Abstract
Chronic Lymphocytic Leukemia (CLL) patients with +12 have been reported to have specific clinical and biologic features. We performed an analysis of the association between demographic; clinical; laboratory; biologic features and outcome in CLL patients with +12 to identify parameters predictive of disease progression; time to treatment; and survival. The study included 487 treatment-naive CLL patients with +12 from 15 academic centers; diagnosed between January 2000 and July 2016; and 816 treatment-naïve patients with absence of Fluorescence In Situ Hybridization (FISH) abnormalities. A cohort of 250 patients with +12 CLL followed at a single US institution was used for external validation. In patients with +12; parameters associated with worse prognosis in the multivariate model were high Lactate DeHydrogenase (LDH) and β-2-microglobulin and unmutated immunoglobulin heavy-chain variable region gene (IGHV). CLL patients with +12 and high LDH levels showed a shorter Progression-Free-Survival (PFS) (30 months vs. 65 months; p < 0.001), Treatment-Free-Survival (TFS) (33 months vs. 69 months; p < 0.001), Overall Survival (OS) (131 months vs. 181 months; p < 0.001) and greater CLL-related mortality (29% vs. 11% at 10 years; p < 0.001) when compared with +12 CLL patients with normal LDH levels. The same differences were observed in the validation cohort. These data suggest that serum LDH levels can predict PFS; TFS; OS and CLL-specific survival in CLL patients with +12.
Keywords: CLL, trisomy 12, LDH, prognosis
1. Introduction
The clinical course of chronic lymphocytic leukemia (CLL) is very heterogeneous and the identification of prognostic and predictive factors for CLL is of great relevance and a field of active investigation [1,2]. During the last decade several laboratory biomarkers have been identified as being correlated with outcomes for risk assessment [3,4,5,6]. The analysis of aberrant chromosomal regions with specific DNA probes by fluorescence in situ hybridization (FISH) resulted in the detection of clonal aberrations and the main recurrent chromosomal abnormalities (del13q, +12, del11q and del17p) define different personal genetic profiling subgroups in CLL [7,8].
Trisomy 12 is the second most frequent cytogenetic abnormality identified by FISH in patients with CLL [7]. It presents as an isolated aberration in about 70% of cases and when it is associated with additional chromosomal abnormalities portends a poor prognosis [7,8,9,10,11].
CLL patients with +12 have unique morphologic and immunophenotypic characteristics [12].
CLL cells with +12 commonly have an atypical morphology, defined as the presence of cleaved nuclei and/or lymphoplasmacytoid features in more than 15% of cells [13,14,15,16]. When analyzing their immunophenotype, cases with +12, in comparison with cases with a normal FISH, show a significantly higher expression of CD19, CD22, CD20, CD79b, CD24, CD27, CD38, CD49d, sIgM, sIgk and sIgλ and a lower expression of CD43 [9].
CLL patients with +12 show a greater percentage of unmutated immunoglobulin heavy-chain variable region gene (IGHV) cases (54%) versus those with del13q and normal karyotype (37% and 31%, respectively) [14,15,16,17]. IGHV studies reported a significantly more frequent expression of stereotyped B-cell receptors compared to patients with CLL and no +12 (44% vs 27% respectively), with a higher prevalence of the IGHV 4–39 gene, particularly in cases that later developed Richter’s syndrome (RS) [17,18,19]. The role of the IGHV mutational status in predicting the clinical course of CLL patients with +12 has been well investigated by Bulian et al. [20]: it proved to be the sole prognostic factor able to stratify overall survival (OS) and time-to-first treatment (TTFT) in +12 CLL.
CLL patients with +12 rarely have TP53 mutations or acquire them over time [6,21]. On the contrary, NOTCH1 mutations are very frequent in +12 CLL patients and are detected in 30–40% of the cases [22,23,24].
In the literature there are few reports that describe in detail the clinical features of CLL cases with +12 [17,25,26,27]: the two largest series were reported by Marin et al., (289 patients) [26] and Strati et al., (250 patients) [27]. We now present a large series of treatment-naive +12 CLL patients and correlate the association between demographic, clinical, laboratory, and biologic features and clinical outcomes.
2. Results
2.1. Patients’ Characteristics
Four-hundred and eighty-seven patients with CLL and +12 and 816 patients with negative FISH were included in the study. +12 patients had a median age at diagnosis of 65.5 years (range 33–90) with a male/female (M:F) ratio of 1.68 (305 males, 63%; and 182 females, 37%). Negative FISH patients had a median age at diagnosis of 63.0 years (range 27–93) and a male/female M:F ratio of 1.58 (500 males, 61%; and 316 females, 39%). All clinical, laboratory, and biologic characteristics at diagnosis are presented in Table 1.
Table 1.
Patients’ baseline characteristics at diagnosis.
| Patients with FISH +12 (487 Patients) | Patients with FISH Negative (816 Patients) | p | ||
|---|---|---|---|---|
| Median age (range) | 65.5 (33–90) | 63.0 (27–93) | 0.002 | |
| Gender M/F (ratio) | 305/182 (1.68) | 500/316 (1.58) | ns | |
| Binet stage |
A
B C |
359 (73.7%) 104 (21.4%) 24 (4.9%) |
663 (81.3%) 103 (12.6%) 50 (6.1%) |
<0.001 |
| Rai stage |
0
I-II III-IV |
237 (48.7%) 225 (46.2%) 25 (5.1%) |
500 (61.3%) 265 (32.4%) 51 (6.3%) |
<0.001 |
| Palpable splenomegaly | 112 (23.0%) | 135 (16.5%) | 0.004 | |
| Palpable hepatomegaly | 64 (13.1%) | 66 (8.1%) | 0.003 | |
| Lymphadenopathies >5 cm | 243 (49.9%) | 298 (36.5%) | <0.001 | |
| White blood cells (mmc) (range) | 15,675 (11,675–24,600) |
15,610 (11,900–22,610) |
ns | |
| Lymphocytes peripheral blood (mmc) (range) | 10,190 (6625–18,965) |
10,800 (7090–16,800) |
ns | |
| Hemoglobin (g/dL) (range) | 13.7 (12.8–14.8) |
13.9 (12.7–14.9) |
ns | |
| Platelets (mmc) (range) | 193,000 (156,000–239,000) |
200,000 (160,000–244,000) |
ns | |
| LDH |
Normal levels
Above the limit |
310/452 (68.6%) 142/452 (31.4%) |
662/767 (86.3%) 105/767 (13.7%) |
<0.001 |
| Lymphocytes bone marrow (%) | 68 (50–80) in 152/487 pts | 60 (40–79) in 197/816 pts | ns | |
| ZAP70 positive (≥20%) | 197/363 (54.3%) | 241/676 (35.6%) | <0.001 | |
| CD38 positive (≥30%) | 222/433 (51.3%) | 213/768 (27.7%) | <0.001 | |
| CD49d positive (≥30%) | 89/113 (78.8%) | 54/201 (26.9%) | <0.001 | |
| β-2-microglobulin |
Normal levels
Above the limit |
195/396 (49.2%) 201/396 (51.8%) |
436/654 (66.7%) 218/654 (33.3%) |
<0.001 |
| IGHV mutational status |
Mutated
Unmutated |
164/384 (42.7%) 220/384 (57.3%) |
430/679 (63.3%) 249/679 (36.7%) |
0.001 |
FISH: Fluorescence In Situ Hybridization; M/F: male/female; LDH: Lactate DeHydrogenase; IGHV: immunoglobulin heavy-chain variable region gene.
Patients with +12 had significantly higher levels of lactate dehydrogenase (LDH) and β-2-microglobulin; more frequently expressed ZAP70, CD38, and CD49d (p < 0.001); and more frequently had an unmutated IGHV status compared to the control group (p < 0.001).
2.2. Outcomes
2.2.1. Patients +12 CLL vs. Negative FISH CLL
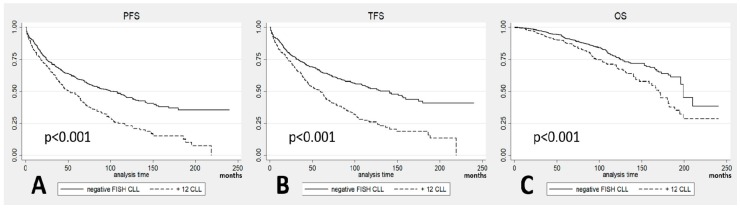
Among the 487 CLL patients with +12, 311 (64%) progressed, 298 received treatment (61%), and 125 (26%) died, resulting in a median progression-free-survival (PFS) of 51 months (confidence interval (CI) 95%: 44 to 64), a median treatment-free-survival (TFS) of 59 months (CI 95%: 47 to 65) and a median OS of 170 months (CI 95%: 147 to 182). We then analysed data regarding the 816 CLL patients with negative FISH: 374 progressed (46%), 328 received treatment (40%), and 145 died (18%), resulting in a median PFS of 100 months (CI 95%: 81 to 118), a median TFS of 141 months (CI 95%: 112 to 160), and a median OS of 199 months (CI 95%: 196 to 210). When comparing the curves, we noted significantly shorter survival of +12 patients than FISH negative in terms of PFS (p < 0.001; Figure 1A), TFS (p < 0.001; Figure 1B) and OS (p < 0.001; Figure 1C). No differences were noted in the occurrence of Richter transformation and second cancers between the two groups.
Figure 1.
(A) Progression-free-survival (PFS) in patients with +12 chronic lymphocytic leukemia (CLL) vs. fluorescence in situ hybridization (FISH) negative CLL; (B) treatment-free-survival (TFS) in patients with +12 CLL vs. FISH negative CLL; (C) overall survival (OS) in patients with +12 CLL vs. FISH negative CLL.
We next performed analysis of all the investigated categorical and continuous variables to identify parameters associated with shorter survival in +12 CLL patients. In univariate analysis, Binet and Rai advanced stage, elevated LDH, and β-2-microglobulin levels and unmutated IGHV were associated with shorter PFS, TFS, and OS; ZAP70 positivity was associated with shorter TFS and OS; whereas older age and positivity for CD38 were associated only with shorter OS. In multivariate analysis, elevated LDH and β-2-microglobulin levels, unmutated IGHV, and Rai advanced stage maintained their association with PFS and TFS, whereas elevated LDH and β-2-microglobulin levels, unmutated IGHV, and older age showed significance for OS (Table S1).
We noticed that elevated LDH levels at diagnosis were more common in CLL patients with +12 compared to negative FISH patients (31% versus 14%, p < 0.001). These levels, as a categorical variable in which each centre declared their ranges and upper limits, were considered elevated if stable over the time. All CLL patients included in the analysis did not show lymphadenomegaly or symptoms suggestive of RS or haemolytic anaemia at the onset of the disease that could cause high LDH levels.
2.2.2. Characteristics of +12 and FISH-Negative CLL Patients Stratified according to LDH Levels
Since LDH is an inexpensive and routinely performed laboratory parameter, we evaluated the frequency of elevated levels of LDH at diagnosis in +12 CLL patients. Among patients with +12, 142 (31.4%) patients showed elevated LDH levels and 310 (68.6%) patients showed levels within normal range. The characteristics of the two subgroups are summarized in Table 2.
Table 2.
Baseline characteristics at diagnosis of CLL patients with +12 divided in two subgroups according to lactate dehydrogenase (LDH) levels.
| Patients with High LDH Levels (142 Patients) | Patients with Normal LDH Levels (310 Patients) | p | ||
|---|---|---|---|---|
| Median age (range) | 65.5 (33–89) | 65.5 (33–90) | ns | |
| Gender M/F (ratio) | 82/60 (1.37) | 201/109 (1.84) | ns | |
| Binet stage |
Stage A
Stage B Stage C |
84 (59.1%) 45 (31.7%) 13 (9.2%) |
249 (80.3%) 51 (16.5%) 10 (3.2%) |
<0.001 |
| Rai stage |
Stage 0
Stage I–II Stage III–IV |
47 (33.1%) 81 (57.0%) 14 (9.9%) |
168 (54.2%) 132 (42.6%) 10 (3.2%) |
<0.001 |
| Palpable splenomegaly | 47 (33.1%) | 58 (18.7%) | 0.001 | |
| Palpable hepatomegaly | 23 (16.2%) | 34 (11.0%) | ns | |
| Lymphadenopathies >5 cm | 89 (62.7%) | 141 (45.5%) | 0.001 | |
| White blood cells (mmc) (range) | 18,500 (12,800–32,370) |
14,680 (11,100–21,100) |
<0.001 | |
| Lymphocytes peripheral blood (mmc) (range) | 12,344 (7811–24,175) |
9245 (5990–15,000) |
<0.001 | |
| Hemoglobin (g/dL) (range) | 13.4 (12.2–14.6) | 13.9 (13.0–15.0) | <0.001 | |
| Platelets (mmc) (range) | 185,000 (153,000–235,000) |
194,000 (159,000–238,000) |
ns | |
| Lymphocytes bone marrow (%) | 70 (50–85) in 63/142 pts | 60 (45–77) in 86/310 pts | 0.03 | |
| ZAP70 positive (≥20%) | 64/107 (59.8%) | 117/231 (50.6%) | ns | |
| CD38 positive (≥30%) | 71/133 (53.4%) | 132/267 (49.4%) | ns | |
| CD49d positive (≥30%) | 31/38 (81.6%) | 58/75 (77.3%) | ns | |
| β-2-microglobulin |
Normal levels
Above the limit |
47/134 (35.1%) 87/134 (64.9%) |
146/258 (56.6%) 112/258 (43.4%) |
<0.001 |
| IGHV mutational status |
Mutated
Unmutated |
40/117 (34.2%) 77/117 (65.8%) |
113/238 (47.5%) 125/238 (52.5%) |
0.017 |
M/F: male/female; LDH: Lactate DeHydrogenase; IGHV: immunoglobulin heavy-chain variable region gene.
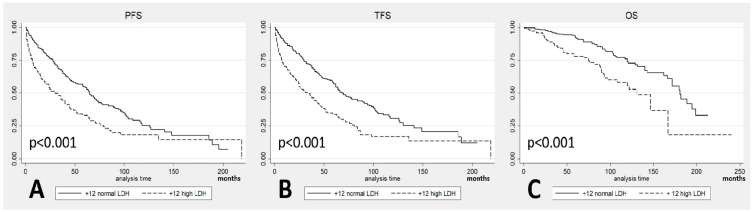
The patients with +12 and high LDH levels progressed in 72% of the cases with a median PFS of 30 months (CI 95% 20–42) and needed treatment in 70% of the cases with a median TFS of 33 months (CI 95% 24–45). In comparison, the patients with +12 and normal LDH levels progressed in 59% of the cases with a median PFS of 65 months (CI 95% 58–73) and needed treatment in 55% of cases with a median TFS of 69 months (CI 95% 62–89). Regarding OS, 113 patients died among the +12 CLL patients, 47 (33%) of those with high LDH and 66 (21%) of those with normal LDH. The median OS was 131 months (CI 95% 97–167) in the patients with high LDH and 181 months (CI 95% 166–199) in the patients with normal LDH. Comparing the outcomes between the two subgroups, statistically significant shorter PFS (p < 0.001; Figure 2A), TFS (p < 0.001; Figure 2B), and OS (p < 0.001; Figure 2C) were observed in the patients with high LDH levels.
Figure 2.
(A) Progression-free-survival (PFS) in patients with +12 stratified according to Lactate DeHydrogenase (LDH) levels; (B) Treatment-free-survival (TFS) in patients with +12 stratified according to LDH levels; (C) Overall survival (OS) in patients with +12 stratified according to LDH levels.
We then compared the causes of death in these patients. Thirty (64% of all the deaths) in the high LDH group and 23 (35%) in the normal LDH group were due to CLL. The median CLL-specific survival was 147 months in the high LDH group and 190 months in the normal LDH group with a different rate of CLL-related mortality at 10 years: 29% vs. 11% (Figure 3).
Figure 3.
Chronic Lymphocytic Leukemia (CLL)-specific survival in patients with +12 stratified according to Lactate DeHydrogenase (LDH) levels.
When performing multivariate analysis in CLL patients with +12 according to CLL-specific survival, the role of LDH was confirmed (p < 0.001, hazard ratio (HR) 3.78, CI 95% 1.73–8.26; Table S1).
We then investigated whether the negative prognostic role of LDH observed in +12 CLL could be extended to CLL patients with negative FISH. Analyzing the same variables and using the same univariate model followed by a multivariate model for the significant variables, LDH had a role for PFS and TFS in univariate analysis but it was not significant in multivariate analysis in which Binet stage, CD38, β-2-microglobulin, and the IGHV status showed a significant role. With respect to OS and CLL-specific survival, LDH was not significant even in univariate analysis (Table S2).
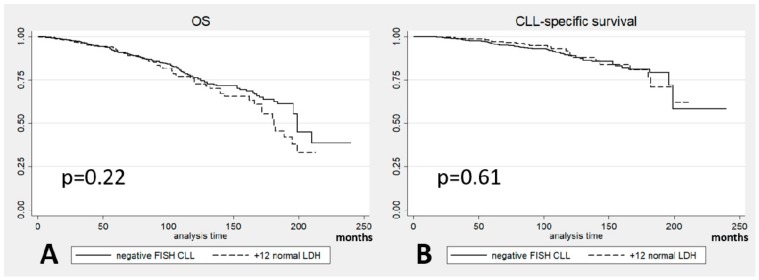
Patients with +12 and normal LDH levels showed no significant differences in survival compared to patients with negative FISH (p = 0.22 for OS and p = 0.61 for CLL-specific survival; Figure 4A,B) indicating that the difference in outcomes is dependent on the patients with +12 and elevated LDH.
Figure 4.
(A) Overall survival (OS) and (B) Chronic Lymphocytic Leukemia (CLL)-specific survival in +12 CLL patients with normal Lactate DeHydrogenase (LDH) levels vs. Fluorescence In Situ Hybridization (FISH) negative CLL patients.
2.2.3. Validation Cohort
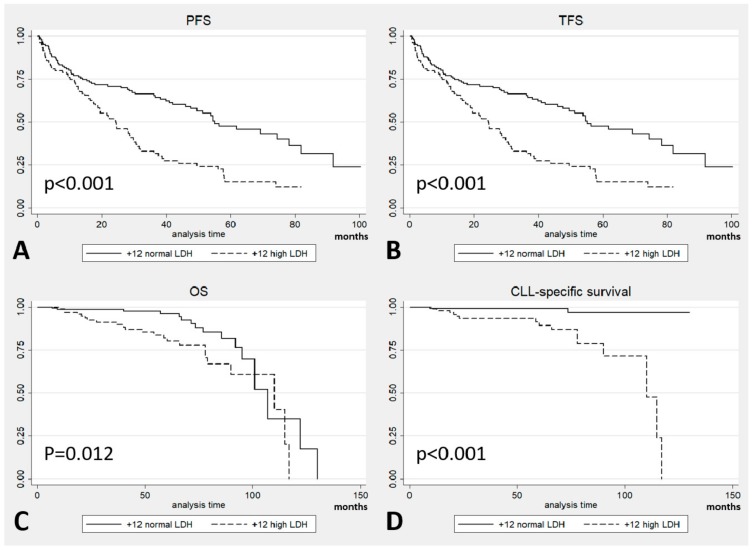
To validate the worse prognosis of +12 CLL patients with high LDH levels, the same analysis was performed on a population of 250 patients with +12 from a single US institution. Baseline patients’ characteristics at presentation are shown in Table S3. This population was divided according to LDH levels available at diagnosis: the two subgroups of 104 and 145 patients with high LDH levels and normal LDH levels, respectively, are presented in Table S4. Differences in the outcomes were found also in these subgroups: patients with high LDH levels showed a shorter median PFS (24 months vs. 55 months in patients with normal LDH levels, p < 0.001; Figure 5A), shorter median TFS (25 months vs. 58 months, p < 0.001; Figure 5B), and higher death rate (22% vs. 11% from all causes and 65% vs. 12% for CLL-related mortality). Also OS (92 months vs. 103 months; p = 0.012; Figure 5C) and CLL-specific survival (99 months vs. 128 months; p < 0.001; Figure 5D) were significantly shorter in the high LDH subgroup.
Figure 5.
Outcomes in the validation cohort by Lactate DeHydrogenase (LDH) levels: (A) Progression-free-survival (PFS); (B) Treatment-free-survival (TFS); (C) Overall survival (OS); and (D) Chronic Lymphocytic Leukemia (CLL)-specific survival.
A univariate analysis was performed in this validation cohort, as in the multicenter cohort: Rai stage, LDH, ZAP70 and β-2-microglobulin resulted significant for PFS and TFS; age, LDH and β-2-microglobulin for OS; LDH and β-2-microglobulin for CLL-specific survival. When these variables were analysed in a multivariate model, LDH was the sole negative independent parameter for PFS, TFS, and CLL-specific survival; LDH and age were significant for OS (Table S5).
3. Discussion
Our retrospective study confirmed that CLL patients showing +12 on FISH analysis have unique clinical and biologic features as reported in the literature [12,25]. The higher expression of CD38 observed in +12 by Athanasiadou et al. [14] was confirmed by our data with a rate of 51%; also the rates of CD49d and ZAP70 were higher in +12 CLL vs. negative FISH CLL (79% and 54% vs. 27% and 36%, respectively). Finally, the higher prevalence of unmutated IGHV in our series of +12 CLL patients (57%) confirmed previously published data [17].
In our series, CLL patients with +12 had a worse prognosis compared to CLL patients with negative FISH disease. The biomarkers associated with shorter PFS, TFS, OS in multivariate analysis were high LDH, unmutated IGHV, and elevated β-2-microglobulin. It resulted that Rai stage was significant only for shorter PFS and shorter TFS, and age only for shorter OS.
Data about the correlation between the IGHV status and outcome in CLL are well known from the literature, including +12 patients [20]. Also in our cohort, high levels of β-2-microglobulin predicted a worse prognosis in terms of PFS, TFS, and OS, as reported in the literature. Advanced Rai stage had an impact on PFS and TFS and older age on OS, as expected.
Analyses about clinicopathologic risk categorization of untreated CLL patients aimed to find predictive outcomes variables: a parameter such as LDH was identified in a study as an independent predictor of TFS independently from FISH categories and the impact of LDH was greater for patients with unmutated IGHV [28], in another case LDH was not included among the variables for a comprehensive prognostic index [29], but our report is the first one about biomarker approaches on the relationship between +12, high LDH levels, and outcome using both a discovery and a validation series of a great size.
When we attempted to analyse the role of LDH by stratifying the +12 CLL cohort according to the LDH levels at diagnosis, patients with high LDH levels showed a significantly worse outcome in terms of PFS, TFS, OS, and CLL-specific survival both in univariate and multivariate analyses. None of the +12 CLL patients, including those with high LDH levels, showed signs or symptoms suggestive of RS at the onset of the disease.
Overall, our data are consistent with previous reports that +12 CLL patients have an intermediate risk of progression compared to the other FISH-defined prognostic subsets [7]. However, stratifying by LDH levels, it appeared clear that +12 CLL patients with high LDH levels showed a worse prognosis compared to +12 CLL patients with normal LDH levels, for whom prognosis was similar to that of patients with negative FISH.
In a wide validation cohort of 250 patients, the parameters which resulted significant were comparable to the ones of the multicenter population, confirming the predictive role of LDH: it resulted that it was the sole negative independent biomarker for PFS, TFS, and CLL-specific survival and it was significant for OS together with age. These data reinforced the impact of LDH in the +12 CLL population, which is marked by unique clinical and biological features that could explain a high rate of LDH levels above the limit, possibly linked also to atypical morphological characteristics of their cells. So it is suggested that LDH, an easily available individualized tool capable of predicting a worse PFS, TFS, and OS, should be taken into account in daily clinical practice.
It would be useful in future studies to investigate outcomes in the LDH-high and LDH-normal +12 CLL patients according to the treatment received, in particular after therapy with the novel targeted agents. Unfortunately, this was not possible in the current study because of the relatively small number of patients treated with targeted agents and short follow-up, precluding a meaningful statistical comparison. In addition, future studies should address the association between high LDH levels and NOTCH1 mutation, which has been detected in 30–40% of +12 cases. Such data were not available for our current study and may provide an explanation for the correlation between high LDH levels and poor prognosis in CLL patients with the +12 abnormality.
4. Materials and Methods
This is a retrospective observational study including treatment-naive CLL patients with +12 as an isolated aberration from 15 academic Italian centers, diagnosed between January 2000 and July 2016. A second cohort of patients who resulted negative for a FISH panel comprising the four common abnormalities, i.e., del13q, +12, del11q, and del17p, was collected from the same centers during the same study period. A previously published overlapping cohort of patients with +12 CLL followed at a single US institution was used for external validation but new and updated data were included [27].
CLL was diagnosed in all the patients according to the 2008 International Workshop on CLL (iwCLL) guidelines [30]. Patients were also screened at baseline with a direct antiglobulin test and radiological examinations and if other possible causes of LDH elevation such as signs or symptoms suggestive of RS or haemolytic anaemia were noted, these patients were excluded from the analysis.
Demographic, clinical, and laboratory data were collected for each patient. Older age was defined as ≥65 years or older, advanced stage as Rai stage III–IV and Binet stage C.
IGHV somatic mutation status and expression of CD38, ZAP70, CD49d, were performed in a standardized fashion by all involved centers, as previously described [31,32]. FISH analysis was performed on interphase nuclei of CLL cells of the peripheral blood and the panel included probes specific to TP53 (17p13.1), ATM (11q22.3), D13S319 (13q14.3), LAMP1 (13q34), and the centromeric region of chromosome 12 (12p11.1–q11) [33]. Disease progression, treatment initiation, and death were recorded to calculate PFS, TFS, and OS.
The study was approved by the Ethics Committee of the ’Fondazione Policlinico Gemelli’ (Protocol No. 0028829/16; Date: 13th July 2016) and was conducted in accordance with the principles of the Declaration of Helsinki. The clinical and laboratory features were obtained by review of the medical records and all the data were centrally collected and analysed.
PFS, TFS, and OS were calculated from the date of diagnosis to the date of progression, treatment, and death, respectively, or the date of last follow-up. We defined CLL-specific death as death secondary to progressive disease, RS, infections, and complications during the treatment or in patients with an active disease.
Normality distributions of all variables were tested by the Shapiro-Wilk and Shapiro-Francia tests. Chi-square test or Fisher’s exact test were used to compare categorical variables (age, gender, Binet and Rai stage, LDH and β-2-microglobulin levels, positivity for ZAP70, CD38 and CD49d, IGHV mutational status), while the Wilcoxon-Mann-Whitney test was applied for continuous variables (white blood and lymphocytes counts, haemoglobin and platelets levels, bone marrow infiltration). Data were summarized as medians, 25th and 75th percentiles. The Kaplan–Meier method was used for survival analyses, and the log-rank test was performed to compare patient subgroups. Univariate and multivariate Cox proportional hazards regression models were fit to assess associations between patients’ characteristics and survival times. The proportional hazards assumption was assessed using the method of Grambsch et al. [34]. Only variables in univariate Cox analysis with a p < 0.01 (or p < 0.05 for validation cohort) were added to the multivariate Cox regression model. p values lower than 0.05 were considered statistically significant and reported as two-sided. All statistics were carried out with the use of STATA/SE 12.0 for Windows.
5. Conclusions
From our multicenter study on 487 patients with +12 and 816 patients with negative FISH and from the analysis of our validation cohort on 250 patients with +12, it emerged that (1) CLL patients with +12 have a significantly higher prevalence of elevated LDH compared to patients without FISH abnormalities, (2) +12 CLL patients have a worse prognosis than negative FISH CLL patients, (3) +12 CLL patients with normal LDH levels have the same prognosis as that of negative FISH patients, and (4) +12 CLL patients with high LDH levels show a worse PFS, TFS, and OS than +12 CLL patients with normal LDH levels or negative FISH patients independently of LDH levels.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/7/896/s1, Table S1: Multivariate analysis in +12 CLL patients (Cox regression analysis); Table S2: Multivariate analysis in negative FISH CLL patients (Cox regression analysis); Table S3: Baseline characteristics of the validation cohort; Table S4: Baseline characteristics of the validation cohort CLL patients with +12 divided in two subgroups according to LDH levels; Table S5: Multivariate analysis in validation cohort patients (Cox regression analysis).
Author Contributions
Conceptualization and methodology, F.A., P.S., I.I., A.F. (Alessandra Ferrajoli) and L.L.; formal analysis, F.C.; investigation, resources and data curation, L.T., A.C. (Agostino Cortelezzi), C.V. (Carlo Visco), M.C., A.C. (Antonio Cuneo), A.G., F.R.M., A.M.F., M.G., F.M., S.M., P.F., G.D.A., R.M., D.V., A.F. (Antonietta Ferretti), G.M.R., C.V. (Candida Vitale), M.C.T., G.R., A.V., S.S. and R.F.; writing original draft preparation and review and editing, F.A., P.S., D.G.E., A.F. (Alessandra Ferrajoli) and L.L; and all authors critically reviewed and approved the final version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Eichhorst B., Dreyling M., Robak T., Montserrat E., Hallek M., ESMO Guidelines Working Group Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2011;22:50–54. doi: 10.1093/annonc/mdr377. [DOI] [PubMed] [Google Scholar]
- 2.Zenz T., Fröhling S., Mertens D., Döhner H., Stilgenbauer S. Moving from prognostic to predictive factors in chronic lymphocytic leukaemia (CLL) Best Pr. Res. Clin. Haematol. 2010;23:71–84. doi: 10.1016/j.beha.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Furman R.R. Prognostic markers and stratification of chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2010;2010:77–81. doi: 10.1182/asheducation-2010.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Pospisilova S., Gonzalez D., Malcikova J., Trbusek M., Rossi D., Kater A.P., Hillmen P. ERIC recommendations on TP53 mutation analysis in chronic lymphocytic leukemia. Leukemia. 2012;26:1458–1461. doi: 10.1038/leu.2012.25. [DOI] [PubMed] [Google Scholar]
- 5.Haferlach C., Dicker F., Schnittger S., Kern W., Haferlach T. Comprehensive genetic characterization of CLL: A study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia. 2007;21:2442–2451. doi: 10.1038/sj.leu.2404935. [DOI] [PubMed] [Google Scholar]
- 6.Rossi D., Rasi S., Spina V., Bruscaggin A., Monti S., Ciardullo C., Forconi F. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121:1403–1412. doi: 10.1182/blood-2012-09-458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Döhner H., Stilgenbauer S., Benner A., Leupolt E., Kröber A., Bullinger L., Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 8.Stilgenbauer S., Dohner K., Bentz M., Lichter P., Dohner H. Molecular cytogenetic analysis of B-cell chronic lymphocytic leukemia. Ann. Hemato. L. 1998;76:101–110. doi: 10.1007/s002770050373. [DOI] [PubMed] [Google Scholar]
- 9.Quijano S., López A., Rasillo A., Sayagués J.M., Barrena S., Sánchez M.L., Romero M. Impact of trisomy 12, del(13q), del(17p), and del(11q) on the immunophenotype, DNA ploidy status, and proliferative rate of leukemic B-cells in chronic lymphocytic leukemia. Cytom. B Clin. Cytom. 2008;74:139–149. doi: 10.1002/cyto.b.20390. [DOI] [PubMed] [Google Scholar]
- 10.Šindelářová L., Michalová K., Zemanová Z., Ransdorfová Š., Březinová J., Peková S., Cmunt E. Incidence of chromosomal anomalies detected with FISH and their clinical correlations in B-chronic lymphocytic leukemia. Cancer Genet. Cytogenet. 2005;160:27–34. doi: 10.1016/j.cancergencyto.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Dohner H., Stilgenbauer S., Fischer K., Bentz M., Lichter P. Cytogenetic and molecular cytogenetic analysis of B cell chronic lymphocytic leukemia: Specific chromosome aberrations identify prognostic subgroups of patients and point to loci of candidate genes. Leukemia. 1997;11(Suppl. 2):S19–S24. [PubMed] [Google Scholar]
- 12.Autore F., Strati P., Laurenti L., Ferrajoli A. Morphological, immunophenotypic, and genetic features of chronic lymphocytic leukemia with trisomy 12: A comprehensive review. Haematologica. 2018;103:931–938. doi: 10.3324/haematol.2017.186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Que T.H., Marco J.G., Ellis J., Matutes E., Babapulle V.B., Boyle S., Catovsky D. Trisomy 12 in chronic lymphocytic leukemia detected by fluorescence in situ hybridization: Analysis by stage, immunophenotype, and morphology. Blood. 1993;82:571–575. [PubMed] [Google Scholar]
- 14.Athanasiadou A., Stamatopoulos K., Tsompanakou A., Gaitatzi M., Kalogiannidis P., Anagnostopoulos A., Tsezou A. Clinical, immunophenotypic, and molecular profiling of trisomy 12 in chronic lymphocytic leukemia and comparison with other karyotypic subgroups defined by cytogenetic analysis. Cancer Genet. Cytogenet. 2006;168:109–119. doi: 10.1016/j.cancergencyto.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Criel A., Verhoef G., Vlietinck R., Mecucci C., Billiet J., Michaux L., Boogaerts M. Further characterization of morphologically defined typical and atypical CLL: A clinical, immunophenotypic, cytogenetic and prognostic study on 390 cases. Br. J. Haematol. 1997;97:383–391. doi: 10.1046/j.1365-2141.1997.402686.x. [DOI] [PubMed] [Google Scholar]
- 16.Matutes E., Oscier D., Garcia-Marco J., Ellis J., Copplestone A., Gillingham R., Catovsky D. Trisomy 12 defines a group of CLL with atypical morphology: Correlation between cytogenetic, clinical and laboratory features in 544 patients. Br. J. Haematol. 1996;92:382–388. doi: 10.1046/j.1365-2141.1996.d01-1478.x. [DOI] [PubMed] [Google Scholar]
- 17.Falisi E., Novella E., Visco C., Guercini N., Maura F., Giaretta I., Neri A. B-cell receptor configuration and mutational analysis of patients with chronic lymphocytic leukaemia and trisomy 12 reveal recurrent molecular abnormalities. Hematol. Oncol. 2014;32:22–30. doi: 10.1002/hon.2086. [DOI] [PubMed] [Google Scholar]
- 18.Rossi D., Spina V., Cerri M., Rasi S., Deambrogi C., De Paoli L., Zucca E. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin. Cancer Res. 2009;15:4415–4422. doi: 10.1158/1078-0432.CCR-08-3266. [DOI] [PubMed] [Google Scholar]
- 19.Maura F., Cutrona G., Fabris S., Colombo M., Tuana G., Agnelli L., Di Raimondo F. Relevance of stereotyped B-cell receptors in the context of the molecular, cytogenetic and clinical features of chronic lymphocytic leukemia. PLoS ONE. 2011;6:e24313. doi: 10.1371/journal.pone.0024313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulian P., Bomben R., Dal Bo M., Zucchetto A., Rossi F.M., Degan M., Cerri M. Mutational status of IGHV is the most reliable prognostic marker in trisomy 12 chronic lymphocytic leukemia. Haematologica. 2017;102:e443–e446. doi: 10.3324/haematol.2017.170340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zenz T., Vollmer D., Trbusek M., Smardova J., Benner A., Soussi T., Denzel T. TP53 mutation profile in chronic lymphocytic leukemia: Evidence for a disease specific profile from a comprehensive analysis of 268 mutations. Leukemia. 2010;24:2072–2079. doi: 10.1038/leu.2010.208. [DOI] [PubMed] [Google Scholar]
- 22.Balatti V., Bottoni A., Palamarchuk A., Alder H., Rassenti L.Z., Kipps T.J., Croce C.M. NOTCH1 mutations in CLL associated with trisomy 12. Blood. 2012;119:329–331. doi: 10.1182/blood-2011-10-386144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balatti V., Lerner S., Rizzotto L., Rassenti L.Z., Bottoni A., Palamarchuk A., Pekarsky Y. Trisomy 12 CLLs progress through NOTCH1 mutations. Leukemia. 2013;27:740–743. doi: 10.1038/leu.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissmann S., Roller A., Jeromin S., Hernandez M., Abaigar M., Hernandez-Rivas J.M., Schnittger S. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): A study on 852 patients. Leukemia. 2013;27:2393–2396. doi: 10.1038/leu.2013.218. [DOI] [PubMed] [Google Scholar]
- 25.Del Giudice I., Rossi D., Chiaretti S., Marinelli M., Tavolaro S., Gabrielli S., Guarini A. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica. 2012;97:437–441. doi: 10.3324/haematol.2011.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Gascón y Marín I., Hernández-Sánchez M., Rodríguez-Vicente A.E., Sanzo C., Aventín A., Puiggros A., Ortega M. A high proportion of cells carrying trisomy 12 is associated with a worse outcome in patients with chronic lymphocytic leukemia. Hematol. Oncol. 2016;34:84–92. doi: 10.1002/hon.2196. [DOI] [PubMed] [Google Scholar]
- 27.Strati P., Abruzzo L.V., Wierda W.G., O’Brien S., Ferrajoli A., Keating M.J. Second cancers and Richter transformation are the leading causes of death in patients with trisomy 12 chronic lymphocytic leukemia. Clin. Lymphoma Myeloma Leuk. 2015;15:420–427. doi: 10.1016/j.clml.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wierda W.G., O’Brien S., Wang X., Faderl S., Ferrajoli A., Do K.A., Burger J.A. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J. Clin. Oncol. 2011;29:4088–4095. doi: 10.1200/JCO.2010.33.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflug N., Bahlo J., Shanafelt T.D., Eichhorst B.F., Bergmann M.A., Elter T., Döhner H. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124:49–62. doi: 10.1182/blood-2014-02-556399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., Kipps T.J. A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanafelt T.D., Witzig T.E., Fink S.R., Jenkins R.B., Paternoster S.F., Smoley S.A., Geyer S. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J. Clin. Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 32.Rassenti L.Z., Huynh L., Toy T.L., Chen L., Keating M.J., Gribben J.G., Kay N.E. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 33.Döhner H., Stilgenbauer S., James M.R., Benner A., Weilguni T., Bentz M., Lichter P. 11q Deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89:2516–2522. [PubMed] [Google Scholar]
- 34.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.