Abstract
While the negative influence of environmental pollution on the respiratory system is well established, especially for people with bronchial hyper-reactivity, the impact of particulate matter on quality of life in asthma patients is not well understood. Three hundred adult asthma patients were recruited for a study; for each patient, the daily concentrations of particulate matter of 2.5 µm or less in diameter (PM2.5) were recorded from air quality monitoring stations. The study was conducted over two weeks. After two weeks, the patients filled out the Asthma Quality of Life Questionnaire (AQLQ), evaluating the quality of their lives throughout the monitored period. Patients exposed to a higher concentration of PM2.5 had significantly lower AQLQ scores. Every 10 µg/m3 of an increase in the concentration of PM2.5 resulted in a decrease of the AQLQ score by 0.16. All domains of quality of life (symptoms, activity limitations, emotional functioning, and environmental stimuli) assessed in the questionnaire were negatively affected by PM2.5. These findings provide an important argument in favor of educating physicians and patients and raising awareness about the detrimental health effects of air pollution. Improving the quality of life of people with asthma requires an immediate and substantial reduction of air pollution.
Keywords: environmental health, PM2.5, quality of life, AQLQ, asthma
1. Introduction
Bronchial asthma is one of the most frequently occurring chronic diseases [1]. Like every chronic disease, it poses a major problem not only in medical terms but also in social and economic terms [2,3,4,5]. As a long-term, incurable disease, it is a source of hardship and limitations for many patients pursuing their everyday activities, negatively impacting the quality of their lives.
The negative influence of particulate matter (PM) on the respiratory system has been known for a long time, especially in the case of patients with bronchial hyper-reactivity [6,7]. However, the evaluation of the impact of particulate matter on the quality of life of patients with bronchial asthma has not yet been widely analyzed. To the best of our knowledge, only limited evidence exists to suggest that fine particles measuring less than 10 µm (PM10) has an impact on the quality of life of patients with bronchial asthma [8], with no conclusive confirmation of the detrimental connection between the quality of life and the concentration of PM10. No studies were conducted concerning particulate matter of 2.5 µm or less in diameter (PM2.5).
PM2.5 (particles which pass through a size-selective inlet with a 50% efficiency cut-off at 2.5 μm aerodynamic diameter) have been classified by International Organization for Standardization as the “high-risk” respirable fraction [9]. PM2.5, also known as respirable dust, is an exceptionally harmful fraction because it penetrates the smallest bronchioles and the alveoli. Therefore, it may interfere with gas exchange inside the lungs and trigger or exacerbate respiratory diseases. Moreover, some fraction of inhaled PM2.5 (most likely the fraction of particles under 0.1 micrometer) may be capable to translocate into blood vessels and then spread with the blood to various tissues and organs [10].
According to WHO, the maximum 24 h average concentration of PM2.5 should not exceed 25 µg/m3. At the same time, while considering PM2.5 as “the most harmful among all atmospheric pollutants”, WHO states there is no safe level, below which we can be sure of the lack of negative effects for human health [11].
In studies conducted to date, researchers evaluated the impact of air pollutants mainly on the aggravation of symptoms of asthma [10,12,13]. However, an evaluation of the relationship between the environment and the quality of life of patients suffering from bronchial asthma has not yet been the subject of a sufficient number of studies. Results of such studies would be important in planning and implementing preventive measures taken in the area of public health, and would lead to the well-being of patients with bronchial asthma. The aim of our study was the evaluation of the impact of PM2.5 air pollution on the self-assessed quality of life of patients with bronchial asthma.
2. Materials and Methods
Patients with bronchial asthma (n = 349) who were treated in two allergy treatment clinics in Krakow, Poland were recruited for the study between June 2013 and May 2015. The diagnosis of asthma was confirmed by a physician (allergy specialist) according to the current guidelines [14]. Participants of the study were adult patients (>18 years old) with partially controlled asthma and were inhabitants of Krakow, in Poland. They declared that they would not leave the city throughout the 14 days of observation. Partly-controlled asthma means that patient had been experiencing one or two of the following features of asthma in the past four weeks: daytime symptoms more than twice a week, night waking due to asthma, rescue medication needed for symptom relief more than twice a week, activity limitation due to asthma [14].
During the first visit, the physician conducted the interview and recorded information about the date of birth, place of residence, education, employment, smoking, having pets, and types of asthma treatment. During the first visit, the patients also signed the informed consent for participation in the study and received a journal for recording the number of hours spent outside and the name of streets that they used for walking or travel each day. Each patient recorded this information every day for two weeks.
For each patient, we recorded the daily concentrations of PM2.5 for each day of observation from all available air quality monitoring stations of the Voivodeship Inspectorate of Environmental Protection in Krakow. Subsequently, each patient was assigned to the station closest to their declared outdoor place of stay, to estimate exposure to PM2.5 during the study. During the second visit (after 14 days) the patients’ quality of life was evaluated using the Standardised Asthma Quality of Life Questionnaire (AQLQ) for adults [15,16,17], which has been commonly used for measuring the asthma-specific quality of life [16,18]. The patients filled out the AQLQ, consisting of 32 questions (concerning the last 14 days). Questions were grouped into four domains: symptoms (12 questions), activity limitations (11 questions), emotional functioning (five questions), and environmental stimuli (four questions).
The patients marked their answers to each question on a seven-degree Likert scale, on which 7 meant “no limitations”, and 1 meant “total limitations” in performing particular activities. The overall score of the AQLQ total was the average of all 32 answers, and the score for a particular domain was the average value for the questions within this domain.
For analysis, we used the data from 300 patients aged 20–80 years old (mean 53, standard deviation/SD/15.3): 145 women (48.3%) and 155 men (51.7%). Forty-nine patients (14%—23 women and 26 men, aged 20–76 years old; mean 51, SD 13.3) were excluded due to leaving the city throughout observation or not filling out/losing the journal of observations. The study was conducted with the approval of the Committee of Bioethics of the Jagiellonian University (number KBET/167/B/2012).
Data were presented as means with standard deviations (SD) or the frequency with percentage distribution, respectively to its measurement scale. Two-week mean PM2.5 concentrations were additionally characterized by their quartile distribution. PM2.5 concentration was analyzed both as a continuous variable and as a categorical variable, according to its quartile distribution. Potential confounders of PM2.5 exposure and AQLQ, such as gender, education, active smoking, employment status, and having pets at home, were controlled in analyses. Associations between these nominal variables and PM2.5 categories were verified using a chi-square test or the exact Fisher test when appropriate. Differences in the age of patients exposed to different concentrations of PM2.5 categories (different quartiles) were tested using one-way ANOVA (equality of variances has been verified with Levene’s test and normal distribution in subgroups with the Kołmogorow–Smirnow test). The nonparametric trend in AQLQ values across PM2.5 categories was checked.
The potential impact of airborne PM2.5 on the quality of life of asthma patients (AQLQ total and its four domains) was estimated with linear regression models using the two-week mean of PM2.5 concentrations both as a categorical variable (according to quartile distribution and by the value of 25 µg/m3—the 24 h threshold suggested by WHO, which we used since there is no two-week guideline value) and as a continuous variable with a unit of 10 µg/m3, both adjusted to age (in years), gender, and university education (yes versus no) smoking (yes versus no) and season of measurement (cold: October to April, versus warm: May–September). Among the variables considered as potential confounders, only age and university education were significantly associated with AQLQ total and its domains in univariate analysis. Gender, smoking, and season of measurement were a priori added to models.
The AQLQ total was classified into three categories: good quality of life (score: 6–7), reduced quality of life (score: 4–5), and poor quality of life (score: 1–3), ordered from good as “1” to poor as ”3”. PM2.5 exposure level (both as a categorical and continuous variable) was used to assess its impact on poor asthma quality of life using ordered logistic regression models adjusted to age, age2, gender, and university education. The same analyses were conducted for all four AQLQ domains, as four separate tests. The proportional odds assumption was verified using the Brant test. The term age2 was added as age had not complied with the proportional odds assumption. The ordered logistic regression models were not adjusted for smoking as it did not affect the risk of poorer asthma life quality. Besides, only 31 (10%) patients were smokers. These patients were attempting to quit smoking and they reported smoking no more than between one to three cigarettes per day at the time of observation. All tests were two-tailed, and significance was set at p < 0.05. All analyses were performed using STATA/IC 13.1 (StataCorp LP, College Station, TX, USA).
3. Results
PM2.5 exposure (mean in a two-week period) averaged 45.1 µg/m3 (median 46; range: 9.6–90.1 µg/m3) and only 75 (25%) patients were exposed to values lower or equal to 25 µg/m3, the 24 h threshold recommended by WHO. PM2.5 measurements were equally distributed across seasons. There were no statistically significant differences between patients in the number of hours spent outdoors. In general, all declared that they spend 2–3 h a day outdoors, on average.
Groups of patients with different average PM2.5 exposure levels (classified by quartiles of PM2.5) did not differ significantly in gender, education level (university versus lower), employment status, or keeping pets at home. Nevertheless, patients exposed to higher levels of PM2.5 were older and the studied subgroups were significantly different according to active smoking prevalence (Table 1). The mean asthma quality of life (AQLQ total) in the studied group was 5.0 (range: 2–7). Declining trends in total and specific domains of asthma quality of life were observed across increasing levels of airborne PM2.5 exposure (Table 1).
Table 1.
Characteristics of the study group: all participants and participants divided in four groups based on quartiles of exposure to PM2.5 levels.
| Characteristics of the Study Group | Total n = 300 |
Low Exposure (≤25 µg/m3) n = 75 |
High Exposure (25.1–46 µg/m3) n = 75 |
Very High Exposure (46.1–58 µg/m3) n = 75 |
Extremely High Exposure (>58 µg/m3) n = 75 |
p |
|---|---|---|---|---|---|---|
| women | 145 (48.3%) | 36 (48.0%) | 30 (40.0%) | 40 (53.3%) | 39 (52.0%) | 0.356 |
| men | 155 (51.7%) | 39 (52.0%) | 45 (60.0%) | 35 (46.7%) | 36 (48.0%) | |
| age (years) | 53 ± 15.3 | 49.8 ± 15.7 | 51.3 ± 15.5 | 52.1 ± 13.3 | 59.0 ± 15.3 | 0.001 |
| university education | 127 (42.3%) | 39 (52%) | 35 (46.7%) | 29 (38.7%) | 24 (32%) | 0.068 |
| active smoker | 31 (10.3%) | 1 (1.3%) | 11 (14.7%) | 16 (21.3%) | 3 (4.0%) | <0.001 |
| current employment | ||||||
| student | 11 (3.7%) | 3 (4.0%) | 4 (5.3%) | 3 (4.1%) | 1 (1.3%) | 0.124 |
| employed | 172 (57.5%) | 47 (62.7%) | 48 (64.0%) | 43 (58.1%) | 34 (45.3%) | |
| unemployed | 27 (9.0%) | 7 (9.3%) | 5 (6.7%) | 9 (12.2%) | 6 (8.0%) | |
| retired | 89 (29.8%) | 18 (24.0%) | 18 (24.0%) | 19 (25.7%) | 34 (45.3%) | |
| missing | 1 | |||||
| keeping pets at home | 133 (43.3%) | 34 (45.3%) | 33 (75%) | 28 (37.3%) | 38 (50.7%) | 0.433 |
| quality of life in asthma | ||||||
| AQLQ * total | 5.0 ± 1.29 | 5.6 ± 1.11 | 5.1 ± 1.14 | 4.8 ± 1.15 | 4.4 ± 1.43 | <0.001 |
| AQLQ symptoms | 5.2 ± 1.34 | 5.7 ± 1.23 | 5.3 ± 1.13 | 5.1 ± 1.28 | 4.6 ± 1.48 | <0.001 |
| AQLQ activity limitation | 4.9 ± 1.37 | 5.7 ± 1.14 | 5.0 ± 1.25 | 4.7 ± 1.18 | 4.3 ± 1.51 | <0.001 |
| AQLQ emotional function | 5.0 ± 1.53 | 5.6 ± 1.21 | 5.1 ± 1.36 | 4.9 ± 1.51 | 4.4 ± 1.78 | <0.001 |
| AQLQ environmental stimuli | 4.6 ± 1.33 | 5.4 ± 1.35 | 4.6 ± 1.22 | 4.3 ± 1.22 | 4.2 ± 1.2 | <0.001 |
* Asthma Quality of Life Questionnaire score.
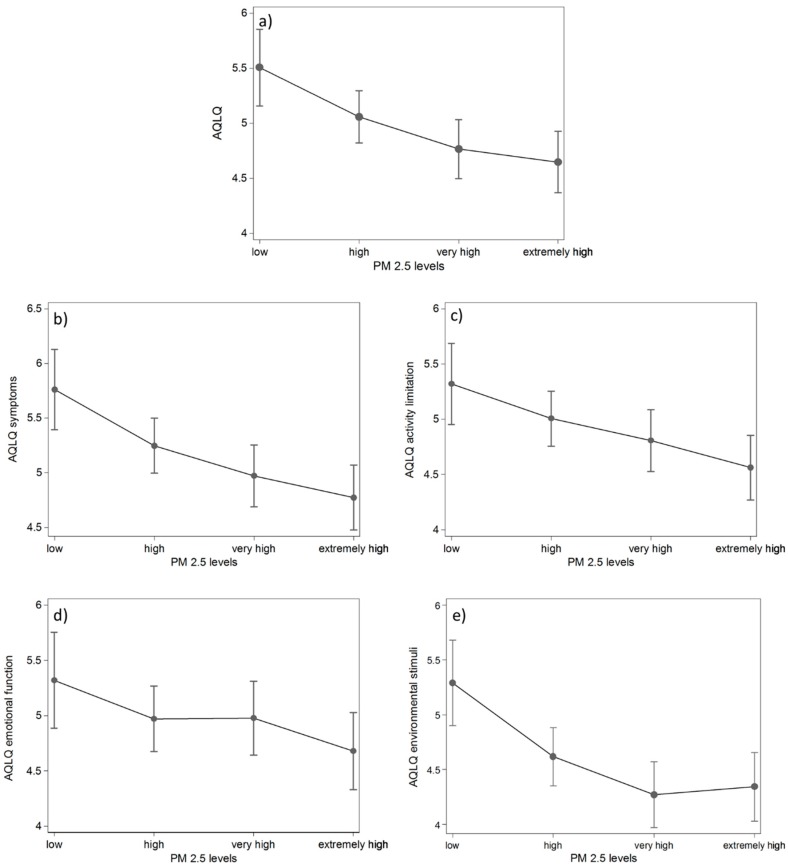
Table 2 shows the effect of categorized PM2.5 exposure on asthma quality of life, adjusted to potential confounders. First, we compared patients exposed to the PM2.5 values above 25 µg/m3 with those exposed to lower pollution levels (≤25 µg/m3). A reduction of 0.6 in the AQLQ total was observed in those exposed to the higher levels compared to those with PM2.5 exposure ≤25 µg/m3, while the deficit in specific domains was statistically significant in the case of AQLQ symptoms and AQLQ environmental stimuli. When quartiles of PM2.5 exposure were compared, after adjustments for the confounders, the effect of exposure became significant in all domains (Table 2). The observed effect of the increased level of exposure was associated with reduced values in the AQLQ total in all higher-level quartiles of exposure, in comparison to low-level quartiles. A similar decrease was observed in AQLQ symptoms and AQLQ environmental stimuli domains. In AQLQ activity limitations and emotional functioning, quality of life differed significantly only between the extremely high-level quartile of exposure and low-level quartile. Predicted margins of AQLQ and its domains according to PM2.5 levels, categorized by quartile distribution, and adjusted to age, gender, active smoking, university education, and season of measurement, are presented in Figure 1.
Table 2.
Impact of categorized concentration of PM2.5 on asthma quality of life (AQLQ) and the risk of poor asthma quality of life, adjusted to potential confounders.
| Concentration of PM2.5 Categorized According to 24 h Threshold (>25 µg/m3, n = 225 versus ≤ 25 µg/m3, n = 75) and According to the Quartiles |
Asthma Quality of Life on Original Scale | Categorized Asthma Quality of Life (Ordered from Good as “1” to Poor as “3”) | ||||
|---|---|---|---|---|---|---|
| B * | 95% CI for B | p a | OR | 95% CI ** for OR | p b | |
| AQLQ total | ||||||
| >25 µg/m3 versus ≤ 25 µg/m3 | −0.56 | −0.98; −0.13 | 0.011 | 5.12 | 2.72; 9.63 | <0.001 |
| high versus low | −0.45 | −0.88; −0.01 | 0.044 | 2.68 | 1.28; 5.60 | 0.009 |
| very high versus low | −0.74 | −1.24; −0.24 | 0.004 | 7.34 | 3.50; 15.42 | <0.001 |
| extremely high versus low | −0.86 | −1.37; −0.34 | <0.001 | 7.17 | 3.39; 15.20 | <0.001 |
| AQLQ symptoms | ||||||
| >25 µg/m3 versus ≤ 25 µg/m3 | −0.63 | −1.08; −0.17 | 0.007 | 3.51 | 1.82; 6.77 | 0.001 |
| high versus low | −0.50 | −0.97; −0.05 | 0.029 | 2.24 | 1.04; 4.84 | 0.039 |
| very high versus low | −0.79 | −1.32; −0.26 | 0.003 | 3.59 | 1.67; 7.72 | 0.001 |
| extremely high versus low | −0.99 | −1.53; −0.44 | <0.001 | 5.30 | 2.48; 11.31 | <0.001 |
| AQLQ activity limitation | ||||||
| >25 µg/m3 versus ≤ 25 µg/m3 | −0.41 | −0.86; 0.04 | 0.074 | 5.74 | 3.07; 10.73 | <0.001 |
| high versus low | −0.31 | −0.77; 0.14 | 0.177 | 3.10 | 1.49; 6.43 | 0.002 |
| very high versus low | −0.51 | −1.03; 0.01 | 0.055 | 8.05 | 3.84; 16.90 | <0.001 |
| extremely high versus low | −0.76 | −1.30; −0.22 | 0.006 | 8.01 | 3.81; 16.85 | <0.001 |
| AQLQ emotional function | ||||||
| >25 µg/m3 versus ≤ 25 µg/m3 | −0.38 | −0.91; 0.14 | 0.155 | 2.30 | 1.30; 4.07 | 0.004 |
| high versus low | −0.35 | −0.89; 0.19 | 0.206 | 1.94 | 0.98; 3.84 | 0.055 |
| very high versus low | −0.34 | −0.96; 0.28 | 0.279 | 2.72 | 1.36; 5.43 | 0.004 |
| extremely high versus low | −0.64 | −1.28; 0.001 | 0.050 | 2.33 | 1.17; 4.62 | 0.016 |
| AQLQ environmental stimuli | ||||||
| >25 µg/m3 versus ≤ 25 µg/m3 | −0.78 | −1.25; −0.30 | <0.001 | 4.78 | 2.72; 8.38 | <0.001 |
| high versus low | −0.67 | −1.16; −0.19 | 0.007 | 3.24 | 1.67; 6.26 | <0.001 |
| very high versus low | −1.02 | −1.58; −0.46 | <0.001 | 6.06 | 3.04; 12.09 | <0.001 |
| extremely high versus low | −0.95 | −1.53; −0.37 | 0.001 | 6.08 | 3.02; 12.24 | <0.001 |
* B—standardized coefficient (beta); ** CI— confidence intervals (a) adjusted to age, gender, active smoking, university education and season of measurement; (b) adjusted to age, age2, gender, and university education.
Figure 1.
Margins with 95% confidence intervals of (a) the Asthma Quality of Life Questionnaire (AQLQ) total, (b) AQLQ symptoms, (c) activity limitations, (d) emotional functioning, and (e) environmental stimuli and its domains according to PM2.5 levels (linear prediction adjusted to age, gender, active smoking, university education, and season of measurement).
To assess the effects of PM2.5 exposure on the risk of poorer outcomes in asthma quality of life (total and at specific domains) multivariable ordinal logistic regression models were applied. It was observed that high levels of PM2.5 exposure were associated with over two-fold higher risk of poorer outcomes in AQLQ total (OR = 2.68, 95% CI = 1.28; 5.60, p = 0.009), while the very high and extremely high exposure were associated with over seven-fold higher (OR = 7.34, 95% CI = 3.50; 15.42, p < 0.001 and OR = 7.17, 95%CI = 3.39; 15.20, p < 0.001, respectively), compared to the low levels of PM2.5 exposure. Similar results were seen in particular domains. The risk of poorer quality of life in AQLQ symptoms reached values from 2.2 times higher for high levels of PM2.5 to 5.3 for the extremely high levels compared to the low levels. Higher risk of poor outcomes in AQLQ environmental stimuli was 3.2 times higher for high levels of exposure and 6 times higher for very and extremely high exposure compared to the exposure to low levels (Table 2).
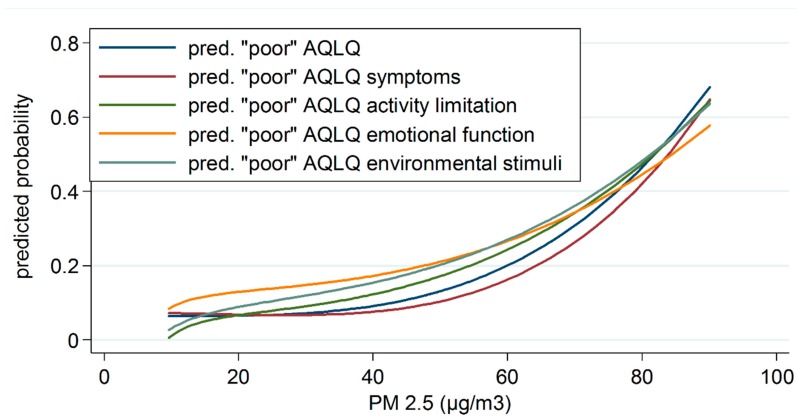
The effect of a continuously expressed level of PM2.5 exposure on asthma quality of life, adjusted to potential confounders, is shown in Table 3. The risk of poorer asthma quality of life increased with higher level of PM2.5 exposure. A 10 µg/m3 increase in PM2.5 was significantly associated with a 46% higher risk of poorer total asthma quality of life (OR = 1.46, 95% CI =1.29; 1.66, p < 0.001) after adjustment for age, gender, and university education. Similar results were observed in each domain, where a 10 µg/m3 increase in PM2.5 increased the risk of poorer outcomes in all AQLQ domains, from 21% in AQLQ emotional functioning to 46% in AQLQ activity limitation (Table 3). Predicted probabilities of “poor” asthma quality of life according to PM2.5 exposure in all AQLQ domains are presented in Figure 2.
Table 3.
Impact of mean 14-day concentration of PM2.5 (increasing unit: 10 µg/m3) on asthma quality of life and the risk of poor asthma quality of life, adjusted to potential confounders.
| PM2.5 Concentration | Asthma Quality of Life on Original Scale | Categorized Asthma Quality of Life (Ordered from Good as “1” to Poor as ”3”) | ||||
|---|---|---|---|---|---|---|
| B * | 95% CI for B | p a | OR | 95% CI for OR | p b | |
| AQLQ total | ||||||
| 14-day concentrations of PM2.5 | −0.16 | −0.24; −0.07 | <0.001 | 1.46 | 1.29; 1.66 | <0.001 |
| AQLQ symptoms | ||||||
| 14-day concentrations of PM2.5 | −0.18 | −0.27; −0.09 | <0.001 | 1.37 | 1.20; 1.55 | <0.001 |
| AQLQ activity limitation | ||||||
| 14-day concentrations of PM2.5 | −0.14 | −0.23; −0.06 | <0.001 | 1.46 | 1.29; 1.66 | <0.001 |
| AQLQ emotional function | ||||||
| 14-day concentrations of PM2.5 | −0.15 | −0.26; −0.05 | 0.004 | 1.21 | 0.91; 1.20 | 0.002 |
| AQLQ environmental stimuli | ||||||
| 14-day concentrations of PM2.5 | −0.14 | −0.24; −0.05 | 0.003 | 1.40 | 1.24; 1.58 | <0.001 |
* B—standardized coefficient (beta); (a) adjusted to age, gender, active smoking, university education, and season of measurement; (b) adjusted to age, age2, gender, and university education.
Figure 2.
Predicted probabilities of “poor” asthma quality of life (total and for specific domains) according to PM2.5 levels.
4. Discussion
In our study, we compared the quality of life of patients exposed to various concentrations of environmental PM2.5 and we observed that increased levels of PM2.5 were related to lower asthma quality of life. Total quality of life and all of its four domains (symptoms, activity limitations, emotional functioning, and environmental stimuli) assessed in the study were significantly reduced as a result of air pollution.
It seems that in evaluating the quality of life it is not just the fact of exceeding the WHO guideline value of PM2.5 exposure that is significant, but also the degree of exceeding the WHO guideline value as well—in situations where the WHO guideline value was exceeded multiple times all domains of quality of life show lower scores. The most easily influenced factors of quality of life were symptoms of asthma and environmental stimuli, which decrease with every case of exceeding the WHO guideline value.
The influence of particulate pollutants, including the PM2.5 fraction, on different aspects of health has been addressed by many studies. The negative impact of PM2.5 on the respiratory system is very well documented by studies, showing connections between concentrations of PM2.5 and longevity [19,20], frequency of lung carcinoma [21,22], the incidence of respiratory diseases, and exacerbations of such diseases and cardiovascular diseases [23,24,25].
Mathematical models estimated that exposure to PM2.5 has decreased average longevity by 8.6 months in Estonia [19], and other studies documented an increase of longevity by as much as 0.35 years for every 10 µg/m3 of reduction in the concentration of this fraction of particles [20]. Long-term exposure to particulate matter PM2.5 leads to increased mortality due to lung cancer [26,27] and an increased incidence of circulatory diseases [28]. In the United States of America, overall mortality and morbidity for cardiovascular diseases and pulmonary diseases (including lung cancer) increased by 4%, 6%, and 8% respectively for every additional 10 µg/m3 of exposure to PM2.5 after excluding other risk factors [29]. It has also been documented that the 15–27% increase in mortality due to lung cancer was connected with the increase in the concentration of PM2.5 by 10 µg/m3, and the risk was even higher for patients with chronic lung diseases [30]. The results of 11 cohort studies in Europe have shown that the hazard ratio for lung adenocarcinoma was 1.55 for every 5 µg/m3 increase in PM2.5 [21], and in six regions of Japan, studies have shown a relationship between exposure to increased concentrations of PM2.5 and the incidence of lung cancer [22].
The negative impact of PM2.5 on the respiratory system is confirmed by a systematic review of epidemiological studies [31]. Other analyses have shown that the increase of PM2.5 by 10 µg/m3 caused a 2.07% increase in the incidence of respiratory diseases and an 8% increase in the hospitalization rate [32,33]. Among the residents of the Chinese canton Guangzhou, a 12.07% increase in the incidence of respiratory diseases for every 100 µg/m3 of increase in PM2.5 was described [34].
Air pollution causes oxidative stress, leading to inflammatory responses of respiratory tracts and bronchial hypersensitivity—typical features of asthma [35]. Moreover, it has been proven that long-term exposure to pollutants from motor vehicle exhaust is related to the development of asthma [10,36,37]. Sudden increases in air pollution cause the aggravation of symptoms of asthma and an increase in the number of hospital admissions in both children and adults [10,12,13]. Studies on the influence of PM2.5 have demonstrated a relationship between the increased concentration of this fraction of particulate matter and the incidence of new cases of bronchial asthma [38], as well as the deterioration of control, with necessary medical intervention in hospital emergency wards [23,24,39,40].
However, previous studies did not investigate the influences of concentrations of PM2.5 on the quality of life of asthma patients. On the one hand, it may seem that widely available diagnostic methods are sufficient to evaluate the control of bronchial asthma, even though they do not reflect a complete evaluation of the patient’s health, which is influenced not only by physical problems but also by psychological and social issues. That is why, on the other hand, the evaluation of the quality of life, especially in chronic diseases, seems to be important, though not always appreciated. It should be emphasized that quality of life is a crucial element of the contemporary understanding of health [41]. Thus, it is important to evaluate various factors that may impact the quality of life. Among such factors are symptoms of illnesses, limitations of activity (physical, social, professional, emotional), and sleep disorders, but also environmental hazards, including particulate pollutants.
Anecdotal evidence suggests that medical doctors specializing in asthma and allergy often register a correlation between a patient’s worse state of being and periods of increased air pollution, but it is difficult to determine whether this is a result of publicity by a media awareness of air pollution, or if the patients’ quality of life is actually decreased at that time. In our study, we recruited patients that were under the regular care of an allergy clinic. It was a fairly homogenous group, that is, patients with partially controlled asthma, well educated about asthma (e.g., recognizing and controlling symptoms of the disease, with knowledge about a type and use of medication) and systematically taking their medication.
Our study had some limitations. We assessed individual exposure based on PM2.5 recorded for the city, while more precise methods of evaluating PM2.5 exposure are potentially available. For example, patients could carry mobile dust monitors. However, technical and financial limitations related to the equipment and patients’ compliance would make such a study expensive and difficult to conduct, especially on a large group of participants. More sophisticated models could be used to assess personal exposure [42,43,44]. Air quality is changing over space and time and people travel and spend time at various locations. Therefore, in future studies more detailed data should be used, for example, the duration of time spent outside in each location and mode and routes of travel would make it easier to capture the full range of personal exposure.
Moreover, in our models, we used two-week mean values of PM2.5 concentration. The aggravation of symptoms of asthma is often caused by sudden increases in air pollution. Therefore, the peak measurements seem more relevant for exacerbations of asthma than the 14 days average. However, it is worth emphasizing that the quality of life of patients with bronchial asthma does not solely depend on acute asthma attacks. Since AQLQ contains questions concerning the last 14 days, we used two-week mean values of PM2.5 concentration as an exposure indicator metric. It is also worth noting that since there is no guideline value for a two-week average PM2.5 concentration, we used the WHO guideline value, which is a 24 h average value as a limit value. Future studies should also consider other pollutants, since there is growing evidence suggesting the synergistic effect of multi-pollutant exposures [45,46]. Finally, people spend most of their time indoors. For example, our participants spent only 2–3 h a day outdoors, on average. For these reasons, indoor sources of air pollution should be taken into account. However, we have recently shown that outdoor PM2.5 concentration strongly predicts indoor concentration [47].
Despite all these limitations, we have been able to show that even relatively simple information about PM2.5 concentration, which is easily accessible for citizens, from air quality monitoring stations may predict the quality of life of asthma patients. Being aware that there is no minimum level of safe exposure to PM2.5, physicians taking care of asthma patients should pay special attention to the course of illness and control of patients with bronchial asthma to air pollution, especially in places where high concentrations of PM2.5 occur during many days over the year.
5. Conclusions
Exposure to increased values of PM2.5 impacts the quality of life among people with exceptional predispositions, such as bronchial asthma patients, which serves as an important argument in favor of educational activities, especially concerning public health, and raising awareness of the importance of the issue of air pollution.
The deterioration in the quality of life of patients with asthma control and medication suggests the need for special control throughout the illness during periods of exposure to increased concentrations of PM2.5, and it is a fact that should be remembered both by doctors and patients themselves.
Besides taking measures for the improvement in the quality of air, it seems equally important to raise awareness about sources of air pollution, factors influencing concentration values, and possible methods of reducing ambient and personal exposure, especially among patients with bronchial hyper-reactivity.
The substantial reduction of air pollution should be the first and immediate request. Appropriate actions should be taken by governmental organizations, local authorities, and inhabitants. Education and raising awareness about the health risk of high-level PM2.5 should be a priority for public health experts.
Acknowledgments
We are grateful to all participants who generously devoted their time to take part in this study. We also thank Magdalena Klimek, Ludwik Odrzywołek, Nikodem Targosz, Jowita Pilch and Iwona Klęk.
Author Contributions
M.Ś., A.G. and G.J. designed the study, M.Ś. collected the data, and wrote the first version of the paper, A.G. and M.Ś. performed the analysis; all authors commented on and approved the manuscript.
Funding
This research was funded by National Science Centre; DEC-2011/03/B/NZ7/00644.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: Executive summary of the GINA Dissemination Committee Report. Allergy Eur. J. Allergy Clin. Immunol. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Ehteshami-Afshar S., Fitzgerald J.M., Doyle-Waters M.M., Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2016;20:11–23. doi: 10.5588/ijtld.15.0472. [DOI] [PubMed] [Google Scholar]
- 3.Ferro M.A., Van Lieshout R.J., Scott J.G., Alati R., Mamun A.A., Dingle K. Condition-specific associations of symptoms of depression and anxiety in adolescents and young adults with asthma and food allergy. J. Asthma. 2016;53:282–288. doi: 10.3109/02770903.2015.1104694. [DOI] [PubMed] [Google Scholar]
- 4.Sundbom F., Malinovschi A., Lindberg E., Alving K., Janson C. Effects of poor asthma control, insomnia, anxiety and depression on quality of life in young asthmatics. J. Asthma. 2016;53:398–403. doi: 10.3109/02770903.2015.1126846. [DOI] [PubMed] [Google Scholar]
- 5.Pawankar R., Mellon M., Parasuraman B., Haahtela T., Tuomisto L., Pietinalho A., Klaukka T., Erhola M., Kaila M., Nieminen M., et al. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014;7:12. doi: 10.1186/1939-4551-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penttinen P., Timonen K.L., Tiittanen P., Mirme A., Ruuskanen J., Pekkanen J. Number concentration and size of particles in urban air: Effects on spirometric lung function in adult asthmatic subjects. Environ. Health Perspect. 2001;109:319–323. doi: 10.1289/ehp.01109319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor G.T., Neas L., Vaughn B., Kattan M., Mitchell H., Crain E.F., Evans R., Gruchalla R., Morgan W., Stout J., et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J. Allergy Clin. Immunol. 2008;121:1133–1139. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Ścibor M., Balcerzak B., Czernecka Ż., Malinowska-Cieślik M. Assessment of life quality in patients with bronchial asthma residing in Krakow in the areas of varying concentrations of particulate matter (PM10) Med. Śr. 2015;18:45–53. [Google Scholar]
- 9.International Organization for Standardization . ISO 7708: 1995-Air Quality-Particle Size Fraction Definitions for Health-Related Sampling. ISO; Geneva, Switzerland: 1995. [Google Scholar]
- 10.Anderson H.R., Favarato G., Atkinson R.W. Long-term exposure to air pollution and the incidence of asthma: Meta-analysis of cohort studies. Air Qual. Atmos. Health. 2013;6:47–56. doi: 10.1007/s11869-011-0144-5. [DOI] [Google Scholar]
- 11.World Health Organization (WHO) Air quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment. World Health Organization; Geneva, Switzerland: 2006. pp. 1–22. [Google Scholar]
- 12.Dick S., Friend A., Dynes K., AlKandari F., Doust E., Cowie H., Ayres J.G., Turner S.W. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4:e006554. doi: 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquemin B., Siroux V., Sanchez M., Carsin A.-E., Schikowski T., Adam M., Bellisario V., Buschka A., Bono R., Brunekreef B., et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE) Environ. Health Perspect. 2015;123:613. doi: 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma—GINA; Fontana, WI, USA: 2015. [Google Scholar]
- 15.Juniper E.F., Sonia Buist A., Cox F.M., Ferrie P.J., King D.R. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 16.Juniper E.F., Norman G.R., Cox F.M., Roberts J.N. Comparison of the standard gamble, rating scale, AQLQ and SF-36 for measuring quality of life in asthma. Eur. Respir. J. 2001;18:38–44. doi: 10.1183/09031936.01.00088301. [DOI] [PubMed] [Google Scholar]
- 17.Juniper E.F., Guyatt G.H., Ferrie P.J., Griffith L.E. Measuring Quality of Life in Asthma. Am. Rev. Respir. Dis. 2013;147:832–838. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 18.Kaambwa B., Chen G., Ratcliffe J., Iezzi A., Maxwell A., Richardson J. Mapping between the Sydney Asthma Quality of Life Questionnaire (AQLQ-S) and five multi-attribute utility instruments (MAUIs) Pharmacoeconomics. 2017;35:111–124. doi: 10.1007/s40273-016-0446-4. [DOI] [PubMed] [Google Scholar]
- 19.Orru H., Maasikmets M., Lai T., Tamm T., Kaasik M., Kimmel V., Orru K., Merisalu E., Forsberg B. Health impacts of particulate matter in five major Estonian towns: Main sources of exposure and local differences. Air Qual. Atmos. Health. 2011;4:247–258. doi: 10.1007/s11869-010-0075-6. [DOI] [Google Scholar]
- 20.Correia A.W., Pope C.A., 3rd, Dockery D.W., Wang Y., Ezzati M., Dominici F. Effect of air pollution control on life expectancy in the United States: An analysis of 545 U.S. counties for the period from 2000 to 2007. Epidemiology. 2013;24:23–31. doi: 10.1097/EDE.0b013e3182770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raaschou-Nielsen O., Andersen Z.J., Beelen R., Samoli E., Stafoggia M., Weinmayr G., Hoffmann B., Fischer P., Nieuwenhuijsen M.J., Brunekreef B., et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 22.Katanoda K., Sobue T., Satoh H., Tajima K., Suzuki T., Nakatsuka H., Takezaki T., Nakayama T., Nitta H., Tanabe K., et al. An Association between Long-Term Exposure to Ambient Air Pollution and Mortality from Lung Cancer and Respiratory Diseases in Japan. J. Epidemiol. 2011;21:132–143. doi: 10.2188/jea.JE20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Y., Xiang X., Juan J., Sun K., Song J., Cao Y., Hu Y. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ. Pollut. 2017;230:227–233. doi: 10.1016/j.envpol.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Ding L., Zhu D., Peng D., Zhao Y. Air pollution and asthma attacks in children: A case–crossover analysis in the city of Chongqing, China. Environ. Pollut. 2017;220:348–353. doi: 10.1016/j.envpol.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation: Available Evidence for the Future Update of the WHO Global Air Quality Guidelines (AQGs) 2015. [(accessed on 12 July 2019)]; Available online: http://www.euro.who.int/__data/assets/pdf_file/0013/301720/Evidence-future-update-AQGs-mtg-report-Bonn-sept-oct-15.pdf.
- 26.Harrison R.M., Smith D.J.T., Kibble A.J. What is responsible for the carcinogenicity of PM2. 5? Occup. Environ. Med. 2004;61:799–805. doi: 10.1136/oem.2003.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin M., Koutrakis P., Schwartz J. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19:680. doi: 10.1097/EDE.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am. J. Epidemiol. 2000;151:440–448. doi: 10.1093/oxfordjournals.aje.a010228. [DOI] [PubMed] [Google Scholar]
- 29.Pope C.A., III, Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K., Thurston G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner M.C., Krewski D., Pope C.A., Chen Y., Gapstur S.M., Thun M.J. Long-term Ambient Fine Particulate Matter Air Pollution and Lung Cancer in a Large Cohort of Never-Smokers. Am. J. Respir. Crit. Care Med. 2011;184:1374–1381. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- 31.Xing Y.F., Xu Y.H., Shi M.H., Lian Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanobetti A., Franklin M., Koutrakis P., Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ. Health A Glob. Access Sci. Source. 2009;8:58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominici F., Peng R.D., Bell M.L., Pham L., McDermott A., Zeger S.L., Samet J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N., Peng X. Relationship between air pollutant and daily hospital visits for respiratory diseases in Guangzhou: A time-series study. J. Environ. Health. 2009;26:1077–1080. [Google Scholar]
- 35.Auerbach A., Hernandez M.L. The effect of environmental oxidative stress on airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2012;12:133–139. doi: 10.1097/ACI.0b013e32835113d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasana J., Dillikar D., Mendy A., Forno E., Ramos Vieira E. Motor vehicle air pollution and asthma in children: A meta-analysis. Environ. Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Searing D.A., Rabinovitch N. Environmental pollution and lung effects in children. Curr. Opin. Pediatr. 2011;23:314–318. doi: 10.1097/MOP.0b013e3283461926. [DOI] [PubMed] [Google Scholar]
- 38.Loftus C., Yost M., Sampson P., Arias G., Torres E., Vasquez V.B., Bhatti P., Karr C. Regional PM2. 5 and asthma morbidity in an agricultural community: A panel study. Environ. Res. 2015;136:505–512. doi: 10.1016/j.envres.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorai A.K., Tuluri F., Tchounwou P.B. A GIS based approach for assessing the association between air pollution and asthma in New York State, USA. Int. J. Environ. Res. Public Health. 2014;11:4845–4869. doi: 10.3390/ijerph110504845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Q., Li X., Wang S., Wang C., Huang F., Gao Q., Wu L., Tao L., Guo J., Wang W., et al. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS ONE. 2016;11:e0153099. doi: 10.1371/journal.pone.0153099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith K.W., Avis N.E., Assmann S.F. Distinguishing between quality of life and health status in quality of life research: A meta-analysis. Qual. Life Res. 1999;8:447–459. doi: 10.1023/A:1008928518577. [DOI] [PubMed] [Google Scholar]
- 42.Crabbe H., Barber A., Bayford R., Hamilton R., Jarrett D., Machin N. The use of a European telemedicine system to examine the effects of pollutants and allergens on asthmatic respiratory health. Sci. Total Environ. 2004;334:417–426. doi: 10.1016/j.scitotenv.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 43.Dewulf B., Neutens T., Lefebvre W., Seynaeve G., Vanpoucke C., Beckx C., Van de Weghe N. Dynamic assessment of exposure to air pollution using mobile phone data. Int. J. Health Geogr. 2016;15:14. doi: 10.1186/s12942-016-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khreis H., Nieuwenhuijsen M. Traffic-related air pollution and childhood asthma: Recent advances and remaining gaps in the exposure assessment methods. Int. J. Environ. Res. Public Health. 2017;14:312. doi: 10.3390/ijerph14030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billionnet C., Sherrill D., Annesi-Maesano I. Estimating the health effects of exposure to multi-pollutant mixture. Ann. Epidemiol. 2012;22:126–141. doi: 10.1016/j.annepidem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Olstrup H., Johansson C., Forsberg B., Tornevi A., Ekebom A., Meister K. A Multi-Pollutant Air Quality Health Index (AQHI) Based on Short-Term Respiratory Effects in Stockholm, Sweden. Int. J. Environ. Res. Public Health. 2019;16:105. doi: 10.3390/ijerph16010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scibor M. Are we safe inside? Indoor air quality in relation to outdoor concentration of PM 10 and PM 2.5 and to characteristics of homes. Sustain. Cities Soc. 2019;48:101537. doi: 10.1016/j.scs.2019.101537. [DOI] [Google Scholar]