Abstract
Objective
Recent anticoagulation trials in all-comer cryptogenic stroke patients have yielded equivocal results, reinvigorating the focus on identifying reproducible markers of an atrial myopathy. We investigated the role of excessive premature atrial complexes (PACs) in ischaemic stroke, including cryptogenic stroke and its association with vascular risk factors.
Methods and results
A case–control study was conducted utilising a multicentre institutional stroke database to compare 461 patients with an ischaemic stroke or transient ischaemic attack (TIA) with a control group consisting of age matched patients without prior history of ischaemic stroke/TIA. All patients underwent 24-hour Holter monitoring during the study period and atrial fibrillation was excluded. An excessive PAC burden, defined as ≥200 PACs/24 hours, was present in 25.6% and 14.7% (p<0.01), of stroke/TIA and control patients, respectively. On multivariate regression, excessive PACs (OR 1.97; 95% CI 1.29 to 3.02; p<0.01), smoking (OR 1.58; 95% CI 1.06 to 2.36; p<0.05) and hypertension (OR 1.53; 95% CI 1.07 to 2.17; p<0.05) were independently associated with ischaemic stroke/TIA. Excessive PACs remained the strongest independent risk factor for the cryptogenic stroke subtype (OR 1.95; 95% CI 1.16 to 3.28; p<0.05). Vascular risk factors that promote atrial remodelling, increasing age (≥75 years, OR 3.64; 95% CI 2.08 to 6.36; p<0.01) and hypertension (OR 1.54; 95% CI 1.01 to 2.34; p<0.05) were independently associated with excessive PACs.
Conclusions
Excessive PACs are independently associated with cryptogenic stroke and may be a reproducible marker of atrial myopathy. Prospective studies assessing their utility in guiding stroke prevention strategies may be warranted.
Keywords: stroke medicine, cardiology
Strengths and limitations of this study.
This study employed a case–control design to compare the burden of premature atrial complexes (PACs) in ischaemic stroke, including the cryptogenic stroke subtype and an age-matched control group.
All patients underwent 24 hours of ambulatory Holter monitoring to exclude atrial fibrillation and to document the burden of PACs.
This study describes the association between vascular risk factors and PACs and used multivariate analysis to reduce confounding.
The study is limited by its cross-sectional, case–control design and causality cannot be inferred from the associations.
Introduction
Approximately 100 000 strokes occur every year in the UK, with 1 in 4 survivors experiencing another stroke.1 While 87% of all strokes are ischaemic in nature, 25%–35% of these are labelled cryptogenic, as a clear cause is not identified.2 3 Subclinical paroxysmal atrial fibrillation (AF) is postulated to be the cause for a significant proportion of these cryptogenic strokes.4 5 However, despite prolonged rhythm monitoring, occult AF occur in only a small proportion of patients.6 7
With the recent equivocal results of randomised controlled anticoagulation trials in all-comer patients with embolic stroke of undetermined source, there has been a heightened focus in identifying reproducible markers of an atrial myopathy.8 Premature atrial complexes (PACs) have been thought to be a benign phenomenon with a prevalence in the general population that ranges from 6% to 29%.9 A limited number of studies have shown a significant association between excessive PACs and ischaemic stroke, suggesting their relevance as a marker of atrial myopathy.10–14 While another study has shown an elevated risk for recurrent stroke in patients with excessive PACs, following a cryptogenic stroke.15 However, these have not delineated whether baseline excessive PACs confer an increased risk for the cryptogenic stroke subtype. In addition, it is unclear whether vascular risk factors that promote stroke, independently and uniformly lead to atrial remodelling that result in excessive PAC burden. We sought to determine the association between excessive PACs and ischaemic stroke, including the cryptogenic stroke subtype and their relationship to conventional risk factors.
Methods
A multicentre case–control study was conducted among consecutive patients who presented with an ischaemic stroke or transient ischaemic attack (TIA) between May 2011 and December 2015. Patients within the stroke/TIA group were identified through a prospectively maintained institutional stroke database that covered three tertiary, university hospitals. Stroke subtypes were determined according to the Trial of Org 10172 in Acute Stroke Treatment classification.16
Inclusion criteria were (1) age ≥18 years; (2) adjudicated to have had an ischaemic stroke/TIA by the stroke service and (3) underwent 24-hour Holter monitoring following their index stroke/TIA. Exclusion criteria were (1) history of AF, atrial flutter or a subsequent diagnosis of these arrhythmias on inpatient telemetry, Holter monitoring or during follow-up; (2) underlying severe cardiomyopathy with an ejection fraction <35%; (3) previous coronary artery bypass grafting; (4) recent myocardial infarction or (5) severe chronic obstructive airways disease.
Eligible patients were compared with a group of age-matched controls, without prior history of stroke or AF and underwent outpatient Holter monitoring. The control group were composed of patients who underwent investigation of chest pain, syncope, presyncope and palpitations. The stroke/TIA and control groups were age matched with the same exclusion criteria applied.
Clinical assessment and outcome measures
All included patients adjudicated as having a stroke/TIA underwent investigation and treatment as per the national stroke guidelines recommendation for standard of care.17 This included physical examination, blood measurements, 12 lead ECG, pulse oximetry, CT of the brain, inpatient cardiac monitoring and vascular assessment. All patients underwent Holter monitoring with 24 hours of continuous rhythm capture utilising the SEER Light Holter Monitor (GE Healthcare, Milwaukee, USA). Data were analysed offline on completion of the monitoring period by cardiac technicians and subsequently reviewed by a cardiac electrophysiologist blinded to the study hypothesis. Rhythm analysis was conducted using MARS Ambulatory ECG Analysis System (GE Healthcare).
Baseline demographic and clinical data were collected from electronic health records along with vascular risk factors to allow for calculation of a CHA2DS2VASc score, medication use and to identify presentations with recurrent stroke or TIA. Based on prior literature, we defined excessive PAC burden as ≥200 PACs/24 hours and a long atrial run as ≥20 beats.10 18 19
Statistical methods
Demographic data, disease status and outcome measures are presented as proportions and summarised by descriptive statistics. Data were tested for normality and parametric or non-parametric tests applied as appropriate. Correlation trends were analysed using Spearman’s rho for non-parametric data. A p value <0.05 was deemed statistically significant and a 95% CI is presented where applicable. Markers associated with stroke/TIA and excessive PACs were identified by univariate and multivariate logistical regression. Any variable with a p value <0.25 on univariate analysis was included in multivariate analyses. All statistical analysis was performed with SPSS Statistics V.24.0.
Patient and public involvement
Public involvement was sought after the methods and outcome measures were identified. The protocol and study design were reviewed by human research ethics committee, 45% of whom were members of the public.
Ethics approval and data sharing
The raw data will be made available by the corresponding author on reasonable request.
Results
In total, 537 patients presented with a stroke/TIA during the study inclusion period and underwent Holter monitoring. Twenty-three out of 537 (4.2%) patients had AF identified on Holter monitoring and were excluded. Four patients with AF had an excessive PAC burden (17%).
Following exclusions, 461 patients with a stroke/TIA were compared against 251 age-matched patients who underwent Holter monitoring during the same time period (figure 1).
Figure 1.
Patient selection: inclusions and exclusions. AF, atrial fibrillation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; TIA, transient ischaemic attack.
The median time to Holter monitoring following the stroke or TIA was 40 days.
Ischaemic stroke and PAC burden
Baseline characteristics stratified according to study groups are shown in table 1. In both groups, the mean age was 70 years and the majority of patients were male. Stroke/TIA patients were significantly more likely to have comorbidities of hypertension, diabetes mellitus, dyslipidaemia, peripheral vascular disease and a prior history of smoking. There were 79 patients with a prior cerebrovascular event in the stroke/TIA group. On admission, there was significantly higher use of statins in the stroke/TIA cohort; however, no difference was evident in the use of antiplatelet therapy or oral anticoagulants. The prevalence of excessive PACs were significantly higher in the stroke/TIA group (25.6% vs 14.7%, p=0.001); however, atrial runs of ≥20 beats were not significantly different (table 1).
Table 1.
Baseline characteristics of study participants
| Characteristics | Control n=251 n (%) |
Stroke/TIA n=461 n (%) |
P value |
| Age, years (SD) | 70.5 (11.6) | 69.8 (12.5) | 0.45 |
| Sex, female | 88 (35.1) | 195 (42.3) | 0.06 |
| Stroke subtypes | |||
| Large vessel atherosclerosis | – | 82 (17.8) | – |
| Small vessel occlusion | – | 86 (18.7) | – |
| Cryptogenic | – | 291 (63.1) | – |
| Stroke of other determined aetiology | – | 2 (0.4) | – |
| Vascular risk factors | |||
| Hypertension | 130 (51.8) | 294 (63.8) | 0.01 |
| Dyslipidaemia | 91 (36.3) | 209 (45.3) | 0.02 |
| Diabetes mellitus | 38 (15.1) | 113 (24.5) | 0.01 |
| Any smoking | 44 (17.5) | 118 (25.6) | 0.01 |
| Previous stroke/TIA | 0 (0) | 79 (17.1) | <0.001 |
| Myocardial infarction | 40 (15.9) | 82 (17.8) | 0.53 |
| Peripheral vascular disease | 5 (2.0) | 26 (5.6) | 0.02 |
| Sleep apnoea | 14 (5.6) | 13 (2.8) | 0.07 |
| History of heart failure | 11 (4.4) | 25 (5.4) | 0.55 |
| CHA2DS2VASc score, median (IQR) | 2 (1–3) | 5 (4–5) | <0.001 |
| Medications | |||
| Warfarin | 4 (1.6) | 4 (0.9) | 0.38 |
| Direct oral anticoagulant | 4 (1.6) | 1 (0.2) | – |
| Antiplatelet therapy | 81 (32.3) | 165 (35.8) | 0.35 |
| Beta blocker | 48 (19.1) | 82 (17.8) | 0.66 |
| Ace inhibitor | 105 (41.8) | 224 (48.6) | 0.08 |
| Statin | 79 (31.5) | 187 (40.6) | 0.02 |
| Premature atrial complexes | |||
| PACs/24 hours, median (IQR) | 37 (13–115) | 62 (20–208) | <0.01 |
| Longest atrial run, median (IQR) | 3 (0–7) | 3 (0–8) | <0.01 |
| Atrial runs >3 beats, median (IQR) | 1 (0–2) | 1 (0–4) | 0.07 |
| ≥200 PACs/24 hours | 37 (14.7) | 118 (25.6) | <0.001 |
| ≥20 beats in runs | 13 (5.2) | 27 (5.9) | 0.71 |
The data are presented as n (%), unless otherwise stated.
PACs, premature atrial complexes; TIA, transient ischaemic attack.
Multivariate analysis showed female sex, hypertension, history of smoking and excessive PACs were significantly associated with a stroke/TIA. Excessive PACs conferred the highest risk for stroke/TIA with an OR of 1.97 (95% CI 1.29 to 3.02), but the difference was not significant when compared with other risk factors associated with stroke/TIA (table 2). Multivariate analysis with various definitions of excessive PACs based on prior literature yielded similar results, with a significant association between excessive PACs and stroke/TIA (online supplementary file 1).
Table 2.
Multivariate analysis: risk factors associated with stroke/TIA and cryptogenic stroke
| Characteristic | Stroke/TIA | Cryptogenic stroke | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Female | 1.51 (1.09 to 2.11) | <0.05 | 1.78 (1.19 to 2.67) | <0.01 |
| Hypertension | 1.53 (1.07 to 2.17) | <0.05 | 1.67 (1.05 to 2.64) | <0.05 |
| Smoking | 1.58 (1.06 to 2.36) | <0.05 | 1.55 (0.95 to 2.53) | 0.08 |
| ≥200 PACs | 1.97 (1.29 to 3.02) | <0.01 | 1.95 (1.16 to 3.28) | <0.05 |
Variables adjusted in the multivariate model: age, sex, excessive premature atrial complexes, hypertension, diabetes mellitus, smoking, dyslipidaemia, peripheral vascular disease and sleep apnoea.
PACs, premature atrial complexes; TIA, transient ischaemic attack.
bmjopen-2019-029164supp001.pdf (92KB, pdf)
Analysis of PAC burden revealed a skewed distribution, median PACs/24 hours and longest atrial ectopic runs were significantly higher in the stroke/TIA group. However, number of beats in runs of >3 beats was not significantly different between the two groups.
Cryptogenic stroke and PAC burden
One hundred and eighty-five patients with cryptogenic stroke, after excluding TIA, were compared with 251 patients in the control group. The mean age was not significantly different between the control and cryptogenic stroke group, 70.5 and 68.9 years, respectively. Cryptogenic stroke was significantly associated with excessive PAC burden, female sex, hypertension, diabetes mellitus, smoking, obstructive sleep apnoea and a higher median CHA2DS2VASc score on univariate analysis. Excessive PACs (OR: 1.95; 95% CI 1.16 to 3.28), female sex (OR: 1.78; 95% CI 1.19 to 2.67) and hypertension (OR: 1.67; 95% CI 1.05 to 2.64) maintained significant and independent associations with cryptogenic stroke subtype on multivariate logistical regression (table 2).
Vascular risk factors and PAC burden
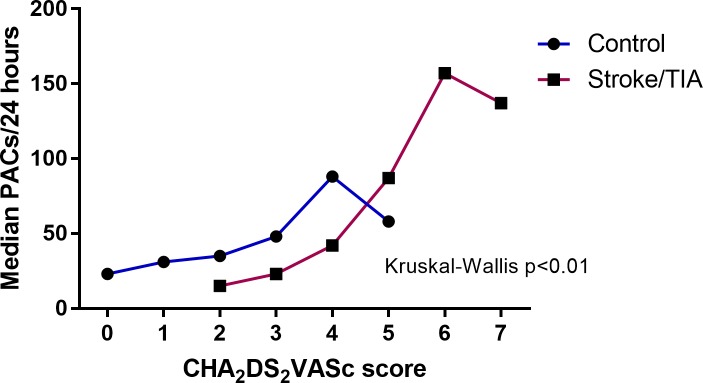
Increasing CHA2DS2VASc score was associated with increasing median PACs in both groups (figure 2). A CHA2DS2VASc score >3 in the control group and two in the stroke/TIA group were associated with an excessive PAC burden. However, only a moderate to weak correlation was evident between increasing CHA2DS2VASc score and increasing PACs/24 hours in all patients (rs=0.32, p<0.001), control patients (rs=0.24, p<0.001) and stroke/TIA patients (rs=0.32, p<0.001), respectively.
Figure 2.
Median PACs by CHA2DS2VASc score for control and stroke/TIA groups. CHA2DS2VASc, risk score for ischaemic stroke; PACs, premature atrial complexes; TIA, transient ischaemic attack.
In all patients, age, hypertension, diabetes mellitus and peripheral vascular disease were significant univariate predictors of excessive PACs. However, only age and hypertension remained independently associated with excessive PACs on regression analysis, with age ≥75 years being the strongest marker associated with excessive PACs (table 3).
Table 3.
Multivariate analysis: vascular risk factors associated with excessive PACs in all patients
| Risk factor | OR (95% CI) | P value |
| Age (years) | ||
| 65–74 | 2.52 (1.42 to 4.45) | <0.01 |
| ≥75 | 3.64 (2.08 to 6.36) | <0.01 |
| Hypertension | 1.54 (1.01 to 2.34) | <0.05 |
| Diabetes mellitus | 1.41 (0.91 to 2.20) | 0.13 |
Variables adjusted in the multivariate model: age: <65, 65–74, ≥75 years, gender, hypertension, diabetes mellitus, smoking, dyslipidaemia, peripheral vascular disease, sleep apnoea.
PACs, premature atrial complexes.
Discussion
In this study, we compared the differences in PAC burden between patients with a stroke/TIA and an age-matched control population, after excluding AF. Excessive PAC burden was significantly more common in the stroke/TIA group. An important new finding in our study was that excessive PACs demonstrated an independent association for the cryptogenic stroke subtype, after adjusting for conventional risk factors (OR: 1.95; 95% CI 1.16 to 3.28). There was a stepwise rise in PAC burden with increasing number of vascular risk factors; age and hypertension were independent risk factors associated with excessive PACs.
Investigators have previously demonstrated a significantly higher PAC burden in patients who develop incident AF and ischaemic stroke.18 20 Existing longitudinal studies from Engström et al that demonstrated a high PAC burden conferred a 1.9 times higher risk for ischaemic stroke.19 Despite the differences in methodology, the current study showed 1.97 times rise in odds for ischaemic stroke. Prior studies have also shown an association between runs of PACs and ischaemic stroke in patients without documented AF.21 The Copenhagen Holter study, a cohort study that analysed the risk for stroke with an elevated PAC burden, defined excessive supraventricular ectopic activity as a composite of either >30 PACs/hour or a run of >20 PACs, and found a positive correlation with increased stroke and death.10 13 In contrast, we did not show a significant difference in PAC runs >20 beats between the two groups.15 This apparent discrepancy is likely due to a lack of standardised definitions for excessive PACs and treating atrial premature runs ≥20 beats as a standalone variable in the current study, instead of a composite measure.
The present study specifically analysed the association between excessive PACs and cryptogenic stroke subtype. It demonstrated an independent association between cryptogenic stroke subtype and excessive PACs with an OR of 1.95. This is an important finding and lends further support to the hypothesis, that excessive PACs may be the manifestation or marker of underlying atrial myopathy, that confers an increased risk for cryptogenic stroke. Further, similar to AF, the risk for ischaemic stroke in the presence of an excessive PAC burden appear to be modulated by vascular risk factors. A CHA2DS2VASc score of 2 conferred a similar risk for ischaemic stroke in patients with excessive PACs, as with AF.10
An increasing CHA2DS2VASc score was significantly associated with increasing median PAC burden in both the control and stroke/TIA group. However, despite the positive correlation between CHA2DS2VASc score and PACs, the strength of the correlation itself remained weak. This was suggestive of differential effects of the various components of CHA2DS2VASc score in contributing to a high PAC burden. The independent contribution of the various risk markers that make up CHA2DS2VASc score have not been assessed previously.10 13 22 Delineation of these specific risk factors that contribute to excessive PACs provides insights into the potential pathophysiological basis for excessive PAC.
Increasing age and hypertension were independently and significantly associated with excessive PACs in the present study. This is consistent with electroanatomical studies that demonstrated slower conduction velocities and both global and regional reduction in atrial voltages with increasing age and hypertension.23 24 Such areas corresponded to delayed enhancement on MRI and histological fibrosis.25 26 Thrombogenesis associated with this underlying atrial remodelling may help explain a significant proportion of strokes currently classified as cryptogenic. Both advancing age and hypertension are also associated with small and large vessel stroke subtypes. In addition to atrial remodelling, it is likely that vascular risk factors promote thrombogenesis and ischaemic stroke through multiple pathways including arterial endothelial dysfunction, and atherosclerosis with localised plaque rupture.27
Our report of the independent association between excessive PACs and cryptogenic stroke further implicates a risk factor driven atrial substrate abnormality in its pathogenesis. These findings are clinically relevant as the results of a recently concluded large multicentre randomised controlled trial failed to demonstrate a benefit for oral anticoagulation in an unselected population with embolic stroke of undetermined source.8 This highlights the heterogeneity of the pathophysiological mechanisms that lead to cryptogenic stroke. There is an unmet clinical need to develop risk markers that identify the subset of patients with cryptogenic stroke that occur as a result of cardioembolism.
Studies have previously described serological and echocardiographic markers associated with the recurrence of cryptogenic stroke.28 Similarly, excessive PACs are readily assessed and may serve as a novel and reproducible marker to identify patients at high risk for the cryptogenic stroke. It is unclear if excessive PACs directly promote thrombogenesis or if they are simply a marker of adverse atrial remodelling that leads to thrombogenesis and stroke. Regardless, the risk conferred by an elevated CHA2DS2VASc score in conjunction with excessive PACs for ischaemic stroke remains significant.10
This study has limitations. The absence of prolonged monitoring with devices such as implantable loop recorders could have led to an underestimation of incident AF. However, we excluded all patients with a diagnosis of AF over 1.9 years of mean follow-up. A higher number of cryptogenic stroke patients were present in our population than previously reported. This is likely due to referral bias, as patients were only included if they underwent Holter monitoring. All patients included in the study had guideline-based referral for Holter monitoring. However, as Holter monitoring was an inclusion criterion, we do not have data on patient who may have received their Holter monitoring at an external institution. However, the higher prevalence of cryptogenic stroke improves the strength of our findings in this specific subset of stroke patients. The time to Holter monitoring following the stroke, based on routine institutional clinical waiting periods, could have introduced unintended variables such as neurologically mediated cardiac modelling with resultant excessive PACs and reverse causality. The higher burden of PACs was noted in a highly selective patient cohort with ischaemic stroke and a high burden of vascular risk factors. Despite the use of multivariate regression analysis, unrecognised confounders cannot be excluded in a cross-sectional case–control study, therefore these findings should not be extrapolated to other patient cohorts.
Conclusions
Excessive PACs are significantly associated with cryptogenic stroke. Vascular risk factors, increasing age and hypertension, were independently associated with excessive PACs. The utility of novel and reproducible cardiac markers to guide preventative strategies in cryptogenic stroke warrant further evaluation.
Supplementary Material
Footnotes
Contributors: JKS led and designed the study, collated and analysed data and wrote the manuscript. ANK, KR, MCT and TF collated and analysed the data and revised the manuscript. HD, JMK, LR, JCC and MW contributed to study design and revised the manuscript. MS analysed the data and revised the manuscript. AWT supervised and designed the study revised the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: The research protocol was approved by the institutional Human Research Ethics Committee and written informed consent was not deemed necessary by the committee (approval number: LR09/2016).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Royal College of Physicians Sentinel Stroke National Audit Programme (SSNAP). National clinical audit annual results portfolio March 2016-April 2017. 2017. http://bit.ly/1NHYlqH (accessed 24 Dec 2018).
- 2. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38. 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 5. Ntaios G, Papavasileiou V, Milionis H, et al. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke 2015;46:176–81. 10.1161/STROKEAHA.114.007240 [DOI] [PubMed] [Google Scholar]
- 6. Liao J, Khalid Z, Scallan C, et al. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke 2007;38:2935–40. 10.1161/STROKEAHA.106.478685 [DOI] [PubMed] [Google Scholar]
- 7. Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016;9:e003333 10.1161/CIRCEP.115.003333 [DOI] [PubMed] [Google Scholar]
- 8. Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–201. 10.1056/NEJMoa1802686 [DOI] [PubMed] [Google Scholar]
- 9. Conen D, Adam M, Roche F, et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation 2012;126:2302–8. 10.1161/CIRCULATIONAHA.112.112300 [DOI] [PubMed] [Google Scholar]
- 10. Larsen BS, Kumarathurai P, Falkenberg J, et al. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol 2015;66:232–41. 10.1016/j.jacc.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 11. Marinheiro R, Parreira L, Amador P, et al. Excessive atrial ectopic activity as an independent risk factor for ischemic stroke. Int J Cardiol 2017;249:226–30. 10.1016/j.ijcard.2017.08.054 [DOI] [PubMed] [Google Scholar]
- 12. Himmelreich JCL, Lucassen WAM, Heugen M, et al. Frequent premature atrial contractions are associated with atrial fibrillation, brain ischaemia, and mortality: a systematic review and meta-analysis. Europace 2018. doi: 10.1093/europace/euy276[Epub ahead of print 1 Dec 2018]. 10.1093/europace/euy276 [DOI] [PubMed] [Google Scholar]
- 13. Binici Z, Intzilakis T, Nielsen OW, et al. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 2010;121:1904–11. 10.1161/CIRCULATIONAHA.109.874982 [DOI] [PubMed] [Google Scholar]
- 14. Sejr MH, Riahi S, Larsen TB, et al. Premature atrial complexes in an ischemic stroke population and risk of recurrent stroke: a systematic review. Expert Rev Cardiovasc Ther 2017;15:447–55. 10.1080/14779072.2017.1332992 [DOI] [PubMed] [Google Scholar]
- 15. Pinho J, Braga CG, Rocha S, et al. Atrial ectopic activity in cryptogenic ischemic stroke and TIA: a risk factor for recurrence. J Stroke Cerebrovasc Dis 2015;24:507–10. 10.1016/j.jstrokecerebrovasdis.2014.09.029 [DOI] [PubMed] [Google Scholar]
- 16. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 17. National Stroke Foundation Melbourne Australia. Clinical guidelines for stroke management 2010, 2010. [Google Scholar]
- 18. Todo K, Moriwaki H, Saito K, et al. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol 2009;61:285–8. 10.1159/000206853 [DOI] [PubMed] [Google Scholar]
- 19. Engström G, Hedblad B, Juul-Möller S, et al. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke 2000;31:2925–9. [DOI] [PubMed] [Google Scholar]
- 20. Acharya T, Tringali S, Bhullar M, et al. Frequent atrial premature complexes and their association with risk of atrial fibrillation. Am J Cardiol 2015;116:1852–7. 10.1016/j.amjcard.2015.09.025 [DOI] [PubMed] [Google Scholar]
- 21. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J 2015;36:170–8. 10.1093/eurheartj/ehu407 [DOI] [PubMed] [Google Scholar]
- 22. Kamel H, Elkind MS, Bhave PD, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke 2013;44:1550–4. 10.1161/STROKEAHA.113.001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 2004;44:109–16. 10.1016/j.jacc.2004.03.044 [DOI] [PubMed] [Google Scholar]
- 24. Medi C, Kalman JM, Spence SJ, et al. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:1317–24. 10.1111/j.1540-8167.2011.02125.x [DOI] [PubMed] [Google Scholar]
- 25. Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. 10.1161/CIRCULATIONAHA.108.811877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens 2007;9:546–50. 10.1111/j.1524-6175.2007.06626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001;104:191–6. 10.1161/01.CIR.104.2.191 [DOI] [PubMed] [Google Scholar]
- 28. Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke 2015;46:1488–93. 10.1161/STROKEAHA.115.008711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029164supp001.pdf (92KB, pdf)