Abstract
The qualitative and quantitative analysis of 16 polycyclic aromatic hydrocarbons (PAHs) in sludge samples from drinking water treatment plants (DWTP) and wastewater treatment plants (WWTP) were established using gas chromatography–mass spectrometry (GC-MS). The method was suitable to quantify PAHs in the sludge of DWTP and WWTP and it was confirmed by the relevant quality assurance/quality control (QA/QC) procedures. The recovery of individual PAHs in the spiked samples ranged from 74.3% to 108.7%. Detection limits of the analytical procedure were 0.0010–0.0046 mg/kg dw for individual PAHs. This method was used to determine the concentration of PAHs in the selected two DWTP and four WWTP sludge samples. The results showed that the total PAHs (∑PAHs) were in low levels which ranged from 0.0668 to 0.1357 mg/kg dw, and 0.5342–1.0666 mg/kg dw for DWTP and WWTP respectively. The 3- & 4-ring PAHs were predominant in DWTP sludge, ranging from 77.4% to 82.7%; the 4-ring PAHs were predominant in WWTP sludge, ranging from 40.7% to 47.6%. The PAHs of DWTP sludge are mainly composed of 3-ring phenanthrene and anthracene and 4-ring pyrene, and chrysene. The PAHs of WWTP sludge are dominated by 4-ring fluoranthene, pyrene, and chrysene. The detected PAHs concentration should be undoubtedly considered for agriculture in sludge applications based on the limits of the EU regulations. The results of this study can be used for regular monitoring to establish a reference for sludge management and application to agriculture.
Keywords: PAHs, GC-MS, sludge, drinking water treatment plants (DWTP), wastewater treatment plants (WWTP)
1. Introduction
With the growth of urbanization, changes in industrial structure and raising awareness of environmental protection, drinking water treatment plants (DWTP) and wastewater treatment plants (WWTP) have been extensively built. The quality of drinking water and rates of sewage treatment has also increased. Although DWTP and WWTP ensure the safety of drinking water and reduce environmental water pollution problems, the sludge they produce is a potential threat to the environment. The sludge may contain hundreds of organic toxic compounds (e.g., polycyclic aromatic hydrocarbons (PAHs), phthalate esters (PAEs), alkylphenol polyethoxylates, synthetic musks, antibiotics, ultraviolet stabilizers, bisphenol analogs, organochlorine pesticides, polybrominated diphenyl ethers (PBDE), pharmaceuticals, hormones, perfluorinated compounds, and polychlorinated biphenyls (PCB)), heavy metals (Pb, Cr, Cu, Ni, Hg, and Cd), and pathogenic bacteria [1,2,3,4]. Improper disposal of sludge may cause secondary pollution to soil, groundwater, surface water and air [4].
PAHs are the hydrophobic organic compounds and tend to be bioaccumulated in the organisms [5,6]. Some PAHs have been identified as carcinogenic, mutagenic, and classified as a priority pollutant by the US Environmental Protection Agency (US EPA) and the European Union (EU). Therefore, PAHs are one of the most common target compounds in sludge-related research, especially the priority 16 PAHs [3]. Stevens et al. [1] surveyed the sludge collected from 14 wastewater treatment plants in the UK and found they contained a total of 24 PAHs concentration of 67–370 mg/kg dw. A review that contains the peer-reviewed literature and official government reports in the US indicated PAHs concentration in sludge ranged from below the detection limit to 199 mg/kg dw [2]. PAHs were one group of the most commonly studied organic compounds in sludge in China. Meng et al. [3] compiled a review and reported that the PAHs concentration of 0.1–170 mg/kg dw in sludge was measured from previous studies that were published during 1999–2012. The variation in PAHs concentration in sludge is considerable, mainly depending on the nature of the wastewater, treatment plant procedures, and geographical differences [7]. The final processing methods of sludge mainly include land application, incineration, and landfill, indicating that PAHs can re-enter the environment through air, water, and soil [3,8]. Therefore, before the final processing of sludge, the concentration level and risk information of pollutants such as PAHs are quite important for evaluating the subsequent fate and impact of specific compounds in the environment.
In Taiwan, about 2.66 million tons of WWTP sludge and 200,000 tons of DWTP sludge are produced each year [9,10]. At present, the final disposal methods of sludge are mainly landfill, incineration, heat treatment, and fertilizer. However, for the specification of various disposal methods, only the basic characteristic and heavy metals of sludge are regulated [11], and the criterion and information on organic pollutants are quite lacking. The purpose of this work was to analyze the PAHs in the sludge by using gas chromatography with mass spectrometer (GC-MS) and to confirm the applicability of analytical procedure by the relevant quality assurance/quality control procedures. In addition, this method was used to determine the concentration of 16 PAHs in the sludge from two DWTP and four WWTP in southwest Taiwan, and to evaluate the PAHs level, composition and potential toxicity in the sludge. The analytical procedures established in this study can be used as routine monitoring of DWTP or/and WWTP sludge to provide important information for sludge management and application strategies.
2. Materials and Methods
2.1. Reagents and Standards
Chromatographic (HPLC) grade n-hexane and acetone were purchased from Echo Chemical Co. Ltd. (Miaoli, Taiwan). Analytical-grade anhydrous sodium sulphate (10–60 mesh) was from Avantor (Center Valley, PA, USA). The Copper powder was supplied by Sigma-Aldrich (Darmstadt, Germany) and washed with dilute nitric acid, reagent water, and acetone, and blown dry with nitrogen before the analysis. Standards of 16 PAHs in a 1000 mg/L mixture solution, deuterated PAHs internal standard solutions (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) at 2000 mg/L, and surrogate standard solutions (2-fluorobiphenyl and 4-terphenyl-d14) at 1000 mg/L were obtained from AccuStandard Chem. Co. (New Haven, CT, USA). The standard working solutions of PAHs mixture, internal standard mixture and surrogate standard mixture were properly diluted with HPLC grade n-hexane and prepared freshly before the analysis. All glassware was rinsed with n-hexane and dried in an oven at 105 °C. Other materials were previously washed with ultrapure water and acetone.
2.2. Sampling
Dewatered sludge samples from two DWTP and four WWTP located in southwestern Taiwan were collected in November 2018. The two selected DWTP (DW1 and DW2) water sources are rivers and reservoirs, respectively; the selected four WWTP (WW1–WW4) inflow raw water is domestic sewage, and the WW2 sewage plant inflow raw water includes domestic sewage and intercepted water from polluted canals (volume ratio 1:2). The collected DWTP sludge sample is subjected to concentration and dehydration procedures, and the WWTP sludge sample is subjected to a concentration, digestion and dehydration process. Among them, WW1 and WW3 sludge are aerobic digestion, while WW2 and WW4 sludge are anaerobic digestion. About 1 kg of sludge was collected in a brown glass container previously washed with n-hexane and transported directly back to the laboratory. In the laboratory, the samples were freeze-dried for 72 h, ground to pass through a 1.0 mm sieve and fully homogenized. The dried sludge was placed in −20 °C in amber glass bottles pre-washed with n-hexane and covered with solvent-rinsed aluminum foil until further processing and analysis [12].
2.3. Sample Preparation
The sludge samples were extracted according to the method of Dong et al. [13] with slight modifications. Briefly, one g of dry and homogenized sludge sample was put into a clean glass test tube, and a 5 mL acetone/n-hexane (1:1), and 10 μL surrogate standard mixture solutions (20 mg/L) were then added. The sample tubes were mixed using the vortex (1 min) and extracted by the ultrasonic treatment (15 min). Mixed sludge and organic phase were separated by centrifugation at 3000 rpm for 10 min. The organic layer containing the extracted compound were collected into another clean glass tube using a Pasteur pipette, and the residual sludge was re-extracted twice with 1:1 (v/v) acetone/n-hexane (5 mL). All extracts were combined, and activated copper was added to the extract for desulphurization. Then, drying over anhydrous sodium sulphate, and concentration to 0.5 mL using a gentle stream of nitrogen. An internal standard mixture solution (200 ng) was added to the extract to be analyzed using GC-MS.
2.4. GC-MS Analysis
A GC-MS system that connects an Agilent 7890B GC (Agilent Technologies, Santa Clara, CA, USA) to an Agilent 5977A mass selective detector (MSD) and equipped with an Agilent 7693A autosampler (Agilent Technologies, Santa Clara, CA, USA) was used to analyse the PAH compounds. The separation column used is a 30 m, 0.25 mm i.d. HP-5MS capillary column (Hewlett-Packard, Palo Alto, CA, USA) coated with 5% phenyl-methylsiloxane (0.25 μm film thickness). The analytical parameter settings for the GC-MS system are listed in Table 1, and the ion mass program used for quantification is detailed in Table 2. Identity of PAHs in the samples was confirmed by the retention time within ±0.06 relative retention time (RRT) units of the RRT of the standard component and the relative intensities of confirmation ions within ±30% of the authentic PAHs standards. Sixteen PAHs were quantified using the response factors related to the respective internal standards based on a five-point calibration curve for individual compounds. In this study, the concentrations of PAHs were expressed on a dry-weight (dw) basis.
Table 1.
Parameters of the GC-MS system.
| Parameter | Set Condition |
|---|---|
| Gas chromatography (GC) | Agilent 7890B (with Agilent 7693A autosampler) |
| Injection volume | 1 µL |
| Inlet temperature | 280 °C |
| Capillary column | HP-5MS (30 m × 0.25 mm i.d. with 0.25 μm film) |
| Injection mode | Splitless |
| Carrier gas | Helium, 1 mL/min |
| Temperature program | 40 °C (1 min) → 120 °C (35 °C/min) → 160 °C (10 °C/min) → 300 °C (5 °C/min, hold for 10 min) |
| Mass selective detector (MS) | Agilent 5977A |
| Ionization mode | Electron ionization (EI) |
| Transfer line temperature | 280 °C |
| Ion source temperature | 230 °C |
| Quadrupole temperature | 150 °C |
| Electronic energy | 70 eV |
| Scan mode | Selective ion monitoring (SIM) (see Table 2) |
| Solvent delay | 4 min |
Table 2.
Selected ion monitoring of each PAHs in GC-MS system.
| Compounds | Abbreviation | Retention Time (min) | Selected Ions (m/z) |
|---|---|---|---|
| Naphthalene-d8 | IS1 a | 4.679 | 136 c |
| Naphthalene | NA | 4.964 | 128, 129, 127 |
| 2-Fluorobiphenyl | SS1 | 6.134 | 172 |
| Acenaphthylene | ACY | 6.845 | 152, 151, 153 |
| Acenaphthene-d10 | IS2 | 7.125 | 164 |
| Acenaphthene | ACE | 7.176 | 154, 153, 152 |
| Fluorene | FL | 8.177 | 166, 165, 167 |
| Phenanthrene-d10 | IS3 | 10.511 | 188 |
| Phenanthrene | PH | 10.557 | 178, 179, 176 |
| Anthracene | AN | 10.700 | 178, 176, 179 |
| Fluoranthene | FLU | 14.624 | 202, 229, 226 |
| Pyrene | PY | 15.399 | 202, 200, 203 |
| 4-Terphenyl-d14 | SS2 b | 16.622 | 244 |
| Benzo[a]anthracene | BaA | 20.582 | 228, 229, 226 |
| Chrysene-d12 | IS4 | 20.626 | 240 |
| Chrysene | CH | 20.724 | 228, 226, 229 |
| Benzo[b]fluoranthene | BbF | 25.085 | 252, 253, 125 |
| Benzo[k]fluoranthene | BkF | 25.188 | 252, 253, 125 |
| Benzo[a]pyrene | BaP | 26.249 | 252, 253, 125 |
| Perylene-d12 | IS5 | 26.500 | 264 |
| Indeno[1,2,3-cd]pyrene | IP | 30.298 | 276, 138, 277 |
| Dibenz[a,h]anthracene | DBA | 30.571 | 278, 139, 279 |
| Benzo[g,h,i]perylene | BP | 31.026 | 276, 138, 277 |
a Internal standard. b Surrogate standard. c Bold indicates quantitative ion.
2.5. Quality Control
To ensure the accuracy and precision of the PAHs analysis process of this study, a five-point calibration standards (0.1 to 2.0 ng/μL) in solution, detection limits, procedural blank, check standard, sample duplicates, and matrix spike standards were carried out. One µL of each calibration standard (containing internal standards) was analysed, and the area of the primary characteristic ion (as indicated in Table 2) was tabulated against concentration for each target analyte. The internal standard method was used to quantify PAHs, calculating response factors (RFs) for each target analyte relative to one of the internal standards and obtaining the relative standard deviation (RSD) of RF. The RSD of RF for each analyte should be less than <20%. The detection limits were estimated from three times standard deviation from repeated (n = 7) analysis of 16 PAHs with a low concentration of 0.01 ng/μL and converted by the concentration factor and sampled mass.
To prevent the contamination during the analyzed procedure, procedural blank samples (n = 3), adding no sludge sample, were prepared by the same procedure from the extraction to the PAHs analysis. The standards used for quality control were prepared by adding the standard solution to 1:1 (v/v) acetone/n-hexane. This study selected sludge samples of DW1 and WW1 for matrix spike standards. The original PAHs concentrations in DW1 and WW1 respectively ranged from 0.0007–0.0119 mg/kg dw and 0.0064–0.1516 mg/kg dw. Then DW1 and WW1 were respectively spiked 50 μL (50 ng) and 200 μL (200 ng) of 1 ng/μL 16 PAHs mix standards. Therefore, the final PAHs concentrations in spiked DW1 and WW1 were respectively in 0.0507–0.0619 mg/kg dw and 0.0264–0.1716 mg/kg dw. The recovery of the spiked samples was determined by the measured concentration dividing by the final concentration of the sample after the addition. The aforementioned procedural blank, check standard, sample duplicates, and matrix spike standard were carried out for every 10 samples.
3. Results
3.1. GC-MS Separation and Identification
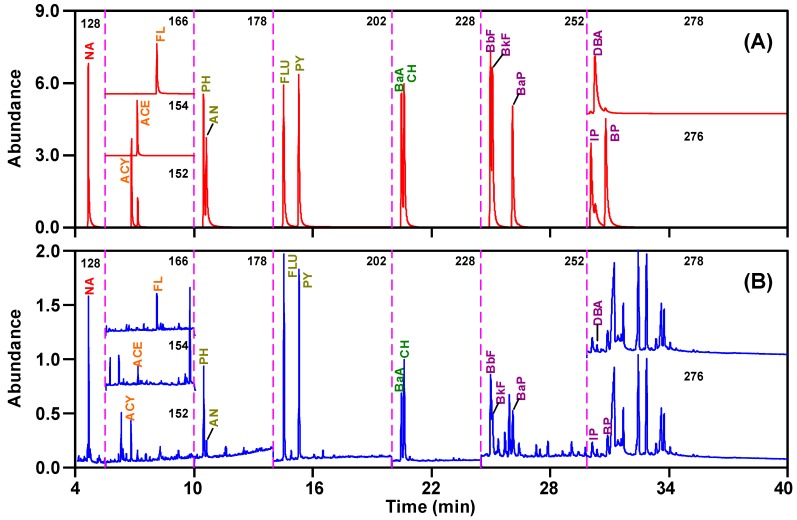
According to the set GC-MS conditions of Table 1 and Table 2, mixed standards of 16 PAHs were analyzed. The results showed that 16 PAHs could be effectively separated (Figure 1A). IS1 of internal standards was used for quantifying naphthalene, IS2 for acenaphthylene, acenaphthene, and fluorine, IS3 for phenanthrene, anthracene, and fluoranthene, IS4 for pyrene, benzo[a]anthracene, and chrysene, and IS5 for benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene. The separation and quantitation of PAHs in the sludge samples were achieved using the same GC-MS conditions as the standards. The 16 PAHs in the sludge samples were defined by the retention time and abundance of quantification/confirmation ions in the 16 PAHs standards. The selected quantification ion chromatograms of 16 PAHs in a standard mixture of 16 PAHs and the WW2 sludge sample was shown in Figure 1A,B. The peaks of 16 PAHs in the WW2 sludge sample were clearly defined and were not disturbed by the peaks of other organic compounds in the sludge (Figure 1 B). Therefore, the 16 PAHs of sludge samples can be quantified using the response factors related to the respective internal standards based on a five-point calibration curve for individual compounds.
Figure 1.
GC-MS selected quantification ion chromatograms of 16 PAHs in (A) standard mixture of 16 PAHs and (B) the WW2 sludge sample. The definitions of compound abbreviation see Table 2.
3.2. Analytical Characteristics
The response factors based on the five-point calibration curve for individual compounds showed an acceptable RSD of 1.5 to 9.4%, the procedural blank values were always less than the detection limit, the recoveries of individual PAHs in check standards ranged from 86 ± 0.6% to 102 ± 2.1% (n = 3) and the relative percent differences of sample duplicates ranged from 1.7 ± 0.9% to 9.4 ± 3.6% (n = 6) for all of the target analyses (Table 3). The surrogate standard recoveries were 93.6 ± 7.1% for 2-fluorobiphenyl and 91 ± 9.3% for 4-terphenyl-d14 with sediment samples (n = 12). In addition, this study performed a matrix spike standard analysis to confirm the presence or absence of matrix interference in the sample and the appropriate analytical method. The recovery of each PAHs in the spiked samples ranged from 74.3 ± 2.3% to 108.7 ± 2.9% (n = 3), indicating that the analytical method of this study is suitable for the analysis of PAHs in sludge samples. The detection limits were 0.0010–0.0046 mg/kg dry weight for individual PAHs (Table 3).
Table 3.
Response factor, detection limits, recoveries of check standards, and relative percent differences of sample duplicates for individual PAHs in this study.
| PAHs a | Response Factor (RF) (n = 5) | Check Standard (n = 3) R b (%) |
Duplicate Sample (n = 6) RPD b (%) |
Spike Sample (n = 3) P b (%) |
Detection Limits (mg/kg dw) |
|
|---|---|---|---|---|---|---|
| Average ± SD a | RSD b (%) | |||||
| NA | 1.11 ± 0.02 | 1.5 | 91 ± 3.5 | 6.1 ± 3.7 | 82.3 ± 0.6 | 0.0010 |
| ACY | 1.26 ± 0.04 | 3.3 | 94 ± 1.0 | 7.1 ± 4.6 | 108.0 ± 3.6 | 0.0012 |
| ACE | 1.17 ± 0.03 | 2.8 | 86 ± 0.6 | 3.4 ± 4.1 | 100.7 ± 3.1 | 0.0017 |
| FL | 0.95 ± 0.04 | 4.6 | 88 ± 1.2 | 7.1 ± 5.3 | 105.3 ± 5.5 | 0.0018 |
| PH | 1.18 ± 0.03 | 2.8 | 98 ± 0.3 | 5.5 ± 4.4 | 108.7 ± 2.9 | 0.0013 |
| AN | 0.64 ± 0.03 | 5.5 | 102 ± 2.1 | 8.1 ± 2.8 | 74.7 ± 2.5 | 0.0028 |
| FLU | 0.86 ± 0.05 | 5.6 | 93 ± 0.6 | 9.4 ± 3.6 | 76.0 ± 1.7 | 0.0022 |
| PY | 1.48 ± 0.11 | 7.4 | 98 ± 4.3 | 1.7 ± 0.9 | 76.3 ± 2.9 | 0.0045 |
| BaA | 0.42 ± 0.03 | 7.6 | 90 ± 3.3 | 4.8 ± 3.9 | 74.3 ± 2.3 | 0.0039 |
| CH | 1.20 ± 0.08 | 6.3 | 90 ± 2.4 | 7.1 ± 1.4 | 87.3 ± 2.1 | 0.0033 |
| BbF | 1.23 ± 0.05 | 4.2 | 96 ± 5.6 | 6.2 ± 3.7 | 94.3 ± 3.8 | 0.0032 |
| BkF | 1.36 ± 0.12 | 8.6 | 98 ± 2.8 | 7.5 ± 6.4 | 88.0 ± 0.0 | 0.0031 |
| BaP | 0.66 ± 0.05 | 8.3 | 92 ± 3.6 | 3.4 ± 1.5 | 93.3 ± 0.6 | 0.0045 |
| IP | 0.44 ± 0.04 | 9.4 | 98 ± 0.9 | 7.3 ± 2.4 | 90.3 ± 7.0 | 0.0046 |
| DBA | 0.59 ± 0.04 | 6.8 | 97 ± 8.1 | 8.5 ± 4.1 | 79.3 ± 1.5 | 0.0045 |
| BP | 1.81 ± 0.17 | 9.5 | 97 ± 2.9 | 5.5 ± 1.7 | 83.0 ± 1.0 | 0.0033 |
| SS1 | 1.54 ± 0.11 | 7.4 | 102 ± 7.1 | 5.1± 2.7 | 92.3 ± 6.7 | - |
| SS2 | 1.11 ± 0.02 | 1.5 | 107 ± 1.8 | 6.7± 3.7 | 89.5 ± 8.8 | - |
a The definitions of compound abbreviation see Table 2; b SD: standard deviation; RSD: Relative standard deviation; R: Recoveries of check standard; RPD: Relative percent differences; P: Recoveries of spike sample.
3.3. Concentration Level of PAHs in Sludge
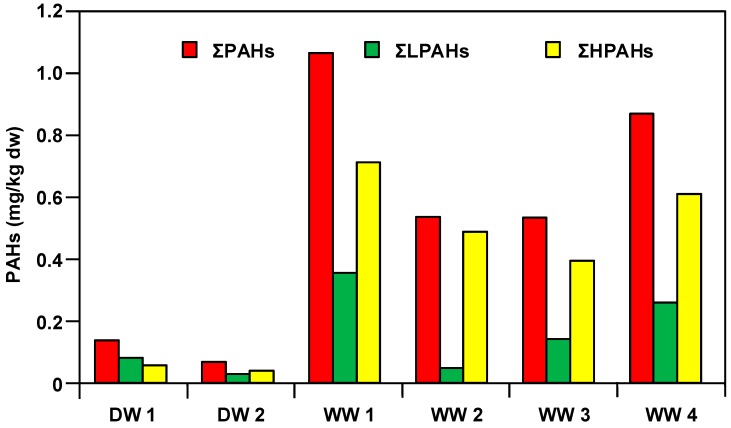
The distribution of 16 PAHs in sludge from selected DWTP (DW1 and DW2) and WWTP (WW1–WW4) is shown in Table 4. The concentrations of ΣPAHs in the WWTP sludge ranged from 0.5332–1.0666 μg/kg dw, which was 4–16 times higher than the DWTP sludge (0.0668–0.1357 mg/kg dw). This result shows that the PAHs of the artificially produced sewage are about one order of magnitude higher than the environmental water. The concentrations of ΣLPAHs (sum of 2- & 3-ring PAHs) and ΣHPAHs (sum of 4-, 5-, and 6-ring PAHs) in DWTP sludge were 0.0279–0.0793 mg/kg dw and 0.0389–0.0564 mg/kg dw, respectively; the concentrations of ΣLPAHs and ΣHPAHs in WWTP sludge were 0.0480–0.3550 mg/kg dw and 0.3931–0.7116 mg/kg dw, respectively. The ΣLPAHs/ΣHPAHs ratio is inconsistent in the two DWTP sludge, DW1 (ΣLPAHs/ΣHPAHs = 1.4) is greater than 1 and DW2 (ΣLPAHs/ΣHPAHs = 0.7) is less than 1. The ratios of ΣLPAHs/ΣHPAHs are consistently less than 1 (ΣLPAHs/ΣHPAHs = 0.1–0.5) in the four WWTP sludge, i.e., ΣHPAHs are significantly higher than ΣLPAHs (Figure 2). The concentration of ΣCPAHs (sum of 7 carcinogenic PAHs) varied in the range of 0.2101–0.3716 mg/kg dw in WWTP sludge, which is significantly higher than that in the DWTP sludge (0.0177 and 0.0409 mg/kg dw) (Table 4). The concentration of ΣCPAHs accounts for about 34.6–45.1% of PAHs in WWTP sludge, which is slightly higher than 26.5–30.1% of DWTP sludge. However, the ΣTEQ (the sum of BaP toxic equivalence quotient of carcinogenic PAHs, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, and dibenz[a,h]anthracene [14,15]) of WWTP sludge (WW1–WW4) is between 0.0210–0.0372 mg/kg TEQ/kg dw, which is about 5–40 times higher than DWTP sludge (DW1: 0.0041 mg TEQ/kg dw and DW2: 0.0018 mg TEQ/kg dw).
Table 4.
PAHs content (mg/kg dw) of sludge from selected drinking water treatment plants (DWTP) and wastewater treatment plants (WWTP) in southwestern Taiwan.
| PAHs a | DWTP | WWTP | |||||
|---|---|---|---|---|---|---|---|
| DW 1 | DW 2 | WW 1 | WW2 | WW3 | WW4 | ||
| 2-ring | NA | 0.0117 | ND (0.0008) e | 0.0981 | 0.0185 | 0.0290 | 0.0518 |
| 3-ring | ACY | ND (0.0007) e | ND (0.0002) e | 0.0285 | 0.0009 | 0.0058 | 0.0146 |
| ACE | 0.0019 | 0.0017 | 0.0064 | 0.0017 | 0.0028 | 0.0117 | |
| FL | 0.0054 | 0.0043 | 0.0104 | 0.0034 | 0.0139 | 0.0097 | |
| PH | 0.0201 | 0.0204 | 0.1291 | 0.0114 | 0.0511 | 0.1018 | |
| AN | 0.0396 | ND (0.0005) e | 0.0827 | 0.0122 | 0.0384 | 0.0683 | |
| 4-ring | FLU | 0.0067 | 0.0075 | 0.1516 | 0.0129 | 0.0829 | 0.1275 |
| PY | 0.0081 | 0.0100 | 0.1475 | 0.1336 | 0.0657 | 0.1195 | |
| BaA b | 0.0119 | ND (0.0014) e | 0.0568 | 0.0163 | 0.0236 | 0.0382 | |
| CH b | 0.0107 | 0.0093 | 0.0884 | 0.0924 | 0.0452 | 0.0622 | |
| 5-ring | BbF b | 0.0015 | 0.0042 | 0.0301 | 0.0087 | 0.0195 | 0.0257 |
| BkF b | 0.0018 | ND (0.0006) e | 0.0297 | 0.0097 | 0.0186 | 0.0255 | |
| BaP b | 0.0059 | ND (0.0011) e | 0.0685 | 0.0070 | 0.0373 | 0.0527 | |
| IP b | 0.0049 | ND (0.0010) e | 0.0754 | 0.0640 | 0.0526 | 0.0750 | |
| 6-ring | DBA b | ND (0.0042) e | ND (0.0002) e | 0.0228 | 0.0434 | 0.0134 | 0.0210 |
| BP | 0.0008 | 0.0037 | 0.0408 | 0.0997 | 0.0344 | 0.0635 | |
| ΣPAHs c | 0.1357 | 0.0668 | 1.0666 | 0.5357 | 0.5342 | 0.8684 | |
| ΣLPAHs c | 0.0793 | 0.0279 | 0.0355 | 0.0480 | 0.1410 | 0.2577 | |
| ΣHPAHs c | 0.0564 | 0.0389 | 0.7116 | 0.4877 | 0.3931 | 0.6107 | |
| ΣLPAHs/ΣHPAHs | 1.40 | 0.72 | 0.50 | 0.10 | 0.36 | 0.42 | |
| ΣCPAHs c | 0.0409 | 0.0177 | 0.3716 | 0.2415 | 0.2101 | 0.3002 | |
| ΣTEQ d | 0.0041 | 0.0018 | 0.0372 | 0.0242 | 0.0210 | 0.0300 | |
a The definitions of compound abbreviation see Table 2; b Carcinogenic PAHs; c ΣPAHs: sum of 2–6-ring PAHs; ΣLPAHs: sum of 2- & 3-ring PAHs; ΣHPAHs: sum of 4-, 5-, and 6-ring PAHs; ΣCPAHs: sum of 7 carcinogenic PAHs; d ΣTEQ: sum of 7 carcinogenic PAHs BaP toxic equivalence quotient; e The measured value is less than the detection limit.
Figure 2.
Distributions of ΣPAHs, ΣLPAHs, and ΣHPAHs in sludge samples of selected DWTP (DW1–DW2) and WWTP (WW1–WW4).
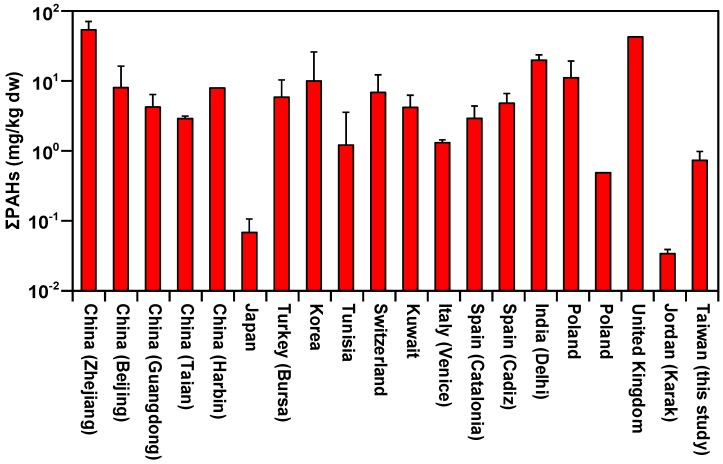
Table 5 shows the comparison of the PAHs concentrations in sludge in this study with that from 19 other studies from around the world. For consistency, only the US EPA priority pollutants of 16 PAHs were selected to estimate ΣPAHs and ΣCPAHs in the 19 studies. The ΣPAHs content (0.53–1.07 mg/kg dw) of WWTP sludge in this study is higher than that of Poland (0.498 mg/kg dw) [16], Jordan (0.034 ± 0.005 mg/kg dw) [17], and Japan (0.069 ± 0.038 mg/kg dw) [18], similar to Italy (1.35 ± 0.13 mg/kg dw) [19] and Tunisia (1.25 ± 2.45 mg/kg dw) [20], but lower than most other countries’ WWTP sludge (Figure 3). The ΣCPAHs accounts for an average of 37% of ΣPAHs and falls within 12–74% of other studies.
Table 5.
Compare the concentrations and composition of PAHs in sludge from other studies around the world.
| Location | Sludge Type | ΣPAHs (mg/kg) | ΣCPAHs (mg/kg) | Dominant PAHs a (percentage) |
Dominant PAHs Ring (percentage) | Ref. |
|---|---|---|---|---|---|---|
| China (Zhejiang) | Sewage (dom/ind) b | 33.73–82.58 56.7 ± 18.5 |
5.8–28.7 15.4 ± 7.5 |
PH (27) d, FLU (12) | 3 (42), 4 (30) | [24] |
| China (Beijing) | Sewage (dom/ind) | 2.47–25.92 8.31 ± 8.79 |
2.08–23.0 6.15 ± 8.24 |
CH (10), BbF (22), BaP (15), BP (18) | 4 (23), 5 (51), 6 (21) | [25] |
| China (Guangdong) | Sewage (dom/ind) | 2.53–6.93 4.40 ± 2.27 |
0.70–1.01 0.87 ± 0.16 |
PH (30), FLU (16), PY (18) | 3 (43), 4 (42) | [26] |
| China (Taian) | Sewage (dom/ind) | 2.81–3.18 3.00 ± 0.26 |
0.12–0.61 0.36 ± 0.35 |
NA (26), PH (22), FLU (13) | 2 (26), 3 (42), 4 (29) | [27] |
| China (Harbin) | Sewage (dom/ind) | 2.2–20 8.2 |
Na c | na | 5&6 (55), 4 (25), 2 (20) | [28] |
| Japan | Sewage | 0.069 ± 0.038 | na | na | na | [18] |
| Turkey (Bursa) | Sewage (dom/ind) | 1.78–19.9 6.08 ± 4.69 |
1.31–11.57 4.18 ± 2.77 |
na | na | [29] |
| Korea | Sewage (urban/rural) | 1.30–44.9 10.4 ± 17.0 |
0.23–25.6 4.8 ± 10.2 |
FLU (14), PY (13), BbF (11) | 4 (39), 5 (32) | [30] |
| Tunisia | Sewage (various) | 0.096–7.72 1.25 ± 2.45 |
0.005–1.37 0.21 ± 0.44 |
PH (28), PY (16), NA (16) | 3 (34), 4 (40), 2 (16) | [20] |
| Switzerland | Sewage (dom/ind/runoff) | 1.01–22.6 7.10 ± 5.73 |
0.46–12.41 3.18 ± 3.25 |
PH (11), FLU (17), PY (14), BbF (11) | 3 (17), 4 (44), 5 (29) | [31] |
| Kuwait | Sewage (urban) | 2.01–7.76 4.33 ± 2.22 |
0.02–2.06 1.42 ± 0.74 |
PH (14), AN (11), DBA (11) | 3 (45), 4 (23), 5 (19) | [32] |
| Italy (Venice) | Sewage (urban) | 1.26–1.44 1.35 ± 0.13 |
0.57–0.73 0.65 ± 0.11 |
PY (8.7), BaA (8.6), CH (8.2) | 3 (28), 4 (32), 5 (26) | [19] |
| Spain (Catalonia) | Sewage (urban) | 1.13–5.52 3.02 ± 1.55 |
0.34–2.25 0.76 ± 0.64 |
PH (25), PY (13), FLU (9.0) | 3 (43), 4 (31) | [33] |
| Spain (Cadiz) | Sewage (urban) | 1.97–10.1 4.97 ± 1.9 |
0.47–4.61 1.93 ± 0.99 |
ACY (11), PH (9.3), PY (19) | 3 (28), 4 (38) | [34] |
| India (Delhi) | Sewage | 14.9–24.2 20.67 ± 4.14 |
9.81 ± 2.35 | BP, DBA. | 6 (33), 5 (31) | [35] |
| Poland | Sewage | 2.04–36.44 11.61 ± 8.72 |
4.30 | ACY (18), FLU (17), BbF (16) | 3 (34), 4 (39) | [36] |
| Poland | Dairy sewage | 0.498 | 0.12 | ACY (2), FL (13), PY (21) | 3 (45), 4 (36) | [16] |
| United Kingdom | Sewage | 18–94 44.8 |
4.5–27.6 13.2 |
FL (13), PH (17), FLU (11) | 3 (39), 4 (30) | [1] |
| Jordan (Karak) | Sewage (dom/ind) | 0.029–0.039 0.034 ± 0.005 |
0.009–0.016 0.013 ± 0.004 |
FL (14), PH (17), BP (17) | 3 (34), 4 (30), 6 (21) | [17] |
| Taiwan | Sewage (urban) | 0.53–1.07 0.75 ± 0.26 |
0.021–0.037 0.028 ± 0.007 |
PY (16), FLU (12), CH (10) | 3 (19), 4 (42), 5 (20) | This study |
a The definitions of compound abbreviation see Table 2; b dom: domestic, ind: industrial; c Not available; d The values in parentheses indicate the percentage to total PAHs.
Figure 3.
The mean concentrations and standard deviation of ΣPAHs in sludge from other studies around the world (Data list in Table 5).
In addition, since land application is one of the major ways for sludge disposal in countries around the world [21]. This study compares the limits of the relevant sludge applied to agricultural soils in the EU and China, due to the lack of relevant PAHs standards in Taiwan. In China, the PAHs contents allowed in sludge used for agriculture are benzo[a]pyrene 2 mg/kg dw and ΣPAHs 5 mg/kg dw [22]. The EU regulations for the use of sludge in agricultural soils are the sum of 9 PAHs (acenaphthene, phenanthrene, fluorene, fluoranthene, pyrene, benzo[b+j+k]fluoranthene, benzo[a]pyrene, benzo[g,h,i]perylene, and indeno[1,2,3-cd]pyrene) which is less than 6 mg/kg dw [23]. In this study, the PAHs of the DWTP and WWTP sludge did not exceed the Chinese standard benzo[a]pyrene 2 mg/kg dw and ΣPAHs 5 mg/kg dw as well as less than the EU limit 6 mg/kg dw of sum of 9 PAHs. This shows that based on the concentration of PAHs, the sludge in this study may be suitable for agricultural applications.
3.4. Composition of PAHs in Sludge
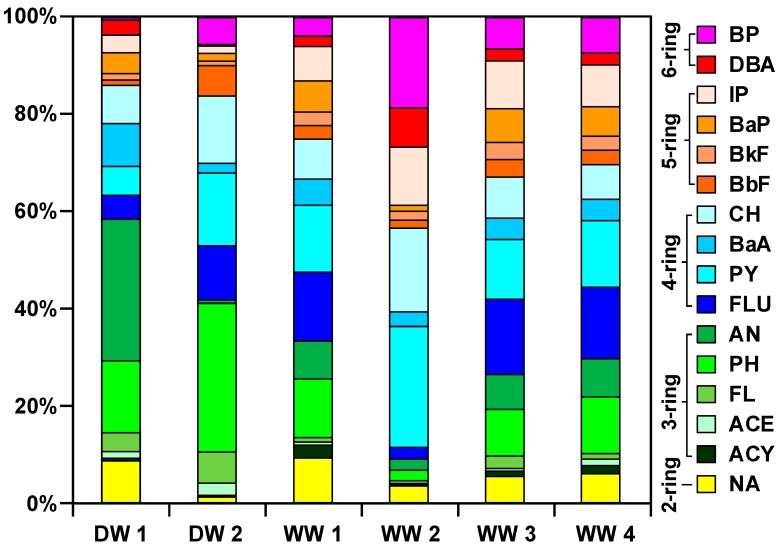
The percentage contribution of 16 PAHs in the two DWTP and four WWTP sludge samples studied is shown in Figure 4. The highest content in DW1 sludge was observed for anthracene (29.2%) and phenanthrene (14.8%), while DW2 sludge was phenanthrene (30.6%), anthracene (14.9%), and chrysene (13.9%). This difference may be due to the fact that the raw water of the two DWTP is river water and reservoir water. The composition of PAHs in sludge may also vary due to the different organic composition of different water sources [7]. In WW1, WW3 and WW4 sludge, phenanthrene (9.6–12.1%), fluoranthene (14.2–15.5%), and pyrene (12.3–13.8%) are the most dominant, which is consistent with the results of previous studies on PAHs composition of municipal wastewater treatment plant sludge [27,37]. The WW2 sludge has the highest content of pyrene (24.9%), benzo[g,h,i]perylene (18.6%), and chrysene (17.3%), which is different from the other three types of sludge studied (Figure 4). Among the four WWTP in this study, the influent water of WW1, WW3, and WW4 is mainly domestic sewage, while the influent water of WW2 includes domestic sewage and polluted river water intercepting the Tainan Canal, and its water is subjected to industrial wastewater, surface runoff, and domestic sewage from the river bank. This may be the reason why the PAHs composition of the WW2 sludge is different from other sludge. Since pyrene and benzo[g,h,i]perylene are designated as traffic-related, and benzo[g,h,i]perylene is identified as a tracer of auto emissions [38,39,40], and chrysene is suggested to indicate industrial waste incinerators [40,41], therefore WW2 sludge has a high proportion of pyrene, benzo[g,h,i]perylene, and chrysene should be affected by surface runoff and industrial wastewater.
Figure 4.
PAHs composition in sludge samples of selected DWTP (DW1–DW2) and WWTP (WW1–WW4). The definitions of compound abbreviation see Table 2.
According to the number of aromatic rings, the 16 PAHs were divided into 2 to 6 ring PAHs [42]. The 3- & 4-ring PAHs were predominant in DW1 and DW2 sludge samples, ranging from 77.4% to 82.7% (Figure 4); the percentage compositions are 1.2–8.6% and 14–16.2% for the 2-ring and 5- & 6-ring PAHs, respectively. The 4-ring PAHs were predominant in WW1, WW3, and WW4 sludge samples, ranging from 40.7% to 41.7% (Figure 4); followed by 3- & 5-ring PAHs accounting for 21.0–24.1% and 19.1–24.0%, respectively. The 2- & 6-ring accounted for the lowest percentages of 5.4–9.2% and 6.0–9.7%, respectively. In the WW2 sludge sample, the percentage of 4-ring PAHs (47.6%) was also the highest. However, the percentage of other ring numbers PAHs to ΣPAHs is different from the other three types of sludge, which are 26.7% of 6-ring, 16.7% of 5-ring, 5.5% of 3-ring and 5.5% of 2-ring. Hua et al. [24] reported that the main components of PAHs in sewage sludge from 12 different industrial and economic development cities in Zhejiang Province (China) were 3- & 4-ring, accounting for 81–97%. Hu et al. [43] also reported that the main composition of PAHs in different types of sludge (including dying, beer-brewing, paper manufacturing, and municipal wastewater treatment plants containing domestic wastewater and industrial wastewater) is 4-ring (43–70%), followed by 3-ring (16–52%), which together accounted for 81–97% of ΣPAHs. Wołejko et al. [37] reported that the 4-ring and 3-ring PAHs in the sludge of the Sokółka WWTP in Poland accounted for 62% and 22%, respectively, accounting for 84% of the ΣPAHs. Overall, the PAHs of sewage sludge is dominated by 3- and 4-ring PAHs, of which 3-ring PAHs is most advantageous with phenanthrene, acenaphthylene, and fluorene, and 4-ring PAHs is most advantageous for fluoranthene, pyrene, and chrysene. However, some sewage sludge has also been found to be dominated by 5-ring benzo[b]fluoranthene, and benzo[a]pyrene or 6-ring benzo[g,h,i]perylene and DAB (Table 5).
4. Conclusions
An appropriate method for the analysis of 16 PAHs in DWTP and WWTP sludge by GC-MS was established. For individual PAHs, the recovery of this method ranged from 74.3 to 108.7% with a detection limit of 0.0010 to 0.0046 mg/kg dw. Using this method for the determination of the PAHs content of two DWTP and four WWTP sludge samples in southwestern Taiwan, the concentration of PAHs in WWTP sludge is between 0.5342–1.0666 mg/kg dw higher than 4–16 times DWTP sludge (0.0668–0.1357 mg/kg dw). These measured concentrations are lower than the PAHs limits applied to agricultural soils in the EU. The PAHs of the DWTP sludge samples in this study were mainly phenanthrene (22.7%) and anthracene (14.9%) of 3-ring PAH and pyrene (10.5%) and chrysene (10.9%) of 4-ring, and the PAHs of WWTP sludge were most advantageous with 4-ring fluoranthene (11.7%), pyrene (16.2%) and chrysene (10.3%). This result can be used for regular monitoring to establish a background for sludge PAHs to provide a reference for future sludge management and applied agriculture.
Acknowledgments
The author thanks Huimin Environmental Tech Corporation (Taiwan) for assisting in sludge sampling.
Author Contributions
Conceptualization, C.-F.C. and C.-D.D.; Methodology, C.-F.C.; Formal Analysis, C.-F.C.; Investigation, Y.C.L.; Resources, Y.C.L., S.-L.H., M.-L.T., P.-P.S. and C.-W.C.; Writing (Original Draft Preparation), C.-F.C. and Y.-R.J.; Writing (Review & Editing), C.-F.C., Y.-R.J. and R.K.; Visualization, C.-F.C.; Supervision, C.-D.D. and C.-W.C.; Project Administration, P.-P.S. and C.-W.C.; Funding Acquisition, C.-D.D.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Stevens J.L., Northcott G.L., Stern G.A., Tomy G.T., Jones K.C. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in UK sewage sludge: Survey results and implications. Environ. Sci. Technol. 2003;37:462–467. doi: 10.1021/es020161y. [DOI] [PubMed] [Google Scholar]
- 2.Harrison E.Z., Oakes S.R., Hysell M., Hay A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006;367:481–497. doi: 10.1016/j.scitotenv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Meng X.Z., Venkatesan A.K., Ni Y.L., Steele J.C., Wu L.L., Bignert A., Bergman Å., Halden R.U. Organic contaminants in Chinese sewage sludge: A meta-analysis of the literature of the past 30 years. Environ. Sci. Technol. 2016;50:5454–5466. doi: 10.1021/acs.est.5b05583. [DOI] [PubMed] [Google Scholar]
- 4.Fijalkowski K., Rorat A., Grobelak A., Kacprzak M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017;203:1126–1136. doi: 10.1016/j.jenvman.2017.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meador J.P., Stein J.E., Reichert W.L., Varanasi U. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev. Environ. Contam. Toxicol. 1995;143:79–165. doi: 10.1007/978-1-4612-2542-3_4. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.F., Dong C.D., Chen C.W. Evaluation of sediment toxicity in Kaohsiung Harbor, Taiwan. Soil Sediment Contam. 2013;22:301–314. doi: 10.1080/15320383.2013.726294. [DOI] [Google Scholar]
- 7.Wu Q., Liu Z., Liang J., Kuo D.T.F., Chen S., Hu X., Deng M., Zhang H., Lu Y. Assessing pollution and risk of polycyclic aromatic hydrocarbons in sewage sludge from wastewater treatment plants in China’s top coal-producing region. Environ. Monit. Assess. 2019;191:102. doi: 10.1007/s10661-019-7225-6. [DOI] [PubMed] [Google Scholar]
- 8.Feng L., Luo J., Chen Y. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015;49:4781–4782. doi: 10.1021/acs.est.5b01455. [DOI] [PubMed] [Google Scholar]
- 9.Taiwan Environmental Protection Agency Environmental Resource Database. [(accessed on 15 Apri1 2019)];2019 Available online: https://erdb.epa.gov.tw/ERDBIndex.aspx.
- 10.Taiwan Water Corporation. [(accessed on 15 Apri1 2019)];2019 Available online: https://www.water.gov.tw/ct.aspx?xItem=%206013&CtNode=990&mp=ep.
- 11.Council of Agriculture, Taiwan . Regulation: Categories, Types and Specifications of Fertilizers (2013/4/3 Revised) Council of Agriculture; Taipei, Taiwan: 2013. [Google Scholar]
- 12.Dong C.D., Chen C.F., Chen C.W. Vertical profile, sources, and equivalent toxicity of polycyclic aromatic hydrocarbons in sediment cores from the river mouths of Kaohsiung Harbor, Taiwan. Mar. Pollut. Bull. 2014;85:665–671. doi: 10.1016/j.marpolbul.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Dong C.D., Chen C.F., Chen C.W. Determination of polycyclic aromatic hydrocarbons in industrial harbor sediments by GC-MS. Int. J. Environ. Res. Public Health. 2012;9:2175–2188. doi: 10.3390/ijerph9062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C.F., Chen C.W., Ju Y.R., Dong C.D. Vertical profile, source apportionment, and toxicity of PAHs in sediment cores of a wharf near the coal-based steel refining industrial zone in Kaohsiung, Taiwan. Environ. Sci. Pollut. Res. Int. 2016;23:4786–4796. doi: 10.1007/s11356-015-5716-8. [DOI] [PubMed] [Google Scholar]
- 15.US Environmental Protection Agency . Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. Office of Research and Development, US Environmental Protection Agency; Washington, DC, USA: 1993. EPA/600/R/089. [Google Scholar]
- 16.Boruszko D. Research on the influence of anaerobic stabilization of various dairy sewage sludge on biodegradation of polycyclic aromatic hydrocarbons PAHs with the use of effective microorganisms. Environ. Res. 2017;155:344–352. doi: 10.1016/j.envres.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Jiries A., Hussain H., Lintelmann J. Determination of polycyclic aromatic hydrocarbons in wastewater, sediments, sludge and plants in Karak province, Jordan. Water Air Soil Pollut. 2000;121:217–228. doi: 10.1023/A:1005257207607. [DOI] [Google Scholar]
- 18.Ozaki N., Takamura Y., Kojima K., Kindaichi T. Loading and removal of PAHs in a wastewater treatment plant in a separated sewer system. Water Res. 2015;80:337–345. doi: 10.1016/j.watres.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Busetti F., Heitz A., Cuomo M., Badoer S., Traverso P. Determination of sixteen polycyclic aromatic hydrocarbons in aqueous and solid samples from an Italian wastewater treatment plant. J. Chromatogr. A. 2006;1102:104–115. doi: 10.1016/j.chroma.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Khadhar S., Higashi T., Hamdi H., Matsuyama S., Charef A. Distribution of 16 EPA-priority polycyclic aromatic hydrocarbons (PAHs) in sludges collected from nine Tunisian wastewater treatment plants. J. Hazard. Mater. 2010;183:98–102. doi: 10.1016/j.jhazmat.2010.06.112. [DOI] [PubMed] [Google Scholar]
- 21.Raheem A., Sikarwar V.S., He J., Dastyar W., Dionysiou D.D., Wang W., Zhao M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018;337:616–641. doi: 10.1016/j.cej.2017.12.149. [DOI] [Google Scholar]
- 22.State Standard of the People’s Republic of China . Control Standards of Pollutants in Sludges from Agricultural Use. Standardization Administration of the People’s Republic of China; Beijing, China: 2018. GB, 4284-2018. [Google Scholar]
- 23.European Commission . Proposal for a Directive of the European Parliament and of the Council on Spreading of Sludge on Land. European Commission; Brussels, Belgium: 2003. [Google Scholar]
- 24.Hua L., Wu W.X., Liu Y.X., Tientchen C.M., Chen Y.X. Heavy metals and PAHs in sewage sludge from twelve wastewater treatment plants in Zhejiang province. Biomed. Environ. Sci. 2008;21:345–352. doi: 10.1016/S0895-3988(08)60053-7. [DOI] [PubMed] [Google Scholar]
- 25.Dai J.Y., Xu M.Q., Chen J.P., Yang X.P., Ke Z.S. PCDD/F, PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing, China. Chemosphere. 2007;66:353–361. doi: 10.1016/j.chemosphere.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 26.Zeng X.Y., Lin Z., Gui H.Y., Shao W.L., Sheng G.Y., Fu J.M., Yu Z.Q. Occurrence and distribution of polycyclic aromatic carbons in sludges from wastewater treatment plants in Guangdong, China. Environ. Monit. Assess. 2010;169:89–100. doi: 10.1007/s10661-009-1153-9. [DOI] [PubMed] [Google Scholar]
- 27.Tian W.J., Bai J., Liu K.K., Sun H.M., Zhao Y.G. Occurrence and removal of polycyclic aromatic hydrocarbons in the wastewater treatment process. Ecotoxicol. Environ. Saf. 2012;82:1–7. doi: 10.1016/j.ecoenv.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Sun S.J., Jia L.R., Li B., Yuan A.N., Kong L.J., Qi H., Ma W.L., Zhang A.P., Wu Y.N. The occurrence and fate of PAHs over multiple years in a wastewater treatment plant of Harbin, Northeast China. Sci. Total Environ. 2018;624:491–498. doi: 10.1016/j.scitotenv.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Salihoglu N.K., Salihoglu G., Tasdemir Y., Cindoruk S.S., Yolsal D., Ogulmus R., Karaca G. Comparison of polycyclic aromatic hydrocarbons levels in sludges from municipal and industrial wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2010;58:523–534. doi: 10.1007/s00244-009-9389-5. [DOI] [PubMed] [Google Scholar]
- 30.Ju J.H., Lee I.S., Sim W.J., Eun H., Oh J.E. Analysis and evaluation of chlorinated persistent organic compounds and PAHs in sludge in Korea. Chemosphere. 2009;74:441–447. doi: 10.1016/j.chemosphere.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 31.Berset J.D., Holzer R. Quantitative determination of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in sewage sludges using supercritical fluid extraction and mass spectrometric detection. J. Chromatogr. A. 1999;852:545–558. doi: 10.1016/S0021-9673(99)00641-X. [DOI] [PubMed] [Google Scholar]
- 32.Helaleh M.I.H., Al-Omair A., Nisar A., Gevao B. Validation of various extraction techniques for the quantitative analysis of polycyclic aromatic hydrocarbons in sewage sludges using gas chromatography-ion trap mass spectrometry. J. Chromatogr. A. 2005;1083:153–160. doi: 10.1016/j.chroma.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 33.Perez S., Guillamon M., Bracelo D. Quantitative analysis of polycyclic aromatic hydrocarbons in sewage sludge from wastewater treatment plants. J. Chromatogr. A. 2001;938:57–65. doi: 10.1016/S0021-9673(01)01338-3. [DOI] [PubMed] [Google Scholar]
- 34.Villar P., Callejon M., Alonso E., Jimenez J.C., Guiraum A. Temporal evolution of polycyclic aromatic hydrocarbons (PAHs) in sludge from wastewater treatment plants: Comparison between PAHs and heavy metals. Chemosphere. 2006;64:535–541. doi: 10.1016/j.chemosphere.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Khillare P.S., Sattawan V.K., Jyethi D.S. Profile of polycyclic aromatic hydrocarbons in digested sewage sludge. Environ. Technol. 2018;17:1–10. doi: 10.1080/09593330.2018.1512654. [DOI] [PubMed] [Google Scholar]
- 36.Baran S., Oleszczuk P. The concentration of polycyclic aromatic hydrocarbons in sewage sludge in relation to the amount and origin of purified sewage. Pol. J. Environ. Stud. 2003;12:523–529. [Google Scholar]
- 37.Wołejko E., Wydro U., Jabłońska-Trypuć A., Butarewicz A., Łoboda T. The effect of sewage sludge fertilization on the concentration of PAHs in urban soils. Environ. Pollut. 2018;232:347–357. doi: 10.1016/j.envpol.2017.08.120. [DOI] [PubMed] [Google Scholar]
- 38.Larsen R.K., Baker J.E. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ. Sci. Technol. 2003;37:1873–1881. doi: 10.1021/es0206184. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Chen L., Huang Q.H., Li W.Y., Tang Y.J., Zhao J.F. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Sci. Total Environ. 2009;407:2931–2938. doi: 10.1016/j.scitotenv.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 40.Chen C.W., Chen C.F., Dong C.D., Tu Y.T. Composition and source apportionment of PAHs in sediments at river mouths and channel in Kaohsiung Harbor, Taiwan. J. Environ. Monit. 2012;14:105–115. doi: 10.1039/C1EM10557D. [DOI] [PubMed] [Google Scholar]
- 41.Yang H.H., Lee W.J., Chen S.J., Lai S.O. PAH emission from various industrial stacks. J. Hazard. Mater. 1998;60:159–174. doi: 10.1016/S0304-3894(98)00089-2. [DOI] [Google Scholar]
- 42.Chen C.F., Chen C.W., Dong C.D., Kao C.M. Assessment of toxicity of polycyclic aromatic hydrocarbons in sediments of Kaohsiung Harbor, Taiwan. Sci. Total Environ. 2013;463–464:1174–1181. doi: 10.1016/j.scitotenv.2012.06.101. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y., Li G., Yan M., Ping C., Ren J. Investigation into the distribution of polycyclic aromatic hydrocarbons (PAHs) in wastewater sewage sludge and its resulting pyrolysis bio-oils. Sci. Total Environ. 2014;473–474:459–464. doi: 10.1016/j.scitotenv.2013.12.051. [DOI] [PubMed] [Google Scholar]