discussion with the patient is vital in this setting.
CONCLUSION
With the indications for immune checkpoint therapy expanding rapidly across tumour types, it is paramount that clinicians be well-versed in the assessment and management of irAEs, including neurological toxicity. As many patients are living longer thanks to the durable remissions induced with ICIs, the implication of toxicity is significant, especially for those considered for adjuvant immune checkpoint treatment. Developing a network of specialist colleagues who are interested in the management of irAEs is very useful to pool expertise and optimise patient outcomes.
Acknowledgments
This article is dedicated to the memory of Professor Martin Gore, a great advocate for the development of research in this area. We are also very grateful to Dr Rachel Brown for her review of the manuscript.
Footnotes
Contributors: LS wrote the first draft of the article and ZT composed the Table and Figure. AC provided specialist neurologist input for the article. JML and ST provided senior oversight, feedback and edited the article. All authors reviewed the final draft prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ST acknowledges research funding and speaker fees from Ventana Medical Systems and consulting fees from Novartis. JL acknowledges institutional research support from: BMS, MSD, Novartis, Pfizer, Achilles Therapeutics, Roche, Nektar Therapeutics, Covance, Immunocore, Pharmacyclics, Aveo and consultancy fees from Achilles Therapeutics, AZ, Boston Biomedical, BMS, Eisai, EUSA Pharma, GSK, Ipsen, Imugene, Incyte, iOnctura, Kymab, Merck Serono, MSD, Nektar, Novartis, Pierre Fabre, Pfizer, Roche/Genentech, Secarna, Vitaccess, as well as support from NIHR RM/ICR Biomedical Research Centre for cancer.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wang DY, Salem J-E, Cohen JV, et al. . Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cuzzubbo S, Javeri F, Tissier M, et al. . Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer 2017;73:1–8. 10.1016/j.ejca.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 3. Kao JC, Liao B, Markovic SN, et al. . Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol 2017;74:1216–22. 10.1001/jamaneurol.2017.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spain L, Walls G, Julve M, et al. . Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol 2017;28:377–85. 10.1093/annonc/mdw558 [DOI] [PubMed] [Google Scholar]
- 5. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017;13:755–63. 10.1038/nrneurol.2017.144 [DOI] [PubMed] [Google Scholar]
- 6. Touat M, Talmasov D, Ricard D, et al. . Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol 2017;30:659–68. 10.1097/WCO.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 7. Larkin J, Chmielowski B, Lao CD, et al. . Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncologist 2017;22:709–18. 10.1634/theoncologist.2016-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Services USDoHaH Common Terminology Criteria for Adverse Events; Version 4.0, 2009. Available: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 9. Suzuki S, Ishikawa N, Konoeda F, et al. . Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–34. 10.1212/WNL.0000000000004359 [DOI] [PubMed] [Google Scholar]
- 10. Touat M, Maisonobe T, Knauss S, et al. . Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–94. 10.1212/WNL.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 11. Hottinger AF, de Micheli R, Guido V, et al. . Natalizumab may control immune checkpoint inhibitor-induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm 2018;5:e439 10.1212/NXI.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haanen JBAG, Carbonnel F, Robert C, et al. . Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28(suppl_4):iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 13. Garcia CR, Jayswal R, Adams V, et al. . Multiple sclerosis outcomes after cancer immunotherapy. Clin Transl Oncol 2019;372 10.1007/s12094-019-02060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerdes LA, Held K, Beltrán E, et al. . CTLA4 as Immunological Checkpoint in the Development of Multiple Sclerosis. Ann Neurol 2016;80:294–300. 10.1002/ana.24715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menzies AM, Johnson DB, Ramanujam S, et al. . Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]



 Dyspnoea/orthopnaea (respiratory muscle involvement)
Dyspnoea/orthopnaea (respiratory muscle involvement) Ptosis/diplopia (ocular involvement)
Ptosis/diplopia (ocular involvement) Dysphagia/dysarthria (bulbar Involvement)
Dysphagia/dysarthria (bulbar Involvement) Dyspnoea/orthopnoea (respiratory involvement)
Dyspnoea/orthopnoea (respiratory involvement) Reduced GCS
Reduced GCS Seizures
Seizures