Abstract
The transformation of normal cells to the cancerous stage involves multiple genetic changes or mutations leading to hyperproliferation, resistance to apoptosis, and evasion of the host immune system. However, to accomplish hyperproliferation, cancer cells undergo profound metabolic reprogramming including oxidative glycolysis and acidification of the cytoplasm, leading to hyperpolarization of the mitochondrial membrane. The majority of drug development research in the past has focused on targeting DNA replication, repair, and tubulin polymerization to induce apoptosis in cancer cells. Unfortunately, these are not cancer-selective targets. Recently, researchers have started focusing on metabolic, mitochondrial, and oxidative stress vulnerabilities of cancer cells that can be exploited as selective targets for inducing cancer cell death. Indeed, the hyperpolarization of mitochondrial membranes in cancer cells can lead to selective importing of mitocans that can induce apoptotic effects. Herein, we will discuss recent mitochondrial-selective anticancer compounds (mitocans) that have shown selective toxicity against cancer cells. Increased oxidative stress has also been shown to be very effective in selectively inducing cell death in cancer cells. This oxidative stress could lead to mitochondrial dysfunction, which in turn will produce more reactive oxygen species (ROS). This creates a vicious cycle of mitochondrial dysfunction and ROS production, irreversibly leading to cell suicide. We will also explore the possibility of combining these compounds to sensitize cancer cells to the conventional anticancer agents. Mitocans in combination with selective oxidative-stress producing agents could be very effective anticancer treatments with minimal effect on healthy cells.
Keywords: mitochondria, apoptosis, oxidative phosphorylation, reactive oxygen species, metabolic reprogramming, electron transport chain, sensitization, chemoresistance
1. Background of Mitochondria and Cancer
Despite many advances in cancer treatment approaches, cancer remains the leading cause of death in both Canada and the United States [1,2]. In Canada, of 206,200 projected cancer incidences, 1 in 4 diagnosed patients are expected to succumb to the disease in 2019 [1]. In the United States, of 1,762,450 projected diagnoses in 2019, about 1 in 3 diagnosed patients are expected to succumb to the disease [2]. Therefore, it is critical to discover new cancer-selective treatments that are both more effective and less toxic to patients.
Cancer cells are notorious for their enhanced ability to proliferate in suboptimal conditions through the accumulation of many genetic mutations. They are able to manipulate metabolic and immunogenic pathways to adapt and operate under difficult conditions [3]. Indeed, metabolic reprogramming in cancer cells has been identified as a hallmark of cancer and a potential vulnerability that can be targeted to fight this disease [4].
Apoptosis, the complex and physiological process of cell death, removes both unwanted cells and those with damaged DNA. In doing so, this process can act as a safeguard against the development of abnormal cells. Apoptosis can be triggered by DNA damage, oxidative stress, growth factor deprivation, and mitochondrial depolarization [5]. Knowledge of the biochemical mechanisms of apoptosis has led to the development of therapies targeting cancer cells to induce cell death [5]. Various processes, including those mentioned previously, can trigger pathways by which apoptosis can initiate and progress. In the intrinsic pathway, internal signals such as DNA damage can lead to a cascade of events, resulting in activation of the Bcl-2 family of proteins, which in turn activate Bax-like proteins to permeabilize the membrane and release cytochrome c [5]. Cytochrome c release will lead to the activation of caspase proteins to induce apoptosis. In the extrinsic pathway, external signals such as tumour necrosis factor (TNF) can lead to caspase activation and apoptosis [5]. The exploitation of vulnerabilities in cancerous cells, including oxidative stress susceptibility and mitochondrial membrane destabilization, could be used to develop novel therapeutic agents that trigger apoptosis and eradicate the disease [6,7]. Researchers have aimed to exploit cancer cell vulnerabilities using a variety of approaches including identification of tumour-suppressor genes, discovery of novel compounds, and development of technologies such as nanoparticles to selectively identify targets that can be effective and selective for cancer [8,9,10]. Unfortunately, cancer cells develop resistance to many chemotherapies by resisting apoptosis and/or exporting the drugs outside the cell. This is achieved by disrupting pro-apoptotic proteins, reducing the function of caspases, and impairing death receptor signaling [5].
Cancer therapeutics including DNA damaging agents (e.g., cisplatin, doxorubicin, or 5-fluorouracil) and tubulin modifying agents (e.g., paclitaxel) have been developed to induce apoptosis in cancer cells. However, these drugs have limitations due to their non-selective nature and extreme toxicity to healthy tissues. Further, cancer cells develop resistance to these drugs and patients may experience severe side effects with no significant impact on the cancer [11,12,13]. In addition, these genotoxic treatments can lead to DNA damage in normal tissues, increasing the risk of further cancer development. Thus, we are essentially using cancer-inducing compounds in our attempts to treat cancer.
In order to proliferate in hostile conditions, cancer cells undergo metabolic reprogramming to meet their energetic needs. In the 1920s, Otto Warburg observed what he termed the ‘Warburg Effect’, which described the production of excessive lactate by cancer cells in the presence of oxygen as a result of ‘oxidative glycolysis’ [14,15]. However, this has led to the misunderstanding that cancer cells have mitochondrial damage and metabolic inefficiencies, forcing them to rely on excessive glycolysis for their energy production [16]. Contrary to this, a large breadth of work has shown that mitochondria and mitochondrial respiration remains undamaged in many cancers [17]. Interestingly, in ovarian cancer, the microenvironment of cancer cells directly impacts their metabolic reprogramming [18]. Peripheral cells of a tumour spheroid exposed to normal oxygen levels relied heavily on aerobic glycolysis and were proliferative cells. In contrast, internal cells were poorly vascularized with poor access to oxygen and glucose, leading to cell quiescence and heavy dependence on mitochondrial oxidative phosphorylation (OxPhos) for the majority of their ATP production [19,20]. This study highlights that the fact that while standard chemotherapy was effective at killing proliferative cells, the quiescent cell population is resistant and causes tumour regeneration. In order to properly combat cancer, a multi-faceted approach targeting mitochondrial vulnerabilities should be considered to ensure complete tumour elimination. In fact, recent observations have indicated that mitochondria actually support and play a critical role in tumourigenesis through their metabolic reprogramming, oxidative signaling, reactive oxygen species generation, and production of oncometabolites [21,22,23,24,25].
Cancer cells have higher metabolic needs and antioxidant defenses compared with healthy cells. They rely heavily on aerobic glycolysis to meet their energy needs and, as a result, upregulate glucose transporters to meet their demands [26,27]. Furthermore, aerobic glycolysis leads to the production of large amounts of lactate and pyruvate, causing increased acidity in the cytoplasm in cancer cells. Thus, their mitochondria are hyperpolarized compared with those of normal cells [27]. This hyperpolarization can also be the result of increased intracellular Ca2+ levels and upregulation of anti-apoptotic Bcl2 protein [28,29] and/or increased apoptosis evasion [30].
While the mitochondria are the organelle responsible for ATP generation, they contain a number of pro-apoptotic factors such as cytochrome c, endonuclease G, and apoptosis inducing factor (AIF), which can induce a cell suicide program if they are released outside. Release of cytochrome c in the cytosol leads to its association with apoptosis protease activating factor 1 (APAF-1) and Caspase-9, eventually forming the apoptosome that activates Caspase-3 and executes apoptosis [31]. In contrast, AIF-initiated apoptosis is caspase-independent, and works through chromatin condensation and DNA fragmentation [32]. The presence of such pro-apoptotic proteins in the mitochondria puts a spotlight on the organelle as an interesting target for cancer therapy research.
Mitochondria play a central role in the apoptosis induction process. It would be appropriate to say that each mitochondrion acts as a “self-destruct” button for the cell. Differences between cancerous and non-cancerous mitochondria can be targeted to allow for the release of pro-apoptotic factors to induce apoptosis selectively in cancer cells. In this review, we will discuss some of the recent advances in the development of therapeutic modulators targeting mitochondrial vulnerabilities.
2. Targeting Mitochondrial Vulnerabilities
Cancer cells may rely heavily on OxPhos in addition to aerobic glycolysis for their excessive energy needs [33,34]. Further, mitochondrial membranes of cancer cells are hyperpolarized relative to normal healthy cells and are poorly assembled [35]. Mitochondrial-targeting treatments thus have the potential to specifically target cancer while selectively sparing normal healthy cells. Mitochondria also play a fundamental role in reactive oxygen species and oxidative stress defense, energy production, and the induction of apoptosis. These mechanisms and functions may be altered in cancer and serve as specific markers to be targeted by therapeutics.
By exploiting cancer cell differences in needs and functions, healthy cells can be spared, leading to treatments that avoid adverse side-effects, as observed in many chemotherapeutics today. For example, Weinberg and Chandel postulate that while cancer cells may simply turn to aerobic glycolysis, there are other reasons supporting targeting the mitochondria [19]. The first being that poorly perfused tumours may have limited access to aerobic conditions and glucose. This forces cancer cells to rely on mitochondrial ATP generation, which can be specifically targeted by therapies [36,37]. The second being that some cancer cells indeed show a heavy dependence on OxPhos for their ATP needs [37,38]. In both scenarios, mitochondrial-targeting treatments can disrupt OxPhos machinery and lead to cancer cell death. Finally, drugs targeting and inhibiting mitochondrial ATP production may sensitize cancer cells to aerobic glycolysis-targeting drugs and enhance their action. Further, when the mitochondria are targeted, leakage of pro-apoptotic factors from mitochondria will regardless result in activation of apoptotic. Further, when the mitochondria are targeted, leakage of pro-apoptotic factors will result in activation of apoptosis, supporting the notion of mitochondrial-targeting drug synergy.
2.1. Induction of Oxidative Stress as a Target for Cancer Treatment
Reactive oxygen species (ROS) are radicals containing a single unpaired electron in their outermost electron shell [39]. At lower levels, ROS can be advantageous in promoting proliferation and signaling [40,41]. However, at higher levels, ROS can induce oxidative stress leading to cell death [40]. Cancer cells, unlike healthy cells, require a higher concentration of ROS to supplement increased proliferation rates. It has been hypothesized that cancer cells can take advantage of the resulting augmented DNA-damage to promote further mutation and tumourigenesis [41,42]. Further, the induction of oxidative stress via chemotherapy or radiation therapy on cancer cells may result in DNA damage-induced cell death [43,44,45,46]. Because of their metabolic changes, a shift to increased ROS in the microenvironment allows for progressive mutation towards a metastatic state [40]. As a result, cancer cells have upregulated antioxidative defenses to survive in their enhanced oxidizing environment.
The production of ROS in the mitochondria is inevitable because of its generation as a by-product in OxPhos. ROS presence plays a role in alteration of mitochondrial dynamics [47] and, as a result, is efficiently quenched in normal cells using an antioxidative defense system that includes superoxide dismutase and glutathione peroxidase [48,49]. If not quenched or eliminated, excessive oxidative stress can cause further dysfunction of mitochondrial proteins, leading to augmented production of ROS, creating a vicious cycle of mitochondrial damage and oxidative stress. This will eventually lead to the collapse of the mitochondrial membrane potential, permeabilization of the membrane, and induction of apoptosis [7].
2.2. Mitochondrial Metabolic Reprogramming as a Target for Cancer Treatment
Cancer cells rely heavily on aerobic glycolysis for the bulk of their energy needs and turn to OxPhos only in situations where oxygen or glucose access is limited. The use of glycolytic targeting therapies may help to reduce the proliferation of cancer cells. Upregulation of aerobic glycolysis is a result of increased expression of oncogenes (such as MYC and KRAS) and deregulation of the P13K signaling pathway [50,51,52]. Owing to excessive glycolysis and lactic acid production, mitochondria are hyperpolarized [26]. As a result of metabolic reprogramming by cancer cells, specific aspects of cancer cell metabolism may be distinct from normal healthy cells. These differences can be targeted by therapies to enhance selectivity for cancer cells and to avoid adverse side effects due to targeting of healthy cells. Specific targets will be discussed in the later sections below.
Recent work on targeting of mitochondrial reprogramming vulnerabilities has focused on many different approaches, including inhibition of upregulated metabolic proteins found only in cancer cells [53], targeting mitochondrial oxidative phosphorylation or respiration [54,55], or through the induction of antibiotics-induced mitochondrial dysfunction [56].
2.3. Sensitization and Reversal of Chemoresistance by Targeting the Mitochondria
Cancer cell mitochondria contain higher amounts of anti-apoptotic Bcl2 family of proteins to evade apoptosis and as such are resistant to anti-cancer drugs. The depletion of mitochondrial DNA (mtDNA) has been reported in numerous cancers in vivo and has been implicated in increasing the expression of anti-apoptotic genes, such as Bcl2. This activates pro-survival enzymes, leading to resistance to chemotherapy-mediated apoptosis [57,58,59,60,61]. Owing to a dependence on aerobic glycolysis, cancer cells are able to resist apoptosis through upregulation of regulatory enzymes [62]. For example, hexokinase II has been shown to increase lactate production, cell proliferation, resistance to drugs, and invasion [63]. Lactate production allows for cancer cells to maintain a slightly acidic micro-environment and enhance their survival through pathways such as using lactate as an antioxidant [64,65,66].
There have been many reported cases of chemosensitization by targeting the mitochondria of cancer cells. One example is the drug metformin, which targets complex I of the electron transport chain in cancer cell mitochondria. Metformin is a novel and exciting development showing promise as an anti-cancer agent and adjuvant through its ability to sensitize cancer cells, particularly in ovarian cancer [67,68,69,70]. As mentioned previously, internal quiescent ovarian cancer cells develop a resistance to chemotherapeutic treatments that are effective against proliferative cells, requiring the addition of OxPhos inhibitor therapy to prevent cancer relapse [18]. In addition, multi-drug resistance to doxorubicin was overcome by utilizing doxorubicin-loaded micelles to allow for the chemotherapy to reach the mitochondria more effectively [71].
3. Strategies to Target Mitochondria
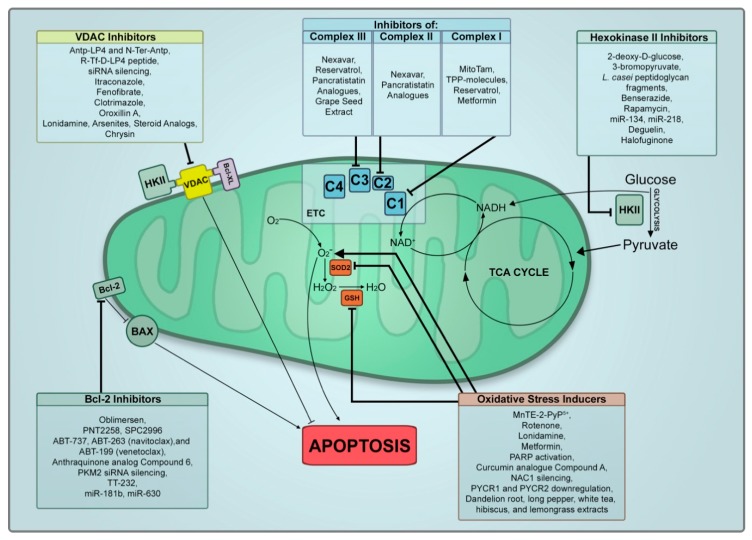
Indeed, a large breadth of work has been put into the identification of mitochondrial vulnerabilities and the identification of mitochondrial differences in cancerous and non-cancerous cells. In doing so, specific targeting approaches of cancer cell mitochondria have been developed. In this section, we will summarize the advances made recently into the development of mitocans as potential therapeutics for cancer. Olivas-Aguirre et al. recently published a great review on mitochondrial-targeted therapies for T cell acute lymphoblastic leukemia focusing on specific targets for mitocans [72]. We will highlight several mitochondrial targets and expand the breadth to include all cancer types. We have tabulated all treatments reviewed in Table 1 and visualized their targets in Figure 1.
Table 1.
Mitocan treatments, targets, and treatment effects. VDAC1—voltage-dependent anion channel 1; 2-DG—2-deoxy-D-glucose; 3-BPA—3-bromopyruvate; Benz—benserazide; STAT3—signal transducer and activator of transcription 3; HK—hexokinases; PBMCs—peripheral blood mononuclear cells; MMP—mitochondrial membrane potential; PKM2—pyruvate kinase M2 isoform; TPP—triphenylphosphonium; ROS—reactive oxygen species; PARP—poly (ADP-ribose) polymerase; NAC1—nucleus-accumbens-1; PYCR—pyrroline-5-carboxylate reductase; GSE—grape seed extract.
| Mitochondrial Targets | Mitocan/Treatment Name | Treatment Effect | Clinical Trial Status | Reference |
|---|---|---|---|---|
| Hexokinase II | 2-DG | Cytotoxicity, sensitization to prednisone | NT * | [73,74] |
| 3-BPA | Cytotoxicity, sensitization to prednisone | NT | [74,75,76] | |
| Lactobacillus casei peptidoglycan fragments (European Patent number 1217005) | Inhibition of entire metabolism of cancer tumour cells | NT | [77] | |
| Benz | Reduces glucose uptake, lactate production, and ATP levels, led to apoptosis | Phase 4 (NCT02741947)) | [78] | |
| Rapamycin/siRNA downregulation of STAT3 | Glycolysis inhibition, reduce glucose consumption | NT | [79] | |
| miR-134 | Knockdown of HKII reduced glucose consumption leading to apoptosis | NT | [80] | |
| miR-218 | Downregulation of HKII and apoptosis | NT | [81,82] | |
| VDAC-1 | VDAC1-based peptides Antp-LP4 and N-Ter-Antp | Highly effective in inducing cell death in leukemia patient PBMCs and cancer cell lines, but not healthy patient PBMCs | NT | [83,84,85] |
| R-Tf-D-LP4 peptide | Targeted transferrin receptor in cancer cells, enhancing specificity of Antp-LP4 and N-Ter-Antp | NT | [86] | |
| VDAC-1 siRNA silencing | Decreased MMP and ATP levels, reducing tumour burden | NT | [87] | |
| Itraconazole | Inhibition of cell proliferation | NT | [88] | |
| Fenofibrate | Reprogramming of metabolism and apoptosis in oral carcinomas | NT | [89] | |
| Clotrimazole | Cytotoxicity, inhibition of glycolysis | NT | [90] | |
| Oroxillin A | Cytotoxicity, apoptosis, cell cycle arrest, and metastasis inhibition | NT | [91] | |
| Lonidamine | Cytotoxicity | NT | [91] | |
| Arsenites | Cytotoxicity | NT | [91] | |
| Steroid Analogs | Cytotoxicity | NT | [91] | |
| Bcl-2 Family | Oblimersen | Downregulation of Bcl-2, synergy with other treatments | (G3139) | [92] |
| PNT2258 | Cell cycle arrest, apoptosis in non-Hodgkin’s lymphoma | Phase 2 (NCT02226965) | [93] | |
| SPC2996 | Leukemic cell clearance, immune system activation and stimulation | Phase 2 (NCT00285103) | [94] | |
| ABT-737 | Apoptosis in lymphoma and leukemia cell lines | NT | [95,96] | |
| ABT-263 (navitoclax) and ABT-199 (venetoclax) | Enhanced effects and specificity compared to ABT-737 | Phase 2 (NCT03504644)Phase 2 (NCT03181126) | [97,98] | |
| Anthraquinone analog Compound 6 | Binds Bcl-2, Mcl-2, and p-Mcl-2 leading to apoptosis induction | NT | [99] | |
| PKM2 siRNA silencing | Regulates oxidative stress induced apoptosis in a variety of cancers | NT | [100,101] | |
| TT-232 | Translocation of PKM2 to nucleus to trigger apoptosis | Phase 2 (NCT00422786) | [102] | |
| miR-181b | Sensitize cancer cells to cisplatin | NT | [103] | |
| miR-630 | Sensitize cancer cells to cisplatin | NT | [104] | |
| Electron Transport Chain | Sorafenib (nexavar) | Inhibition of ATP synthase leading to Parkin-mediated apoptosis | Phase 3 (NCT00105443) | [105] |
| MitoTam | Increased localization of tamoxifen to mitochondria, leading to increased specificity | Clinical trials to begin shortly | [106] | |
| TPP-Peptide Artemisinin-TPP Green titania ((G-TiO2-x) conjugated to TPP |
Selectively kill anticancer cells | NT | [107,108,109] | |
| Reservatrol | Act as a pro-oxidant leading to cancer cell death | NT | [110,111] | |
| Metformin | Selective mitochondrial targeting, acts as an adjuvant with many cancer therapies | Phase 1 (NCT03477162) | [112,113,114] | |
| Pancratistatin analogues SVTH-6 and SVTH-7 | Highly selective cytotoxicity on cancer cells in 2D and 3D culture models | NT | [115] | |
| Oxidative Stress | MnTE-2-PyP5+ | Enhance chemotherapeutic effect by mitochondrial environment modulation | NT | [116] |
| Rotenone | Activates NOX2 resulting in increased ROS and cell death | NT | [117] | |
| Lonidamine | Cytotoxicity through ROS generation | NT | [118] | |
| Metformin | Additionally exert oxidative stress | Phase 1 (NCT03477162) | [112,113,114] | |
| PARP activation | Enhances ROS production leading to apoptosis | NT | [119] | |
| Curcumin analogue Compound A | Selective apoptosis through the generation of significant ROS in a variety of cancers | NT | [120] | |
| NAC1 silencing | Removal of oxidative stress defense mechanism, sensitization | NT | [121] | |
| PYCR1 and PYCR2 downregulation | Sensitizes cancer cells to ROS by inhibiting stress-response proteins | NT | [122] | |
| Natural Health Products Targeting Mitochondria | Chrysin | Inhibits HKII binding to VDAC1 leading to apoptosis | NT | [123] |
| Deguelin | Downregulates HKII leading to apoptosis | NT | [124] | |
| Halofuginone | Downregulates HKII | Phase 1 (NCT00027677) | [125] | |
| GSE | Targets complex III and depletes glutathione antioxidant leading to apoptosis in cancer | NT | [126] | |
| Dandelion root, long pepper, white tea, hibiscus, and lemongrass extracts | Highly effective induction of apoptosis and excessive ROS generation | Phase 1 (OCT1226, DRE) | [127,128,129,130] |
* NT = not tested in clinical trials.
Figure 1.
Mitocan treatments and mitochondria targets. Summary of reviewed mitocan treatments and their targets in the mitochondria. HKII, hexokinase II; C1, complex I; C2, complex II; C3, complex III; C4, complex IV; ETC, electron transport chain; VDAC, voltage-dependent anion channel 1; TPP, triphenylphosphonium; PARP, poly (ADP-ribose) polymerase; NAC1, nucleus-accumbens-1; PYCR, pyrroline-5-carboxylate reductase.
3.1. Targeting Mitochondrial Interacting Hexokinase II
Cancer cells rely heavily on aerobic glycolysis and, as a result, over-express hexokinases (HK) that catalyze the first step of glucose metabolism [131,132]. Of the four HK isoforms, hexokinase II (HKII) plays a critical role in cancer cell survival and proliferation. At the outer mitochondrial membrane, HKII binds to voltage-dependent anion channel 1 (VDAC1) and facilitates its interaction with adenine nucleotide translocase on the inner mitochondrial membrane [133,134]. This interaction is able to couple aerobic glycolysis with OxPhos and create a working relationship between the two metabolic processes, allowing HKII to exchange ADP for ATP from the mitochondria and increase the rate of glycolysis [72,134]. Targeting HKII could lead to uncoupling and stunting of aerobic glycolysis, leading to cancer cell death.
Recent research has focused on agents that are capable of inhibiting HKII and preventing the progression of glucose metabolism. Some commonly used inhibitors include glucose analogs 2-deoxy-D-glucose (2-DG) and 3-bromopyruvate (3-BPA), which have been shown to induce cytotoxic effects on acute lymphoblastic leukemia while enhancing the efficacy of prednisone treatment [73,74]. An epidermal growth factor receptor-targeted liposomal formulation of 3-BPA showed improved permeability, HKII inhibition, and cytotoxicity compared with conventional aqueous 3-BPA formulations [75]. However, a recent study indicated that 3-BPA was still effective in killing HKII knockout cells, thus suggesting that in addition to HKII, the efficacy of 3-BPA may be also dependent on tumour microenvironment and glucose availability [76]. A cytotoxic peptidoglycan complex and a synthetic peptide derived from Lactobacillus casei were both able to impair the entire metabolism of tumour cells via displacement of HKII from the mitochondrial membrane [77]. Importantly, these compounds were cytotoxic to cancer cells while stimulating glycolysis in healthy noncancerous cells, presumably because of the cancer cell dependence on HKII binding to the mitochondrial membrane. Benserazide (Benz), originally designed to treat Parkinson’s Disease, is another inhibitor of HKII that was able to selectively reduce glucose uptake, lactate production, and intracellular ATP levels, leading to the dissipation of the mitochondrial membrane potential (MMP) and apoptosis [78].
There have also been efforts to genetically target HKII to kill cancer cells. Signal transducer and activator of transcription 3 (STAT3) is an oncogene playing critical roles in tumour development, angiogenesis, and metastasis [135]. STAT3 is known as a downstream factor from rapamycin (mTOR) and a regulator of HKII; thus, the mTOR–STAT3–HKII pathway is an interesting target for glycolysis inhibition in cancer cells [79]. Rapamycin treatment and siRNA downregulation of STAT3 were shown to directly decrease glucose consumption and downregulate HKII [79]. Knockdown of HKII was also achieved using microRNA-143 (miR-143) overexpression, and led to the promotion of cancer cell apoptosis through inhibition of glucose metabolism and proliferation [80]. In the same regard, overexpression of miR-218 downregulated HKII expression, facilitated by the cell-promoting oncogene Bmi1, in a novel miR-218/Bmi1/HKII axis [81,82].
Interestingly, HKII has also been implicated as a target to sensitize cancer cells to chemotherapy and radiotherapy. Upregulation of HKII has been shown to modulate resistance to rituximab, and inhibition of HKII (via 2-DG, discussed above) resulted in sensitization of these cells to rituximab, as indicated by decreased MMP and cell viability [136]. HKII depletion has also pushed cancer cells to resort to OxPhos, sensitizing cancer cells to be targeted by complex I inhibitor metformin to facilitate apoptosis [112]. Other studies have shown that targeting both HKII and its regulators led to radiation treatment sensitization [137,138].
Of these HKII targeting compounds, Benz (clinical trial number: NCT02741947) and metformin usage in human cancers (clinical trial number: NCT03477162) has progressed to clinical studies. However, it is important to note that Benz completed phase 4 clinical trials testing for usage in Parkinson’s Disease and not cancer.
3.2. Targeting Voltage-Dependent Anion Channel 1
Voltage-dependent anion channel 1 (VDAC1) is situated on the outer mitochondrial membrane and allows for the transfer of many compounds including metabolites, fatty acids, Ca2+, ROS, and cholesterol across the mitochondrial membrane [139].
By targeting VDAC1, it is possible to hinder cancer cell apoptosis evading mechanisms. VDAC1 plays a central role in mitochondrial apoptosis, interacting with anti-apoptotic proteins such as Bcl2 or Bcl-xL, as well as apoptosis-suppressing HKII. Synthetic peptides based on VDAC1 binding sites were used to inhibit Bcl2, Bcl-xL, and HKII, preventing their association with VDAC1 and thus inhibiting cancer cells’ ability to evade apoptosis [83]. VDAC1-based peptides, Antp-LP4 and N-Ter-Antp, were engineered and were highly efficacious on peripheral blood mononuclear cells (PBMCs) from leukemia patients, but spared healthy patient PBMCs [84]. These peptides were also highly effective in inducing cell death in a variety of cancer cell lines [85]. The same research team further designed peptides with increased specificity, including the R-Tf-D-LP4 peptide, which contain a transferrin receptor internalization sequence (Tf) to promote the targeting of overexpressed transferrin receptor in cancer cells [86]. This peptide was widely successful, however, common metabolic enzymes such as GLUT-1, GAPDH, and citrate synthase were targeted, potentially leading to adverse side effects in healthy cells and complications in clinical trials.
Taking a genetic approach to targeting this protein, siRNA silencing of VDAC1 resulted in decreased MMP and ATP levels in cancer cell lines, as well as a drastic reduction of tumour burden on lung cancer xenografted mice [87]. An antifungal drug, itraconazole, was shown to target VDAC1 and inhibit mTOR activity and cell proliferation through modulation of mitochondrial metabolism, leading to apoptosis [88]. However, VDAC1 knockdown may have toxic effects owing to their expression in healthy cells, and more studies on toxicity should be done [139].
In addition, the VDAC1–HKII complex can be targeted to trigger apoptosis in cancer cells overexpressing HKII. In a recent study, fenofibrate interrupted the binding of HKII to VDAC1 and reprogrammed the metabolic pathway in oral squamous cell carcinoma [89]. Many other compounds that are capable of destroying the VDAC–HKII bond have been highlighted by Magri et al. [140], including clotrimazole [90], 3-BR [141], 2DG [142], oroxillin A, lonidamine, arsenites, and steroid analogs [91].
Fenofibrate (clinical trial number: NCT01965834) and lonidamine (clinical trial number: NCT00435448) entered clinical trials, but were terminated at phase II and III, respectively.
3.3. Targeting Bcl2
The family of anti-apoptotic B-cell lymphoma 2 (Bcl-2) proteins present an interesting target for cancer therapies because of their role in promoting cell survival inhibition of apoptosis [143]. Initial attempts to develop agents to target Bcl-2 aimed to decrease Bcl-2 levels through the delivery of RNA antisense molecules [144]. This study highlighted molecules that have significant anticancer effects and have entered clinical trials, including Oblimersen (G3139/Genasense) [92], PNT2258 (NCT0222696) [93], and SPC2996 (NCT00285103) [94]. BH-3 mimetics imitate protein–protein interactions between BH-3 domains and Bcl-2 family members, and have been used to displace bound Bcl-2 protein from pro-apoptotic partners, leading to cancer cell death [144]. ABT-737, the first BH-3 mimetic, efficiently induced apoptosis in lymphoma and leukemia cell lines [95,96], leading to the development of orally bioavailable ABT-263 (navitoclax) and ABT-199 (venetoclax), selective to Bcl2, which have shown promise in clinical studies [97,98]. A novel anthraquinone BH-3 mimetic named compound 6 was identified to be the first small molecule to induce apoptosis in melanoma cancer cells by binding to Bcl-2, Mcl-2, and phosphorylated Mcl-2 [99].
Mitochondrial pyruvate kinase M2 isoform (PKM2) can translocate to the mitochondria and interact with Bcl-2 to regulate oxidative stress-induced apoptosis via stabilization of Bcl-2 [100]. siRNA knockdown of PKM2 resulted in decreased viability and increased apoptosis in hepatocellular carcinoma, ovarian carcinoma, and colon cancer cells [101]. On the other hand, somatostatin structural analogue TT-232 can interact with PKM2, translocate it to the nucleus, and trigger caspase-independent cell death [102].
Owing to their role as anti-apoptotic proteins, the Bcl-2 family of proteins has attracted great attention for their potential to be targeted to reverse chemoresistance. With platinum-based drugs such as cisplatin, the usage of Bcl-2 inhibitors such as ABT-737 led to the reversal of platinum resistance in ovarian cancer, sensitizing these cells to cisplatin and leading to apoptosis [145]. MicroRNAs (miRNAs) have also been used to modulate multidrug resistance in cancer cells to sensitize them to chemotherapies, such as ectopically expressed miR-181b [103] and mir-630 [104], showing the ability to overcome chemoresistance and leading to a favourable response to cisplatin treatment. Bcl-2 inhibition by ABT-199 treatment showed synergistic viability decreases when used in combination with doxorubicin in breast cancer, supporting the notion that Bcl-2 inhibition can sensitize cancer cells to chemotherapy [146].
ABT-199 (clinical trial number: NCT03181126), ABT-263 (clinical trial number: NCT03504644), and TT-232 (clinical trial number: NCT00422786) are all currently undergoing phase I or II clinical trials.
3.4. Targeting Electron Transport Chain Complexes
An interesting approach to treatment involves engineering mitocans (selective, mitochondrial-targeting anticancer agents) that are able to target electron transport chain (ETC) complex proteins [147]. Treatments designed to target cancer cell OxPhos may shut down important machinery in cancer cell metabolism, sensitizing them to aerobic glycolysis targeting agents or potentially facilitating the leakage of mitochondrial pro-apoptotic proteins.
Many mitocans targeting complexes I, II, and III have shown great efficacy and selectivity for cancer. Sorafenib (nexavar) inhibits the activity of complex II and complex III of the ETC along with ATP synthase, leading to the stabilization of serine-threonine protein kinase PINK1, which in turn induces Parkin-mediated apoptosis [105]. A derivative of tamoxifen, MitoTam, was conjugated with membrane-permeable cation triphenylphosphonium (TPP), allowing for the localization of MitoTam in the mitochondria to target complex I [106]. MitoTam suppressed Her2high tumours and avoided toxicity to healthy cells. Similarly, other mitocans like TPP-peptide [107], artemisinin-TPP [108], and green titania (G-TiO2-x) conjugated to TPP [109] have been shown to selectively kill cancer cells. Reservatrol has been shown to act on and inhibit complexes I and III and act as a pro-oxidant, leading to cell death in cancer [110,111].
Metformin has gained a great deal of interest for its anti-cancer efficacy. Metformin directly inhibits complex I of the mitochondria ETC [113]. Originally a drug used to treat diabetes, metformin has burst onto the scene as a new and exciting mitocan capable of targeting cancer very selectively [148]. Of great interest is the ability of metformin to act as an adjuvant and sensitize cancer cells to other treatments, including therapies such as ABT-737 [67,68,69,70,149,150,151]. As such, metformin provides solid evidence that targeting ETC complexes allows for an efficacious mono or adjuvant treatment for cancer.
We have previously demonstrated that pancrasitatin (PST) analogs induced apoptosis at extremely low EC50 levels in a wide variety of cancers with a much higher efficacy than standard chemotherapeutics [115]. Importantly, our mitocans were highly selective for cancer, with minimal toxicity on normal healthy cells in vitro (both 2D and 3D spheroid culture models) and in vivo. In order for these mitocans to induce apoptosis, they required a functional complex II and III as the pro-apoptotic effects of PST analog SVTH-6 were abolished with the inhibition of ETC complexes II and III. This indicates that SVTH-6 must interact with complex II or III in order to exert its anti-cancer effects. It may be possible that PST analogs interact or bind with complex II or III in order to exploit an unidentified mechanism of cancer cell metabolism or potentially affect downstream pathways of these complexes. Another PST analog we analyzed, SVTH-7, was more efficacious than gemcitabine and any other anti-cancer agent on several cancer cell lines with EC50 in the nM range.
Sorafenib (clinical trial number: NCT00105443) has completed phase III clinical trials and MitoTam is expected to begin clinical trials shortly.
3.5. Oxidative Stress Targeting
It follows that if mitochondrial OxPhos is targeted, mitochondrial stress may lead to increased ROS production, leading to oxidative stress. Increased oxidative stress can affect mitochondrial proteins, leading to further dysfunction of mitochondria, resulting in generation of additional ROS in a positive feedback loop. Targeting mitochondria or oxidative stress defense mechanisms can initiate this cycle, ultimately leading to cell death [152].
The use of pro-oxidant agents allows for increasing ROS in cancer cells to cytotoxic levels, leading to apoptosis. Cancer cells thrive under the presence of ROS for proliferative signaling, increased mutation rate to further tumourigenesis, DNA damage, and genome instability [41,42,153]. Thus, cancer cells may already have higher, but sublethal oxidative stress presenting a selective vulnerability not found in normal healthy cells. The manganese porphyrin, manganese (III) meso-tetrakis N-ethylpyridinium-2-yl porphyrin (MnTE-2-PyP5+), is able to respond to increased ROS levels in cancer cells to modulate the mitochondrial environment and enhance the effects of chemotherapeutics dexamethasone and 2-DG [116]. Rotenone, a complex I inhibitor, activates NOX2 through the PI3K/Akt/mTORC1 signaling pathway, resulting in the release of excessive ROS generation and increased cancer cell death [117]. Similarly, lonidamine induces ROS generation through complex II to promote cell death [118]. Metformin, discussed previously, also exerts its effects on cancer through the induction of oxidative stress [114]. This could presumably be because of inhibition of complex I and interruption of the ETC, resulting in the leakage of electrons to oxygen [73]. Activation of poly (ADP-ribose) polymerase (PARP) will enhance the PARP-1–ATF4–MKP-1–JNK/p38-MAPK retrograde pathway, leading to the generation of ROS followed by cell death [119]. We have also demonstrated that a novel curcumin analogue, Compound A, showed high efficacy and induced selective apoptosis through the generation of ROS in a variety of cancer cell lines alone and in combination with another pro-oxidant, piperlongumine [120].
In contrast, the inhibition of proteins implicated in cancer cells oxidative stress defense mechanisms could be targeted. In doing so, cancer cells may lose their ability to thrive under environments containing a higher concentration of ROS compared with normal healthy cells. One example is the silencing of nucleus-accumbens-1 (NAC1), which has been shown to facilitate oxidative stress resistance, sensitizing cancer cells to oxidative stress generating chemotherapeutics [121]. Pyrroline-5-carboxylate reductase 1 and 2 (PYCR1, PYCR2) downregulation or silencing could potentially sensitize cancer cells to ROS by inhibiting stress-response protein ribonucleotide reductase small subunit B (RRM2B), a protein implicated in protection from oxidative stress [122]. However, this method of oxidative stress targeting may not be ideal because of the necessity and high expression of ROS-protecting mechanisms in healthy noncancerous cells. Piperlongumine has also been implicated in targeting of glutathione S—transferase PI (GSTP1), which inhibits its oxidative stress protection mechanism, leading to cancer cell death [49,154]. Further research should look at the assessment of resulting toxicity or developing targeted downregulation of cancer cells.
3.6. Combination Therapies to Target Multiple Vulnerabilities
By using agents capable of targeting different vulnerabilities of mitochondria, effective treatment approaches using well-tolerated concentrations of chemotherapies may be developed. Targeting multiple facets of cancer cell vulnerabilities will additionally prevent the relapse of cancers thanks to targeting cancer cells in multiple environments. For example, targeting both aerobic glycolysis and OxPhos may lead cancer cells to apoptosis.
Our studies on PST analog SVTH-7 indicated that combination treatment with oxidative stress inducing piperlongumine (PL) led to a more efficacious treatment that dissipated the MMP, decreased oxygen consumption, and induced the release of many apoptogenic factors from the mitochondria [155]. Because of the hyperpolarization of the mitochondria in cancer cells, the PST analogs were able to selectively target cancer, possibly through the processing and uptake of PST analogs in positively charged molecules to be brought to only cancer cell mitochondria. In this case, combining a complex II and III targeting agent to induce oxidative stress synergized greatly with ROS, generating PL as an extremely efficacious and selective treatment for cancer.
In this review, we have additionally highlighted many studies wherein one mitochondrial-targeting agent sensitized cancer cells to another treatment [67,68,69,70,103,104,112,136,137,138,145,146,149,150,151]. The ability of mitocans to synergize well with other therapeutics is crucial because of the many conventional treatment approaches utilizing multi-drug treatments to target multiple vulnerabilities of cancer cells.
4. Natural Health Products Targeting the Mitochondria
Natural health products (NHPs) are materials isolated from various food and plant sources that have exhibited medicinal properties [156]. NHPs are complex mixtures of various bioactive components, including mitocans, that have the potential for selective and non-toxic treatment of cancer.
Natural health products can target the same mitochondrial vulnerabilities as described previously with other therapies. Chrysin, a natural flavone from plant extracts traditionally used in herbal Chinese medicine, drastically inhibited HKII binding to VDAC1, resulting in extensive apoptosis in hepatocellular carcinoma [123]. Similarly, deguelin [124] and halofuginone (clinical trial number: NCT00027677) [125], natural compounds isolated from Mundulea sericea and Dichroa febrifuga, respectively, downregulated HKII leading to apoptosis in in vitro and in vivo studies on lung and colon cancer, respectively. Grape seed extract (GSE) was shown to target ETC complex III, deplete levels of glutathione antioxidant, and result in the loss of MMP in cancer [126]. An interesting review from Sehrawat et al. highlights the usage of various NHPs in chemoprevention to cause mitochondrial dysfunction [157]
We conducted research into developing NHPs as selective anticancer therapeutics with high efficacy. These extracts contain multiple active phytochemicals that could target different biochemical pathways to induce apoptosis in cancer cells. Natural extracts of dandelion root [127], long pepper [128], hibiscus [129], lemongrass, and white tea [130] were shown to be highly effective in inducing apoptosis via the generation of excessive ROS and dissipation of MMP. These extracts were non-toxic to healthy cells and were well-tolerated in animals over a long period of time. Oral administration of these extracts in nude mice xenografted with human tumours resulted in tumour burden reduction. Indeed, the dandelion root extract has moved to clinical trials (clinical trial number: OCT1226, DRE) in Canada.
Research into targeting mitochondria via NHPs highlights the potential of these compounds to be used as safe treatment options capable of also being selective for cancer. Many NHPs may also serve as effective partners in combination treatment to allow for the targeting of many cancer vulnerabilities.
5. Conclusions
Mitochondria are central to apoptosis, to the generation of ATP via OxPhos, and as a source of ROS particularly in the event of mitochondrial dysfunction. This presents a very interesting organelle to target in cancer cells for the induction of cell death. The development of mitochondria-targeting therapies is interesting and promising, warranting further investigation of these therapies. Targeting mitochondria would be far more selective than targeting DNA replication and repair because of differences in cancer cell and healthy cell mitochondria. By using a selective treatment, approaches can avoid adverse side effects such as organ toxicity that arise from cytotoxicity to normal healthy cells. Mitochondrial-targeting drugs are able to target mitochondrial vulnerabilities in cancer cells, but not healthy cells. Indeed, several current research studies that have specific cancer vulnerabilities are able to be targeted by small molecules, resulting in mitochondrial depolarization and cell death. The usage of mitocans as an adjuvant with other therapies can serve as a deterrent to cancer drug resistance, leading to the efficient elimination of cancer cells. Oxidative stress vulnerabilities combined with mitochondrial differences in cancer cells present an excellent opportunity to kill cancer cells, using mitocans in combination with pro-oxidative modulators.
This strategy is in the early stage of development as an anticancer therapy approach, but has the potential to bring transformational change in cancer treatment. Although scientific research indicates that these compounds are generally nontoxic and well-tolerated, more work is needed to observe the long-term effects of mitocans and pro-oxidant usage on the health and physiology of vital organs. Accelerated studies pertaining to safety, efficacy, and human clinical trials are urgently needed to bring some of these compounds to general cancer therapy regimens.
Acknowledgments
The authors would all like to acknowledge Dennis Ma, Christopher Pignanelli, Pamela Ovadje, Cory Philion, Ivan Ruvinov, and Krishan Parashar for their work and support. The authors would also like to thank Caleb Vegh, Darcy Wear, and Anshika Jain for their work in manuscript review.
Author Contributions
C.N. and S.P. both were fully involved in the literature survey, review design, and preparation of the manuscript.
Funding
The authors are grateful to NSERC, the Rasch Foundation, the Lotte and John Hecht Foundation, the Couvillon family, and Windsor Mold Group for their support for this work.
Conflicts of Interest
The authors declare that they have no competing interests, financial or otherwise.
References
- 1.Canadian Cancer Statistics Advisory Committee . Canadian Cancer Statistics 2018. Canadian Cancer Society; Toronto, ON, Canada: 2018. [(accessed on 8 April 2019)]. Available online: Cancer.ca/Canadian-Cancer-Statistics-2018-EN. [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panieri E., Santoro M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha-Bhattarai T., Rangnekar V.M. Cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene. 2010;29:3873–3880. doi: 10.1038/onc.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes L.M., Hossain M., Varela-Ramirez A., Das U., Ayala-Marin Y.M., Dimmock J.R., Aguilera R.J. A novel class of piperidones exhibit potent, selective and pro-apoptotic anti-leukemia properties. Oncol. Lett. 2016;11:3842–3848. doi: 10.3892/ol.2016.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhtar M.J., Alhadlaq H.A., Kumar S., Alrokayan S.A., Ahamed M. Selective cancer-killing ability of metal-based nanoparticles: Implications for cancer therapy. Arch. Toxicol. 2015;89:1895–1907. doi: 10.1007/s00204-015-1570-1. [DOI] [PubMed] [Google Scholar]
- 11.Cheung-Ong K., Giaever G., Nislow C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Katsetos C.D., Dráber P. Tubulins as therapeutic targets in cancer: From bench to bedside. Curr. Pharm. Des. 2012;18:2778–2792. doi: 10.2174/138161212800626193. [DOI] [PubMed] [Google Scholar]
- 13.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburg O., Wind F., Negelein E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace D.C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 16.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 17.Potter M., Newport E., Morten K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016;44:1499–1505. doi: 10.1042/BST20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emmings E., Mullany S., Chang Z., Landen C.N., Linder S., Bazzaro M. Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer. Int. J. Mol. Sci. 2019;20:229. doi: 10.3390/ijms20010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyfried T.N. Cancer as a mitochondrial metabolic disease. Front. Cell Dev. Biol. 2015;3:43. doi: 10.3389/fcell.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badrinath N., Yoo S.Y. Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis. 2018;39:1419–1430. doi: 10.1093/carcin/bgy148. [DOI] [PubMed] [Google Scholar]
- 25.Fogal V., Richardson A.D., Karmali P.P., Scheffler I.E., Smith J.W., Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang M., Kim S.S., Lee J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013;45:e45. doi: 10.1038/emm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat T.A., Kumar S., Chaudhary A.K., Yadav N., Chandra D. Restoration of mitochondria function as a target for cancer therapy. Drug Discov. Today. 2015;20:635–643. doi: 10.1016/j.drudis.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 29.Heerdt B.G., Houston M.A., Augenlicht L.H. Growth properties of colonic tumor cells are a function of the intrinsic mitochondrial membrane potential. Cancer Res. 2006;66:1591–1596. doi: 10.1158/0008-5472.CAN-05-2717. [DOI] [PubMed] [Google Scholar]
- 30.Sun L., Shukair S., Naik T.J., Moazed F., Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell. Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X., Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 32.Candé C., Cohen I., Daugas E., Ravagnan L., Larochette N., Zamzami N., Kroemer G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/S0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 33.Zu X.L., Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-de-Cossio-Diaz J., Vazquez A. Limits of aerobic metabolism in cancer cells. Sci. Rep. 2017;7:13488. doi: 10.1038/s41598-017-14071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolfi S.C., Chan L.L., Qiu J., Tedeschi P.M., Bertino J.R., Hirshfield K.M., Oltvai Z.N., Vazquez A. The metabolic demands of cancer cells are coupled to their size and protein synthesis rates. Cancer Metab. 2013;1:20. doi: 10.1186/2049-3002-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain R.K., Munn L.L., Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer. 2002;2:266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 37.Viale A., Pettazzoni P., Lyssiotis C.A., Ying H., Sánchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V., et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viale A., Corti D., Draetta G.F. Tumors and mitochondrial respiration: A neglected connection. Cancer Res. 2015;75:3685–3686. doi: 10.1158/0008-5472.CAN-15-0491. [DOI] [PubMed] [Google Scholar]
- 39.Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill J.G., Piskounova E., Morrison S.J. Cancer, Oxidative Stress, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016;81:163–175. doi: 10.1101/sqb.2016.81.030791. [DOI] [PubMed] [Google Scholar]
- 41.Sosa V., Moliné T., Somoza R., Paciucci R., Kondoh H., LLeonart M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Sotgia F., Martinez-Outschoorn U.E., Lisanti M.P. Mitochondrial oxidative stress drives tumor progression and metastasis: Should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011;9:62. doi: 10.1186/1741-7015-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 44.Yang H., Villani R.M., Wang H., Simpson M.J., Roberts M.S., Tang M., Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018;37:266. doi: 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azzam E.I., de Toledo S.M., Little J.B. Stress signaling from irradiated to non-irradiated cells. Curr. Cancer Drug Targets. 2004;4:53–64. doi: 10.2174/1568009043481641. [DOI] [PubMed] [Google Scholar]
- 46.Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose Response. 2006;5:26–38. doi: 10.2203/dose-response.06-112.Pollycove. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim B., Song Y.S. Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic. Res. 2016;50:1065–1070. doi: 10.1080/10715762.2016.1210141. [DOI] [PubMed] [Google Scholar]
- 48.Afonso V., Champy R., Mitrovic D., Collin P., Lomri A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Jt. Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Aynacioglu A.S., Nacak M., Filiz A., Ekinci E., Roots I. Protective role of glutathione S-transferase P1 (GSTP1) Val105Val genotype in patients with bronchial asthma. Br. J. Clin. Pharmacol. 2004;57:213–217. doi: 10.1046/j.1365-2125.2003.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 51.Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L., et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Courtnay R., Ngo D.C., Malik N., Ververis K., Tortorella S.M., Karagiannis T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 53.Heerma van Voss M.R., Kammers K., Vesuna F., Brilliant J., Bergman Y., Tantravedi S., Wu X., Cole R.N. Global Effects of DDX3 Inhibition on Cell Cycle Regulation Identified by a Combined Phosphoproteomics and Single Cell Tracking Approach. Transl. Oncol. 2018;11:755–763. doi: 10.1016/j.tranon.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian S., Chen H., Tan W. Targeting mitochondrial respiration as a therapeutic strategy for cervical cancer. Biochem. Biophys. Res. Commun. 2018;499:1019–1024. doi: 10.1016/j.bbrc.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 55.Kuntz E.M., Baquero P., Michie A.M., Dunn K., Tardito S., Holyoake T.L., Helgason G.V., Gottlieb E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017;23:1234–1240. doi: 10.1038/nm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esner M., Graifer D., Lleonart M.E., Lyakhovich A. Targeting cancer cells through antibiotics-induced mitochondrial dysfunction requires autophagy inhibition. Cancer Lett. 2017;384:60–69. doi: 10.1016/j.canlet.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Guerra F., Arbini A.A., Moro L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta Bioenerg. 2017;1858:686–699. doi: 10.1016/j.bbabio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Lee H.C., Yin P.H., Lin J.C., Wu C.C., Chen C.Y., Wu C.W., Chi C.W., Tam T.N., Wei Y.H. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann. N. Y. Acad. Sci. 2005;1042:109–122. doi: 10.1196/annals.1338.011. [DOI] [PubMed] [Google Scholar]
- 59.Arbini A.A., Guerra F., Greco M., Marra E., Gandee L., Xiao G., Lotan Y., Gasparre G., Hsieh J.T., Moro L. Mitochondrial DNA depletion sensitizes cancer cells to PARP inhibitors by translational and post-translational repression of BRCA2. Oncogenesis. 2013;2:e82. doi: 10.1038/oncsis.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu M., Zhou Y., Shi Y., Ning L., Yang Y., Wei X., Zhang N., Hao X., Niu R. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life. 2007;59:450–457. doi: 10.1080/15216540701509955. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan S., Guha M., Avadhani N.G. Mitochondrial respiratory defects promote the Warburg effect and cancer progression. Mol. Cell. Oncol. 2015;3:e1085120. doi: 10.1080/23723556.2015.1085120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Y., Cao M., Liu J., Yang Q., Miao X., Go V.L.W., Lee P.W.N., Xiao G.G. Metabolic Regulation in Mitochondria and Drug Resistance. Adv. Exp. Med. Biol. 2017;1038:149–171. doi: 10.1007/978-981-10-6674-0_11. [DOI] [PubMed] [Google Scholar]
- 63.Anderson M., Marayati R., Moffitt R., Yeh J.J. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget. 2016;8:56081–56094. doi: 10.18632/oncotarget.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi S.Y., Collins C.C., Gout P.W., Wang Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013;230:350–355. doi: 10.1002/path.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazzio E.A., Boukli N., Rivera N., Soliman K.F. Pericellular pH homeostasis is a primary function of the Warburg effect: Inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci. 2012;103:422–432. doi: 10.1111/j.1349-7006.2012.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirschhaeuser F., Sattler U.G., Mueller-Klieser W. Lactate: A metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 67.Cicero A.F., Tartagni E., Ertek S. Metformin and its clinical use: New insights for an old drug in clinical practice. Arch. Med. Sci. 2012;8:907–917. doi: 10.5114/aoms.2012.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero I.L., McCormick A., McEwen K.A., Park S., Karrison T., Yamada S.D., Pannain S., Lengyel E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012;119:61–67. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei D.H., Wang Y., Shi H.R. Association of p53 and mitochondrial gene with chemosensitization by metformin in ovarian cancer. Oncotarget. 2017;9:2971–2976. doi: 10.18632/oncotarget.22863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bishnu A., Sakpal A., Ghosh N., Choudhury P., Chaudhury K., Ray P. Long term treatment of metformin impedes development of chemoresistance by regulating cancer stem cell differentiation through taurine generation in ovarian cancer cells. Int. J. Biochem. Cell Biol. 2019;107:116–127. doi: 10.1016/j.biocel.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Xu J., Zhang B.C., Li X.L., Xu W.H., Zhou J., Shen L., Wei Q.C. Chemosensitization and radiosensitization of a lung cancer cell line A549 induced by a composite polymer micelle. Discov. Med. 2016;22:7–17. [PubMed] [Google Scholar]
- 72.Olivas-Aguirre M., Pottosin I., Dobrovinskaya O. Mitochondria as emerging targets for therapies against T cell acute lymphoblastic leukemia. J. Leukoc. Biol. 2019;105:935–946. doi: 10.1002/JLB.5VMR0818-330RR. [DOI] [PubMed] [Google Scholar]
- 73.Arora K.K., Pedersen P.L. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J. Biol. Chem. 1988;263:17422–17428. [PubMed] [Google Scholar]
- 74.Hulleman E., Kazemier K.M., Holleman A., VanderWeele D.J., Rudin C.M., Broekhuis M.J., Evans W.E., Pieters R., Den Boer M.L. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113:2014–2021. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gandham S.K., Talekar M., Singh A., Amiji M.M. Inhibition of hexokinase-2 with targeted liposomal 3-bromopyruvate in an ovarian tumor spheroid model of aerobic glycolysis. Int. J. Nanomed. 2015;10:4405–4423. doi: 10.2147/IJN.S82818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho N., Morrison J., Silva A., Coomber B.L. The effect of 3-bromopyruvate on human colorectal cancer cells is dependent on glucose concentration but not hexokinase II expression. Biosci. Rep. 2016;36:e00299. doi: 10.1042/BSR20150267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fichera G.A., Fichera M., Milone G. Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anti-Cancer Drugs. 2016;27:609–619. doi: 10.1097/CAD.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li W., Zheng M., Wu S., Gao S., Yang M., Li Z., Min Q., Sun W., Chen L., Xiang G., et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017;36:58. doi: 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C., Darnell J.E., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 80.Sun X., Zhang L. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci. Rep. 2017;37:BSR20160404. doi: 10.1042/BSR20160404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tu Y., Gao X., Li G., Fu H., Cui D., Liu H., Jin W., Zhang Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046–6055. doi: 10.1158/0008-5472.CAN-13-0358. [DOI] [PubMed] [Google Scholar]
- 82.Liu H., Liu N., Cheng Y., Jin W., Zhang P., Wang X., Yang H., Xu X., Wang Z., Tu Y., et al. Hexokinase 2 (HK2), the tumor promoter in glioma, is downregulated by miR-218/Bmi1 pathway. PLoS ONE. 2017;12:e0189353. doi: 10.1371/journal.pone.0189353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shoshan-Barmatz V., Mizrachi D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012;2:164. doi: 10.3389/fonc.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prezma T., Shteinfer A., Admoni L., Raviv Z., Sela I., Levi I., Shoshan-Barmatz V. VDAC1-based peptides: Novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia. Cell Death Dis. 2013;4:e809. doi: 10.1038/cddis.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shoshan-Barmatz V., Krelin Y., Chen Q. VDAC1 as a Player in Mitochondria-Mediated Apoptosis and Target for Modulating Apoptosis. Curr. Med. Chem. 2017;24:4435–4446. doi: 10.2174/0929867324666170616105200. [DOI] [PubMed] [Google Scholar]
- 86.Shteinfer-Kuzmine A., Amsalem Z., Arif T., Zooravlov A., Shoshan-Barmatz V. Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. Mol. Oncol. 2018;12:1077–1103. doi: 10.1002/1878-0261.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arif T., Vasilkovsky L., Refaely Y., Konson A., Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Mol. Ther. Nucleic Acid. 2014;3:e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Head S.A., Shi W., Zhao L., Gorshkov K., Pasunooti K., Chen Y., Deng Z., Li R.J., Shim J.S., Tan W., et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc. Natl. Acad. Sci. USA. 2015;112:e7276–e7285. doi: 10.1073/pnas.1512867112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jan C.I., Tsai M.H., Chiu C.F., Huang Y.P., Liu C.J., Chang N.W. Fenofibrate Suppresses Oral Tumorigenesis via Reprogramming Metabolic Processes: Potential Drug Repurposing for Oral Cancer. Int. J. Biol. Sci. 2016;12:786–798. doi: 10.7150/ijbs.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kadavakollu S., Stailey C., Kunapareddy C.S., White S. Clotrimazole as a Cancer Drug: A Short Review. Med. Chem. 2014;4:722–724. doi: 10.4172/2161-0444.1000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belzacq A.S., El Hamel C., Vieira H.L., Cohen I., Haouzi D., Métivier D., Marchetti P., Brenner C., Kroemer G. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene. 2001;20:7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- 92.Advani P.P., Paulus A., Masood A., Sher T., Chanan-Khan A. Pharmacokinetic evaluation of oblimersen sodium for the treatment of chronic lymphocytic leukemia. Expert Opin. Drug Metab. Toxicol. 2011;7:765–774. doi: 10.1517/17425255.2011.579105. [DOI] [PubMed] [Google Scholar]
- 93.Ebrahim A.S., Kandouz M., Liddane A., Sabbagh H., Hou Y., Li C., Al-Katib A. PNT2258, a novel deoxyribonucleic acid inhibitor, induces cell cycle arrest and apoptosis via a distinct mechanism of action: A new class of drug for non-Hodgkin’s lymphoma. Oncotarget. 2016;7:42374–42384. doi: 10.18632/oncotarget.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dürig J., Dührsen U., Klein-Hitpass L., Worm J., Hansen J.B., Ørum H., Wissenbach M. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia. 2011;25:638–647. doi: 10.1038/leu.2010.322. [DOI] [PubMed] [Google Scholar]
- 95.Anderson M.A., Huang D., Roberts A. Targeting BCL2 for the Treatment of Lymphoid Malignancies. Semin. Hematol. 2014;51:219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 96.Vogler M., Dinsdale D., Sun X.M., Young K.W., Butterworth M., Nicotera P., Dyer M.J., Cohen G.M. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15:820–830. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 97.Tse C., Shoemaker A.R., Adickes J., Anderson M.G., Chen J., Jin S., Johnson E.F., Marsh K.C., Mitten M.J., Nimmer P., et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 98.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y., Xie M., Song T., Sheng H., Yu X., Zhang Z. A novel BH3 mimetic efficiently induces apoptosis in melanoma cells through direct binding to anti-apoptotic Bcl-2 family proteins, including phosphorylated Mcl-1. Pigment Cell Melanoma Res. 2015;28:161–170. doi: 10.1111/pcmr.12325. [DOI] [PubMed] [Google Scholar]
- 100.Liang J., Cao R., Wang X., Zhang Y., Wang P., Gao H., Li C., Yang F., Zeng R., Wei P. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2016;27:329–351. doi: 10.1038/cr.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldberg M.S., Sharp P.A. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J. Exp. Med. 2012;209:217–224. doi: 10.1084/jem.20111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steták A., Veress R., Ovádi J., Csermely P., Kéri G., Ullrich A. Nuclear translocation of the tumor marker pyruvate kinase M2 induces programmed cell death. Cancer Res. 2007;67:1602–1608. doi: 10.1158/0008-5472.CAN-06-2870. [DOI] [PubMed] [Google Scholar]
- 103.Liu H.N., Qie P., Yang G., Song Y.B. miR-181b inhibits chemoresistance in cisplatin-resistant H446 small cell lung cancer cells by targeting Bcl-2. Arch. Med. Sci. 2018;14:745–751. doi: 10.5114/aoms.2018.73131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen M.J., Wu D.W., Wang G.C., Wang Y.C., Chen C.Y., Lee H. MicroRNA-630 may confer favorable cisplatin-based chemotherapy and clinical outcomes in non-small cell lung cancer by targeting Bcl-2. Oncotarget. 2018;9:13758–13767. doi: 10.18632/oncotarget.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang C., Liu Z., Bunker E., Ramíretz A., Lee S., Peng Y., Tan A.-C., Eckhardt S.G., Chapnick D.A., Liu X. Sorafenib targets the mitochondrial electron transport chain complexes and ATP synthase to activate the PINK1-Parkin pathway and modulate cellular drug response. J. Biol. Chem. 2017;292:15105–15120. doi: 10.1074/jbc.M117.783175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohlenova K., Sachaphibulkij K., Stursa J., Bezawork-Geleta A., Blecha J., Endaya B., Werner L., Cerny J., Zobalova R., Goodwin J. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2high Breast Cancer. Antioxid. Redox Signal. 2017;26:84–103. doi: 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang H., Feng Z., Wang Y., Zhou R., Yang Z., Xu B. Integrating Enzymatic Self-Assembly and Mitochondria Targeting for Selectively Killing Cancer Cells without Acquired Drug Resistance. J. Am. Chem. Soc. 2016;138:16046–16055. doi: 10.1021/jacs.6b09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang C.J., Wang J., Zhang J., Lee Y.M., Feng G., Lim T.K., Shen H.M., Lin Q., Liu B. Mechanism-Guided Design and Synthesis of a Mitochondria-Targeting Artemisinin Analogue with Enhanced Anticancer Activity. Angew. Chem. Int. Ed. Engl. 2016;55:13770–13774. doi: 10.1002/anie.201607303. [DOI] [PubMed] [Google Scholar]
- 109.Mou J., Lin T., Huang F., Shi J., Chen H. A New Green Titania with Enhanced NIR Absorption for Mitochondria-Targeted Cancer Therapy. Theranostics. 2017;7:1531–1542. doi: 10.7150/thno.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zini R., Morin C., Bertelli A., Bertelli A.A., Tillement J.P. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp. Clin. Res. 1999;25:87–97. [PubMed] [Google Scholar]
- 111.Sassi N., Mattarei A., Azzolini M., Bernardi P., Szabo’ I., Paradisi C., Zoratti M., Biasutto L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr. Pharm. Des. 2014;20:172–179. doi: 10.2174/13816128113199990034. [DOI] [PubMed] [Google Scholar]
- 112.DeWaal D., Nogueira V., Terry A.R., Patra K.C., Jeon S.M., Guzman G., Au J., Long C.P., Antoniewicz M.R., Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. Correction in 2018, 9, 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andrzejewski S., Gravel S.P., Pollak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Queiroz E.A., Puukila S., Eichler R., Sampaio S.C., Forsyth H.L., Letes S.J., Barbosa A.M., Dekker R.F.H., Fortes Z.B., Khaper N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS ONE. 2014;9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma D., Pignanelli C., Tarade D., Gilbert T., Noel M., Mansour F., Adams S., Dowhayko A., Stokes K., Vshyvenko S., et al. Cancer Cell Mitochondria Targeting by Pancratistatin Analogs is Dependent on Functional Complex II and III. Sci. Rep. 2017;7:42957. doi: 10.1038/srep42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaramillo M.C., Briehl M.M., Batinic-Haberle I., Tome M.E. Manganese (III) meso-tetrakis N-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radic. Biol. Med. 2015;83:89–100. doi: 10.1016/j.freeradbiomed.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu W., Tian H., Yue W., Li L., Li S., Gao C., Si L., Qi L., Lu M., Hao B., et al. Rotenone induces apoptosis in human lung cancer cells by regulating autophagic flux. IUBMB Life. 2016;68:388–393. doi: 10.1002/iub.1493. [DOI] [PubMed] [Google Scholar]
- 118.Nath K., Guo L., Nancolas B., Nelson D.S., Shestov A.A., Lee S.-C., Roman J., Zhou R., Leeper D.B., Halestrap A.P., et al. Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta. 2016;1866:151–162. doi: 10.1016/j.bbcan.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hocsak E., Szabo V., Kalman N., Antus C., Cseh A., Sumegi K., Eros K., Hegedus Z., Gallyas F., Jr., Sumegi B., et al. PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic. Biol. Med. 2017;108:770–784. doi: 10.1016/j.freeradbiomed.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 120.Pignanelli C., Ma D., Noel M., Ropat J., Mansour F., Curran C., Pupulin S., Larocque K., Wu J., Liang G., et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017;7:1105. doi: 10.1038/s41598-017-01230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ren Y.J., Wang X.H., Ji C., Guan Y.-D., Lu X.-J., Liu X.-R., Zhang H.-H., Guo L.-C., Xu Q.-H., Zhu W.-D., et al. Silencing of NAC1 Expression Induces Cancer Cells Oxidative Stress in Hypoxia and Potentiates the Therapeutic Activity of Elesclomol. Front. Pharm. 2017;8:804. doi: 10.3389/fphar.2017.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuo M.L., Lee M.B., Tang M., Den Besten W., Hu S., Sweredoski M.J., Hess S., Chou C.M., Changou C.A., Su M., et al. PYCR1 and PYCR2 Interact and Collaborate with RRM2B to Protect Cells from Overt Oxidative Stress. Sci. Rep. 2016;6:18846. doi: 10.1038/srep18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu D., Jin J., Yu H., Zhao Z., Mal D., Zhang C., Jiang H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017;36:44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li W., Gao F., Ma X., Wang R., Dong X., Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating Hexokinases II-mediated glycolysis. Oncotarget. 2017;8:32586–32599. doi: 10.18632/oncotarget.15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen G.-Q., Tang C.-F., Shi X.-K., Lin C.-Y., Fatima S., Pan X.-H., Yang D.-J., Zhang G., Lu A.-P., Lin S.-H., et al. Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism. Oncotarget. 2015;6:24148–24162. doi: 10.18632/oncotarget.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shrotriya S., Deep G., Lopert P., Patel M., Agarwal R., Agarwal C. Grape seed extract targets mitochondrial electron transport chain complex III and induces oxidative and metabolic stress leading to cytoprotective autophagy and apoptotic death in human head and neck cancer cells. Mol. Carcinog. 2014;54:1734–1747. doi: 10.1002/mc.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ovadje P., Hamm C., Pandey S. Efficient induction of extrinsic cell death by dandelion root extract in human chronic myelomonocytic leukemia (CMML) cells. PLoS ONE. 2012;7:e30604. doi: 10.1371/journal.pone.0030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ovadje P., Ma D., Tremblay P., Roma A., Steckle M., Guerrero J.-A., Arnason J.T., Pandey S. Evaluation of the efficacy & biochemical mechanism of cell death induction by Piper longum extract selectively in in-vitro and in-vivo models of human cancer cells. PLoS ONE. 2014;9:e113250. doi: 10.1371/journal.pone.0113250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nguyen C., Baskaran K., Pupulin A., Ruvinov I., Zaitoon O., Gretwal S., Scaria B., Mehaidli A., Vegh C., Pandey S. Hibiscus flower extract selectively induces apoptosis in breast cancer cells and positively interacts with common chemotherapeutics. BMC Complement. Altern. Med. 2019;19:98. doi: 10.1186/s12906-019-2505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Philion C., Ma D., Ruvinov I., Mansour F., Pignanelli C., Noel M., Saleem A., Arnason J., Rodrigues M., Singh I., et al. Cymbopogon citratus and Camellia sinensis extracts selectively induce apoptosis in cancer cells and reduce growth of lymphoma xenografts in vivo. Oncotarget. 2017;8:110756–110773. doi: 10.18632/oncotarget.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson J.E. Hexokinases. Rev. Physiol. Biochem. Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 132.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 133.Vyssokikh M.Y., Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim. Pol. 2003;50:389–404. [PubMed] [Google Scholar]
- 134.Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2014;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li M., Jin R., Wang W., Zhang T., Sang J., Li N., Han Q., Zhao W., Li C., Liu Z. STAT3 regulates glycolysis via targeting hexokinase 2 in hepatocellular carcinoma cells. Oncotarget. 2017;8:24777–24784. doi: 10.18632/oncotarget.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gu J.J., Singh A., Xue K., Mavis C., Barth M., Yanamadala V., Lenz P., Grau M., Lenz G., Czuczman M.S., et al. Up-regulation of hexokinase II contributes to rituximab-chemotherapy resistance and is a clinically relevant target for therapeutic development. Oncotarget. 2017;9:4020–4033. doi: 10.18632/oncotarget.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y., Murray-Stewart T., Casero R.A., Jr., Kagiampakis I., Jrin L., Zhang J., Wang H., Che Q., Tong H., Ke J., et al. Targeting hexokinase 2 inhibition promotes radiosensitization in HPV16 E7-induced cervical cancer and suppresses tumor growth. Int. J. Oncol. 2017;50:2011–2023. doi: 10.3892/ijo.2017.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vartanian A., Agnihotri S., Wilson M.R., Burrell K.E., Tonge P.D., Alamsahebpour A., Jalali S., Taccone M.S., Mansouri S., Golbourn B., et al. Targeting hexokinase 2 enhances response to radio-chemotherapy in glioblastoma. Oncotarget. 2016;7:69518–69535. doi: 10.18632/oncotarget.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A., Arif T. Voltage-Dependent Anion Channel 1 As an Emerging Drug Target for Novel Anti-Cancer Therapeutics. Front. Oncol. 2017;7:154. doi: 10.3389/fonc.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magrì A., Reina S., De Pinto V. VDAC1 as Pharmacological Target in Cancer and Neurodegeneration: Focus on Its Role in Apoptosis. Front. Chem. 2018;6:108. doi: 10.3389/fchem.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galluzzi L., Kepp O., Vander Heiden M.G., Kroemer G. Metabolic targets for cancer therapy. Nat. Rev. Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 142.Ben Sahra I., Laurent K., Giuliano S., Larbret F., Ponzio G., Gounon P., Le Marchand-Brustel Y., Giorgetti-Peraldi S., Cormont M., Bertolotto C., et al. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 143.Ruefli-Brasse A., Reed J.C. Therapeutics targeting Bcl-2 in hematological malignancies. Biochem. J. 2017;474:3643–3657. doi: 10.1042/BCJ20170080. [DOI] [PubMed] [Google Scholar]
- 144.Adams C.M., Clark-Garvey S., Porcu P., Eischen C.M. Targeting the Bcl-2 Family in B Cell Lymphoma. Front. Oncol. 2019;8:636. doi: 10.3389/fonc.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Y., Li S., Sun Y., Zhang D., Zhao Z., Liu L. Reversing platinum resistance in ovarian cancer multicellular spheroids by targeting Bcl-2. OncoTargets Ther. 2019;12:897–906. doi: 10.2147/OTT.S187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Inao T., Iida Y., Moritani T., Okimoto T., Talnino R., Kotani H., Harada M. Bcl-2 inhibition sensitizes triple-negative human breast cancer cells to doxorubicin. Oncotarget. 2018;9:25545–25556. doi: 10.18632/oncotarget.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vatrinet R., Iommarini L., Kurelac I., De Luise M., Gasparre G., Porcelli A.M. Targeting respiratory complex I to prevent the Warburg effect. Int. J. Biochem. Cell Biol. 2015;63:41–45. doi: 10.1016/j.biocel.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 148.Pierotti M.A., Berrino F., Gariboldi M., Melani C., Mogavero A., Negri T., Pasanisi P., Pilotti S. Targeting metabolism for cancer treatment and prevention: Metformin, an old drug with multi-faceted effects. Oncogene. 2013;32:1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 149.Park S.H., Lee D.H., Kim J.L., Kim B.R., Na Y.J., Jo M.J., Jeong Y.A., Lee S.-Y., Lee S.I., Lee Y.Y., et al. Metformin enhances TRAIL-induced apoptosis by Mcl-1 degradation via Mule in colorectal cancer cells. Oncotarget. 2016;7:59503–59518. doi: 10.18632/oncotarget.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sesen J., Dahan P., Scotland S.J., Saland E., Dang V.-T., Lemarié A., Tyler B.M., Brem H., Toulas C., Moyal E.C.-J., et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS ONE. 2015;10:e0123721. doi: 10.1371/journal.pone.0123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Velez J., Pan R., Lee J.T., Enciso L., Suarez M., Duque J.E., Jaramillo D., Lopez C., Morales L., Bornmann W., et al. Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget. 2016;7:51435–51449. doi: 10.18632/oncotarget.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis. Dis. 2016;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Visconti R., Grieco D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Dev. 2009;12:240–245. [PubMed] [Google Scholar]
- 154.Raj L., Ide T., Gurkar A.U., Foley M., Schenone M., Li X., Tolliday N.J., Golub T.R., Carr S.A., Shamji A.F., et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2018;561:231–234. doi: 10.1038/s41586-018-0284-y. Retracted in 2018, 561, 420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Ma D., Gilbert T., Pignanelli C., Tarade D., Noel M., Mansour F., Gupta M., Ma S., Ropat J., Curran C., et al. Exploiting mitochondrial and oxidative vulnerabilities with a synthetic analog of pancratistatin in combination with piperlongumine for cancer therapy. FASEB J. 2018;32:417–430. doi: 10.1096/fj.201700275R. [DOI] [PubMed] [Google Scholar]
- 156.Trottier G., Boström P.J., Lawrentschuk N., Fleshner N.E. Nutraceuticals and prostate cancer prevention: A current review. Nat. Rev. Urol. 2010;7:21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 157.Sehrawat A., Roy R., Pore S.K., Hahm E.R., Samanta S.K., Singh K.B., Kim S.H., Singh K., Singh S.V. Mitochondrial dysfunction in cancer chemoprevention by phytochemicals from dietary and medicinal plants. Semin. Cancer Biol. 2016;47:147–153. doi: 10.1016/j.semcancer.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]