Abstract
The use of targeted agents and immunotherapy for the treatment of advanced non-small-cell lung cancer (NSCLC) has made it mandatory to characterize tumor tissue for patient selection. Moreover, the development of agents that are active against specific resistance mechanisms arising during treatment make it equally important to characterize the tumor tissue at progression by performing tissue re-biopsy. Given that tumor tissue is not always available for molecular characterization due to the paucity of diagnostic specimens or problems relating to the carrying out of invasive procedures, the use of liquid biopsy represents a valid approach to overcoming these difficulties. The most common material used for liquid biopsy in this setting is plasma-derived cell free DNA (cfDNA), which originates from cells undergoing apoptosis or necrosis. However, other sources of tumor material can be considered, such as extracellular vesicle (EV)-derived nucleic acids, which are actively secreted from living cells and closely correspond to tumor dynamics. In this review, we discuss the role of liquid biopsy in the therapeutic management of NSCLC with particular regard to targeted therapy and immunotherapy, and analyze the pros and cons of the different types of samples used in this context.
Keywords: liquid biopsy, diagnostic biomarkers, targeted therapy, acquired resistance, lung cancer
1. Introduction
The use of non-invasive methods to characterize tumors is a valuable tool for the management and assessment of cancer patients, e.g., at diagnosis, for the prediction of prognosis and response to therapy, and in the monitoring of response to treatment. The introduction of targeted therapy into clinical practice has made it mandatory to characterize tumor tissue for patient selection, increasing the need for biological material for molecular analysis. In patients with advanced non-small-cell lung cancer (NSCLC) for whom diagnosis is normally made on small biopsies or cytology specimens, the frequent paucity of available tumor tissue is a serious problem. Moreover, if the biological sample available derives from a bone biopsy, there is a higher risk of bad quality material that can compromise the molecular analysis. In addition, the tumor may be heterogeneous and a biopsy performed at a specific point may consequently not represent the overall molecular landscape of the cancer. Liquid biopsy represents an optimal strategy to overcome such problems. Another important use of liquid biopsy is to monitor response to treatment and the onset of resistance mechanisms. Given that tumor re-biopsy is an invasive procedure and that not all patients are amenable to surgery, the only remaining option is to characterize tumor alterations by liquid biopsy. Molecular analysis of liquid biopsy normally takes less time than that of tumor tissue, which involves several steps and evaluations performed by a pathologist, with a consequent lengthening of the turnaround time.
As the amount of tumor material present in biological fluids is very limited, it is crucial to use highly sensitive methodologies to avoid false-negative results and guarantee the best treatment options for patients. Although different types of biological samples are used for liquid biopsies, cell free DNA (cfDNA) is currently the most widely used material in clinical practice.
In this review, we report on the use of liquid biopsy in NSCLC treatment, in particular, targeted therapy and immunotherapy, and focus on the different types of biological samples used for molecular analyses, weighing the pros and cons of each approach.
2. Targeted Therapy in NSCLC
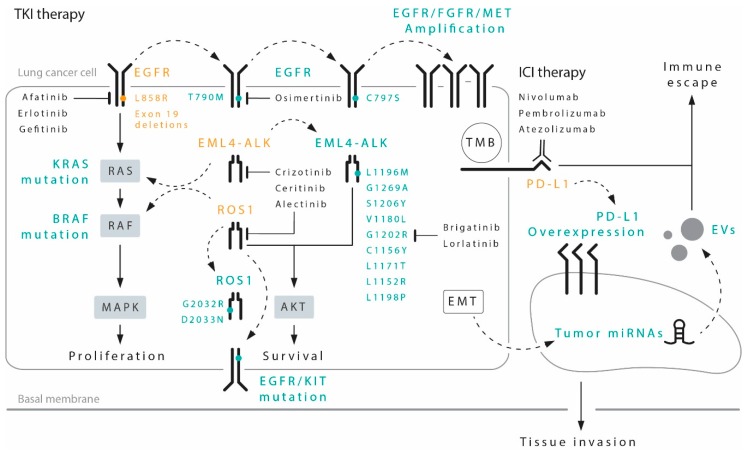
Tyrosine kinase inhibitors (TKIs) represent the treatment of choice for subgroups of patients carrying specific gene alterations (Figure 1). In particular, patients harboring mutations of the epidermal growth factor receptor (EGFR), as well as rearrangements of the anaplastic lymphoma kinase (ALK) or the ROS proto-oncogene 1 (ROS1), have significantly improved clinical outcomes when treated with TKI therapy.
Figure 1.
Most relevant targeted therapies and immune checkpoint inhibitor (ICIs) in non-small-cell lung cancer (NSCLC), with the principal targets highlighted (in orange) and the most recurrent resistance mechanisms (in green) potentially detectable by liquid biopsy. EGFR, epidermal growth factor receptor; KIT, c-Kit proto-oncogene receptor tyrosine kinase; MET, c-Met proto-oncogene; EML4-ALK, echinoderm microtubule associated protein-like 4-anaplastic lymphoma kinase; ROS1, ROS1 proto-oncogene receptor tyrosine kinase; EV, extracellular vesicles.
Specific driver mutations can identify patients that are sensitive to certain target inhibitors, whereas the expression of the programmed death-ligand 1 (PD-L1) and elevated tumor mutational burden (TMB), may help define the patients with higher probability to respond to immune checkpoint inhibitors (ICIs). A series of FDA-approved tyrosine kinase inhibitor (TKIs) and ICIs are now available for the first-line treatment of NSCLC. Acquired resistance, however, can promote downstream activation (straight arrowed lines) or bypass (dotted arrows) of signaling pathways, via secondary mutations in the same target gene, as well as parallel mutations or amplification of membrane receptors (e.g., EGFR, FGFR, MET, KIT), and transcription of tumor-specific miRNAs. Emergence of epithelial-to-mesenchymal transition (EMT) is a common trait of advanced-cancer clones that have developed pharmacological resistance. All these mechanisms of pharmacological resistance are potentially detectable via liquid biopsy.
2.1. Anti-Epidermal Growth Factor Receptor
A new era in NSCLC treatment started in 2004 when it was discovered that mutations of EGFR gene are predictive of sensitivity to therapy with anti-EGFR TKIs [1,2]. Following the disappointing results of the IDEAL-1 and IDEAL-2 trials for gefitinib [3,4], and the TALENT and TRIBUTE trials for erlotinib [5,6], both drugs continued to produce contrasting results until the success of several studies in which EGFR-TKIs were compared to chemotherapy in patients with activating EGFR mutations [7,8,9]. In this setting, both drugs were shown to prolong progression-free survival (PFS) but not overall survival (OS) compared with platinum-doublet chemotherapy (median PFS of 9.2–13.1 months vs. 4.6–6.3 months, respectively).
Afatinib, a second-generation EGFR-TKI that irreversibly blocks EGFR and other ErbB-family receptors, demonstrated a benefit in PFS with respect to chemotherapy in two phase III trials, (LUX-Lung 3 and LUX-Lung 6) [10,11]. It also showed a significant advantage in terms of OS in patients carrying EGFR exon 19 deletions [12]. Subsequently, osimertinib, a third-generation EGFR-TKI developed for the second-line treatment of patients progressing on first- or second-generation TKIs and developing an EGFR-T790M mutation [13], prolonged PFS with respect to gefitinib and erlotinib in previously untreated EGFR-mutated patients. It has since been approved for use in a first-line setting [14].
However, although a clinical benefit is indubitably observed with the use of these drugs, resistance mechanisms inevitably appear after around 12 months of treatment [7,8,9,10,11]. The most common resistance mechanism to first- and second-generation TKIs is the gatekeeper T790M mutation occurring at exon 20 of EGFR (Figure 1) in about 50–70% of cases. It has been seen that patients developing this alteration remain sensitive to osimertinib, thus explaining why this drug was approved for second-line therapy in this subgroup [7]. This has led to the need for tumor characterization after progression on first- or second-line TKIs, which poses the problem of having sufficient tumor tissue available for molecular analysis. As tumor re-biopsy is not always possible due to tumor localization or patients’ health conditions, liquid biopsy has become a key element in the determination of resistance mutations.
In contrast to first- and second-generation TKIs, no predominant acquired resistant mechanisms have been observed for osimertinib in the first-line setting, for which very few data are still available. A number of in vitro studies and case reports have shown that secondary EGFR mutations [15,16], activation of AXL [17] and ERK [18] signaling, and small cell transformation [19] may emerge as acquired resistance mechanisms in this setting. More data are available on resistance mechanisms to second-line osimertinib in T790M-positive patients. In these patients, C797S mutation is observed in around 20–40% of cases, while amplification of the c-Met proto-oncogene (MET) in 14% of cases; at lower frequency, transformation into small-cell phenotype, amplification of the erb-B2 receptor tyrosine kinase 2 (HER2) or the fibroblast growth factor receptor 1 (FGFR1) genes, and mutations in the b-raf proto-oncogene, serine/threonine kinase (BRAF) is also observed [20,21].
2.2. Anti-Anaplastic Lymphoma Kinase
In 2007, aberrant gene fusion of the echinoderm microtubule associated protein-like 4 (EML4) with ALK was documented in NSCLC [22]. Genetic rearrangement of ALK is present in about 3–7% of NSCLC patients who are usually young, non-smokers and have tumors with adenocarcinoma (ADC) histology [23]. The first drug developed against this alteration was crizotinib, an oral ATP-competitive selective inhibitor of the ALK, MET, and ROS1 tyrosine kinase. Two phase III trials, PROFILE 1007 [24] and PROFILE 1014 [25] showed a significant advantage of crizotinib over chemotherapy in first- and second-line settings, respectively. Based on these results, the drug was approved for treatment of patients with advanced ALK-rearranged tumors, becoming the treatment of choice in untreated NSCLC patients carrying this driver alteration. However, after about 12 months’ treatment the majority of patients progressed by developing both ALK-dependent and -independent resistance mechanisms. The most common secondary ALK mutations are L1196M and G1269A [26,27,28], both of which interfere with crizotinib binding. Other mutant variants that reduce the ATP-binding affinity of crizotinib include S1206Y, V1180L and G1202R, whereas other secondary mutations, such as C1156Y, L1171T, L1152R and L1198P promote ATP binding and stabilize the ALK active conformation [29,30] (Figure 1). These resistance mechanisms occur even more frequently after treatment with second-generation ALK inhibitors such as ceritinib and alectinib, reflecting their greater potency and selectivity [31]. Although these mutations also induce resistance to both first- and second-generation ALK inhibitors [32], brigatinib, another second-generation ALK inhibitor, is mainly active against secondary resistance mutations, with the exception of the G1202R [33]. Subsequently, the third-generation ALK inhibitor lorlatinib was developed to overcome ALK secondary resistance mutations, including G1202R, and has a potential indication for use after failure of other ALK inhibitors [34]. The identification of different ALK mutations when progression occurs on TKI treatment is crucial to enable the right drug to be offered to the right patient at the right time. Within this context, the use of liquid biopsy to monitor the development of mutations has become necessary.
2.3. Anti-ROS Proto-Oncogene 1
Chromosomal rearrangements involving the ROS1 gene were first described in NSCLC in 2007 [35]. Subsequently, a frequency of 1–2% was documented in this type of cancer, with a prevalence in younger patients, never or light smokers and ADC histology [36]. ROS1-rearranged lung tumors are “addicted” to ROS1 for growth and survival [35], leading to a pharmacological sensitivity of the tumor to ROS1-directed TKIs [36,37]. After preclinical studies showed that crizotinib potently inhibits ROS1 [37], the phase I PROFILE 1001 study of crizotinib in ALK-translocated patients was amended to include ROS1-rearranged patients, reporting an objective response rate (ORR) and disease control rate (DCR) to crizotinib of 72% and 90%, respectively [37]. Following these results, crizotinib was fully approved by the Food and Drug Administration (FDA) in March 2016 for the treatment of advanced ROS1-rearranged NSCLC. However, as happens in EGFR-mutated and ALK-translocated patients, ROS1-rearranged tumors typically develop resistance mechanisms to crizotinib involving secondary mutations in the ROS1 kinase domain (50–60% of cases) or “off target” alterations in parallel pathways [38,39].
The most frequent ROS1 resistance mutation is the G2032R, which causes steric hindrance to drug binding, whilst not altering the oncogenic kinase activity [40]. Other resistance mutations include D2033N, S1986Y/F, L2026M and L1951R. G2032R and L1951R have been shown to confer the highest level of crizotinib-resistant phenotype in vitro [41]. Less is known about “off target” mechanisms. Alterations of the KIT proto-oncogene receptor tyrosine kinase (KIT), the KRAS proto-oncogene GTPase (KRAS), EGFR and BRAF genes have also been reported [42,43,44]. Phenotypic changes, such as epithelial-to-mesenchymal transition (EMT), have also been described [39]. Several other agents have proven active in patients with ROS1-rearranged tumors and in those developing ROS1 resistance mutations. In particular, ceritinib and brigatinib have shown comparable activity against L2026M but not against G2032R, D2033N or L1951R [38]. Whilst lorlatinib has proven effective against different resistance mutations [45], its activity against G2032R is limited. Despite cabozantinib being one of the few agents with confirmed activity against the G2032R mutation, its high toxicity severely restricts its use in this setting [46].
3. Immune Checkpoint Inhibitors
The development of immune checkpoint inhibitors (ICIs) introduced a new era in the treatment of lung cancer. Two agents directed against the programmed cell death protein 1 (PD-1), nivolumab and pembrolizumab, and one agent directed against the programmed death-ligand 1 (PD-L1), atezolizumab, have been approved for second-or-more line treatment of advanced lung ADC (Figure 1), but generate a durable clinical response in only 30% of patients [47,48,49,50]. Pembrolizumab is also approved for the first-line treatment of patients with PD-L1 expression >50%, with a 45% response rate and a median progression-free survival (PFS) of 10.3 months (95% confidence interval [CI] 6.7-not reached) [51]. In addition, recent evidence has highlighted the efficacy of a combination of chemotherapy (CT) and ICIs in the first-line setting, with a median PFS of 8.8 months (95% CI 7.6 to 9.2), independently of PD-L1 expression [52]. There are still no accurate predictive biomarkers to select patients who are more likely to respond to ICI therapy. PD-L1 expression is currently the only biomarker used but is unreliable as PD-L1-negative patients sometimes respond to therapy [47,48,49,50]. Although tumor mutational burden (TMB) represents another parameter capable of identifying patients who are more likely to respond to ICIs [53], a fully standardized method for its detection has not yet been established [54]. Liquid biopsy could potentially be of help in identifying biomarkers of resistance to ICI treatment during the course of treatment, thus bypassing the problem of tumor heterogeneity. However, there are numerous difficulties involved in developing targeted sequencing panels to for the assessment of TMB starting from cfDNA, and further research is needed to validate this approach [54].
Numerous studies have also reported a correlation between neutrophil-to-lymphocyte ratio (NLR) and immunotherapy [55,56,57]. In a recent report, Jiang et al. performed a comprehensive online search to explore the relation between blood NLR and OS and PFS in NSCLC patients treated with ICIs. They considered 16 studies, and found a significant association between elevated NLR values and patients’ prognosis, with shorter PFS and OS in those patients treated with ICIs and with elevated blood NLR [58].
An important role has also been reported in determining ICIs resistance by tumor associated macrophages (TAMs). In particular, Muraoka et al. demonstrated that in immune-resistant tumors, TAMs remained inactive and did not serve as antigen-presenting cells, and their manipulation, by inducing their antigen-presenting activity, could re-sensitize cells to treatment [59]. Moreover, TAMs enhance tumor hypoxia and aerobic glycolysis, contributing to resistance to therapy. Depletion of TAMs restores drug sensitivity and increases PD-L1 expression in aerobic cancer cells as well as T-cell infiltration in tumors, resulting in antitumor efficacy by anti-PD-L1 treatment [60].
4. Liquid Biopsy in Monitoring Response to Treatment
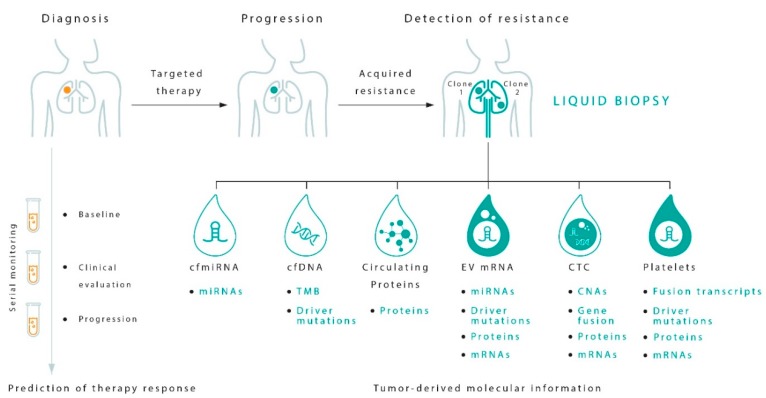
The main use of liquid biopsy in NSCLC is that monitoring resistance mechanisms that develop with targeted agents or ICIs. Different types of samples are used for this purpose (Figure 2). The possibility of identifying resistance mechanisms during treatment could help to identify disease progression earlier and improve treatment personalization.
Figure 2.
Potentials of tumor circulating biomarkers for prediction and monitoring response to therapy in NSCLC by liquid biopsy. TMB, tumor mutational burden; miRNA, microRNA; mRNA, messenger RNA; CNAs, genomic copy number alterations. A range of tumor components can be isolated from the blood of patients with NSCLC, such as cell-free DNA (cfDNA) and cell-free microRNAs (cfmiRNAs), circulating tumor cells (CTCs), extracellular vesicles (EVs), platelets and proteins released in the circulation. All these circulating cancer biomarkers are most suitable for different diagnostic applications to derive specific clinically relevant molecular information that may help predict the risk of relapse and the development of resistance mechanisms. While sequencing of cfDNA is the gold standard to determine the mutational status of the tumor, new technologies have enabled the advantage to be taken of CTCs, obtaining a complete and complementary view of tumor biology. EVs are a reservoir of miRNAs, and can integrate the information derived from cfmiRNAs. Platelets can often contain larger oncogenic transcripts and gene-fusion mRNAs. Analysis of circulating proteins may provide in a not-far future a wealth of information on tumor development. These methods pose liquid biopsy as a comprehensive diagnostic practice to yield the molecular heterogeneity of cancer. As a foreseeable application of liquid biopsy, serial blood testing holds great potential for minimally invasive dynamic monitoring of therapy efficacy, allowing early diagnosis of progression to metastasis.
4.1. Cell Free DNA
4.1.1. Cell Free DNA Biology
There is evidence that tumor cells release their DNA into the circulation [61]. The challenge in detecting cfDNA lies in the fact that cfDNA is markedly dilute compared to background circulating germline DNA (0.01–10%) [62]. This is one of the main reasons why highly sensitive methodologies are needed to determine cfDNA. A growing number of studies have investigated the usefulness of cfDNA in cancer management and, among these, the assessment of cfDNA for treatment monitoring and detection of resistance mechanisms is one of the most promising (Table 1).
Table 1.
Principal resistance biomarkers detectable by cell free DNA (cfDNA).
| Biomarkers | N Patients | Methodology | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| EGFR T790M | 23 | PNAClamp™ | 71.4 | 100 | Hur et al. [63] |
| 21 | SARMS | 39 | 100 | Maheswaran et al. [64] | |
| 208 | ddPCR™ | - | 57 | Jenkins et al. [65] | |
| 226 | cobas® | - | 51 | Jenkins et al. [65] | |
| 227 | NGS | - | 65 | Jenkins et al. [65] | |
| 216 | BEAMing | 69 | 70 | Oxnard et al. [66] | |
| ALK resistance mutations | 7 | ddPCR™ | 2 mutations detected | - | Yoshida et al. [67] |
| 20 | ddPCR™ | 10 mutations detected | - | Bordi P et al. [68] | |
| ROS1 resistance mutations | 18 | NGS | 6 mutations detected | - | Dagogo et al. [69] |
Abbreviations: PNAClamp™, peptide nucleic acid (PNA)-mediated PCR-EGFR Mutation Detection Kit; SARMS, Scorpion Amplification Refractory Mutation System; ddPCR™, Droplet Digital ™ PCR; cobas®, cobas® EGFR Mutation Test v2; NGS, next generation sequencing; BEAMing, Beads Emulsion Amplification Magnetic digital PCR.
4.1.2. Clinical Application of cfDNA
A great deal of research has been performed to identify EGFR T790M mutations and monitor EGFR-sensitive mutations in cfDNA during the course of TKI treatment, using methodologies with different sensitivity [70,71]. These methods include real-time PCR-based approaches, digital droplet PCR, BEAMing and next generation sequencing (NGS) methodologies [72]. However, although all of these are high-sensitivity methodologies, sensitivity is fairly low (60–70%). Conversely, specificity is very high (90–100%) [10,73,74,75]. Hence, the absence of a specific mutation, as detected in cfDNA, does not exclude the possibility of that mutation being present in tumor tissue. Recent advances in NGS technology have demonstrated the possibility of obtaining high levels of both specificity and sensitivity [76,77], suggesting that the low sensitivity will probably be overcome.
The guidelines from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology established that the use of cfDNA is indicated at diagnosis when tissue is limited and/or insufficient for molecular testing, and also for the monitoring of T790M mutation [78]. However, in either case, if the EGFR mutation test is negative in cfDNA, tissue biopsy should be considered.
It was recently shown that NGS analysis of cfDNA in lung cancer with insufficient tumor samples for tissue sequencing could be extremely useful, facilitating the detection of actionable variants that frequently co-occur with other potentially clinically relevant genomic alterations and thus permitting the timely initiation of genotype-matched therapies [79].
Analysis of the T790M mutation should be always accompanied by evaluation of the sensitizing EGFR mutation to confirm that tumor DNA is being shed into the circulation. Oxnard et al. [80] reported a higher percentage of responders in patients with a double-negative cfDNA analysis (i.e., absence of T790M and EGFR-sensitivity mutation), than in those with negative T790M but positive EGFR-sensitivity mutation. This suggests that the absence of mutations in the former group of patients was mainly due to a “non-shedding” tumor, rather than to the actual absence of mutation. However, in both cases, tumor re-biopsy is recommended [72].
In addition to its proven usefulness in EGFR mutation analysis, cfDNA could also help to monitor secondary ALK resistance mutations during treatment with an anti-ALK agent [66,68,72,81]. As observed in patients progressing on EGFR-TKIs, those progressing during anti-ALK treatments may develop secondary ALK resistance mutations for which more specific anti-ALK agents might be effective. Given the wide range of mutations that need to be determined, NGS would seem to be the best methodology [72,76,77]. However, there is still insufficient evidence to support the use of testing ALK mutational status in lung ADC patients with sensitizing ALK translocation who have progressed after treatment with an ALK-targeted TKI [78]. Similarly, cfDNA analysis could potentially be used for ROS1-positive patients treated with crizotinib as they too may develop resistance mechanisms that could be used to drive subsequent targeted treatment [67,76,77]. However, there are still no clinical indications to indicate the ROS1 test in cfDNA.
With regard to ICIs, the only potentially detectable biomarker in cfDNA that could drive the choice of treatment is the TMB test. Gandara et al. demonstrated that it is possible to use cfDNA to determine TMB, also reporting a relationship between clinical outcome and cfDNA TMB [82]. However, further studies are needed to establish the conditions required for using this test in routine clinical practice, especially the choice of cut-off value to be used [54].
4.2. Circulating Tumor Cells
4.2.1. Circulating Tumor Cell Biology
Although extremely rare, circulating tumor cells (CTCs) isolated from the peripheral blood of cancer patients represent a promising alternative to invasive biopsies as a source of tumor tissue for the detection, characterization, and monitoring of all non-hematologic cancers [69]. Most importantly, compared to single-site biopsy CTC analysis gives a comprehensive picture of the overall tumor content and of the intratumoral heterogeneity arising from branched clonal evolution [83,84]. The usefulness of CTC single-cell analysis lies mainly in its providing parallel information on the mutational profile of the tumor cell, copy number alteration (CNA), genomic rearrangement, and gene expression [85].
A crucial technical challenge to detecting CTCs stems from their rarity, e.g., one cell per milliliter of blood, among millions of background leukocytes [86]. To date, CTC-enrichment by immunomagnetic capture coupled with microfluidic devices has proven the most successful and most widely-used approach to isolate CTCs thanks to the presence of CTC-specific antigens and tumor markers of the tissue of origin that make them clearly distinguishable from leukocytes [87]. Downstream single-cell genetic and phenotypic profiling has made it possible to explore the mechanisms of metastasis and tumor-initiating capacity of CTCs [88]. Downregulation of the major histocompatibility complex (MHC) to avoid immune detection and survive the migration in the bloodstream [89], along with the expression of genes involved in EMT, would seem to be prerequisites of CTCs to initiate organ infiltration [90]. Detection of CTCs with EMT signature is associated with metastasis in NSCLC patients [91], where the presence of certain subpopulations of CTCs defines different tumor characteristics, and the total number of CTCs in the blood correlates with the onset of EGFR-mutated metastasis [92]. The phenotypic evolution of CTCs can be traced during treatment, revealing the appearance of stem cell features and resistance markers that denote the latency of CTCs in the circulation and their resistance to anticancer therapies [93].
4.2.2. Potential Clinical Application of CTCs
In patients with lung cancer, complete CNA profiling of single CTCs generates a molecular classifier capable of predicting chemoresistance and clinical outcome [94]. Furthermore, combining genome-wide DNA methylation and RNA sequencing at single-cell resolution with functional drug screening assays has provided a useful insight into the association between CTCs and different types of drug resistance [95].
The clinical usefulness of CTCs in NSCLC has been further tested with various approaches in an attempt to raise the number of detectable CTCs per milliliter of patient’s blood and consequently increase the sensitivity threshold for the analysis of resistance mutations [69]. Using microfluidic CTC pre-enrichment followed by a PCR-based assay, it is possible to identify EGFR-mutation-positive patients by tissue biopsy with an accuracy of 94%. During anti-EGFR therapy, CTCs can also detect the emergence of the T790M resistance mutation [96]. However, cfDNA analysis has a clear advantage over CTCs in whole-exome sequencing for the detection of a broad range of resistance mutations to EGFR-directed therapy [93]. Pailler et al. detected EML4-ALK rearrangement in CTCs using size-exclusion microfiltration coupled with fluorescence in situ hybridization (FISH). The authors found a strong correlation between ALK-fusion status in CTCs and that of the tumor biopsy, detecting at least four ALK-rearranged CTCs per ml of blood in each patient tested. The CTCs expressed an homogenous pattern of ALK rearrangements, constituting in a unique population of cells with enhanced EMT phenotype [97]. Ilie et al. reported that ALK gene rearrangement carried by CTCs was confirmed by a strong ALK protein expression on the CTC surface and accompanied by the corresponding ALK rearrangement in the primary tumor [64]. Furthermore, real-time blood monitoring of ALK-rearranged CTCs during the course of therapy with crizotinib showed great promise in predicting the outcome of NSCLC patients with ALK-rearranged tumors [98].
It may be possible to evaluate resistance to immunotherapy in NSCLC by recovering CTCs that carry the PD-L1 protein on their plasma membrane. In a study by Ilié et al., the expression of PD-L1 on CTCs was isolated from advanced NSCLC patient blood samples, measured by immunocytochemistry (ICC) and matched with primary tumor staining, revealing 93% concordance between CTCs and tissue, and 55% specificity in detecting PD-L1-positive CTCs. Patients who had PD-L1-positive CTCs in circulation also had a trend for worse prognosis when undergoing chemotherapy [99]. Interestingly, the presentation of PD-L1 on CTC surface has been associated with disease progression and lack of response to anti-PD-1 therapy, which could reflect advanced disease status and immune escape mediated by other checkpoint pathways, probably activated by CTCs that have already begun transformation to an EMT-like phenotype [100]. These findings strongly suggest that PD-L1 measured in CTCs adequately reflects PD-L1 status in the primary tumor. However, the detection of PD-L1 protein in CTCs can also be used to select patients who are more likely to respond to anti-PD-1/PD-L1 treatment, as immunofluorescence has shown that PD-L1 is expressed, together with the epithelial cell adhesion molecule (EpCAM), in over 80% of CTCs derived from metastatic lung cancer [101]. Monitoring PD-L1-positive CTCs at baseline and during the course of therapy in NSCLC patients treated with nivolumab could thus help to predict clinical outcome.
Recent advances in isolating CTCs have enabled precise genetic analysis of drug-resistant CTC clones with specific signatures of direct clinical relevance. Such data support the notion that CTCs can simultaneously provide an overview of tumor genomics and gene expression, and that the characterization of CTCs may be a key factor as these cells are ultimately responsible for metastasis [90]. CTC counts work as independent prognostic factors at both baseline and during follow-up in patients with EGFR-mutated or ALK-rearranged NSCLCs undergoing standard chemotherapy or targeted therapies [102]. In patients with metastatic cancer receiving systemic treatment, temporal changes in the number of CTCs have been shown to correlate with clinical outcome, as measured by standard radiography [103]. Thus, the study of CTCs could have broad implications in elucidating the functional applicability of novel biomarkers, at the same time improving the clinical management of cancer. Nonetheless, CTC technology needs to be reliable, reproducible and robust, with clinical validation to ensure standardization of the readout. For this reason, the use of cfDNA remains preferable for mutational assays that seek to define biomarkers of immediate clinical utility. Table 2 summarizes the most relevant studies on detection of predictive biomarkers by the use of CTCs.
Table 2.
Principal clinical biomarkers detectable by circulating tumor cells (CTCs).
| Biomarkers | N Patients | Methodology | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| EGFR T790M | 21 | SARMS | 94 | 100 | Maheswaran et al. [64] |
| EML4-ALK | 39 | FISH | 100 | 100 | Pailler et al. [100] |
| 32 | FA-FISH | 100 | 100 | Pailler et al. [98] | |
| 87 | FISH | 100 | 100 | Ilie et al. [99] | |
| PD-L1 expression | 71 | ICC | 55 | 100 | Ilie et al. [101] |
Abbreviations: SARMS, Scorpion Amplification Refractory Mutation System; FISH, fluorescence in situ hybridization; FA-FISH, filter-adapted fluorescence in situ hybridization; ICC, immunocytochemistry.
4.3. Extracellular Vesicles
4.3.1. Extracellular Vesicle Biology
The discovery of the ubiquitous presence of a large number of extracellular vesicles (EVs) in the body fluids, coupled with the technical upgrades for EVs purification, is destined to make a substantial improvement in the detection of novel tumor biomarkers, from routine blood tests [104]. The investigation of the functional relevance of EVs flow into the circulation is ongoing, although their key role in cell-to-cell communication and in the transfer of biological material, typically functional microRNAs (miRNAs), messenger RNAs (mRNAs), or proteins, inside the recipient cells, is now widely accepted [105,106].
There is evidence that EVs may be directly involved in cancer resistance to therapy, and tumor EV-derived information could be used for the early diagnosis of the metastatic propensity of a primary tumor [107]. Tumor EVs can promote the organ-specific metastasization of the cancer cell type they have originated from [108], by preparing the pre-metastatic niche through the engagement of the signaling pathway of normal cells [109,110], while also allowing further uptake of malignant EVs [111]. For example, the conversion of quiescent cells to a pro-tumorigenic phenotype by tumor EVs could be stimulated by the transfer of cellular protein kinases, and the correlation between phosphorylation of protein kinases in EVs and that in the tumor tissue could serve as a predictive biomarker of tumor evolution during TKI treatment [112].
4.3.2. Potential Clinical Application of EVs
Tumor EVs are capable of transferring the oncogenic ALK isoform from cancer cells, conferring drug resistance to sensitive cells, via activation of the MAPK pathway, which indicates that EVs could be a vehicle for the onset of therapeutic resistance [113]. Likewise, NSCLC cells can alter the function of adjacent cells by the exchange of EVs that transport active EGFR [114,115]. Interestingly, nuclear translocation of EGFR seems to be mainly dependent on EV fusion mechanism, rather than specific nuclear localization signals [116]: once in the nucleus, EGFR activates distinct signaling pathways that are associated with tumor resistance to therapy-induced DNA damage and anti-EGFR treatment [117,118]. Detection of circulating EGFR protein has long been envisaged as an important prognostic marker in many cancers, including NSCLC [119], and now this information might finally be complemented with a more accurate analysis of EV protein content, to achieve the best prediction to anti-EGFR therapy [120]. EVs can also transport nucleic acids molecules of wild-type EGFR and mutated EGFR reflecting the genetic signature of the original tumor [121]. In a recent study by Hur et al. EVs isolated from plasma and bronchoalveolar lavage fluid (BALF) of NSCLC patients were used to genotype the EGFR status via liquid biopsy, with high accuracy, demonstrating improved specificity and sensitivity, compared to cfDNA analysis [122]. The DNA of EGFR, extracted from both plasma and BALF EVs, contained the L858R mutation and the exon 19 deletion, showing 100% accordance with tissue biopsy, where sensitivity of cfDNA was around 70%. EV liquid biopsy also enabled the authors to detect the T790M mutation with high sensitivity, later confirmed by tissue re-biopsy, in patients who developed resistance to TKIs [122].
In addition to influencing the phenotype of adjacent cells by sharing EV cargo and preparing the pre-metastatic niche in distant sites, cancer cells can also release a continuous amount of immunomodulatory EVs [123] that increase substantially during cytotoxic treatment [63]. The fact that EVs modulate immune cells has been known for a long time [124], as they are a central element in the antigen presentation process, via both the MHC classes I and II [125], and in the receptor–ligand interaction via the tetraspanin network [106]. At the same time, tumor-derived EVs may be the conduit for immune surveillance escape by cancer cells, leading to the failure of immunotherapies [126]. Tumor EVs probably succeed in deceiving lymphocyte activation through the exposure of inhibitory ligands that elicit the immune checkpoint response. Upregulation of PD-L1 expression on the plasma membrane is indeed a common adaptation used by cancer cells [127], with levels of circulating PD-L1 clearly correlating with tumor aggressiveness [128]. PD-L1 positive EVs inhibit CD8+ T cell activation, and their presence in the blood of patients with metastatic cancer identifies those who are more likely to respond to anti-PD-1 therapy [129]. The positive response to anti-PD-1 antibodies can also be predicted by measuring the expression PD-L1 mRNA in the EVs of NSCLC patients [130].
Mechanisms of acquired resistance to ICI treatments can emerge at different molecular levels, and tumor-derived EVs have been shown to play a crucial part in this process [131]. Tumor EVs impair T cell function and cytotoxic activity of natural killer (NK) cells by releasing TGF-β into the microenvironment [132] or by delivering miRNAs into T cells, which results it the downregulation of genes required for the activation of the inflammatory process [65]. Tumor EVs also substantially reduce proliferation of CD8+ T cells and NK cells [133]. They contribute to a tumor suppressive microenvironment by delivering a non-coding RNA that induces the expression of PD-L1 on antigen-presenting cells, also stimulating concurrent cancer-promoting inflammation by the release of cytokines by monocytes [134].
Monitoring the presence of tumor-derived EVs, by blood tests and the investigation of the vesicle-specific content could prove to be crucial for rapid and accurate diagnosis or for early tumor detection [135]. Parallel screening for antigenic EV and analysis of EV biomarkers during the course of follow-up could help to define the best personalized treatment and also predict resistance. Although key technical and practical issues result mainly in the collection of heterogeneous populations of vesicles of unknown origin [136], limiting the use of the EVs in routine clinical practice, emerging technologies will probably overcome these limitations in the not-too-far future [135,137]. Table 3 summarizes the most relevant studies on detection of predictive biomarkers by the use of EVs.
Table 3.
Principal clinical biomarkers detectable by extracellular vesicles (EVs).
| Biomarkers | N Patients | Methodology | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| EGFR T790M | 23 | PNAClamp™ | 100 | 100 | Hur et al. [63] |
| PD-L1 expression | 8 | ddPCR™ | - | - | Del Re et al. [132] |
Abbreviations: PNAClamp™, peptide nucleic acid (PNA)-mediated PCR-EGFR Mutation Detection Kit; ddPCR™, Droplet Digital ™ PCR.
4.4. Circulating miRNA
4.4.1. Circulating miRNA Biology
Extracellular miRNAs are detected in all body fluids and account for over 50% of all the RNA species in the blood plasma [138]. These molecules are major modulator of gene expression, by guiding the degradation and translational repression of specific mRNA targets [139]. Most of the circulating cell-free miRNAs (cfmiRNAs) involved in cell-to-cell communication are selectively delivered to target cells by loading them into EVs, although functional miRNAs may also be released into the blood circulation by dying cells [140]. These cfmiRNAs have proven their diagnostic and prognostic relevance, in NSCLC, as in many other solid tumors [140].
In the course of the past ten years a multitude of alternative approaches have been used for cfmiRNAs extraction from blood, with a different impact on the quality of the results and the sensitivity of target detection. However, none have been designated as the effective standard procedure [141]. The most widely used techniques to analyze blood-recovered miRNAs are quantitative PCR, microarrays and deep sequencing (miRNA-seq), the last one having consistently improved the characterization of miRNA profiles of patients [140]. Expression profiling of total blood miRNAs can help the detection of early stage tumors [142] and could provide useful prognostic and predictive information [143,144]. For example, detection of circulating miR-21 and miR-10b, two of the most widely investigated cfmiRNAs, facilitates the diagnosis of lung cancer [145]. miR-21, targeting the phosphatase and tensin homolog (PTEN) gene, among others, is substantially overexpressed in patients with advanced-stage diseases and poor survival [146], while high concentrations of miR-10b can also correlate with disease progression and metastasis in NSCLC [147].
4.4.2. Potential for Use of miRNAs in Clinical Practice
Circulating miRNAs have an important role in the development of drug resistance in many cancers, and can be used as efficient biomarkers to predict therapeutic response to TKIs in NSCLC [148]. For examples, blood levels of miR-21, increases from baseline to the time of acquired resistance in patients treated with first-line anti-EGFR TKIs [149]. Li et al. also found that miR-21 levels were higher in lung cancer cells with acquired resistance to TKIs than in TKI-sensitive cells. By deregulating the expression of PTEN and the programmed cell death 4 (PDCD4), miR-21 could induced TKI-resistance via activation of the AKT pathway. These cells had no T790M mutations or MET alterations, suggesting that the upregulation of miR-21 could represent a new mechanism of acquired resistance in NSCLC, as confirmed also in animal models [149]. In a cohort of 105 non-smoker patients with lung ADC, circulating miR-195 and miR-122 expression levels were found to be associated with mutant-EGFR tumors and were also independent predictors of survival [150]. Elevated levels of miR-127 promote shift to EMT in lung cancer cells treated with anti-EGFR TKIs: in a regulatory loop involving the inflammatory signals of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) downstream to EGFR, miR-127 was found as a self-reinforcing factor capable of sustaining the oncogenic transition [151]. Kwok et al. assayed serum EV-RNA serially by quantitative PCR, and revealed that circulating miR-21-5p and miR-486-3p transported by EVs correlate with disease progression in EML4-ALK-translocated NSCLC patients treated with anti-ALK agents. These two miRNAs were differentially expressed by EVs secreted by resistant subclones, which in turn induced drug resistance in recipient cell [152].
Numerous miRNAs transferred via EVs have a role in anti-tumor immunity [153], and can exert key immune-modulatory and pro-oncogenic functions by targeting a multitude of NK and T cell receptor mRNAs [154], or downregulating the expression of genes involved in the inflammatory response [65]. However, miRNAs transported by EVs, like those secreted by lung cancer cells in hypoxic conditions, can induce tumor infiltration of pro-oncogenic macrophages via transfer of miR-103a, and subsequent reduction of PTEN levels [155]. Recently, characteristic EV-miRNA signatures of cancer-related inflammation have been associated with prediction of response to anti PD-1/PD-L1 therapy in NSCLC [156]. Finally, the relative enrichment of certain miRNAs can be specific for each EV subtype, classified on the basis of the presence of characteristic surface markers, thus defining a functional signature of cancer EV-miRNAs that could enable improved screening for drug resistance [157].
Although much of the research carried out to date on circulating RNA remains exploratory, blood-based RNA profiling of cancer patients provides a valuable source of information to improve our understanding of tumor evolution and its adaptation to therapeutic pressure in clinical studies [158].
4.5. Platelets
4.5.1. Platelet Biology and Involvement in Cancer
Analysis of platelet RNA content has recently emerged as an alternative, highly sensitive and accurate method for blood-based diagnostics in cancer. Platelets are anucleate blood cells deriving from megakaryocytes after a cellular redistribution of organelles and vesicular structures; they have multiple functions and very short lifespan [159]. Hence, although platelets do not contain their own RNAs, they are capable of capturing those RNAs that are released into the circulation by other cells, through both EV-dependent and -independent mechanisms. Platelets are highly receptive cells that can adapt easily to the environment, and they are very active in secreting regulatory factors and EVs [159]. By virtue of these properties, platelets are thought to be a major contributor to cancer progression and metastasization, not only because of their role in the coagulation process, but also because they create a microenvironment that protects tumor cells from immune elimination and facilitate their extravasation into the connective tissues [160]. Cancer cells can prepare their niche by secreting EVs that will transfer oncogenic RNAs into circulating platelets, which could then release these RNAs at the metastatic site [161]. These so-called tumor-educated platelets change their RNA expression profile in response to contact with tumor cells, reflecting the molecular signature of the tumor tissue, with important diagnostic consequences. Best et al. reported that the sequencing of platelet-associated RNA yielded about 5000 differently expressed RNAs between cancer patients and healthy controls, with an overall sensitivity of 97%, a specificity of 94% and an accuracy of 95% [162]. This analysis discriminated NSCLC patients with localized and metastasized tumors from healthy individuals with 75% accuracy, providing useful information about the primary tumor. The authors also accurately identified mutated EGFR, KRAS, and amplified MET tumors, with a specificity of 81%, 85% and 75%, respectively, and with a sensitivity of 90%, 94% and 100%, respectively [162].
4.5.2. Clinical Potentiality of Platelets in NSCLC
In NSCLC, platelets are associated with resistance to TKI therapy, as they can also transport growth factors and cytokines that induce a bypass of EGFR signaling [163], while the number of blood platelets is, in itself, an independent predictor of survival for patients having EGFR-mutant tumors that are treated with anti-EGFR TKIs [164]. Analysis of blood platelets can also predict resistance to crizotinib and PFS of NSCLC patients with EML4-ALK rearrangements [165]. Transcripts of EML4-ALK are transferred into blood platelets by EVs released from cancer cells. Nilsson et al. detected EML4-ALK-translocated RNA in platelets can be achieved with 65% sensitivity and 100% specificity, permitting the monitoring of response to crizotinib and defining patient outcome. By measuring EML4-ALK transcript in platelets, crizotinib resistance was discovered two months before the radiographic detection of disease progression [165]. Table 4 reports the main studies that analyze platelets biomarkers.
Table 4.
Principal clinical biomarkers detectable by platelets.
| Biomarkers | N Patients | Methodology | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| EGFR T790M | 283 | NGS | 96 | 92 | Best et al. [164] |
| EML4-ALK | 77 | RT-PCR | 65 | 100 | Nilsson et al. [163] |
Abbreviations: NGS, next generation sequencing; RT-PCR, reverse transcription PCR.
Despite the risk of false positive and the possibility of non-cancer-related systemic factors affecting results, the analysis of platelets RNA content shows promise for improving current NSCLC diagnostic tests, and for predicting the course of disease during the development of drug resistance mutations. Given that platelets are, per se, huge collectors of EV-derived RNA, the detection of tumor-derived RNA in platelets could help to bypass the problems of the rapid degradation of circulating RNAs or low number of EVs containing mutated or rearranged transcripts.
4.6. Circulating Proteins
Secreted proteins from cancer cells have a key role in the oncogenic process, while serving as useful tumor biomarkers. Hence, a great effort has been carried out in the recent years to define the cancer secretome [166]. Proteomic profiling of cancer tissue and liquid biopsy, coupled with mutational cfDNA analysis, incorporated into a logistic regression algorithm, has been successfully applied to track lung cancer development and gain information on early disease detection [167]. So far, however, large-scale approaches in NSCLC have been confined to tissue analysis and cell lines, and there is a paucity of information about the correlation between clinical response and the levels of circulating protein markers [168,169]. Zhang and colleagues used proteomics of lung cancer cells and tissues from in vivo models to define a detailed map of post-transcriptional modifications of receptor proteins and downstream kinase pathways, modulated by afatinib and erlotinib, and found that quantification of chances in major sites of phosphorylation in mutant EGFR signaling could predict the degree of response to TKI treatment [170]. It would be interesting if this information could be translated to circulating proteins. However, the identification of relevant protein markers in liquid biopsy is, at the moment, further limited by a lack of the adequate sensitivity and specificity that are required for an optimal clinical biomarker [171]. Initially, quantitative mass spectrometry helped identify differentially expressed proteins in body fluids and predict clinical outcome during chemotherapy or radiotherapy [172,173]. Proteomic analysis extended to serum samples of NSCLC patients treated with erlotinib demonstrated that the presence of distinct subsets of protein biomarkers are associated with OS and PFS [174]. Most relevant predictive protein biomarkers were components of the MET pathway, including the hepatocyte growth factor (HGF), and pro-inflammatory cytokines. Growth factors and cytokines were indeed among the first serum biomarkers being studied by traditional methods, like enzyme-linked immunosorbent assay (ELISA), in association with NSCLC therapeutic resistance [175]. For example, dynamic evaluation of the serum levels of HGF, as wells as those of interleukin-6 and vascular endothelial growth factor (VEGF), during the course of therapy, could be used to predict the efficacy of anti-EGFR-TKI treatment in NSCLC patients with mutant EGFR [176,177]. The predictive value of serum proteins emerged also in the evaluation of the clinical response of NSCLC patients to nivolumab: changes in the levels of several cytokines, chemokines, growth factors and angiogenesis factor, from baseline to the first clinical evaluation, showed clear association with the outcome [178].
It is likely that proteins found in circulation are not always free, but transported by extracellular structures, or are part of CTC content. As discussed above, proteins that can have clinical relevance for prediction of therapy response in NSCLC, like mutant EGFR and ALK, or PD-L1, are frequently found associated with EVs [179]. Proteomic approaches have shown a differential abundance in protein content between normal and lung cancer-derived EVs, with an enrichment for proteins involved in signal transduction, including EGFR [180]. In future, combined proteomics of tissue and body fluids with improved bioinformatics analysis may help overcome the limitation of this approach in sensitivity for the use in routine liquid biopsy.
5. Conclusions
Since cfDNA-based assays are relatively low in complexity, in terms of purification and analysis, they are currently used in the clinical practice of NSCLC, and are considered the gold standard for defining the tumor mutational status and predicting treatment response by liquid biopsy. Analysis of CTCs can provide, however, an unparalleled mean to interrogate tumor biology and metastatic evolution. At the same time, the development of more reliable and low-cost technologies for the isolation of EVs and purification of their nucleic acid content makes probably EV analysis the new frontier of cancer diagnostics. Biologically speaking, EV release represent an active and most pliant mechanism that tumor cell can use to influence the surrounding microenvironment and communicate with distant sites, preparing the metastatic niche and shutting down the immune response. Secondly, investigating EV contents and surface markers that can track back to the cell of origin of primary tumors would make diagnosis and prediction of therapy response more precise and accurate, given the relative abundance of tumor-derived EVs in circulation compared to CTCs. On the other side, release of cfmiRNAs may account for a specific and more dynamic mechanism of resistance adopted by cancer cells, therefore it can bring up unique information of disease progression. Recent advances in proteomics have provided novel means for adding circulating proteins to the arsenal of the liquid biopsy biomarkers. So far, proteomics has provided a wealth of information from tissue specimen, but its potential use for blood-based tests is limited by the insufficiency in sensitivity and specificity needed of the clinics. It is quite evidently tough that each biomarker comes with its own limitations. Potentially, it would be the complementation of all different methodologies of analysis to provide the most accurate prediction of therapy response and define the best biomarker utility, depending on tumor stage and patient’s condition. Although at the moment only EGFR mutation analysis on cfDNA is used and has a role in the clinical practice, other determinations will probably have a clinical impact on treatment decision making in the near future, such as ALK and ROS1 mutation analyses, especially during the course of therapy to monitor the onset of resistance mechanisms. Moreover, while mutation analysis based on cfDNA is the easier and cheaper approach in liquid biopsy, other biological sources (CTC, EVs and platelets) might better perform in the detection of gene fusions and genomic rearrangements. However, the issue remains that all current methodologies must be improved and standardized for their potential usefulness in the clinical practice.
Author Contributions
L.P. and P.U. contributed equally to the conceptualization, writing, and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez J.G., Jänne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F.J., Lindeman N., Boggon T.J., et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M., Yano S., Giaccone G., Tamura T., Nakagawa K., Douillard J.-Y., Nishiwaki Y., Vansteenkiste J., Kudoh S., Rischin D., et al. Multi-Institutional Randomized Phase II Trial of Gefitinib for Previously Treated Patients with Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Kris M.G., Natale R.B., Herbst R.S., Lynch T.J., Jr., Prager D., Belani C.P., Schiller J.H., Kelly K., Spiridonidis H., Sandler A., et al. Efficacy of Gefitinib, an Inhibitor of the Epidermal Growth Factor Receptor Tyrosine Kinase, in Symptomatic Patients with Non–Small Cell Lung Cancer. JAMA. 2003;290:2149. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 5.Herbst R.S., Prager D., Hermann R., Fehrenbacher L., Johnson B.E., Sandler A., Kris M.G., Tran H.T., Klein P., Li X., et al. TRIBUTE: A Phase III Trial of Erlotinib Hydrochloride (OSI-774) Combined with Carboplatin and Paclitaxel Chemotherapy in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 6.Gatzemeier U., Pluzanska A., Szczesna A., Kaukel E., Roubec J., De Rosa F., Milanowski J., Karnicka-Mlodkowski H., Pesek M., Serwatowski P., et al. Phase III Study of Erlotinib in Combination with Cisplatin and Gemcitabine in Advanced Non–Small-Cell Lung Cancer: The Tarceva Lung Cancer Investigation Trial. J. Clin. Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 7.Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Sequist L.V., Yang J.C.-H., Yamamoto N., O’Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.-M., Boyer M., et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y.-L., Zhou C., Hu C.-P., Feng J., Lu S., Huang Y., Li W., Hou M., Shi J.H., Lee K.Y., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 12.Yang J.C.-H., Sequist L.V., Geater S.L., Tsai C.-M., Mok T.S.K., Schuler M., Yamamoto N., Yu C.-J., Ou S.-H.I., Zhou C., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 13.Mok T.S., Wu Y.-L., Ahn M.-J., Garassino M.C., Kim H.R., Ramalingam S.S., Shepherd F.A., He Y., Akamatsu H., Theelen W.S.M.E., et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 15.Nishino M., Suda K., Kobayashi Y., Ohara S., Fujino T., Koga T., Chiba M., Shimoji M., Tomizawa K., Takemoto T., et al. Effects of secondary EGFR mutations on resistance against upfront osimertinib in cells with EGFR-activating mutations in vitro. Lung Cancer. 2018;126:149–155. doi: 10.1016/j.lungcan.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Uchibori K., Inase N., Nishio M., Fujita N., Katayama R. Identification of Mutation Accumulation as Resistance Mechanism Emerging in First-Line Osimertinib Treatment. J. Thorac. Oncol. 2018;13:915–925. doi: 10.1016/j.jtho.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Namba K., Shien K., Takahashi Y., Torigoe H., Sato H., Yoshioka T., Takeda T., Kurihara E., Ogoshi Y., Yamamoto H., et al. Activation of AXL as a Preclinical Acquired Resistance Mechanism Against Osimertinib Treatment in EGFR -Mutant Non–Small Cell Lung Cancer Cells. Mol. Cancer Res. 2019;17:499–507. doi: 10.1158/1541-7786.MCR-18-0628. [DOI] [PubMed] [Google Scholar]
- 18.Ku B.M., Choi M.K., Sun J.-M., Lee S.-H., Ahn J.S., Park K., Ahn M.-J. Acquired resistance to AZD9291 as an upfront treatment is dependent on ERK signaling in a preclinical model. PLoS ONE. 2018;13:e0194730. doi: 10.1371/journal.pone.0194730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minari R., Bordi P., Del Re M., Facchinetti F., Mazzoni F., Barbieri F., Camerini A., Comin C.E., Gnetti L., Azzoni C., et al. Primary resistance to osimertinib due to SCLC transformation: Issue of T790M determination on liquid re-biopsy. Lung Cancer. 2018;115:21–27. doi: 10.1016/j.lungcan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Le X., Puri S., Negrao M.V., Nilsson M.B., Robichaux J., Boyle T., Hicks J.K., Lovinger K.L., Roarty E., Rinsurongkawong W., et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR -Mutant NSCLC. Clin. Cancer Res. 2018;24:6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxnard G.R., Hu Y., Mileham K.F., Husain H., Costa D.B., Tracy P., Feeney N., Sholl L.M., Dahlberg S.E., Redig A.J., et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with EGFR T790M–Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018;4:1527. doi: 10.1001/jamaoncol.2018.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H., Kurashina K., Hatanaka H., et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 23.Katayama R., Lovly C.M., Shaw A.T. Therapeutic Targeting of Anaplastic Lymphoma Kinase in Lung Cancer: A Paradigm for Precision Cancer Medicine. Clin. Cancer Res. 2015;21:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak E.L., Bang Y.-J., Camidge D.R., Shaw A.T., Solomon B., Maki R.G., Ou S.-H.I., Dezube B.J., Jänne P.A., Costa D.B., et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon B.J., Mok T., Kim D.-W., Wu Y.-L., Nakagawa K., Mekhail T., Felip E., Cappuzzo F., Paolini J., Usari T., et al. First-Line Crizotinib versus Chemotherapy in ALK -Positive Lung Cancer. N. Engl. J. Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 26.Shaw A.T., Engelman J.A. ALK in Lung Cancer: Past, Present, and Future. J. Clin. Oncol. 2013;31:1105–1111. doi: 10.1200/JCO.2012.44.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama R., Friboulet L., Koike S., Lockerman E.L., Khan T.M., Gainor J.F., Iafrate A.J., Takeuchi K., Taiji M., Okuno Y., et al. Two Novel ALK Mutations Mediate Acquired Resistance to the Next-Generation ALK Inhibitor Alectinib. Clin. Cancer Res. 2014;20:5686–5696. doi: 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doebele R.C., Pilling A.B., Aisner D.L., Kutateladze T.G., Le A.T., Weickhardt A.J., Kondo K.L., Linderman D.J., Heasley L.E., Franklin W.A., et al. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non–Small Cell Lung Cancer. Clin. Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama R., Shaw A.T., Khan T.M., Mino-Kenudson M., Solomon B.J., Halmos B., Jessop N.A., Wain J.C., Yeo A.T., Benes C., et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci. Transl. Med. 2012;4:ra17–ra120. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyokawa G., Hirai F., Inamasu E., Yoshida T., Nosaki K., Takenaka T., Yamaguchi M., Seto T., Takenoyama M., Ichinose Y. Secondary Mutations at I1171 in the ALK Gene Confer Resistance to Both Crizotinib and Alectinib. J. Thorac. Oncol. 2014;9:e86–e87. doi: 10.1097/JTO.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 31.Gainor J.F., Dardaei L., Yoda S., Friboulet L., Leshchiner I., Katayama R., Dagogo-Jack I., Gadgeel S., Schultz K., Singh M., et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana D., Ceccon M., Gambacorti-Passerini C., Mologni L. Activity of second-generation ALK inhibitors against crizotinib-resistant mutants in an NPM-ALK model compared to EML4-ALK. Cancer Med. 2015;4:953–965. doi: 10.1002/cam4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S., Anjum R., Squillace R., Nadworny S., Zhou T., Keats J., Ning Y., Wardwell S.D., Miller D., Song Y., et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin. Cancer Res. 2016;22:5527–5538. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 34.Shaw A.T., Felip E., Bauer T.M., Besse B., Navarro A., Postel-Vinay S., Gainor J.F., Johnson M., Dietrich J., James L.P., et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K., Reeves C., Li Y., et al. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Bergethon K., Shaw A.T., Ignatius Ou S.-H., Katayama R., Lovly C.M., McDonald N.T., Massion P.P., Siwak-Tapp C., Gonzalez A., Fang R., et al. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. J. Clin. Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw A.T., Ou S.-H.I., Bang Y.-J., Camidge D.R., Solomon B.J., Salgia R., Riely G.J., Varella-Garcia M., Shapiro G.I., Costa D.B., et al. Crizotinib in ROS1 -Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gainor J.F., Tseng D., Yoda S., Dagogo-Jack I., Friboulet L., Lin J.J., Hubbeling H.G., Dardaei L., Farago A.F., Schultz K.R., et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1 -Positive Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2017;2017:25957–25970. doi: 10.1200/PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song A., Kim T.M., Kim D.-W., Kim S., Keam B., Lee S.-H., Heo D.S. Molecular Changes Associated with Acquired Resistance to Crizotinib in ROS1-Rearranged Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015;21:2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 40.Awad M.M., Katayama R., McTigue M., Liu W., Deng Y.-L., Brooun A., Friboulet L., Huang D., Falk M.D., Timofeevski S., et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N. Engl. J. Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katayama R., Kobayashi Y., Friboulet L., Lockerman E.L., Koike S., Shaw A.T., Engelman J.A., Fujita N. Cabozantinib Overcomes Crizotinib Resistance in ROS1 Fusion–Positive Cancer. Clin. Cancer Res. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dziadziuszko R., Le A.T., Wrona A., Jassem J., Camidge D.R., Varella-Garcia M., Aisner D.L., Doebele R.C. An Activating KIT Mutation Induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J. Thorac. Oncol. 2016;11:1273–1281. doi: 10.1016/j.jtho.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaishnavi A., Schubert L., Rix U., Marek L.A., Le A.T., Keysar S.B., Glogowska M.J., Smith M.A., Kako S., Sumi N.J., et al. EGFR Mediates Responses to Small-Molecule Drugs Targeting Oncogenic Fusion Kinases. Cancer Res. 2017;77:3551–3563. doi: 10.1158/0008-5472.CAN-17-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cargnelutti M., Corso S., Pergolizzi M., Mévellec L., Aisner D.L., Dziadziuszko R., Varella-Garcia M., Comoglio P.M., Doebele R.C., Vialard J., et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget. 2015;6:5182–5194. doi: 10.18632/oncotarget.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Facchinetti F., Loriot Y., Kuo M.-S., Mahjoubi L., Lacroix L., Planchard D., Besse B., Farace F., Auger N., Remon J., et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin. Cancer Res. 2016;22:5983–5991. doi: 10.1158/1078-0432.CCR-16-0917. [DOI] [PubMed] [Google Scholar]
- 46.Drilon A., Rekhtman N., Arcila M., Wang L., Ni A., Albano M., Van Voorthuysen M., Somwar R., Smith R.S., Montecalvo J., et al. Cabozantinib in patients with advanced RET -rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 50.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 53.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenizia F., Pasquale R., Roma C., Bergantino F., Iannaccone A., Normanno N. Measuring tumor mutation burden in non-small cell lung cancer: Tissue versus liquid biopsy. Transl. Lung Cancer Res. 2018;7:668–677. doi: 10.21037/tlcr.2018.09.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezquita L., Auclin E., Ferrara R., Charrier M., Remon J., Planchard D., Ponce S., Ares L.P., Leroy L., Audigier-Valette C., et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagley S.J., Kothari S., Aggarwal C., Bauml J.M., Alley E.W., Evans T.L., Kosteva J.A., Ciunci C.A., Gabriel P.E., Thompson J.C., et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;4:351–357. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Diem S., Schmid S., Krapf M., Flatz L., Born D., Jochum W., Templeton A.J., Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Jiang T., Bai Y., Zhou F., Li W., Gao G., Su C., Ren S., Chen X., Zhou C. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer. 2019;130:76–83. doi: 10.1016/j.lungcan.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Muraoka D., Seo N., Hayashi T., Tahara Y., Fujii K., Tawara I., Miyahara Y., Okamori K., Yagita H., Imoto S., et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J. Clin. Investig. 2019;129:1278–1294. doi: 10.1172/JCI97642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong H., Kim S., Hong B.J., Lee C.J., Kim Y.E., Bok S., Oh J.M., Gwak S.H., Yoo M.Y., Lee M.S., et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 2019;79:795–806. doi: 10.1158/0008-5472.CAN-18-2545. [DOI] [PubMed] [Google Scholar]
- 61.Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic Characteristics of the DNA Found in the Plasma of Cancer Patients. Oncology. 1989;46:318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- 62.Diaz L.A., Bardelli A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aubertin K., Silva A.K.A., Luciani N., Espinosa A., Djemat A., Charue D., Gallet F., Blanc-Brude O., Wilhelm C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep35376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ilie M., Long E., Butori C., Hofman V., Coelle C., Mauro V., Zahaf K., Marquette C.H., Mouroux J., Paterlini-Bréchot P., et al. ALK-gene rearrangement: A comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann. Oncol. 2012;23:2907–2913. doi: 10.1093/annonc/mds137. [DOI] [PubMed] [Google Scholar]
- 65.Muller L., Mitsuhashi M., Simms P., Gooding W.E., Whiteside T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bordi P., Tiseo M., Rofi E., Petrini I., Restante G., Danesi R., Del Re M. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin. Lung Cancer. 2017;18:692–697. doi: 10.1016/j.cllc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 67.Dagogo-Jack I., Rooney M., Nagy R.J., Lin J.J., Chin E., Ferris L.A., Ackil J., Lennerz J.K., Lanman R.B., Gainor J.F., et al. Molecular Analysis of Plasma From Patients With ROS1-Positive NSCLC. J. Thorac. Oncol. 2019;14:816–824. doi: 10.1016/j.jtho.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding M., Deng L., Yu R., Lu D., Bai Y., Wu X., Shao Y.W., Yang Y. Case Report: Temporal Heterogeneity of ALK Activating Mutations in Sequential ALK TKI–Treated Non–Small-Cell Lung Cancer Revealed Using NGS-Based Liquid Biopsy. Clin. Lung Cancer. 2019;20:e229–e232. doi: 10.1016/j.cllc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Krebs M.G., Sloane R., Priest L., Lancashire L., Hou J.M., Greystoke A., Ward T.H., Ferraldeschi R., Hughes A., Clack G., et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 70.Ulivi P. Non-Invasive Methods to Monitor Mechanisms of Resistance to Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancer: Where Do We Stand? Int. J. Mol. Sci. 2016;17:1186. doi: 10.3390/ijms17071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim M., Kim C.-J., Sunkara V., Kim M.-H., Cho Y.-K. Liquid Biopsy in Lung Cancer: Clinical Applications of Circulating Biomarkers (CTCs and ctDNA) Micromachines. 2018;9:100. doi: 10.3390/mi9030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rolfo C., Mack P.C., Scagliotti G.V., Baas P., Barlesi F., Bivona T.G., Herbst R.S., Mok T.S., Peled N., Pirker R., et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 73.Oxnard G.R., Paweletz C.P., Kuang Y., Mach S.L., O’Connell A., Messineo M.M., Luke J.J., Butaney M., Kirschmeier P., Jackman D.M., et al. Noninvasive Detection of Response and Resistance in EGFR-Mutant Lung Cancer Using Quantitative Next-Generation Genotyping of Cell-Free Plasma DNA. Clin. Cancer Res. 2014;20:1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douillard J.-Y., Ostoros G., Cobo M., Ciuleanu T., Cole R., McWalter G., Walker J., Dearden S., Webster A., Milenkova T., et al. Gefitinib Treatment in EGFR Mutated Caucasian NSCLC: Circulating-Free Tumor DNA as a Surrogate for Determination of EGFR Status. J. Thorac. Oncol. 2014;9:1345–1353. doi: 10.1097/JTO.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mok T., Wu Y.-L., Lee J.S., Yu C.-J., Sriuranpong V., Sandoval-Tan J., Ladrera G., Thongprasert S., Srimuninnimit V., Liao M., et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin. Cancer Res. 2015;21:3196–3203. doi: 10.1158/1078-0432.CCR-14-2594. [DOI] [PubMed] [Google Scholar]
- 76.Plagnol V., Woodhouse S., Howarth K., Lensing S., Smith M., Epstein M., Madi M., Smalley S., Leroy C., Hinton J., et al. Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS One. 2018;13:e0193802. doi: 10.1371/journal.pone.0193802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leighl N.B., Page R.D., Raymond V.M., Daniel D.B., Divers S.G., Reckamp K.L., Villalona-Calero M.A., Dix D., Odegaard J.I., Lanman R.B., et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non–small Cell Lung Cancer. Clin. Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-19-0624. [DOI] [PubMed] [Google Scholar]
- 78.Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors. J. Mol. Diagn. 2018;20:129–159. doi: 10.1016/j.jmoldx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Zugazagoitia J., Ramos I., Trigo J.M., Palka M., Gómez-Rueda A., Jantus-Lewintre E., Camps C., Isla D., Iranzo P., Ponce-Aix S., et al. Clinical utility of plasma-based digital next-generation sequencing in patients with advance-stage lung adenocarcinomas with insufficient tumor samples for tissue genotyping. Ann. Oncol. 2019;30:290–296. doi: 10.1093/annonc/mdy512. [DOI] [PubMed] [Google Scholar]
- 80.Oxnard G.R., Thress K.S., Alden R.S., Lawrance R., Paweletz C.P., Cantarini M., Yang J.C.-H., Barrett J.C., Jänne P.A. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016;34:3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida R., Sasaki T., Umekage Y., Tanno S., Ono Y., Ogata M., Chiba S., Mizukami Y., Ohsaki Y. Highly sensitive detection of ALK resistance mutations in plasma using droplet digital PCR. BMC Cancer. 2018;18:1136. doi: 10.1186/s12885-018-5031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gandara D.R., Paul S.M., Kowanetz M., Schleifman E., Zou W., Li Y., Rittmeyer A., Fehrenbacher L., Otto G., Malboeuf C., et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 83.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P., et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B.K., Veeriah S., Shafi S., Johnson D.H., Mitter R., Rosenthal R., et al. Tracking the Evolution of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 85.Neumann M.H.D., Bender S., Krahn T., Schlange T. ctDNA and CTCs in Liquid Biopsy – Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018;16:190–195. doi: 10.1016/j.csbj.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alix-Panabières C., Pantel K. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 2014;14:623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 87.De Wit S., Van Dalum G., Lenferink A.T.M., Tibbe A.G.J., Hiltermann T.J.N., Groen H.J.M., Van Rijn C.J.M., Terstappen L.W.M.M. The detection of EpCAM + and EpCAM − circulating tumor cells. Sci. Rep. 2015;5:1–10. doi: 10.1038/srep12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poudineh M., Sargent E.H., Pantel K., Kelley S.O. Biomarkers of Invasive Cancers. Nat. Biomed. Eng. 2018;2:72–84. doi: 10.1038/s41551-018-0190-5. [DOI] [PubMed] [Google Scholar]
- 89.Ramsköld D., Luo S., Wang Y.C., Li R., Deng Q., Faridani O.R., Daniels G.A., Khrebtukova I., Loring J.F., Laurent L.C., et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 2012;30:777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massagué J., Obenauf A.C. Metastatic colonization by circulating tumour cells. Nat. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lecharpentier A., Vielh P., Perez-Moreno P., Planchard D., Soria J.C., Farace F. Detection of circulating tumour cells with a hybrid (epithelial/ mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer. 2011;105:1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanssen A., Wagner J., Gorges T.M., Taenzer A., Uzunoglu F.G., Driemel C., Stoecklein N.H., Knoefel W.T., Angenendt S., Hauch S., et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsao S.C.H., Wang J., Wang Y., Behren A., Cebon J., Trau M. Characterising the phenotypic evolution of circulating tumour cells during treatment. Nat. Commun. 2018;9:1482. doi: 10.1038/s41467-018-03725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carter L., Rothwell D.G., Mesquita B., Smowton C., Leong H.S., Fernandez-Gutierrez F., Li Y., Burt D.J., Antonello J., Morrow C.J., et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017;23:114–119. doi: 10.1038/nm.4239. [DOI] [PubMed] [Google Scholar]
- 95.Gkountela S., Castro-Giner F., Szczerba B.M., Vetter M., Landin J., Scherrer R., Krol I., Scheidmann M.C., Beisel C., Stirnimann C.U., et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell. 2019;176:98–112.e14. doi: 10.1016/j.cell.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maheswaran S., Sequist L.V., Nagrath S., Ulkus L., Brannigan B., Collura C.V., Inserra E., Diederichs S., Iafrate A.J., Bell D.W., et al. Detection of Mutations in EGFR in Circulating Lung-Cancer Cells. N. Engl. J. Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pailler E., Adam J., Barthélémy A., Oulhen M., Auger N., Valent A., Borget I., Planchard D., Taylor M., André F., et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non–small-cell lung cancer. J. Clin. Oncol. 2013;31:2273–2281. doi: 10.1200/JCO.2012.44.5932. [DOI] [PubMed] [Google Scholar]
- 98.Pailler E., Oulhen M., Borget I., Remon J., Ross K., Auger N., Billiot F., Ngo Camus M., Commo F., Lindsay C.R., et al. Circulating tumor cells with aberrant ALK copy number predict progression-free survival during crizotinib treatment in ALK-rearranged non-small cell lung cancer patients. Cancer Res. 2017;77:2222–2230. doi: 10.1158/0008-5472.CAN-16-3072. [DOI] [PubMed] [Google Scholar]
- 99.Ilié M., Szafer-Glusman E., Hofman V., Chamorey E., Lalvée S., Selva E., Leroy S., Marquette C.H., Kowanetz M., Hedge P., et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018;29:193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 100.Nicolazzo C., Raimondi C., Mancini M., Caponnetto S., Gradilone A., Gandini O., Mastromartino M., Del Bene G., Prete A., Longo F., et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schott D.S., Pizon M., Pachmann U., Pachmann K. Sensitive detection of PD-L1 expression on circulating epithelial tumor cells (CETCs) could be a potential biomarker to select patients for treatment with PD-1/PD-L1 inhibitors in early and metastatic solid tumors. Oncotarget. 2017;8:72755–72772. doi: 10.18632/oncotarget.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tong B., Xu Y., Zhao J., Chen M., Zhong W., Xing J., Wang M. Prognostic role of circulating tumor cells in patients with EGFR -mutated or ALK -rearranged non-small cell lung cancer. Thorac. Cancer. 2018;9:640–645. doi: 10.1111/1759-7714.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagrath S., Sequist L.V., Maheswaran S., Bell D.W., Irimia D., Ulkus L., Smith M.R., Kwak E.L., Digumarthy S., Muzikansky A., et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nat. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 105.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 106.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 107.Syn N., Wang L., Sethi G., Thiery J.P., Goh B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016;37:606–617. doi: 10.1016/j.tips.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Boriachek K., Islam M.N., Möller A., Salomon C., Nguyen N.T., Hossain M.S.A., Yamauchi Y., Shiddiky M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2018;14:1–21. doi: 10.1002/smll.201702153. [DOI] [PubMed] [Google Scholar]
- 109.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nat. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ortiz A., Gui J., Zahedi F., Yu P., Cho C., Bhattacharya S., Carbone C.J., Yu Q., Katlinski K.V., Katlinskaya Y.V., et al. An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles. Cancer Cell. 2019;35:33–45. doi: 10.1016/j.ccell.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Mijn J.C., Sol N., Mellema W., Jimenez C.R., Piersma S.R., Dekker H., Schutte L.M., Smit E.F., Broxterman H.J., Skog J., et al. Analysis of AKT and ERK1/2 protein kinases in extracellular vesicles isolated from blood of patients with cancer. J. Extracell. Vesicles. 2014;3:1–11. doi: 10.3402/jev.v3.25657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cesi G., Philippidou D., Kozar I., Kim Y.J., Bernardin F., Van Niel G., Wienecke-Baldacchino A., Felten P., Letellier E., Dengler S., et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol. Cancer. 2018;17:1–14. doi: 10.1186/s12943-018-0886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 115.Perez-Torres M., Valle B.L., Maihle N.J., Negron-Vega L., Nieves-Alicea R., Cora E.M. Shedding of epidermal growth factor receptor is a regulated process that occurs with overexpression in malignant cells. Exp. Cell Res. 2008;314:2907–2918. doi: 10.1016/j.yexcr.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Read J., Ingram A., Al Saleh H.A., Platko K., Gabriel K., Kapoor A., Pinthus J., Majeed F., Qureshi T., Al-Nedawi K. Nuclear transportation of exogenous epidermal growth factor receptor and androgen receptor via extracellular vesicles. Eur. J. Cancer. 2017;70:62–74. doi: 10.1016/j.ejca.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 117.Hsu S.C., Miller S.A., Wang Y., Hung M.C. Nuclear EGFR is required for cisplatin resistance and DNA repair. Am. J. Transl. Res. 2009;1:249–258. [PMC free article] [PubMed] [Google Scholar]
- 118.Li C., Iida M., Dunn E.F., Ghia A.J., Wheeler D.L. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jantus-Lewintre E., Sirera R., Cabrera A., Blasco A., Caballero C., Iranzo V., Rosell R., Camps C. Analysis of the prognostic value of soluble epidermal growth factor receptor plasma concentration in advanced non-small-cell lung cancer patients. Clin. Lung Cancer. 2011;12:320–327. doi: 10.1016/j.cllc.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 120.Maramotti S., Paci M., Manzotti G., Rapicetta C., Gugnoni M., Galeone C., Cesario A., Lococo F. Soluble epidermal growth factor receptors (sEGFRs) in cancer: Biological aspects and clinical relevance. Int. J. Mol. Sci. 2016;17:593. doi: 10.3390/ijms17040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Figueroa J.M., Skog J., Akers J., Li H., Komotar R., Jensen R., Ringel F., Yang I., Kalkanis S., Thompson R., et al. Detection of wild-Type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro. Oncol. 2017;19:1494–1502. doi: 10.1093/neuonc/nox085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hur J.Y., Kim H.J., Lee J.S., Choi C.M., Lee J.C., Jung M.K., Pack C.G., Lee K.Y. Extracellular vesicle-derived DNA for performing EGFR genotyping of NSCLC patients. Mol. Cancer. 2018;17:1–6. doi: 10.1186/s12943-018-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruivo C.F., Adem B., Silva M., Melo S.A. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017;77:6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 126.Parmiani G., Filipazzi P., Iero M., Huber V., Valenti R., Rivoltini L. Tumor-Released Microvesicles as Vehicles of Immunosuppression: Figure 1. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 127.Balaj L., Chen C.C., Lawler S.E., Lee H., Lamszus K., Speranza M.C., Bronisz A., Lee K., Chiocca E.A., Hayes J.L., et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018;4:eaar2766. doi: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang J., Gao J., Li Y., Nie J., Dai L., Hu W., Chen X., Han J., Ma X., Tian G., et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac. Cancer. 2015;6:534–538. doi: 10.1111/1759-7714.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herlyn M., Lu Y., Chen G., McGettigan S., Zhong W., Dong H., Xia H., Wu M., Yang J., Man Q., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nat. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Del Re M., Marconcini R., Pasquini G., Rofi E., Vivaldi C., Bloise F., Restante G., Arrigoni E., Caparello C., Grazia Bianco M., et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer. 2018;118:820–824. doi: 10.1038/bjc.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clayton A., Mitchell J.P., Court J., Linnane S., Mason M.D., Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 133.Clayton A., Mitchell J.P., Court J., Mason M.D., Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 134.Warnken U., Seiffert M., Schulz R., Nessling M., Paggetti J., Lichter P., Cid L.L., Worst T., Stilgenbauer S., Diederichs S., et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci. Immunol. 2017;2:eaah5509. doi: 10.1126/sciimmunol.aah5509. [DOI] [PubMed] [Google Scholar]
- 135.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willms E., Johansson H.J., Mäger I., Lee Y., Blomberg K.E.M., Sadik M., Alaarg A., Smith C.I.E., Lehtiö J., El Andaloussi S., et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoshioka Y., Kosaka N., Konishi Y., Ohta H., Okamoto H., Sonoda H., Nonaka R., Yamamoto H., Ishii H., Mori M., et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Godoy P.M., Bhakta N.R., Barczak A.J., Cakmak H., Fisher S., MacKenzie T.C., Patel T., Price R.W., Smith J.F., Woodruff P.G., et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018;25:1346–1358. doi: 10.1016/j.celrep.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwarzenbach H., Nishida N., Calin G.A., Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014;11:145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 141.El-Khoury V., Pierson S., Kaoma T., Bernardin F., Berchem G. Assessing cellular and circulating miRNA recovery: The impact of the RNA isolation method and the quantity of input material. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Halvorsen A.R., Bjaanæs M., LeBlanc M., Holm A.M., Bolstad N., Rubio L., Peñalver J.C., Cervera J., Mojarrieta J.C., López-Guerrero J.A., et al. A unique set of 6 circulating microRNAs for early detection of non-small cell lung cancer. Oncotarget. 2016;7:37250–37259. doi: 10.18632/oncotarget.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cui E.H., Li H.J., Hua F., Wang B., Mao W., Feng X.R., Li J.Y., Wang X. Serum microRNA 125b as a diagnostic or prognostic biomarker for advanced NSCLC patients receiving cisplatin-based chemotherapy. Acta Pharmacol. Sin. 2013;34:309–313. doi: 10.1038/aps.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y., Gu J., Roth J.A., Hildebrandt M.A.T., Lippman S.M., Ye Y., Minna J.D., Wu X. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73:4801–4809. doi: 10.1158/0008-5472.CAN-12-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen J., Liu Z., Todd N.W., Zhang H., Liao J., Yu L., Guarnera M.A., Li R., Cai L., Zhan M., et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X.G., Zhu W.Y., Huang Y.Y., Ma L.N., Zhou S.Q., Wang Y.K., Zeng F., Zhou J.H., Zhang Y.K. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med. Oncol. 2012;29:618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 147.Roth C., Kasimir-Bauer S., Pantel K., Schwarzenbach H. Screening for circulating nucleic acids and caspase activity in the peripheral blood as potential diagnostic tools in lung cancer. Mol. Oncol. 2011;5:281–291. doi: 10.1016/j.molonc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han F., He J., Li F., Yang J., Wei J., Cho W.C., Liu X. Emerging Roles of MicroRNAs in EGFR-Targeted Therapies for Lung Cancer. BioMed Res. Int. 2015;2015:672759. doi: 10.1155/2015/672759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li B., Ren S., Li X., Wang Y., Garfield D., Zhou S., Chen X., Su C., Chen M., Kuang P., et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer. 2014;83:146–153. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 150.Zhang H., Su Y., Xu F., Kong J., Yu H., Qian B. Circulating microRNAs in relation to EGFR status and survival of lung adenocarcinoma in female non-smokers. PLoS ONE. 2013;8:e81408. doi: 10.1371/journal.pone.0081408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shi L., Wang Y., Lu Z., Zhang H., Zhuang N., Wang B., Song Z., Chen G., Huang C., Xu D., et al. MIR-127 promotes EMT and stem-like traits in lung cancer through a feed-forward regulatory loop. Oncogene. 2017;36:1631–1643. doi: 10.1038/onc.2016.332. [DOI] [PubMed] [Google Scholar]
- 152.Kwok H.H., Ning Z., Chong P.W.C., Wan T.S.K., Ng M.H.L., Ho G.Y.F., Ip M.S.M., Lam D.C.L. Transfer of extracellular vesicle-associated-RNAs induces drug resistance in ALK-translocated lung adenocarcinoma. Cancers (Basel) 2019;11:104. doi: 10.3390/cancers11010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsitsiou E., Lindsay M.A. microRNAs and the immune response. Curr. Opin. Pharmacol. 2009;9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jasinski-Bergner S., Mandelboim O., Seliger B. The Role of MicroRNAs in the control of innate immune response in cancer. J. Natl. Cancer Inst. 2014;106:1–13. doi: 10.1093/jnci/dju257. [DOI] [PubMed] [Google Scholar]
- 155.Hsu Y.L., Hung J.Y., Chang W.A., Jian S.F., Lin Y.S., Pan Y.C., Wu C.Y., Kuo P.L. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol. Ther. 2018;26:568–581. doi: 10.1016/j.ymthe.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shukuya T., Amann J., Ghai V., Wang K., Carbone D.P. Circulating miRNA and extracellular vesicle containing miRNA as response biomarkers of anti PD-1/PD-L1 therapy in non-small-cell lung cancer. J. Clin. Oncol. 2018;36:3058. doi: 10.1200/JCO.2018.36.15_suppl.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaur S., Elkahloun A.G., Arakelyan A., Young L., Myers T.G., Otaizo-Carrasquero F., Wu W., Margolis L., Roberts D.D. CD63, MHC class 1, and CD47 identify subsets of extracellular vesicles containing distinct populations of noncoding RNAs. Sci. Rep. 2018;8:1–17. doi: 10.1038/s41598-018-20936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 159.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 160.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nilsson R.J.A., Balaj L., Hulleman E., Van Rijn S., Pegtel D.M., Walraven M., Widmark A., Gerritsen W.R., Verheul H.M., Vandertop W.P., et al. Blood platelets contain tumor-derived RNA biomarkers. Blood. 2011;118:3680–3683. doi: 10.1182/blood-2011-03-344408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Best M.G., Sol N., Kooi I., Tannous J., Westerman B.A., Rustenburg F., Schellen P., Verschueren H., Post E., Koster J., et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yano S., Wang W., Li Q., Matsumoto K., Sakurama H., Nakamura T., Ogino H., Kakiuchi S., Hanibuchi M., Nishioka Y., et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 164.Watanabe K., Yasumoto A., Amano Y., Kage H., Goto Y., Yatomi Y., Takai D., Nagase T. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS ONE. 2018;13:e0203625. doi: 10.1371/journal.pone.0203625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nilsson R.J.A., Karachaliou N., Berenguer J., Gimenez-Capitan A., Schellen P., Teixido C., Tannous J., Kuiper J.L., Drees E., Grabowska M., et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2015;7:1066. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Feizi A., Banaei-Esfahani A., Nielsen J. HCSD: The human cancer secretome database. Database. 2015;2015:bav051. doi: 10.1093/database/bav051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L., Douville C., Javed A.A., Wong F., Mattox A., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Sci. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cho W.C.S. Application of proteomics in non-small-cell lung cancer. Expert Rev. Proteomics. 2016;13:1–4. doi: 10.1586/14789450.2016.1121813. [DOI] [PubMed] [Google Scholar]
- 169.Cheung C.H.Y., Juan H.F. Quantitative proteomics in lung cancer. J. Biomed. Sci. 2017;24:37. doi: 10.1186/s12929-017-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X., Maity T., Kashyap M.K., Bansal M., Venugopalan A., Singh S., Awasthi S., Marimuthu A., Charles Jacob H.K., Belkina N., et al. Quantitative Tyrosine Phosphoproteomics of Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor-treated Lung Adenocarcinoma Cells Reveals Potential Novel Biomarkers of Therapeutic Response. Mol. Cell. Proteom. 2017;16:891–910. doi: 10.1074/mcp.M117.067439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hench I.B., Hench J., Tolnay M. Liquid Biopsy in Clinical Management of Breast, Lung, and Colorectal Cancer. Front. Med. 2018;5:9. doi: 10.3389/fmed.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walker M.J., Zhou C., Backen A., Pernemalm M., Williamson A.J.K., Priest L.J.C., Koh P., Faivre-Finn C., Blackhall F.H., Dive C., et al. Discovery and Validation of Predictive Biomarkers of Survival for Non-small Cell Lung Cancer Patients Undergoing Radical Radiotherapy: Two Proteins With Predictive Value. EBioMedicine. 2015;2:841–850. doi: 10.1016/j.ebiom.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jia H., Tian Y., Jiang C.G., Han W. Evaluation of 29 indicators for the prognosis of advanced non-small cell lung cancer with cytokine-induced killer cell therapy combined with chemotherapy. Exp. Ther. Med. 2016;11:1601–1610. doi: 10.3892/etm.2016.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fidler M.J., Frankenberger C., Seto R., Lobato G.C., Fhied C.L., Sayidine S., Basu S., Pool M., Karmali R., Batus M., et al. Differential expression of circulating biomarkers of tumor phenotype and outcomes in previously treated non-small cell lung cancer patients receiving erlotinib vs. cytotoxic chemotherapy. Oncotarget. 2017;8:58108–58121. doi: 10.18632/oncotarget.17510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Matsumoto K., Umitsu M., De Silva D.M., Roy A., Bottaro D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017;108:296–307. doi: 10.1111/cas.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kasahara K., Arao T., Sakai K., Matsumoto K., Sakai A., Kimura H., Sone T., Horiike A., Nishio M., Ohira T., et al. Impact of serum hepatocyte growth factor on treatment response to epidermal growth factor receptor tyrosine kinase inhibitors in patients with non-small cell lung adenocarcinoma. Clin. Cancer Res. 2010;16:4616–4624. doi: 10.1158/1078-0432.CCR-10-0383. [DOI] [PubMed] [Google Scholar]
- 177.Jia Y., Li X., Zhao C., Jiang T., Zhao S., Zhang L., Liu X., Shi J., Qiao M., Luo J., et al. Impact of serum vascular endothelial growth factor and interleukin-6 on treatment response to epidermal growth factor receptor tyrosine kinase inhibitors in patients with non-small-cell lung cancer. Lung Cancer. 2018;125:22–28. doi: 10.1016/j.lungcan.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 178.Oyanagi J., Koh Y., Sato K., Mori K., Teraoka S., Akamatsu H., Kanai K., Hayata A., Tokudome N., Akamatsu K., et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer. 2019;132:107–113. doi: 10.1016/j.lungcan.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 179.Rolfo C. Exosomal Proteins in Lung Cancer: The Last Frontier in Liquid Biopsies. J. Thorac. Oncol. 2016;11:1609–1611. doi: 10.1016/j.jtho.2016.08.122. [DOI] [PubMed] [Google Scholar]
- 180.Clark D.J., Fondrie W.E., Yang A., Mao L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J. Proteom. 2016;133:161–169. doi: 10.1016/j.jprot.2015.12.023. [DOI] [PubMed] [Google Scholar]