Abstract
Diabetic retinopathy (DR) is reaching epidemic levels globally due to the increase in prevalence of diabetes mellitus (DM). DR also has detrimental effects to quality of life, as it is the leading cause of blindness in the working-age population and the most common cause of vision loss in individuals with DM. Over several decades, many studies have recognized the role of inflammation in the development and progression of DR; however, in recent years, accumulating evidence has also suggested that non-coding RNAs, especially long non-coding (lncRNAs), are aberrantly expressed in diabetes and may play a putative role in the development and progression of DR through the modulation of gene expression at the transcriptional, post-transcriptional, or epigenetic level. In this review, we will first highlight some of the key inflammatory mediators and transcription factors involved in DR, and we will then introduce the critical roles of lncRNAs in DR and inflammation. Following this, we will discuss the implications of lncRNAs in other epigenetic mechanisms that may also contribute to the progression of inflammation in DR.
Keywords: diabetic retinopathy, inflammation, lncRNAs, epigenetics, histone modifications, DNA methylation, miRNAs
1. Introduction
The increase in the global prevalence of diabetic retinopathy is intimately connected to the soaring prevalence of diabetes mellitus (DM) to an epidemic proportion [1,2,3,4,5,6]. Diabetic retinopathy (DR) is the leading cause of blindness in the working-age population and the most common cause of vision loss in individuals with DM [7,8,9]. In 2015, 2.6 million people suffered from visual impairment due to DR; this figure is projected to reach 3.2 million people in 2020 [6,10]. As the life expectancy of individuals with diabetes increases due to medical advances, the prevalence of DR is expected to further magnify unless improvements are made in the current diagnosis, management, and treatment of this disease through understanding of the underlying pathogenetic mechanisms. Moreover, a persistent and complex problem surrounding the adherence to diabetic eye care guidelines among patients with diabetes due to the lack of knowledge of diabetic complications exist, as well as the asymptomatic features of the disease in the presence of major microvasculature changes, which likely perpetuates this increase in prevalence [11,12,13]. DR is a chronic microvascular complication of DM and is associated with a longer duration of diabetes and poor control of blood sugar, lipids, and blood pressure [14,15]. The majority of diabetics with type 1 and greater than 60% of diabetics with type 2 develop signs of DR within 20 years of diagnosis of DM [16,17,18,19,20,21]. DR progresses from non-proliferative diabetic retinopathy (NPDR) to more advanced forms of vision-threatening diabetic retinopathy that include proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME). Clinically, NPDR is differentiated from more advanced forms of DR by the lack of neovascularization; however, an eye with NPDR may present with classic DR signs such as microaneurysms, intraretinal hemorrhages, venous beading, intraretinal microvascular abnormalities, and hard exudates [22]. PDR is characterized by the presence of retinal neovascularization due to ischemia that results from vascular occlusion [22]. DME occurs as a result of the accumulation of fluid in the neural retina, which leads to thickening of the retina and cystoid macular edema. DME is another important factor to be considered, as it may be present in any of the stages of DR [22]. Hyperglycemia is implicated in the pathogenesis of DR and induces a variety of biochemical pathways including genetic and epigenetic factors, advanced glycation end-products formation, polyol pathway, protein kinase C pathway, hexosamine pathway, retinal renin-angiotensin system, and numerous inflammatory mechanisms. With the prevalence of DR reaching paramount levels and the risk this disease poses to vision and thus quality of life, understanding the molecular mechanisms implicated in the pathogenesis of DR becomes critical. In this review, we explore the current state of knowledge surrounding the roles of long non-coding RNAs (lncRNAs) and inflammation in DR. Further, we will critically discuss the relevance of this knowledge to the pathogenesis of DR and the importance of novel approaches to diagnosis, treatment, and management of DR that go beyond the current standards of care.
2. Inflammation and Diabetic Retinopathy (DR)
Microvasculature instability in DR is a result of the combination of increased vascular permeability and vascular occlusion [22]. Vascular endothelial growth factor (VEGF) is a known contributor to vascular dysfunction in later stages of DR through increasing vascular leakage and angiogenesis [23,24]. Anti-VEGF therapy is useful for the treatment of DME as well as later stages of DR, when significant alterations (angiogenesis) to the retina have occurred. Moreover, anti-VEGF treatment has significant side-effects, requires repeated intraocular injections, and most importantly, only approximately 50% of patients respond to therapy [25]. There has been increasing evidence for the role of inflammation in the development and progression of DR; however, the detailed mechanisms initiating these inflammatory changes have yet to be elucidated [26,27]. Multiple inflammatory mediators and transcription factors work in concert to mediate such effects.
2.1. NF-κB
NF-κB is a ubiquitous transcription factor regulating the expression of cytokines, chemokines, growth factors, and cell adhesion. Most commonly, NF-κB is composed of p65 and p50 subunits, which when activated translocates into the nucleus as a p50-p65 heterodimer and initiates pro-inflammatory protein transcription (notably, iNOS2, ICAM-1, IL-1β, and TNF-α) [28,29,30,31,32,33,34,35,36,37]. Due to this, NF-κB is suggested to play a critical role in the development and progression of DR by inducing an overt-inflammatory response [31,33]. Hyperglycemic induced activation of NF-κB occurs very early in the development of DR and is an important signaling pathway that induces apoptosis in retinal endothelial cells [38,39]. Retinal capillaries of diabetic eye donors show increased numbers of retinal pericytes with activated NF-κB relative to non-diabetic donors, while endothelial cells in both were negative [40]. In addition, NF-κB activation induced by hyperglycemia may have pro-apoptotic consequences in retinal pericytes by accelerating loss of these cells in DR [40]. Interestingly, selective inhibition of NF-κB activation with dehydroxymethylepoxyquinomicin inhibited diabetes-induced retinal leukostasis and retinal expressions of ICAM-1 and VEGF in vivo [41]. Nevertheless, less specific therapies such as salicylates (aspirin, sodium salicylate, and sulfasalazine) have been shown to inhibit NF-κB activation in diabetes; thus, inhibiting degeneration of retinal capillaries and preventing ganglion cell death in diabetic rats [42]. In addition, non-specific therapy using multiple antioxidants including ascorbic acid, β-carotene, and selenium has been demonstrated to impede the development of DR through inhibition of NF-κB in diabetic rats [43].
2.2. Cytokines and Chemokines
A number of inflammatory cytokines and chemokines including IL-6, IL-8, IL-1β, and TNF-α have been shown to be elevated in diabetic vitreous samples [44,45,46]. Interestingly, one study showed higher concentrations of IL-8 and TNF-α in vitreous samples from eyes with NPDR than eyes with PDR [44]. However, another study demonstrated increasing concentrations of IL-1β, IL-2, IFN-γ, TNF-α, IL-4, IL-5, IL-6, and IL-10 in the aqueous humor associated with increasing severity of DR [47]. Similarly, in the context of DME, significant differences have been reported for several cytokines in the aqueous humor of DME patients and intravitreal administration of aflibercept (an anti-angiogenic agent) was shown to decrease the concentration of certain cytokines (including VEGF, IL-6, and IL-1β) [48]. Of note, the total vitreous protein concentration between patients with NPDR and PDR is comparable, which likely suggests that increased protein levels found in these samples is likely attributed to secretion rather than vascular leakage into the vitreous due to increased permeability [44]. These inflammatory mediators are produced by activated microglia, macroglia, endothelial cells, and even neurons at more advanced stages of DR [47]. Regardless, inflammatory cytokines are seen in early DR and the inflammatory response progresses throughout all cell types of the retina and mediate DR progression [44,47]. IL-1β is likely a crucial mediator associated with early damage in DR and the increasing concentration throughout the development of DR might promote the inflammatory process and initiate the production of other inflammatory mediators [49,50]. The accumulation of these inflammatory mediators has been proposed to contribute to angiogenesis and neurodegeneration in DR. Angiogenic responses of endothelial cells is induced directly by inflammatory cytokines such as IL-1β, IFN-γ, and TNF-α and indirectly by inducing endothelial cells to produce growth factors rather than a direct effect of hyperglycemia on endothelial cells [51,52,53,54]. Inflammatory cytokines also stimulate endothelial cell secretion of adhesion molecules such as ICAM-1; thus, promoting leukostasis [55]. Cumulatively, these processes lead to microvascular instability comprised of increased vascular permeability which allows increased leakage of vascular fluid and migration of immune cells into the retina as well as vascular occlusion due to endothelial cell degeneration [56,57,58,59]. The resulting ischemia and hypoxia of the retina promotes VEGF expression and further pro-inflammatory cytokine and chemokine production resulting in angiogenesis in PDR. VEGF is a double-edged sword in not only promoting inflammation and angiogenesis, but also promoting neuronal growth, differentiation, and survival [60,61,62]. Early in DR, cytokines and growth factor production may be a way to maintain neuronal function through increasing VEGF levels; however, as levels of proinflammatory mediators increase, they become detrimental and impair the positive effect of VEGF leading to eventual neuronal death in the retina.
2.3. Complement System
The complement system, a part of the innate immune system with a central role in host defense against infectious pathogens, has been shown to be dysregulated in DR. A fully activated complement pathway leads to C3/C5 convertase generation and, ultimately, to the formation of the membrane attack complex (MAC), which can kill pathogens, and in some instances, host cells; thus, potentially contributing to neurodegeneration in DR. Moreover, some studies suggest that dysregulation through elevated complement protein (C5b-9) deposition in the retinal vascular lumen and reduction of complement inhibitor proteins (CD55 and CD59) may be related to DR progression [63,64]. Additionally, hyperglycemic by-products, such as methylglyoxal greatly impair the function of complement regulatory proteins including the C1 inhibitor [65,66]. Deposition of C5b-9, the terminal product of the complement pathway, is present within the retinal blood vessels of diabetic patient donors, while notably not present in non-diabetic patient donors [65]. Another study found extensive deposition of C5b-9, C3d, and vitronectin (acts by forming a stable complex with extracellular C5b-9) in the retinal vascular lumen of patient donors with clinically evident DR, but the absence of the above in the majority of control patient donors [63]. Extensive deposition of complement factors in the retinal vascular lumen leads to MAC formation, which likely contributes to retinal endothelial cell death and increased retinal vascular permeability in diabetes [64]. Key players in the complement pathway (C4b, factor B, C3, and C9) are also elevated in the vitreous of patients with PDR relative to non-diabetic controls [67,68]. Complement pathway factors such as C3a and C5a are chemotactic and activate neutrophils, which parallels with findings from studies demonstrating increased numbers of neutrophils in diabetic retinal vessels [69]. Activated neutrophils worsen microvascular instability in DR via incurring damage to the endothelium, which leads to increased levels of plasma components in the connective tissue matrix, potentially exacerbating the inflammatory response found in diabetes [69]. Lastly, C5aR is constitutively expressed on Müller cells, the expression of which is up-regulated by prostaglandin E2, and most critically, hyperglycemia, and is associated with upregulation of IL-6 and VEGF leading to increased retinal endothelial cell proliferation and permeability [70].
Given the complex nature of cellular environments, the above summary of inflammatory processes involved in the development and progression of DR are by no means exhaustive. Though a detailed discussion of other DR-related molecular alterations (i.e., in matrix metalloproteinases [71], toll-like receptors [72], and α-crystallins [73]) goes beyond the scope of this review, future research is expected to provide additional knowledge on the inflammatory pathways contributing to DR. Considering the impact inflammatory pathways have in the pathogenesis of DR, inhibition of these inflammatory processes may be an appealing option to integrate into the future standard of care. However, further understanding on the particular initiators propagating inflammation in DR is needed. In the last few years, accumulating evidence has suggested that non-coding RNAs, especially lncRNAs may be aberrantly expressed in diabetes and may play a putative role in the development and progression of DR through modulation of gene expression at the transcriptional, post-transcriptional, or epigenetic level [74]. In addition, the role of lncRNAs in DR deserves to be investigated as they may also have a role as new biomarkers offering diagnostic value or in future novel treatments of DR. Thus, in the next section(s), we will explore the role of lncRNAs in the pathogenesis of DR and the crucial nature of their role in stability and maintenance of gene expression patterns, especially relating to inflammatory pathways.
3. LncRNAs: Novel, Emerging, Regulatory RNA Molecules
As novel sequencing technologies continue to rapidly emerge [75], the identification of non-coding loci, in parallel, grows at an unprecedented rate. Non-coding DNA regions constitute more than 98% of the human genome [76] and due to the pervasiveness of transcription [77], the transcriptional products from certain non-coding RNA genes can serve critical roles in a diverse array of biological processes, ranging from embryonic development [78] to proper maintenance of the immune system [79]. Amongst the various non-coding RNAs, long non-coding RNAs (lncRNAs) are a class of fundamental RNA transcripts that are larger than 200 base-pairs and generally do not have protein-coding potential. Mechanistically, lncRNAs are capable of governing gene expressions through a number of different means: (i) Serving as a decoy for transcription factors, which can enable gene inactivation [80], (ii) guiding certain proteins, such as chromatin-modifying enzymes, to certain regions of the DNA [81], (iii) acting as a scaffold for the assembly of multiple protein subunits into complexes [82], (iv) functioning as a molecular sponge that sequesters pertinent microRNAs (miRNAs) to allow or prevent the translation of distinct messenger RNAs (mRNAs) [83,84], or by (v) directly enhancing the activation of neighboring genes [85]. Given the complex regulatory nature of these transcripts, certain lncRNAs may follow more than one of the above mechanisms and present with distinct functionalities and structural features depending on their subcellular localization. For example, nuclear-retained lncRNAs are typically implicated in transcriptional regulation [86], alternative splicing [87], and in the organization of nuclear architecture [88]; while, cytoplasmic lncRNAs are involved in post-transcriptional modifications that determine the stability and translation potential of mRNAs [89]. Remarkably, other lncRNAs have been documented to be present in both cellular compartments (the nucleus and cytoplasm), where these transcripts have versatile roles in shaping the epigenome and influencing pertinent biological processes such as transcription and translation [90,91]. Notably, recent reports are beginning to demonstrate that lncRNAs can also be present in the mitochondria [92]—alluding to the unique diversification of these RNA molecules.
In addition to their subcellular localization, the site of biogenesis can further classify lncRNAs. For example, recent updates in the classification system broadly categorize lncRNAs as either intergenic (not intersecting with any protein-coding genes) or intragenic/genic (overlapping protein-coding genes) [93,94,95]. In particular, long intergenic ncRNAs (lincRNAs) arise from intergenic regions (a span of DNA sequences situated between two genes), and albeit variably, possess a greater degree of evolutionary conservation at the sequence and RNA secondary structure level compared to intragenic lncRNAs [93,94,95,96]. Conversely, intragenic lncRNAs are transcribed in distinct regions that intersect with protein-coding loci and depending on their genomic location, these lncRNAs can be further defined as bidirectional (transcribed in a divergent manner from the promoter of a protein-coding gene on the opposite strand), intronic (originates from only the intronic regions of a protein-coding gene in either direction), antisense (transcribed on the anti-sense/non-coding strand of a protein-coding gene and may overlap the coding exons of the gene), and sense (transcribed from the sense/coding strand of the protein-coding gene and may overlap the coding exons of the gene) [93,94,95]. Despite the numerous transcriptional orientations existing for lncRNAs, it is likely that many of these RNA molecules share comparable mechanistic and functional properties that are involved in governing the genomic landscape, whether through cis (nearby) or trans (distant)-acting mechanisms.
Nevertheless, although thousands of lncRNAs continue to be annotated on a daily basis, only a small subset of these RNA molecules is functionally characterized. In comparison to their miRNA counterparts, a lot less is known about the mechanistic abilities of lncRNAs in certain disease contexts, particularly in diabetes. Therefore, in this review, we will first present the putative roles of lncRNAs in DR and inflammation, and then discuss their implications in other epigenetic mechanisms that may also contribute to the progression of inflammation in DR. Of note, we understand that the lncRNAs we will be examining in this review are non-exhaustive; however, we hope that by acknowledging the interconnectedness between these key players in inflammation and other epigenetic mechanisms, we will be able to promote novel exploratory studies that will help better understand the intricacies behind this coordinated molecular network.
4. LncRNAs and DR
Recent studies have made it evident that lncRNAs are dysregulated during DR [97,98,99]. As a matter of fact, in a study by Yan et al., microarray analyses of retinal tissues from 2-month old (streptozotocin-induced) diabetic mice demonstrated differential expressions of lncRNAs when compared to non-diabetic retinas; in particular, 89 lncRNAs were upregulated and 214 lncRNAs were downregulated during early DR [98]. To better understand the interactive capabilities of lncRNAs in DR, Yan et al. constructed a co-expression network between lncRNAs and mRNAs using their microarray findings and bioinformatics tools; this network comprised of 100 mRNAs and 79 differentially expressed lncRNAs that collectively contributed to 2675 network nodes. Gene ontology (GO) analyses further revealed that this regulatory network was implicated in a number of biological processes, which included cellular stress and DNA damage responses, epithelium and tube development, and tube morphogenesis. In addition to GO, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses also indicated that this co-expression network is connected to a number of signaling pathways (i.e., MAPK, chemokine signalling, pyruvate metabolism, and complement and coagulation cascades) that are involved in the progression of DR (i.e., inflammation and neovascularization). Moreover, in a separate study by Wang et al., fibrovascular membranes were obtained, through pars plana vitrectomy, from PDR patients who did or did not receive intravitreal pre-treatment with conbercept (an anti-VEGF drug), and these membranes were then subjected to RNA extraction and microarray profiling [99]. The microarray results indicated that nearly 427 lncRNAs (263 upregulated and 164 downregulated) and 571 mRNAs (192 upregulated and 379 downregulated) were differentially expressed between the two PDR patient groups. Furthermore, following the construction of a lncRNA—mRNA co-expression network, GO and KEGG analyses revealed that several of the dysregulated lncRNAs and mRNAs were involved in numerous pathways, including inflammatory signaling (i.e., TNF-α, IL-17, and nucleotide-binding and oligomerization domain (NOD)-like receptors), HIF-1 signalling, membrane trafficking (interactions with SNARE proteins) and various metabolic processes such as gluconeogenesis. Nevertheless, the findings from both studies suggest that lncRNAs are capable of participating in a variety of biological processes and any changes in the cellular milieu, whether through acute or chronic stimulus such as diabetes, can have profound effects on the global molecular network.
To further emphasize that the dysregulation of lncRNAs plays a critical role in DR, researchers performed a three-stage genome-wide association study (GWAS) involving type II diabetic Japanese patients with DR (N = 837) and without DR (N = 1149) [100]. Interestingly, after using a meta-analysis model to combine select single nucleotide polymorphisms (SNPs) from all three stages, the SNP rs9362054 was found to be strongly associated with DR. More specifically, rs9362054 is situated in an intron of the RP1-90L14.1 gene, which encodes a lincRNA (lnc-KIAA1009-1) and is located between two protein-coding genes (KIAA1009 and TBX18) on chromosome 6. Due to the distinct genomic localization of RP1-90L14.1 and the fact that KIAA1009 (also known as CEP162 and QN1) is vital for ciliogenesis [101], the investigators suspect that the lnc-KIAA1009-1 may interconnect these genes via cis-regulation and contribute to defective ciliogenesis; raising the possibility that aberrations in key lncRNAs may facilitate the pathogenesis of certain diseases. Although additional loss-of-function and gain-of-function experiments are required to validate this hypothesis, these unique findings raise new questions regarding the molecular basis of lncRNAs in DR. In the following sections, we will take an in-depth look at some of the well-characterized lncRNAs in DR with respect to their regulatory roles in inflammation (summarized in Table 1).
Table 1.
Pertinent lncRNAs that are involved in DR.
| lncRNA | Reported Functions in DR | Implications in Other Epigenetic Mechanisms |
|---|---|---|
| ANRIL |
|
|
| BDNF-AS |
|
|
| H19 |
|
|
| HOTTIP |
|
|
| MALAT1 |
|
|
| MEG3 |
|
|
| MIAT |
|
|
| RNCR3 |
|
|
STZ = streptozotocin; DR = diabetic retinopathy; HG = high glucose; NG = normal glucose; HRECs = human retinal endothelial cells; KO = knockout; RGCs = retinal ganglion cells; EndMT = endothelial-to-mesenchymal transition.
5. LncRNAs as Novel Regulators of Inflammation in DR
5.1. MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is one of the earliest lncRNAs to be identified in DR. Originally discovered in non-small cell lung carcinoma (NSCLC) [115], this highly studied intergenic lncRNA has been implicated in various cancers [116,117,118], cardiovascular disease [119], neurological disorders [120,121], skeletal myogenesis [122], and neural development [123]. Considering the ubiquitous expressions of MALAT1 in tissues and its high degree of evolutionary conservation [124], further exploration of MALAT1, in particular disease contexts, will provide useful insights into the interactions between lncRNAs and the genome. Indeed, in recent years, the importance of MALAT1 is being recognized by researchers in the field of diabetes and several functional studies are being carried out that specifically examine the dynamics of this lncRNA in diabetic complications [125,126,127]. Particularly, in DR, Yan et al. were the first to document significant aberrations in the expression of MALAT1 in the retinas of STZ-induced diabetic mice (a type I diabetes model), RF/6A (choroid-retinal endothelial) cells cultured in high glucose, and aqueous humors and fibrovascular membranes of type II diabetic patients [98]. In addition to their in vitro and in vivo observations, in silico analysis further revealed that the MALAT1 sequence contains transcription factor binding sites for NF-κB, which is a critical mediator of immune and inflammatory responses [98]. Moreover, in a separate study by the same group, elevated MALAT1 expressions were also evident in the retinas of STZ-induced diabetic rats and db/db mice (a type II diabetes model) [106]. Additionally, several vasoactive and inflammatory markers accompanied the elevated expressions of MALAT1 in the diabetic retina, which included VEGF, PEDF, ICAM, and TNF-α—suggesting a potential pathogenetic association for MALAT1 in DR. Interestingly, intraocular injections of a MALAT1 short hairpin RNA (shRNA) in the diabetic rats significantly alleviated diabetes-induced retinal inflammation, retinal cell apoptosis, vascular leakage, and electroretinogram abnormalities. Similarly, small interfering RNA (siRNA)-mediated knockdown of MALAT1 in RF/6A cells treated with exogenous VEGF or TNF-α significantly diminished the migration and tube formation potential of endothelial cells compared to scrambled controls. The researchers in the study further determined that the MAPK pathway is critically implicated in MALAT1′s ability to augment proliferation in RF/6A cells during hyperglycemic stress. Specifically, western blot analyses demonstrated that MALAT1 knockdown in HG-treated retinal cells could directly reduce the expressions of phosphorylated p38 levels; however, this knockdown did not have any visible reductions on phosphorylated JNK1/2 or ERK1/2 proteins. In order to confirm their observations, Liu et al. overexpressed MALAT1 in RF/6A cells and then subsequently treated these cells with either chemical inhibitors or siRNAs for p38, JNK, and ERK for 48 h [106]. As expected, the p38 chemical inhibitor (SB203580) or siRNA was able to significantly impede the proliferative potential of MALAT1, whereas the JNK and ERK-specific treatments were unable to hinder MALAT1-induced hyper-proliferation—ultimately suggesting that MALAT1 can exert its proliferative capabilities specifically through the p38 MAPK signaling pathway. Since p38 MAPK has been previously implicated in diabetes-induced retinal inflammation [128], the collective findings by Liu et al. demonstrate that other novel epigenetic molecules, such as lncRNAs, can play critical regulatory roles in the inflammatory pathways involved in the progression of DR.
To further extend the inflammatory functionalities of MALAT1, a recent study from our laboratory found that MALAT1 is capable of epigenetically regulating a number of inflammatory cytokines in DR: IL-6, IL-1β, MCP-1, and TNF-α [107]. While the precise epigenetic mechanisms will be discussed in more detail in Section 6, our initial in vitro experiments determined that MALAT1 silencing, via siRNA transfection, in human retinal endothelial cells (HRECs) significantly alleviated high glucose-induced upregulations of inflammatory cytokines. Corresponding to our observed in vitro patterns, the genetic ablation of Malat1 (i.e., a global knockout) also diminished diabetes-induced vascular leakage and inflammation in the retinal tissues of Malat1 knockout diabetic mice compared to wild-type diabetic controls. Similarly, in the vitreous humors of PDR patients, MALAT1 transcript levels were significantly upregulated and pathogenetically associated with two other pro-inflammatory cytokines, IL-6 and TNF-α. Further supporting the mechanistic relationship between MALAT1 and these inflammatory cytokines, a previous study by us demonstrated that MALAT1 is capable of mediating IL-6 and TNF-α through the activation of its inflammatory ligand, known as serum amyloid antigen 3 (SAA3), in large vessel endothelial cells during hyperglycemia [129]. Taken together, our data indicates that the heightened production of MALAT1 promotes an inflammatory phenotype in diabetes. Aside from inflammation, it is important to note that MALAT1 has also been shown to regulate angiogenesis in the neonatal retina [130] and in high glucose-treated HRECs [131]; after all, angiogenesis and inflammation are dynamic processes that are both actively involved in DR [132].
5.2. MIAT
Myocardial infarction-associated transcript (MIAT; also referred to as RNCR2, Gomafu, or AK028326) was originally identified in a case-control GWAS, where 6 SNPs in the MIAT locus conferred susceptibility to myocardial infarction (MI) [133]. Following this initial study, several experimental studies have emerged that shed light on the functional roles of MIAT in various biological and pathological processes, including schizophrenia [134], NSCLC [135], retinal and brain development [136,137], cataract formation [138], diabetic cardiomyopathy [139], and diabetic nephropathy [140]. In the context of DR, MIAT is significantly upregulated in the retinas of STZ-induced diabetic rats and db/db mice, and in the fibrovascular membranes of diabetic patients compared to non-diabetic controls [111]. Similar to the patterns observed in their in vivo experiments, Yan et al. also observed upregulated expressions of MIAT in several HG-treated retinal cell lines (i.e., RF/6A, microvascular endothelial cells, Müller cells and retinal ganglion cells) in vitro [111]. Interestingly, HG-induced upregulations of MIAT were also evident in two other non-retinal endothelial cell lines (HUVECs and EA.hy.926), which suggests that MIAT may be critically involved in endothelial cell functions during hyperglycemic stress. Furthermore, intravitreal injections of MIAT shRNA in the diabetic rats diminished diabetes-induced electroretinogram abnormalities, apoptosis of retinal cells and pericytes, and retinal vascular leakage. Additionally, retinal inflammation in the diabetic retinas was significantly alleviated after the knockdown of MIAT; in particular, Western blots demonstrated that MIAT shRNA is capable of downregulating TNF-α, VEGF, and ICAM proteins when compared to diabetic controls.
A recent study by Zhang et al. suggests that NF-κB and MIAT may share an intricate mechanistic relationship under hyperglycemic environments [112]. In fact, chromatin immunoprecipitation (ChIP) assays revealed that NF-κB (p65, the pertinent subunit of NF-κB) selectively binds to the promoter of MIAT and high glucose stimulation of primary rat retinal Müller cells subsequently heightens the binding activation of NF-κB with MIAT, compared to normal glucose controls. Moreover, pre-treatment of rat retinal Müller cells with an IKK inhibitor (Bay 11-7082) significantly downregulated the HG-induced expression levels of MIAT, suggesting that NF-κB may directly facilitate the regulation of MIAT under cellular stress. The researchers further examined the effects of MIAT knockdown on cultured Müller cells and observed that MIAT can also directly regulate HG-induced apoptosis by inhibiting the expressions and functions of miR-29b, ultimately allowing increases in the transcription factor Sp1 (lncRNA-miRNA interactions will be briefly discussed in Section 6.3) [112]. Nevertheless, the overall findings for MIAT strongly demonstrate that lncRNAs are implicated in several cellular networks and further research into their contributions in each network will provide new functional and mechanistic insights behind these molecules under select cellular stress responses.
5.3. ANRIL
Consisting of 19 exons and spanning nearly 126 kilobases (kb) [141], the antisense RNA to INK4 locus (ANRIL; also known as CDKN2B-AS1) gene gives rise to a 3.8-kb lncRNA that is prominently deregulated in cardiovascular disease [142], several cancers [143], diabetic nephropathy [144], diabetic cardiomyopathy [144], and primary open-angle glaucoma [145]. Not only is ANRIL deregulated in several pathologies, ANRIL also shares a close connection with inflammation. For example, as evidenced by Zhou et al., several isoforms of ANRIL are markedly upregulated in large vessel endothelial cells (HUVECs) following TNF-α treatments [146]. Additional experiments from this study determined that a putative NF-κB binding site exists in the ANRIL promoter sequence and TNF-α treatment is capable of augmenting the binding between NF-κB and the ANRIL promoter. To further support this direct binding relationship, p65 was silenced via siRNAs and it was evident that the suppression of NF-κB impeded the TNF-α-induced ANRIL expressions—suggesting that NF-κB can mediate the transcriptional activity of the ANRIL gene. Interestingly, more downstream of NF-κB signalling, ANRIL is capable of regulating IL-6 and IL-8 expressions in TNF-α-treated HUVECs by directly interacting with YY1 (a RNA-binding protein and transcription factor that binds to the promoter loci of several proinflammatory genes). Namely, the authors observed that silencing ANRIL led to reduced YY1 binding with its IL-6 and IL-8 promoters, which ultimately suppressed the TNF-α-induced upregulations of IL-6 and IL-8 at both RNA and protein levels [146]. Despite the significance of ANRIL’s link with inflammation in large vessel endothelial cells, whether ANRIL exerts similar functional and mechanistic capabilities in inflammation during DR remains to be determined. However, a recent study, demonstrated by our laboratory, alludes to the angiogenic capabilities of this lncRNA in advancing DR, which becomes critical for discussion. As evident by the findings from both in vitro and in vivo experiments, hyperglycemia can significantly induce the upregulation of ANRIL in HG-treated HRECs and in the retinas of STZ-induced diabetic mice [97]. Not only is ANRIL heightened in such hyperglycemic environments, but blocking the expressions of ANRIL greatly hampers glucose-induced retinal angiogenesis. In particular, suppressing the expressions of ANRIL, via siRNAs, dramatically reduces high glucose-induced increases in endothelial cell tube formation, cellular proliferation, and VEGF RNA and protein expressions in HRECs. While, the retinal tissues from ANRIL knockout diabetic mice exhibited dramatic reductions in VEGF mRNA and protein levels, and retinal microvascular permeability compared to wild-type diabetic retinas. Furthermore, mechanistically, ANRIL was shown to govern VEGF expressions through its possible interactions with important epigenetic mediators, such as histone methylation (polycomb repressive complex 2; PRC2) and acetylation enzymes (p300), and miR-200b, during DR (these interactions will be elaborated further in Section 6.2 below).
5.4. H19
H19, a conserved and paternally imprinted lncRNA, is one of the earliest identified lncRNAs [147]. Aside from its involvement in a number of cancers [148,149,150], emerging evidence in recent years demonstrates that H19 can influence several other pathophysiological processes such as preeclampsia [151], neural inflammation/stroke [152,153], seizure-induced brain injury [154], and corneal neovascularization [155]. H19 can also impart its inflammatory capabilities in atherosclerosis [156]. Notably, overexpressing H19 in HUVECs and vascular smooth muscle cells (VSMCs) restricts apoptosis and promotes the proliferative and migratory potential of both cells by upregulating the expressions of p38 and p65 (critical factors in the MAPK and NF-κB pathways, respectively) [156]. Similarly, H19′s inflammatory properties also extend into ischemic cerebral injury, where H19 silencing can directly inhibit the levels of IL-1β and TNF-α, while increasing the production of IL-10 in the cerebral tissues and plasma of ischemic mice [152]. While the inflammatory-mediated mechanisms are evident for H19 in atherosclerosis and neuroinflammation, the influence of H19 on inflammation during the progression of DR is not known. Despite the absence of literature that document this particular relationship in DR, our laboratory recently confirmed a role for H19 in mediating the glucose-induced phenotypic switch (also known as endothelial-to-mesenchymal transition; EndMT) of endothelial cells in the diabetic retina [104]. In fact, since HG promotes the upregulation of mesenchymal markers (i.e., FSP-1, SM22, and α-SMA) and the downregulation of H19 and endothelial cell markers (i.e., CD-31 and VE-CAD), the overexpression of H19 in HG-treated HRECs dramatically reversed the trends evoked by hyperglycemia, which is suggestive of a protective role for H19 in preventing EndMT in DR. Further confirming our in vitro findings, H19 RNA levels were significantly reduced in the vitreous humors of PDR patients, and H19 knockout control mice exhibited an EndMT retinal phenotype that were comparable to wild-type and H19 knockout diabetic retinas. Moreover, using additional in vitro experiments, we mechanistically demonstrated that H19 mediates EndMT through its regulation of the MAPK-ERK1/2 pathway via Smad-independent TGF-β signaling. Nevertheless, the discussion of this initial study on H19 provides novel insights into the pathogenesis of DR and further research is warranted to explore H19′s implications in other DR-related molecular pathways. After all, the presence of altered extracellular matrix proteins, neovascularization, and inflammation are critical processes that collectively contribute to fibrosis and retinal tissue damage, and subsequent ocular complications [157,158].
5.5. BDNF-AS
The low levels of nerve growth factor BDNF (brain-derived neurotrophic factor) is linked to several neurodegenerative disorders [159]. Additionally, BDNF is implicated in the retina, where this neurotrophin is capable of promoting cellular differentiation and exerting anti-inflammatory effects in LPS-stimulated retinal pigment epithelial cells [160,161]. In the context of DR, previous studies have confirmed that the levels of BDNF are significantly reduced in the vitreous, serum, and plasma of PDR patients [161,162], while downregulated BDNF protein levels are also evident in the serum and retinal tissues of STZ-induced diabetic rats [162]. Since it is apparent that BDNF is decreased in diabetic environments, one plausible mechanism for this downregulation may be mediated by BDNF-AS, the natural antisense lncRNA of BDNF. In a study by Xu et al., oxygen and glucose-deprived primary retinal ganglion cells (RGCs) exhibited an inverse relationship between BDNF and BDNF-AS expression levels: Increases in BDNF-AS and reductions in BDNF [102]. This regulatory relationship was then confirmed by luciferase assays, where it was observed that BDNF-AS directly targets the complementary sequences of the BDNF mRNA, ultimately inhibiting the expression of this neurotrophin. Furthermore, using transduction approaches, shRNA-mediated knockdown of BDNF-AS in RGCs prevented ischemia-induced increases in cell apoptosis and TNF-α expressions—signifying that BDNF-AS plays an important role in augmenting ischemic injury. In accordance with these findings, Li et al. also demonstrate a similar phenomenon between BDNF-AS and BDNF expression levels in human retinal pigment epithelial cells (ARPE-19) cultured in high glucose conditions [103]. Accordingly, siRNA-mediated knockdown of BDNF-AS in ARPE-19 cells alleviated glucose-induced elevations in cell apoptosis and reductions in BDNF levels; thereby, conferring protection to RGCs against diabetes-associated damage.
5.6. MEG3
The maternally expressed gene 3 (MEG3) is a lncRNA gene that belongs to the DLK1—MEG3 imprinting locus and exerts critical developmental properties [163]. Several lines of evidence also suggest that the inactivation of this gene and the subsequent loss of the MEG3 lncRNA are frequently documented in numerous cancers, suggesting important tumour-suppressive properties of this gene [164]. In diabetic environments, similar reductions of MEG3 have been reported in both diabetic animal and in vitro models [108,109,110]. Notably, reduced serum levels of MEG3 were observed in patients with DR compared to controls; whereas, overexpression of MEG3 in ARPE-19 cells markedly downregulated HG-induced increases of VEGF and TGF-β1 at both mRNA and protein levels [108]. Furthermore, Tong et al. report that MEG3 overexpression in ARPE-19 cells significantly alleviates glucose-induced apoptosis and upregulations of IL-6, IL-1β, and TNF-α [109]. Following additional mechanistic experiments, the researchers concluded that MEG3 can exert its anti-inflammatory and anti-apoptotic effects through the NF-κB and Bcl-2/Bax signaling pathways by specifically targeting two important epigenetic regulators, SIRT1 and miR-34a (these interactions will be further elaborated in Section 6.3. In a separate study by Qiu et al., intravitreal injections of MEG3 shRNA in STZ-induced diabetic mice dramatically aggravated acellular capillaries, retinal vascular leakage, and retinal inflammation (i.e., elevated expressions of TNF-α, VEGF, IL-6, IL-1, and CCL2 were observed) [110]. Additionally, mechanistic exploratory studies demonstrated that MEG3 knockdown is capable of decreasing apoptosis and improving cell viability in HG-treated RF/6A cells, which is further mediated by MEG3′s involvement in PI3K/Akt signaling [110].
5.7. RNCR3
Retinal non-coding RNA3 (RNCR3) is an intergenic lncRNA that was first documented in the developing mouse retina [136]. In addition to its role in the eye, RNCR3 is implicated in atherosclerosis [165] and in the differentiation of oligodendrocytes and neurons [166]. Alternatively, in DR, Liu et al. demonstrates that hyperglycemia upregulates RNCR3 and the subsequent administration of intravitreal RNCR3 shRNA greatly impedes glial cell reactivity, as well as inducing significant reductions in many cytokines, including MCP-1, TNF-α, and VEGF-A, in the retinas of diabetic mice [113]. Additionally, the knockdown of RNCR3 evoked neuroprotective effects on diabetic retinal tissues by improving visual function and RGC survival in the diabetic mice, while conversely decreasing glucose-induced apoptosis of retinal cells. Consistent with these findings, Shan et al. also reported similar increasing patterns of RNCR3 in HG-treated RF/6A cells, diabetic mice retinas, and in the fibrovascular membranes of diabetic patients [114]. As well, shRNA-mediated knockdown of RNCR3, via intravitreal injections, reduced acellular capillaries, and retinal vascular leakage in the diabetic retinas. Interestingly, although the knockdown of RNCR3 was shown to decrease viability, migratory potential, and tube formation of HG-treated RF/6A cells in vitro, the researchers proposed that a complex cross-talk exists, involving the RNCR3/KLF2 (Kruppel-like factor 2)/miR-185-5p regulatory network, which facilitates the regulation of RF/6A cells.
5.8. HOTTIP
HOXA transcript at the distal tip (HOTTIP) is a newly emerging lincRNA that resides near the 5′-end of the HOXA locus and is actively involved in the coordination of various HOXA genes, which are important in embryonic development [167]. While its disease-specific functions are being annotated, recent evidences demonstrate that HOTTIP dysregulation is associated with many cancers [168]. Similarly, significant aberrations of HOTTIP are evident in the retinas of STZ-induced diabetic rats and db/db mice [105]. Indeed, using diabetic animal models, Sun and Xu demonstrate for the first time that diabetes can induce the upregulation of HOTTIP. To better understand the implications of this lncRNA in DR, the researchers administered an intraocular HOTTIP shRNA in diabetic rats and found that the knockdown of HOTTIP can directly attenuate diabetes-induced electroretinogram abnormalities and retinal inflammation, which was evident through reduced expressions of VEGF and ICAM-1 proteins. Furthermore, the siRNA-mediated downregulation of HOTTIP dramatically reduced cell viability in RF/6A cells treated with HG or hydrogen peroxide, compared to cells only treated with HG or hydrogen peroxide—indicating that HOTTIP can also influence the degree of cellular apoptosis under hyperglycemic or oxidative stress conditions. In addition to their in vitro findings, HOTTIP silencing directly decreased phosphorylated p38 protein expressions, but did not have an impact on the phosphorylation levels of ERK1/2 and JNK1/2. Conversely, HOTTIP-induced cellular proliferation can be prevented by the administration of an inhibitor (SB203580) or siRNA for p38 and not by inhibitors of JNK or ERK, which alludes to the dynamic relationship between the p38-MAPK signaling pathway and HOTTIP.
6. Other Epigenetic Players Involved in the Cross-Talk between lncRNAs and Inflammation: The Missing Puzzle Pieces?
As alluded to earlier, the molecular network is complex and precisely coordinated during homeostasis. In the event of chronic hyperglycemia, the activity of various genes go awry—particularly genes associated with oxidative stress and inflammation—and damaging environments are generated that can evoke long-lasting effects despite the normalization of glucose [169,170,171]. Epigenetic mechanisms, which modify the expression of genes without changing the underlying nucleotide composition, are critically implicated in diabetes [172,173,174]. Nevertheless, presently, very few studies exist that take into consideration the complex crosstalk between lncRNAs and other epigenetic mechanisms during inflammation in DR. Therefore, in the sections below, we will discuss the three major epigenetic mechanisms in relation to lncRNAs and inflammation in the diabetic retina.
6.1. DNA Methylation
One of the earliest discovered epigenetic mechanisms is DNA methylation [175], which involves the interactions between two opposing enzymes that facilitate the methylation status of cytosine residues in CpG dinucleotides: either through the addition (via DNA methyltransferases; DNMTs) or removal (via DNA demethylases) of methyl groups [176]. Further, genomic regions that contain a high frequency of CpG dinucleotides are referred to as ‘CpG islands’ (CGIs), which reside in the regulatory/promoter regions of genes, and the CGIs can ultimately determine the transcriptional activity of a gene based on its degree of methylation [176,177]. For example, promoter CGIs that are hypermethylated are associated with gene silencing, while conversely hypomethylation is associated with gene activation [176,177]. Indeed, in recent years, the impact of DNA methylation has been documented in DR, where previous reports suggest that hyperglycemia can evoke distinct methylation patterns in the promoters of miRNAs [178] and several DR-related genes (i.e., MMP-9 and TNF) [179,180,181], furthering the progression of DR. Adding to these results, findings from our recent study demonstrate for the first time that DNA methylation is closely connected with MALAT1 and its inflammatory mediators in DR pathogenesis [107]. In fact, blocking DNMTs (through the administration of pan-DNMT inhibitors or a DNMT1 siRNA, which is a constitutively expressed DNMT) in HRECs cultured in NG or HG conditions further exacerbated glucose-induced RNA expressions of MALAT1, IL-6, TNF-α, MCP-1, and IL-1β—indicating that DNMTs actively participate in the transcriptional regulation of several genes. Moreover, using a DNA methylation array, we then closely examined the CpG sites across the MALAT1 gene in both NG and HG-treated HRECs. Intriguingly, we observed that transient glucose treatments (48 h) did not significantly alter the methylation status of the CGI in the MALAT1 promoter. While we conducted our DNA methylation experiment at one particular time-point, it would be intriguing to see whether initial hyperglycemic treatments can provoke persistent, long-lasting changes in the methylation status of the CGI in the MALAT1 promoter. Constructing such an in vitro cell culture model involving multiple time-points and alternating glucose treatments will provide unique insights behind metabolic memory and the regulatory nature of DNA methylation on the biogenesis of lncRNAs during the progression of DR.
6.2. Histone Modifications
Another fundamental and well-studied epigenetic mechanism that is involved in the coordination of gene expression is histone modifications. Histone-modifying enzymes, such as histone methyltransferases, histone demethylases, histone acetyltransferases, and histone deacetylases, coordinate their actions by chemically modifying particular amino acid residues within the histone proteins (H2A, H2B, H3, and H4), which subsequently governs the overall conformation of the chromatin and its accessibility to transcription factors for gene transcription at that modified region [182,183,184,185,186]. For example, a euchromatin (open) configuration is induced by histone acetyltransferases through the acetylation of lysine residues, which generally leads to active gene transcription [182,183,184]; whereas, depending on the degree of methylation and specific residue, histone methyltransferases facilitate the methylation of lysine residues that can drive gene silencing (a heterochromatin state) or activation [185,186]. Changes in histone modifications have been extensively reported in multiple cancers [187] and in recent years, several studies have also documented the presence of aberrant histone modifications in diabetic environments [188,189,190,191,192,193,194]. Despite the breadth of information, very few studies have addressed the involvement of histone modifications on lncRNA-mediated mechanisms in DR. In fact, presently, only histone methylation and acetylation have been shown by our laboratory to influence lncRNAs in DR, which will be the topic of discussion in the paragraphs below.
Polycomb repressive complex 2 (PRC2) is a multimeric histone methyltransferase complex that catalyzes the tri-methylation of lysine 27 on histone 3 (H3K27me3), a distinct chromatin mark linked with gene repression [195]. A previous study from our laboratory demonstrated that the core components of PRC2 (EZH2, SUZ12, and EED) were significantly elevated in HG-treated HRECs and retinal tissues of diabetic rats and mice, which were also accompanied by increased VEGF expressions and reduced miR-200b levels (a negative regulator of VEGF) [196]. Furthermore, using ChIP-qPCR analyses to confirm the initial observations between PRC2 and miR-200b, HG-treated HRECs exhibited increased H3K27me3 and decreased RNA polymerase 2 associations in the promoter region of miR-200b when compared to NG controls. Interestingly, in vitro disruption of PRC2 with 3-Deazaneplanocin (DZNep) dramatically prevented HG-induced reductions of miR-200b, while VEGF RNA and protein levels were dramatically decreased in parallel—suggesting that PRC2 can negatively regulate miR-200b, while indirectly promoting the expressions of VEGF, in hyperglycemic environments. Further extending the regulatory mechanisms of PRC2, in vitro and in vivo analyses from our recent studies also revealed that lncRNAs are intimately connected with PRC2 functions in diabetes [97,107]. Beginning with ANRIL, retinal tissues from ANRIL knockout diabetic mice revealed depressed expressions of EZH2 and EED RNA levels (and no changes in SUZ12 expressions), when compared to wild-type diabetic retinas. Similar observations were additionally reported in HG-treated HRECs following ANRIL silencing—confirming ANRIL’s direct impact on the EZH2 and EED subunits of PRC2. On the other hand, administration of DZNep in HG-treated HRECs significantly reduced both ANRIL and VEGF RNA expressions, which suggests that a highly interactive network may exist between these molecules; after all, RNA immunoprecipitation analyses demonstrated that HG could promote a strong binding association between EZH2 and ANRIL [97]. Of note, ANRIL also shared a similar relationship with p300 [97], which is a prominent histone acetyltransferase involved in the regulation of several glucose-related genes [197,198,199]. Generally, HG-induced upregulations of p300 were corrected following ANRIL silencing in HRECs and ANRIL knockout diabetic mice exhibited reduced retinal p300 levels compared to wild-type diabetic retinas. Interestingly, transfection with siP300 in HG-treated HRECs did not alter ANRIL expressions [97].
Nearly analogous to ANRIL, the reductions of MALAT1, through silencing or knockout strategies, significantly prevented diabetes-induced increases in EZH2, SUZ12, and EED RNA levels [107]. Interestingly, DZNep pre-treatment was capable of reducing glucose-induced upregulations of MALAT1 and TNF-α, but conversely exacerbated the expressions of IL-6, MCP-1, and IL-1β; these findings may allude to the context-specific regulation of PRC2 on target gene expressions [200]. Moreover, the relationship between MALAT1 and PRC2 was quite evident in HRECs, as strong binding associations were also observed and the in vitro silencing of MALAT1 directly reduced EZH2 protein levels in HG environments. Collectively, these findings allude to the potential abilities of lncRNAs to form scaffolds or act as guides with certain chromatin-modifying enzymes in diabetic environments. Further mechanistic-based studies are warranted that closely examine the relationship between these key epigenetic players in mediating the pathogenesis of DR.
6.3. miRNAs
miRNAs (miRs) have emerged as critical post-transcriptional regulators of gene expression [201,202]. Despite being ~22 nucleotides in length, these small ncRNAs exert their powerful functions by binding to the 3′ untranslated region (3′-UTR) of their target mRNAs, which subsequently leads to mRNA degradation and/or the inhibition of protein translation [203]. MiRs have been implicated in cancers [204], cardiovascular disease [205], neurodegenerative diseases [206] and within the last decade, numerous miRs have been identified in DR [199,207,208,209,210]. For the purposes of this review, we will briefly look at few of the documented miRs that are known to interact with lncRNAs during the progression DR.
Tong et al. shed novel insights into the regulatory capabilities of MEG3 on a molecular axis involving SIRT1 (a histone deacetylase) and miR-34a in retinal epithelial cells [109]. With HG environments promoting the upregulation of miR-34a and downregulations of MEG3 and SIRT1, the subsequent overexpression of MEG3 in ARPE-19 cells dramatically reversed the HG-induced effects—confirming the inverse relationships shared between MEG3, SIRT1, and miR-34a. Furthermore, incorporating miR-34a mimics and inhibitors into their in vitro experiments, the authors confirmed that miR-34a could negatively regulate SIRT1. In silico analyses and luciferase experiments were then carried out that confirmed MEG3′s ability to positively regulate SIRT1 by directly sponging its negative regulator, miR-34a. Additionally, MEG3 is capable of reducing HG-induced apoptosis and inflammation by downregulating miR-34a levels. Tong et al. also indicated that either MEG3 overexpression or miR-34a knockdown is capable of upregulating the levels of SIRT1 by reducing the HG-induced activation of the NF-κB signaling pathway.
Shan et al. also established that the lncRNA RNCR3 is upregulated in RF/6A cells cultured in HG and associated with retinal vascular dysfunction in vivo [114]. In addition to their initial findings, the authors wanted to examine the regulatory role of miR-185-5p on RNCR3 and KLF2 expressions in RF/6A cells, since their previous atherosclerosis-based study determined that a feedback loop existed between these molecules [165]. It was determined that miR-185-5p directly regulates RNCR3 and KLF2 expressions, since the levels of RNCR3 and KLF2 decreased after the administration of miR-185-5p mimics. Furthermore, the knockdown of RNCR3 or the presence of miR-185-5p mimics both contributed to reduced cell viability and proliferation, whereas KLF2 overexpression increased cell viability and proliferation—alluding to the potential regulatory network in RF/6A cells.
Moreover, MIAT was shown to function as a molecular decoy/sponge that sequesters miR-29b and miR-150-5p, which subsequently promotes the expression of their target mRNAs [111,112]. In particular, Yan et al. first used bioinformatics tools to identify predicted binding sites of miR-150-5p on its target mRNA, VEGF, and its target lncRNA, MIAT [111]. Using this information, the authors then cloned the specific regions to luciferase vectors and consequently, transfected RF/6A endothelial cells with these vectors and miR-150-5p mimics. Interestingly, the luciferase assays demonstrated that miR-150-5p directly targets VEGF and MIAT. Following these findings, the authors wanted to gain a better understanding of the decoy/sponge functions of MIAT in vitro, so increasing levels of miR-150-5p were administered to RF/6A cells in the absence or presence of MIAT. It was observed that VEGF expressions were dramatically upregulated during MIAT overexpression, while conversely VEGF levels significantly decreased in MIAT-overexpressing endothelial cells with increasing levels of miR-150-5p. To determine whether similar patterns exist vice-versa, the authors also administered increasing levels of MIAT in the presence or absence of miR-150-5p. Indeed, the gradual increases in MIAT were capable of restoring the miR-150-5p-induced downregulations of VEGF in miR-150-5p-overexpressing RF/6A cells, which confirms the interplay between these molecules. This regulatory cross-talk between MIAT, miR-150-5p, and VEGF was also implicated in the critical functions of endothelial cells during high glucose stress [111]. Additional findings by Zhang et al. demonstrate that MIAT is also capable of promoting Sp1 through the suppression of its negative regulator, miR-29b, in HG-treated rat retinal Müller cells—ultimately leading to heightened levels of apoptosis [112]. In fact, MIAT knockdown was capable of elevating miR-29b levels and cell viability, while decreasing the increased levels of Sp1 and apoptosis in HG-treated Müller cells.
7. Conclusions
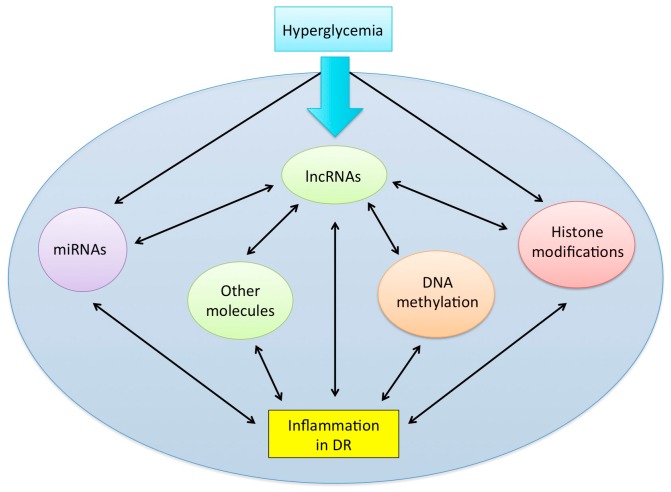
Undoubtedly, the recent emergence of lncRNAs has evolved our understanding of pathogenetic mechanisms in inflammatory-driven diseases including DR. Gain-of-function and loss-of-function experiments have also made it evident that lncRNAs are dynamic regulators of gene expression. As fields continue to annotate lncRNAs, a dire need remains for DR research that elucidate the underpinnings of the epigenome network in order to understand the driving factors that initiate inflammatory changes. Integrated analyses that take into consideration this complex molecular landscape (please see Figure 1) will bring new questions to light regarding the epigenetic paradigm and provide novel avenues for better-targeted therapeutic and diagnostic options. In this review, not only have we highlighted the inflammatory-based roles of lncRNAs in DR, but we have also attempted to address other epigenetic mechanisms that are implicated in this coordinated regulation of inflammation. While the comprehension of this network is a work in progress in the field of DR, we hope that future studies will take these considerations into account when examining the epiphenomena. Nevertheless, continuing to explore the genomic landscape and its intricacies will provide novel mechanistic insights and discussions for the functions of lncRNAs in DR. To conclude, as DR prevalence reaches epidemic levels globally, understanding the inflammatory-mediated roles of lncRNAs in DR development and progression is critical for improving the diabetic standard of care in not only diagnostic testing, but also in the management and treatment of DR.
Figure 1.
A schematic depicting the dynamic, coordinated network involving epigenetic modifications in inflammation during DR. Several key epigenetic mechanisms are involved in the progression of inflammation. LncRNAs may serve as critical regulators of inflammation, through their effects on other epigenetic mechanisms, such as DNA methylation, histone modifications, and the activity of other non-coding RNAs (i.e., miRNAs). Furthermore, since the molecular network is heavily coordinated, several individual components of this network may inter-regulate one another, indicated by the double arrows in the figure, and future research should keep these interactions in mind.
Acknowledgments
The authors of this review would like to thank all past and current members of the Chakrabarti Lab, who have contributed to the advancement of diabetes research.
Author Contributions
S.B., M.S., and S.C. equally contributed to the writing and editing of the manuscript, and drawing of the figures and table.
Funding
The research presented in this review was supported by Diabetes Canada and the Heart and Stroke Foundation of Ontario.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chan J.C.N., Malik V., Jia W., Kadowaki T., Yajnik C.S., Yoon K., Hu F. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Werfalli M., Engel M.E., Musekiwa A., Kengne A.P., Levitt N.S. The prevalence of type 2 diabetes among older people in Africa: A systematic review. Lancet Diabetes Endocrinol. 2016;4:72–84. doi: 10.1016/S2213-8587(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 4.Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H., Zimmet P., Son H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N., Gill J.M. Type 2 diabetes in migrant south Asians: Mechanisms, mitigation, and management. Lancet Diabetes Endocrinol. 2015;3:1004–1016. doi: 10.1016/S2213-8587(15)00326-5. [DOI] [PubMed] [Google Scholar]
- 6.Leasher J.L., Bourne R.A.A., Flaxman S.R., Jonas J.B., Keefe J., Kovin N., Pesudovs K., Price H., White R.A., Wong T.Y., et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–1649. doi: 10.2337/dc15-2171. [DOI] [PubMed] [Google Scholar]
- 7.Yau J.W.Y., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S., Dekker J.M., Fletcher A., Grauslund J., et al. For the Meta-analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nentwich M.M., Ulbig M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes. 2015;6:489–499. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prokofyeva E., Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: A literature review. Ophthalmic Res. 2012;7:171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 10.Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:1221–1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 11.Wong T.Y., Mwamburi M., Klein R., Larsen M., Flynn H., Hernandez-Medina M., Ranganathan G., Wirostko B., Pleil A., Mitchell P. Rates of progression in diabetic retinopathy during different time periods: A systematic review and meta-analysis. Diabetes Care. 2009;32:2307–2313. doi: 10.2337/dc09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 14.Tan G.S., Cheung N., Simo R., Cheung G.C., Wong T.Y. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5:143–155. doi: 10.1016/S2213-8587(16)30052-3. [DOI] [PubMed] [Google Scholar]
- 15.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 16.Keenan H.A., Costacou T., Sun J.K., Doria A., Cavellerano J., Coney J., King G.L. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration. Diabetes Care. 2007;30:1995–1997. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- 17.Fong D.S., Aiello L., Gardner T.W., Kin G.L., Blankenship G., Cavallerano J.D., Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27:S84–S87. doi: 10.2337/diacare.27.2007.S84. [DOI] [PubMed] [Google Scholar]
- 18.Aiello L.P., Gardner T.W., King G.L., Blankenship G., Cavallerano J.D., Ferris R.L., Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Kristinsson J.K. Diabetic retinopathy. Screening and prevention of blindness. A doctoral thesis. Acta Ophthalmol. Scand. Suppl. 1997;223:1–76. [PubMed] [Google Scholar]
- 20.Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 21.Stefánsson E., Bek T., Porta M., Larsen N., Kristinsson J.K., Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol. Scand. 2000;78:374–385. doi: 10.1034/j.1600-0420.2000.078004374.x. [DOI] [PubMed] [Google Scholar]
- 22.Garg S.J., Maguire J.I., Regillo C.D., Spirn M.J., Tasman W. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 7th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2017. pp. 610–621. [Google Scholar]
- 23.Miller J.W., Adamis A.P., Shima D.T., D’Amore P.A., Moulton R.S., O’Reilly M.S., Folkman J., Dvorak H.F., Brown L.F., Berse B., et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994;145:574–584. doi: 10.1097/00006982-199515020-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 25.Singer M.A., Kermany D.S., Waters J., Jansen M.E., Tyler L. Diabetic macular edema: It is more than just VEGF. F1000Research. 2016;5 doi: 10.12688/f1000research.8265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamis A.P. Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern T.S. Contributions of Inflammatory Processes to the Development of the Early Stages of Diabetic Retinopathy. Exp. Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y., Sarthy V., Kern T. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am. J. Physiol. 2004;287:R735–R741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 29.Ellis E.A., Guberski D.L., Hutson B., Grant M.B. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide. 2002;6:295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- 30.Du Y., Smith M.A., Miller C.M., Kern T.S. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L., Szabo C., Kern T.S. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-B. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto K., Khosrof S., Bursell S.E., Rohan R., Murata T., Clermont A.C., Aiello L.P., Ogura Y., Adamis A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joussen A.M., Poulaki V., Qin W., Kirchhof B., Mitsiades N., Wiegand S.J., Rudge J., Yancopoulos G.D., Adamis A.P. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am. J. Pathol. 2002;160:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowluru R.A., Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: Effect of antioxidants. Investig. Ophthalmol. Vis. Sci. 2004;45:4161–4166. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- 35.Kowluru R.A., Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004;88:1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmo A., Cunha-Vaz J.G., Carvalho A.P., Lopes M.C. L-arginine transport in retinas from streptozotocin diabetic rats: Correlation with the level of IL-1 beta and NO synthase activity. Vis. Res. 1999;39:3817–3823. doi: 10.1016/S0042-6989(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 37.Gerhardinger C., Costa M.B., Coulombe M.C., Toth I., Hoehn T., Grosu P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Investig. Ophthalmol. Vis. Sci. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 38.Joussen A.M., Huang S., Poulaki V., Camphausen K., Beecken W.D., Kirchhof B., Adamis A.P. In vivo retinal gene expression in early diabetes. Investig. Ophthalmol. Vis. Sci. 2001;42:3047–3057. [PubMed] [Google Scholar]
- 39.Du X., Stocklauser-Farber K., Rosen P. Generation of reactive oxygen intermediates, activation of NF- kappaB, and induction of apoptosis in human endothelial cells by glucose: Role of nitric oxide synthase? Free Radic. Biol. Med. 1999;27:752–763. doi: 10.1016/S0891-5849(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 40.Romeo G., Liu W., Asnaghi V., Kern T.S., Lorenzi M. Activation of nuclear factor-kappa B induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 41.Nagai N., Izumi-Nagai K., Oike Y., Koto T., Satofuka S., Ozawa Y., Yamashiro K., Inoue M., Tsubota K., Umezawa K., et al. Suppression of Diabetes-Induced Retinal Inflammation by Blocking the Angiotensin II Type 1 Receptor or Its Downstream Nuclear Factor- B Pathway. Retin. Cell Biol. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- 42.Zheng L., Howell S.J., Hatala D.A., Huang K., Kern T.S. Salicylate-Based Anti-Inflammatory Drugs Inhibit the Early Lesion of Diabetic Retinopathy. Diabetes. 2007;56:337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 43.Kowluru R.A., Koppolu P., Chakrabarti S., Chen S. Diabetes-induced Activation of Nuclear Transcriptional Factor in the Retina, and its Inhibition by Antioxidants. Free Radic. Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 44.Boss J.D., Singh P.K., Pandya H.K., Tosi J., Kim C., Tewari A., Juzych M.S., Abrams G.W., Kumar A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017;58:5594–5603. doi: 10.1167/iovs.17-21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuukia T., Kandab T., Kimuraa Y., Kotajimac N., Tamurac J., Kobayashic I., Kishia S. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J. Diabetes Complicat. 2001;15:257–259. doi: 10.1016/S1056-8727(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 46.Doganay S., Evereklioglu C., Er H., Türköz Y., Sevinç A., Mehmet N., Savli H. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002;16:163–170. doi: 10.1038/sj/eye/6700095. [DOI] [PubMed] [Google Scholar]
- 47.Wu H., Hwang D., Song X., Tao Y. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. J. Ophthalmol. 2017;2017:9402198. doi: 10.1155/2017/9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mastropasqua R., D’Aloisio R., Di Nicola M., Di Martino G., Lamolinara A., Di Antonio L., Tognetto D., Toto L. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-35036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scuderi S., D’amico A.G., Federico C., Saccone S., Magro G., Bucolo C., Drago F., D’Agata V. Different retinal expression patterns of IL-1α, IL-1β, and their receptors in a rat model of type 1 STZ-induced diabetes. J. Mol. Neurosci. 2015;56:431–439. doi: 10.1007/s12031-015-0505-x. [DOI] [PubMed] [Google Scholar]
- 50.Endo H., Naito T., Asahara T., Kajima M., Shiota H. Cytokines in the vitreous fluid of patients with proliferative diabetic retinopathy—Vascular endothelial growth factor and platelet-derived growth factor are elevated in proliferative diabetic retinopathy. Nippon Ganka Gakkai Zasshi. 2000;104:711–716. doi: 10.1167/iovs.12-9766. [DOI] [PubMed] [Google Scholar]
- 51.Voronov E., Shouval D.S., Krelin Y., Cagnano E., Benharroch D., Iwakura Y., Dinarello C.A., Apte R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leali D., Dell’Era P., Stabile H., Sennino B., Chambers A.F., Naldini A., Sozzani S., Nico B., Ribatti D., Presta M. Osteopontin (ETA-1) and fibroblast growth factor-2 cross-talk in angiogenesis. J. Immunol. 2003;171:1085–1093. doi: 10.4049/jimmunol.171.2.1085. [DOI] [PubMed] [Google Scholar]
- 53.Busik J.V., Mohr S., Grant M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aplin A.C., Gelati M., Fogel E., Carnevale E., Nicosia R.F. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol. Genom. 2006;27:20–28. doi: 10.1152/physiolgenomics.00048.2006. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Esselman W.J., Jump D.B., Busik J.V. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2005;46:4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valle A., Giamporcaro G.M., Scavini M., Stabilini A., Grogan P., Bianconi E., Sebastiani G., Masini M., Maugeri N., Porretti L., et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–2077. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stitt A.W., Curtis T.M., Chen M., Medina R.J., McKay G.J., Jenkins A., Gardiner T.A., Lyons T.J., Hammes H.P., Simo R., et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Engerman R.L. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 59.Durham J.T., Herman I.M. Microvascular modifications in diabetic retinopathy. Curr. Diab. Rep. 2001;11:25–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 60.Imai H., Singh R.S., Fort P.E., Gardner T.W. Neuroprotection for diabetic retinopathy. Dev. Ophthalmol. 2009;44:56–68. doi: 10.1159/000223946. [DOI] [PubMed] [Google Scholar]
- 61.Lange C., Storkebaum E., de Almodovar C.R., Dewerchin M., Carmeliet P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016;12:439–454. doi: 10.1038/nrneurol.2016.88. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert R.E., Vranes D., Berka J.L., Kelly D.J., Cox A., Wu L.L., Stacker S.A., Cooper M.E. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab. Investig. 1998;78:1017–1027. [PubMed] [Google Scholar]
- 63.Gerl V., Bohl J., Pitz S., Stoffelns B. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2002;43:1104–1108. [PubMed] [Google Scholar]
- 64.Zhang J., Gerhardinger C., Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]
- 65.Qin X., Goldfine A., Krumrei N., Grubissich L., Acosta J., Chorey M., Hays A.P., Halperin J.A. Glycation inactivation of the complement regulatory protein CD59: A possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes. 2004;53:2653–2661. doi: 10.2337/diabetes.53.10.2653. [DOI] [PubMed] [Google Scholar]
- 66.Aleksandrovskii Y.A. Antithrombin III, C1 inhibitor, methylglyoxal, and poly- morphonuclear leukocytes in the development of vascular complications in diabetes mellitus. Thromb. Res. 1992;67:179–189. doi: 10.1016/0049-3848(92)90137-Y. [DOI] [PubMed] [Google Scholar]
- 67.Gao B.B., Chen X., Timothy N., Aiello L.P., Feener E.P. Characterization of the Vitreous Proteome in Diabetes without Diabetic Retinopathy and Diabetes with Proliferative Diabetic Retinopathy. J. Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 68.García-Ramírez M., Canals F., Hernández C., Colomé N., Ferrer C., Carrasco E., García-Arumí J., Simó R. Proteomic analysis of human vitreous fluid by fluorescence- based difference gel electrophoresis (DIGE): A new strategy for identifying potential candidates in the pathogenesis. Diabetologia. 2007;50:1294–1303. doi: 10.1007/s00125-007-0627-y. [DOI] [PubMed] [Google Scholar]
- 69.Cao J., McLeod S., Merges C.A., Lutty G.A. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch. Ophthalmol. 1998;116:589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 70.Cheng L., Bu H., Portillo J.A., Li Y., Subauste C.S., Huang S.S., Kern T.S., Lin F. Modulation of retinal Müller cells by complement receptor C5aR. Investig. Ophthalmol. Vis. Sci. 2013;54:8191–8198. doi: 10.1167/iovs.13-12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowluru R.A., Zhong Q., Santos J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert Opin. Investig. Drugs. 2012;21:797–805. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L., Wang J., Fang J., Zhou H., Liu X., Su S.B. High glucose induces and activates Toll-like receptor 4 in endothelial cells of diabetic retinopathy. Diabetol. Metab. Syndr. 2015;7:1–10. doi: 10.1186/s13098-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Losiewicz M.K., Fort P.E. Diabetes Impairs the neuroprotective properties of retinal Alpha-crystallins. Investig. Ophthalmol. Vis. Sci. 2011;52:5034–5042. doi: 10.1167/iovs.10-6931. [DOI] [PubMed] [Google Scholar]
- 74.Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Gloss B.S., Dinger M.E. Realizing the significance of noncoding functionality in clinical genomics. Exp. Mol. Med. 2018;50:1–8. doi: 10.1038/s12276-018-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birney E., Stamatoyannopoulos J.A., Dutta A., Guigó R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., Thurman R.E., et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Djebali S., Davis C., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perry R.B.-T., Ulitsky I. The functions of long noncoding RNAs in development and stem cells. Development. 2016;143:3882–3894. doi: 10.1242/dev.140962. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y.G., Satpathy A.T., Chang H.Y. Gene regulation in the immune system by long noncoding RNAs. Nat. Immunol. 2017;18:962–972. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kino T., Hurt D.E., Ichijo T., Nader N., Chrousos G.P. Noncoding RNA Gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010;3:1–33. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zappulla D.C., Cech T.R. RNA as a flexible scaffold for proteins: Yeast telomerase and beyond. Cold Spring Harb. Symp. Quant. Biol. 2006;71:217–224. doi: 10.1101/sqb.2006.71.011. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Sun L., Wang L., Liu Z., Li Q., Yao B., Wang C., Chen T., Tu K., Liu Q. Long non-coding RNA DSCR8 acts as a molecular sponge for miR-485-5p to activate Wnt/β-catenin signal pathway in hepatocellular carcinoma. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-018-0937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olgun G., Sahin O., Tastan O. Discovering lncRNA mediated sponge interactions in breast cancer molecular subtypes. BMC Genom. 2018;19:1–12. doi: 10.1186/s12864-018-5006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ørom U.A., Derrien T., Beringer M., Gumireddy K., Gardini A., Bussotti G., Lai F., Zytnicki M., Notredame C., Huang Q., et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vance K.W., Ponting C.P. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30:348–355. doi: 10.1016/j.tig.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutchinson J.N., Ensminger A.W., Clemson C.M., Lynch C.R., Lawrence J.B., Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom. 2007;8:1–16. doi: 10.1186/1471-2164-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai F., Orom U.A., Cesaroni M., Beringer M., Taatjes D.J., Blobel G.A., Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon J.H., Abdelmohsen K., Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J. Mol. Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miao H., Wang L., Zhan H., Dai J., Chang Y., Wu F., Liu T., Liu Z., Gao C., Li L., et al. A long noncoding RNA distributed in both nucleus and cytoplasm operates in the PYCARD-regulated apoptosis by coordinating the epigenetic and translational regulation. PLoS Genet. 2019;15:1–24. doi: 10.1371/journal.pgen.1008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilk R., Hu J., Blotsky D., Krause H.M. Diverse and pervasive subcellular distributions for both coding and long noncoding RNAs. Genes Dev. 2016;30:594–609. doi: 10.1101/gad.276931.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mercer T.R., Neph S., Dinger M.E., Crawford J., Smith M.A., Shearwood A.M., Haugen E., Bracken C.P., Rackham O., Stamatoyannopoulos J.A., et al. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jarroux J., Morillon A., Pinskaya M. History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 95.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ransohoff J.D., Wei Y., Khavari P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomas A.A., Feng B., Chakrabarti S. ANRIL: A regulator of VEGF in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017;58:470–480. doi: 10.1167/iovs.16-20569. [DOI] [PubMed] [Google Scholar]
- 98.Yan B., Tao Z.F., Li X.M., Zhang H., Yao J., Jiang Q. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014;55:941–951. doi: 10.1167/iovs.13-13221. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Gao X., Liu J., Wang J., Zhang Y., Zhang T., Zhang H. Effect of intravitreal conbercept treatment on the expression of Long Noncoding RNAs and mRNAs in Proliferative Diabetic Retinopathy Patients. Acta Ophthalmol. 2019:1–11. doi: 10.1111/aos.14083. [DOI] [PubMed] [Google Scholar]
- 100.Awata T., Yamashita H., Kurihara S., Morita-Ohkubo T., Miyashita Y., Katayama S., Mori K., Yoneya S., Kohda M., Okazaki Y., et al. A genome-wide association study for diabetic retinopathy in a Japanese population: Potential association with a long intergenic non-coding RNA. PLoS ONE. 2014;9:1–9. doi: 10.1371/journal.pone.0111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W.J., Tay H.G., Soni R., Perumal G.S., Goll M.G., Macaluso F.P., Asara J.M., Amack J.D., Tsou M.F. CEP162 is an axoneme-recognition protein promoting ciliary transition zone assembly at the cilia base. Nat. Cell Biol. 2013;15:591–601. doi: 10.1038/ncb2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu L., Zhang Z., Xie T., Zhang X., Dai T. Inhibition of BDNF-AS provides neuroprotection for retinal ganglion cells against ischemic injury. PLoS ONE. 2016;11:1–13. doi: 10.1371/journal.pone.0164941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Xu F., Xiao H., Han F. Long noncoding RNA BDNF-AS inversely regulated BDNF and modulated high-glucose induced apoptosis in human retinal pigment epithelial cells. J. Cell Biochem. 2018;119:817–823. doi: 10.1002/jcb.26245. [DOI] [PubMed] [Google Scholar]
- 104.Thomas A.A., Biswas S., Feng B., Chen S., Gonder J., Chakrabarti S. lncRNA H19 prevents endothelial–mesenchymal transition in diabetic retinopathy. Diabetologia. 2019;62:517–530. doi: 10.1007/s00125-018-4797-6. [DOI] [PubMed] [Google Scholar]
- 105.Sun Y., Liu Y.X. LncRNA HOTTIP improves diabetic retinopathy by regulating the p38-MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2941–2948. doi: 10.26355/eurrev_201805_15048. [DOI] [PubMed] [Google Scholar]
- 106.Liu J.Y., Yao J., Li X.M., Song Y.C., Wang X.Q., Li Y.J., Yan B., Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:1–10. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Biswas S., Thomas A.A., Chen S., Aref-Eshghi E., Feng B., Gonder J., Sadikovic B., Chakrabarti S. MALAT1: An Epigenetic Regulator of Inflammation in Diabetic Retinopathy. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-24907-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang D., Qin H., Leng Y., Li X., Zhang L., Bai D., Meng Y., Wang J. LncRNA MEG3 overexpression inhibits the development of diabetic retinopathy by regulating TGF-β1 and VEGF. Exp. Ther. Med. 2018;16:2337–2342. doi: 10.3892/etm.2018.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tong P., Peng Q.H., Gu L.M., Xie W.W., Li W.J. LncRNA-MEG3 alleviates high glucose induced inflammation and apoptosis of retina epithelial cells via regulating miR-34a/SIRT1 axis. Exp. Mol. Pathol. 2019;107:102–109. doi: 10.1016/j.yexmp.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Qiu G.Z., Tian W., Fu H.T., Li C.P., Liu B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem. Biophys. Res. Commun. 2016;471:135–141. doi: 10.1016/j.bbrc.2016.01.164. [DOI] [PubMed] [Google Scholar]
- 111.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. LncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 112.Zhang J., Chen M., Chen J., Lin S., Cai D., Chen C., Chen Z. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci. Rep. 2017;37:1–10. doi: 10.1042/BSR20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C., Li C.P., Wang J.J., Shan K., Liu X., Yan B. RNCR3 knockdown inhibits diabetes mellitus-induced retinal reactive gliosis. Biochem. Biophys. Res. Commun. 2016;479:198–203. doi: 10.1016/j.bbrc.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 114.Shan K., Li C.P., Liu C., Liu X., Yan B. RNCR3: A regulator of diabetes mellitus-related retinal microvascular dysfunction. Biochem. Biophys. Res. Commun. 2017;482:777–783. doi: 10.1016/j.bbrc.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 115.Ji P., Diederichs S., Wang W., Böing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E., et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Z., Chen C., Liu Y., Wu C. 17β-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level. Biochem. Biophys. Res. Commun. 2014;445:388–393. doi: 10.1016/j.bbrc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 117.Hirata H., Hinoda Y., Shahryari V., Deng G., Nakajima K., Tabatabai Z.L., Ishii N., Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75:1322–1331. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren S., Liu Y., Xu W., Sun Y., Lu J., Wang F., Wei M., Shen J., Hou J., Gao X., et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J. Urol. 2013;190:2278–2287. doi: 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Vausort M., Wagner D.R., Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ. Res. 2014;115:668–677. doi: 10.1161/CIRCRESAHA.115.303836. [DOI] [PubMed] [Google Scholar]
- 120.Yao J., Wang X.Q., Li Y.J., Shan K., Yang H., Wang Y.N., Yao M.D., Liu C., Li X.M., Shen Y., et al. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol. Med. 2016;8:1–17. doi: 10.15252/emmm.201606749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Q.S., Wang Z.H., Zhang J.L., Duan Y.L., Li G.F., Zheng D.L. Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting α-synuclein protein expression. Biomed. Pharmacother. 2016;83:153–159. doi: 10.1016/j.biopha.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 122.Watts R., Johnsen V.L., Shearer J., Hittel D.S. Myostatin-induced inhibition of the long noncoding RNA Malat1 is associated with decreased myogenesis. Am. J. Physiol. Physiol. 2013;304:C995–C1001. doi: 10.1152/ajpcell.00392.2012. [DOI] [PubMed] [Google Scholar]
- 123.Bernard D., Prasanth K.V., Tripathi V., Colasse S., Nakamura T., Xuan Z., Zhang M.Q., Sedel F., Jourdren L., Coulpier F., et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–3093. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eißmann M., Gutschner T., Hämmerle M., Günther S., Caudron-Herger M., Groβ M., Schirmacher P., Rippe K., Zörnig M., Braun T., et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076–1087. doi: 10.4161/rna.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gordon A.D., Biswas S., Feng B., Chakrabarti S. MALAT1: A regulator of inflammatory cytokines in diabetic complications. Endocrinol. Diabetes Metab. 2018;1:1–11. doi: 10.1002/edm2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu M., Wang R., Li X., Fan M., Lin J., Zhen J., Chen L., Lv Z. LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with β-catenin. J. Cell Mol. Med. 2017;21:2732–2747. doi: 10.1111/jcmm.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang M., Gu H., Chen J., Zhou X. Involvement of long noncoding RNA MALAT1 in the pathogenesis of diabetic cardiomyopathy. Int. J. Cardiol. 2016;202:753–755. doi: 10.1016/j.ijcard.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 128.Du Y., Tang J., Li G., Berti-Mattera L., Lee C.A., Bartkowski D., Gale D., Monahan J., Niesman M.R., Alton G., et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Investig. Ophthalmol. Vis. Sci. 2010;51:2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Puthanveetil P., Chen S., Feng B., Gautam A., Chakrabarti S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell Mol. Med. 2015;19:1418–1425. doi: 10.1111/jcmm.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Michalik K.M., You X., Manavski Y., Doddaballapur A., Zörnig M., Braun T., John D., Ponomareva Y., Chen W., Uchida S., et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 131.Liu P., Jia S.B., Shi J.M., Li W.J., Tang L.S., Zhu X.H., Tong P. LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci. Rep. 2019;39:1–26. doi: 10.1042/BSR20181469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Capitão M., Soares R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell Biochem. 2016;117:2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 133.Ishii N., Ozaki K., Sato H., Mizuno H., Saito S., Takahashi A., Miyamoto Y., Ikegawa S., Kamatani N., Hori M., et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J. Hum. Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 134.Barry G., Briggs J.A., Vanichkina D.P., Poth E.M., Beveridge N.J., Ratnu V.S., Nayler S.P., Nones K., Hu J., Bredy T.W., et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol. Psychiatry. 2014;19:486–494. doi: 10.1038/mp.2013.45. [DOI] [PubMed] [Google Scholar]
- 135.Zhang H.Y., Zheng F.S., Yang W., Lu J. Bin. The long non-coding RNA MIAT regulates zinc finger E-box binding homeobox 1 expression by sponging miR-150 and promoteing cell invasion in non-small-cell lung cancer. Gene. 2017;633:61–65. doi: 10.1016/j.gene.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 136.Blackshaw S., Harpavat S., Trimarchi J., Cai L., Huang H., Kuo W.P., Weber G., Lee K., Fraioli R.E., Cho S.H., et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:1–21. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aprea J., Prenninger S., Dori M., Ghosh T., Monasor L.S., Wessendorf E., Zocher S., Massalini S., Alexopoulou D., Lesche M., et al. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32:3145–3160. doi: 10.1038/emboj.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen Y., Dong L.F., Zhou R.M., Yao J., Song Y.C., Yang H., Jiang Q., Yan B. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: A clinical and in vitro study. J. Cell Mol. Med. 2016;20:537–548. doi: 10.1111/jcmm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou X., Zhang W., Jin M., Chen J., Xu W., Kong X. lncRNA MIAT functions as a competing endogenous RNA to upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy. Cell Death Dis. 2017;8:1–8. doi: 10.1038/cddis.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou L., Xu D.Y., Sha W.G., Shen L., Lu G.Y., Yin X. Long non-coding MIAT mediates high glucose-induced renal tubular epithelial injury. Biochem. Biophys. Res. Commun. 2015;468:726–732. doi: 10.1016/j.bbrc.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 141.Pasmant E., Laurendeau I., Héron D., Vidaud M., Vidaud D., Bièche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: Identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67:3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 142.Broadbent H.M., Peden J.F., Lorkowski S., Goel A., Ongen H., Green F., Clarke R., Collins R., Franzosi M.G., Tognoni G., et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum. Mol. Genet. 2008;17:806–814. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 143.Li Z., Yu X., Shen J. ANRIL: A pivotal tumor suppressor long non-coding RNA in human cancers. Tumor Biol. 2016;37:5657–5661. doi: 10.1007/s13277-016-4808-5. [DOI] [PubMed] [Google Scholar]
- 144.Thomas A.A., Feng B., Chakrabarti S. ANRIL regulates production of extracellular matrix proteins and vasoactive factors in diabetic complications. Am. J. Physiol. Metab. 2017;314:E191–E200. doi: 10.1152/ajpendo.00268.2017. [DOI] [PubMed] [Google Scholar]
- 145.Pasquale L.R., Loomis S.J., Kang J.H., Yaspan B.L., Abdrabou W., Budenz D.L., Chen T.C., Delbono E., Friedman D.S., Gaasterland D., et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am. J. Ophthalmol. 2013;155:1–17. doi: 10.1016/j.ajo.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou X., Han X., Wittfeldt A., Sun J., Liu C., Wang X., Gan L.M., Cao H., Liang Z. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol. 2016;13:98–108. doi: 10.1080/15476286.2015.1122164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pachnis V., Belayew A., Tilghman S.M. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc. Natl. Acad. Sci. USA. 2006;81:5523–5527. doi: 10.1073/pnas.81.17.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang X., Yan Y., Hu M., Chen X., Wang Y., Dai Y., Wu D., Wang Y., Zhuang Z., Xia H. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J. Neurosurg. 2017;2016:129–136. doi: 10.3171/2014.12.JNS1426.test. [DOI] [PubMed] [Google Scholar]
- 149.Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 150.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:1–11. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song X., Luo X., Gao Q., Wang Y., Gao Q., Long W. Dysregulation of LncRNAs in Placenta and Pathogenesis of Preeclampsia. Curr. Drug Targets. 2017;18:115–1170. doi: 10.2174/1389450118666170404160000. [DOI] [PubMed] [Google Scholar]
- 152.Wang J., Zhao H., Fan Z., Li G., Ma Q., Tao Z., Wang R., Feng J., Luo Y. Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1-Dependent M1 Microglial Polarization. Stroke. 2017;48:2211–2221. doi: 10.1161/STROKEAHA.117.017387. [DOI] [PubMed] [Google Scholar]
- 153.Wang J., Cao B., Han D., Sun M., Feng J. Long Non-coding RNA H19 Induces Cerebral Ischemia Reperfusion Injury via Activation of Autophagy. Aging Dis. 2017;8:71–84. doi: 10.14336/AD.2016.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Han C.L., Ge M., Liu Y.P., Zhao X.M., Wang K.L., Chen N., Hu W., Zhang J.G., Li L., Meng F.G. Long non-coding RNA H19 contributes to apoptosis of hippocampal neurons by inhibiting let-7b in a rat model of temporal lobe epilepsy. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-018-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun B., Ding Y., Jin X., Xu S., Zhang H. Long Non-coding RNA H19 Promotes Corneal Neovascularization by Targeting MicroRNA-29c. Biosci. Rep. 2019;39:1–12. doi: 10.1042/BSR20182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan J.X. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017;21:322–328. [PubMed] [Google Scholar]
- 157.Biswas S., Chakrabarti S. Increased Extracellular Matrix Protein Production in Chronic Diabetic Complications: Implications of Non-Coding RNAs. Non-Coding RNA. 2019;5:30. doi: 10.3390/ncrna5010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roy S., Amin S., Roy S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016;142:71–75. doi: 10.1016/j.exer.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zuccato C., Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 160.Hackett S.F., Friedman Z., Freund J., Schoenfeld C., Curtis R., DiStefano P.S., Campochiaro P.A. A splice variant of trkB and brain-derived neurotrophic factor are co- expressed in retinal pigmented epithelial cells and promote differentiated characteristics. Brain Res. 1998;789:201–212. doi: 10.1016/S0006-8993(97)01440-6. [DOI] [PubMed] [Google Scholar]
- 161.Kaviarasan K., Jithu M., Arif Mulla M., Sharma T., Sivasankar S., Das U.N., Angayarkanni N. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism. 2015;64:958–966. doi: 10.1016/j.metabol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 162.Ola M.S., Nawaz M.I., El-Asrar A.A., Abouammoh M., Alhomida A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol. Neurobiol. 2013;33:359–367. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.da Rocha S.T., Edwards C.A., Ito M., Ogata T., Ferguson-Smith A.C. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–316. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Y., Zhang X., Klibanski A. MEG3 noncoding RNA: A tumor suppressor. J. Mol. Endocrinol. 2012;48:1–16. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shan K., Jiang Q., Wang X.Q., Wang Y.N., Yang H., Yao M.D., Liu C., Li X.M., Yao J., Liu B., et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016;7:1–13. doi: 10.1038/cddis.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mercer T.R., Qureshi I.A., Gokhan S., Dinger M.E., Li G., Mattick J.S., Mehler M.F. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:1–15. doi: 10.1186/1471-2202-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B.R., Protacio A., Flynn R.A., Gupta R.A., et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–126. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lian Y., Cai Z., Gong H., Xue S., Wu D., Wang K. HOTTIP: A critical oncogenic long non-coding RNA in human cancers. Mol. Biosyst. 2016;12:3247–3253. doi: 10.1039/C6MB00475J. [DOI] [PubMed] [Google Scholar]
- 169.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ceriello A., Esposito K., Ihnat M., Thorpe J., Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J. Clin. Endocrinol. Metab. 2009;94:2751–2756. doi: 10.1210/jc.2009-0762. [DOI] [PubMed] [Google Scholar]
- 171.Aiello L.P. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:17–23. doi: 10.2337/dc13-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Villeneuve L.M., Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Physiol. 2010;299:F14–F25. doi: 10.1152/ajprenal.00200.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miao F., Chen Z., Genuth S., Paterson A., Zhang L., Wu X., Li S.M., Cleary P., Riggs A., Harlan D.M., et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63:1748–1762. doi: 10.2337/db13-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kowluru R.A., Santos J.M., Mishra M. Epigenetic Modifications and Diabetic Retinopathy. Biomed. Res. Int. 2013;2013:1–9. doi: 10.1155/2013/635284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Felsenfeld G. A brief history of epigenetics. Cold Spring Harb. Perspect. Biol. 2014;6:1–10. doi: 10.1101/cshperspect.a018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miranda T.B., Jones P.A. DNA methylation: The nuts and bolts of repression. J. Cell Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 177.Deaton A.M., Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dos Santos N.M., Silva A.S., Wanderley D.Q.E.I., Modesto F.J., Alves P.G.C., Ferreira D.N.R., Pordeus L.R., de Carvalho C.M., Paulo D.O.N., Camati P.D. Analysis of the DNA methylation profiles of miR-9-3, miR-34a, and miR-137 promoters in patients with diabetic retinopathy and nephropathy. J. Diabetes Complicat. 2018;32:593–601. doi: 10.1016/j.jdiacomp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 179.Kowluru R.A., Shan Y., Mishra M. Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab. Investig. 2016;96:1040–1049. doi: 10.1038/labinvest.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mishra M., Kowluru R.A. The role of DNA methylation in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2016;57:5748–5757. doi: 10.1167/iovs.16-19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agardh E., Lundstig A., Perfilyev A., Volkov P., Freiburghaus T., Lindholm E., Rönn T., Agardh C.D., Ling C. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:1–9. doi: 10.1186/s12916-015-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 183.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 184.Eberharter A., Becker P. Histone acetylation: A switch between repressive and permissive chromatin. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Greer E.L., Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martin C., Zhang Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 187.Sawan C., Herceg Z. Histone Modifications and Cancer. Adv. Genet. 2010;70:57–85. doi: 10.1016/B978-0-12-380866-0.60003-4. [DOI] [PubMed] [Google Scholar]
- 188.El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P.L., Roeder R.G., Cooper M.E., Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun G., Reddy M.A., Yuan H., Lanting L., Kato M., Natarajan R. Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J. Am. So. Nephrol. 2010;21:2069–2080. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Villeneuve L.M., Reddy M.A., Lanting L.L., Wang M., Meng L., Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miao F., Wu X., Zhang L., Yuan Y.C., Riggs A.D., Natarajan R. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J. Biol. Chem. 2007;282:13854–13863. doi: 10.1074/jbc.M609446200. [DOI] [PubMed] [Google Scholar]
- 192.Zhong Q., Kowluru R.A. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–1313. doi: 10.2337/db10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mishra M., Zhong Q., Kowluru R.A. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014;55:7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang W., Sidoli S., Zhang W., Wang Q., Wang L., Jensen O.N., Guo L., Zhao X., Zheng L. Abnormal levels of histone methylation in the retinas of diabetic rats are reversed by minocycline treatment. Sci. Rep. 2017;7:1–14. doi: 10.1038/srep45103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Holoch D., Margueron R. Mechanisms Regulating PRC2 Recruitment and Enzymatic Activity. Trends Biochem. Sci. 2017;42:531–542. doi: 10.1016/j.tibs.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 196.Ruiz M.A., Feng B., Chakrabarti S. Polycomb repressive complex 2 regulates MiR-200b in retinal endothelial cells: Potential relevance in diabetic retinopathy. PLoS ONE. 2015;10:1–21. doi: 10.1371/journal.pone.0123987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen S., Feng B., George B., Chakrabarti R., Chen M., Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am. J. Physiol. Metab. 2009;298:E127–E137. doi: 10.1152/ajpendo.00432.2009. [DOI] [PubMed] [Google Scholar]
- 198.Cao Y., Feng B., Chen S., Chu Y., Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Investig. Ophthalmol. Vis. Sci. 2014;55:7321–7331. doi: 10.1167/iovs.14-15167. [DOI] [PubMed] [Google Scholar]
- 199.Feng B., Cao Y., Chen S., Chu X., Chu Y., Chakrabarti S. MiR-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65:768–779. doi: 10.2337/db15-1033. [DOI] [PubMed] [Google Scholar]
- 200.Lee S.T., Li Z., Wu Z., Aau M., Guan P., Karuturi R.K., Liou Y.C., Yu Q. Context-Specific Regulation of NF-κB Target Gene Expression by EZH2 in Breast Cancers. Mol. Cell. 2011;43:798–810. doi: 10.1016/j.molcel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 201.Shivdasani R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood. 2006;108:3646–3653. doi: 10.1182/blood-2006-01-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ivey K.N., Srivastava D. microRNAs as developmental regulators. Cold Spring Harb. Perspect. Biol. 2015;7:1–9. doi: 10.1101/cshperspect.a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cai Y., Yu X., Hu S., Yu J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee Y.S., Dutta A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Romaine S.P.R., Tomaszewski M., Condorelli G., Samani N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart. 2015;101:921–928. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nelson P.T., Wang W.X., Rajeev B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18:130–138. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kovacs B., Lumayag S., Cowan C., Xu S. microRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011;52:4402–4409. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 208.McArthur K., Feng B., Wu Y., Chen S., Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen S., Feng B., Thomas A.A., Chakrabarti S. MiR-146a regulates glucose induced upregulation of inflammatory cytokines extracellular matrix proteins in the retina and kidney in diabetes. PLoS ONE. 2017;12:1–17. doi: 10.1371/journal.pone.0173918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mortuza R., Feng B., Chakrabarti S. MiR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia. 2014;57:1037–1046. doi: 10.1007/s00125-014-3197-9. [DOI] [PubMed] [Google Scholar]