Abstract
For many years, an area-wide fruit fly control campaign against the Mexican fruit fly, Anastrepha ludens (Loew) has been implemented in some regions of Mexico and Texas, using the sterile insect technique (SIT) as its principal component. To improve the efficiency of the SIT, a genetic sexing strain based on black pupae mutation (bp) was developed for A. ludens, namely, ‘Tapachula-7’ (Tap-7 genetic sexing strains [GSSs]). This strain was introduced into the AW-IPM program recently and allows male-only releases for SIT applications. Here, we report the genetic and biological characterization of a new mutation, slow larvae (sl), which was introduced to the original translocation of the Tap-7 GSS resulting in two new GSS (slow-7 and Tap/slow-7). In both GSSs, the translocated wild-type males emerge from brown pupae that develop faster than females. The females are homozygous for sl mutation in the slow-7 GSS and homozygous for sl and bp mutations in the Tap/slow-7 GSS, reaching larval maturity 2 d after most of the wild-type males, allowing the separation of most males during pupariation. The potential use of the slow-7 and Tap/slow-7 GSSs in mass rearing and large-scale population suppression programs is discussed.
Keywords: Mexican fruit fly, mass rearing, sterile insect technique, mutation, genetic sexing strain
The Mexican fruit fly (Mexfly), Anastrepha ludens (Loew), is the most serious economic pest among fruit flies in the south of United States and Central America. Since 1992, an area-wide insect pest management (AW-IPM) campaign against this pest has been implemented in some regions of Mexico and Texas. The sterile insect technique (SIT) is the principal component of this population suppression campaign (Gutiérrez 2010). The SIT application consists of rearing, irradiation, marking, and release of a large number of insects into the field, which after mating will transfer sterile sperm to wild females and inhibit their reproduction, thus introducing sterility into the target wild population (Knipling 1966).
The Mexfly SIT program has been releasing both females and males into target areas during several years. However, it has been shown that the exclusive release of males by using genetic sexing strains (GSSs) is much more effective, e.g., avoids preferential mating among released flies, documented in bisexal strains (Robinson et al. 1986, McInnis et al. 1994, Rendon et al. 2000, 2004), and reduces postproduction handling costs to the control programs as the marking, irradiation, transport, and release activities are reduced by half. All GSSs used so far in SIT projects have been based on the same sex-specific pseudo-linkage principle (Y-autosome translocation), using different recessive markers. The combination of Y-autosome translocation and a selectable marker results in heterozygous males (wild-type phenotype) and homozygous females for the marker (mutant phenotype; Robinson et al. 1999). The markers used to construct the sexing mechanism and determine the sex separation strategy can be functional at any developmental stage. Thus, several GSSs have been developed using a variety of gene markers such as: wing mutation (bent wing) where the females are unable to fly and only males are active in the field (Meats et al. 2002), pupal color markers which allow the separation of male from female pupae through a sorting machine (Rössler 1979, Busch-Petersen and Kafu 1989, McCombs and Saul 1995, McInnis et al. 2004), as well as mutations affecting embryonic and larval development (Cladera 1995). However, the most successful GSS has been developed in the Mediterranean fruit fly Ceratitis capitata using two mutations: white pupae (wp; Rössler 1979) and temperature-sensitive lethal (tsl; Franz et al. 1994). In this GSS, males emerge from brown pupae and are resistant to elevated temperatures (34 to 35°C), whereas females emerge from white pupae and are sensitive to high temperatures. The thermal sensitivity allows male-only production by killing all females through an embryonic heat shock treatment. Additionally, the tsl marker has been associated with slow larval development phenotype (Franz 2005), thus inducing an additional self-sexing mechanism in the GSS, since males develop 1 or 2 d faster than females (Cáceres 2002).
Using the same sex-specific pseudo-linkage principle (Y-autosome translocation) and as selectable marker the black pupae mutation (bp) located on chromosome 2, a GSS for Mexfly has been recently developed, the Tapachula-7 (Tap-7 GSS; Zepeda-Cisneros et al. 2014). In this GSS, males emerge from brown pupae (wild-type), whereas the females emerge from black pupae (mutant), thus allowing the separation of males from females at the pupal stage using a color-sorting machine. The Tap-7 GSS has been introduced into action programs in Mexico and United States for male-only releases. As is the case for Mediterranean fruit fly, the integrity of this genetic sexing system is maintained through a filter rearing system (FRS) which eliminates any recombinant insects (mutant males emerging from black pupae and wild-type females emerging from brown pupae; Fisher and Caceres 2000).
In this study, we report the genetics of slow larvae (sl), a new morphological mutant for A. ludens, which displays two traits: slow development during the larval stage and light pigmentation at the pupal and adult stage. The new mutation was integrated into the current black pupal color-based sexing system of the Mexfly, allowing self-sexing during the pupal stage. The potential use of the new GSS developed in the frame of this study in SIT programs is discussed.
Materials and Methods
Insect Strains and Rearing Conditions
All seven strains used in the present study are maintained at the Insect Pest Control Laboratory of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Seibersdorf, Vienna, Austria (Table 1). During the screening for new phenotypes in several established wild-type (WT) isolines, one of them yielded 16 light brown pupae. Emerged adults of these pupae (4 males and 12 females) showed light pigmentation and were interbred. All F1 offspring displayed light pigmentation and delayed larval development time (sl mutation) and were used to establish the purebred colony used in this study. Using the original T:Y(2bp+)/bp translocation from Tap-7 GSS, two new GSSs were constructed by backcrossing wild males to mutant females: Slow-7 GSS [T:Y(2sl+)/sl] and Tap/slow-7 GSS [T:Y(2bp+sl+)/bp sl]. Flies were maintained in small cages (1,500 cm3 of volume) with traditional food used under mass rearing condition (sugar:protein, ration 3:1) and water ad libitum at a density of 30 pairs per cage. Once the flies reached sexual maturity (10 d after emergence), one oviposition substrate (egg collector) was placed on top of the cage for egg collection. To collect fresh eggs only, the females had access to the egg collector only 5 h per day. In all experiments no especial conditions were used, the eggs were transferred to the larval carrot diet and the temperature used in all stages was 25°C (Tanaka et al. 1969).
Table 1.
List of Mexfly, A. ludens strains used during the study
| Name of the strain | Genotype | Reference |
|---|---|---|
| Wild-type | bp + sl + /bp + sl + | |
| Black pupa mutation | bp/bp | Zepeda et al. 2014 |
| Slow larvae mutation | sl/sl | This study |
| Black –pupa–slow larvae double mutation | bp sl/bp sl | This study |
| Tapachula 7 genetic sexing strain (Tap-7 GSS) | T(Y;2bp+)-7 | Zepeda et al. 2014 |
| Slow 7 genetic sexing strain (Slow-7 GSS) | T(Y;2sl+)-7 | This study |
| Tapachula/slow 7 genetic sexing strain (Tap/slow-7 GSS) | T(Y;2bp+sl+)-7 | This study |
Genetics of sl Mutant
Reciprocal crosses, in single mating pairs, were made between sl mutants and WT insects in order to determine the mode of inheritance of the delayed larval development phenotype. The F1 progeny were interbred in groups of five pairs and the resulted F2 phenotypes were observed and recorded. Crosses between the two mutant lines, bp and sl, were also carried out to assess their potential linkage. The F1 progeny were interbred and the F2 phenotypes were observed and recorded. To determine the genetic distance between the bp and sl loci in males and females, heterozygous insects in repulsion phase (bp+sl/bp sl+) and in coupling phase (bp sl/bp+sl+) were reciprocally backcrossed to double mutant insects (bp sl/bp sl).
Biological Characteristics
The biological characteristics of wild-type, single mutants (slow larvae and black pupae), double mutant (slow larvae and black pupae), and GSSs (Tap-7, Slow-7 GSS, and Tap/slow-7 GSS) were assessed in a comparative way. For determining the survival from egg to different development stages (larva, pupa, and adult) and the male ratio (number of males by females produced), 100 eggs from all the evaluated strains were placed on a net over a wet blotting black filter for 4 d at 26°C. The net with the eggs was then transferred to the larval diet in a Petri dish. After 3 d, the hatch rate was recorded and the Petri dishes were opened and placed inside a plastic container with sawdust. Pupae were collected from the sawdust and recorded by phenotype. Finally, the percentage of adults from each sex emerged from pupae was recorded. Twelve replicates of 100 eggs each were carried out per strain (n = 12 × 7 strains = 84 experimental units).
For estimating the time required for embryonic development, 100 eggs per replicate were placed on a net over a wet black filter paper in a small Petri dish (100 × 15 mm). The hatch rate was recorded daily until reaching maximum hatching. Fifteen replicates per strain were carried out. The larval development time was estimated as the number of days from when eggs were placed on the larval diet to the larval pupariation phase (Fraenkel and Bhaskaran 1973, Meza et al. 2005). For timing the larva stage, a sample of 0.1 ml of eggs of uniform age, close to hatching, was transferred to the larval diet, contained into a large Petri dish (150 × 15 mm). To avoid collecting immature larvae, 9-d-old larvae were recovered using a sieve and diluting the larval diet in water. The larvae collected were returned to another large Petri dish with fresh carrot diet and placed inside a plastic box with sawdust. The postfeeding larvae that came out of the fresh diet during the wandering larvae phase were collected daily from the sawdust as immobile larvae and prepupa (pupariation phase) and recorded (Denlinger and Zdárek 1994). Six replicates per strain were carried out.
The same procedure was used for the GSSs recording the brown pupae (males) and mutant pupae (females).
Data Analysis
The genetic crosses data were evaluated by contingency tables followed by Pearson chi-squared tests. The recombination distance was estimated as and the standard error was calculated using the formula (Serra 1965), where R is the number of recombinants phenotype and n is the total number of individuals observed. Each biological characteristic was analyzed by one-way analysis of variance (ANOVA) using the ‘strain’ as predictor of survivorship across development stages (larva, pupa, and adult) and the male ratio (number of males by females produced). The Tukey’s HSD test was used as a post hoc method to compare means between strains on significant factors. To normalize the data distribution and stabilize the variances, the data in percentages were transformed following arcsin (Zar 2010). The genetic load of the pure mutations was estimated as the difference in average survival of the transformation from egg to adult between mutant and wild-type insects. Embryonic developmental time data were corrected based on maximum egg hatch using Abbott’s formula (Abbott 1925) to avoid the effect of different fitness between them. The embryonic and larval developmental time were analyzed using a generalized linear model with Poisson distribution and a log-link function (Agresti 1996). A matched pairs analysis was performed to analyze the difference in larval developmental time between males and females for each GSS. All data were analyzed with Statistical Discovery JMP 11.0.0 software (SAS Institute).
Results
The Morphology and the Genetics of the sl Mutation
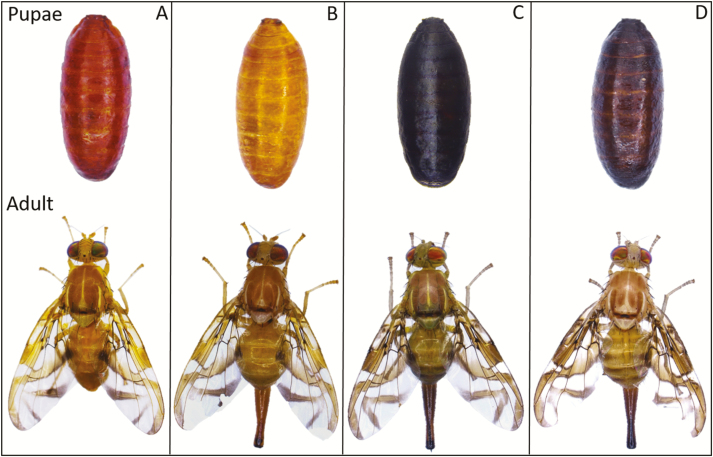
The typical yellowish-brown pigmentation in A. ludens wild-type insects (Fig. 1A) is significantly attenuated in the new sl mutation. The puparium of sl mutation has a golden-like color and the adults exhibit colorless dorsal fringes, which can be clearly distinguished from the wild-type phenotype by visual examination (Fig. 1B).
Fig. 1.
Adult and pupal phenotypes of the selectable markers involved in the A. ludens GSS. (A) Wild-type male. (B) Slow larvae female mutant (sl) in slow-7 GSS. (C) Black pupae female mutant (bp) in Tap-7 GSS. (D) Double mutant female (bp sl) in Tap/slow-7 GSS.
The mode of inheritance of sl in A. ludens was tested by appropriate genetic crosses between the mutant and the wild-type line. As shown in Table 2, all F1 offspring of the reciprocal crosses between the mutant and the wild-type lines were wild-type. In F2 offspring of reciprocal crosses, the proportion of the wild-type and the sl phenotypes was according to the segregation of a recessive autosomal gene (3WT:1sl), although significant deviation was observed in some replicates, explained by the low viability of sl, perhaps accentuated when both phenotypes compete each other as larvae (Table 2). It is worth noting that, as expected, sl individuals were having a golden-like puparium and exhibited colorless dorsal fringes at the adult stage which significantly facilitates the monitoring of the sl phenotype.
Table 2.
Mode of inheritance of slow larvae (sl) in A. ludens
| Cross | Pair | F1 (genotype) | F2 phenotype | TOTAL | X2 (3:1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | WT | sl | ||||||
| male | female | male | female | ||||||
| WT | sl | 1 | WT (sl/sl+) | 414 | 428 | 119 | 121 | 1082 | 4.59 |
| 2 | 328 | 342 | 92 | 103 | 865 | 2.78 | |||
| 3 | 375 | 368 | 109 | 92 | 944 | 6.92 | |||
| sl | WT | 1 | WT (sl+/sl) | 283 | 279 | 90 | 83 | 735 | 0.84 |
| 2 | 368 | 372 | 108 | 92 | 940 | 6.95 | |||
| 3 | 264 | 212 | 63 | 72 | 611 | 2.75 | |||
X20.05,1 = 3.841.
The potential genetic linkage between sl and bp in A. ludens was assessed by appropriate genetic crosses. All F1 offspring of the reciprocal crosses between the two homozygous mutant lines sl and bp were 100% wild-type. In both reciprocal crosses, the proportion of the F2 offspring phenotypes was significantly deviated from the expected (9WT:3bp:3sl:1bp sl), if the two genetic loci (sl and bp) were segregating independently. In addition, there was complete absence of double recessive homozygous insects (bp sl). Taken together, these data strongly suggest that the two genes, sl and bp, are linked, being located on the same chromosome. Due to crossing-over event during the F1 interbred, some F2sl insects were heterozygous for bp and some F2bp insects were heterozygous for sl; thus, double mutant insects (bp sl) were obtained after the interbreeding of F2sl and F2bp insects. Interestingly, the phenotype of the bp sl insects showed an additive interaction between the two mutations resulting in a dark pupal color phenotype which is lighter than the typical of the bp mutant (Fig. 1C and D).
The genetic distance between the bp and sl genetic loci was determined by appropriate genetic crosses involving the F1 wild-type progeny of the direct cross between the two homozygous mutant lines. These F1 wild-type insects, which are heterozygous for both genes in the repulsion phase, were reciprocally test-crossed to bp sl and again a significant deviation from the proportion expected (1WT:1bp:1sl:1bp sl) for independent segregation was observed. The same result was obtained when the recombinant wild-type (WT Tc) insects, in coupling phase, were test-crossed to bp sl insects (Table 3). In both experiments (repulsion and coupling), recombinant individuals were observed only in the female offspring while no recombination was observed in males. Based on the female recombinants, the estimated distance between the bp and sl genetic loci was estimated to be, in average, 0.43 cM.
Table 3.
Linkage analysis of the bp and sl genetic loci of A. ludens
| Crosses Phenotype (Genotype) |
Generation | WT | bp | sl | bp sl | TOTAL | X2 | Recombination r (SE) | |
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
|
sl
(bp+sl/bp+sl) |
bp
(bp sl+/bp sl+) |
F2 | 603 | 261 | 170 | 0 | 1034 | 91.6a | – |
|
bp
(bp sl+/bp sl+) |
sl
(bp+sl/bp+sl) |
362 | 156 | 99 | 0 | 617 | 55.7a | – | |
| WT F1 (bp sl+/bp+sl) |
Double mutant (bp sl/bp sl) |
Test-crossed | 0 | 385 | 240 | 0 | 625 | 692.3b | 0.00 |
|
Double mutant
(bp sl/bp sl) |
WT F1 (bp sl+/bp+sl) |
249 | 350 | 247 | 130 | 976 | 99.5b | 0.39 (0.015) | |
| WT Tc (bp sl/bp+sl+) |
Double mutant (bp sl/bp sl) | 658 | 0 | 0 | 429 | 1087 | 1183.5b | 0.00 | |
| Double mutant (bp sl/bp sl) | WT Tc (bp sl/bp+sl+) |
663 | 537 | 364 | 307 | 1871 | 170.0b | 0.48 (0.011) | |
a9:3:3:1 hypothesis; b1:1:1:1 hypothesis; X20.05, df = 3 = 7.82.
Tc = Insect from test-cross.
Comparative Analysis of the Biological Characteristics
Significant differences were detected between the strains tested with respect to hatch rate (F6,75 = 108.32, P < 0.001), egg to pupa survival (F6,75 = 211.75, P < 0.001), and egg to adult survival (F6,75 = 190.98, P < 0.001). Comparative analysis between the wild-type and the mutant lines regarding the survival showed that the genetic load was minimal for single mutant individuals (2.13% for bp and 2.17% for and sl), with no significant difference between the bp and sl mutations. However, in the double mutant line (bp sl), the genetic load increased up to 14.25%, significantly affecting the survival in the Tap/slow-7 GSS (Table 4).
Table 4.
Survival from egg to different developmental stages and sex ratio (number of males by female produced) (Mean ± SE)
| Strain | Larva neonatea (%) | Pupa (%) |
Adult (%) |
Males/females |
|---|---|---|---|---|
| WT | 88.50 ± 0.66a | 84.58 ± 0.65a | 82.83 ± 0.60a | 1.05 ± 0.03a |
| bp | 86.30 ± 0.89a | 84.40 ± 1.00a | 80.70 ± 1.75a | 0.98 ± 0.04a |
| sl | 84.91 ± 1.11a | 83.33 ± 0.97a | 80.66 ± 1.02a | 0.99 ± 0.02a |
| bp sl | 78.50 ± 0.89b | 70.50 ± 0.75b | 68.58 ± 1.75b | 1.03 ± 0.02a |
| Tap-7 GSS [T(Y;2bp+)-7] | 66.25 ± 1.2c | 40.58 ± 1.74c | 37.25 ± 1.66c | 1.21 ± 0.05a |
| Slow-7 GSS [T(Y;2sl+)-7] | 65.75 ± 1.57c | 39.66 ± 1.72c | 36.41 ± 1.69c | 1.86 ± 0.12b |
| Tap/slow-7 GSS [T(Y;2bp+sl+)-7] | 56.16 ± 1.29d | 33.41 ± 1.36d | 28.66 ± 1.48d | 2.13 ± 0.14c |
aEgg hatch; for each column, lower case letters represent significant differences between strains (P < 0.05).
Significant differences were also detected between the strains tested with respect to the sex ratio (F6,75 =34.98, P < 0.001) and as shown in Table 4, it is clearly in favor of males in the slow-7 GSS and even more pronounced in the Tap/slow-7 GSS. So, the male production was almost similar in all GSS (Tap-7 = 20.33%, Slow-7 = 23.50% and Tap/slow-7 = 19.33%); however, the female production was more reduced in the GSS that integrated the sl marker (Tap-7 = 16.92%, Slow-7 = 12.92% and Tap/slow-7 = 9.33%).
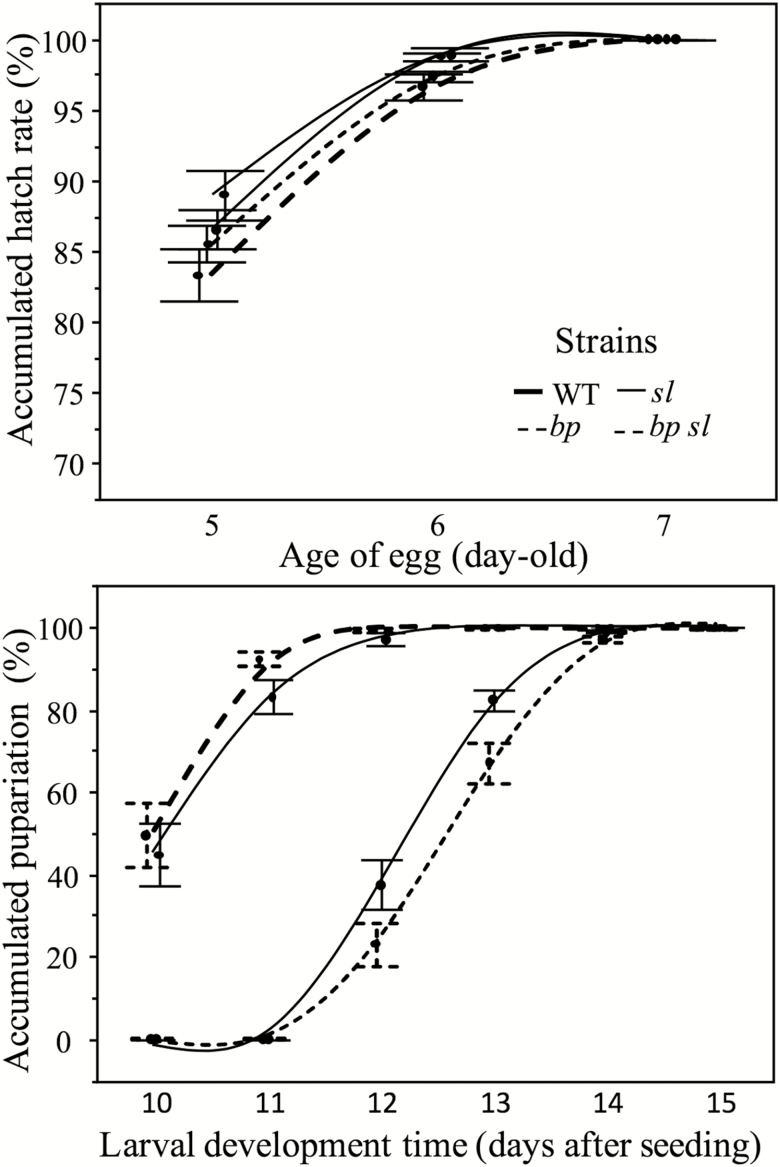
As shown in Table 5, comparative analysis between the wild-type and the purebred mutant (bp, sl and bp sl) lines did not reveal any significant differences with respect to the embryonic developmental time. For all strains tested, the neonate larvae hatching started when the eggs were 4 d old, more than 80% of the eggs had hatched when they were 5 d, whereas the rest of them (up to 100%) when the eggs were 7 d old (Fig. 2). By contrast, significant differences were detected between the wild-type and the purebred mutant (bp, sl and bp sl) lines regarding the larval development time (Table 5). Wild-type and bp mutant insects started their pupariation phase at 10 d after the eggs were put on the larval diet (egg seeding) and it took 12 d in total for all individuals to achieve their pupariation phase. On the other hand, the single mutant line sl and double mutant line bp sl were delayed by almost 2 d to start the pupariation phase (12 d), and it took 15 d for all individuals to reach it (Fig. 2).
Table 5.
Generalized linear model analysis of embryonic and larval developmental time (Poisson distribution, log-link)
| Factor | Source | df | X2 | P |
|---|---|---|---|---|
| Egg hatch | Strain | 3 | 0.001 | 0.99 |
| Egg hatching day | 1 | 0.056 | 0.81 | |
| Strain*Egg hatching day | 3 | 0.001 | 0.99 | |
| Larval development | Strain | 3 | 12.27 | 0.006 |
| day/ time | 1 | 16.96 | <0.001 | |
| Strain*Pupariation day | 3 | 11.74 | 0.008 |
Fig. 2.
Daily pattern of egg hatching and pupariation of wild-type (WT) as well as black pupae (bp), slow larvae (sl), and double homozygous mutant (bp sl) lines of A. ludens.
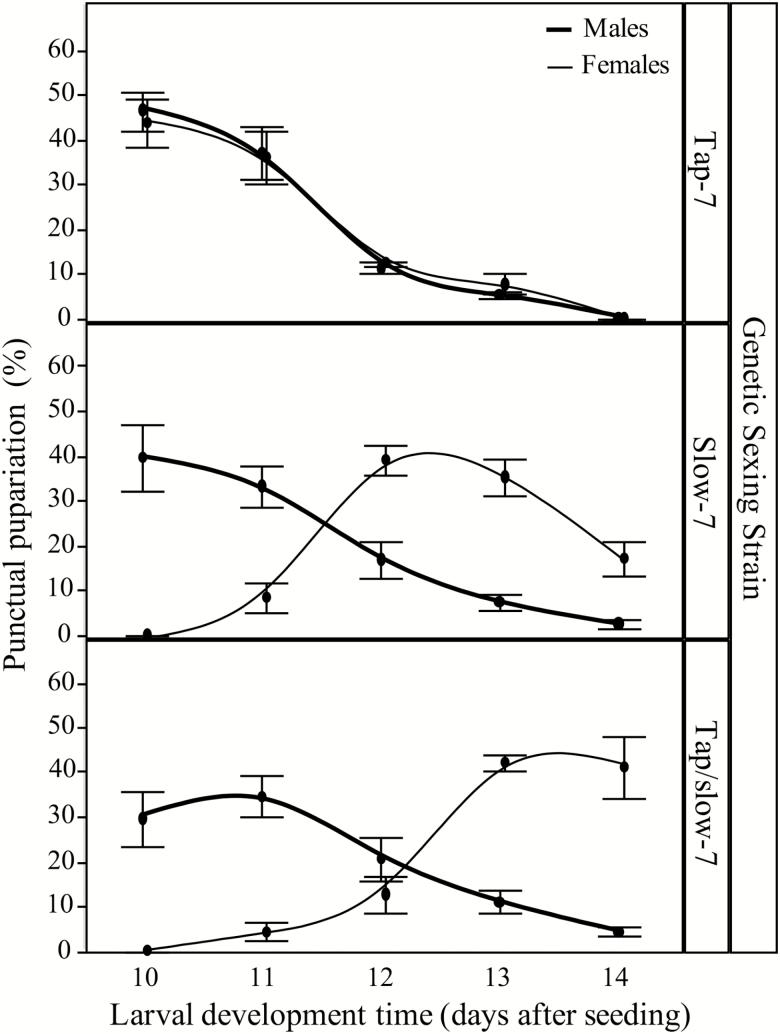
Regarding the genetic sexing strains, the pupariation started 10 d after egg seeding for all of them. No significant difference was detected in the pupariation rate between males and females of Tap-7 GSS (F = 1.55; P = 0.217); however, significant differences were detected for the slow-7 GSS and Tap/slow-7 GSS (F = 90.44; P < 0.001 and F = 47.41; P < 0.001, respectively).
In the Tap-7 GSS, the percentage of pupariation was daily 50:50 for males and females and it took 4 d for all individuals achieve the pupariation phase. However, this was not the case for the Slow-7 and Tap/slow-7 GSSs in which it was observed that only males achieve the pupariation phase at 10 d after egg seeding. At the 12 d, about 89.70% and 84.74% of the male achieve the pupariation phase and only 47.51% and 16.84% of the females for slow-7 GSS Tap/slow-7 GSS, respectively. In general, the pupariation process for the two new GSS required 1 d more (5 d) and in the last day, the percentage of female in pupariation in both slow-7 GSS and Tap/slow-7 GSS was higher than the Tap-7 GSS (Fig. 3).
Fig. 3.
Male and female daily pupariation patterns of A. ludens GSS.
Discussion
This work presents evidence that the sl mutation of the Mexfly, A. ludens, is due to a recessive and autosomal gene (sl) which is linked to the black pupae (bp) locus located on the mitotic chromosome 2 (Zepeda et al. 2014). The linked segregation of light pigmentation and slow larval development traits in the sl mutation suggests that both traits are either pleiotropic effects of a single gene or they are due to two genes which are extremely close to each other. The integration of the sl mutation into the current pupal color-based GSS used in mass rearing and SIT applications resulted in two new GSSs for A. ludens, which were named slow-7 and Tap/slow-7. Both GSSs produce wild-type males emerging from brown pupae, which develop faster than females. In the slow-7 GSS, the females are homozygous for the sl mutation and emerge from golden-color pupae, whereas in the Tap/slow-7 GSS, the females are homozygous for both selectable markers (bp sl) and emerge from light black pupae.
It is worth noting that the females of both GSS reach larval maturity significantly later (hence the name ‘slow larvae’) than most of the wild-type males, conferring self-sexing and allowing the separation of the majority of males from during late larval—early pupal stage. A similar pupariation process has been observed in two GSS for C. capitata: Vienna 8 and Cast 191. The Vienna 8 GSS carries a translocation (Y;5) marked with two mutations: white pupae (wp) and temperature-sensitive lethal (tsl). This GSS is also characterized by the presence of a slow larval development trait which was attributed to a pleiotropic effect of the tsl gene (Franz 2005). The Cast 191 GSS was also shown to carry the mutation slow (sw) located on chromosome 2, which affects the rate of embryonic and larval development and has pleiotropic effects on eye color and iridescence (Manso and Lifschitz 1992, Cladera 1995). In contrast with the C. capitata sw mutation, the A. ludens sl mutant does not show a delayed embryonic development, and it is similar to the effect induced by the tsl mutation in the Mediterranean fruit fly Vienna 8 GSS, where slow developmental rate has been reported only at the larval stage (Caceres 2002). However, this finding could be influenced by the observation period (daily), a reduction of this period (e.g., 8 h) and the use of different temperatures in the incubation could be addressed in the future to confirm whether sl mutation in A. ludens do not have any effect in the embryo stage. In the case of slow-7 GSS (brown pupae = male, golden pupae = female), the efficiency to distinguish male from female pupae was low, because brown and golden spectral colors are close resulting in some wild-type pupae being misclassified as sl pupae, because of displayed depigmentation (phenocopy). Nevertheless, to maintain the integrity of the sexing system, all wild-type phenocopies can be detected through the adult observation, because unlike sl adults, the phenocopies do not show depigmentation. Detecting phenocopy in adults stage is a strategy frequently used in the FRS for the mass production of Tap-7 GSS, as the black pupae mutation affects pigmentation at several stages, the observation of adult pigmentation conferring additional reliability to the pupal color-based sexing system (Zepeda et al. 2014). The puparium depigmentation phenomenon in wild-type insects can be explained as an effect of poor protein ingestion during the larval stage in some individuals, as dietary protein is associated with sclerotization and cuticular melanization (Lee et al. 2008, Andersen 2010), which can contribute to the presence of phenocopies in this slow-7 GSS.
In Tap/slow-7 GSS (brown pupae = male, clear dark pupae = female), the wild-type phenocopies were scarce and the two genders were easily sorted. Additionally, the self-separation during the pupation could be more efficient because the bp sl double mutants exhibited a longer delay than the sl mutants alone (e.g., in the GSS at 12 d of the larval development time, females bp sl showed a 16.81% of accumulated pupariation, whereas sl females already 47.51%). In both slow-7 and Tap/slow-7 GSSs, a small proportion of pupae recovered consists of a mixture of males and females, which in the case of the Tap/slow-7 GSS can be separated by the sorting machine currently used in the mass-rearing production of Tap-7 GSS in the Moscafrut SAGARPA-IICA facility (Flores et al. 2015). However, due to the long genetic distance between the alleles bp and sl, and if the translocation is not between them, the risk of recombination type 1b (recombination between the two genetic loci) could be high (Franz 2002). Nevertheless, this constrain can be minimized if the FRS is fed with pupae from the individuals that achieve their pupariation earlier, which will be wild-type males only, as well as pupae from individuals that achieve their pupariation later, which will be highly enriched in sl females in the case of slow-7 GSS and bp sl females in Tap/slow GSS. So, in the FRS the recombinant individuals that need to be removed for Slow-7 GSS are wild-type females and sl males, whereas for Tap/slow-7 are three phenotypes of females (wild-type, bp, and sl) and males (sl, bp, and sl bp). In addition, the induction of homozygous viable chromosomal inversions into the chromosome 2 and its introduction in the GSS could drastically reduce recombination rate and increase the genetic stability, ideally the inversion should cover the translocation break point and the bp and sl genetic loci (Franz 2005).
The large-scale production of a GSS which integrated the sl mutation could have some advantages. For example, in comparison with the Tap-7 GSS, the lower fitness observed in the GSS with sl marker is mainly due to the low production of female mutants, which significantly increases the rate of male production, thus decreasing the production cost of male for release, but will need to be evaluated the colony reproduction. Differing from C. capitata mass rearing, where the larva left the diet by themselves once they are matured and ready to enter in the pupariation phase, in A. ludens mass rearing, the whole larvae have to be separated from the diet using a mechanical sieve (Orozco-Davila et al. 2017). The competition for food among larvae is quite high under this rearing method. Therefore, under mass rearing conditions, the slow larval development exhibited by females in the GSS would favor males because they could fed in competition with youngers larva females, which demand less food and, interestingly, it has been reported that individuals with faster larval development are heavier and more sexually competitive (Meza et al. 2005). Further research should be conducted to evaluate the potential use to the SIT, e.g., survival in the field, mating success, dispersal ability, and to develop practical protocols for rearing the Tap/slow-7 GSS that can allow the separation of both genders by combining critical time-points during development with mechanical and optical sorting based on pupal color to ensure the delivery and release of only sterile males. Excess female larvae or black pupae females could be used for the production of larval or pupal parasitoids, respectively (López et al. 2006).
Acknowledgments
We thank Diana Pérez-Staples for comments on an earlier draft of the manuscript. We are also thankful to Martha Guillen and Ulysses Sto. Tomas from the IPCL for their assistance in insect rearing the flies and the experimental process.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Agresti A. 1996. An introduction to categorical data analysis. John Wiley, New York. [Google Scholar]
- Andersen S. O. 2010. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 40: 166–178. [DOI] [PubMed] [Google Scholar]
- Busch-Petersen E., and Kafu A. N. D. A.. . 1989. Stability of two mass-reared genetic sexing strains of Ceratitis capitata (Diptera: Tephritidae) based on pupal color dimorphisms. Environ. Entomol. 18: 315–322. [Google Scholar]
- Cáceres C. 2002. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica. 116: 107–116. [DOI] [PubMed] [Google Scholar]
- Cladera J. L. 1995. Self-sexing strain of Ceratitis capitata (Diptera: Tephritidae) based on a gene that affects the rate of development. Ann. Entomol. Soc. Am. 88: 353–356. [Google Scholar]
- Davidowitz G., D’Amico L. J., and Nijhout H. F.. . 2003. Critical weight in the development of insect body size. Evol. Dev. 5: 188–197. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., and Zdárek J.. . 1994. Metamorphosis behavior of flies. Ann. Rev. Entomol. 39: 243–266. [DOI] [PubMed] [Google Scholar]
- Fisher K., and Caceres C.. . 2000. A filter rearing system for mass reared genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae), pp. 543–550. InFifth International Symposium on Fruit Flies of Economic Importance: Area-wide control of fruit flies and other insect pests, 28 May-2 June, 1998, Penang, Malaysia, Penerbit Universiti Sains Malaysia. [Google Scholar]
- Flores S., Campos S., Gómez E., Espinoza E., Wilson W., and Montoya P.. . 2015. Evaluation of field dispersal and survival capacity of the genetic sexing strain Tapachula-7 of Anastrepha ludens (Diptera: Tephritidae). Fla. Entomol. 98: 209–214. [Google Scholar]
- Fraenkel G., and Bhaskaran G.. 1973. Pupariation and pupation in cyclorrhaphous flies (Diptera): terminology and interpretation. Ann. Entomol. Soc. Am. 66: 418–422. [Google Scholar]
- Franz G. 2002. Recombination between homologous autosomes in medfly (Ceratitis capitata) males: type-1 recombination and the implications for the stability of genetic sexing strains. Genetica. 116: 73–84. [DOI] [PubMed] [Google Scholar]
- Franz G. 2005. Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing as required for the sterile insect technique, pp. 427–451. InDyck V. A., Hendrichs J., and Robinson A. S. (eds.), Sterile insect technique principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Franz G., Gencheva E., and Kerremans P.. 1994. Improved stability of genetic sex-separation strains for the Mediterranean fruit fly, Ceratitis capitata. Genome. 37: 72–82. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M. 2010. El programa moscas de la fruta en México, pp. 3–9. InMontoya P., Toledo J. and Hernández E. (eds.), Moscas de la Fruta: fundamentos y procedimientos para su manejo. S y G, México. [Google Scholar]
- Knipling E. F. 1966. Some basic principles in insect population suppression. ESA Bull. 12: 7–15. [Google Scholar]
- Lee K. P., Simpson S. J., and Wilson K.. 2008. Dietary protein‐quality influences melanization and immune function in an insect. Funct. Ecol. 22: 1052–1061. [Google Scholar]
- López S. N., Viscarret M., Lanzavecchia S., Goenaga S., and Cladera J. L.. . 2006. Producción masiva y simultánea de machos de Ceratitis capitata (Diptera: Tephritidae) y parasitoides Dichasmimorpha longicaudata (Hymenoptera: Braconidae). Rev. Soc. Entomol. Arg. 65: 99–109. [Google Scholar]
- Manso F. C., and Lifschitz E.. . 1992. Nueva metodología genética para el mejoramiento de la efciencia de la técnica del macho estéril en el control de la mosca del Mediterráneo Ceratitis capitata. Cienc. Invest. 44:225–228. [Google Scholar]
- McCombs S. D., and Saul S. H.. 1995. Translocation-based genetic sexing system for the oriental fruit fly (Diptera: Tephritidae) based on pupal color dimorphism. Ann. Entomol. Soc. Am. 88: 695–698. [Google Scholar]
- McInnis D. O., Tam S., Grace C., and Miyashita D.. . 1994. Population suppression and sterility rates induced by variable sex ratio, sterile insect releases of Ceratitis capitata (Diptera: Tephritidae) in Hawaii. Ann. Entomol. Soc. Am. 87: 231–240. [Google Scholar]
- McInnis D. O., Tam S., Lim R., Komatsu J., Kurashima R., and Albrecht C.. . 2004. Development of a pupal color-based genetic sexing strain of the melon fly, Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae). Ann. Entomol. Soc. Am. 97:1026–1033. [Google Scholar]
- Meats A., Maheswaran P., Frommer M., and Sved J.. . 2002. Towards a male-only release system for SIT with the Queensland fruit fly, Bactrocera tryoni, using a genetic sexing strain with a temperature-sensitive lethal mutation. Genetica. 116: 97–106. [DOI] [PubMed] [Google Scholar]
- Meza J. S., Fleischer F. D., and Orozco D.. . 2005. Pupariation time as a source of variability in mating performance in mass-reared Anastrepha ludens (Diptera: Tephritidae). J. Econ. Entomol. 98: 1930–1936. [DOI] [PubMed] [Google Scholar]
- Orozco‐Dávila D., Quintero L., Hernández E., Solís E., Artiaga T., Hernández R., and Montoya P. 2017. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico–a review. Entomol. Exp. Appl. 164: 176–187. [Google Scholar]
- Rendon P., McInnis D., Lance D., and Stewart J.. . 2000. Comparison of medfly male-only and bisexual releases in large scale field trials, pp. 517–525. InJoint proceedings of the international conference on area-wide control of insect pests, Fifth International Symposium on Fruit Flies of Economic Importance: Area-wide control of fruit flies and other insect pests, 28 May-2 June 1998, Penang, Malaysia, Penerbit Universiti Sains Malaysia. [Google Scholar]
- Rendón P., McInnis D., Lance D., and Stewart J.. 2004. Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 97: 1547–1553. [DOI] [PubMed] [Google Scholar]
- Robinson A. S., Cirio U., Hooper G. H. S., and Caparella M.. 1986. Field cage studies with a genetic sexing strain in Ceratitis capitata. Entomol. Exp. Appl. 41: 231–235. [Google Scholar]
- Robinson A. S., Franz G., and Fisher K... 1999. Genetic sexing strains in the medfly, Ceratitis capitata: development, mass rearing and field application. Trends Entomol. 2: 81–104. [Google Scholar]
- Rössler Y. 1979. Automated sexing of Ceratitis capitata [Dip.: Tephritidae]: the development of strains with inherited, sex-limited pupal color dimorphism. Entomophaga. 24: 411–416. [Google Scholar]
- Serra J. A. 1965. Modern genetics. 2. Academic Press, New York, pp. 616. [Google Scholar]
- Tanaka N., Steiner L. F., Ohinata K., and Okamoto R.. 1969. Low-cost larval rearing medium for mass production of oriental and Mediterranean fruit flies. J. Econ. Entomol. 62: 967–968. [Google Scholar]
- Zar J. H. 2010. Biostatistical analysis 5th ed. Pearson Prentice Hall Inc., Upper Saddle River, NJ, USA. [Google Scholar]
- Zepeda-Cisneros C. S., Meza Hernández J. S., García-Martínez V., Ibañez-Palacios J., Zacharopoulou A., and Franz G.. 2014. Development, genetic and cytogenetic analyses of genetic sexing strains of the Mexican fruit fly, Anastrepha ludens Loew (Diptera: Tephritidae). BMC Genet. 15(Suppl 2): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]