Abstract
Inflammatory bowel disease (IBD) is a chronic and relapsing intestinal inflammatory condition, hallmarked by a disturbance in the bidirectional interaction between gut and brain. In general, the gut/brain axis involves direct and/or indirect communication via the central and enteric nervous system, host innate immune system, and particularly the gut microbiota. This complex interaction implies that IBD is a complex multifactorial disease. There is increasing evidence that stress adversely affects the gut/microbiota/brain axis by altering intestinal mucosa permeability and cytokine secretion, thereby influencing the relapse risk and disease severity of IBD. Given the recurrent nature, therapeutic strategies particularly aim at achieving and maintaining remission of the disease. Alternatively, these strategies focus on preventing permanent bowel damage and concomitant long-term complications. In this review, we discuss the gut/microbiota/brain interplay with respect to chronic inflammation of the gastrointestinal tract and particularly shed light on the role of stress. Hence, we evaluated the therapeutic impact of stress management in IBD.
Keywords: IBD, gastrointestinal tract, microbiota, brain, interplay, stress
1. Introduction
Inflammatory bowel disease (IBD) is a chronic and relapsing disorder [1], including Crohn’s disease and ulcerative colitis. While Crohn’s disease is characterized by transmural inflammation in any part of the gastrointestinal tract, ulcerative colitis is affecting the mucosal layer of the colon and rectum. Similar to other immune-mediated chronic diseases, such as rheumatoid arthritis, IBD is hallmarked by periods of remission interspersed with periods of acute flare. During disease course, symptoms like abdominal pain, cramping, loose stools or bloody diarrhea, fatigue, anemia and/or weight loss can manifest. The prevalence and incidence of IBD are increasing enormously [2]. Together with its early onset, relapsing nature, and life-threatening complications, IBD is currently a major health issue. Although the exact pathogenesis is unclear, IBD is certainly driven by disturbed crosstalk between a variety of parameters, i.e., genetic susceptibility and internal and external factors [3], which will be discussed in more detail. Although treatment options mainly focus on reducing intestinal inflammation [1], achieving/maintaining remission or improving the patient’s quality-of-life, no cure for IBD is currently available. Given that IBD is a systemic disease, often associated with comorbidities such as anxiety and depression, this narrative review aims to evaluate the mutual interplay between stress and the gut/microbiota/brain axis, particularly with regard to chronic inflammation of the gastrointestinal tract. These insights set the basis for better understanding the impact of stress management on disease activity in IBD.
2. Search Strategy
For this narrative review, we selected peer-reviewed preclinical and clinical articles as well as meta-analyses and important reviews from the PubMed database between January 1980 and June 2019. The following search terms were used: inflammatory bowel disease/IBD, Crohn’s disease, ulcerative colitis, irritable bowel syndrome, gut/intestines, microbiota/microbiome, brain, interplay/axis, psychological/chronic/acute/cognitive stress, lifestyle factors, stress management, stress resilience, inflammation, disease activity, therapies/interventions/therapeutic strategies.
3. Gut/Microbiota/Brain Interplay
In this section, we will focus on the tight association between the brain and gut, discussing the involvement of endocrine, immune, and neural pathways as well as the gut microbiota.
3.1. Brain/Gut Interaction
The hypothalamic pituitary adrenal (HPA) axis is an endocrine pathway belonging to the limbic system of the brain. In response to stress [4], the activated HPA axis causes the secretion of corticotropin-releasing factor (CRF) from the hypothalamus, which stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH). In turn, ACTH triggers the immunosuppressive stress-hormone cortisol from the adrenal cortex [5], which ordinarily induces the synthesis of anti-inflammatory cytokines. However, in response to stress, sustained cortisol activity has also been associated with pro-inflammatory responses [6]. Likewise, stress-induced cortisol was shown to increase intestinal barrier dysfunction, as recently shown by crypt analyses from rodents and humans [7]. Moreover, administration of cortisol in a porcine model caused a shift in microbiota composition, [8], pointing towards a role for cortisol in regulating intestinal inflammation and altering microbiota composition.
In addition to the HPA axis, the autonomic nervous system (ANS) coordinates the function of the gastrointestinal tract. The ANS is known to trigger efferent signals from the central nervous system (CNS; i.e., brain and spinal cord) to the intestinal wall to regulate mucosal immune responses [9] and other intestinal functions, such as nutrient absorption [10]. Vice versa, via enteric, spinal, and vagal nerves, afferent signals from the intestinal lumen are also known to regulate behavior, sleep, and stress reactivity [11,12]. Upon receiving stimuli from the diet and gut microbiota [13,14], the enteric nervous system (ENS, “second brain”), which is part of the peripheral nervous system, mainly communicates with the CNS in a bidirectional manner. However, the ENS is also capable of intrinsically innervating the gut [15] in an autonomous manner [16].
3.2. Gut Microbiota
The gastrointestinal tract serves as a dynamic and local ecosystem for gut microbiota. Whereas, often being classified into two major phyla, i.e., Bacteroidetes and Firmicutes [17], the gut microbiota is composed of over 35,000 bacterial species [18]. Besides playing a role in metabolism [19], it is also essential for controlling processes related to barrier function against pathogenic microorganism colonization, such as mucosal integrity [20], immunomodulation [21], and pathogen protection [22]. Recently, preclinical [23], translational [24], and clinical [25] studies suggested that alterations in the structural composition or function of the microbiome can contribute to the development of mental illness, including depression-like behavior, and thus, is a vital component linking the gut/brain axis. In line, data have indicated strong correlations between alterations in gut microbiota and the development of multifactorial chronic inflammatory disorders, such as IBD [26,27], suggesting that dysbiosis is an important factor in both gastrointestinal and mental health.
Intestinal bacteria and their metabolites are also involved in gut-associated neuroimmune mechanisms that influence mood and behavior leading to depression. These mechanisms include tryptophan metabolism as well as neural signaling within the ENS [28]. Tryptophan is an essential amino acid, derived from the diet. While crossing the blood–brain barrier and acting as a precursor of the neurotransmitter serotonin, tryptophan can also be degraded in the gut through the kynurenine and serotonin synthesis pathways. This degradation can affect its availability to pass the blood–brain barrier. Thus, by modulating tryptophan levels, microbiota can affect the brain, resulting in behavioral changes [29].
By fermentation of dietary fibers, the gut microbiota is also responsible for producing short-chain fatty acids (SCFAs), including butyric acid, propionic acid, and acetic acid, which are typically found to be reduced in mucosa and feces of patients with IBD [30]. As extensively reviewed by Parada Venegas et al. [31], these metabolic products have shown to play an important role in promoting epithelial cell proliferation [32], barrier function [33], and cellular metabolism [34]. In addition, SCFAs have been involved in controlling intestinal inflammation through activation of G-protein coupled receptor signaling pathways [35], thereby regulating intestinal homeostasis and inhibiting pathogen colonization. Relevantly, SCFAs are also known to exert neuroprotective properties. For instance, gamma-aminobutyric acid is an inhibitory neurotransmitter involved in anxiety and depression and can therefore modulate behavior [36]. Other mechanisms by which the intestinal microbiota affect neural responses include alterations in bacterial neurometabolites or bacterial cell wall sugars. These products can either act directly on primary afferent axons or trigger epithelial cells to release molecules that modulate neural signaling within the ENS [28].
Altogether, the multifaceted interplay between the gut, microbiota, and brain allows for intestinal and extraintestinal homeostasis, thereby coordinating gastrointestinal functions and modulating mood and higher cognitive functions, respectively.
4. Gut/Microbiota/Brain Interplay in IBD Development
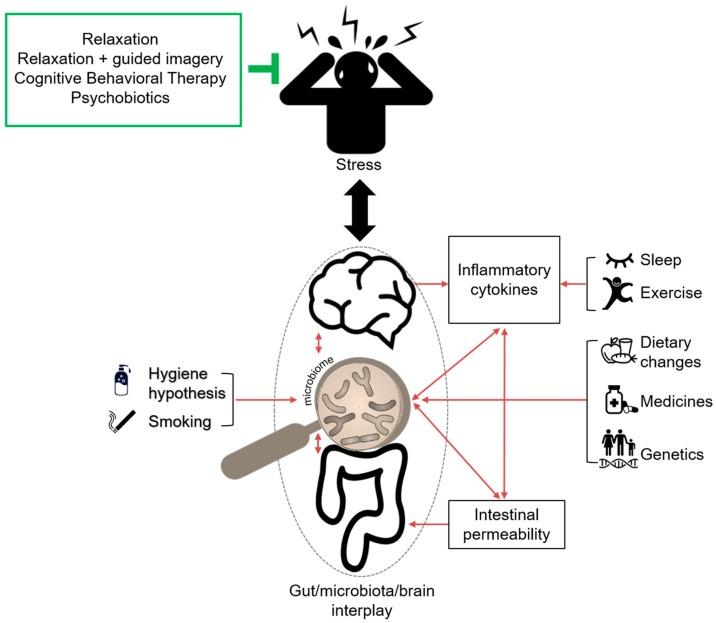
It has become evident that factors such as genetics, environment, diet, and lifestyle are involved in dysregulation of the gut/microbiota/brain interplay, which in this section, will be discussed in the context of IBD development (Figure 1 and Table 1) [37,38].
Figure 1.
The gut/microbiota/brain interplay and its interactions upon exposure to stress. Under conditions of psychological stress including lack of sleep and physical inactivity, the brain (HPA axis) stimulates the production of pro-inflammatory cytokines. This can result in increased intestinal permeability and altered gut microbiota. In addition, fat- and sugar-enriched foods, long-term usage of medicines, as well as genetic predisposition can directly affect the gut microbiota composition, and subsequently, intestinal permeability. Furthermore, personal habits, such as hygiene and smoking, can also have an impact on the gut microbiome. Altogether, the multitude of stress-related factors can perturb the gut/microbiota/brain interplay, which contributes to the development of IBD. Relevantly, several stress management techniques have been proven to greatly alleviate IBD symptoms and improve the quality of life of IBD patients. Given that the exact underlying mechanisms in the context of IBD are not yet fully understood, therapeutic options aimed at improving stress management deserve further investigation.
Table 1.
Factors involved in the gut/microbiota/brain interplay in inflammatory bowel disease (IBD) development.
| Factor | Type of study | N | Intervention/Methodology | Outcome | Author(s) | Reference |
|---|---|---|---|---|---|---|
| Genetics | Clinical study | 637 | Questionnaire | First-degree relatives have 10-fold increased risk of IBD development | Orholm, M. et al. | 37 |
| Meta-analysis | >75,000 cases & controls | GWAS | Identified 30 gene loci for CD and 23 for UC | Jostins, L. et al. | 39 | |
| Trans-ancestry association studies | 238,401 | GWAS | Identified 38 susceptibility loci for IBD | Liu, J.Z. et al. | 40 | |
| Genetic association study | 6228 | Association studies | Identified UPS and CYLD gene are important in IBD pathogenesis | Cleynen, I. et al. | 41 | |
| Genotype association study | 34,819 | Association studies | Insights into genetic heterogeneity between ileal and colonic CD | Cleynen, I. et al. | 42 | |
| Clinical study | 323 | Array-based transcriptome data | Identified 99 strong positional candidate genes in 63 risk loci | Momozawa, Y. et al. | 43 | |
| Clinical study | 189 twin pairs | Questionnaire | Results highlight the importance of environmental triggers | Spehlmann, ME. et al. | 45 | |
| Clinical study | 80 twin pairs | Questionnaire | Genetic influence is stronger in CD than in UC | Halfvarson, J. et al. | 46 | |
| Diet | Interventional, open-label, pilot study | 16 | Vitamin D3 supplementation | Vitamin D3 modulates the gut microbiome | Bashir, M. et al. | 48 |
| In-vivo mouse study | 4–8 mice per group | Oral antibiotics | Increased levels of intracellular zinc led to bacterial clearance | Lahiri, A. et al. | 49 | |
| In-vivo mouse study | 4–5 mice per group | High-fat diet and oral antibiotics | High-fat diet alters gut microbiome composition | Hildebrandt, MA. | 55 | |
| In-vivo mouse study | >100 inbred mouse strains | High-fat/high-sucrose diet | High-fat/high-sucrose diet influences gut microbiota composition | Parks, BW. et al. | 56 | |
| Case-control study | 86 | Dietary intake | Mono- and polyunsaturated fats consumption is a risk factor for IBD | Geerling, BJ. et al. | 57 | |
| In-vivo mouse study | 4–5 mice per group | Diet enriched with phytosterols | Phytosterols are protective against IBD | Aldini, R. et al. | 58 | |
| Environment | Population-based case-control study | 1382 | Questionnaire on 25 different topics | Altered intestinal microbiota may modulate risk of IBD | Ng, SC. et al. | 62 |
| Retrospective case study | 1194 | Clinical history and questionnaire | Higher prevalence of CD in urban areas and UC in inland areas | Carpio, D. et al. | 68 | |
| Smoking | Meta-analysis | 245 articles | Smoking | Smoking is a risk factor for IBD | Mahid, SS. et al. | 51 |
| Prospective case-control study | 160 | Transdermal nicotine or placebo patches | Smoking effects gut microbiota composition | Richardson, CE. et al. | 52 | |
| Medication | Meta-analysis | 11 observational studies | Antibiotic exposure | Antibiotics increases the risk of new-onset CD than UC | Ungaro, R. et al. | 70 |
| Meta-analysis | 20 studies | OCP | Increased risk for development of CD and UC | Ortizo, R. et al. | 71 | |
| Case-control study | 122 | NSAIDs | Provoked disease activity in IBD | Felder, JB. et al. | 72 | |
| Exercise | In-vivo mouse study | 4 mice per group | Exercise training | Alleviated symptoms of acute colitis | Saxena, A. et al. | 60 |
| Prospective cohort study | 194,711 | Physical activity | Inversely associated with risk of CD | Khalili, H. | 61 | |
| Uncontrolled pilot study | 12 | 12-week walking program | Beneficial for IBD patients | Loudon, CP. et al. | 63 | |
| Sleep disturbances | Longitudinal,internet-based cohort data | 3173 | Questionnaire | Increased risk of disease flares in CD but not UC | Ananthakrishnan, AN. et al. | 75 |
| Clinical study | 10 | Disturbances in sleep-wake cycle | Sleep disturbances led to immunologic alterations | Born, J. et al. | 76 | |
| Clinical study | 47 | Questionnaire assessing sleep quality | Impaired sleep quality is associated with pediatric IBD | Mahlmann, L. et al. | 77 | |
| Clinical study | 32 | Questionnaire assessing sleep quality | Impaired quality of life in IBD | Keefer, L. et al. | 78 | |
| Prospective observational cohort study | 41 | Pittsburgh sleep quality index (PSQI) | Strong association between poor sleep quality and IBD | Ali, T. et al. | 79 | |
| In-vivo mouse study | 33 | Diet and sleep disturbances | Circadian disorganization impacts intestinal microbiota | Voigt, RM. et al. | 80 |
CD = Crohn’s disease; GWAS = genome-wide association studies; NSAIDs = nonsteroidal anti-inflammatory drugs; OCP = oral contraceptive pill; SNP = single nucleotide polymorphism; UC = ulcerative colitis; UPS = ubiquitin protease system.
To date, genome-wide association studies revealed more than 200 susceptibility gene loci in IBD [39,40,41,42,43]. First, based on a model selection analysis, 163 susceptibility gene loci were identified, of which 23 and 30 loci were shown to be specific for ulcerative colitis and Crohn’s disease, respectively [39]. These data were further completed with a more recent association study identifying 38 novel risk loci [40]. Based on another large genetic association study in IBD patients, it was shown that ten single nucleotide polymorphisms, in a total of four genes, were found to be significantly correlated with Crohn’s disease [41]. Strongest correlations were found with CYLD, a de-ubiquitinating enzyme, pointing towards the ubiquitin proteasome system as a major contributor to IBD pathogenesis [41]. A more recent and very large study of 34,819 IBD patients investigating genotype–phenotype associations across 156,154 genetic variants also provided important insights into the genetic heterogeneity between ileal and colonic Crohn’s disease, thereby rejecting the current classification of Crohn’s disease versus ulcerative colitis [42].
In addition to genetics, the association between non-coding single nucleotide polymorphisms and IBD risk has gained major interest [44]. Moreover, based on several twin studies, the importance of non-genetic environmental factors [45,46] on IBD disease manifestation has become clear. One example is the implication of micronutrients in IBD progression. Patients with IBD are commonly diagnosed with a vitamin D deficiency [47], which can be related to lowered oral intake of vitamin D or decreased sunlight exposure. A more recent study in healthy volunteers showed that, specifically, the gut microbiome of the upper gastrointestinal tract is positively influenced in response to vitamin D3 treatment [48], suggesting that vitamin D plays a protective role in IBD pathogenesis. Alternatively, intracellular levels of zinc [49] and iron [50] have been associated with bacterial clearance and consequent intestinal permeability and increased risk of IBD, respectively.
Another environmental, lifestyle-related factor is smoking, which has been shown to cause a two-fold increased risk for IBD [51]. Besides affecting the nicotinic acetylcholine receptors present on gastrointestinal mucosal epithelial cells [52], smoking can modulate the human gut microbiota composition, thereby affecting the course of the disease in IBD. Although in the context of Crohn’s disease, smoking has adverse effects, in ulcerative colitis patients, it may play a protective role, implying that smoking may be a disease-specific modifier [53].
As mentioned, dietary fiber intake is able to prolong IBD remission through subsequent increase in luminal production of short-chain fatty acids [31,54]. In addition, it is well-established that high intake of fat- and sugar-enriched foods are capable of regulating intestinal microbiota composition and diversity [55,56], thereby also initiating and sustaining inflammation in patients with IBD [57]. Furthermore, high intake of n-3 polyunsaturated fatty acids and plant sterols have been shown to be protective [58], suggesting that dietary changes play a role in IBD pathogenesis.
It is also well known that low-to-moderate intensity exercise positively affects immune function [59]. Indeed, preclinical studies showed that moderate intensity exercise decreased the expression of pro-inflammatory cytokines, thereby improving acute colitis [60]. Human data regarding the beneficial effects of exercise on the development of intestinal inflammation are mixed, mainly due to the variations in type and rate of exercise. Several studies suggested an inversed correlation between physical activity and the risk or onset of IBD [61]; however, these effects have shown to be disease specific [62]. Nevertheless, other studies focusing on the association between exercise and disease course or the quality of life of IBD patients found a beneficial effect on well-being, sleep, confidence, and mood [63,64].
Further evidence also suggests that geographic location and socioeconomic status are associated with the risk of IBD, thereby supporting the “hygiene hypothesis” of Bloomfield [65]. This hypothesis postulates that the recent rapid rise in IBD, especially in industrialized regions [66], may be due to the lower rate of infection during childhood. The lower infection rate may evolve from reduced exposure to enteric bacteria and improved sanitation during early life [67]. Although this reasoning might indeed explain the higher incidence of IBD in urban areas, the environmental location has been shown to differently affect the prevalence of Crohn’s disease and ulcerative colitis. Whereas Crohn’s disease has shown to be more frequent in urban/coastal areas, ulcerative colitis is more prevalent in inland municipalities [68].
Given that the gut microbiota is relatively diverse and unstable during early childhood, any kind of alteration is likely to affect the intestinal immune responses and predispose individuals to IBD. For instance, medications, including antibiotics, contraceptives as well as non-steroidal anti-inflammatory drugs (NSAIDs) are known to increase the risk of IBD, likely through altering the commensal flora and/or the intestinal barrier [69]. More specifically, based on a meta-analysis, antibiotics were shown to associate with increased risk of new-onset Crohn’s disease rather than ulcerative colitis [70]. In line, a multiple database search revealed that individuals exposed to oral contraceptives had a 24% and 30% increased risk for developing Crohn’s disease and ulcerative colitis, respectively, compared with those not exposed to the medication [71]. Likewise, high doses and long-term treatments with NSAIDs [72] resulted in the exacerbation of IBD [73], potentially acting via non-selective inhibition of the cyclo-oxygenase [74].
Relevantly, IBD disease activity and its risk of relapse has also been associated with sleep disturbances [75]. Sleep disturbances can induce the levels of inflammatory cytokines, thereby activating an inflammatory cascade [76]. Furthermore, sleep disturbances have been shown to occur in IBD patients, including pediatric patients [77] as well as those with inactive disease [78], and can negatively impact quality of life. Indeed, optimized sleep duration (i.e., six to nine hours/day) was able to decrease the risk of ulcerative colitis. Further, based on a prospective study, a strong inversed correlation between sleep quality and the activity of IBD was demonstrated [79]. Also, disruptions of the circadian organization, a form of long-term biological stress, are known to affect health [80]. It has been suggested that the adverse effects of the host’s circadian rhythm, including sleep disruption, can alter the circadian rhythm of the intestinal microbiota, thereby changing its community structure [80]. Given that the gut microbiota plays a key role in the development of IBD, it is also likely that circadian disorganization, through dysbiosis of the intestinal microbiota, negatively impacts the course of the disease.
5. Stress and Intestinal Microbiota: Bidirectional Relationship in IBD
5.1. Influence of Stress on Gut Microbiota
Several lines of evidence suggest that stress, induced by dietary, environmental or neuroendocrine factors, can adversely affect the gut/microbiota/brain axis [81,82] (Table 2).
Table 2.
Studies investigating the link between stress and gut microbiota.
| Factor | Type of Study | N | Intervention/Methodology | Outcome | Author(s) | Reference |
|---|---|---|---|---|---|---|
| Prenatal/early life stress | In-vivo mouse study | 6–20 mice per group | Maternal high-fat diet | Dysbiosis and low-grade inflammation in the intestine | Xie, R. et al. | 83 |
| In-vivo rat study | 6–10 per group | Prenatal stress | Long-lasting alterations in the intestinal microbiota composition | Golubeva, AV. et al. | 84 | |
| In-vivo mouse study | 21–23 mice per group | Prenatal stress | Alterations in vaginal microbiota contributed to reprogramming of the developing brain | Jasarevic, E. et al. | 85 | |
| In vivo primates study | 20 | Maternal separation | Maternal separation-induced psychological disturbances altered intestinal microflora | Bailey, MT. et al. | 91 | |
| In-vivo rat study | 22 | Maternal separation | Early life stress induced alterations in gut-brain axis contributing to IBD symptoms | O’Mahony, SM. et al. | 92 | |
| Longitudinal clinical study | 192 children | Questionnaire | Prenatal stress is associated with microbial colonization patterns in infants | Zijlmans, MA. et al. | 104 | |
| Chronic/social/environmental stress | In-vivo mouse study | 7–20 mice per group | Chronic social defeat | Stress induced complex structural changes in the gut microbiota | Bharwani, A. et al. | 81 |
| In-vivo mouse study | 10 mice per group | Lactation | Cellular transfer of bacterial translocation occurred in pregnant and lactating mice | Donnet-Hughes, A. et al. | 87 | |
| In-vivo mouse study | 5 mice per group | SDR | Stress led to significant changes in intestinal microbiota colonization | Bailey, MT. et al. | 88 | |
| In-vivo mouse study | 10–12 (3 independent experiments) | Unpredictable chronic mild stress | Altered intestinal microbiota composition, specifically the lactobacillus compartment | Marin, IA. et al. | 89 | |
| In-vivo mouse study | 5 mice per group | SDR | Affected microbial populations that are closely associated with the colonic mucosa | Galley, JD. et al. | 90 | |
| In-vivo mouse study | 4–6 mice per group | Chronic restraint stress | Disturbed gut microbiota and subsequent activation of immune system led to colitis | Gao, X. et al. | 93 | |
| In-vivo rat study | 7–8 rats per group | WAS | Intestinal inflammation by impaired mucosal defenses against luminal bacteria | Soderholm, JD. et al. | 94 | |
| In-vivo rat study | 6 | Cold-restraint stress or WAS | Exacerbated intestinal inflammation due to increased uptake of immunogenic substances | Saunders, PR. et al. | 95 | |
| In-vivo rat study | not specified | Stress induction | Increased gastrointestinal permeability, allowing luminal constituents to the mucosal immune system | Meddings, JB. et al. | 96 | |
| In-vivo rat study | 4 rats per group | WAS | Stress-induced epithelial mitochondrial damage and mucosal mast cell activation | Santos, J. et al. | 97 | |
| Field experiment in wild birds | 64 | Corticosterone-implant | Altered gut microbiome in free-living birds | Noguera, JC. et al. | 101 | |
| In-vivo rat study | 13–14 rats per group | WAS | Altered intestinal mucus composition | Da Silva, S et al. | 102 | |
| In-vivo mouse study | 18–24 mice per group | Germ-free and specific-pathogen free; acute restraint stress | Commensal microbiota can affect the postnatal development of the HPA stress response | Sudo, N. et al. | 108 | |
| In-vivo mouse study | 12 mice per group | Germ-free and specific-pathogen free | Conventional intestinal microbiota influenced the development of behavior | Neufeld, KM. et al. | 109 | |
| In-vivo mouse study | 7–14 mice per group | Germ-free and specific-pathogen free | Gut microbiota affected mammalian brain development and subsequent adult behavior | Diaz Heijtz, R. et al. | 110 | |
| Clinical study | 263 | Daily interview assessment | Stress is associated with digestive problems and gastrointestinal health | Walker, LS. et al. | 103 | |
| Clinical study | 40 | Depression | Increased bacterial translocation and activated immune responses against commensal bacteria | Maes, M. et al. | 105 | |
| Clinical study | 65 | Coping style instrument | IBD adolescents used more avoidant coping styles compared to healthy controls | Van der Zaag-Loonen, HJ. et al. | 106 | |
| Pro/prebiotics | In-vivo rat study | 4–5 rats per group | WAS and probiotics | Probiotics prevented chronic stress-induced intestinal abnormalities | Zareie, M. et al. | 99 |
| In-vivo rat study | 84 | Maternal separationand prebiotics/probiotics/LC-PUFA | Nutritional intervention at weaning normalized gut permeability and restored growth rate | Garcia-Rodenas, CL. et al. | 100 | |
| In-vivo mouse study | 36 | Probiotic formulation | Suggested the importance of probiotics in gut/brain axis in stress-related disorders | Bravo, JA. et al. | 111 | |
| In-vivo mouse study | 8 mice per group | Chronic mild stress and probiotics | Decreased pro-inflammatory cytokines and altered stress-related behaviors | Li, N. et al. | 113 | |
| In-vivo rat study | 36 rats; | Probiotic formulation | Anxiolytic-like activity in rats | Messaoudi, M. et al. | 112 | |
| Double-blind, placebo-controlled, randomized parallel group study | 66 individuals | Probiotic formulation | Beneficial psychological effects in healthy human volunteers | Messaoudi, M. et al. | 112 | |
| Systematic review | 11 RCTs | Prebiotic supplementation | Short-term beneficial effects in intestinal microbiota composition | Rao, S. et al. | 114 |
HPA = hypothalamic pituitary adrenal axis; LC-PUFA = long-chain poly-unsaturated fatty acids; RCT = randomized controlled trials; SDR = social disruption; WAS = water-avoidance stress.
5.1.1. Preclinical Studies
It was recently shown in mice that maternal nutrition can negatively affect offspring intestinal development and function. For instance, maternal high-fat diet caused a shift in microbiota composition, thereby predisposing the offspring to develop intestinal inflammation [83]. In line, early prenatal stress in rodents was shown to increase the Oscillibacter, Anaerotruncus, and Peptococcus genera [84] and induce a loss of Lactobacilli transmission to the neonate [84,85], pointing towards the involvement of birth canal delivery in gut microbiota colonization [86,87]. These data were further supported by other preclinical studies showing that short-term or mild chronic stress caused a reduction in Lactobacilli [88,89,90]. These data suggest that stressor-induced changes have important health implications [90]. It has also been shown, both in primates [91] and in rodents [92], that maternal separation, a form of chronic stress, was able to induce a change in fecal microbiota in new-born animals. A more recent study demonstrated that chronic stress resulted in dysbiosis of the murine gut microbiota, thereby inducing an immune system response and facilitating experimentally induced colitis [93]. Other genetically susceptible rodent models revealed that chronic psychological stress induced mucosal dysfunction, intestinal abnormalities, and subsequently intestinal inflammation [94]. Likewise, it was shown in rats that acute [95], environmental [96] as well as chronic stress [97] increased intestinal permeability, and hence, luminal molecule delivery to the mucosal immune system, thereby triggering pro-inflammatory responses. In this context, probiotics (living organisms yielding benefits on the host’s microbiome [98]) were shown to revert chronic stress-induced abnormalities of the intestinal tract [99]. Also, it was shown that diets containing a combination of specific long-chain polyunsaturated fatty acids, prebiotics, and probiotics restored the rat intestinal microbiota composition [100]. More recently, it was even demonstrated that stress hormones, through manipulation of basal corticosterone levels, were able to alter the gut microbiome of free-living birds [101]. Further, using a sophisticated rat model, water avoidance stress was shown to alter the mucus composition [102], which is known as the host’s primary innate defense. Given that changes in the production of mucosal proteins have been associated with dysbiosis of the gut microbiota, it is likely that stress indirectly affects the intestinal microbiota via inflammation of the mucosal protein layer [94].
5.1.2. Clinical Studies
Few clinical studies also revealed that stress is associated with digestive problems and gastrointestinal health [103]. For instance, by means of a phylogenetic microarray, one study showed that exposure to stress during pregnancy resulted in aberrant microbiota colonization patterns in pediatrics, which likely increased inflammation and gastrointestinal symptoms [104]. In line with these findings, stress-related psychiatric disorders, such as depression, were also associated with increased bacterial translocation, thereby activating immune responses against commensal bacteria [105]. Although these data imply that stress has a potent influence on intestinal microbiota, stress is a subjective experience, which makes it challenging to objectively evaluate the effects of stress. Therefore, further human studies should be performed to verify that stress results in dysbiosis of the gut microbiota.
5.2. Impact of Intestinal Microbiota on Stress Responsiveness
Appropriate physiological responses to stress and/or immunity are necessary for survival. As such, aberrant responsiveness can be detrimental to the host, leading to the development of chronic disorders, including IBD [106] and brain disorders [107].
5.2.1. Preclinical Studies
Preclinical studies using germ-free animals, specific pathogen-free animals, and animals exposed to pathogens, probiotics or antibiotics have been performed to better understand how gut microbiota can regulate stress response, cognition, and behavior [28]. Previously, it has become clear that intestinal colonization with conventional microbiota at an early developmental stage is important for stress responsiveness in adult mice [108]. This study showed that the HPA stress response was exaggerated in the absence of normal gut microbiota reconstitution, whereas it could be partially corrected by reconstitution of feces from specific pathogen-free mice at an early, but not at a later stage. In line with these results, other murine studies demonstrated the influence of conventional gut microbiota on the development of behavior [109,110], and showed that this effect occurred along with neurochemical changes in the brain [109]. Another study based on a rat model of acute psychological stress demonstrated that the probiotic Lactobacillus farciminis reduced intestinal leakiness, thereby decreasing plasma levels of lipopolysaccharides, and consequently, diminishing the HPA axis response to stress. Moreover, by reducing stress-induced plasma corticosterone levels, Bravo et al. [111] showed that the probiotic Lactobacillus rhamnosus was able to reduce the stress response as well as anxiety-related behaviors and cognition in mice. Furthermore, it has been shown that the combination of probiotics, such as Lactobacillus helveticus and Bifidobacterium longum, resulted in a reduction of anxiety-like behaviors in rodents [112,113].
5.2.2. Clinical Studies
Messaoudi et al. [112] also validated their preclinical findings in healthy human volunteers. Their findings suggested that probiotic formulation attenuated psychological distress in healthy volunteers, which may be linked to decreased urinary free cortisol levels [112]. In agreement with these data, a randomized, double-blind, placebo-controlled trial suggested that the administration of probiotics helped in reducing anxiety-like behavior among patients with chronic fatigue syndrome [114]. Altogether, these data further confirm that the intestinal microbiota plays a role in controlling stress responsiveness, behavior, and cognition.
5.3. Stress and Its Impact on Inflammation
While not having discussed in detail yet, the impact of stress on the immune system appears to be quite complex. Depending on the type of stress (short-term or chronic) and/or hormones being released, a stressor may either suppress or enhance immune function [115].
5.3.1. Preclinical Studies
Several preclinical studies have shown that short-term stress induces significant changes in absolute numbers and composition of blood leukocytes [116,117]. Likewise, short-term stress was shown to increase the circulating levels of interleukin-6 (IL6) and pro-inflammatory monocyte chemotactic protein-1 (MCP1/CCL2) [118]. These findings were also further confirmed by others showing that social disruption reduced microbial diversity and richness in mice, which correlated with increased circulating levels of the pro-inflammatory cytokines MCP1 and IL6 [88]. Relevantly, based on data demonstrating that administration of antibiotics was able to abolish social stress-mediated increases in pro-inflammatory cytokines [88], it is likely that intestinal microbiota plays a role in stressor-induced pro-inflammatory responses.
5.3.2. Clinical Studies
Previous studies in humans provided similar evidence that stress induces an increase of pro-inflammatory Th1 cytokines [119,120,121]. For instance, academic stress, referred to as the body’s response to academic-related workload that goes beyond the adaptive capabilities of students [122], was shown to significantly increase the production of interferon-gamma (IFNγ) and tumor necrosis factor alpha (TNFα) [119]. Acute stress can also upregulate anti-inflammatory cytokines including IL10, while independently inhibiting pro-inflammatory cytokines such as TNFα [123]. These effects can induce a shift towards Th2-mediated humoral response [124], which may be essential to prevent hyperactivation of the stress system. Alternatively, chronic stress is known to increase the release of cortisol levels for several days, an effect that may be associated with immunosuppression, as shown by a reduction in circulating CD8+ lymphocytes, natural killer cells, and macrophages [125]. Nevertheless, as chronic psychological stress was also associated with increased levels of serum C-reactive protein (CRP) [126], these data suggest that chronic stress may also exert pro-inflammatory effects. Obviously, it should be noted that the gastrointestinal tract per se, including its local microbiota, may serve as an essential organ mediating immune responses [127]. This is not only of relevance in the context of human IBD, but also in irritable bowel syndrome (IBS) [128] as well as major depressive disorders [129].
5.4. Stress and Inflammation in IBD
Inflammatory bowel disease is a complex disease that likely does not only consist of Crohn’s disease and ulcerative colitis. Extensive translational research has been conducted to better understand the role of stress and inflammation in IBD [130].
5.4.1. Preclinical Studies
Previously, using rodent models of spontaneous colitis, it was shown that intestinal inflammation is associated with defects in mucosal barrier or dysfunctional regulatory T lymphocytes [131]. Other studies using mice fed a dextran–sulfate–sodium diet to induce colitis revealed the importance of intestinal adhesion molecules, such as ICAM-1, in the development of intestinal inflammation [132]. Relevantly, when dextran–sulfate–sodium-treated mice were injected with enterotoxigenic Bacteroides fragilis, increased colitis and colonic inflammation was observed [133]. Similarly, in cellular models, Clostridium difficile toxin A was shown to induce apoptosis and inflammation in enterocytes [134,135]. Moreover, using a mouse model of depression, it was shown that stress-induced release of corticosteroids can reactivate IBD [136], likely via increased production of pro-inflammatory cytokines. These data imply that stress may affect the course of the disease.
5.4.2. Clinical Studies
In humans, it was shown that infections with Bacteroides fragilis, through secretion of its pro-inflammatory toxin, is associated with development of ulcerative colitis [137]. Likewise, infections with Clostridium difficile is thought to be involved in the reactivation of IBD in patients [138]. Within a similar context, it was also shown that patients with ileal Crohn’s disease had a higher percentage of invasive Escherichia coli in the mucosa as compared to healthy controls, and these percentages even correlated with severity of the disease [139]. In line, another study demonstrated that Escherichia coli can replicate inside macrophages of patients with Crohn’s disease and subsequently secrete large amounts of TNFα, thereby contributing to inflammation [140]. Collectively, these data point towards the strong relationship between the gut microbiota composition and intestinal inflammation.
It is also important to note that psychosocial stress, including psychological distress, anxiety, and depression, can induce low-grade chronic inflammation in the gut. For instance, in humans, it was shown that depression correlated with elevated levels of TNFα and CRP, pro-inflammatory cytokines that that are known to trigger inflammation in patients with IBD [141,142]. Hence, it has been proposed that stress gradually contributes to the development or exacerbation of IBD (as reviewed elsewhere [28,143]). Vice versa, compared to the general population, young patients with IBD displayed higher rates of psychological stress, and these results were further confirmed in gastrointestinal disorders such as IBS [144,145]. Together, these data imply that the disease itself may have a direct impact on the quality of life of patients.
Yet, it is not clear whether individuals with higher stress also experience more IBD symptoms. A large cross-sectional, population-based study of IBD patients showed that the relationship between intestinal inflammation and symptomatic disease activity differed between Crohn’s disease and ulcerative colitis [146]. Whereas Crohn’s disease did not associate with intestinal inflammation and disease activity symptoms, an association was found for ulcerative colitis. These findings suggest that the duration and intensity of stress factors may have differential influences on chronic inflammatory diseases. Furthermore, this study showed that perceived stress in both diseases correlated with disease activity symptoms, while not with inflammation [146]. Although the majority of conventional therapies in IBD focus on tackling intestinal inflammation, these insights open new venues for stress reduction in the management of IBD.
6. Managing Stress in IBD: Does It Make the Gut Feel Better?
In recent years, significant progress has been made in the treatment of IBD, focusing either on targeted therapies [147] or on alternative and complementary strategies [148], which has recently been extensively reviewed [147,148]. Nevertheless, there is no certain cure for IBD due to the limited effectiveness of current therapies, which often even goes hand in hand with significant side effects. Quality of life as well as anxiety and depression are known predictors of negative medical outcomes in many chronic conditions, and as reviewed, stress has a profound impact on these variables in patients with IBD. Hence, a number of approaches have focused on relieving stress as a potential therapeutic option in IBD (Table 3) [148,149].
Table 3.
Clinical studies investigating stress management in IBD.
| Factor | Type of Study | N | Intervention | Outcome in IBD Patients | Author(s) | Reference |
|---|---|---|---|---|---|---|
| Guided imagery/Relaxation training | Pilot RCT | 28 | Guided imagery with relaxation (GIR) | Improved QL in elderly women with osteoarthritis | Baird, CL. et al. | 150 |
| Prospective RCT | 39 | Relaxation-training | Beneficial effects on anxiety, pain and stress in IBD patients | Mizrahi, MC. et al. | 163 | |
| Self-directed stress management | Clinical study | 45 | 3 types of stress management, including self-directed and conventional medical treatment | Trained CD patients showed reduced fatigue, constipation and abdominal pain, whereas no beneficial effects in conventional-treated CD patients | García-Vega, E. et al. | 151 |
| Lifestyle management | Clinical study | 60 | 60-h training program in lifestyle modification over a period of 10 weeks | Short-term benefits in the QL in UC patients, whereas no long-term effects | Langhorst, J. et al. | 157 |
| Clinical study | 49 | 8-session information about QL and stress management | No effect on anxiety levels 6 months post-intervention | Larsson, K. et al. | 160 | |
| Prospective, randomized waiting-control group design | 30 | 60-h training program on life style management | Improved QL in patients with UC remission | Elsenbruch, S. et al. | 167 | |
| Prospective, randomized study | 32 | Low-intensity walking program | Improved QL of CD patients | Ng, V. et al. | 179 | |
| Prospective RCT | 30 | Moderate-intensity running | Improved QL of IBD patients | Klare, P. et al. | 180 | |
| Psychotherapy | Two clinical trials | 36 | 7-session behavioral protocol | 57% reduction in IBD relapse in the following 12 months | Keefer, L. et al. | 152 |
| RCT | 41 | Primary and Secondary Control Enhancement Therapy-Physical Illness | Beneficial effects on depression in IBD adolescents | Szigethy, E. et al. | 158 | |
| Clinical study | 178 | Nurse-led counselling | Improved QL over 6 rather than 12 months in IBD patients | Smith, GD. et al. | 161 | |
| Prospective, uncontrolled open trial | 30 | Supportive-expressive group psychotherapy | No changes in QL, anxiety, or depression over the course of treatment in UC/CD | Maunder, RG. et al. | 162 | |
| Meta-analysis | 1824 studies with 14 RCTs | Psychological therapy | Small short-term beneficial effects on QL and depression in IBD patients | Gracie, DJ. et al. | 166 | |
| RCT | 29 | Breath-Body-Mind Workshop; questionnaire | Significant long-lasting benefits for IBD symptoms, anxiety, depression and QL | Gerbarg, PL. et al. | 155 | |
| Control study | 60 | Mindfulness-based stress reduction | Improved mood and QL after six months of intervention | Neilson, K. et al. | 156 | |
| RCT | 36 | Gut-directed hypnotherapy | Gut-directed hypnotherapy may be one aspect in a disease-management program for IBD | Keefer, L. et al. | 159 | |
| Clinical trial | 66 | Multi-convergent therapy (psychotherapy) | Therapy is beneficial in the management of IBD symptoms | Berrill, JW. et al. | 164 | |
| Medication | Retrospective observational study | 30 | Herbal treatment | Positive effect of cannabis on disease activity in CD | Naftali, T. et al. | 171 |
| Prospective, placebo-controlled study | 21 | Herbal treatment | Short course of cannabis had beneficial effects in CD patients | Naftali, T. et al. | 172 | |
| Double-blind RCT | 108 | Placebo or vitamin D3 | Reduced relapse risk in CD | Jorgensen, SP. et al. | 174 | |
| Prospective | 37 | Active or plain vitamin D | Active form of vitamin D has short-term beneficial effects in CD | Miheller, P. et al. | 175 | |
| Meta-analysis | 12 studies | Serum folate and vitamin B12 | Low concentration of serum folate is a risk factor for IBD and supplementation may be beneficial | Pan, Y. et al. | 177 | |
| Double-blind RCT | 10 per group | Placebo/ phylloquinone/ vitamin D3 | No significant beneficial effects of phylloquinone on bone health in CD patients | O’Connor EM. et al. | 178 |
CD = Crohn’s disease; QL = quality of life; RCT = randomised controlled trial; UC = ulcerative colitis.
Stress management is a technique used to diminish the physiological effects of stress and tension, and to help the individual to improve his/her coping skill. One variant of such therapy is relaxation, by which the individual is trained by a therapist or in a self-directed manner to create physiological and mental rest. Several studies showed the effectiveness of relaxation training in a variety of physical illnesses, including cardiovascular disease, arthritis [150], and IBD [151,152,153]. Previously, it was shown that stress management could significantly improve IBD symptoms such as pain and fatigue [151]. In line, behavioral self-management therapy resulted in a 57% decrease in 1 year risk to relapse in IBD patients [152], supporting the beneficial effects of stress management in IBD. More recently, two clinical studies pointed towards the beneficial effects of mindfulness on psychological and physical symptoms, quality of life, and C-reactive protein, an established biomarker [154], in patients with IBD [155,156]. However, based on a systematic review, McCombie et al. [153] concluded that the effect of psychotherapy led to inconsistent results in IBD patients. It should be noted that the studies included in this study focused on a wide range of therapies. Thus, although meditation and relaxation may have beneficial effects on inflammatory activity and quality of life in IBD patients, the effectiveness of mindfulness-based interventions on disease activity remains to be elucidated.
Whereas several studies demonstrated that psychotherapy had a beneficial impact on anxiety [157], depression [158], and quality of life of IBD patients [159], other studies were not able to find any effect [160,161]. Yet, two other studies investigated the impact of combining a variety of techniques as a treatment option in IBD [161,162]. Indeed, when combining relaxation with guided imagery, a method focusing on mind-relaxing images to replace stressful thoughts, both anxiety status and quality of life appeared to be improved among patients with IBD [163]. Similarly, multi-convergent therapy, which combines mindfulness meditation with cognitive behavioral therapy, has been used as a therapeutic option in patients with tinnitus and IBS. Therefore, its applicability and efficacy were investigated in an IBD population that received conventional therapy [164]. This study revealed that multi-convergent therapy improved quality of life mainly in IBD patients suffering from IBS-like symptoms [164], suggesting that this strategy has beneficial effects only in a subgroup of IBD patients. Although IBS and IBD are medically distinct from each other, symptoms compatible with IBS indeed often co-exist in patients with IBD [165], and should therefore not be underestimated. Within this context, it was recently shown that 36% and 37% of CD and UC patients, respectively, met IBS diagnostic criteria [165], confirming that the presence of IBS-like symptoms in IBD is common [148]. Furthermore, patients with IBS in quiescent IBD were shown to have significantly more anxiety and depression than patients without IBS [165].
More recent data suggested that short-term cognitive behavioral therapy improved quality of life and depression scores in patients with IBD, while not affecting disease activity or other measures of psychological well-being [166]. Others particularly investigated the impact of mind–body therapy, a combination of moderate exercise, diet, stress management training, behavioral techniques and self-care strategies on patients with ulcerative colitis. Results suggested that this approach has a positive effect on IBD development by improving quality of life and mental/physical health scores [167].
As reviewed in detail elsewhere, other complementary and alternative medicines, such as herbal medicine, vitamin supplementation, and exercise have also gained attention for its anti-inflammatory properties and usefulness in the treatment of IBD [148,168]. Within this context, preclinical data from mice suggested that cannabinoid receptor activation mediates protective mechanisms in experimental colitis [169,170]. In line with these data, two clinical studies reported that cannabis was able to reduce IBD symptoms [171,172], pointing towards its ability to treat IBD. Nevertheless, whether cannabis is able to positively affect the course of disease requires further investigation. Other preclinical studies in mice showed an important role for vitamin D and its receptor in the regulation of inflammation of the gastrointestinal tract [173]. Indeed, IBD patients often lack this vitamin [47], and hence, studies investigated the role of vitamin D [174,175,176] in treatment of IBD patients. Although these studies pointed towards beneficial effects on disease activity and risk of relapse, other clinical studies on the use of vitamin B [177] or K [178] in IBD treatment were inconsistent. These data imply that there is a lack of evidence to support positive effects of vitamins on IBD disease course [148]. Likewise, whereas preclinical [60] and clinical studies [179,180] suggested that low-to-moderate intensity exercise exerted beneficial effects on intestinal inflammation, overall health, and quality of life of IBD patients, further studies investigating the impact of exercise on disease activity and/or prevention of IBD are warranted [148].
Given that stress orchestrates an important influence on structural and functional aspects of the microbiome, multiple studies have also investigated the role of psychobiotics in stress-related diseases. Psychobiotics refer to probiotics or prebiotics that can manipulate commensal gut microbiota, and when ingested at adequate quantities, may indirectly have positive psychiatric effects in psychopathology [181]. As extensively reviewed, both in experimental colitis and human IBD, pre- and probiotics have shown beneficial effects in the prevention of IBD by modulating the trophic functions of the microbiota, improving the intestinal mucosal barrier and mediating anti-inflammatory responses [182]. Given that the intake of psychobiotics also seem to exert antidepressant effects, including improvements in mood and decreases in stress-related plasma and urinary free cortisol [181], it may be postulated that psychobiotics might serve as therapeutic modulators of the gut/microbiota axis and positively influence psychological functions in the context of IBD.
Altogether, the quality of life and course of IBD are regulated by psychological conditions, and as such, the implementation of stress management may play an important role in IBD disease regression (see also Figure 1). Nevertheless, current studies are limited in sample size and study design, and hence, may lack proper controls. Therefore, future research is needed to validate current treatment options and/or to explore novel therapeutic opportunities in order to prevent IBD onset or improve stress-affected well-being of IBD patients.
7. Conclusions
In summary, this review summarized the tight connection between the gut, microbiota, and brain in the context of IBD and has particularly shed light on the impact of stress on this interplay. It should be noted, however, that it is rather challenging to investigate the impact of stress on IBD, as stress can arise from totally different origins and may be closely connected to potential individual confounding factors, including (mental) health status and inter-individual variability in stress responsiveness and/or vulnerability. Therefore, future research involving preclinical studies as well as large-scale, controlled clinical trials should not only focus on unravelling the exact mechanisms through which stress affects IBD. However, it is also of great interest to further investigate the exact mechanisms of how stress management can orchestrate beneficial effects in IBD and how stress-relieving therapies should be implemented in IBD care.
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| ANS | Autonomic nervous system |
| CNS | Central nervous system |
| CRF | Corticotropin-releasing factor |
| CRP | C-reactive protein |
| ENS | Enteric nervous system |
| HPA | Hypothalamic pituitary adrenal |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IFNγ | Interferon-gamma |
| IL | Interleukin |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| MCP1 | Monocyte chemotactic protein-1 |
| SCFA | Short-chain fatty acids |
| TNFα | Tumor necrosis factor alpha |
Author Contributions
Conceptualization, Y.O., C.M.C.O. and R.S.; writing—original draft preparation, Y.O.; writing—review and editing, Y.O., T.Y., T.H., R.S.-S.; funding acquisition, R.S.-S.
Funding
This research was supported by the Dutch Organization for Scientific Research (NWO; Vidi grant no. 016.126.327), ASPASIA (grant no. 015.008.043), and TKI-LSH (grant no. 40-41200-98-9306).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tontini G.E., Vecchi M., Pastorelli L., Neurath M.F., Neumann H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World J. Gastroenterol. 2015;21:21–46. doi: 10.3748/wjg.v21.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat Rev. Gastroenterol. Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 3.Ye Y., Pang Z., Chen W., Ju S., Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J. Clin. Exp. Med. 2015;8:22529–22542. [PMC free article] [PubMed] [Google Scholar]
- 4.Camara R.J., Ziegler R., Begre S., Schoepfer A.M., von Kanel R., Swiss Inflammatory Bowel Disease Cohort Study Group The role of psychological stress in inflammatory bowel disease: Quality assessment of methods of 18 prospective studies and suggestions for future research. Digestion. 2009;80:129–139. doi: 10.1159/000226087. [DOI] [PubMed] [Google Scholar]
- 5.Mawdsley J.E., Rampton D.S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeager M.P., Pioli P.A., Guyre P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response. 2011;9:332–347. doi: 10.2203/dose-response.10-013.Yeager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng G., Victor Fon G., Meixner W., Creekmore A., Zong Y., M K.D., Colacino J., Dedhia P.H., Hong S., Wiley J.W. Chronic stress and intestinal barrier dysfunction: Glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep. 2017;7:4502. doi: 10.1038/s41598-017-04755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrosus E., Silva E.B., Lay D., Jr., Eicher S.D. Effects of orally administered cortisol and norepinephrine on weanling piglet gut microbial populations and Salmonella passage. J. Anim. Sci. 2018;96:4543–4551. doi: 10.1093/jas/sky312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Ariki S., Husband A.J. The role of sympathetic innervation of the gut in regulating mucosal immune responses. Brain Behav. Immun. 1998;12:53–63. doi: 10.1006/brbi.1997.0509. [DOI] [PubMed] [Google Scholar]
- 10.Mourad F.H., Saade N.E. Neural regulation of intestinal nutrient absorption. Prog. Neurobiol. 2011;95:149–162. doi: 10.1016/j.pneurobio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Cryan J.F., O’Mahony S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 12.Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017;7:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo B.B., Mazmanian S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furness J.B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5:1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- 15.Schneider S., Wright C.M., Heuckeroth R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019;81:235–259. doi: 10.1146/annurev-physiol-021317-121515. [DOI] [PubMed] [Google Scholar]
- 16.Gershon M.D. The enteric nervous system: A second brain. Hosp. Pract. 1999;34:31–52. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 17.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haiser H.J., Turnbaugh P.J. Developing a metagenomic view of xenobiotic metabolism. Pharmacol. Res. 2013;69:21–31. doi: 10.1016/j.phrs.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuethe J.W., Armocida S.M., Midura E.F., Rice T.C., Hildeman D.A., Healy D.P., Caldwell C.C. Fecal Microbiota Transplant Restores Mucosal Integrity in a Murine Model of Burn Injury. Shock. 2015 doi: 10.1097/SHK.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamada N., Chen G.Y., Inohara N., Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guida F., Turco F., Iannotta M., De Gregorio D., Palumbo I., Sarnelli G., Furiano A., Napolitano F., Boccella S., Luongo L., et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry. 2016 doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 25.Mi G.L., Zhao L., Qiao D.D., Kang W.Q., Tang M.Q., Xu J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van Leeuwenhoek. 2015;107:1547–1553. doi: 10.1007/s10482-015-0448-9. [DOI] [PubMed] [Google Scholar]
- 26.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M., Sun K., Wu Y., Yang Y., Tso P., Wu Z. Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease. Front. Immunol. 2017;8:942. doi: 10.3389/fimmu.2017.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 29.Waclawikova B., El Aidy S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals. 2018;11:63. doi: 10.3390/ph11030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., Inatomi O., Bamba S., Sugimoto M., Andoh A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- 31.Parada Venegas D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J.H., Kotani T., Konno T., Setiawan J., Kitamura Y., Imada S., Usui Y., Hatano N., Shinohara M., Saito Y., et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE. 2016;11:e0156334. doi: 10.1371/journal.pone.0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng L., Kelly C.J., Battista K.D., Schaefer R., Lanis J.M., Alexeev E.E., Wang R.X., Onyiah J.C., Kominsky D.J., Colgan S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 36.Lydiard R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry. 2003;64(Suppl. 3):21–27. [PubMed] [Google Scholar]
- 37.Orholm M., Munkholm P., Langholz E., Nielsen O.H., Sorensen T.I., Binder V. Familial occurrence of inflammatory bowel disease. N. Engl. J. Med. 1991;324:84–88. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- 38.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J.Z., van Sommeren S., Huang H., Ng S.C., Alberts R., Takahashi A., Ripke S., Lee J.C., Jostins L., Shah T., et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleynen I., Vazeille E., Artieda M., Verspaget H.W., Szczypiorska M., Bringer M.A., Lakatos P.L., Seibold F., Parnell K., Weersma R.K., et al. Genetic and microbial factors modulating the ubiquitin proteasome system in inflammatory bowel disease. Gut. 2014;63:1265–1274. doi: 10.1136/gutjnl-2012-303205. [DOI] [PubMed] [Google Scholar]
- 42.Cleynen I., Boucher G., Jostins L., Schumm L.P., Zeissig S., Ahmad T., Andersen V., Andrews J.M., Annese V., Brand S., et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet. 2016;387:156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Momozawa Y., Dmitrieva J., Theatre E., Deffontaine V., Rahmouni S., Charloteaux B., Crins F., Docampo E., Elansary M., Gori A.S., et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat. Commun. 2018;9:2427. doi: 10.1038/s41467-018-04365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barthel C., Spalinger M.R., Brunner J., Lang S., Fried M., Rogler G., Scharl M. A distinct pattern of disease-associated single nucleotide polymorphisms in IBD risk genes in a family with Crohn’s disease. Eur J. Gastroenterol. Hepatol. 2014;26:803–806. doi: 10.1097/MEG.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 45.Spehlmann M.E., Begun A.Z., Burghardt J., Lepage P., Raedler A., Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: Results of a nationwide study. Inflamm. Bowel Dis. 2008;14:968–976. doi: 10.1002/ibd.20380. [DOI] [PubMed] [Google Scholar]
- 46.Halfvarson J., Bodin L., Tysk C., Lindberg E., Jarnerot G. Inflammatory bowel disease in a Swedish twin cohort: A long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124:1767–1773. doi: 10.1016/S0016-5085(03)00385-8. [DOI] [PubMed] [Google Scholar]
- 47.Ardesia M., Ferlazzo G., Fries W. Vitamin D and inflammatory bowel disease. Biomed. Res. Int. 2015;2015:470805. doi: 10.1155/2015/470805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashir M., Prietl B., Tauschmann M., Mautner S.I., Kump P.K., Treiber G., Wurm P., Gorkiewicz G., Hogenauer C., Pieber T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016;55:1479–1489. doi: 10.1007/s00394-015-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahiri A., Abraham C. Activation of pattern recognition receptors up-regulates metallothioneins, thereby increasing intracellular accumulation of zinc, autophagy, and bacterial clearance by macrophages. Gastroenterology. 2014;147:835–846. doi: 10.1053/j.gastro.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogler G., Vavricka S. Anemia in inflammatory bowel disease: An under-estimated problem? Front. Med. (Lausanne) 2014;1:58. doi: 10.3389/fmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahid S.S., Minor K.S., Soto R.E., Hornung C.A., Galandiuk S. Smoking and inflammatory bowel disease: A meta-analysis. Mayo Clin. Proc. 2006;81:1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 52.Richardson C.E., Morgan J.M., Jasani B., Green J.T., Rhodes J., Williams G.T., Lindstrom J., Wonnacott S., Peel S., Thomas G.A. Effect of smoking and transdermal nicotine on colonic nicotinic acetylcholine receptors in ulcerative colitis. QJM. 2003;96:57–65. doi: 10.1093/qjmed/hcg007. [DOI] [PubMed] [Google Scholar]
- 53.Khor B., Gardet A., Xavier R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pituch-Zdanowska A., Banaszkiewicz A., Albrecht P. The role of dietary fibre in inflammatory bowel disease. Prz. Gastroenterol. 2015;10:135–141. doi: 10.5114/pg.2015.52753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hildebrandt M.A., Hoffmann C., Sherrill-Mix S.A., Keilbaugh S.A., Hamady M., Chen Y.Y., Knight R., Ahima R.S., Bushman F., Wu G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parks B.W., Nam E., Org E., Kostem E., Norheim F., Hui S.T., Pan C., Civelek M., Rau C.D., Bennett B.J., et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geerling B.J., Dagnelie P.C., Badart-Smook A., Russel M.G., Stockbrugger R.W., Brummer R.J. Diet as a risk factor for the development of ulcerative colitis. Am. J. Gastroenterol. 2000;95:1008–1013. doi: 10.1111/j.1572-0241.2000.01942.x. [DOI] [PubMed] [Google Scholar]
- 58.Aldini R., Micucci M., Cevenini M., Fato R., Bergamini C., Nanni C., Cont M., Camborata C., Spinozzi S., Montagnani M., et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE. 2014;9:e108112. doi: 10.1371/journal.pone.0108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brolinson P.G., Elliott D. Exercise and the immune system. Clin. Sports Med. 2007;26:311–319. doi: 10.1016/j.csm.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Saxena A., Fletcher E., Larsen B., Baliga M.S., Durstine J.L., Fayad R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J. Inflamm. 2012;9:30. doi: 10.1186/1476-9255-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khalili H., Ananthakrishnan A.N., Konijeti G.G., Liao X., Higuchi L.M., Fuchs C.S., Spiegelman D., Richter J.M., Korzenik J.R., Chan A.T. Physical activity and risk of inflammatory bowel disease: Prospective study from the Nurses’ Health Study cohorts. BMJ. 2013;347:f6633. doi: 10.1136/bmj.f6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng S.C., Tang W., Leong R.W., Chen M., Ko Y., Studd C., Niewiadomski O., Bell S., Kamm M.A., de Silva H.J., et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut. 2015;64:1063–1071. doi: 10.1136/gutjnl-2014-307410. [DOI] [PubMed] [Google Scholar]
- 63.Loudon C.P., Corroll V., Butcher J., Rawsthorne P., Bernstein C.N. The effects of physical exercise on patients with Crohn’s disease. Am. J. Gastroenterol. 1999;94:697–703. doi: 10.1111/j.1572-0241.1999.00939.x. [DOI] [PubMed] [Google Scholar]
- 64.Engels M., Cross R.K., Long M.D. Exercise in patients with inflammatory bowel diseases: Current perspectives. Clin. Exp. Gastroenterol. 2018;11:1–11. doi: 10.2147/CEG.S120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bloomfield S.F., Stanwell-Smith R., Crevel R.W., Pickup J. Too clean, or not too clean: The hygiene hypothesis and home hygiene. Clin. Exp. Allergy. 2006;36:402–425. doi: 10.1111/j.1365-2222.2006.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng S.C., Bernstein C.N., Vatn M.H., Lakatos P.L., Loftus E.V., Jr., Tysk C., O’Morain C., Moum B., Colombel J.F., Epidemiology et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 67.Koloski N.A., Bret L., Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: A critical review of the literature. World J. Gastroenterol. 2008;14:165–173. doi: 10.3748/wjg.14.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carpio D., Barreiro-de Acosta M., Echarri A., Pereira S., Castro J., Ferreiro R., Lorenzo A., Group E. Influence of urban/rural and coastal/inland environment on the prevalence, phenotype, and clinical course of inflammatory bowel disease patients from northwest of Spain: A cross-sectional study. Eur J. Gastroenterol. Hepatol. 2015;27:1030–1037. doi: 10.1097/MEG.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 69.Piovani D., Danese S., Peyrin-Biroulet L., Nikolopoulos G.K., Lytras T., Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 70.Ungaro R., Bernstein C.N., Gearry R., Hviid A., Kolho K.L., Kronman M.P., Shaw S., Van Kruiningen H., Colombel J.F., Atreja A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: A meta-analysis. Am. J. Gastroenterol. 2014;109:1728–1738. doi: 10.1038/ajg.2014.246. [DOI] [PubMed] [Google Scholar]
- 71.Ortizo R., Lee S.Y., Nguyen E.T., Jamal M.M., Bechtold M.M., Nguyen D.L. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: A meta-analysis of case-controlled and cohort studies. Eur J. Gastroenterol. Hepatol. 2017;29:1064–1070. doi: 10.1097/MEG.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 72.Felder J.B., Korelitz B.I., Rajapakse R., Schwarz S., Horatagis A.P., Gleim G. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: A case-control study. Am. J. Gastroenterol. 2000;95:1949–1954. doi: 10.1111/j.1572-0241.2000.02262.x. [DOI] [PubMed] [Google Scholar]
- 73.Singh S., Graff L.A., Bernstein C.N. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am. J. Gastroenterol. 2009;104:1298–1313. doi: 10.1038/ajg.2009.15. [DOI] [PubMed] [Google Scholar]
- 74.Guslandi M. Exacerbation of inflammatory bowel disease by nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors: Fact or fiction? World J. Gastroenterol. 2006;12:1509–1510. doi: 10.3748/wjg.v12.i10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ananthakrishnan A.N., Long M.D., Martin C.F., Sandler R.S., Kappelman M.D. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 2013;11:965–971. doi: 10.1016/j.cgh.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Born J., Lange T., Hansen K., Molle M., Fehm H.L. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 77.Mahlmann L., Gerber M., Furlano R.I., Legeret C., Kalak N., Holsboer-Trachsler E., Brand S. Impaired objective and subjective sleep in children and adolescents with inflammatory bowel disease compared to healthy controls. Sleep Med. 2017;39:25–31. doi: 10.1016/j.sleep.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Keefer L., Stepanski E.J., Ranjbaran Z., Benson L.M., Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J. Clin. Sleep Med. 2006;2:409–416. [PubMed] [Google Scholar]
- 79.Ali T., Madhoun M.F., Orr W.C., Rubin D.T. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013;19:2440–2443. doi: 10.1097/MIB.0b013e3182a0ea54. [DOI] [PubMed] [Google Scholar]
- 80.Voigt R.M., Forsyth C.B., Green S.J., Mutlu E., Engen P., Vitaterna M.H., Turek F.W., Keshavarzian A. Circadian disorganization alters intestinal microbiota. PLoS ONE. 2014;9:e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bharwani A., Mian M.F., Foster J.A., Surette M.G., Bienenstock J., Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Bengmark S. Gut microbiota, immune development and function. Pharmacol Res. 2013;69:87–113. doi: 10.1016/j.phrs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Xie R., Sun Y., Wu J., Huang S., Jin G., Guo Z., Zhang Y., Liu T., Liu X., Cao X., et al. Maternal High Fat Diet Alters Gut Microbiota of Offspring and Exacerbates DSS-Induced Colitis in Adulthood. Front. Immunol. 2018;9:2608. doi: 10.3389/fimmu.2018.02608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golubeva A.V., Crampton S., Desbonnet L., Edge D., O’Sullivan O., Lomasney K.W., Zhdanov A.V., Crispie F., Moloney R.D., Borre Y.E., et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. doi: 10.1016/j.psyneuen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Jasarevic E., Howerton C.L., Howard C.D., Bale T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collado M.C., Cernada M., Bauerl C., Vento M., Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 2012;3:352–365. doi: 10.4161/gmic.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donnet-Hughes A., Perez P.F., Dore J., Leclerc M., Levenez F., Benyacoub J., Serrant P., Segura-Roggero I., Schiffrin E.J. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr Soc. 2010;69:407–415. doi: 10.1017/S0029665110001898. [DOI] [PubMed] [Google Scholar]
- 88.Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marin I.A., Goertz J.E., Ren T., Rich S.S., Onengut-Gumuscu S., Farber E., Wu M., Overall C.C., Kipnis J., Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017;7:43859. doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galley J.D., Nelson M.C., Yu Z., Dowd S.E., Walter J., Kumar P.S., Lyte M., Bailey M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey M.T., Coe C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35:146–155. doi: 10.1002/(SICI)1098-2302(199909)35:2<146::AID-DEV7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 92.O’Mahony S.M., Marchesi J.R., Scully P., Codling C., Ceolho A.M., Quigley E.M., Cryan J.F., Dinan T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 93.Gao X., Cao Q., Cheng Y., Zhao D., Wang Z., Yang H., Wu Q., You L., Wang Y., Lin Y., et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA. 2018;115:E2960–E2969. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soderholm J.D., Yang P.C., Ceponis P., Vohra A., Riddell R., Sherman P.M., Perdue M.H. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 95.Saunders P.R., Santos J., Hanssen N.P., Yates D., Groot J.A., Perdue M.H. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig. Dis. Sci. 2002;47:208–215. doi: 10.1023/A:1013204612762. [DOI] [PubMed] [Google Scholar]
- 96.Meddings J.B., Swain M.G. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 97.Santos J., Yang P.C., Soderholm J.D., Benjamin M., Perdue M.H. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gareau M.G., Sherman P.M., Walker W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zareie M., Johnson-Henry K., Jury J., Yang P.C., Ngan B.Y., McKay D.M., Soderholm J.D., Perdue M.H., Sherman P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia-Rodenas C.L., Bergonzelli G.E., Nutten S., Schumann A., Cherbut C., Turini M., Ornstein K., Rochat F., Corthesy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J. Pediatr. Gastroenterol. Nutr. 2006;43:16–24. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- 101.Noguera J.C., Aira M., Perez-Losada M., Dominguez J., Velando A. Glucocorticoids modulate gastrointestinal microbiome in a wild bird. R. Soc. Open Sci. 2018;5:171743. doi: 10.1098/rsos.171743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Da Silva S., Robbe-Masselot C., Ait-Belgnaoui A., Mancuso A., Mercade-Loubiere M., Salvador-Cartier C., Gillet M., Ferrier L., Loubiere P., Dague E., et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: Prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G420–G429. doi: 10.1152/ajpgi.00290.2013. [DOI] [PubMed] [Google Scholar]
- 103.Walker L.S., Garber J., Smith C.A., Van Slyke D.A., Claar R.L. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J. Consult. Clin. Psychol. 2001;69:85–91. doi: 10.1037/0022-006X.69.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zijlmans M.A., Korpela K., Riksen-Walraven J.M., de Vos W.M., de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Maes M., Kubera M., Leunis J.C., Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 106.Van der Zaag-Loonen H.J., Grootenhuis M.A., Last B.F., Derkx H.H. Coping strategies and quality of life of adolescents with inflammatory bowel disease. Qual. Life Res. 2004;13:1011–1019. doi: 10.1023/B:QURE.0000025598.89003.0c. [DOI] [PubMed] [Google Scholar]
- 107.McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255-e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 110.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Bjorkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.F., Rougeot C., Pichelin M., Cazaubiel M., et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 113.Li N., Wang Q., Wang Y., Sun A., Lin Y., Jin Y., Li X. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2018;12:266. doi: 10.3389/fnbeh.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao S., Srinivasjois R., Patole S. Prebiotic supplementation in full-term neonates: A systematic review of randomized controlled trials. Arch. Pediatr. Adolesc. Med. 2009;163:755–764. doi: 10.1001/archpediatrics.2009.94. [DOI] [PubMed] [Google Scholar]
- 115.Dhabhar F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 116.Viswanathan K., Dhabhar F.S. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc. Natl. Acad. Sci. USA. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dhabhar F.S., Miller A.H., McEwen B.S., Spencer R.L. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J. Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 118.Viswanathan K., Daugherty C., Dhabhar F.S. Stress as an endogenous adjuvant: Augmentation of the immunization phase of cell-mediated immunity. Int. Immunol. 2005;17:1059–1069. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- 119.Maes M., Christophe A., Bosmans E., Lin A., Neels H. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol. Psychiatry. 2000;47:910–920. doi: 10.1016/S0006-3223(99)00268-1. [DOI] [PubMed] [Google Scholar]
- 120.Larson M.R., Ader R., Moynihan J.A. Heart rate, neuroendocrine, and immunological reactivity in response to an acute laboratory stressor. Psychosom. Med. 2001;63:493–501. doi: 10.1097/00006842-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 121.Schmid-Ott G., Jaeger B., Meyer S., Stephan E., Kapp A., Werfel T. Different expression of cytokine and membrane molecules by circulating lymphocytes on acute mental stress in patients with atopic dermatitis in comparison with healthy controls. J. Allergy Clin. Immunol. 2001;108:455–462. doi: 10.1067/mai.2001.117800. [DOI] [PubMed] [Google Scholar]
- 122.Alsulami S., Al Omar Z., Binnwejim M.S., Alhamdan F., Aldrees A., Al-Bawardi A., Alsohim M., Alhabeeb M. Perception of academic stress among Health Science Preparatory Program students in two Saudi universities. Adv. Med. Educ. Pract. 2018;9:159–164. doi: 10.2147/AMEP.S143151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Connor T.J., Brewer C., Kelly J.P., Harkin A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J. Neuroimmunol. 2005;159:119–128. doi: 10.1016/j.jneuroim.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 124.Elenkov I.J. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 125.Frank M.G., Frank J.L.W., Hendricks S.E., Burke W.J., Johnson D.R. Age at onset of major depressive disorder predicts reductions in NK cell number and activity. J. Affect. Disord. 2002;71:159–167. doi: 10.1016/S0165-0327(01)00395-0. [DOI] [PubMed] [Google Scholar]
- 126.Danner M., Kasl S.V., Abramson J.L., Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom. Med. 2003;65:347–356. doi: 10.1097/01.PSY.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 127.Chang Y.M., El-Zaatari M., Kao J.Y. Does stress induce bowel dysfunction? Expert. Rev. Gastroenterol. Hepatol. 2014;8:583–585. doi: 10.1586/17474124.2014.911659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vrakas S., Mountzouris K.C., Michalopoulos G., Karamanolis G., Papatheodoridis G., Tzathas C., Gazouli M. Intestinal Bacteria Composition and Translocation of Bacteria in Inflammatory Bowel Disease. PLoS ONE. 2017;12:e0170034. doi: 10.1371/journal.pone.0170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slyepchenko A., Maes M., Jacka F.N., Kohler C.A., Barichello T., McIntyre R.S., Berk M., Grande I., Foster J.A., Vieta E., et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2017;86:31–46. doi: 10.1159/000448957. [DOI] [PubMed] [Google Scholar]
- 130.Mizoguchi A., Takeuchi T., Himuro H., Okada T., Mizoguchi E. Genetically engineered mouse models for studying inflammatory bowel disease. J. Pathol. 2016;238:205–219. doi: 10.1002/path.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singh B., Read S., Asseman C., Malmstrom V., Mottet C., Stephens L.A., Stepankova R., Tlaskalova H., Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 2001;182:190–200. doi: 10.1034/j.1600-065X.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 132.Tlaskalova-Hogenova H., Tuckova L., Stepankova R., Hudcovic T., Palova-Jelinkova L., Kozakova H., Rossmann P., Sanchez D., Cinova J., Hrncir T., et al. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann. N. Y. Acad. Sci. 2005;1051:787–798. doi: 10.1196/annals.1361.122. [DOI] [PubMed] [Google Scholar]
- 133.Rabizadeh S., Rhee K.J., Wu S., Huso D., Gan C.M., Golub J.E., Wu X., Zhang M., Sears C.L. Enterotoxigenic bacteroides fragilis: A potential instigator of colitis. Inflamm. Bowel Dis. 2007;13:1475–1483. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahida Y.R., Makh S., Hyde S., Gray T., Borriello S.P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: Induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brito G.A., Fujji J., Carneiro-Filho B.A., Lima A.A., Obrig T., Guerrant R.L. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 2002;186:1438–1447. doi: 10.1086/344729. [DOI] [PubMed] [Google Scholar]
- 136.Ghia J.E., Blennerhassett P., Deng Y., Verdu E.F., Khan W.I., Collins S.M. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 137.Zamani S., Hesam Shariati S., Zali M.R., Asadzadeh Aghdaei H., Sarabi Asiabar A., Bokaie S., Nomanpour B., Sechi L.A., Feizabadi M.M. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017;9:53. doi: 10.1186/s13099-017-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Issa M., Vijayapal A., Graham M.B., Beaulieu D.B., Otterson M.F., Lundeen S., Skaros S., Weber L.R., Komorowski R.A., Knox J.F., et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2007;5:345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 139.Baumgart M., Dogan B., Rishniw M., Weitzman G., Bosworth B., Yantiss R., Orsi R.H., Wiedmann M., McDonough P., Kim S.G., et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 140.Darfeuille-Michaud A., Boudeau J., Bulois P., Neut C., Glasser A.L., Barnich N., Bringer M.A., Swidsinski A., Beaugerie L., Colombel J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 141.Ford D.E., Erlinger T.P. Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 142.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 143.De Punder K., Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015;6:223. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Whitehead W.E., Palsson O., Jones K.R. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 145.Loftus E.V., Jr., Guerin A., Yu A.P., Wu E.Q., Yang M., Chao J., Mulani P.M. Increased risks of developing anxiety and depression in young patients with Crohn’s disease. Am. J. Gastroenterol. 2011;106:1670–1677. doi: 10.1038/ajg.2011.142. [DOI] [PubMed] [Google Scholar]
- 146.Targownik L.E., Sexton K.A., Bernstein M.T., Beatie B., Sargent M., Walker J.R., Graff L.A. The Relationship Among Perceived Stress, Symptoms, and Inflammation in Persons With Inflammatory Bowel Disease. Am. J. Gastroenterol. 2015;110:1001–1012. doi: 10.1038/ajg.2015.147. [DOI] [PubMed] [Google Scholar]
- 147.Coskun M., Vermeire S., Nielsen O.H. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol. Sci. 2017;38:127–142. doi: 10.1016/j.tips.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 148.Torres J., Ellul P., Langhorst J., Mikocka-Walus A., Barreiro-de Acosta M., Basnayake C., Ding N.J.S., Gilardi D., Katsanos K., Moser G., et al. European Crohn’s and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J. Crohns Colitis. 2019;13:673–685e. doi: 10.1093/ecco-jcc/jjz051. [DOI] [PubMed] [Google Scholar]
- 149.Regueiro M., Greer J.B., Szigethy E. Etiology and Treatment of Pain and Psychosocial Issues in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:430–439 e434. doi: 10.1053/j.gastro.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 150.Baird C.L., Sands L.P. Effect of guided imagery with relaxation on health-related quality of life in older women with osteoarthritis. Res. Nurs. Health. 2006;29:442–451. doi: 10.1002/nur.20159. [DOI] [PubMed] [Google Scholar]
- 151.Garcia-Vega E., Fernandez-Rodriguez C. A stress management programme for Crohn’s disease. Behav. Res. Ther. 2004;42:367–383. doi: 10.1016/S0005-7967(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 152.Keefer L., Kiebles J.L., Martinovich Z., Cohen E., Van Denburg A., Barrett T.A. Behavioral interventions may prolong remission in patients with inflammatory bowel disease. Behav. Res. Ther. 2011;49:145–150. doi: 10.1016/j.brat.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCombie A.M., Mulder R.T., Gearry R.B. Psychotherapy for inflammatory bowel disease: A review and update. J. Crohns Colitis. 2013;7:935–949. doi: 10.1016/j.crohns.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 154.Vermeire S., Van Assche G., Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 155.Gerbarg P.L., Jacob V.E., Stevens L., Bosworth B.P., Chabouni F., DeFilippis E.M., Warren R., Trivellas M., Patel P.V., Webb C.D., et al. The Effect of Breathing, Movement, and Meditation on Psychological and Physical Symptoms and Inflammatory Biomarkers in Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm. Bowel Dis. 2015;21:2886–2896. doi: 10.1097/MIB.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 156.Neilson K., Ftanou M., Monshat K., Salzberg M., Bell S., Kamm M.A., Connell W., Knowles S.R., Sevar K., Mancuso S.G., et al. A Controlled Study of a Group Mindfulness Intervention for Individuals Living With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016;22:694–701. doi: 10.1097/MIB.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 157.Langhorst J., Mueller T., Luedtke R., Franken U., Paul A., Michalsen A., Schedlowski M., Dobos G.J., Elsenbruch S. Effects of a comprehensive lifestyle modification program on quality-of-life in patients with ulcerative colitis: A twelve-month follow-up. Scand. J. Gastroenterol. 2007;42:734–745. doi: 10.1080/00365520601101682. [DOI] [PubMed] [Google Scholar]
- 158.Szigethy E., Kenney E., Carpenter J., Hardy D.M., Fairclough D., Bousvaros A., Keljo D., Weisz J., Beardslee W.R., Noll R., et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J. Am. Acad. Child. Adolesc Psychiatry. 2007;46:1290–1298. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 159.Keefer L., Kiebles J.L., Kwiatek M.A., Palsson O., Taft T.H., Martinovich Z., Barrett T.A. The potential role of a self-management intervention for ulcerative colitis: A brief report from the ulcerative colitis hypnotherapy trial. Biol. Res. Nurs. 2012;14:71–77. doi: 10.1177/1099800410397629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Larsson K., Sundberg Hjelm M., Karlbom U., Nordin K., Anderberg U.M., Loof L. A group-based patient education programme for high-anxiety patients with Crohn disease or ulcerative colitis. Scand. J. Gastroenterol. 2003;38:763–769. doi: 10.1080/00365520310003309. [DOI] [PubMed] [Google Scholar]
- 161.Smith G.D., Watson R., Roger D., McRorie E., Hurst N., Luman W., Palmer K.R. Impact of a nurse-led counselling service on quality of life in patients with inflammatory bowel disease. J. Adv. Nurs. 2002;38:152–160. doi: 10.1046/j.1365-2648.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- 162.Maunder R.G., Esplen M.J. Supportive-expressive group psychotherapy for persons with inflammatory bowel disease. Can. J. Psychiatry. 2001;46:622–626. doi: 10.1177/070674370104600706. [DOI] [PubMed] [Google Scholar]
- 163.Mizrahi M.C., Reicher-Atir R., Levy S., Haramati S., Wengrower D., Israeli E., Goldin E. Effects of guided imagery with relaxation training on anxiety and quality of life among patients with inflammatory bowel disease. Psychol Health. 2012;27:1463–1479. doi: 10.1080/08870446.2012.691169. [DOI] [PubMed] [Google Scholar]
- 164.Berrill J.W., Sadlier M., Hood K., Green J.T. Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. J. Crohns Colitis. 2014;8:945–955. doi: 10.1016/j.crohns.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 165.Perera L.P., Radigan M., Guilday C., Banerjee I., Eastwood D., Babygirija R., Massey B.T. Presence of Irritable Bowel Syndrome Symptoms in Quiescent Inflammatory Bowel Disease Is Associated with High Rate of Anxiety and Depression. Dig. Dis. Sci. 2019 doi: 10.1007/s10620-019-05488-8. [DOI] [PubMed] [Google Scholar]
- 166.Gracie D.J., Irvine A.J., Sood R., Mikocka-Walus A., Hamlin P.J., Ford A.C. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017;2:189–199. doi: 10.1016/S2468-1253(16)30206-0. [DOI] [PubMed] [Google Scholar]
- 167.Elsenbruch S., Langhorst J., Popkirowa K., Muller T., Luedtke R., Franken U., Paul A., Spahn G., Michalsen A., Janssen O.E., et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother. Psychosom. 2005;74:277–287. doi: 10.1159/000086318. [DOI] [PubMed] [Google Scholar]
- 168.Cheifetz A.S., Gianotti R., Luber R., Gibson P.R. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:415–429 e415. doi: 10.1053/j.gastro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Kimball E.S., Schneider C.R., Wallace N.H., Hornby P.J. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- 170.Massa F., Marsicano G., Hermann H., Cannich A., Monory K., Cravatt B.F., Ferri G.L., Sibaev A., Storr M., Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Investig. 2004;113:1202–1209. doi: 10.1172/JCI200419465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Naftali T., Lev L.B., Yablecovitch D., Half E., Konikoff F.M. Treatment of Crohn’s disease with cannabis: An observational study. Isr Med. Assoc. J. 2011;13:455–458. [PubMed] [Google Scholar]
- 172.Naftali T., Bar-Lev Schleider L., Dotan I., Lansky E.P., Sklerovsky Benjaminov F., Konikoff F.M. Cannabis induces a clinical response in patients with Crohn’s disease: A prospective placebo-controlled study. Clin. Gastroenterol. Hepatol. 2013;11:1276–1280 e1271. doi: 10.1016/j.cgh.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 173.Froicu M., Weaver V., Wynn T.A., McDowell M.A., Welsh J.E., Cantorna M.T. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol. Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 174.Jorgensen S.P., Agnholt J., Glerup H., Lyhne S., Villadsen G.E., Hvas C.L., Bartels L.E., Kelsen J., Christensen L.A., Dahlerup J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 175.Miheller P., Muzes G., Hritz I., Lakatos G., Pregun I., Lakatos P.L., Herszenyi L., Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm. Bowel Dis. 2009;15:1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 176.Ananthakrishnan A.N. Vitamin D and Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016;12:513–515. [PMC free article] [PubMed] [Google Scholar]
- 177.Pan Y., Liu Y., Guo H., Jabir M.S., Liu X., Cui W., Li D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients. 2017;9:382. doi: 10.3390/nu9040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.O’Connor E.M., Grealy G., McCarthy J., Desmond A., Craig O., Shanahan F., Cashman K.D. Effect of phylloquinone (vitamin K1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn’s disease. Br. J. Nutr. 2014;112:1163–1174. doi: 10.1017/S0007114514001913. [DOI] [PubMed] [Google Scholar]
- 179.Ng V., Millard W., Lebrun C., Howard J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin. J. Sport Med. 2007;17:384–388. doi: 10.1097/JSM.0b013e31802b4fda. [DOI] [PubMed] [Google Scholar]
- 180.Klare P., Nigg J., Nold J., Haller B., Krug A.B., Mair S., Thoeringer C.K., Christle J.W., Schmid R.M., Halle M., et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion. 2015;91:239–247. doi: 10.1159/000371795. [DOI] [PubMed] [Google Scholar]
- 181.Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akram W., Garud N., Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov. Ther. 2019;13:1–8. doi: 10.5582/ddt.2019.01000. [DOI] [PubMed] [Google Scholar]