Abstract
Purpose
This study investigated the safety and clinical activity of lumretuzumab, a humanised antihuman epidermal growth factor receptor 3 (HER3) monoclonal antibody, in combination with carboplatin and paclitaxel in first-line treatment of patients with squamous non-small cell lung cancer (sqNSCLC). HER3 ligand heregulin and HER3 protein expression were evaluated as potential biomarkers of clinical activity.
Patients and methods
This open-label, phase Ib/II study enrolled patients receiving lumretuzumab at 800 mg (flat) in combination with carboplatin (area under the curve (AUC) 6 mg/mL×min) and paclitaxel (200 mg/m2) administered intravenously on a every 3-week schedule. Adverse event (AE) rates and tumour responses were determined. Heregulin messenger RNA (mRNA) and HER3 protein expression were investigated in archival tumour biopsies.
Results
Altogether, 12 patients received lumretuzumab in combination with carboplatin and paclitaxel. The most frequent AEs were gastrointestinal, haematological and nervous system toxicities, which were generally mild and manageable. Partial responses were observed in 3 of 12 patients lasting 81, 177 and 207 days. All responses were achieved in tumours expressing higher heregulin mRNA levels.
Conclusion
Lumretuzumab in combination with carboplatin and paclitaxel was well tolerated. Objective responses were enriched in tumours expressing higher heregulin mRNA levels.
Keywords: human epidermal growth factor receptor 3 (HER3), ErbB3, phase i, heregulin, non-small cell lung cancer (NSCLC), squamous, biomarker
Key questions.
What is already known about this subject?
Human epidermal growth factor receptor 3 (HER3) is associated with tumour development and poor clinical prognosis in different cancers.
HER3 ligand heregulin may serve as a response prediction marker for HER3-targeting therapy.
Heregulin expression levels are higher and more frequent in squamous non-small cell lung cancer (sqNSCLC).
What does this study add?
HER3-targeting lumretuzumab in combination with carboplatin and paclitaxel was well tolerated.
High heregulin expression levels enriched for patients more likely to show a clinical response.
Clinically meaningful contribution to efficacy by lumretuzumab could not be demonstrated as compared with chemotherapy and immunotherapy.
How might this impact on clinical practice?
HER3-targeting therapy may not add meaningful clinical benefit as to what has been shown for platinum-containing treatment regimens in the first-line treatment setting of sqNSCLC.
Introduction
Lung cancer is one of the most frequent types of cancer worldwide both in terms of cases (2.1 million cases, 11.6% of total) and deaths (1.8 million deaths, 18.4%).1 Among lung cancer subtypes, non-small cell lung cancer (NSCLC) is the most prevalent and about 30% of NSCLC are squamous (sqNSCLC) cell carcinomas. Approximately 80% of lung cancer cases are diagnosed at stages III and IV.2
Until the advent of cancer immunotherapy, that is, checkpoint inhibition, platinum-based combination chemotherapy was the standard of care in patients with newly diagnosed advanced/metastatic sqNSCLC.3 Targeted agents such as epidermal growth factor receptor (EGFR) inhibitors and anaplastic lymphoma kinase (ALK) inhibitors have shown clinical efficacy in molecular subgroups such as EGFR-mutant and ALK-rearranged NSCLC particularly of the non-squamous subtype4–6 but less so for all-comers treated with cetuximab, cisplatin and vinorelbine7 or for patients with sqNSCLC treated with necitumumab, gemcitabine and cisplatin.8 In the meantime, phase III studies have established antiprogrammed cell-death protein 1 (ligand) (PD-(L)1) compounds as the new standard of care in both sqNSCLC and non-squamous NSCLC. Pembrolizumab monotherapy has been approved as first-line therapy for patients with high PD-L1 expression (≥50%)9 and for non-squamous histology in combination with platinum and pemetrexed10 and also for squamous histology in combination with carboplatin together with paclitaxel or nab-paclitaxel11 independently of PD-L1 expression.
HER3 is a key dimerisation partner of HER family members which activates several signal transduction pathways, particularly the phosphoinositide-3-kinase (PI3K)/Akt pathway and is associated with tumour development and poor clinical prognosis in different cancers.12 Lumretuzumab is a humanised, glycoengineered immunoglobulin G1 antibody which binds with high affinity and specificity to the extracellular domain of HER3. Prevention of the ligand heregulin binding to HER3 by lumretuzumab resulted in almost complete inhibition of HER3 heterodimerisation and phosphorylation as well as inhibition of tumour growth in cell-line-based mouse models.13 In a phase I study, the safety of lumretuzumab in patients with advanced solid tumours was evaluated; no dose-limiting toxicity was observed at doses up to 2000 mg every 2 weeks and the maximum tolerated dose was not reached. Lumretuzumab doses from 200 mg led to downregulation of membranous HER3 and a target-independent pharmacokinetic profile was observed from 400 mg doses and above.14
HER3 is widely expressed in NSCLC including sqNSCLC.15 16 In addition, higher expression levels of heregulin, the ligand of HER3, were associated with improved antitumour efficacy in preclinical models.17–19 Internal data from tumour bank samples as well as published data20 provided evidence that heregulin expression levels are higher and more frequent in sqNSCLC as compared with NSCLC adenocarcinoma. Hence, we hypothesised that deprivation of HER3/PI3K-mediated cell survival signals by HER3-targeting lumretuzumab might provide improved treatment benefit especially in patients with sqNSCLC. At the time of study initiation, carboplatin and paclitaxel were considered a standard of care with an objective response rate of ~25%.21 Therefore, carboplatin and paclitaxel were included as backbone chemotherapy regimen for the treatment of advanced/metastatic sqNSCLC.
Methods
Study design
The study reported here was a phase Ib/II, open-label, non-randomised, multicentre study (ClinicalTrials.gov Identifier: NCT02204345) of lumretuzumab in combination with paclitaxel and carboplatin in patients with metastatic or advanced sqNSCLC. The primary objectives of the study were to evaluate the safety and tolerability of lumretuzumab in combination with carboplatin and paclitaxel and to estimate the efficacy of the combination, as measured by the objective response rate (ORR, defined as complete response (CR) rate+partial response (PR) rate).
As previously shown, pharmacokinetics of lumretuzumab was linear from ≥400 mg per patient, indicative of saturated target-mediated drug disposition, and maximum pharmacodynamic activity was reached in monotherapy ≥400 mg 14. Therefore, a dose of 800 mg was defined as a fixed dose of lumretuzumab in this study.
Ethics
All patients provided written informed consent. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki in five centres in Spain, Canada and Denmark.
Patients
Patients had a histologically confirmed diagnosis of advanced or metastatic (stage IIIb or IV22) sqNSCLC. Patients had not received prior chemotherapy or targeted therapy for NSCLC. Eligible patients were ≥18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and had adequate haematology, blood chemistry and renal and liver function. Prior radiotherapy to control local symptoms was allowed if target lesions outside the radiotherapy field existed. Patients eligible for enrolment had to provide archival tumour biopsy tissue or underwent a fresh (pretreatment) tumour biopsy that was used to assess the level of HER3 protein expression by immunohistochemistry (IHC) and central pathology review.
Study drug administration
All patients were administered 800 mg of lumretuzumab intravenously in combination with 6 mg/mL×min AUC of carboplatin and 200 mg/m2 of paclitaxel every 3 weeks for 4–6 cycles. Thereafter, patients could receive lumretuzumab as a monotherapy (carboplatin and paclitaxel could be continued at the investigator’s discretion) until disease progression, death, unacceptable toxicity, withdrawal of consent or at the investigator’s decision, whichever occurred first. For carboplatin and paclitaxel, premedication was administered according to the manufacturer’s instructions. For lumretuzumab, no premedication was foreseen prior to the first administration but could be introduced for subsequent cycles based on the patient’s tolerability.
Tumour response and safety
Tumour response assessment using Response Evaluation Criteria in Solid Tumours V.1.123 was conducted at screening and every 6 weeks thereafter by the investigators. CRs and PRs had to be confirmed with a second assessment.
Safety assessments included physical (ECOG performance status, vital signs) and laboratory examinations and ECG. AEs were defined according to the Common Terminology Criteria for Adverse Events, V.4.0 (CTCAE V.4.0).
Biomarker assessments
Squamous cell carcinoma antigen (SCC) and cytokeratin fragment (CYFRA) 21-1 from peripheral blood were locally assessed by immunoassay every 6 weeks.
Fresh or archival tumour biopsies were collected during screening. HER3 protein expression was assessed using an IHC assay as described previously14. Heregulin mRNA expression was measured by quantitative real-time PCR assay, as a potential predictive biomarker for lumretuzumab activity, in formalin-fixed paraffin-embedded (FFPE) sections obtained from fresh tumour biopsies collected from all patients at screening prior to initiation of treatment.24 A prototype diagnostic assay was used for which reagents were prepared in a good manufacturing practice facility, the assay run using a z480 PCR system and calculation of Ct values was performed using validated diagnostic software (Q2 Solutions (Livingston, UK)). Patients were assigned as having high heregulin, if mRNA concentrations were greater than median heregulin expression previously determined using 150 primary FFPE tumour biopsy samples obtained from patients diagnosed with sqNSCLC.
Statistical considerations
All patients who received at least one dose of study medication were included in the statistical analyses. Descriptive statistics were used for demographics and safety as well as efficacy. In addition, in order to explore the relation between heregulin levels and response as well as whether responders and non-responders have different tumour marker dynamics, additional plots are shown.
Results
Patients
Patient demographics and baseline characteristics are presented in table 1. Altogether, 12 patients were enrolled. None of them had prior surgery for their disease and only two patients (16.7%) had prior radiotherapy. All 12 patients (100%) received at least one dose of the study treatment. The most common reason for discontinuation from the study was disease progression (9/12 patients (75%)). Other reasons for discontinuation were AE (general physical health deterioration; 1/12 patients (8.3%)), physician decision (1/12 patients (8.3%)) and other reason (1/12 patients (8.3%)). The median number of treatment cycles administered per patient was 5.5 cycles (range 1–16) for lumretuzumab and four cycles (range 1–6) for both carboplatin and paclitaxel. HER3 was present on tumour cells in 9/12 patients (75%), although median expression was low (mean immunoreactive score (IRS): 0.25, range 0–1.47). HER3 expression was not detectable by IHC in 2/3 (66%) patients with a PR.
Table 1.
Baseline patient demographics and characteristics
| Characteristic | Patients (N=12) |
| Sex, n (%) | |
| Male | 10 (83.3) |
| Female | 2 (16.7) |
| Age (years), median (range) | 66.5 (52–74) |
| ECOG score, n (%) | |
| 0 | 5 (41.7) |
| 1 | 7 (58.3) |
| Prior radiotherapy, n (%) | 2 (16.7) |
| Prior surgery, n (%) | 0 |
ECOG, Eastern Cooperative Oncology Group.
Safety
All 12 patients (100%) experienced at least one AE in the study (table 2). There were no deaths due to AEs. The most common AEs (>40% of patients) were diarrhoea (9/12 patients (75%)), asthenia (8/12 patients (66.7%)) and neurotoxicity (5/12 patients (41.7%)). Five of 12 patients (41.7%) had nine AEs of CTCAE grade 3 (anaemia (two events), neutropenia (two events), thrombocytopenia; respiratory tract infection, loss of consciousness, dyspnoea and general physical health deterioration (one event each)). Two patients also experienced two AEs of grade 4 (neutrophil count decreased and platelet count decreased). One of 12 patients (8.3%) had an AE (deterioration of general physical health, considered unrelated to lumretuzumab) leading to withdrawal from the study treatment. A total of 3/12 patients (25%) experienced four serious adverse events (SAEs). The four SAEs were respiratory tract infection and loss of consciousness experienced by one patient and infected neoplasm and dyspnoea experienced by one patient each all considered unrelated to study treatment.
Table 2.
Summary of adverse events of any grade and of grade ≥3 adverse events
| Adverse event | Patients (n) having an adverse event (%) (N=12) | |
| All grades | Grade ≥3 | |
| Diarrhoea | 9 (75.0) | 0 |
| Asthenia | 8 (66.7) | 0 |
| Platelet count decreased | 5 (41.7) | 2 (16.7) |
| Neurotoxicity | 5 (41.7) | 0 |
| Infusion-related reaction* | 4 (33.3) | 0 |
| Nausea | 4 (33.3) | 0 |
| Neutropenia | 3 (25.0) | 2 (16.7) |
| Anaemia | 3 (25.0) | 2 (16.7) |
| Dyspnoea | 3 (25.0) | 1 (8.3) |
| Respiratory tract infection | 3 (25.0) | 1 (8.3) |
| Constipation | 3 (25.0) | 0 |
| Weight decreased | 3 (25.0) | 0 |
| Decreased appetite | 3 (25.0) | 0 |
| Alopecia | 3 (25.0) | 0 |
| Insomnia | 3 (25.0) | 0 |
| Rash | 3 (25.0) | 0 |
| Abdominal pain | 2 (16.7) | 0 |
| Abdominal pain upper | 2 (16.7) | 0 |
| Stomatitis | 2 (16.7) | 0 |
| Vomiting | 2 (16.7) | 0 |
| Fatigue | 2 (16.7) | 0 |
| Musculoskeletal pain | 2 (16.7) | 0 |
| Pain in the extremity | 2 (16.7) | 0 |
| Hypomagnesemia | 2 (16.7) | 0 |
Only adverse events reported by >10% of the patients are shown.
*Four patients had infusion-related reactions; three of which the investigator considered as related to lumretuzumab and one patient had an infusion-related reaction related to paclitaxel.
Antitumour activity
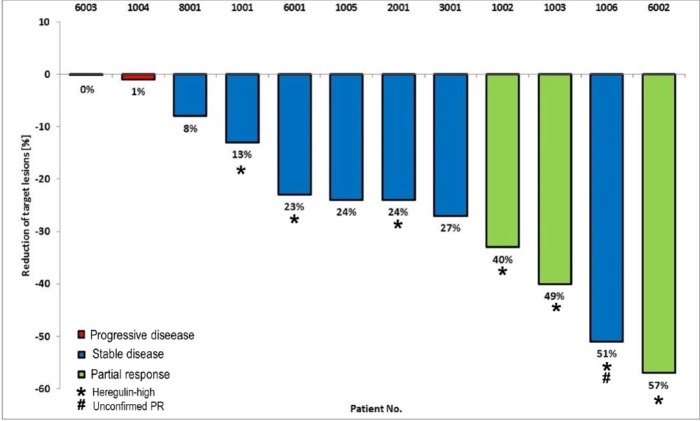
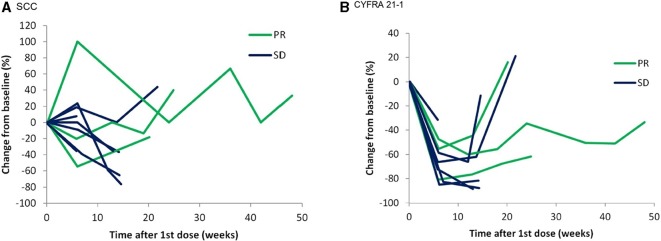
The ORR and disease control rate (DCR) were 25% (90% CI 4.44 to 45.56) and 91.7% (90% CI 78.54 to 100), respectively (table 3). The best response was a PR seen in 3/12 patients (25%), and 8/12 patients (75%) had stable disease as a best response (figure 1). The median duration of PFS was 122 days (95% CI 81 to 217) with 10/12 patients (83.3%) showing disease progression during the study. There were 2/12 patients (16.7%) without any progression events, who were censored from the analysis of progression-free survival (PFS). The duration of the PR in patients was 81, 177 and 207 days, respectively. Tumour shrinkage in patients was accompanied by decreasing levels of CYFRA 21-1 but less so for SCC (figure 2).
Table 3.
Tumour response to treatment (RECIST)
| HRG low (N=5) |
HRG high (N=7) |
All patients (N=12) |
|
| Objective response rate, N (%) | 0 | 3 (42.9) | 3 (25.0) |
| Disease control rate, N (%) | 4 (80.0) | 7 (100) | 11 (91.7) |
| PFS, median (95% CI), days | 100 (38, 196) | 122 (65, 217) | 122 (81, 217) |
HRG, heregulin;PFS, progression-free survival; RECIST, response evaluation criteria in solid tumours.
Figure 1.
Best percentage change from baseline in sum of target lesions.
Figure 2.
Tumour marker dynamics: percentage change from baseline, measured in plasma.
Heregulin mRNA–response relationships
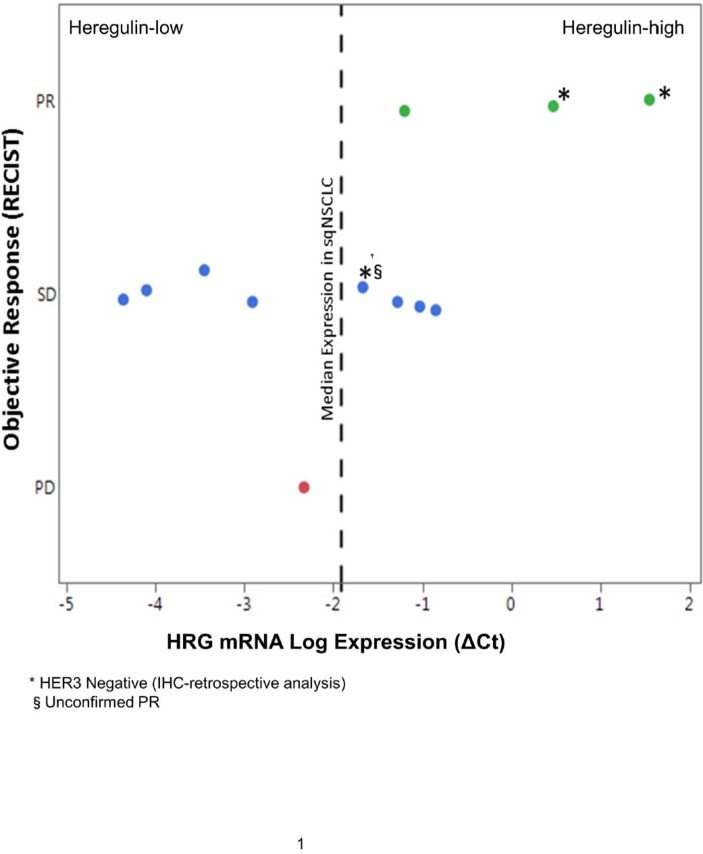
Heregulin mRNA expression and response evaluation criteria in solid tumours response data was available for all patients (figure 3). Seven patients were considered heregulin high using the median ΔCt as a cut-off, determined from the analysis of a cohort of 150 FFPE tumour samples from patients with sqNSCLC as reported previously.24 All three patients with a PR were in the heregulin-high group. Hence, the ORR in the heregulin-high population was 42.9%, the DCR was 100% (7/7 patients) and the median PFS (95% CI) was 122 (65 to 217) days compared with an ORR of 0% (0/5 patients), a DCR of 80.0% (4/5 patients) and a median PFS (95% CI) of 100 (38 to 196) days for the remaining heregulin-low patients.
Figure 3.
Objective response and HRG mRNA log expression in patients with first-line sqNSCLC (n=12). Green indicates partial response; blue indicates stable disease and red indicates progressive disease. HRG mRNA log expression determined using the prototype diagnostic assay. HER3, human epidermal growth factor receptor 3; HRG, heregulin; PR, partial response; mRNA, messenger RNA; sqNSCLC, squamous non-small cell lung cancer.
Discussion
This is the first study to describe the combination of an anti-HER3 monoclonal antibody, lumretuzumab, with standard-of-care chemotherapy, that is, paclitaxel and carboplatin, as a first-line treatment for patients with sqNSCLC. In addition, the study aimed to generate signals in patients with elevated heregulin expression levels.
Overall, the safety profile of lumretuzumab in combination with carboplatin and paclitaxel in patients with advanced sqNSCLC is acceptable and consistent with carboplatin/paclitaxel chemotherapy toxicities. The most common AEs in this study were gastrointestinal, haematological and nervous system toxicities similar to the well-known side effects of carboplatin/paclitaxel chemotherapy alone.25–28 The addition of lumretuzumab to carboplatin and paclitaxel may have caused an increase in the incidence of diarrhoea compared with chemotherapy alone. Overall, this combination appeared to be tolerable as the median number of cycles given for chemotherapy was similar to the one seen by others.25 26 28 An increased incidence of diarrhoea has been previously described for lumretuzumab monotherapy and combinations of lumretuzumab with cetuximab, erlotinib and pertuzumab.14 24 29 An increased incidence of diarrhoea was also observed with other HER3-targeting agents, seribantumab and patritumab, when combined with erlotinib, for the treatment of NSCLC in phase II studies.30 31 Nevertheless, no diarrhoea grade ≥3 event has been reported for the combination of lumretuzumab with chemotherapy in the present study.
The efficacy seen with the combination of lumretuzumab in combination with carboplatin and paclitaxel, ORR=25% and a median PFS=122 days, is similar to what has been published for chemotherapy alone in recent phase III studies.25–28 Certainly, due to the low number of patients treated in this study, the efficacy data should be interpreted cautiously.
Clinical phase II studies using HER3-targeting therapies32–37 have suggested heregulin mRNA expression levels as a response-predictive biomarker for HER3-targeting therapy. In particular, patients with sqNSCLC have been shown to express higher levels of heregulin per se.20 Indeed, all three partial responders described in this study were considered to have higher heregulin expression levels; hence, the level of heregulin expression may enrich for patients with objective responses and a higher DCR. However, the number of patients responding to treatment were too small to draw firm conclusions in this regard and duration of response in patients with high tumour heregulin levels was in the range of what can be expected for chemotherapy alone.38 This is in line with phase II studies in NSCLC, colorectal cancer and head and neck cancer that could not show any treatment benefit of HER3-targeting therapies in heregulin-high subgroups.31 39 40 An ongoing phase II study of HER3-targeting seribantumab in combination with docetaxel or pemetrexed in second to third-line treatment, prospectively heregulin-positive tested and selected patients with NSCLC might shed more light on this issue.41 The clinical activity in this small phase I patient set is overall inferior to the recent phase III trials including checkpoint inhibition in advanced/metastatic NSCLC. Pembrolizumab monotherapy in patients with PD-L1 expression (≥50%) with sqNSCLC or non-squamous NSCLC achieved an ORR of 44.8%,9 pembrolizumab in combination with chemotherapy in patients with sqNSCLC had an ORR of 57.9% with a median duration of response of 7.7 months11 and atezolizumab in combination with chemotherapy in patients with sqNSCLC showed an ORR of 49% and a median duration of response of 7.2 months.38 Eventually, the present data and the outcome of a sister study testing the combination of lumretuzumab plus erlotinib in patients with metastatic/advanced NSCLC showing similar limited efficacy24 led to the decision not to execute the phase II portion of this study.
In conclusion, combination treatment of lumretuzumab with carboplatin and paclitaxel was well tolerated, but clinically meaningful contribution to efficacy by lumretuzumab could not be demonstrated. High heregulin expression levels may be used to enrich for patients more likely to benefit, although the number of patients responding to treatment were too small to verify this hypothesis. Overall, the results of the present study indicate that HER3 is not a strong enough driver for sqNSCLC to warrant further clinical development of lumretuzumab in this indication.
Acknowledgments
The authors would like to thank the patients and their families for their participation in this study, and the staff at the study sites.
Footnotes
J-MC and WJ contributed equally.
Previous presentation of data: Annals of Oncology, Volume 27, Issue suppl_6, 1 October 2016, 372P.
Contributors: All authors contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Funding: This study was funded by F. Hoffmann–La Roche Ltd.
Competing interests: WJ, MC, MH and MW are the sponsor employees and have sponsor stock ownership. CA and FM are also the sponsor employees. IJ is the sponsor consultant from A4P. AC is the member of the Speaker Bureau of Roche and got research support from Roche.
Patient consent for publication: Not required.
Ethics approval: Local ethics committee approval was obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 3. Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7):vii56–64. 10.1093/annonc/mds226 [DOI] [PubMed] [Google Scholar]
- 4. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 5. Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 6. Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 7. Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525–31. 10.1016/S0140-6736(09)60569-9 [DOI] [PubMed] [Google Scholar]
- 8. Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–74. 10.1016/S1470-2045(15)00021-2 [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 10. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 11. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 12. Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 2010;16:1373–83. 10.1158/1078-0432.CCR-09-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirschberger C, Schiller CB, Schräml M, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013;73:5183–94. 10.1158/0008-5472.CAN-13-0099 [DOI] [PubMed] [Google Scholar]
- 14. Meulendijks D, Jacob W, Martinez-Garcia M, et al. First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin Cancer Res 2016;22:877–85. 10.1158/1078-0432.CCR-15-1683 [DOI] [PubMed] [Google Scholar]
- 15. Ocana A, Vera-Badillo F, Seruga B, et al. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013;105:266–73. 10.1093/jnci/djs501 [DOI] [PubMed] [Google Scholar]
- 16. Yi ES, Harclerode D, Gondo M, et al. High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 1997;10:142–8. [PubMed] [Google Scholar]
- 17. Schoeberl B, Faber AC, Li D, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 2010;70:2485–94. 10.1158/0008-5472.CAN-09-3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawakami H, Okamoto I, Yonesaka K, et al. The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells. Oncotarget 2014;5:11847–56. 10.18632/oncotarget.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meetze K, Vincent S, Tyler S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res 2015;21:1106–14. 10.1158/1078-0432.CCR-14-2407 [DOI] [PubMed] [Google Scholar]
- 20. Lee E-S, Son D-S, Kim S-H, et al. Prediction of recurrence-free survival in postoperative non-small cell lung cancer patients by using an integrated model of clinical information and gene expression. Clin Cancer Res 2008;14:7397–404. 10.1158/1078-0432.CCR-07-4937 [DOI] [PubMed] [Google Scholar]
- 21. Socinski MA, Bondarenko IN, Karaseva NA, et al. Results of a randomized, phase III trial of nab-paclitaxel (nab-P) and carboplatin (C) compared with cremophor-based paclitaxel (P) and carboplatin as first-line therapy in advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28(18 Suppl). 10.1200/jco.2010.28.18_suppl.lba7511 [DOI] [Google Scholar]
- 22. Nair A, Klusmann MJ, Jogeesvaran KH, et al. Revisions to the TNM staging of non-small cell lung cancer: rationale, clinicoradiologic implications, and persistent limitations. Radiographics 2011;31:215–38. 10.1148/rg.311105039 [DOI] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24. Meulendijks D, Jacob W, Voest EE, et al. Phase Ib study of lumretuzumab plus cetuximab or erlotinib in solid tumor patients and evaluation of HER3 and heregulin as potential biomarkers of clinical activity. Clin Cancer Res 2017;23:5406–15. 10.1158/1078-0432.CCR-17-0812 [DOI] [PubMed] [Google Scholar]
- 25. Langer CJ, Novello S, Park K, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 2014;32:2059–66. 10.1200/JCO.2013.54.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Douillard J-Y, Douillard J-Y, Nakagawa K, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2965–71. 10.1200/JCO.2011.35.0660 [DOI] [PubMed] [Google Scholar]
- 27. Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1835–42. 10.1200/JCO.2009.26.1321 [DOI] [PubMed] [Google Scholar]
- 28. Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911–7. 10.1200/JCO.2009.21.9618 [DOI] [PubMed] [Google Scholar]
- 29. Schneeweiss A, Park-Simon T-W, Albanell J, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drug 2018;36:848–59. 10.1007/s10637-018-0562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sequist LV, Gray JE, Harb WA, et al. Randomized phase II trial of seribantumab in combination with erlotinib in patients with EGFR Wild‐type non‐small cell lung cancer. Oncologist 2019. 10.1634/theoncologist.2018-0695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paz-Arez L, Serwatowski P, Szczęsna A, et al. P3.02b-045 Patritumab plus Erlotinib in EGFR Wild-Type Advanced Non–Small Cell Lung Cancer (NSCLC): Part a Results of HER3-Lung Study. J Thorac Oncol 2017;12:S1214–S1215. 10.1016/j.jtho.2016.11.1712 [DOI] [Google Scholar]
- 32. Von Pawel J, Tseng J, Dediu M, et al. Phase 2 HERALD study of patritumab (P) with erlotinib (E) in advanced NSCLC subjects (SBJs). J Clin Oncol 2014;32(15 Suppl). 10.1200/jco.2014.32.15_suppl.8045 [DOI] [Google Scholar]
- 33. Sequist LV, Lopez-Chavez A, Doebele RC, et al. A randomized phase 2 trial of MM-121, a fully human monoclonal antibody targeting ErbB3, in combination with erlotinib in EGFR wild-type NSCLC patients. J Clin Oncol 2014;32(15 Suppl). 10.1200/jco.2014.32.15_suppl.8051 [DOI] [Google Scholar]
- 34. Higgins MJ, Doyle C, Paepke S, et al. A randomized, double-blind phase II trial of exemestane plus MM-121 (a monoclonal antibody targeting ErbB3) or placebo in postmenopausal women with locally advanced or metastatic ER+/PR+, HER2-negative breast cancer. J Clin Oncol 2014;32(15 Suppl). 10.1200/jco.2014.32.15_suppl.587 [DOI] [Google Scholar]
- 35. Liu JF, Ray-Coquard I, Selle F, et al. Randomized phase II trial of seribantumab in combination with paclitaxel in patients with advanced platinum-resistant or -refractory ovarian cancer. J Clin Oncol 2016;34:4345–53. 10.1200/JCO.2016.67.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmes FA, McIntyre KJ, Krop IE, et al. A randomized, phase 2 trial of preoperative MM-121 with paclitaxel in triple negative (TN) and hormone receptor (HR) positive, HER2-negative breast cancer. Cancer Res 2015;75. [Google Scholar]
- 37. Yonesaka K, Hirotani K, von Pawel J, et al. Circulating heregulin level is associated with the efficacy of patritumab combined with erlotinib in patients with non-small cell lung cancer. Lung Cancer 2017;105:1–6. 10.1016/j.lungcan.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 38. Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab plus carboplatin plus paclitaxel or nab-paclitaxel vs carboplatin plus nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36(18 Suppl). 10.1200/JCO.2018.36.18_suppl.LBA9000 [DOI] [Google Scholar]
- 39. Fayette J, Wirth L, Oprean C, et al. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Front Oncol 2016;6 10.3389/fonc.2016.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill AG, Findlay MP, Burge ME, et al. Phase II study of the dual EGFR/HER3 inhibitor duligotuzumab (MEHD7945A) vs. cetuximab in combination with FOLFIRI in Second-Line RAS wild-type metastatic colorectal cancer. Clin Cancer Res 2018;24:2276–84. 10.1158/1078-0432.CCR-17-0646 [DOI] [PubMed] [Google Scholar]
- 41. Sequist LV, Anderson I, Bauer TM, et al. A phase 2 study of seribantumab (MM-121) in combination with docetaxel or pemetrexed versus docetaxel or pemetrexed alone in patients with heregulin positive (HRG+), locally advanced or metastatic non-small cell lung cancer (NSCLC). Ann Oncol 2016;27(Suppl 6). 10.1093/annonc/mdw383.96 [DOI] [Google Scholar]