Abstract
Garlic (Allium sativum L.) is a widely consumed spice in the world. Garlic contains diverse bioactive compounds, such as allicin, alliin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and S-allyl-cysteine. Substantial studies have shown that garlic and its bioactive constituents exhibit antioxidant, anti-inflammatory, antibacterial, antifungal, immunomodulatory, cardiovascular protective, anticancer, hepatoprotective, digestive system protective, anti-diabetic, anti-obesity, neuroprotective, and renal protective properties. In this review, the main bioactive compounds and important biological functions of garlic are summarized, highlighting and discussing the relevant mechanisms of actions. Overall, garlic is an excellent natural source of bioactive sulfur-containing compounds and has promising applications in the development of functional foods or nutraceuticals for the prevention and management of certain diseases.
Keywords: garlic, phytochemicals, organic sulfides, health benefits, antioxidant, anticancer, cardiovascular protection, antimicrobial
1. Introduction
Garlic (Allium sativum L.) is a common spice with many health benefits, mainly due to its diverse bioactive compounds, such as organic sulfides, saponins, phenolic compounds, and polysaccharides [1,2,3]. Garlic is commonly consumed and has a long history of being utilized as a traditional medicine in China [4]. In recent decades, numerous studies have demonstrated the remarkable biological functions of garlic, including antioxidant, cardiovascular protective, anticancer, anti-inflammatory, immunomodulatory, anti-diabetic, anti-obesity, and antibacterial properties [5,6,7,8,9,10,11]. Investigations have increasingly focused on black garlic, a processed garlic product with increased polyphenol and flavonoid contents, as well as better antioxidant properties, compared to the fresh garlic [12]. In order to highlight the significance of garlic in human health, we searched high-quality studies from the last five years from the Web of Science Core Collection and reviewed the main bioactive compounds and biological functions of garlic, with special attention paid to the relevant mechanisms of actions. We hope that this review paper will attract more interest in garlic and provide updated scientific evidence for the better utilization of garlic in human health and disease management.
2. Bioactive Compounds of Garlic
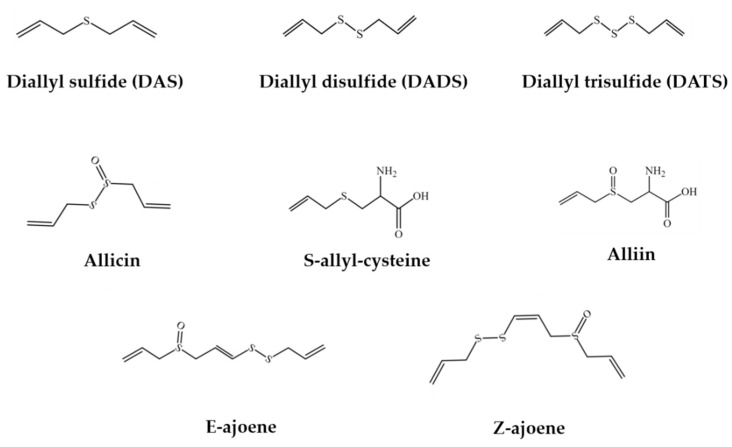
Garlic has a variety of bioactive compounds, including organosulfur compounds, saponins, phenolic compounds, and polysaccharides [2,3,13,14]. The major active components of garlic (Figure 1) are its organosulfur compounds, such as diallyl thiosulfonate (allicin), diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), E/Z-ajoene, S-allyl-cysteine (SAC), and S-allyl-cysteine sulfoxide (alliin) [15,16,17,18]. In general, organosulfur compounds in raw garlic have higher digestibility than those in cooked garlic [19]. In addition, saponins were found to be more stable in the cooking process [20]. The total amount of saponin in purple garlic was almost 40 times higher than that in white garlic, and several saponin compounds were only found to exist in purple garlic, such as desgalactotigonin-rhamnose, proto-desgalactotigonin, proto-desgalactotigonin- rhamnose, voghieroside D1, sativoside B1-rhamnose, and sativoside R1 [2]. Moreover, garlic contained more than 20 phenolic compounds, with higher contents than many common vegetables [21]. The main phenolic compound was β-resorcylic acid, followed by pyrogallol, gallic acid, rutin, protocatechuic acid, as well as quercetin [22]. Furthermore, garlic polysaccharides were reported to contain 85% fructose, 14% glucose, and 1% galactose [23].
Figure 1.
The chemical structures of the main organosulfur compounds in garlic.
The effects of different processing methods on bioactive components of garlic have been also studied. For example, it was found that the 38 components of garlic changed after thermal treatment during the processing of black garlic [24]. In addition, the polysaccharide degraded and the content of reducing sugar increased during the thermal processing of black garlic [25]. Also, increasing temperature and decreasing humidity enhance the contents of polyphenols and the total flavonoids in black garlic [26,27]. In the future, more bioactive compounds produced in the processing of garlic should be separated and identified.
3. Biological functions of Garlic
3.1. Antioxidant Activity
The antioxidant activities of natural products have been widely evaluated, such as fruits, vegetables, mushrooms, cereal, flowers, and wild fruits [28,29,30,31,32,33,34]. Accumulating studies have found that garlic has strong antioxidant properties. A study evaluated the antioxidant capacities of both raw and cooked garlic, and found that the raw garlic exhibited stronger antioxidant activity (by 1,1-diphenyl-2-picrilhydrazyl (DPPH) radical scavenging assay, 2,2’-Azino-bis(3-ethyl- benzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay, and ferric ion reducing antioxidant power (FRAP) assay). Stir-fried garlic was also shown to have stronger antioxidant capacities (by β-carotene bleaching), indicating that the processing could affect the antioxidant property of garlic [35]. In another study, the results of DPPH and oxygen radical absorption capacity (ORAC) assays showed that the ethanolic extract of garlic sprouts exhibited stronger antioxidant activities than the ethanolic extract of raw garlic [36]. In addition, the antioxidant properties of aged garlic were found to be higher than fresh garlic by DPPH, ABTS, FRAP, H2O2 scavenging, and Fe2+ chelating assays [37]. Compared with multi clove garlic extract, single clove garlic extract had a higher amount of phenolic compounds and showed stronger antioxidant activity [38]. Moreover, the antioxidant activity of black garlic increased with thermal treatment, and the highest antioxidant activity was obtained on the 21st day of processing [39,40]. Also, the increased pressure improved the antioxidant activity of garlic paste [41]. However, the antioxidant activity of “Laba” garlic, a traditional Chinese garlic product, decreased during fermentation [42].
The aged garlic extract (AGE) induced the expression of several antioxidant enzymes, such as heme oxygenase-1 (HO-1) and the glutamate-cysteine ligase modifier (GCLM) subunit through the nuclear factor erythrobia-2 related factor 2 (Nrf2)-antioxidant response element (ARE) pathway, which protected human endothelial cells against oxidative stress [43]. Garlic saponins were reported to protect mouse-derived C2C12 myoblasts against growth inhibition and DNA damage induced by H2O2, and to scavenge intracellular reactive oxygen species (ROS) [44].
In summary, garlic and its active ingredients (such as phenols and saponins) have certain antioxidant effects. Different processing methods also affected the antioxidant activity of garlic. Usually, raw garlic had a stronger antioxidant activity than cooked garlic, and the antioxidant activity of fermented garlic, such as black garlic, was stronger than that of crude garlic. In addition, the cellular experiment showed that the mechanism of antioxidative action of garlic might be involved with the enhancement of antioxidant enzyme activities and the regulation of the Nrf2-ARE pathway.
3.2. Anti-Inflammatory Activity
Garlic and its bioactive compounds have also been shown to exhibit anti-inflammatory properties. In a study, the ethyl linoleate in garlic reduced the production of nitric oxide (NO) and prostaglandin E-2 by down-regulating the expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX2) in lipopolysaccharide-stimulated RAW 264.7 macrophages [45]. Another study revealed that the garlic 14-kDa protein inhibited the inflammatory mediators including NO, TNF-α, and interleukin (IL) -1β by inhibiting the transcription factor nuclear factor-kappa B (NF-κB) signaling pathway in lipopolysaccharide-stimulated J774A.1 macrophages [46]. In addition, AGE inhibited inflammation in apolipoprotein E-knockout mice. The treatment of AGE reduced the level of tumor necrosis factor-α (TNF-α) and the IL-1 receptor-associated kinase 4 and enhanced the activity of the adenosine monophosphate-activated protein kinase (AMPK) in the liver [47]. Moreover, allicin could be used as a potential complementary treatment against the inflammatory response induced by schistosome infection in BALB/c mice [48]. Furthermore, garlic supplements alleviated osteoarthritis in obese or overweight patients by reducing resistin [49].
Collectively, in both in vitro and in vivo experiments, garlic could inhibit inflammation mainly by inhibiting inflammatory mediators, such as NO, TNF-α, and IL-1. Garlic has great potential to treat inflammatory diseases, such as arthritis, in humans, because of its low or absent toxicity.
3.3. Antimicrobial Activity
Garlic has a broad spectrum of antibacterial and antifungal properties [50,51,52]. The antibacterial activities of two special varieties of garlic, “Rosato” and “Caposele”, from the Campania region in Italy were analyzed. It was found that the Caposele variety could significantly suppress the growth of Aspergillus versicolor and Penicillum citrinum, while the Rosato variety had a stronger inhibitory effect on Penicillium expansum [53]. Moreover, AGE was effective in inhibiting Burkholderia cepacian [54]. Garlic oil also had antibacterial activities and could restrict the growth of Staphylococcus aureus, Escherichia coli, and Bacillus subtilis [50]. It was found that garlic oil inhibited the fungus Penicillium funiculosum, probably by penetrating into cells and organelles, destroying the cell structure, and inducing the leakage of cytoplasm and macromolecules [55]. Additionally, garlic oil was found to disrupt the normal metabolism of Candida albicans, which is associated with the induction of key genes involved in oxidative phosphorylation, the cell cycle, and protein processing in the endoplasmic reticulum [56]. Furthermore, in a clinical trial, the treatment of raw garlic inhibited Helicobacter pylori in the stomach of patients with H. pylori infection [57].
In a word, the antibacterial effects of garlic are related to its varieties and processing methods. Garlic oil was demonstrated to be the main antibacterial ingredient that destroys the structure and the metabolic process of bacterial cells.
3.4. Modulating Immune System
Garlic contains many bioactive compounds that are beneficial for the immune system. Garlic polysaccharides have an immunomodulatory effect and regulate the expressions of IL-6, IL-10, TNF-α, and interferon-γ in RAW 264.7 macrophages. Compared with black garlic, polysaccharides in fresh garlic exhibit a more potent activity in immunomodulation. This is probably due to the degradation of fructan constituents during processing [58]. In an in vivo study, the treatment of garlic oil 30 minutes before the administration of diazinon to Wistar rats can normalize several immunological parameters of rats, such as their serum total immunoglobulin concentration and T-cell subtype CD4+ [59]. In addition, the combination of garlic oil and levamisole can significantly balance the T-helper 1 / T-helper 2 response in Wistar rats [60]. Moreover, selenylation modification of garlic polysaccharides significantly improves its immune-enhancing activity, and selenizing garlic polysaccharides promotes lymphocyte proliferation, enhances interferon-γ and IL-2, and increases the serum antibody titer in 14-day-old chickens [61]. Moreover, the consumption of AGE was found to reduce the occurrence and severity of the cold and flu and improve the immune system functions in humans [8]. Overall, polysaccharides appear to be the main immune-modulating components in garlic.
3.5. Cardiovascular Protection
Recently, the numbers of deaths from cardiovascular diseases have significantly risen [62]. There has been a growing interest in natural products to protect the cardiovascular system, and garlic is one of the most promising candidates [63,64,65,66,67]. It has been demonstrated that the intake of garlic powder can effectively reduce blood pressure, total cholesterol, low-density lipoprotein cholesterol, and other risk factors related to cardiovascular diseases [68].
3.5.1. Antihypertensive Activity
Garlic can also reduce oxidative stress, increase the production of NO and hydrogen sulfide (H2S), and inhibit the angiotensin converting enzyme, thereby lowering hypertension [69,70,71,72,73,74]. A study showed that AGE could stimulate the production of NO, leading to endothelial-dependent vasodilation in the isolated rat aortic rings. Moreover, l-arginine in AGE was crucial in the NOS-mediated NO production [71]. In addition, S-1-propylenecysteine was shown to be the key antihypertensive compound in the AGE. S-1-propylenecysteine was shown to improve peripheral blood circulation and reduce the systolic blood pressure in spontaneous hypertension rats, without affecting the systolic blood pressure of control rats [75]. In another study, the nitrites in the fermented garlic extract (FGE) could be converted into NO in vivo by Bacillus subtilis. Further, NO reduced the systolic blood pressure in spontaneous hypertension rats through the soluble guanylyl cyclase (sGC)-cyclic guanosine monophosphate (cGMP)-protein kinases G (PKG) pathway [76]. Also, FGE was shown to alleviate pulmonary hypertension by decreasing the expression of vascular endothelial cell adhesion molecule-1 and matrix metalloproteinase-9 (MMP-9) and increasing the expression of PKG and endothelial nitric oxide synthase (eNOS) in monocrotaline-induced pulmonary hypertension rats [77]. Moreover, the combination of garlic and its bioactive compound alliin and captopril increased the activity of captopril on inhibiting angiotensin-converting enzyme (ACE) and hypertension in rats [72]. In a placebo-controlled trial, 44 patients with hypertension were given enzymatic browning processed garlic, and their systolic blood pressure and the diastolic blood pressure were significantly reduced [78]. The anti-hypertensive mechanisms of garlic are shown in Figure 2.
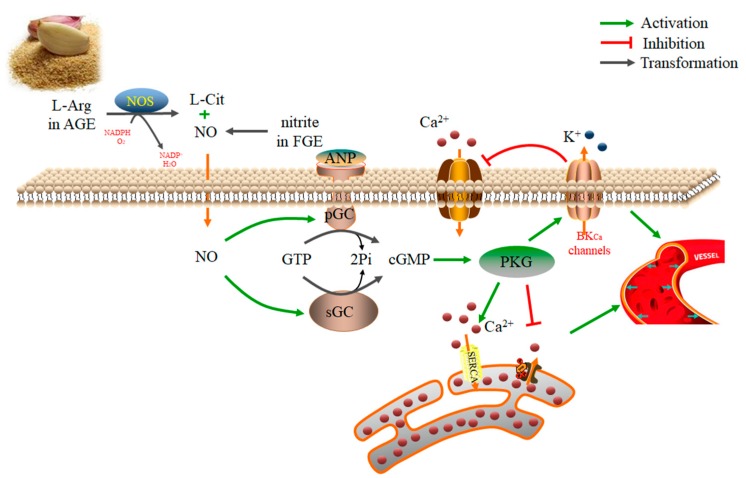
Figure 2.
The mechanisms of the antihypertensive properties of garlic extract via increasing the production of nitric oxide (NO) in vascular smooth muscle cells. The l-arginine (L-Arg) in aged garlic extract (AGE) could be transformed into NO and L-citruline (L-Cit) mediated by nitric oxide synthase (NOS). Moreover, the nitrite in the fermented garlic extract (FGE) could be converted into NO in vivo by Bacillus subtilis. NO and atrial natriuretic peptide (ANP) activated particulate guanylyl cyclase (pGC) and soluble guanylyl cyclase (sGC), thus catalyzing the transform of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). The elevated cGMP activated PKG, and PKG decreased intracellular Ca2+ concentration by increasing intracytoplasmic Ca2+ transport into the sarcoplasmic reticulum through the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pathway, thereby preventing the release of Ca2+ from the sarcoplasmic reticulum to the cytoplasm, and stimulating the Ca2+-activated K+ (BKCa) channel on the cell membrane, as well as reducing the Ca2+ influx. As a result, the vascular smooth muscle relaxed, and the blood vessels dilated.
3.5.2. Anti-hyperlipidemic Activity
Studies demonstrate that garlic can lower blood lipids in animals and people. A study showed that high temperature and high pressure processing could remove the pungency of garlic, and this garlic effectively reduced the levels of total cholesterol, low-density lipoprotein cholesterol, and triglyceride in high-cholesterol diet-fed Sprague–Dawley rats [79]. Another study found that adding 1.5% black garlic extract in high-fat diet for male Sqrague-Dawley rats could significantly modulate the metabolism of lipids and cholesterol and decrease the total levels of blood lipids, triglyceride, and cholesterol, which could be due to the reduction of the mRNA expression of sterol regulatory element binding protein-1c [80]. In a cross sectional study, the intake of garlic (300 mg/day, 8 weeks) was shown to reduce the levels of cholesterol and low-density lipoprotein and elevate the level of high-density lipoprotein, but garlic had no effect on the level of triglycerides in patients with diabetic dyslipidemia [81]. Additionally, a supplement of aged garlic for 13 weeks was found to reduce the activities of myeloperoxidase and lipid hydroperoxide in serum and to decrease the concentrations of F2-isoprostanes in plasma and urine in 41 patients with hypercholesterolemia. Moreover, aged garlic had better effects than raw garlic [82].
3.5.3. Heart Protection
Garlic can also protect the heart. Garlic has been shown to increase Na+/K+-ATPase protein levels and reduce cardiac hypertrophy and remodeling induced by isoproterenol in rats [83]. Another study indicated that garlic extract activated the sirtuin 3-manganese superoxide dismutase pathway by deacetylating manganese superoxide dismutase, thus protecting the heart function in streptomycin-induced diabetic rats [84]. In addition, adding garlic and fenugreek into the diet improved pathological changes of heart tissues in rats [85]. Garlic extract had protective effects on heart rate variability and improved cardiac, as well as mitochondrial, dysfunction in insulin-resistant obese rats [86]. Moreover, garlic extract treatment ameliorated the heart tissue in a rat model of gentamicin-induced chronic renal failure and induced a reduction in oxidative stress and controlled Na+/K+-ATPase activity and Ca2+ levels [87]. Furthermore, AGE had a dose-dependent protective effect against isoproterenol-induced cardiotoxicity. SAC combined with atenolol was more effective against isoproterenol-induced myocardial dysfunction in rats [88]. Notably, allicin was easily degraded into organic diallyl polysulfide in the presence of thiols, which was able to effectively provide H2S to protect the heart [13].
3.5.4. Other Cardiovascular Protective effects
Garlic also has other cardiovascular protective effects. Garlic was reported to inhibit platelet aggregation, which might be related to the antioxidant activity of garlic and its antioxidant compounds [13,89]. The polyphenols in aged black garlic extract had a relaxing effect on coronary arteries before and after ischemia-reperfusion (I/R) in rat hearts and improved myocardial contractility [90]. Moreover, AGE treatment inhibited inflammatory response to prevent atherosclerosis by reducing the serum level of C-reactive protein and thromboxane B-2, the protein level of TNF-α and IL-1 receptor-associated kinase 4, and increasing AMPK activity in the liver of apolipoprotein E-knockout mice [47]. Furthermore, AGE inhibited the vascular inflammation and lipid deposition in progress of atherosclerosis at an early stage in the apolipoprotein E-knockout mice, and AGE also inhibited the development of coronary artery calcification in humans [91].
In short, numerous studies have found that garlic exhibits protective cardiovascular effects and can alleviate hypertension, hyperlipidemia, and heart disease. Garlic’s mechanisms of action could be mainly related to the reduction of oxidative stress, suppression of angiotensin converting enzymes, a reduction of lipid peroxidation, and an increase of NO and H2S production.
3.6. Anticancer Activity
Cancer is acknowledged to be a primary cause of death in the world, and various natural products like berries, cruciferous vegetables, tomatoes, and ginger have been demonstrated to possess anticancer properties [92,93,94,95,96,97,98,99,100]. Recent studies have also shown that garlic and its active constituents can protect against diverse cancers, such as colorectal, lung, gastric, and bladder cancers [101,102,103,104,105,106].
3.6.1. Regulating Metabolism of Carcinogenic Substances
People are exposed to various carcinogens in their daily lives [107]. A study revealed that garlic and its sulfur compounds can diminish the activation of carcinogens, thus reducing the risk of cancer [108]. In addition, garlic and its organic allyl sulfides can inhibit the generation of nitrosamines, a kind of carcinogen produced during cooking and storage [109,110]. Moreover, garlic allyl sulfides can block DNA alkylation, which is an early step in nitrosamine carcinogenesis [108].
3.6.2. Suppressing Cell Growth and Proliferation
Cancer cells have the characteristic of infinite proliferation [111]. It was reported that crude garlic extract exhibited an anti-proliferative effect on human cancer cell lines, including liver (HepG2), colon (Caco2), prostate (PC-3), and breast (MCF-7) cancer cells [112]. Garlic extract induced G2/M-phase cell cycle arrest in EJ bladder cancer cells by activating ataxia-telangiectasia mutated and checkpoint kinase 2, and then inhibiting the phosphorylation of Cdc25C (Ser216) and Cdc2 (Thr14/Tyr15), down-regulating the expression of Cyclin B1, and up-regulating p21WAF1 [113]. The bioactive compounds of garlic, DATS, can suppress the proliferation of SGC-7901 gastric cancer cells and block the cell cycle in the G2/M-phase [114]. Moreover, SAC can induce G1/S-phase cell cycle arrest in A2780 human epithelial ovarian cancer cells [115]. S-propargyl-l-cysteine (SPRC), an analogue of SAC, reduced the proliferation of human pancreatic ductal adenocarcinoma cells and induced cell cycle arrest in the G2/M-phase [116]. The garlic derived S-allylmercaptocysteine (SAMC) suppressed the proliferation of hepatocellular carcinoma cells and negatively affected the cell cycle. It decreased the percentage of the S phase and increased the percentage of the G0/G1 phase [117]. Another study showed that SAMC could inhibit the proliferation of human colorectal carcinoma SW620 cells [118]. Moreover, allicin was found to inhibit the proliferation of gastric adenocarcinoma cells. It induced cell cycle arrest in the S-phase, without affecting normal intestinal cells (INT-407) [18]. Furthermore, ajoene was shown to restrain the growth of glioblastoma multiforme cancer stem cells and human breast cancer cells [119,120].
In addition, AGE had anti-tumor effects on 1,2-dimethylhydrazine (DMH)-induced colon cancer in rats and could delay cell cycle progression during the G2/M-phase by inactivating the NF-κB signaling pathway and down-regulating the expression of Cyclin B1 and Cyclin-dependent kinase 1 [121]. Another study found that ethanol-based garlic extract suppressed the growth of multiple myeloma and prostate cancer cells in vitro. The growth of mammary tumor cells was also suppressed in vivo by ethanol-based garlic extract by increasing stress on the endoplasmic reticulum [122]. Figure 3 shows the mechanisms of cell cycle inhibition by garlic.
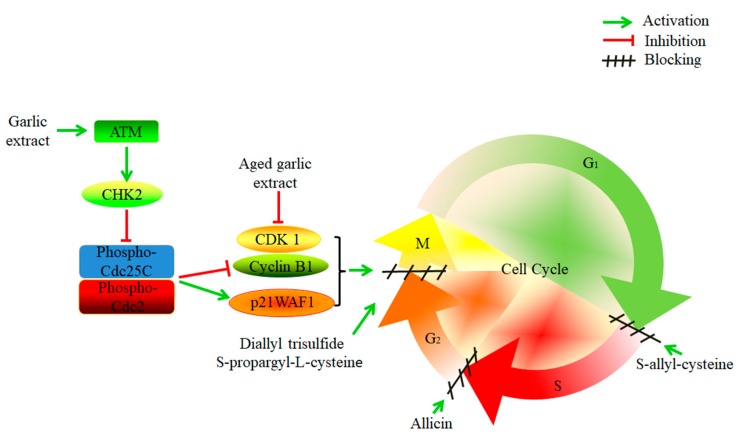
Figure 3.
The mechanisms of garlic and its active compounds on the inhibition of the cell cycle in cancer cells. Garlic extract activated ataxia-telangiectasia mutated (ATM) and checkpoint kinase 2 (CHK2), and inhibited the phosphorylation of Cdc25C and Cdc2, which down-regulated cyclin B1 and up-regulating p21WAF1, thereby inhibiting the cell cycle in the G2/M-phase. Aged garlic extract can down-regulate Cyclin B1 and Cyclin-dependent kinase 1 (CDK1) and block the cell cycle in the G2/M-phase. Diallyl trisulfide and S-propargyl-l-cysteine can also block the cell cycle in the G2/M-phase. Moreover, S-allyl-cysteine induced cell cycle arrest in the G1/S-phase, and allicin induced cell cycle arrest during the S-phase.
3.6.3. Inducing Apoptosis
The intake of raw and crushed garlic was found to upregulate apoptotic-related genes, such as aryl hydrocarbon receptor, hypoxia-inducible factor 1α, and proto-oncogene c-Jun, which influenced the expression of genes related to immunity and cancer in the blood of human beings [123]. DATS induced apoptosis via the accumulation of B cell lymphoma-2 (Bcl-2) associated X (Bax), p53 and cytochrome C, and the decrease of Bcl-2 expression in SGC-7901 gastric cancer cells. DATS also significantly induced tumor apoptosis in a mouse model with SGC-7901 gastric cancer cells [114]. In addition, SAC could induce the apoptosis of A2780 human epithelial ovarian cancer cells, decrease pro-caspase-3, poly(ADP-ribose) polymerase-1 (PARP-1) and Bcl-2, and increase the expression of active caspase-3 and Bax protein [115]. SAMC induced apoptosis via the Jun N-terminal kinase (JNK) and p38 mitogen activated protein kinase (p38 MAPK) pathways in SW620 human colorectal carcinoma cells [118]. Moreover, alliin was shown to induce the apoptosis of gastric adenocarcinoma cells by producing ROS and could decrease the membrane potential of mitochondria through down-upregulating the protein level of Bax/Bcl-2 and up-regulating cytochrome C [18]. Furthermore, S-propargyl-l-cysteine led to apoptosis in human pancreatic ductal adenocarcinoma cells and limited tumor growth in the Panc-1 xenograft model in vivo by activating the JNK pathway [116].
3.6.4. Suppressing Angiogenesis
It was reported that AGE inhibited cell motility, proliferation, and tube formation of ECV304 endothelial cells and the transformed rat lung endothelial cells [124]. Further, DATS effectively inhibit the angiogenesis in MDA-MB-231 human breast cancer cells [125]. Furthermore, the combination of garlic and lemon aqueous extracts had an inhibitory effect on EMT6/P breast cancer cells in BALB/c mice by inhibiting the expression of vascular endothelial growth factor (VEGF) and, finally, angiogenesis [126].
3.6.5. Inhibiting Invasion and Migration
Both invasion and migration are malignant behaviors of tumor cells [111]. Garlic extract inhibited the expression of MMP-9, reduced the binding activity of the transcription factor activator protein 1 (AP-1) (specificity the protein-1 and NF-κB motifs), and increased the expression of heat shock protein A6, which blocked the migration and invasion of bladder cancer EJ cells [113]. AGE decreased the invasive capacity of SW480 and SW620 colorectal cancer cells by inhibiting cell motility and cell proliferation [124]. In addition, SAC reduced the migration of A2780 epithelial ovarian cancer cells by significantly reducing the expression of wingless-type MMTV integration site family member 5A (Wnt5a), phosphor-protein kinase B, and c-Jun proteins [115]. In another study, DATS inhibited SGC-7901 gastric tumor cell migration and invasion by regulating the protein expressions of MMP-9 and E-cadherin in BALB/c-nude mice [114].
3.6.6. Alleviating the Adverse Effects of Anticancer Therapies
Garlic has been indicated to mitigate the adverse effects of several anticancer therapies [127]. In one study, AGE improved the renal histological, ultrastructural, and biochemical changes induced by cisplatin therapy, such as hemorrhaging, glomerular atrophy, tubular necrosis, and degeneration in adult male rats [128]. Furthermore, allicin enhanced the anticancer effect of tamoxifen in mice and reduce the liver injury caused by tamoxifen treatment. Allicin improved the tamoxifen-induced changes in the levels of superoxide dismutase, glutathione, aspartate aminotransferase, alkaline phosphatase, and alanine aminotransferase [129]. Additionally, garlic had a protective effect against febrile neutropenia in patients who received chemotherapy for hematological malignancies in the lower-risk febrile neutropenia subgroup [130].
3.6.7. Other Anti-Cancer Actions
AGE was shown to enhance the in vitro production of interferon-γ in splenocytes and increase the ratio of CD4+/CD8+ on implanted fibrosarcoma tumors in BALB/c mice, which improved the immune responses of mice to fibrosarcoma and inhibited tumor growth [131]. Additionally, the garlic and lemon aqueous extract were demonstrated to activate the immune system against implanted breast cancer in mice by increasing interferon-γ, IL-2, and IL-4 levels [126]. Moreover, DADS prevented colorectal tumorigenesis induced by azoxymethane and dextran sulfate in FVB/N mice. The treatment of DADS reduced inflammation by suppressing glycogen-synthase kinase-3β and the nuclear localization of NF-κB [132].
In summary, garlic and its active components can prevent and manage different cancers (Table 1). These anticancer mechanisms include the regulation of carcinogen metabolism, inhibition of cell growth and proliferation, induction of apoptosis, suppression of angiogenesis, and inhibition of invasion and migration. Garlic can also diminish the negative effects of anticancer therapies.
Table 1.
The biological activities of garlic and its active components.
| Product | Compounds | Study Type | Subjects/ Cell Lines |
Main Effects | Possible Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Antioxidant Activity | ||||||
| Aged garlic extract | In vitro | Human endothelial cells | Protecting cells against oxidative stress | Inducing the expression of several antioxidant enzymes, HO-1 and GCLM subunit, through Nrf2- ARE pathway | [43] | |
| Saponins | In vitro | Mouse-derived C2C12 myoblasts | Protecting cells against the growth inhibition and DNA damage induced by H2O2 | Scavenging intracellular reactive oxygen species | [44] | |
| Anti-inflammatory Activity | ||||||
| Ethyl linoleate | In vitro | Lipopolysaccharide-stimulated RAW 264.7 macrophages | Reducing the production of NO and prostaglandin E-2 | Down regulating the expression of iNOS and COX2 | [45] | |
| Garlic 14-kDa protein | In vitro | Lipopolysaccharide-stimulated J774A.1 macrophages | Inhibiting the inflammatory mediators such as NO, TNF-α, and IL-1β | Inhibiting the transcription factor NF-κB signaling pathway | [46] | |
| Aged garlic extract | In vivo | Apolipoprotein E-knockout mice | Inhibiting inflammation | Reducing the level of TNF-α and interleukin IL-1 receptor-associated kinase 4 Increasing the activity of AMPK in the liver |
[47] | |
| Allicin | In vivo | BALB/c mice | Protecting against the inflammatory response induced by schistosome infection | [48] | ||
| Garlic tablets (equal to 2.5 g fresh garlic daily) | Clinical trial | 40 post-menopausal obese or overweight patients | Alleviating osteoarthritis | Reducing resistin | [49] | |
| Antimicrobial Activity | ||||||
| Garlic “Rosato” and “Caposele” | In vitro | Aspergillus versicolor, Penicillum citrinum and Penicillium expansum | Inhibiting the growth of bacteria | [53] | ||
| Aged garlic extract | Allicin | In vitro | Burkholderia cepacian | Inhibiting the growth of bacteria | [54] | |
| Garlic oil | In vitro | Staphylococcus aureus, Escherichia coli and Bacillus subtilis | Inhibiting the growth of bacteria | [50] | ||
| In vitro | Penicillium funiculosum | Inhibiting the growth of bacteria | Penetrating into cells and organelles Destroying the cell structure Leading to the leakage of cytoplasm and macromolecules |
[55] | ||
| In vitro | Candida albicans | Disrupting the normal metabolism of bacteria | Inducing key genes involved in oxidative phosphorylation, the cell cycle, and protein processing in the endoplasmic reticulum | [56] | ||
| Raw garlic | Clinical trial | 15 patients with H. pylori infection | Inhibiting Helicobacter pylori in the stomach | [57] | ||
| Modulating Immune System | ||||||
| Fresh garlic | Polysaccharides/ Fructan |
In vitro | RAW 264.7 macrophages | Exerting immunomodulatory effect | Regulating the expressions of IL-6, IL-10, TNF-α, and interferon-γ | [58] |
| Garlic oil | In vivo | Wistar rats | Normalizing several immunological parameters of rats, such as the serum total immunoglobulin concentration and T-cell subtype CD4+ Combination of garlic oil and levamisole could balance the T-helper 1/ T-helper 2 response |
[59,60] | ||
| Selenizing garlic polysaccharides | In vivo | 14-day-old chickens | Promoting lymphocyte proliferation Enhancing interferon-γ and IL-2 Increase the serum antibody titer |
[61] | ||
| Aged garlic extract | Clinical trial | 56 healthy human participants | Reducing the occurrence and severity of cold and flu Improving the immune system functions |
[8] | ||
| Cardiovascular Protection | ||||||
| Aged garlic extract | In vitro | Isolated rat aortic rings | Leading to endothelial-dependent vasodilation | Stimulating the production of NO | [71] | |
| Aged black garlic extract | Polyphenols | In vivo | Isolated hearts of male Sprague–Dawley rats | Relaxing coronary arteries before and after ischemia-reperfusion in rat Preventing the decrease of myocardial contractility |
[90] | |
| Aged garlic extract | S-1-propylenecysteine | In vivo | Spontaneous hypertension rats | Improving peripheral blood circulation Reducing the systolic blood pressure |
[75] | |
| Fermented garlic extract by Bacillus subtilis | In vivo | Spontaneous hypertension rats | Reducing the systolic blood pressure | Modulating the sGC-cGMP-PKG pathway | [76] | |
| Fermented garlic extract | In vivo | Monocrotaline-induced pulmonary hypertension rats | Alleviating pulmonary hypertension | Decreasing the expression of vascular endothelial cell adhesion molecule-1 and MMP- 9Increasing the expression of PKG and eNOS |
[77] | |
| Garlic | Alliin | In vivo | Female Wistar albino rats | Increasing the activity of captopril on inhibiting ACE and hypertension | [72] | |
| 1.5% black garlic extract | In vivo | High-fat diet-fed male Sprague–Dawley rats | Modulating the metabolism of lipid and cholesterol Decreasing the levels of blood total lipids, triglyceride, and cholesterol |
Reducing the mRNA expression of sterol regulatory element binding protein-1c | [80] | |
| Raw garlic | Allyl methyl sulfide/Allyl methyl sulfoxide | In vivo | Male Sprague–Dawley rats | Reduce cardiac hypertrophy remodeling induced by isoproterenol | Increasing Na+/K+-ATPase protein level | [83] |
| Raw garlic | In vivo | Streptomycin-induced diabetic rats | Protecting the heart function Activating sirtuin 3-manganese superoxide dismutase pathway |
Deacetylating manganese superoxide dismutase | [84] | |
| Garlic extract | In vivo | Insulin-resistant obese rats | Protecting heart rate variability, cardiac dysfunction, and mitochondrial dysfunction | [86] | ||
| In vivo | Rat model of gentamicin-induced chronic renal failure | Protecting the heart tissue | Reducing oxidative stress Controlling Na+/K+-ATPase activity and Ca2+ levels |
[87] | ||
| Aged garlic extract | SAC | In vivo | Rats with myocardial dysfunction induced by isoproterenol | Protecting against cardiotoxicity | [88] | |
| Aged garlic extract | In vivo | Apolipoprotein E-knockout mice | Inhibiting inflammatory response to prevent atherosclerosis | Reducing serum level of C-reactive protein and thromboxane B-2, protein level of TNF-α and IL-1 receptor-associated kinase 4 Increasing AMPK activity in the liver |
[47] | |
| In vivo | Apolipoprotein E-knockout mice | Inhibiting the vascular inflammation and lipid deposition in the early stage of atherosclerosis development | [91] | |||
| High temperature and high pressure-processed garlic | In vivo | High-cholesterol diet-fed Sprague–Dawley rats | Reducing the levels of total cholesterol, low-density lipoprotein cholesterol and triglyceride | [79] | ||
| Garlic | Cohort study | 30 patients with diabetic dyslipidemia | Decreasing the level of cholesterol and low-density lipoprotein Increase the level of high-density lipoprotein |
[81] | ||
| Aged garlic | Clinical trial | 41 patients with hypercholesterolemia | Reducing the activity of myeloperoxidase and lipid hydroperoxide in serum Decreasing the concentration of F2-isoprostanes in plasma and urine | [82] | ||
| Enzymatic browning processed garlic | Clinical trial | 44 patients with hypertension | Reducing systolic blood pressure and diastolic blood pressure | [78] | ||
| Anticancer Activity | ||||||
| Garlic extract | In vitro | Bladder cancer EJ cells | Inducing G2/M-phase cell cycle arrest Inhibiting cell growth Inhibiting cell migration and invasion |
Activating the ATM pathway and CHK2; Inhibiting the expression of MMP-9 Reducing the binding activity of transcription factors AP-1, specificity protein-1 and NF-κB motifs Increasing the expression of heat shock protein A6 |
[113] | |
| Aged garlic extract | In vitro | Colorectal cancer cell lines (SW480 and SW620) ECV304 cells and the transformed rat lung endothelial cells |
Decreasing invasive activity Inhibiting cell proliferation Decreasing invasive activity Inhibiting the tube formation of endothelial cells |
Inhibiting cell motility | [124] | |
| In vitro | DLD-1 human colon cancer cells (ATCC CCL-221) | Inhibiting cell proliferation | Down regulating the expression of cyclin B1 and CDK1 Inhibiting of activation of NF-κB |
[121] | ||
| Crude garlic extract | Lipid bioactive compounds | In vitro | Human liver cancer (Hep-G2) Colon cancer (Caco-2) Prostate cancer (PC-3) Breast cancer (MCF-7) Mouse macrophage cell (TIB-71) lines |
Inhibiting the growth rate of Hep-G2, PC-3, MCF-7, and TIB-71 cells by 80%–90% at 72 h (P < 0.05). | Inhibiting cell proliferation Inducing cell cycle arrest Inducing apoptosis |
[112] |
| Allicin | In vitro | Human gastric adenocarcinoma cell line | Inhibiting cell proliferation | Inducing cell cycle arrest at S-phase | [18] | |
| DATS | In vitro | Human gastric carcinoma cell line (SGC-7901) | Inhibiting cell proliferation Blocking cell cycle Increasing apoptotic cell death |
Accumulating Bax, p53, and cytochrome C and decreasing the expression of Bcl-2 | [114] | |
| In vitro | Human breast cancer cell line (MDA-MB-231) | Inhibiting angiogenesis | [125] | |||
| Z-ajoene | In vitro | Glioblastoma multiforme cells | Inhibiting the growth of the cancer stem cells population | [119] | ||
| In vitro | Human breast cancer cells (MDA-MB-231) | Inhibiting cell growth Inducing cell apoptosis |
Targeting the folding of proteins in the endoplasmic reticulum of cancer cells | [120] | ||
| SAC | In vitro | Human epithelial ovarian cancer cell line (A2780) | Inhibiting cell proliferation Inducing G1/S-phase cell cycle arrest Increasing apoptosis Reducing the migration of cells |
Decreasing the expression of pro-caspase-3, Parp-1 Bcl-2 and increasing active caspase-3 and Bax Reducing the expression of Wnt5a, phosphorylation protein kinase B and c-Jun proteins |
[115] | |
| SPRC | In vitro | Human pancreatic ductal adenocarcinoma cells (Panc-1) | Inhibiting cell proliferation Inducing apoptosis |
Inducing G2/M-phase cell cycle arrest Regulating the level of JNK protein |
[116] | |
| SAMC | In vitro | Human colorectal carcinoma cell line (SW620) | Inhibiting cell proliferation Inducing cell apoptosis |
Regulating JNK and p38 MAPK pathways | [118] | |
| In vitro | Hepatoma cell lines (Hep3B and Huh-7) | Reducing the cell viability Shortening the S phase and increasing the G0/G1 phase |
[117] | |||
| Alliin | In vitro | Gastric adenocarcinoma cells | Regulating cell apoptosis | Generating reactive oxygen species Decreasing mitochondrial membrane potential by Bax/Bcl-2 Up-regulating cytochrome C |
[18] | |
| Aged garlic extract | In vivo | Adult male Wister albino rats treated with cisplatin | Improving the renal histological, ultrastructural and biochemical changes, such as hemorrhage, glomerular atrophy, tubular necrosis and degeneration | [128] | ||
| In vivo | Fibrosarcoma tumors implanted BALB/c mice | Improving the immune responses of mice to fibrosarcoma Inhibiting tumor growth |
Increasing the ratio of CD4+/CD8+ Producing interferon-γ in splenocytes |
[131] | ||
| Garlic and lemon aqueous extract | In vivo | BALB/c mice xenograft model of breast cancer EMT6/P cells | Reducing tumor size Inhibiting angiogenesis Inducing apoptosis Activating the immune system |
Inhibiting the expression of vascular endothelial growth factor Increasing interferon-γ, IL-2, and IL-4 levels |
[126] | |
| Allicin | In vivo | Female Swiss albino mice | Alleviating liver injury induced by tamoxifen | Changing the decrease of superoxide dismutase, glutathione and total protein and the increase of aspartate aminotransferase, alkaline phosphatase and alanine aminotransferase levels | [129] | |
| DADS | In vivo | FVB/N mice | Preventing colorectal tumorigenesis induced by azoxymethane and dextran sulfate | Inhibiting inflammation Inhibiting glycogen-synthase kinase-3β Reducing the nuclear localization of NF-κB |
[132] | |
| DATS | In vivo | Female BALB/c-nude mouse xenograft model of human gastric carcinoma SGC-7901 cells | Inhibiting tumor growth Promoting tumor apoptosis |
Regulating the expressions of MMP-9 and E-cadherin protein | [114] | |
| SPRC | In vivo | Xenograft model of pancreatic ductal adenocarcinoma Panc-1 cells | Inhibiting tumor growth | Regulating the level of JNK protein | [116] | |
| SAMC | In vivo | Mouse xenograft model of hepatoma Huh-7 cells | Inhibiting tumor growth | Interacting with the Wnt-pathway co-receptor LRP6 on the cell membrane | [117] | |
| Raw, crushed garlic | Cohort study | 17 volunteers from Beltsville, Maryland | Up-regulating seven genes including AHR, ARNT, HIF1A, JUN, NFAM1, OSM and REL | [123] | ||
| Garlic extract | Cohort study | Patients who received chemotherapy for hematological malignancies | Protective effect on febrile neutropenia in lower-risk subgroup | [130] | ||
| Hepatoprotective Activity | ||||||
| Black garlic extract | In vitro | Rat clone-9 hepatocytes | Inhibiting apoptosis, lipid peroxidation, oxidative stress, and inflammation | [136] | ||
| Garlic extract | In vivo | Wistar rats | Attenuating the liver damage induced by alloxan Improving plasma biochemical factors of hepatic function, such as urea, creatinine, aspartate transaminase, and alanine transaminase |
[137] | ||
| Single clove garlic extract | In vivo | Male rabbits | Protecting against CCl4-induced acute liver injury | [38] | ||
| LAFGE | In vivo | C57/BL6 J mice | Reducing the liver lipid level Ameliorating the hepatic steatosis |
[142] | ||
| In vivo | rats | Inhibiting liver cell apoptosis Protecting liver from acetaminophen-induced liver injury |
Suppressing MAPK phosphorylation Down regulating p53 |
[143] | ||
| Garlic oil | In vivo | 1,3-Dichloro-2-propanol-treated rats | Protecting liver | Enhancing the activities of hepatic antioxidant enzymes Blocking metabolic activation of 1, 3-dichloro-2-propanol Reducing the apoptosis in liver |
[139] | |
| DADS | In vivo | Wistar rats | Protecting mice from nonalcoholic fatty liver disease induced by long-term high-fat diet. | Reducing the release of pro-inflammatory cytokines in the liver Increasing antioxidant activity by inhibiting the expression of cytochrome P450 2E1 |
[141] | |
| LAFGE | Clinical trial | 36 adults with mildly high level of serum gamamyl glutamyl transpeptiase | Improving the levels of gamamyl glutamyl transpeptias and alanine aminotransferase without adverse effects | [144] | ||
| Digestive System Protection | ||||||
| Black garlic extract | In vitro | Small intestine | Stimulating gastrointestinal peristalsis Promoting gastrointestinal emptying and facilitates defecation. |
[146] | ||
| DADS DAS |
In vitro | Interferon-γ-stimulated intestinal cells | Reducing interferon-inducible protein-10, IL-6 Inhibiting NO and the expression of STAT-1 |
[70] | ||
| In vivo | Male ICR mice | Improving the colitis induced by dinitrobenzenesulfonic acid | [70] | |||
| Garlic and cabbage extract | In vivo | Sprague–Dawley rats | Reducing the length of gastric ulcer, the total gastric acid, gastric juice volume, total bacteria count, and histopathological changes caused by aspirin Improving the pH value of gastric juice |
[147] | ||
| Aged garlic extract | In vivo | Male albino rats | Healing the gastric mucosal injury induced by indomethacin Reducing the total microbial amount in stomach |
[148] | ||
| In vivo | Male Wistar rats | Preventing the indomethacin-induced ulcer | Reducing oxidative stress Elevating the level of prostaglandin E-2, glutathione, and NO in gastric tissue |
[149] | ||
| Allicin | In vivo | Dextran sulfate sodium-induced colitis mice | Alleviating the ulcerative colitis induced by dextran sulfate sodium | Inhibiting the activation of AP-1/NF-κB/signal transducer and activator of transcription-1 Inhibiting the phosphorylation of p38, JNK, and extracellular signal-regulated kinase 1/2 -regulated PPAR-γ | [150] | |
| Raw garlic | Clinical trial | 15 patients with H. pylori infection | Decreasing the bacterial urease activity Reducing the residing of Helicobacter pylori in the stomach | [57] | ||
| Anti-Diabetic Activity | ||||||
| Garlic | In vivo | Diabetic rats | Protecting against diabetic retinopathy Improving weight, blood glucose, and morphological changes of retinal tissue | [152] | ||
| Clinical trial | 768 patients with type 2 diabetes mellitus | Reducing fructosamine and glycosylated hemoglobin | [154] | |||
| Anti-Obesity Activity | ||||||
| LAFGE | In vivo | High-fat diet-fed male C57BL/6J mice | Reducing the weight Reducing the epididymal, retroperitoneal, and mesenteric adipose tissue mass |
Inhibiting the lipogenesis by down-regulating the mRNA and protein expression of PPAR-γ, C/EBPα, and lipogenic proteins | [9] | |
| Methanolic extract of black garlic | In vivo | High-fat diet-fed male Wistar rats | Reducing the weight Regulating lipid metabolism |
Upregulating the expression of AMPK, forkhead box protein O1, perilipin, and adiponectin in the adipose tissue Down-regulating cluster of differentiation 36, plasminogen activator inhibitor 1, resistin, and TNF-α |
[156] | |
| Garlic oil | In vivo | High-fat diet-fed male Sprague–Dawley rats | Counteracting the influence of high-fat diet on the body weight and adipose tissue weight | [155] | ||
| Neuroprotection | ||||||
| Aged garlic extract | FruArg | In vitro | Lipopolysaccharide-activated murine BV-2 microglial cells | Alleviating neuroinflammation | Inhibiting the production of NO Regulating the expression of multiple protein targets related to oxidative stress |
[157] |
| Garlic extract | In vivo | Female Wistar rats | Reducing the concentration of Pb in the blood and brain Preventing the Pb-induced apoptosis of neurons |
[159] | ||
| Aged garlic extract | In vivo | Adult male Wistar rats | Attenuating the damage of working memory | Improving the loss of cholinergic neurons Increasing the level of vesicular glutamate transporter 1 and glutamate decarboxylase in the hippocampal area |
[161] | |
| Ethanol extract of garlic | In vivo | Diabetic Wistar rats | Improving memory | Increasing the activity of Na+/K+ ATPase, Ca2+ ATPase, and glutamine synthetase in the hippocampus | [162] | |
| Z-ajoene | In vivo | Male gerbils | Preventing I/R-induced delayed neuronal death and gliosis region of the hippocampus |
Reducing lipid peroxidation in the CA1 | [15] | |
| SAC | In vivo | Male albino Wistar rats | Ameliorating the cognitive impairment | Reducing oxidative stress, neuroinflammation, astrogliosis, and acetylcholinesterase activity | [164] | |
| Renal Protection | ||||||
| Aqueous extract of garlic | In vivo | Type 1 diabetic rats | Reducing the oxidative stress in the kidneys | [165] | ||
| In vivo | Wistar rats | Improving the renal plasma biochemical factors induced by alloxan | [137] | |||
| DATS | In vivo | Male albino rats | Protecting the kidney from oxidative stress injury induced by As | Activating the Nrf2-ARE pathway | [166] | |
Abbreviations: ACE, angiotensin-converting enzyme; AHR, aryl hydrocarbon receptor; allicin, diallyl thiosulfonate; alliin, S-allyl-cysteine sulfoxide; AMPK, adenosine monophosphate-activated protein kinase; AP-1, activator protein 1; ARE, antioxidant response element; ARNT, aryl hydrocarbon receptor nuclear translocator; ATM, ataxia-telangiectasia mutated; Bax, Bcl-2 associated X; Bcl-2, B cell lymphoma-2; CDK1, cyclin-dependent kinase 1; cGMP, cyclic guanosine monophosphate; CHK2, checkpoint kinase 2; COX2, cyclooxygenase-2; DADS, diallyl disulfide; DAS, diallyl sulfide; DATS, diallyl trisulfide; eNOS, endothelial nitric oxide synthase; FruArg, N-α-(1-deoxy-D-fructos-1-yl)-l-arginine; GCLM, glutamate-cysteine ligase modifier; HIF1A, hypoxia-inducible factor 1α; HO-1, heme oxygenase-1; IL, interleukin; iNOS, inducible NO synthase; JNK, Jun N-terminal kinase; JUN, proto-oncogene c-Jun; LAFGE, fermented garlic extract by Lactobacillus plantarum BL2; MMP-9, matrix metalloproteinase-9; NFAM1, nuclear factor of activated T cells (NFAT) activating protein with immunoreceptor tyrosine-based activation motif 1; NF-κB, nuclear factor-kappa B; NO, nitric oxide; Nrf2, nuclear factor erythrobia-2 related factor 2; OSM, oncostatin M; Parp-1, poly (ADP-ribose) polymerase-1; PKG, protein kinases G; PPAR-γ, peroxisome proliferator-activated receptor-γ; p38 MAPK, p38 mitogen activated protein kinase; REL, V-relavian reticuloendotheliosis viral oncogene homolog; SAC, S-allyl-cysteine; sGC, soluble guanylyl cyclase; SAMC, S-allylmercaptocysteine; SPRC, S-propargyl-l-cysteine; STAT-1, signal transducer and activator of transcription-1; TNF-α, tumor necrosis factor-α; Wnt5a, wingless-type MMTV integration site family member 5A.
3.7. Hepatoprotective Activity
Accumulating studies have revealed that several natural products, including garlic, had hepatoprotective effects [133,134,135]. In an in vitro study, the black garlic extract reduced the damage of tert-butyl hydroperoxide in rat clone-9 hepatocytes by inhibiting apoptosis, lipid peroxidation, oxidative stress, and inflammation [136]. In another study, garlic attenuated liver damage induced by alloxan in rats and improved the biochemical plasma factors of hepatic functions, such as urea, creatinine, aspartate transaminase, and alanine transaminase [137]. In addition, the combination of garlic and ascorbic acid protected against the liver toxicity induced by Cd in albino mice [138]. Moreover, single clove garlic had a stronger protective effect than multi-clove garlic on CCl4-induced acute liver injury in male rabbits [38].
Moreover, garlic oil was shown to enhance the activities of hepatic antioxidant enzymes, block metabolic activation of 1,3-dichloro-2-propanol, and reduce apoptosis in the liver, indicating a protective effect against liver injury in rats [139]. Also, it was reported that the active compounds of garlic, such as DAS, DADS, and S-methyl-l-cysteine, could prevent and treat liver damage, such as acute and chronic ethanol-induced liver damage [140]. DADS in garlic essential oil was found to attenuate nonalcoholic fatty liver disease, which was induced by a high-fat diet in rats. DADS significantly reduced the release of pro-inflammatory cytokines in the liver and increased antioxidant activity by inhibiting the expression of cytochrome P450 2E1 [141].
Compared with unfermented garlic extract, the fermented garlic extract by Lactobacillus plantarum BL2 (LAFGE) was able to more effectively reduce liver lipid levels and ameliorate hepatic steatosis in mice [142]. Another study revealed that LAFGE inhibited liver cell apoptosis partly by suppressing MAPK phosphorylation and down-regulating p53, which protected the liver from acetaminophen-induced injury in rats [143]. Furthermore, LAFGE was considered as a potential treatment for mild hepatic dysfunction. In a double-blind, randomized, placebo-controlled study, 36 adults with mildly high level of serum gamamyl glutamyl transpeptiase (GGT) received LAFGE, and the levels of GGT and alanine aminotransferase were improved without adverse effects [144]. However, an animal study showed that an overdose of garlic has negative morphological effects on the liver. After 30 days of injecting fresh garlic extract at 500 mg and 1000 mg/kg via a gastric tube in Wistar albinism rats, bleeding and nodular swelling appeared on the external surfaces of the liver, and the weight of the liver increased [145].
Generally speaking, garlic can effectively alleviate acute or chronic liver injury, but the side effects of excessive consumption of garlic also need to be considered. It is necessary to evaluate the safe dose and duration of garlic usage in humans.
3.8. Digestive System Protection
Garlic has been reported to have therapeutic efficacy against gastric tissue injury. A study showed that black garlic extract can stimulate gastrointestinal peristalsis, promote gastrointestinal emptying, and facilitate defecation. The water fraction of black garlic had a better effect on improving gastrointestinal functions compared with the n-butanol fraction and ethyl acetate fraction in the small intestine in vitro [146]. Additionally, the treatment by garlic and cabbage extract reduced the length of gastric ulcers, total gastric acid, gastric juice volume, total bacteria count, and changes in histopathology. This treatment also improved the pH value of gastric juice in rats [147]. Furthermore, the oral administration of AGE was shown to heal gastric mucosal injury induced by indomethacin in male rats and reduce the total microbial amount in the stomach [148]. AGE was effective in preventing indomethacin-induced ulcers in rats via the reduction in oxidative stress and the elevation of prostaglandin E-2, glutathione, and NO in gastric tissue [149].
The bioactive compounds in garlic were also crucial in the protection of the digestive system. Allicin was demonstrated to inhibit the activation of the AP-1/NF-κB/signal transducer and activator of transcription-1 (STAT-1) by inhibiting the phosphorylation of p38, JNK, and the extracellular signal-regulated kinase 1/2 (ERK1/2)-regulated peroxisome proliferator-activated receptor (PPAR)-γ, which alleviated ulcerative colitis in mice [150]. DADS reduced interferon-inducible protein-10, IL-6, and DAS inhibited NO, as well as the expression of STAT-1 in interferon-γ-stimulated intestinal cells. Further, DAS and DADS improved the colitis induced by dinitrobenzenesulfonic acid in mice [70]. In addition, intake of raw garlic was shown to decrease bacterial urease activity and reduce Helicobacter pylori in the stomach of 15 patients [57].
In general, garlic and its bioactive compounds can improve gastrointestinal functions and alleviate colitis, gastric ulcers, and other gastrointestinal diseases by reducing oxidative stress, inhibiting inflammation, and decreasing Helicobacter pylori.
3.9. Anti-Diabetic Activity
Garlic has been shown to reduce pancreatic cell injury, oxidative stress, and pathological changes in streptomycin-induced type 1 diabetic rats [151]. In addition, garlic had a protective effect on diabetic retinopathy in diabetic rats. The weight, blood glucose, and morphological changes of retinal tissue in the group treated with garlic improved after 7 weeks of gastric gavage of raw garlic extract in rats [152]. Moreover, AGE had a dose-dependent anti-diabetic effect on streptomycin-induced diabetic rats [153]. Furthermore, a meta-analysis was performed on 768 patients with type 2 diabetes mellitus in nine randomized controlled trials, and the result showed that garlic supplements significantly reduced fructosamine and glycosylated hemoglobin. This study demonstrated that garlic supplements were effective in the management of type 2 diabetes mellitus [154]. Thus, garlic and its bioactive components might be effective agents to help treat diabetes and diabetic complications.
3.10. Anti-Obesity Activity
Garlic oil has anti-obesity properties and has been shown to counteract the influence of a high-fat diet on the weight of body and adipose tissue in hyperlipidemia rats [155]. In addition, the oral administration of LAFGE reduced the weight of high-fat diet male C57BL/6J mice. LAFGE also reduced their epididymal, retroperitoneal, and mesenteric adipose tissue mass. The possible mechanism of action was that LAFGE inhibited lipogenesis by down-regulating the mRNA and protein expression of PPAR-γ, C/EBPα, and lipogenic proteins [9]. Moreover, the methanolic extract of black garlic was found to reduce the weight of rats fed with a high-fat diet. This treatment regulated lipid metabolism by up-regulating the expression of AMPK, forkhead box protein O1, perilipin, and adiponectin in the adipose tissue of the rats and down-regulating the cluster of differentiation 36 (CD36), plasminogen activator inhibitor 1, resistin, and TNF-α [156].
Collectively, the studies demonstrate that fermented garlic products have certain positive effects on obesity by inhibiting lipogenesis and regulating lipid metabolism.
3.11. Neuroprotection
A study revealed that AGE and its carbohydrate derivative N-α-(1-deoxy-D-fructos-1-yl)- l-arginine alleviated neuroinflammation by inhibiting the production of NO and regulating the expression of multiple protein targets related to oxidative stress in lipopolysaccharide-activated murine BV-2 microglial cells [157]. In another study, the anti-neuritis activity of garlic was related to the organosulfur compounds in lipopolysaccharide-stimulated BV2 microglia cells [158]. The treatment of garlic during pregnancy and lactation was shown to decrease the concentration of Pb in the blood and brain and partially prevent the Pb-induced apoptosis of neurons during the hippocampal development in rats [159]. The Basso, Beattie, and Bresnahan (BBB) scoring system was used to conduct a neurological evaluation on the influence of AGE on a spinal cord I/R model of rats. Compared with the I/R group, the BBB score of the AGE group was markedly higher, thus demonstrating that AGE had a significant neuroprotective effect [160]. Additionally, AGE attenuated the loss of cholinergic neurons and enhanced the level of vesicular glutamate transporter 1 and glutamate decarboxylase in the hippocampal area of rats, which attenuated the damage of working memory [161]. Moreover, the ethanol extract of garlic was shown to improve memory. Garlic activated Na+/K+ ATPase, Ca2+ ATPase, and glutamine synthetase in the hippocampus of diabetic Wistar rats [162]. Furthermore, the ethanol extract of fermented garlic effectively prevented working memory via the damage induced by monosodium glutamate [163]. Z-ajoene was able to prevent I/R-induced delayed neuronal death and gliosis and reduce the lipid peroxidation in the CA1 region of the hippocampus [15]. Moreover, SAC ameliorated cognitive impairment in rats by reducing oxidative stress, neuroinflammation, astrogliosis, and acetylcholinesterase activity [164].
In conclusion, both in vivo and in vitro experiments showed that garlic has significant neuroprotective properties and mainly acts on the hippocampus. Organic sulfur compounds were shown to play a major role in neuroprotection.
3.12. Renal Protection
Garlic was shown to effectively alleviate nephrotoxicity [10]. The aqueous extract of garlic was shown to reduce the oxidative stress in the kidneys of diabetic rats [165]. In addition, the aqueous extract of garlic improved the renal plasma biochemical factors induced by alloxan in Wistar rats [137]. Moreover, DATS was reported to activate the Nrf2-ARE pathway, protecting the kidney from oxidative stress injury induced by arsenic in rats [166].
4. Conclusions
Garlic is a widely consumed spice with a characteristic odor. It contains many bioactive components, such as organic sulfides, saponins, phenolic compounds, and polysaccharides. The organic sulfides, such as allicin, alliin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and S-allyl-cysteine, are major bioactive components in garlic. Garlic and its bioactive components show many biological functions, such as antioxidant, anti-inflammatory, immunomodulatory, cardiovascular protective, anticancer, hepatoprotective, digestive system protective, anti-diabetic, anti-obesity, neuroprotective, renal protective, antibacterial, and antifungal activities. Generally, garlic is non-toxic or has low toxicity. Therefore, garlic and its bioactive compounds are promising as functional foods or nutraceuticals for the prevention and treatment of different diseases. In the future, more biological functions of garlic should be evaluated, and the relative compounds of garlic need to be separated and identified. More investigations should be conducted to deeply illustrate garlic’s mechanisms of action. In addition, the effects of the processing, such as fermentation and heat, on garlic should be further studied because they could impact the biological functions and safety of garlic. Furthermore, more clinic trials should be carried out to confirm the health benefits of garlic on humans, and special attention should be paid to the side effects/safety of garlic.
Author Contributions
Conceptualization, A.S., R.-Y.G., and H.-B.L.; writing—original draft preparation, A.S., S.-Y.C., X.-Y.X., and G.-Y.T.; writing—review and editing, R.-Y.G., H.C., V.M., and H.-B.L.; supervision, R.-Y.G. and H.-B.L.; funding acquisition, R.-Y.G. and H.-B.L.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1600100), the Shanghai Basic and Key Program (18JC1410800), the Agri-X Interdisciplinary Fund of Shanghai Jiao Tong University (Agri-X2017004), and the Key Project of Guangdong Provincial Science and Technology Program (2014B020205002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bose S., Laha B., Banerjee S. Quantification of allicin by high performance liquid chromatography-ultraviolet analysis with effect of post-ultrasonic sound and microwave radiation on fresh garlic cloves. Pharmacogn. Mag. 2014;10:288–293. doi: 10.4103/0973-1296.133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diretto G., Rubio-Moraga A., Argandona J., Castillo P., Gomez-Gomez L., Ahrazem O. Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules. 2017;22:1359. doi: 10.3390/molecules22081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szychowski K.A., Rybczynska-Tkaczyk K., Gawel-Beben K., Swieca M., Karas M., Jakubczyk A., Matysiak M., Binduga U.E., Gminski J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018;68:73–81. doi: 10.1515/pjfns-2017-0005. [DOI] [Google Scholar]
- 4.Jacob B., Narendhirakannan R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotechnol. 2019;9:4. doi: 10.1007/s13205-018-1528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonpeng S., Siripongvutikorn S., Sae-Wong C., Sutthirak P. The antioxidant and anti-cadmium toxicity properties of garlic extracts. Food Sci. Nutr. 2014;2:792–801. doi: 10.1002/fsn3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee D.Y., Li H., Lim H.J., Lee H.J., Jeon R., Ryu J.-H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food. 2012;15:992–999. doi: 10.1089/jmf.2012.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayat S., Cheng Z., Ahmad H., Ali M., Chen X., Wang M. Garlic, from remedy to stimulant: Evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants. Front. Plant Sci. 2016;7:1235. doi: 10.3389/fpls.2016.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Percival S.S. Aged garlic extract modifies human immunity. J. Nutr. 2016;146:433S–436S. doi: 10.3945/jn.115.210427. [DOI] [PubMed] [Google Scholar]
- 9.Lee H.S., Lim W.C., Lee S.J., Lee S.H., Lee J.H., Cho H.Y. Antiobesity effect of garlic extract fermented by lactobacillus plantarum bl2 in diet-induced obese mice. J. Med. Food. 2016;19:823–829. doi: 10.1089/jmf.2016.3674. [DOI] [PubMed] [Google Scholar]
- 10.Seckiner I., Bayrak O., Can M., Mungan A.G., Mungan N.A. Garlic supplemented diet attenuates gentamicin nephrotoxicity in rats. Int. Braz. J. Urol. 2014;40:562–567. doi: 10.1590/S1677-5538.IBJU.2014.04.17. [DOI] [PubMed] [Google Scholar]
- 11.Yun H.M., Ban J.O., Park K.R., Lee C.K., Jeong H.S., Han S.B., Hong J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol Ther. 2014;142:183–195. doi: 10.1016/j.pharmthera.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kimura S., Tung Y.C., Pan M.H., Su N.W., Lai Y.J., Cheng K.C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017;25:62–70. doi: 10.1016/j.jfda.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley J.M., Organ C.L., Lefer D.J. Garlic-derived organic polysulfides and myocardial protection. J. Nutr. 2016;146:403S–409S. doi: 10.3945/jn.114.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y.C., Guan M., Zhao X., Li X.L. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018;56:325–332. doi: 10.1080/13880209.2018.1479868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo D.Y., Kim W., Nam S.M., Yoo M., Lee S., Yoon Y.S., Won M.H., Hwang I.K., Choi J.H. Neuroprotective effects of Z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal CA1 region after transient forebrain ischemia. Food Chem. Toxicol. 2014;72:1–7. doi: 10.1016/j.fct.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kodera Y., Ushijima M., Amano H., Suzuki J., Matsutomo T. Chemical and biological properties of S-1-propenyl-l-cysteine in aged garlic extract. Molecules. 2017;22:570. doi: 10.3390/molecules22040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo M., Lee S., Kim S., Hwang J.B., Choe J., Shin D. Composition of organosulfur compounds from cool- and warm-type garlic (Allium sativum L.) in Korea. Food Sci. Biotechnol. 2014;23:337–344. doi: 10.1007/s10068-014-0047-y. [DOI] [Google Scholar]
- 18.Mansingh D.P., Dalpati N., Sali V.K., Vasanthi A.H.R. Alliin the precursor of allicin in garlic extract mitigates proliferation of gastric adenocarcinoma cells by modulating apoptosis. Pharmacogn. Mag. 2018;14:S84–S91. [Google Scholar]
- 19.Torres-Palazzolo C., Ramirez D., Locatelli D., Manucha W., Castro C., Camargo A. Bioaccessibility and permeability of bioactive compounds in raw and cooked garlic. J. Food Compos. Anal. 2018;70:49–53. doi: 10.1016/j.jfca.2018.03.008. [DOI] [Google Scholar]
- 20.Lanzotti V., Bonanomi G., Scala F. What makes allium species effective against pathogenic microbes? Phytochem. Rev. 2013;12:751–772. doi: 10.1007/s11101-013-9295-3. [DOI] [Google Scholar]
- 21.Liu J., Ji F., Chen F.M., Guo W., Yang M.L., Huang S.X., Zhang F., Liu Y.S. Determination of garlic phenolic compounds using supercritical fluid extraction coupled to supercritical fluid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018;159:513–523. doi: 10.1016/j.jpba.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Nagella P., Thiruvengadam M., Ahmad A., Yoon J.Y., Chung I.M. Composition of polyphenols and antioxidant activity of garlic bulbs collected from different locations of Korea. Asian J. Chem. 2014;26:897–902. doi: 10.14233/ajchem.2014.16143A. [DOI] [Google Scholar]
- 23.Hang X. Isolation and identification of garlic polysaccharide. Food Sci. 2005;26:48–51. [Google Scholar]
- 24.Liang T.F., Wei F.F., Lu Y., Kodani Y., Nakada M., Miyakawa T., Tanokura M. Comprehensive nmr analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015;63:683–691. doi: 10.1021/jf504836d. [DOI] [PubMed] [Google Scholar]
- 25.Lu X.M., Li N.Y., Qiao X.G., Qiu Z.C., Liu P.L. Effects of thermal treatment on polysaccharide degradation during black garlic processing. Lwt-Food Sci. Technol. 2018;95:223–229. doi: 10.1016/j.lwt.2018.04.059. [DOI] [Google Scholar]
- 26.Sun Y.E., Wang W.D. Changes in nutritional and bio-functional compounds and antioxidant capacity during black garlic processing. J. Food Sci. Technol. 2018;55:479–488. doi: 10.1007/s13197-017-2956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.S., Kang O.J., Gweon O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods. 2013;5:80–86. doi: 10.1016/j.jff.2012.08.006. [DOI] [Google Scholar]
- 28.Li Y., Zhang J.J., Xu D.P., Zhou T., Zhou Y., Li S., Li H.B. Bioactivities and health benefits ofwild fruits. Int. J. Mol. Sci. 2016;17:1258. doi: 10.3390/ijms17081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu L., Xu B.T., Xu X.R., Gan R.Y., Zhang Y., Xia E.Q., Li H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 30.Deng G.F., Lin X., Xu X.R., Gao L.L., Xie J.F., Li H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods. 2013;5:260–266. doi: 10.1016/j.jff.2012.10.015. [DOI] [Google Scholar]
- 31.Guo Y.J., Deng G.F., Xu X.R., Wu S., Li S., Xia E.Q., Li F., Chen F., Ling W.H., Li H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195–1205. doi: 10.1039/c2fo30110e. [DOI] [PubMed] [Google Scholar]
- 32.Deng G.F., Xu X.R., Guo Y.J., Xia E.Q., Li S., Wu S., Chen F., Ling W.H., Li H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods. 2012;4:906–914. doi: 10.1016/j.jff.2012.06.008. [DOI] [Google Scholar]
- 33.Li A.N., Li S., Li Y., Xu D.P., Li H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the osmanthus fragrans flower. Molecules. 2016;21:218. doi: 10.3390/molecules21020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Tang G.Y., Zhao C.N., Feng X.L., Xu X.Y., Cao S.Y., Meng X., Li S., Gan R.Y., Li H.B. Comparison of antioxidant activities of different grape varieties. Molecules. 2018;23:2432. doi: 10.3390/molecules23102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locatelli D.A., Nazareno M.A., Fusari C.M., Camargo A.B. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017;220:219–224. doi: 10.1016/j.foodchem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Zakarova A., Seo J.Y., Kim H.Y., Kim J.H., Shin J.H., Cho K.M., Lee C.H., Kim J.S. Garlic sprouting is associated with increased antioxidant activity and concomitant changes in the metabolite profile. J. Agric. Food Chem. 2014;62:1875–1880. doi: 10.1021/jf500603v. [DOI] [PubMed] [Google Scholar]
- 37.Jang H.J., Lee H.J., Yoon D.K., Ji D.S., Kim J.H., Lee C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018;27:219–225. doi: 10.1007/s10068-017-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naji K.M., Al-Shaibani E.S., Alhadi F.A., Al-Soudi S.A., D’Souza M.R. Hepatoprotective and antioxidant effects of single clove garlic against ccl4-induced hepatic damage in rabbits. BMC Complement. Altern. Med. 2017;17:411. doi: 10.1186/s12906-017-1916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z.S., Lei M.M., Liu R., Gao Y.F., Xu M.Y., Zhang M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015;39:39–47. doi: 10.1111/jfbc.12102. [DOI] [Google Scholar]
- 40.Choi I.S., Cha H.S., Lee Y.S. Physicochemical and antioxidant properties of black garlic. Molecules. 2014;19:16811–16823. doi: 10.3390/molecules191016811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiramatsu K., Tsuneyoshi T., Ogawa T., Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nutr. Res. 2016;36:143–149. doi: 10.1016/j.nutres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Unni L.E., Chauhan O.P., Raju P.S. High pressure processing of garlic paste: Effect on the quality attributes. Int. J. Food Sci. Technol. 2014;49:1579–1585. doi: 10.1111/ijfs.12456. [DOI] [Google Scholar]
- 43.Liu J., Guo W., Yang M.L., Liu L.X., Huang S.X., Tao L., Zhang F., Liu Y.S. Investigation of the dynamic changes in the chemical constituents of chinese “laba” garlic during traditional processing. Rsc Adv. 2018;8:41872–41883. doi: 10.1039/C8RA09657K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang J.S., Kim S.O., Kim G.Y., Hwang H.J., Kim B.W., Chang Y.C., Kim W.J., Kim C.M., Yoo Y.H., Choi Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016;37:149–156. doi: 10.3892/ijmm.2015.2398. [DOI] [PubMed] [Google Scholar]
- 45.Park S.Y., Seetharaman R., Ko M.J., Kim D.Y., Kim T.H., Yoon M.K., Kwak J.H., Lee S.J., Bae Y.S., Choi Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW 264.7 cells. Int. Immunopharmacol. 2014;19:253–261. doi: 10.1016/j.intimp.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Rabe S.Z.T., Ghazanfari T., Siadat Z., Rastin M., Rabe S.Z.T., Mahmoudi M. Anti-inflammatory effect of garlic 14-kDa protein on LPS-stimulated-J774A.1 macrophages. Immunopharmacol. Immunotoxicol. 2015;37:158–164. doi: 10.3109/08923973.2015.1005229. [DOI] [PubMed] [Google Scholar]
- 47.Morihara N., Hino A., Miki S., Takashima M., Suzuki J. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol. Nutr. Food Res. 2017;61:1700308. doi: 10.1002/mnfr.201700308. [DOI] [PubMed] [Google Scholar]
- 48.Metwally D.M., Al-Olayan E.M., Alanazi M., Alzahrany S.B., Semlali A. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in vivo. BMC Complement. Altern. Med. 2018;18:135. doi: 10.1186/s12906-018-2191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehghani S., Alipoor E., Salimzadeh A., Yaseri M., Hosseini M., Feinle-Bisset C., Hosseinzadeh-Attar M.J. The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-α, and on pain severity, in overweight or obese women with knee osteoarthritis. Phytomedicine. 2018;48:70–75. doi: 10.1016/j.phymed.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y.J. Experimental study on the optimization of extraction process of garlic oil and its antibacterial effects. Afr. J. Tradit. Complement. Altern. Med. 2014;11:411–414. doi: 10.4314/ajtcam.v11i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li S., Gan R.Y., Li H.B. Natural products for the prevention and management of Helicobacter pylori infection. Compr. Rev. Food Sci. F. 2018;17:937–952. doi: 10.1111/1541-4337.12355. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017;18:1283. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fratianni F., Riccardi R., Spigno P., Ombra M.N., Cozzolino A., Tremonte P., Coppola R., Nazzaro F. Biochemical characterization and antimicrobial and antifungal activity of two endemic varieties of garlic (Allium sativum L.) of the campania region, southern Italy. J. Med. Food. 2016;19:686–691. doi: 10.1089/jmf.2016.0027. [DOI] [PubMed] [Google Scholar]
- 54.Wallock-Richards D., Doherty C.J., Doherty L., Clarke D.J., Place M., Govan J.R.W., Campopiano D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE. 2014;9:e112726. doi: 10.1371/journal.pone.0112726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W.R., Shi Q.S., Liang Q., Huang X.M., Chen Y.B. Antifungal effect and mechanism of garlic oil on penicillium funiculosum. Appl. Microbiol. Biot. 2014;98:8337–8346. doi: 10.1007/s00253-014-5919-9. [DOI] [PubMed] [Google Scholar]
- 56.Li W.R., Shi Q.S., Dai H.Q., Liang Q., Xie X.B., Huang X.M., Zhao G.Z., Zhang L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zardast M., Namakin K., Kaho J.E., Hashemi S.S. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J. Phytomed. 2016;6:495–501. [PMC free article] [PubMed] [Google Scholar]
- 58.Li M., Yan Y.X., Yu Q.T., Deng Y., Wu D.T., Wang Y., Ge Y.Z., Li S.P., Zhao J. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 macrophages. J. Food Sci. 2017;82:765–771. doi: 10.1111/1750-3841.13589. [DOI] [PubMed] [Google Scholar]
- 59.Hassouna I., Ibrahim H., Gaffar F.A., El-Elaimy I., Latif H.A. Simultaneous administration of hesperidin or garlic oil modulates diazinon-induced hemato- and immunotoxicity in rats. Immunopharmacol. Immunotoxicol. 2015;37:442–449. doi: 10.3109/08923973.2015.1081932. [DOI] [PubMed] [Google Scholar]
- 60.Mohamed E.H., Baiomy A.A.A., Ibrahim Z.S., Soliman M.M. Modulatory effects of levamisole and garlic oil on the immune response of wistar rats: Biochemical, immunohistochemical, molecular and immunological study. Mol. Med. Rep. 2016;14:2755–2763. doi: 10.3892/mmr.2016.5551. [DOI] [PubMed] [Google Scholar]
- 61.Qiu S.L., Chen J., Qin T., Hu Y.L., Wang D.Y., Fan Q., Zhang C.S., Chen X.Y., Chen X.L., Liu C., et al. Effects of selenylation modification on immune-enhancing activity of garlic polysaccharide. PLoS ONE. 2014;9:e86377. doi: 10.1371/journal.pone.0086377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart disease and stroke statistics-2018 update a report from the American Heart Association. Circulation. 2018;137:E67–E492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food. Sci. Nutr. 2014;54:352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- 64.Tang G.Y., Meng X., Li Y., Zhao C.N., Liu Q., Li H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 2017;9:857. doi: 10.3390/nu9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao C.N., Meng X., Li Y., Li S., Liu Q., Tang G.Y., Li H.B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9:598. doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng J., Zhou Y., Li S., Zhang P., Zhou T., Xu D.P., Li H.B. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int. J. Mol. Sci. 2017;18:555. doi: 10.3390/ijms18030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayan L., Koulivand P.H., Gorji A. Garlic: A review of potential therapeutic effects. Avicenna. J. Phytomed. 2014;4:1–14. [PMC free article] [PubMed] [Google Scholar]
- 68.Kwak J.S., Kim J.Y., Paek J.E., Lee Y.J., Kim H.R., Park D.S., Kwon O. Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 2014;8:644–654. doi: 10.4162/nrp.2014.8.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz C., Correa-Rotter R., Sanchez-Gonzalez D.J., Hernandez-Pando R., Maldonado P.D., Martinez-Martinez C.M., Medina-Campos O.N., Tapia E., Aguilar D., Chirino Y.I., et al. Renoprotective and antihypertensive effects of S-allylcysteine in 5/6 nephrectomized rats. Am. J. Physiol. Renal Physiol. 2007;293:F1691–F1698. doi: 10.1152/ajprenal.00235.2007. [DOI] [PubMed] [Google Scholar]
- 70.Fasolino I., Izzo A.A., Clavel T., Romano B., Haller D., Borrelli F. Orally administered allyl sulfides from garlic ameliorate murine colitis. Mol. Nutr. Food Res. 2015;59:434–442. doi: 10.1002/mnfr.201400347. [DOI] [PubMed] [Google Scholar]
- 71.Takashima M., Kanamori Y., Kodera Y., Morihara N., Tamura K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine. 2017;24:56–61. doi: 10.1016/j.phymed.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Asdaq S.M., Inamdar M.N. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17:1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Sausbier M., Schubert R., Voigt V., Hirneiss C., Pfeifer A., Korth M., Kleppisch T., Ruth P., Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ. Res. 2000;87:825–830. doi: 10.1161/01.RES.87.9.825. [DOI] [PubMed] [Google Scholar]
- 74.Li X., Kim H.Y., Cui H.Z., Cho K.W., Kang D.G., Lee H.S. Water extract of zanthoxylum piperitum induces vascular relaxation via endothelium-dependent NO-cGMP signaling. J. Ethnopharmacol. 2010;129:197–202. doi: 10.1016/j.jep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Ushijima M., Takashima M., Kunimura K., Kodera Y., Morihara N., Tamura K. Effects of S-1-propenylcysteine, a sulfur compound in aged garlic extract, on blood pressure and peripheral circulation in spontaneously hypertensive rats. J. Pharm. Pharmacol. 2018;70:559–565. doi: 10.1111/jphp.12865. [DOI] [PubMed] [Google Scholar]
- 76.Park B.M., Cha S.A., Kim H.Y., Kang D.K., Yuan K., Chun H., Chae S.W., Kim S.H. Fermented garlic extract decreases blood pressure through nitrite and sGC-cGMP-PKG pathway in spontaneously hypertensive rats. J. Funct. Foods. 2016;22:156–165. doi: 10.1016/j.jff.2016.01.034. [DOI] [Google Scholar]
- 77.Park B.M., Chun H., Chae S.W., Kim S.H. Fermented garlic extract ameliorates monocrotaline-induced pulmonary hypertension in rats. J. Funct. Foods. 2017;30:247–253. doi: 10.1016/j.jff.2017.01.024. [DOI] [Google Scholar]
- 78.Han C.H., Liu J.C., Chen K.H., Lin Y.S., Chen C.T., Fan C.T., Lee H.L., Liu D.Z., Hou W.C. Antihypertensive activities of processed garlic on spontaneously hypertensive rats and hypertensive humans. Bot. Stud. 2011;52:277–283. [Google Scholar]
- 79.Sohn C.W., Kim H., You B.R., Kim M.J., Kim H.J., Lee J.Y., Sok D.E., Kim J.H., Lee K.J., Kim M.R. High temperature- and high pressure-processed garlic improves lipid profiles in rats fed high cholesterol diets. J. Med. Food. 2012;15:435–440. doi: 10.1089/jmf.2011.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ha A.W., Ying T., Kim W.K. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr. Res. Pract. 2015;9:30–36. doi: 10.4162/nrp.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siddiqui N.A., Haider S., Misbah-ur-Rehman M., Perveen T. Role of herbal formulation of garlic on lipid profile in patients with type 2 diabetes related dyslipidemia. Pak. Heart J. 2016;49:146–150. [Google Scholar]
- 82.Ho X.L., Tsen S.Y., Ng M.Y., Lee W.N., Low A., Loke W.M. Aged garlic supplement protects against lipid peroxidation in hypercholesterolemic individuals. J. Med. Food. 2016;19:931–937. doi: 10.1089/jmf.2016.3693. [DOI] [PubMed] [Google Scholar]
- 83.Khatua T.N., Borkar R.M., Mohammed S.A., Dinda A.K., Srinivas R., Banerjee S.K. Novel sulfur metabolites of garlic attenuate cardiac hypertrophy and remodeling through induction of Na+/K+-ATPase expression. Front. Pharmacol. 2017;8:18. doi: 10.3389/fphar.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sultana M.R., Bagul P.K., Katare P.B., Mohammed S.A., Padiya R., Banerjee S.K. Garlic activates SIRT-3 to prevent cardiac oxidative stress and mitochondrial dysfunction in diabetes. Life Sci. 2016;164:42–51. doi: 10.1016/j.lfs.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 85.Mukthamba P., Srinivasan K. Hypolipidemic influence of dietary fenugreek (Trigonella foenum-graecum) seeds and garlic (Allium sativum) in experimental myocardial infarction. Food Funct. 2015;6:3117–3125. doi: 10.1039/C5FO00240K. [DOI] [PubMed] [Google Scholar]
- 86.Supakul L., Pintana H., Apaijai N., Chattipakorn S., Shinlapawittayatorn K., Chattipakorn N. Protective effects of garlic extract on cardiac function, heart rate variability, and cardiac mitochondria in obese insulin-resistant rats. Eur. J. Nutr. 2014;53:919–928. doi: 10.1007/s00394-013-0595-6. [DOI] [PubMed] [Google Scholar]
- 87.Gomaa A.M.S., Abdelhafez A.T., Aamer H.A. Garlic (Allium sativum) exhibits a cardioprotective effect in experimental chronic renal failure rat model by reducing oxidative stress and controlling cardiac Na+/K+-ATPase activity and Ca2+ levels. Cell Stress Chaperones. 2018;23:913–920. doi: 10.1007/s12192-018-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Avula P.R., Asdaq S.M., Asad M. Effect of aged garlic extract and S-allyl cysteine and their interaction with atenolol during isoproterenol induced myocardial toxicity in rats. Indian J. Pharmacol. 2014;46:94–99. doi: 10.4103/0253-7613.125185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Torres I., Torres-Narvaez J.C., Pedraza-Chaverri J., Rubio-Ruiz M.E., Diaz-Diaz E., Del Valle-Mondragon L., Martinez-Memije R., Lopez E.V., Guarner-Lans V. Effect of the aged garlic extract on cardiovascular function in metabolic syndrome rats. Molecules. 2016;21:1425. doi: 10.3390/molecules21111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Villalon A.L., Amor S., Monge L., Fernandez N., Prodanov M., Munoz M., Inarejos-Garcia A.M., Granado M. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J. Funct. Foods. 2016;27:189–200. doi: 10.1016/j.jff.2016.08.062. [DOI] [Google Scholar]
- 91.Morihara N., Hino A., Yamaguchi T., Suzuki J. Aged garlic extract suppresses the devegopment of atherosclerosis in apollipoprotein E-knockout mice. J. Nutr. 2016;146:460S–463S. doi: 10.3945/jn.114.206953. [DOI] [PubMed] [Google Scholar]
- 92.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 93.Zhao C.N., Li Y., Meng X., Li S., Liu Q., Tang G.Y., Gan R.Y., Li H.B. Insight into the roles of vitamins C and D against cancer: Myth or truth? Cancer Lett. 2018;431:161–170. doi: 10.1016/j.canlet.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 94.Gan R.Y., Li H.B., Sui Z.Q., Corke H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018;58:924–941. doi: 10.1080/10408398.2016.1231168. [DOI] [PubMed] [Google Scholar]
- 95.Tao J., Li Y., Li S., Li H.B. Plant foods for the prevention and management of colon cancer. J. Funct. Foods. 2018;42:95–110. doi: 10.1016/j.jff.2017.12.064. [DOI] [Google Scholar]
- 96.Xu X.Y., Meng X., Li S., Gan R.Y., Li Y., Li H.B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients. 2018;10:1553. doi: 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Li S., Meng X., Gan R.Y., Zhang J.J., Li H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728. doi: 10.3390/nu9070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng J., Zhou Y., Li Y., Xu D.P., Li S., Li H.B. Spices for prevention and treatment of cancers. Nutrients. 2016;8:495. doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Y., Zheng J., Li Y., Xu D.P., Li S., Chen Y.M., Li H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8:515. doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Y., Li Y., Zhou T., Zheng J., Li S., Li H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8:156. doi: 10.3390/nu8030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y., Wu Z.R., Chen P., Yang L., Deng W.R., Wang Y.Q., Li H.Y. Effect of the tyrosinase inhibitor (S)-N-trans-feruloyloctopamine from garlic skin on tyrosinase gene expression and melanine accumulation in melanoma cells. Bioorg. Med. Chem. Lett. 2015;25:1476–1478. doi: 10.1016/j.bmcl.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 102.Myneni A.A., Chang S.C., Niu R.G., Liu L., Swanson M.K., Li J.W., Su J., Giovino G.A., Yu S.Z., Zhang Z.F., et al. Raw garlic consumption and lung cancer in a chinese population. Cancer Epidemiol. Biomarkers Prev. 2016;25:624–633. doi: 10.1158/1055-9965.EPI-15-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim W.T., Seo S.P., Byun Y.J., Kang H.W., Kim Y.J., Lee S.C., Jeong P., Seo Y., Choe S.Y., Kim D.J., et al. Garlic extract in bladder cancer prevention: Evidence from T24 bladder cancer cell xenograft model, tissue microarray, and gene network analysis. Int. J. Oncol. 2017;51:204–212. doi: 10.3892/ijo.2017.3993. [DOI] [PubMed] [Google Scholar]
- 104.Cao H.X., Zhu K.X., Fan J.G., Qiao L. Garlic-derived allyl sulfides in cancer therapy. Anticancer Agents Med. Chem. 2014;14:793–799. doi: 10.2174/1871520614666140521120811. [DOI] [PubMed] [Google Scholar]
- 105.Kodali R.T., Eslick G.D. Meta-analysis: Does garlic intake reduce risk of gastric cancer? Nutr. Cancer. 2015;67:1–11. doi: 10.1080/01635581.2015.967873. [DOI] [PubMed] [Google Scholar]
- 106.Bom J., Gunutzmann P., Hurtado E.C.P., Maragno-Correa J.M.R., Kleeb S.R., Lallo M.A. Long-term treatment with aqueous garlic and/or tomato suspensions decreases Ehrlich ascites tumors. Evid. Based Complement. Altern. Med. 2014;2014:381649. doi: 10.1155/2014/381649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith M.T., Guyton K.Z., Gibbons C.F., Fritz J.M., Portier C.J., Rusyn I., DeMarini D.M., Caldwell J.C., Kavlock R.J., Lambert P.F., et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ. Health Perspect. 2016;124:713–721. doi: 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicastro H.L., Ross S.A., Milner J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015;8:181–189. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jakszyn P., Agudo A., Ibanez R., Garcia-Closas R., Pera G., Amiano P., Gonzalez C.A. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J. Nutr. 2004;134:2011–2014. doi: 10.1093/jn/134.8.2011. [DOI] [PubMed] [Google Scholar]
- 110.Milner J.A. Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation—Garlic and carcinogenesis. Adv. Exp. Med. Biol. 2001;492:69–81. doi: 10.1007/978-1-4615-1283-7_7. [DOI] [PubMed] [Google Scholar]
- 111.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Bagul M., Kakumanu S., Wilson T.A. Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J. Med. Food. 2015;18:731–737. doi: 10.1089/jmf.2014.0064. [DOI] [PubMed] [Google Scholar]
- 113.Shin S.S., Song J.H., Hwang B., Noh D.H., Park S.L., Kim W.T., Park S.S., Kim W.J., Moon S.K. HSPA6 augments garlic extract-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; implication for cell cycle dysregulation, signaling pathway alteration, and transcription factor-associated mmp-9 regulation. PLoS ONE. 2017;12:e0171860. doi: 10.1371/journal.pone.0171860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang X.Y., Zhu X.S., Huang W.Z., Xu H.Y., Zhao Z.X., Li S.Y., Li S.Z., Cai J.H., Cao J.M. Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int. Immunopharmacol. 2017;48:135–145. doi: 10.1016/j.intimp.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Xu Y.S., Feng J.G., Zhang D., Zhang B., Luo M., Su D., Lin N.M. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta. Pharmacol. Sin. 2014;35:267–274. doi: 10.1038/aps.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang W., Cheng J.W., Zhu Y.Z. The JNK signaling pathway is a novel molecular target for S-propargyl-l-cysteine, a naturally-occurring garlic derivatives: Link to its anticancer activity in pancreatic cancer in vitro and in vivo. Curr. Cancer Drug Targets. 2015;15:613–623. doi: 10.2174/1568009615666150602143943. [DOI] [PubMed] [Google Scholar]
- 117.Xiao J., Xing F.Y., Liu Y.X., Lv Y., Wang X.G., Ling M.T., Gao H., Ouyang S.Y., Yang M., Zhu J., et al. Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta Pharm. Sin. B. 2018;8:575–586. doi: 10.1016/j.apsb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., Li H.Y., Zhang Z.H., Bian H.L., Lin G. Garlic-derived compound S-allylmercaptocysteine inhibits cell growth and induces apoptosis via the JNK and p38 pathways in human colorectal carcinoma cells. Oncol. Lett. 2014;8:2591–2596. doi: 10.3892/ol.2014.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jung Y., Park H., Zhao H.Y., Jeon R., Ryu J.H., Kim W.Y. Systemic approaches identify a garlic-derived chemical, Z-ajoene, as a glioblastoma multiforme cancer stem cell-specific targeting agent. Mol. Cells. 2014;37:547–553. doi: 10.14348/molcells.2014.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaschula C.H., Hunter R., Cotton J., Tuveri R., Ngarande E., Dzobo K., Schafer G., Siyo V., Lang D., Kusza D.A., et al. The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol. Carcinog. 2016;55:1213–1228. doi: 10.1002/mc.22364. [DOI] [PubMed] [Google Scholar]
- 121.Jikihara H., Qi G.Y., Nozoe K., Hirokawa M., Sato H., Sugihara Y., Shimamoto F. Aged garlic extract inhibits 1,2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol. Rep. 2015;33:1131–1140. doi: 10.3892/or.2014.3705. [DOI] [PubMed] [Google Scholar]
- 122.Petrovic V., Nepal A., Olaisen C., Bachke S., Hira J., Sogaard C.K., Rost L.M., Misund K., Andreassen T., Melo T.M., et al. Anti-cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients. 2018;10:450. doi: 10.3390/nu10040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Charron C.S., Dawson H.D., Albaugh G.P., Solverson P.M., Vinyard B.T., Solano-Aguilar G.I., Molokin A., Novotny J.A. A single meal containing raw, crushed garlic influences expression of immunity- and cancer-related genes in whole blood of humans. J. Nutr. 2015;145:2448–2455. doi: 10.3945/jn.115.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsuura N., Miyamae Y., Yamane K., Nagao Y., Hamada Y., Kawaguchi N., Katsuki T., Hirata K., Sumi S.I., Ishikawa H. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells(1–3) J. Nutr. 2006;136:842S–846S. doi: 10.1093/jn/136.3.842S. [DOI] [PubMed] [Google Scholar]
- 125.Wei Z.H., Shan Y.L., Tao L., Liu Y.P., Zhu Z.J., Liu Z.G., Wu Y.Y., Chen W.X., Wang A.Y., Lu Y. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1 synthesis, and decreases breast cancer metastasis. Mol. Carcinog. 2017;56:2317–2331. doi: 10.1002/mc.22686. [DOI] [PubMed] [Google Scholar]
- 126.Talib W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition. 2017;43:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 127.Raisuddin S., Ahmad S., Fatima M., Dabeer S. Toxicity of anticancer drugs and its prevention with special reference to role of garlic constituents. Ann. Phytomed. 2018;7:13–26. doi: 10.21276/ap.2018.7.1.3. [DOI] [Google Scholar]
- 128.Nasr A.Y., Saleh H.A.M. Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell Int. 2014;14:92. doi: 10.1186/s12935-014-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suddek G.M. Allicin enhances chemotherapeutic response and ameliorates tamoxifen- induced liver injury in experimental animals. Pharm. Biol. 2014;52:1009–1014. doi: 10.3109/13880209.2013.876053. [DOI] [PubMed] [Google Scholar]
- 130.Gatt M.E., Strahilevitz J., Sharon N., Lavie D., Goldschmidt N., Kalish Y., Gural A., Paltiel O.B. A randomized controlled study to determine the efficacy of garlic compounds in patients with hematological malignancies at risk for chemotherapy-related febrile neutropenia. Integr. Cancer Ther. 2015;14:428–435. doi: 10.1177/1534735415588928. [DOI] [PubMed] [Google Scholar]
- 131.Tabari M.A., Ebrahimpour S. Effect of aged garlic extract on immune responses to experimental fibrosarcoma tumor in BALB/c mice. Indian J. Cancer. 2014;51:609–613. doi: 10.4103/0019-509X.175359. [DOI] [PubMed] [Google Scholar]
- 132.Saud S.M., Li W.D., Gray Z., Matter M.S., Colburn N.H., Young M.R., Kim Y.S. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev. Res. 2016;9:607–615. doi: 10.1158/1940-6207.CAPR-16-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qamar A., Siddiqui A., Kumar H. Fresh garlic amelioration of high-fat-diet induced fatty liver in albino rats. J. Pak. Med. Assoc. 2015;65:1102–1107. [PubMed] [Google Scholar]
- 134.Meng X., Li Y., Li S., Gan R.Y., Li H.B. Natural products for prevention and treatment of chemical-induced liver injuries. Compr. Rev. Food Sci. Food Saf. 2018;17:472–495. doi: 10.1111/1541-4337.12335. [DOI] [PubMed] [Google Scholar]
- 135.Meng X., Li S., Li Y., Gan R.Y., Li H.B. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients. 2018;10:1457. doi: 10.3390/nu10101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee K.C., Teng C.C., Shen C.H., Huang W.S., Lu C.C., Kuo H.C., Tung S.Y. Protective effect of black garlic extracts on tert-Butyl hydroperoxide-induced injury in hepatocytes via a c-Jun N-terminal kinase-dependent mechanism. Exp. Ther. Med. 2018;15:2468–2474. doi: 10.3892/etm.2018.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aprioku J.S., Amah-Tariah F.S. Garlic (Allium sativum L.) protects hepatic and renal toxicity of alloxan in rats. Br. J. Pharm. Res. 2017;17:34909. doi: 10.9734/JPRI/2017/34909. [DOI] [Google Scholar]
- 138.Kaur S., Sharma S. A histometric study to assess preventive action of ascorbic acid and garlic on cadmium induced hepatotoxicity in albino mice. Int. J. Pharm. Phytopharmacol. Res. 2015;5:398. [Google Scholar]
- 139.Ko J.W., Park S.H., Lee I.C., Lee S.M., Shin I.S., Kang S.S., Moon C., Kim S.H., Heo J.D., Kim J.C. Protective effects of garlic oil against 1,3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol. Cell. Toxicol. 2016;12:185–195. doi: 10.1007/s13273-016-0023-0. [DOI] [Google Scholar]
- 140.Guan M.J., Zhao N., Xie K.Q., Zeng T. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem. Toxicol. 2018;111:467–473. doi: 10.1016/j.fct.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 141.Lai Y.S., Chen W.C., Ho C.T., Lu K.H., Lin S.H., Tseng H.C., Lin S.Y., Sheen L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014;62:5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]
- 142.Lee H.S., Lee S.J., Yu H.J., Lee J.H., Cho H.Y. Fermentation with Lactobacillus enhances the preventive effect of garlic extract on high fat diet-induced hepatic steatosis in mice. J. Funct. Foods. 2017;30:125–133. doi: 10.1016/j.jff.2016.12.043. [DOI] [Google Scholar]
- 143.Lee H.S., Lim W.C., Lee S.J., Lee S.H., Yu H.J., Lee J.H., Cho H.Y. Hepatoprotective effects of lactic acid-fermented garlic extract against acetaminophen-induced acute liver injury in rats. Food Sci. Biotechnol. 2016;25:867–873. doi: 10.1007/s10068-016-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim H.N., Kang S.G., Roh Y.K., Choi M.K., Song S.W. Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: A double-blind, randomized, placebo-controlled trial. Eur. J. Nutr. 2017;56:1993–2002. doi: 10.1007/s00394-016-1318-6. [DOI] [PubMed] [Google Scholar]
- 145.Siddique A., Iqbal J., Sheikh M.A. Effects of garlic (Allium sativum) on the weights of liver in albino rats. Pak. J. Med. Health Sci. 2015;9:1051–1054. [Google Scholar]
- 146.Chen Y.A., Tsai J.C., Cheng K.C., Liu K.F., Chang C.K., Hsieh C.W. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res. Int. 2018;107:102–109. doi: 10.1016/j.foodres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 147.Ben Hadda T., ElSawy N.A., Header E.A.M., Mabkhot Y.N., Mubarak M.S. Effect of garlic and cabbage on healing of gastric ulcer in experimental rats. Med. Chem. Res. 2014;23:5110–5119. doi: 10.1007/s00044-014-1092-z. [DOI] [Google Scholar]
- 148.Badr G.M., Al-Mulhim J.A. The protective effect of aged garlic extract on nonsteroidal anti-inflammatory drug-induced gastric inflammations in male albino rats. Evid. Based Complement. Altern. Med. 2014;2014:759642. doi: 10.1155/2014/759642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El-Ashmawy N.E., Khedr E.G., El-Bahrawy H.A., Selim H.M. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition. 2016;32:849–854. doi: 10.1016/j.nut.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 150.Shi L.M., Lin Q.L., Li X.H., Nie Y., Sun S.G., Deng X.Y., Wang L., Lu J., Tang Y.P., Luo F.J. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-7 activation. Mol. Nutr. Food Res. 2017;61:1601013. doi: 10.1002/mnfr.201601013. [DOI] [PubMed] [Google Scholar]
- 151.Kaur G., Padiya R., Adela R., Putcha U.K., Reddy G.S., Reddy B.R., Kumar K.P., Chakravarty S., Banerjee S.K. Garlic and resveratrol attenuate diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front. Pharmacol. 2016;7:360. doi: 10.3389/fphar.2016.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Al-brakati A.Y. Protective effect of garlic against diabetic retinopathy in adult albino rats. Res. J. Pharm. Biol. Chem. Sci. 2016;7:2748–2759. [Google Scholar]
- 153.Thomson M., Al-Qattan K.K., Divya J.S., Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016;16:17. doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang J., Zhang X.M., Lan H.L., Wang W.J. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): A meta-analysis of randomized controlled trials. Food Nutr. Res. 2017;61:1377571. doi: 10.1080/16546628.2017.1377571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang C., Li L.H., Yang L.G., Lu H., Wang S.K., Sun G.J. Anti-obesity and hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018;15:43. doi: 10.1186/s12986-018-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y.C., Kao T.H., Tseng C.Y., Chang W.T., Hsu C.L. Methanolic extract of black garlic ameliorates diet-induced obesity via regulating adipogenesis, adipokine biosynthesis, and lipolysis. J. Funct. Foods. 2014;9:98–108. doi: 10.1016/j.jff.2014.02.019. [DOI] [Google Scholar]
- 157.Zhou H., Qu Z., Mossine V.V., Nknolise D.L., Li J.L., Chen Z.Z., Cheng J.L., Greenlief C.M., Mawhinney T.P., Brown P.N., et al. Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells. PLoS ONE. 2014;9:e113531. doi: 10.1371/journal.pone.0113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ho S.C., Su M.S. Evaluating the anti-neuroinflammatory capacity of raw and steamed garlic as well as five organosulfur compounds. Molecules. 2014;19:17697–17714. doi: 10.3390/molecules191117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ebrahimzadeh-Bideskan A.R., Hami J., Alipour F., Haghir H., Fazel A.R., Sadeghi A. Protective effects of ascorbic acid and garlic extract against lead-induced apoptosis in developing rat hippocampus. Metab. Brain Dis. 2016;31:1123–1132. doi: 10.1007/s11011-016-9837-7. [DOI] [PubMed] [Google Scholar]
- 160.Cemil B., Gokce E.C., Kahveci R., Gokce A., Aksoy N., Sargon M.F., Erdogan B., Kosem B. Aged garlic extract attenuates neuronal injury in a rat model of spinal cord ischemia/reperfusion injury. J. Med. Food. 2016;19:601–606. doi: 10.1089/jmf.2016.0018. [DOI] [PubMed] [Google Scholar]
- 161.Thorajak P., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B. Effects of aged garlic extract on cholinergic, glutamatergic and gabaergic systems with regard to cognitive impairment in a β-induced rats. Nutrients. 2017;9:686. doi: 10.3390/nu9070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Semuyaba I., Safiriyu A.A., Tiyo E.A., Niurka R.F. Memory improvement effect of ethanol garlic (A. Sativum) extract in streptozotocin-nicotinamide induced diabetic wistar rats is mediated through increasing of hippocampal sodium-potassium ATPase, glutamine synthetase, and calcium ATPase activities. Evid. Based Complement. Altern. Med. 2017;2017:3720380. doi: 10.1155/2017/3720380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nurmasitoh T., Sari D.C.R., Partadiredja G. The effects of black garlic on the working memory and pyramidal cell number of medial prefrontal cortex of rats exposed to monosodium glutamate. Drug Chem. Toxicol. 2018;41:324–329. doi: 10.1080/01480545.2017.1414833. [DOI] [PubMed] [Google Scholar]
- 164.Zarezadeh M., Baluchnejadmojarad T., Kiasalari Z., Afshin-Majd S., Roghani M. Garlic active constituent S-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur. J. Pharmacol. 2017;795:13–21. doi: 10.1016/j.ejphar.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 165.Nasiri A., Ziamajidi N., Abbasalipourkabir R., Goodarzi M.T., Saidijam M., Behrouj H., Asl S.S. Beneficial effect of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of type 1 diabetic rats. Indian J. Clin. Biochem. 2017;32:329–336. doi: 10.1007/s12291-016-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miltonprabu S., Sumedha N.C., Senthilraja P. Diallyl trisulfide, a garlic polysulfide protects against as-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017;50:107–120. doi: 10.1016/j.intimp.2017.06.011. [DOI] [PubMed] [Google Scholar]