Abstract
Breast cancer (BC) is the most frequent oncologic cause of death among women and the improvement of its treatments is compelling. Platinum salts (e.g., carboplatin, cisplatin, and oxaliplatin) are old drugs still used to treat BC, especially the triple-negative subgroup. However, only a subset of patients see a concrete benefit from these drugs, raising the question of how to select them properly. Therefore, predictive biomarkers for platinum salts in BC still represent an unmet clinical need. Here, we review clinical and preclinical works in order to summarize the current evidence about predictive or putative platinum salt biomarkers in BC. The association between BRCA1/2 gene mutations and platinum sensitivity has been largely described. However, beyond the mutations of these two genes, several other proteins belonging to the homologous recombination pathways have been linked to platinum response, defining the concept of BRCAness. Several works, here reviewed, have tried to capture BRCAness through different strategies, such as homologous recombination deficiency (HRD) score and genetic signatures. Moreover, p53 and its family members (p63 and p73) might also be used as predictors of platinum response. Finally, we describe the mounting preclinical evidence regarding base excision repair deficiency as a possible new platinum biomarker.
Keywords: platinum, breast cancer, BRCA, BRCAness, homologous recombination repair, base excision repair
1. Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women and the first cause of cancer death in women [1]. Despite recent therapeutic advances, metastatic BC (mBC) remains a lethal disease, and there is fervent interest in the discovery of new drugs that may change the natural history of the disease [2]. Currently, BC is classified into different subtypes according to the expression of estrogen and progesterone receptors and the overexpression of HER2 protein. This classification has a practical implication, since each cancer subtype requires different medical treatments [2]. Of note, triple-negative BC (TNBC) is characterized by negative hormonal receptor status and negative HER2 status [3].
Platinum salts (e.g., carboplatin (CBDCA) and cisplatin (CDDP)) are long-standing compounds used in several cancer types [4]. Moreover, these agents are part of the therapeutic armamentarium for BC, especially TNBC [5,6,7]. Recently, the discovery that a defective DNA repair system (a quite common feature of cancer cells) can increase the efficacy of DNA damaging agents, has renewed interest in platinum salts [8]. The cytotoxicity of CDDP and other platinum agents is exerted through different cellular mechanisms [9,10,11,12]. Platinum salts enter into the cells through the High Affinity Copper Uptake Protein 1 (SLC31A1), whereas their efflux is guaranteed by both the ABCC2 and the Copper-Transporting P-type Adenosine Triphosphate (ATP7B) [13,14,15]. Interestingly, it has been hypothesized that platinum and copper could compete for their influx and efflux, causing a mutual interference of their transport [16,17]. Once inside the cells, platinum salts undergo aquation becoming highly reactive to many cellular targets, especially DNA [18]. At this level, platinum molecules are vulnerable to inactivation by antioxidant molecules like glutathione and metallothionein [19,20]. The aquated platinum salts react with DNA, generating monoadducts, inter- (ICL) and intra-DNA strand cross-links, and are able to distort the double helix of DNA causing single-strand breaks (SSBs) and double-strand breaks (DSBs) [11]. The principal effect of this structural distortion is the blockage of both DNA replication and DNA transcription, that if permanent, causes severe cell cycle arrest and induces cell apoptosis or necrosis [10]. In particular, triggering cell death is concentration dependent. Indeed, high CDDP doses cause necrosis, whereas chronic low doses induce apoptosis [21]. Notably, Becker et al. [22] recently demonstrated that CDDP and other platinum-containing regimens were also able to directly interfere with mRNA translation, interacting with the nascent transcript and generating similar RNA adducts. Generally, DNA bulky lesions generated by platinum drugs are efficiently repaired by nucleotide excision repair and homologous recombination (HR) pathways [18]. Moreover, several studies have demonstrated that base excision repair (BER) also plays a role in mediating cisplatin cytotoxicity [23,24,25,26,27,28,29,30,31,32]. Though not being directly active on bulky DNA lesions, different studies have highlighted a role for BER in repairing the platinum-induced DNA ICLs and in modulating other indirect effects generated by the CDDP exposure.
It is, therefore, clear that a defect in one of these DNA repair pathways, particularly HR and BER, could represent a useful predictive biomarker for platinum salt sensitivity. Patient selection is crucial when using platinum salts, and the identification of predictive biomarkers still represents an unmet clinical need. Therefore, the main purpose of this review is to summarize the currently available evidence about predictive biomarkers of platinum salt sensitivity in BC, in order to integrate them into future clinical trials, and hopefully, in clinical practice.
This review will address both translational data regarding HR deficiency (HRD) as a marker of platinum salt sensitivity and new emerging preclinical biomarkers of platinum salt sensitivity.
2. HRD as a Biomarker of Platinum Sensitivity
2.1. The HR Pathway
Once a DSB occurs, cells activate two different DNA repair mechanisms, depending on the phase of the cell cycle [33]: the HR system or the non-homologous end joining (NHEJ) system. Homologous recombination, which is mostly active during S- and G2-phases of the cell cycle [34], repairs the DSBs by using a homologous DNA molecule, therefore acting as an error-free repair mechanism [35]. In contrast, NHEJ, which is active through the whole cell cycle [34], ligates the ends of a DSB in an error-prone way, increasing DNA mutagenicity and genomic instability [36].
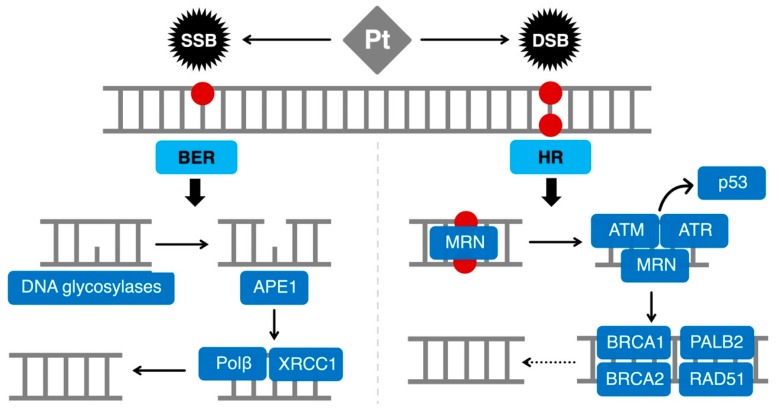
The HR pathway consists of a high number of proteins (Figure 1) [35,37,38,39]. In a simplified view of the process, the first step involves the MRN complex (MRE11-RAD50-NBS1) that detects the DSB and recruits ATM and ATR which, in turn, activate the cell cycle checkpoints and induce cell cycle arrest through p53 [40]. Subsequently, ATM phosphorylates histone H2AX causing the recruitment of 53BP1 and the Breast Cancer Type 1 Susceptibility Protein (BRCA1) to the damaged area. At the beginning of DSB repair, BRCA1 has a crucial role. Indeed, BRCA1 controls the DSB resection and helps the transition from DSB resection to PALB2/Breast Cancer Type 2 Susceptibility Protein (BRCA2)-mediated RAD51 loading [41]. The protein RAD51 eventually forms a filament of nucleic acid and proteins allowing the alignment of broken DNA with the normal one and the subsequent synthesis of new DNA to fill the genomic gap [42].
Figure 1.
Representation of the main DNA repair pathways involved in platinum salts-induced DNA damage. SSBs are mainly repaired by the BER pathway, which needs proficient DNA glycosylases that recognize and cleave the damaged base. Then, APE1 removes the abasic site that can be sealed by Polβ and ligases. In the case of DSBs, HR plays a crucial role. The MRN complex recognizes the DSB and recruits ATM and ATR, which can eventually induce cell cycle arrest through p53. Subsequently, ATM can cause the recruitment of BRCA1, BRCA2, and PALB2 which determine the RAD51 loading and the subsequent DNA synthesis. BER: base excision repair; DBS: double-strand break; HR: homologous recombination repair; Pt: platinum salts; SSB: single-strand break.
In the case of HR deficiency, the error-prone NHEJ system becomes the central axis of DSB repair, creating genomic instability and virtual cell death [33]. As platinum salts induce DSBs, cells would need a proficient HR to survive. Therefore, cancer cells bearing an HR deficiency might be sensitive to platinum salts.
2.2. BRCA1/2 Mutations
The BRCA1/2 proteins represent an essential component of HR and germline mutations of their genes are detected in about 5% of unselected BC and in up to 10–20% of TNBC [43,44]. The BRCA1/2 proteins, along with others (e.g., PALB2, RAD51, and CHEK2), play a critical role in ensuring genomic stability and efficient DNA repair through the HR machinery [45,46]. On these bases, platinum compounds have been tested as a therapeutic option for BRCA-mutated tumors and TNBC, leading to promising results observed among BRCA1 mutation carriers, treated with CDDP, in both neoadjuvant and metastatic setting [47,48] (Table 1).
Table 1.
Predictive biomarkers of platinum salts efficacy in BC.
| Reference | Setting | Biomarker | Treatments | Outcomes/Observations |
|---|---|---|---|---|
| BRCA1/2 Mutations | ||||
| TNT [49] (phase III) |
Stage IV TNBC | BRCA1/2m | CBDCA versus Docetaxel | Increased ORR and PFS with Carboplatin versus Docetaxel |
| TBCRC009 [50] (phase II) |
Stage IV TNBC | BRCA1/2m | CBDCA or CDDP | Increased ORR in BRCA1/2m versus BRCA1/2 wt |
| BROCADE [51] (phase II) |
Stage IV BC with BRCA1/2m |
BRCA1/2m | CP versus CPV versus TV | Increased ORR and PFS with CP and CPV versus TV |
| GeparSixto [53] (phase II) |
Stage II–III TNBC | BRCA1/2m | P+A+Bev ± CBDCA | No additive effect on pCR for carboplatin |
| BrighTNess [57] (phase III) |
Stage II–III TNBC | BRCA1/2m | CP ± V then AC | No additive effect on pCR for carboplatin |
| BRCAness | ||||
| Telli et al. [58] (pooled analysis) |
Localized TNBC | HRD score > 41 | Various platinum containing regimens | Increased pCR in HRD versus HRD < 41 |
| Kaklamani et al. [59] (phase II) |
Stage I–III TNBC | HR status (HRD score + BRCA1/2m) | CBDCA + E | HRD status and the HRD score predict pCR |
| GeparSixto [53] (phase II) |
Stage II–III TNBC | HRD status * | P+A+Bev ± CBDCA | HRD positive status associated with increased pCR versus HRD negative Adding carboplatin increased pCR in HRD positive but not in HRD negative tumors |
| SWOG9313 [60] (phase III) |
Stage I–II TNBC | HRD status * | Concomitant versus sequential AC | HRD positive status associated with DFS. No significative trend observed with OS |
| Gene Signatures and p53 Family | ||||
| Lehmann et al. [61] (in vitro analysis) |
TNBC cell lines | BL | Platinum salts | Increased sensitivity |
| TNT [49] (phase III) |
Stage IV TNBC | BL, core-basal | CBDCA versus Docetaxel | Reduced ORR and PFS in not-BL and not-core basal |
| Silver et al. [62] | Stage II–III TNBC | p53 NSM | Cisplatin | Increased pCR versus not p53 NSM |
| Silver et al. [62] | Stage II–III TNBC | ∆Np63/TAp73 ratio > 2 | Cisplatin | Numerical increased pCR vs. ∆Np63/TAp73 ratio < 2 |
| BER | ||||
| Kothandapani et al. [26] (in vitro analysis) |
MDA-MB-231 TNBC cell line | PolB | CDDP | Upon KO, cells are resistant to CDDP treatment |
| Kothandapani et al. [26] (in vitro analysis) |
MDA-MB-231 TNBC cell line | UNG | CDDP | Upon KO, cells are resistant to CDDP treatment |
| Kothandapani et al. [26] (in vitro analysis) |
MDA-MB-231 TNBC cell line | APE1 | CDDP | Upon MX, APE1 inhibitor, cells are resistant to CDDP treatment |
A: non-pegylated liposomal doxorubicin; AC: doxorubicin + cyclophosphamide; APE1: apurinic/apyrimidinic endonuclease 1; BC: breast cancer; Bev: bevacizumab; BL: basal-like gene signature; BRCA1/2m: BRCA1/2 mutation; CBDCA: carboplatin; CDDP: cisplatin; CP: carboplatin + paclitaxel; CPV: carboplatin + paclitaxel + veliparib; DOR: duration of the response; E: eribuline; HRD: homologous recombination deficiency; KO: knock-out; MX: Methoxyamine; NSM: non-sense or frameshift mutation; ORR: objective response rate; P: paclitaxel; PFS: progression-free survival; PolB polymerase beta; TNBC: triple-negative breast cancer; TV: temozolomide + veliparib; V: veliparib; wt: wild type. * HRD status was defined positive as either a deleterious tumor BRCA1/2 (tBRCA) mutation or a pre-defined HRD score ≥ 42. HRD status was defined negative as either an absence of deleterious tumor BRCA1/2 (tBRCA) mutation or a pre-defined HRD score < 42.
The first randomized phase III study, investigating the role of platinum salts in unselected TNBC was the Triple-Negative Breast Cancer Trial (TNT) [49]. Patients with metastatic TNBC or carrying a BRCA mutation were equally randomized to receive six to eight cycles of first-line carboplatin or docetaxel. In the overall population, no difference was observed among the two arms in terms of objective response rate (ORR) (ORR: 31.4% for carboplatin versus 34.0% for docetaxel; absolute difference −2.6%, 95% confidence interval (CI) −12.1 to 6.9; p = 0.66). Nevertheless, when looking at the pre-specified subgroup analysis according to BRCA mutational status, the use of carboplatin in the BRCA-mutated cohort led to a doubling in ORR compared to docetaxel (ORR: 68% for carboplatin versus 33.3% for docetaxel; absolute difference 34.7%; p = 0.03), with a significant heterogeneity of treatment effect for BRCA-mutated patients (interaction test: p = 0.01). Furthermore, longer progression-free survival (PFS) was detected for BRCA-mutated patients (median PFS: 6.8 months versus 4.4 months; interaction p = 0.002), even if no advantage was observed in terms of overall survival (OS) [49]. Consistently, in the phase II, non-randomized TBCRC009 trial [50], among 86 metastatic TNBC patients treated with cisplatin or carboplatin as first- or second-line therapy, BRCA-mutated patients showed higher ORR compared to those without BRCA mutations (ORR: 54.5% versus 19.7%; p = 0.022). Taken together, these findings confirm the biological heterogeneity of TNBC and its differential sensitivity to platinum salts, that seems to be much greater for BRCA-mutated patients. A further contribution came from the phase 2 study BROCADE, in which 290 patients, having metastatic breast cancer carrying a BRCA1/2 mutation were randomized to receive the combination of veliparib, a poly(ADP-ribose) polymerase (PARP) inhibitor, with carboplatin plus paclitaxel versus carboplatin plus paclitaxel and versus temozolomide plus veliparib [51]. Although no difference was found in terms of PFS and OS with the addition of veliparib to carboplatin plus paclitaxel, it is noteworthy to consider the clinically relevant performance of the carboplatin plus paclitaxel arm (PFS: 12.3 months; OS: 25.9 months), that may represent a reasonable treatment for these patients, as it will be further evaluated in the phase III BROCADE 3 trial (NCT02163694).
Dealing with the neoadjuvant setting, the role of platinum salts is still controversial. A recent meta-analysis, considering data from nine randomized clinical trials, confirmed an absolute 15% increased pathologic complete response (pCR) rate, when adopting platinum-based regimens in TNBC (pCR rate from 37.0% to 52.1%; OR 1.96, 95% CI 1.46–2.62; p < 0.0001), even though high heterogeneity was detected among the included studies. Nevertheless, no statistical benefit was observed on both event-free survival (EFS) and OS within the same pooled analysis [6]. This discrepancy represents one of the most-debated arguments when discussing the implementation of platinum salts in the neoadjuvant treatment of TNBC, together with toxicity issues and a missing standard combination regimen. However, it is important to consider that none of these studies were designed to detect any impact on long-term outcomes. Hence, the power of the studies may not be adequate for these speculations. The two most representative randomized phase II studies conducted in this setting are the GeparSixto and the CALGB 40603 trials [52,53,54]. Both studies confirmed a pCR benefit with the addition of platinum agents to a neoadjuvant chemotherapy with taxanes and anthracyclines [52,53,54]. Nevertheless, the GeparSixto is the only study detecting a significant increase in disease-free survival (DFS) in this setting (DFS at 3 years: 86.1% versus 75%; HR: 0.56, 95% CI 0.34–0.93; p = 0.0244) [53], while no significant EFS difference was observed in the CALGB 40603 trial (EFS at 3 years: 76.5% versus 71.6%; HR: 0.84, 95% CI 0.58–1.22, p = 0.36) [55]. When looking at the BRCA-mutated subgroup of the GeparSixto trial (representing only the 17.4% of the overall population), high pCR rates were observed irrespectively of carboplatin use (pCR rates: 66.7% for non-carboplatin arm versus 65.4% for carboplatin arm; HR: 0.94, 95% CI 0.29–3.05; p = 0.92), confirming high chemo-sensitivity of BRCA1/2 carriers. However, no additive effect was observed for carboplatin (interaction test: p = 0.58) in this subgroup [56]. Surprisingly, the BRCA wild-type cohort benefited the most from the addition of carboplatin, in terms of pCR (pCR rates: 36.4% for non-carboplatin arm versus 55% for carboplatin arm; HR: 2.14, 95% CI 1.28–3.58; p = 0.004) [55]. This observation may be justified by the presence of BRCA-like phenotypes among sporadic TNBC, but also by the use of an intensified chemotherapy regimen. Additionally, the BrighTNess trial, a randomized clinical trial evaluating the role of neoadjuvant carboplatin alone or in combination with veliparib for TNBC patients, reported similar pCR data according to BRCA-mutational status [57]. However, these post-hoc subgroup analyses were performed among a very limited number of BRCA-mutated patients (50 patients in the GeparSixto trial and 46 patients in the BrighTNess study), and their relevance remains exploratory.
In conclusion, the neoadjuvant management of BRCA-mutated patients is still a matter of debate, with no definitive data supporting the utility or futility of platinum salts in this setting. Interestingly, a significant contribution will be provided by the ongoing INFORM study (NCT01670500), a randomized, phase II trial comparing four cycles of neoadjuvant cisplatin versus four cycles of doxorubicin plus cyclophosphamide among BRCA-mutated patients with early breast cancer.
2.3. BRCAness
Although BRCA1 and BRCA2 gene products are considered the major players in the HR system, cancers harboring a pathological variant of BRCA1/2 genes represent only the tip of the iceberg of the overall BC characterized by a homologous recombination deficiency, due to the emerging subgroup of tumors that share clinico–biological features of BRCA-mutant tumors in the absence of a BRCA1 or BRCA2 mutation, a condition known as BRCAness [63]. Since therapies, such as platinum-based chemotherapy and PARP inhibitors, have revealed their efficacy in BRCA1/2 mutation carriers [64,65], searching for BRCAness has become more appealing especially for those tumors characterized by poor outcomes and treatment options (Table 1). In TNBC, for example, BRCAness is found in more than 25% of cases [66]. Unfortunately, an unequivocal BRCAness biomarker is currently lacking, due to the wide spectrum of genetic and epigenetic alterations that may be involved. Among them, the most frequent genetic alteration involve ATM, ATR, PALB2, CHEK1, CHEK2, RAD51, Nijmegen breakage syndrome protein 1 (NBS1) and the Fanconi anaemia complementation group (FANC) family [63]. In addition, the modification of the cellular transcriptional activity, like those induced by BRCA1 promoter hypermethylation, can be a cause of epigenetic BRCAness. Given the evidence that cancer with an HRD system shows a typical mutational signature produced by the error-prone NHEJ activity in repairing DSBs [67], two commercial assays detecting the main three genomic structural rearrangements founded in HRD tumors has been developed. In “myChoice HRD” (Myriad genetics) test, loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale transition (LTS) are measured and can be combined in an HRD score. The predictive power of a specific HRD threshold derived from the combined HRD score has been evaluated retrospectively by Telli et al. [58]. In this study, HR deficiency (defined as an HRD score ≥ 42 and/or the presence of BRCA1 or BRCA2 mutation) was evaluated as a predictor of response to neoadjuvant platinum-based chemotherapy for TNBC in two different clinical cohorts. The dichotomized HRD score was significantly associated with both RCB 0/I (no residual cancer burden or minimal residual disease) and pCR in both cohorts. Moreover, HRD was still able to significantly predict RCB 0/I and pCR also when adjusted for clinical variables [58]. Concordant results have been found in a small trial addressed to patients with early stage TNBC enrolled to receive neoadjuvant platinum-based therapy. In the exploratory analysis of this trial, HRD status and the HRD score could predict pCR (HRD status p = 0.0012; HRD score p = 0.0024) [59]. Additionally, a post-hoc analysis of the GeparSixto study tried to explore the role of HRD score in predicting pCR to neoadjuvant chemotherapy in 193 patients treated for TNBC. Homologous recombination deficiency was defined as either a high HRD score (≥42) or a BRCA mutation in the primary tumor. Tumors with HRD were more likely to achieve pCR than HR proficient ones (55.9% versus 29.8%, p = 0.001). Moreover, patients with HRD tumors showed higher pCR rates with the addition of carboplatin to the chemotherapy backbone (64.9% versus 45.2%; p = 0.025) [68]. In a retrospective analysis of 425 patients with TNBC treated with adjuvant doxorubicin and cyclophosphamide in the SWOG9313 trial, a high HRD score (≥42) was observed in more than a half of BRCA wild-type patients and it was independently associated with better DFS when adjusted for treatment and nodal status (HR 0.64, 95% CI 0.43–0.94; p = 0.023) as well as OS (HR 0.65, 95% CI 0.47–1.53; p = 0.59). Moreover, BRCA1 promoter methylation was associated with higher HRD scores but no predictive value was established [60]. Conversely, in the previously discussed TNT trial, HRD score and BRCA1 promoter methylation were not associated to a better response to first-line platinum salts [49]. However, since HR biomarkers in TNT were evaluated on archival tissue, a possible explanation of these findings is that the “soft BRCAness” of HRD tumors is easily revertible under the selective pressure of neo/adjuvant DNA-damaging therapy. Therefore, when tumors relapse, cancer cells may be no longer methylated and sensitive to platinum agents [49,63].
Moreover, BRCAness has not only been investigated as a predictive biomarker, but also as a new therapeutic strategy through its pharmacological induction. Intriguing results suggest that the induction of a BRCA-mutant-like phenotype could be achieved through the epigenetic silencing of BRCA1, enhancing platinum salts’ activity and enabling the use of targeted drugs such as PARP inhibitors [69,70].
3. New Emerging Biomarkers
3.1. Gene Signatures
Lehmann et al. [61] showed that TNBCs can be further sub-classified into six different molecular entities. Among these, basal-like 1 (BL1) and basal-like 2 (BL2) subtypes had a peculiar sensitivity to platinum salts, irrespectively to BRCA1 mutation status, suggesting the presence of other defects in the HR pathway [61]. Concordant data came from a recent study on 465 Chinese TNBCs [71]. In that study, the authors classified TNBCs into four main classes through a multi-omics approach. Among these classes, the basal-like subtype was characterized by an HR defect, and thus, may be especially sensitive to DNA damaging agents like platinum salts.
Another genomic classification is PAM50, which differentiates at least five BC entities [72]. Among these, the basal-like can be ideally assimilated to TNBC. However, it is important to note that not all TNBCs display a basal phenotype [73,74,75,76] and basal-like cancers account for 60–90% of TNBCs [77,78]. The basal-like molecular subtype can be approximated to the core-basal breast cancer, which is only defined by the absence of ER, PgR, HER2, and the presence of EGFR and cytokeratin 5/6 [79]. In the TNT trial reported above [49], the basal-like molecular subtype and the core-basal type were investigated as possible predictive biomarkers of CBDCA sensitivity. Interestingly, in both of these types, carboplatin performs as well as docetaxel in terms of ORR and PFS. On the contrary, in non-basal-like BCs, docetaxel outperforms carboplatin in terms of ORR and PFS. A trend toward a better ORR and PFS for docetaxel was seen in non-core-basal BCs.
3.2. p53 Family
Other putative biomarkers of platinum sensitivity may be represented by the p53 status and the levels of p53 family members (p63 and p73). The protein p53 represents a crucial node in the DNA repair pathway [80], and its defects could be causative of platinum salt cytotoxicity [81,82]. Until now, only p53-nonsense or frameshift mutations have been associated to response to cisplatin in TNBCs [62]. Moreover, a high incidence of p53-truncating mutations in BRCA1-mutated BCs has been observed [83]. Therefore, p53-truncating mutations may represent an alternative marker of BRCA1 deficiency.
Both p63 and p73 proteins belong to the p53 family and govern a variety of cellular functions [84]. From a molecular point of view, the p63 isoform ∆Np63 antagonizes the pro-apoptotic activity of the p73 isoform TAp73 through direct physical sequestration [85]. Cisplatin showed to promote ∆Np63 dissociation from TAp73 in vitro [86]. Free TAp73 can, therefore, trigger the pro-apoptotic cascade. In light of this evidence, it has been hypothesized that ∆Np63/TAp73 levels might represent a proxy for cisplatin sensitivity. Indeed, some data suggest that a ∆Np63/TAp73 ratio > 2 may predict a favorable outcome for cisplatin [62], though the predictive role of the ∆Np63/TAp73 ratio needs further confirmation [50].
3.3. BER
Generally, BER is involved in processing non-bulky DNA lesions, usually induced by oxidative, alkylating, and methylation agents [87,88,89]. Although BER is composed of few sequential steps acting to guarantee the correct DNA repair, it involves a great number of non-classical DNA-repair proteins and post-translational modifications that are finely regulated [90,91,92,93]. Among BER modulators, nucleophosmin (NPM1), p53, and XRCC1 are the most effective [88,94,95], mostly in CDDP response.
The first BER step involves lesion-specific DNA glycosylases, including 8-oxoguanine DNA glycosylase (OGG1) [96] and uracil-DNA-glycosylase (UNG) [97], that recognize and cleave the damaged base or Uracil, thus generating an abasic site (AP) (Figure 1). Then, a specific apurinic/apyrimidinic endonuclease, called APE1, cleaves the abasic site [98,99]. Finally, the SSB generated by APE1 is sealed by other BER factors, including Polymerase β (Polβ) and ligases [100,101]. Although all the enzymes are needed for the good success of the entire BER, APE1 represents the core of the pathway, being able to coordinate every step.
As previously proposed [23,32], the clear documentation of a novel role of BER in mediating cisplatin cytotoxicity was produced by Kothandapani et al. [26]. The authors have finely demonstrated how Polβ- and UNG-deficient MDA-MB-231 TNBC cells are more resistant to CDDP treatment. The same effect was obtained by treating cells with Methoxyamine, an inhibitor of the endonuclease activity of APE1. Although the authors specified that BER enzymes are not directly involved in the removal of cisplatin-produced ICLs on DNA, they demonstrated that BER is active in damages indirectly induced by CDDP.
A part of CDDP cytotoxicity requires the generation of reactive oxygen species (ROS) [102]. Reactive oxygen species are mainly generated as a by-product of the aerobic mitochondrial respiration or by following continuous exposition of chemical agents [103]. When ROS levels increase, thus destabilizing the redox homeostasis of the cell, DNA, proteins, and lipids can be severely damaged. Reactive oxygen species-induced DNA damages, including the oxidation of the guanine (8oxoG), are efficiently repaired by the BER pathway. Notably, the most important side effect characterizing the use of CDDP, and to a lesser extent, of other platinum drugs, is the development of a severe peripheral neuropathy. In 2009, Preston et al. [29] demonstrated that both cisplatin and oxaliplatin increase ROS and 8oxoG levels, and then later, Kelley et al. [24] supported the hypothesis that this phenomenon might be considered a secondary effect of DNA damage induced by platinum drugs in sensory neurons. Remarkably, Kelley et al.’s [24] work has demonstrated that neuron cells, silenced for APE1 expression, are more sensitized to CDDP and oxaliplatin, but not carboplatin, observing a decrease of cell viability and a parallel increase of apoptosis. The cause of this high mortality is associated with an increased production of ROS levels within the cells that, by destabilizing the redox cell balance, induces an increase amount of 8oxoG. On the contrary, the stimulation of the APE1 endonuclease activity significantly decreases the toxicity of CDDP on neuronal cells [104]. Moreover, the treatment with the APE1-targeted first-generation (E3330) and the novel second-generation (APX2009) agonists protect against neurotoxicity induced by platinum compounds [105,106]. This should explain the role of APE1 in mediating inflammation induced by platinum drugs, principally through its redox activity [107].
Concordantly, by applying a CRISPR-based genetics screening, it has been shown how both BER-factors XRCC1 and OGG1 are involved in restoring ICLs and ROS-mediated oxidative damages induced by platinum drugs [31], highlighting again the role of BER enzymes in protecting against platinum-induced cytotoxicity.
Taken together, these observations explain how CDDP and platinum drugs may induce DNA damage by formation of bulky DNA lesions that are efficiently repaired by the cooperation of several DNA repair pathways, including BER. At the same time, the observed increase in ROS levels and the increased sensitization effect in BER-deficient cells could explain the primary involvement of the BER pathway in response to platinum drug cytotoxicity. These important observations led to consider BER enzymes as new anti-tumoral targets to be used in combination treatments with platinum drugs. The protein APE1 can be considered a new promising target for the combination of CDDP-based chemotherapy as proposed by Wang et al. [108] in NCSLC patients. Moreover, APE1 protein expression quantification revealed higher levels in CDDP-resistant patients and was correlated with a lower OS and event-free interval (EFI). Recently, we have improved the knowledge of the role of APE1 and NPM1 in mediating the response of CDDP and carboplatin in TNBC cells lines, differing for APE1 and NPM1 protein expression [109]. As already demonstrated by Poletto et al. [28], for other cell models, CDDP induced a shuttling of APE1 and NPM1 from nucleolus to nucleus compartments, which is possibly regulated to the non-DNA repair functions of APE1 on RNA metabolism. Particularly, TNBC cells, characterized by lower levels of both APE1 and NPM1 proteins, were much more sensitized to the combined treatment of platinum drugs and inhibitors of APE1 endonuclease activity as well as inhibitors of APE1–NPM1 interaction.
4. Conclusions
Despite being widely used for the treatment of multiple cancer types, platinum salts are only recently gaining momentum for the treatment of breast cancer, especially TNBC. Due to the great molecular heterogeneity of this subtype, several efforts have been made to identify predictive factors capable of effectively guiding patients’ stratification, but to date, the most solid one is the BRCA1/2 mutational status. Alternative biomarkers, such as BRCAness and BER enzymes are currently under investigation to further develop the role of platinum salts in breast cancer, not just for treatment benefit prediction but also as targets for new therapeutic strategies aimed at synergizing with this class of compounds.
Abbreviations
| BC | Breast cancer |
| mBC | Metastatic breast cancer |
| TNBC | Triple-negative breast cancer |
| CBDCA | Carboplatin |
| CDDP | Cisplatin |
| SLC31A1 | High Affinity Copper Uptake Protein 1 |
| ATP7B | Copper-Transporting P-type Adenosine Triphosphate |
| ICL | Inter-DNA cross-link |
| SSB | Single-strand break |
| DSB | Double-strand break |
| HR | Homologous recombination repair |
| BER | Base excision repair |
| HRD | Homologous recombination deficiency |
| NHEJ | Non-homologous end joining |
| BRCA1 | Breast Cancer Type 1 Susceptibility Protein |
| BRCA2 | Breast Cancer Type 2 Susceptibility Protein |
| ORR | Objective response rate |
| OS | Overall survival |
| PFS | Progression-free survival |
| PARP | Poly(ADP-ribose) polymerase |
| pCR | Pathologic complete response |
| EFS | Event-free survival |
| DFS | Disease-free survival |
| RCB | Residual cancer burden |
| NPM1 | Nucleophosmin |
| OGG1 | 8-Oxoguanine DNA glycosylase |
| UNG | Uracil-DNA-glyucosylase |
| Polβ | Polymerase β |
| ROS | Reactive oxygen species |
| 8oxoG | Oxidized guanine |
| EFI | Event-free survival |
Funding
This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant number IG19862) to G.T.
Conflicts of Interest
Puglisi has received honoraria and advisory roles from Celgene, Eli Lilly, Ipsen, MSD, Novartis, Roche, Takeda; travel grants from Celgene, Roche, and Servier; research grants from AstraZeneca, EISAI, and Roche. Gerratana reports non-financial support from Menarini Silicon Biosystems (travel grants); honoraria from Elly Lilly; research grants from Eisai Co.; outside the submitted work. The other authors declare no conflicts of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Waks A.G., Winer E.P. Breast Cancer Treatment. JAMA. 2019;321:288. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Rakha E.A., Green A.R. Molecular classification of breast cancer: What the pathologist needs to know. Pathology. 2017;49:111–119. doi: 10.1016/j.pathol.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone T.C., Suntharalingam K., Lippard S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016;116:3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerratana L., Fanotto V., Pelizzari G., Agostinetto E., Puglisi F. Do platinum salts fit all triple negative breast cancers? Cancer Treat. Rev. 2016;48:34–41. doi: 10.1016/j.ctrv.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Poggio F., Bruzzone M., Ceppi M., Pondé N.F., La Valle G., Del Mastro L., de Azambuja E., Lambertini M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018;29:1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli F., Barni S., Bregni G., de Braud F., Di Cosimo S. Platinum salts in advanced breast cancer: A systematic review and meta-analysis of randomized clinical trials. Breast Cancer Res. Treat. 2016;160:425–437. doi: 10.1007/s10549-016-4025-3. [DOI] [PubMed] [Google Scholar]
- 8.Rocha C., Silva M., Quinet A., Cabral-Neto J., Menck C. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics. 2018;73:e478s. doi: 10.6061/clinics/2018/e478s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florea A.M., Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwelling L.A., Kohn K.W. Effects of cisplatin on dna and the possible relationships to cytotoxicity and mutagenicity in mammalian cells. Cisplatin. 2013;73:21–35. [Google Scholar]
- 11.Rosenberg B. Cisplatin. Academic Press; New York, NY, USA: 2013. Cisplatin: Its history and possible mechanisms of action; pp. 9–20. [Google Scholar]
- 12.Bradner W.T., Rose W.C., Huftalen J.B. Cisplatin. Academic Press; New York, NY, USA: 2013. antitumor activity of platinum analogs; pp. 171–182. [Google Scholar]
- 13.Cui Y., König J., Buchholz J.K., Spring H., Leier I., Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- 14.Komatsu M., Sumizawa T., Mutoh M., Chen Z.S., Terada K., Furukawa T., Yang X.L., Gao H., Miura N., Sugiyama T., et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- 15.Holzer A.K., Samimi G., Katano K., Naerdemann W., Lin X., Safaei R., Howell S.B. The Copper Influx Transporter Human Copper Transport Protein 1 Regulates the Uptake of Cisplatin in Human Ovarian Carcinoma Cells. Mol. Pharmacol. 2004;66:817–823. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- 16.Safaei R., Katano K., Samimi G., Naerdemann W., Stevenson J.L., Rochdi M., Howell S.B. Cross-resistance to cisplatin in cells with acquired resistance to copper. Cancer Chemother. Pharmacol. 2004;53:239–246. doi: 10.1007/s00280-003-0736-3. [DOI] [PubMed] [Google Scholar]
- 17.Katano K., Kondo A., Safaei R., Holzer A., Samimi G., Mishima M., Kuo Y.-M., Rochdi M., Howell S.B. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 18.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 19.Eastman A. Cross-linking of glutathione to DNA by cancer chemotherapeutic platinum coordination complexes. Chem. Biol. Interact. 1987;61:241–248. doi: 10.1016/0009-2797(87)90004-4. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara K., Fujiwara Y., Nishio K., Ohmori T., Sugimoto Y., Komiya K., Matsuda T., Saijo N. Metallothionein content correlates with the sensitivity of human small cell lung cancer cell lines to cisplatin. Cancer Res. 1991;51:3237–3242. [PubMed] [Google Scholar]
- 21.Gonzalez V.M., Fuertes M.A., Alonso C., Perez J.M. Is Cisplatin-Induced Cell Death Always Produced by Apoptosis? Mol. Pharmacol. 2001;59:657–663. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- 22.Becker J.P., Weiss J., Theile D. Cisplatin, oxaliplatin, and carboplatin unequally inhibit in vitro mRNA translation. Toxicol. Lett. 2014;225:43–47. doi: 10.1016/j.toxlet.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Horton J.K., Srivastava D.K., Zmudzka B.Z., Wilson S.H. Strategic down-regulation of DNA polymerase β by antisense RNA sensitizes mammalian cells to specific DNA damaging agents. Nucleic Acids Res. 1995;23:3810–3815. doi: 10.1093/nar/23.19.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley M.R., Jiang Y., Guo C., Reed A., Meng H., Vasko M.R. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H.S., Guo C., Thompson E.L., Jiang Y., Kelley M.R., Vasko M.R., Lee S.H. APE1, the DNA base excision repair protein, regulates the removal of platinum adducts in sensory neuronal cultures by NER. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2015;779:96–104. doi: 10.1016/j.mrfmmm.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothandapani A., Dangeti V.S.M.N., Brown A.R., Banze L.A., Wang X.H., Sobol R.W., Patrick S.M. Novel role of base excision repair in mediating cisplatin cytotoxicity. J. Biol. Chem. 2011;286:14564–14574. doi: 10.1074/jbc.M111.225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kothandapani A., Sawant A., Dangeti V.S.M.N., Sobol R.W., Patrick S.M. Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity. Nucleic Acids Res. 2013;41:7332–7343. doi: 10.1093/nar/gkt479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poletto M., Lirussi L., Wilson D.M., Tell G. Nucleophosmin modulates stability, activity, and nucleolar accumulation of base excision repair proteins. Mol. Biol. Cell. 2014;25:1641–1652. doi: 10.1091/mbc.e13-12-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preston T.J., Henderson J.T., McCallum G.P., Wells P.G. Base excision repair of reactive oxygen species-initiated 7,8-dihydro-8-oxo-2′-deoxyguanosine inhibits the cytotoxicity of platinum anticancer drugs. Mol. Cancer Ther. 2009;8:2015–2026. doi: 10.1158/1535-7163.MCT-08-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawant A., Floyd A.M., Dangeti M., Lei W., Sobol R.W., Patrick S.M. Differential role of base excision repair proteins in mediating cisplatin cytotoxicity. DNA Repair. 2017;51:46–59. doi: 10.1016/j.dnarep.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slyskova J., Sabatella M., Ribeiro-Silva C., Stok C., Theil A.F., Vermeulen W., Lans H. Base and nucleotide excision repair facilitate resolution of platinum drugs-induced transcription blockage. Nucleic Acids Res. 2018;46:9537–9549. doi: 10.1093/nar/gky764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J., Parsons J., Nicolay N.H., Caporali S., Harrington C.F., Singh R., Finch D., Datri S., Farmer P.B., Johnston P.G., et al. Cells deficient in the base excision repair protein, DNA polymerase beta, are hypersensitive to oxaliplatin chemotherapy. Oncogene. 2010;29:463–468. doi: 10.1038/onc.2009.327. [DOI] [PubMed] [Google Scholar]
- 33.Ceccaldi R., Rondinelli B., D’Andrea A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Z., Bozzella M., Seluanov A., Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright W.D., Shah S.S., Heyer W.-D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018;293:10524–10535. doi: 10.1074/jbc.TM118.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang H.H.Y., Pannunzio N.R., Adachi N., Lieber M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krejci L., Altmannova V., Spirek M., Zhao X. Homologous recombination and its regulation. Nucleic Acids Res. 2012;40:5795–5818. doi: 10.1093/nar/gks270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasin M., Rothstein R. Repair of Strand Breaks by Homologous Recombination. Cold Spring Harb. Perspect. Biol. 2013;5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Heyer W.-D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 41.Chen C.-C., Feng W., Lim P.X., Kass E.M., Jasin M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu. Rev. Cancer Biol. 2018;2:313–336. doi: 10.1146/annurev-cancerbio-030617-050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godin S.K., Sullivan M.R., Bernstein K.A. Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem. Cell Biol. 2016;94:407–418. doi: 10.1139/bcb-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F., Fulton L.L., Dooling D.J., Ding L., Mardis E.R., et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma P., Klemp J.R., Kimler B.F., Mahnken J.D., Geier L.J., Khan Q.J., Elia M., Connor C.S., McGinness M.K., Mammen J.M.W., et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res. Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin D.S., Chahwan C., Huffman J.L., Tainer J.A. Structure and function of the double-strand break repair machinery. DNA Repair. 2004;3:863–873. doi: 10.1016/j.dnarep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Scully R., Chen J., Ochs R.L., Keegan K., Hoekstra M., Feunteun J., Livingston D.M. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/S0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 47.Byrski T., Gronwald J., Huzarski T., Grzybowska E., Budryk M., Stawicka M., Mierzwa T., Szwiec M., Wiśniowski R., Siolek M., et al. Pathologic Complete Response Rates in Young Women with BRCA1-Positive Breast Cancers after Neoadjuvant Chemotherapy. J. Clin. Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 48.Byrski T., Dent R., Blecharz P., Foszczynska-Kloda M., Gronwald J., Huzarski T., Cybulski C., Marczyk E., Chrzan R., Eisen A., et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012;14:R110. doi: 10.1186/bcr3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tutt A., Tovey H., Cheang M.C.U., Kernaghan S., Kilburn L., Gazinska P., Owen J., Abraham J., Barrett S., Barrett-Lee P., et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isakoff S.J., Mayer E.L., He L., Traina T.A., Carey L.A., Krag K.J., Rugo H.S., Liu M.C., Stearns V., Come S.E., et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy with Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yardley D.A., Coleman R., Conte P., Cortes J., Brufsky A., Shtivelband M., Young R., Bengala C., Ali H., Eakel J., et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: Results from the tnAcity trial. Ann. Oncol. 2018;29:1763–1770. doi: 10.1093/annonc/mdy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikov W.M., Berry D.A., Perou C.M., Singh B., Cirrincione C.T., Tolaney S.M., Kuzma C.S., Pluard T.J., Somlo G., Port E.R., et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance) J. Clin. Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loibl S., Weber K.E., Timms K.M., Elkin E.P., Hahnen E., Fasching P.A., Lederer B., Denkert C., Schneeweiss A., Braun S., et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—Final results from GeparSixto. Ann. Oncol. 2018;29:2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 54.Von Minckwitz G., Schneeweiss A., Loibl S., Salat C., Denkert C., Rezai M., Blohmer J.U., Jackisch C., Paepke S., Gerber B., et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 55.Sikov W., Berry D., Perou C., Singh B., Cirrincione C., Tolaney S., Somlo G., Port E., Qamar R., Sturtz K., et al. Abstract S2-05: Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/-carboplatin and/or bevacizumab in triple-negative breast cancer: Outcomes from CALGB 40603 (Alliance) Cancer Res. 2016;76:S2-05. doi: 10.1158/1538-7445.SABCS15-S2-05. [DOI] [Google Scholar]
- 56.Hahnen E., Lederer B., Hauke J., Loibl S., Kröber S., Schneeweiss A., Denkert C., Fasching P.A., Blohmer J.U., Jackisch C., et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer. JAMA Oncol. 2017;3:1378. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loibl S., O’Shaughnessy J., Untch M., Sikov W.M., Rugo H.S., McKee M.D., Huober J., Golshan M., von Minckwitz G., Maag D., et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 58.Telli M., McMillan A., Ford J., Richardson A., Silver D., Isakoff S., Kaklamani V., Gradishar W., Stearns V., Connolly R., et al. Abstract P3-07-12: Homologous recombination deficiency (HRD) as a predictive biomarker of response to neoadjuvant platinum-based therapy in patients with triple negative breast cancer (TNBC): A pooled analysis. Cancer Res. 2016;76:625–630. doi: 10.1158/1538-7445.SABCS15-P3-07-12. [DOI] [Google Scholar]
- 59.Kaklamani V.G., Jeruss J.S., Hughes E., Siziopikou K., Timms K.M., Gutin A., Abkevich V., Sangale Z., Solimeno C., Brown K.L., et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer ( NCT01372579) Breast Cancer Res. Treat. 2015;151:629–638. doi: 10.1007/s10549-015-3435-y. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P., Barlow W.E., Godwin A.K., Pathak H., Isakova K., Williams D., Timms K.M., Hartman A.R., Wenstrup R.J., Linden H.M., et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313) Ann. Oncol. 2018;29:654–660. doi: 10.1093/annonc/mdx821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehmann B.D., Bauer J.A., Chen X., Sanders M.E., Chakravarthy A.B., Shyr Y., Pietenpol J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silver D.P., Richardson A.L., Eklund A.C., Wang Z.C., Szallasi Z., Li Q., Juul N., Leong C.-O., Calogrias D., Buraimoh A., et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lord C.J., Ashworth A. BRCAness revisited. Nat. Rev. Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 64.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 66.De Summa S., Pinto R., Sambiasi D., Petriella D., Paradiso V., Paradiso A., Tommasi S. BRCAness: A deeper insight into basal-like breast tumors. Ann. Oncol. 2013;24:viii13–viii21. doi: 10.1093/annonc/mdt306. [DOI] [PubMed] [Google Scholar]
- 67.Lieber M.R. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End-Joining Pathway. Annu. Rev. Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Von Minckwitz G., Timms K., Untch M., Elkin E.P., Fasching P.A., Schneeweiss A., Salat C., Rezai M., Blohmer J.U., Zahm D.M., et al. Prediction of pathological complete response (pCR) by Homologous Recombination Deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: Results from GeparSixto. J. Clin. Oncol. 2015;33:1004. doi: 10.1200/jco.2015.33.15_suppl.1004. [DOI] [Google Scholar]
- 69.Min A., Im S.-A., Kim D.K., Song S.-H., Kim H.-J., Lee K.-H., Kim T.-Y., Han S.-W., Oh D.-Y., Kim T.-Y., et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015;17:33. doi: 10.1186/s13058-015-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mio C., Gerratana L., Bolis M., Caponnetto F., Zanello A., Barbina M., Di Loreto C., Garattini E., Damante G., Puglisi F. BET proteins regulate homologous recombination-mediated DNA repair: BRCAness and implications for cancer therapy. Int. J. Cancer. 2019;144:755–766. doi: 10.1002/ijc.31898. [DOI] [PubMed] [Google Scholar]
- 71.Jiang Y.-Z., Ma D., Suo C., Shi J., Xue M., Hu X., Xiao Y., Yu K.-D., Liu Y.-R., Yu Y., et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell. 2019;35:428–440.e5. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jumppanen M., Gruvberger-Saal S., Kauraniemi P., Tanner M., Bendahl P.-O., Lundin M., Krogh M., Kataja P., Borg Å., Fernö M., et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007;9:R16. doi: 10.1186/bcr1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calza S., Hall P., Auer G., Bjöhle J., Klaar S., Kronenwett U., Liu E.T., Miller L., Ploner A., Smeds J., et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rouzier R., Perou C.M., Symmans W.F., Ibrahim N., Cristofanilli M., Anderson K., Hess K.R., Stec J., Ayers M., Wagner P., et al. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clin. Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 76.Bertucci F., Finetti P., Cervera N., Esterni B., Hermitte F., Viens P., Birnbaum D. How basal are triple-negative breast cancers? Int. J. Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 77.Hubalek M., Czech T., Müller H. Biological Subtypes of Triple-Negative Breast Cancer. Breast Care. 2017;12:8–14. doi: 10.1159/000455820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan C., Oh D.S., Wessels L., Weigelt B., Nuyten D.S.A., Nobel A.B., van’t Veer L.J., Perou C.M. Concordance among Gene-Expression–Based Predictors for Breast Cancer. N. Engl. J. Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 79.Cheang M.C.U., Voduc D., Bajdik C., Leung S., McKinney S., Chia S.K., Perou C.M., Nielsen T.O. Basal-Like Breast Cancer Defined by Five Biomarkers Has Superior Prognostic Value than Triple-Negative Phenotype. Clin. Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 80.Marcel V., Nguyen Van Long F., Diaz J.-J. 40 Years of Research Put p53 in Translation. Cancers. 2018;10:152. doi: 10.3390/cancers10050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pestell K.E., Hobbs S.M., Titley J.C., Kelland L.R., Walton M.I. Effect of p53 status on sensitivity to platinum complexes in a human ovarian cancer cell line. Mol. Pharmacol. 2000;57:503–511. doi: 10.1124/mol.57.3.503. [DOI] [PubMed] [Google Scholar]
- 83.Holstege H., Joosse S.A., van Oostrom C.T.M., Nederlof P.M., de Vries A., Jonkers J. High Incidence of Protein-Truncating TP53 Mutations in BRCA1-Related Breast Cancer. Cancer Res. 2009;69:3625–3633. doi: 10.1158/0008-5472.CAN-08-3426. [DOI] [PubMed] [Google Scholar]
- 84.Allocati N., Di Ilio C., De Laurenzi V. p63/p73 in the control of cell cycle and cell death. Exp. Cell Res. 2012;318:1285–1290. doi: 10.1016/j.yexcr.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 85.Rocco J.W., Leong C.-O., Kuperwasser N., DeYoung M.P., Ellisen L.W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Leong C.-O., Vidnovic N., DeYoung M.P., Sgroi D., Ellisen L.W. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J. Clin. Investig. 2007;117:1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Limpose K.L., Corbett A.H., Doetsch P.W. BERing the burden of damage: Pathway crosstalk and posttranslational modification of base excision repair proteins regulate DNA damage management. DNA Repair. 2017;56:51–64. doi: 10.1016/j.dnarep.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dianov G.L., Hübscher U. Mammalian base excision repair: The forgotten archangel. Nucleic Acids Res. 2013;41:3483–3490. doi: 10.1093/nar/gkt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antoniali G., Malfatti M.C., Tell G. Unveiling the non-repair face of the Base Excision Repair pathway in RNA processing: A missing link between DNA repair and gene expression? DNA Repair. 2017 doi: 10.1016/j.dnarep.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Allinson S.L., Sleeth K.M., Matthewman G.E., Dianov G.L. Orchestration of base excision repair by controlling the rates of enzymatic activities. DNA Repair. 2004;3:23–31. doi: 10.1016/j.dnarep.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Almeida K.H., Sobol R.W. A unified view of base excision repair: Lesion-dependent protein complexes regulated by post-translational modification. DNA Repair. 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Déry U., Masson J.-Y. Twists and turns in the function of DNA damage signaling and repair proteins by post-translational modifications. DNA Repair. 2007;6:561–577. doi: 10.1016/j.dnarep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Wilson S.H., Kunkel T.A. Passing the baton in base excision repair. Nat. Struct. Biol. 2000;7:176–178. doi: 10.1038/82818. [DOI] [PubMed] [Google Scholar]
- 94.Fan J., Wilson D.M. Protein-protein interactions and posttranslational modifications in mammalian base excision repair. Free Radic. Biol. Med. 2005;38:1121–1138. doi: 10.1016/j.freeradbiomed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Vascotto C., Fantini D., Romanello M., Cesaratto L., Deganuto M., Leonardi A., Radicella J.P., Kelley M.R., D’Ambrosio C., Scaloni A., et al. APE1/Ref-1 Interacts with NPM1 within Nucleoli and Plays a Role in the rRNA Quality Control Process. Mol. Cell. Biol. 2009;29:1834–1854. doi: 10.1128/MCB.01337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faucher F., Doubli S., Jia Z. 8-oxoguanine DNA glycosylases: One lesion, three subfamilies. Int. J. Mol. Sci. 2012;13:6711–6729. doi: 10.3390/ijms13066711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zharkov D.O., Mechetin G.V., Nevinsky G.A. Uracil-DNA glycosylase: Structural, thermodynamic and kinetic aspects of lesion search and recognition. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010;685:11–20. doi: 10.1016/j.mrfmmm.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tell G., Lirussi L., Antoniali G., Wilson III D.M., Poletto M. The Base Excision Repair Pathway. World Scientific; Singapore: 2016. The Abasic Endonuclease APE1: Much more than a DNA Repair Enzyme; pp. 219–251. [Google Scholar]
- 99.Wilson D.M. Ape1 abasic endonuclease activity is regulated by magnesium and potassium concentrations and is robust on alternative DNA structures. J. Mol. Biol. 2005;345:1003–1014. doi: 10.1016/j.jmb.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 100.Sobol R.W., Horton J.K., Kühn R., Gu H., Singhal R.K., Prasad R., Rajewsky K., Wilson S.H. Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature. 1996;379:183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 101.Sung J.-S., DeMott M.S., Demple B. Long-patch base excision DNA repair of 2-deoxyribonolactone prevents the formation of DNA-protein cross-links with DNA polymerase beta. J. Biol. Chem. 2005;280:39095–39103. doi: 10.1074/jbc.M506480200. [DOI] [PubMed] [Google Scholar]
- 102.Brozovic A., Ambriović-Ristov A., Osmak M. The relationship between cisplatin-Induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit. Rev. Toxicol. 2010;40:347–359. doi: 10.3109/10408441003601836. [DOI] [PubMed] [Google Scholar]
- 103.Gul T., Mehraj Balkhi H., Haq E. Evaluation of Cellular Processes by in Vitro Assays. Bentham Science Publishers; Soest, The Netherlands: 2018. Reactive Oxygen Species (ROS) pp. 58–67. [Google Scholar]
- 104.Duarte D.B., Vasko M.R. DNA Repair in Cancer Therapy. Elsevier; Amsterdam, The Netherlands: 2012. The Role of DNA Damage and Repair in Neurotoxicity Caused by Cancer Therapies; pp. 283–299. [Google Scholar]
- 105.Kelley M.R., Wikel J.H., Guo C., Pollok K.E., Bailey B.J., Wireman R., Fishel M.L., Vasko M.R. Identification and Characterization of New Chemical Entities Targeting Apurinic/Apyrimidinic Endonuclease 1 for the Prevention of Chemotherapy-Induced Peripheral Neuropathy. J. Pharmacol. Exp. Ther. 2016;359:300–309. doi: 10.1124/jpet.116.235283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vasko M.R., Guo C., Thompson E.L., Kelley M.R. The repair function of the multifunctional DNA repair/redox protein APE1 is neuroprotective after ionizing radiation. DNA Repair. 2011;10:942–952. doi: 10.1016/j.dnarep.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frossi B., Antoniali G., Yu K., Akhtar N., Kaplan M.H., Kelley M.R., Tell G., Pucillo C.E.M. Endonuclease and redox activities of human apurinic/apyrimidinic endonuclease 1 have distinctive and essential functions in IgA class switch recombination. J. Biol. Chem. 2019:jbc.RA118.006601. doi: 10.1074/jbc.RA118.006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang D., Xiang D.-B., Yang X.-Q., Chen L.-S., Li M.-X., Zhong Z.-Y., Zhang Y.-S. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer. 2009;66:298–304. doi: 10.1016/j.lungcan.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 109.Malfatti M.C., Gerratana L., Dalla E., Isola M., Damante G., Di Loreto C., Puglisi F., Tell G. APE1 and NPM1 protect cancer cells from platinum compounds cytotoxicity and their expression pattern has a prognostic value in TNBC. J. Exp. Clin. Cancer Res. 2019 doi: 10.1186/s13046-019-1294-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]