Abstract
Chronic inflammation and oxidative stress are common and co-substantial pathological processes accompanying and contributing to cancers. Numerous epidemiological studies have indicated that non-steroidal anti-inflammatory drugs (NSAIDs) could have a positive effect on both the prevention of cancer and tumor therapy. Numerous hypotheses have postulated that NSAIDs could slow tumor growth by acting on both chronic inflammation and oxidative stress. This review takes a closer look at these hypotheses. In the cancer process, one of the major signaling pathways involved is the WNT/β-catenin pathway, which appears to be upregulated. This pathway is closely associated with both chronic inflammation and oxidative stress in cancers. The administration of NSAIDs has been observed to help in the downregulation of the WNT/β-catenin pathway and thus in the control of tumor growth. NSAIDs act as PPARγ agonists. The WNT/β-catenin pathway and PPARγ act in opposing manners. PPARγ agonists can promote cell cycle arrest, cell differentiation, and apoptosis, and can reduce inflammation, oxidative stress, proliferation, invasion, and cell migration. In parallel, the dysregulation of circadian rhythms (CRs) contributes to cancer development through the upregulation of the canonical WNT/β-catenin pathway. By stimulating PPARγ expression, NSAIDs can control CRs through the regulation of many key circadian genes. The administration of NSAIDs in cancer treatment would thus appear to be an interesting therapeutic strategy, which acts through their role in regulating WNT/β-catenin pathway and PPARγ activity levels.
Keywords: non-steroidal anti-inflammatory drug, cancer, WNT, inflammation, oxidative stress, PPARγ
1. Introduction
The complex process of cancer can be defined in terms of three stages: initiation, promotion, and progression [1,2,3]. Many cancers are initiated by chronic inflammation, involving numerous physical, chemical, and biological determinants [4]. Several studies have examined the association between chronic inflammation and cancer [5,6] and indeed have highlighted the promising role of anti-inflammatory treatments for cancer [7]. Chronic inflammation is responsible for the different stages observed in cancers, such as invasion, angiogenesis, proliferation, and metastasis [8,9,10].
In parallel, oxidative stress causes DNA damage in cancers [11]. In the past few years, the combined effect of oxidative stress and chronic inflammation has been the subject of several studies [12]. Reactive oxygen species production (ROS) is increased by the activation of inflammatory factors [13,14,15] and thus also participates in the processes of invasion, proliferation, angiogenesis, and then metastasis [16]. The canonical WNT/β-catenin pathway controls numerous other pathways involved in cancer development and tissue homeostasis. This pathway is regulated from transcription-level regulations to post-transcriptional modifications. In cancers, an aberrant WNT/β-catenin pathway is generally observed and leads to oxidative stress and inflammation [12,17,18].
Several epidemiological studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) could have a positive effect on both the prevention of cancer and tumor therapy. Moreover, the regular administration of aspirin, a NSAID, has been found to be correlated with a reduction in cancer incidence [19]. A regular therapy of more than 75 mg/day of aspirin diminishes the incidence of several cancers and tumor metastases, leading to an improvement in survival rates [20]. Regular use of NSAIDs is associated with a reduced incidence of several cancers, such as breast cancer, lung cancer, and gliomas [21,22]. Recent data have shown that the use of aspirin is associated with a reduction in the incidence of death from cancer, as well as in metastatic spread [19,20,23]. Anti-inflammatory drugs are commonly used in clinical practice due to their analgesic, anti-inflammatory and antipyretic effects. Furthermore, NSAIDs are often used in conjunction with other drugs in treating a number of diseases. Numerous hypotheses have postulated that NSAIDs could decrease tumor growth by acting on both chronic inflammation and oxidative stress [24]. Anti-inflammatory drugs could be used to target the chronic inflammatory microenvironment of tumors. It is well known that the human body is capable of self-healing after a short-term inflammatory response, but a long-term chronic inflammation could lead to initiation of the cancer process. Many studies have shown that inflammatory factors, including interleukins, TNF-α, nuclear factor-κB (NF-κB) and ROS production-induced inflammation, infiltrate the inflammatory microenvironment, leading to DNA damage and ultimately the initiation of the cancer process [25,26].
NSAIDS act as peroxisome proliferator-activated receptor gamma (PPARγ) agonists and could thus downregulate the aberrant WNT/β-catenin pathway in cancers [22]. PPARγ agonists offer an interesting therapeutic solution in cancers by acting on both oxidative stress and inflammation [27,28]. Indeed, in several tissues, canonical WNT/β-catenin pathway activation leads to inactivate PPARγ, while PPARγ activation inhibits the canonical WNT/β-catenin pathway. In cancers, the canonical WNT/β-catenin pathway is overactivated while PPARγ is decreased [12]. In parallel, the disruption of circadian rhythms (CRs) has been shown in cancers [29]. This dysregulation upregulates the canonical WNT/β-catenin pathway, which participates in the cancer process. PPARγ modulates CRs by regulating some circadian genes, such as Bmal1 (brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1) [30], and can directly target the WNT pathway [31]. Numerous evidence points to the anti-cancer benefits of NSAIDs, even if these benefits remain unclear and poorly understood. Nevertheless, data from experiments suggest a potential role for NSAIDs in the treatment of cancer through the regulation of the WNT/β-catenin pathway [32].
This review focuses on the interest of using NSAIDs in cancer therapy through their capacity to regulate the aberrant canonical WNT/β-catenin pathway and PPARγ, two systems that respond in an opposite manner.
2. Benefits and Disadvantages of NSAIDs-Based Cancer Prevention
2.1. NSAIDs and Cancer Prevention
A strategy for reducing cancer risks could involve the use of NSAIDs (such as aspirin, naproxen, or ibuprofen) [20]. Some studies have presented a correlation between the long-term use of aspirin and a reduction in both the incidence and mortality of cancers, a reduction that can vary from 20% to 75% [20]. The most marked effects have been observed in colorectal cancers, stomach cancers, and esophageal adenocarcinoma, while less marked effects have been found in lung, prostate, and breast cancers [20]. In contrast, little benefit has been observed in pancreatic and endometrial cancers [20]. Numerous molecular mechanisms could explain the link between NSAIDs and cancer prevention, such as COX inhibition, immune response, PI3K/Akt pathway downregulation, pro-inflammatory response, and decreased glycolytic signaling in tumor cells [22,33]. COX inhibition is associated with the reduction in inflammatory mediators including prostaglandins [34]. The activation of COX in the cancer process leads to the expression of prostaglandin E3 (PGE2), which induces angiogenesis, tumor growth and metastasis [35]. Furthermore, PGE2 stimulates several signaling pathways, such as the PI3K/Akt and NF-κB, which induce tumorigenesis [35]. Finally, recent studies have shown that NSAIDs could also act on other signaling pathways, such as iNOS, TNF-alpha and interleukins [33].
2.2. NSAIDs Lead to Cancer Cell Apoptosis
The use of NSAID (aspirin) in ovarian cancer cells decreases Bcl-2 expression and increases Bax gene expression [24]. The role of Bcl-2 in cancer is to inhibit apoptosis by changing mitochondria thiol, affecting mitochondria membrane permeability, and translocating to the mitochondria membrane the apoptotic protein precursor Apaf-1 to inhibit the role of the latter. The NSAID sulindac diminishes the expression of both the protein Bcl-XL and the Bcl-XL antagonist of Bax to induce activation of caspase cascade, which stimulates the apoptosis process [36]. Aspirin can change mitochondrial permeability to downregulate Bcl-2 expression, block ATP synthesis, and release cytochrome C, which triggers apoptosis [37]. The NSAID celecoxib activates the p53-upegulated modulator of apoptosis (PUMA) to increase p53 expression and thus initiate apoptosis [38].
2.3. NSAIDs Inhibit COX-2
NSAIDs are known to protect cells from one step on the path to cancer through the inhibition of COX [39]. COX presents three subtypes: COX-1, COX-2, and COX-3 (in the nervous system) [40,41]. COX-1 catalyzes the production of prostaglandins (PGs) to maintain physiological functions. COX-2, a membrane-bound protein, is not expressed in normal cells but overexpressed in inflammation and tumors [42]. The PG overexpression is induced by COX-2 catalysis during inflammation that leads to neovascularization to provide nutrition for tumor proliferation [43]. In parallel, in tumors, COX-2 upregulates Bcl-2 expression to initiate the anti-apoptotic process [44], while it modulates MMP-2 expression, which induces tumor invasion and metastasis [45]. Celecoxib, a NSAID and a COX-2 enzyme inhibitor, can downregulate tumor proliferation and can induce apoptosis in a variety of tumor cells [24]. By directly blocking COX-2 expression, NSAIDS could prevent cancer initiation [24].
2.4. NSAIDs and the Akt Pathway
Colorectal cancer presents epidermal growth factor receptor (EGFR) overexpression and the use of aspirin can downregulate EGFR [46]. EGFR is involved in several pathophysiological responses in cancer, such as migration, proliferation, and invasion [47,48]. The Akt pathway is stimulated by EGFR [49]. An aberrant WNT/β-catenin pathway activates EGFR activity [18]. Furthermore, NSAIDs dephosphorylate Akt signaling and decrease MMP-2 gene expression to stop invasion and cell growth [50,51].
2.5. NSAIDs and Their Side Effects
The long-term use of NSAIDs could result in the appearance of side effects such as renal failure and gastro-intestinal symptoms (bleeding, mucosal lesions, inflammation leading to intestinal strictures and perforation, peptic ulcers) [52]. The administration of NSAIDs also increases the risk of deep vein thrombosis and pulmonary embolism, myocardial infarction, and stroke [53,54,55]. Numerous COX inhibitors have been withdrawn because of the associated increased risk of thromboembolic events. Celecoxib remains the only selective COX inhibitor available in the US and Europe [56]. COX inhibitor herbal medicines, such as Cordia myxa fruit, would appear to be promising “NSAID-like” agents in that they inhibit cancer and inflammation [52].
2.6. PPARγ: A Therapeutic Solution Induced by NSAIDs
NSAIDS act as PPARγ agonists by inhibiting COX-2 in gliomas [57] and colon cancer [58]. In parallel, NSAIDs present a COX independent anti-carcinogenic action through the direct control of the PPARγ expression [59,60]. Several studies have shown that NSAID action is modulated by PPARγ [61,62]. Studies have shown the potential impact of NSAIDs through the interplay of PPARγ and the WNT/β-catenin pathway [22].
2.7. PPARγ in Cancers
The ligand-activated transcriptional factor peroxisome proliferator receptor γ (PPARγ) is a component of the nuclear hormone receptor super family. It makes a heterodimer with retinoid X receptor (RXR), forming a PPARγ-RXR complex that binds to specific peroxisome proliferator response element (PPRE) regions in the DNA. It also activates numerous target genes involved in fatty acid transport (FABP3), cholesterol metabolism (CYP7A1, LXRα, CYP27), glucose homeostasis (PEPCK, GyK), and lipid catabolism (SCD-1). This dimer acts on other coactivator proteins like PGC-1α, and leads to specific gene overexpressions [63]. Glucose homeostasis, insulin sensitivity, lipid metabolism, immune responses, cell fate, and inflammation are regulated by PPARγ activation [64,65]. Circadian variations in blood pressure and heart rate are controlled by PPARγ expression by its interaction on Bmal1 [30,66]. PPARγ controls the expression of numerous genes implicated in inflammation, and it diminishes the activity of inflammation-related transcription factors such as NF-κB [67]. Several studies have shown decreased PPARγ expression in association with chronic inflammation in cancers [12,68].
2.8. Benefits and Disadvantages of PPAR Gamma Agonists in Cancers
Some positive effects have been observed with the administration of PPAR gamma agonists in the cancer process. PPAR gamma stimulation could reduce cancer development by the arrest of cell proliferation and the inhibition of the tumor growth factor [69]. The decrease in cyclin D1, a WNT target, is associated with the downregulation of cyclin-dependent kinase (CDK) and thus attenuates the phosphorylation of the retinoblastoma (Rb) protein leading to the arrest of the cell cycle [69]. Moreover, PPAR gamma agonists could induce apoptosis through intrinsic and extrinsic apoptosis pathways [70]. PPAR gamma activation is associated with the diminution in anti-apoptotic proteins, including Bcl-2, and the increase in p53 and the Bcl-2-associated death promoter (BAD) protein (B-4) [71]. TNF pathway activity is decreased by PPAR gamma agonists, leading to apoptosis [72]. In pancreatic cancer, invasiveness is affected by PPAR gamma activation, leading to the improvement of MMP-2 and the expression of plasminogen activation inhibitor-1 [72]. PPAR gamma agonists inhibit VEGF, IL-8, COX and thus suspend tumor angiogenesis [73]. In addition, PPAR gamma agonists reduce glycolytic pathway activity by altering the nutrient pathway and WNT signaling [74].
However, the use of PPAR gamma agonists does have some side effects, even if new molecules now have fewer disadvantages [53]. Rosiglitazone has been correlated with an augmentation of myocardial ischemia [75], but results from other studies remain unclear, showing no significant increase in cardiovascular events [76]. PPAR gamma agonist therapies appear to be correlated with an augmented risk of heart failure [77]. Rosiglitazone therapy enhances the risk of fatal and non-fatal heart failure ([76], and similar results have been observed with pioglitazone therapy [78]. Weight gain, edema formation, and fluid retention are other side effects of PPAR gamma agonists [79]. The administration of PPAR gamma agonists could also be associated with increased vascular permeability, leading to the appearance of peripheral edema [80].
3. Two Major Mechanisms Involved in Cancers: Chronic Inflammation and Oxidative Stress
3.1. Chronic Inflammation in Cancers
Some studies have presented that chronic inflammation leads to DNA damage and tissue injury [81]. Chronic inflammation impairs cell homeostasis and metabolism initiating the development of cancer [82]. Moreover, DNA damage from chronic inflammation provides a point of origin for the development of malignancy sites [83,84]. The relationship between cancer and chronic inflammation has been well documented by numerous studies [12,85]. Chronic inflammation stimulates ROS and reactive nitrogen species (RNS) production, leading to DNA damage [86]. Thus, genomic instabilities are caused by DNA damage and lead to the cancer process [87]. Several sites of common pathogenic infections are related to cancer initiation [88,89].
The immune system is also regulated by several inflammatory factors, such as the tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and tumor growth factor-β (TGF-β) [90,91]. TNF-α expression leads to DNA damage and cytokine stimulation (such as IL-17 [92]), which are responsible for tumor growth, invasion, and angiogenesis [93]. Interleukins IL6 and IL-17 stimulate the signal transducer and activator transcription (STAT) signaling involved in the cancer process [94].
Chronic inflammation is also responsible for an augmentation in cyclooxygenase 2 (COX-2, a prostaglandin-endoperoxidase synthase) [95]. Several cytokines (TNF-α, IL-1) activate COX-2 [90]. COX-2 stimulates ROS and RNS production [95,96]. NF-κB stimulates several pro-inflammatory factors that activate COX-2 and inducible nitric oxide synthase (iNOS) [82]. NF-κB is one of the major factors implicated in chronic inflammation in the cancer process [82,97]. Numerous studies have shown that NF-κB stimulates the expression of TNF-α, IL-6, IL-8, STAT3, COX-2, BCL-2 (B-cell lymphoma 2), metalloproteinases (MMPs), VEGF [82], and thus ROS production [98]. Il-6 and VEGF stimulate the STAT-3 pathway, which is involved in proliferation, angiogenesis and metastasis [99]. Numerous cancers show an over-activation of the STAT-3 pathway [100]. Furthermore, during chronic inflammation, iNOS, an enzyme catalyzing nitric oxide (NO), is stimulated [101], resulting in an increase in p53 gene mutations [90].
3.2. Oxidative Stress in Cancers
Oxidative stress is considered as an imbalance between the production and elimination of ROS and RNS [11,102]. Cell damage from oxidation and nitration of macromolecules enhances ROS production by activation of the NADPH oxidase (NOX) enzyme. This phenomenon leads to the reduction of the transfer of electrons through the mitochondrial membrane to reduce the molecular oxidative metabolism. ROS production has a key role in numerous signaling pathways that are involved in changes in the microenvironment [103]. Thus, dysfunctions in the respiratory chain of mitochondria are responsible for ROS production [104]. The inflammation observed in sites where there is damage involves the uptake of oxygen leading to the release of ROS and its accumulation [8,105]. NF-κB, STAT, hypoxia-inducible factors (HIF) and both activator protein-1 (AP-1) play a major role in stimulating this process [82]. Moreover, in a vicious circle, COX-2, TNF-α, IL-6, and iNOS are induced by oxidative stress [96]. NADPH-oxidase (NOX) is activated by chronic inflammation and increases oxidative stress, resulting in changes in nuclear signaling [106].
3.3. Interaction between Oxidative Stress and Inflammation
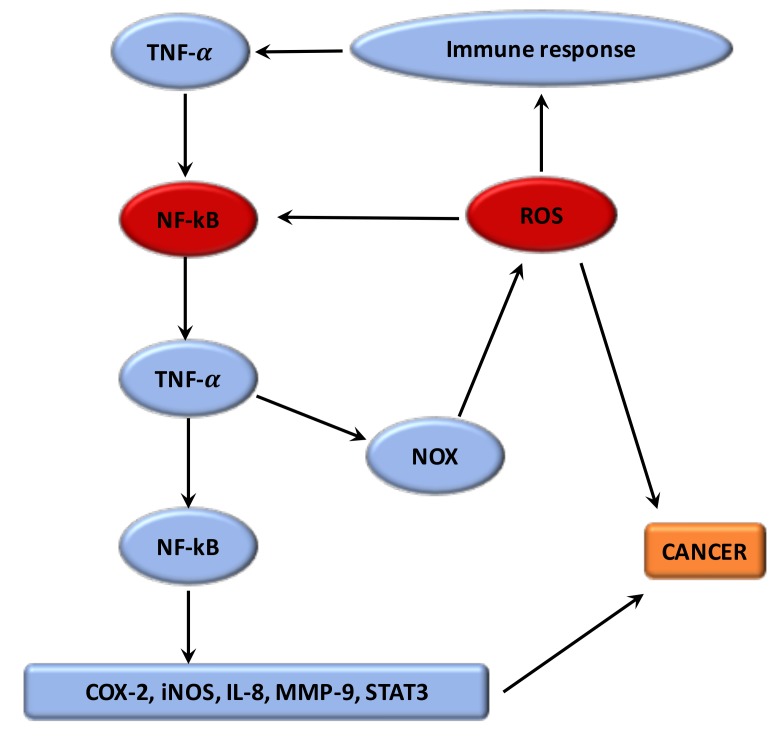
Numerous studies have shown that the phenomenon by which oxidative stress can enhance chronic inflammation, which in a negative feedback could lead to cancers [11] (Figure 1). The imbalance caused by oxidative stress leads to damage in the signaling in cells [102]. ROS have a main role both upstream and downstream of the NF-κB and TNF-α signaling pathways, which are the main mediators of the inflammation. The hydroxyl radical is the most harmful of all the ROS. A vicious circle is observed between ROS and these pathways. ROS are formed by the NOX system. Moreover, the proteins modified by ROS could result in initiation of the auto-immune response to stimulate TNF-α and thus NOX [107]. Nuclear factor erythroid-2 related factor 2 (Nrf2) is mainly associated with oxidative stress in inflammation [11]. Nrf2 is a transcription factor which binds with the antioxidant response element (ARE) [108]. The protective role of Nrf2 in cancer relates to its capacity to reduce inflammation and oxidative stress [109]. Several studies have presented that Nrf2 can have an anti-inflammatory role by regulating MAPK (mitogen-activated protein kinases), NF-κB, and PI3K pathways [110]. Thus, Nrf2 may play a major role in reducing oxidative damage [111]. Evidence suggests that mitochondrial dysregulation has a significant role in the cancer process [11].
Figure 1.
Relationship between ROS and chronic inflammation. The imbalance caused by oxidative stress leads to damage in the signaling in cells. ROS have a key role both upstream and downstream of the NF-κB and TNF-α signaling pathways, which are the main mediators of the inflammation. A vicious circle is observed between ROS and these pathways. ROS are generated by the NOX system. Proteins modified by ROS could result in activation of the auto-immune response to stimulate TNF-α and thus NOX. The dysregulation of these targets leads to the activation of several pathways involved in cancer initiation.
4. The WNT/β-Catenin Pathway
4.1. The WNT Pathway, Chronic Inflammation, and Oxidative Stress
Many studies have observed that the canonical WNT/β-catenin pathway increases the inflammatory process [81]. Furthermore, infection pathogens activate the WNT/β-catenin pathway, thereby enhancing inflammation [112]. ROS, stimulated by NOX, activates the canonical WNT/β-catenin pathway through the oxidization and inhibition of the nucleoredoxin (a redox-sensitive regulator), thus stimulating the cancer process [82]. ROS production stimulates c-Myc [113], STAT [114] and phosphatidylinositol-3-kinase (PI3K/Akt) [115], and the inhibition of PPARγ [116]. ROS production stimulates Akt signaling by inhibiting the phosphatase and tensin homolog deleted from chromosome (PTEN) [117,118]. The canonical WNT/β-catenin pathway may thus have a key role in the cancer process by stimulating both oxidative stress and inflammation [12]. The WNT pathway is the target of several inhibitors in therapeutic strategies to counteract tumorigenesis, such as OMP-54F28 [119,120,121], frizzled antibodies [122], tankyrase inhibitors [123], CBP inhibitors [124], and PORCN inhibitors [125].
4.2. The Sanonical WNT/β-Catenin Pathway, a Major Factor in Cancer Development
The name WNT is derived from wingless drosophila melanogaster and its mouse homolog Int. The WNT pathway is implicated in several pathways and regulating signaling pathways, like embryogenesis, cell proliferation, migration and polarity, apoptosis, and organogenesis [126]. During the adult stage, the WNT pathway is non-activated or silent. However, in numerous mechanisms and pathologies, such as inflammatory, metabolic and neurological disorders, and cancers, the WNT pathway may become dysregulated [127]. Some studies have utilized the WNT pathway for the cell therapy-bioengineering processes [128].
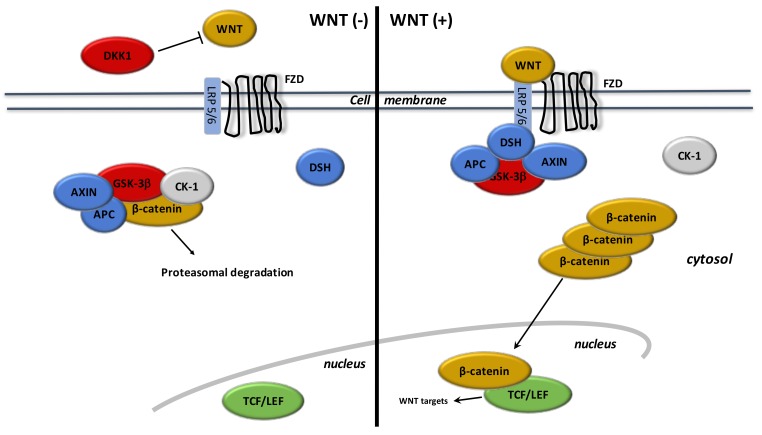
WNT ligands are lipoproteins that stimulate specific co-receptors (Figure 2). These WNT ligands stimulate the canonical WNT pathway by activation of β-catenin. WNT ligands stimulate frizzled (FZD) receptors and low-density lipoprotein receptor-related protein 5 and 6 (LRP 5/6) [129,130]. The complex formed by these extracellular WNT ligands and FZD/LRP5/6 activates the intracellular disheveled (DSH). This stimulation leads to inactivate the destruction complex of β-catenin in the cytoplasm. B-catenin accumulates in the cytoplasm and then migrates into the nucleus. Nuclear β-catenin links to T-Cell factor/lymphoid enhancer factor (TCF/LEF) to activate target gene transcriptors, such as c-Myc and cyclin D1 [131].
Figure 2.
The canonical WNT/β-catenin pathway. WNT (−). Under physiologic circumstances, the cytoplasmic β-catenin is linked to its destruction complex, consisting of APC, AXIN, and GSK-3β. After CK-1 phosphorylates on Ser45 residue, β-catenin is phosphorylated on Thr41, Ser37, and Ser33 residues by GSK-3β. Thus, phosphorylated β-catenin is destroyed into the proteasome. Then, cytoplasmic level of β-catenin is kept low in the non-presence of WNT ligands. If β-catenin is not accumulated in the nucleus, the TCF/LEF complex does not stimulate the target genes. DKK1 inhibits the WNT/β-catenin pathway through the bind to WNT ligands or LRP5/6. WNT (+). When WNT ligands activate both FZD and LRP5/6, DSH is stimulated and phosphorylated by FZD. Phosphorylated DSH in turn activates AXIN, which comes off β-catenin destruction complex. Thus, β-catenin escapes from phosphorylation and then accumulates in the cytoplasm. The accumulated cytosolic β-catenin moves into the nucleus, where it interacts with TCF/LEF and stimulates the transcription of target genes.
During the “off-state” of the WNT/β-catenin pathway, WNT ligands do not interact with FZD and LRP 5/6. The β-catenin destruction complex, composed by AXIN, APC (adenomatous polyposis coli) and GSK-3β (glycogen synthase kinase 3β), phosphorylates β-catenin. Then, phosphorylated β-catenin is destroyed into the proteasome.
Many WNT inhibitors downregulate the canonical WNT/β-catenin pathway. GSK-3β is the main inhibitor of the WNT pathway. GSK-3β is a neuron-specific intracellular serine-threonine kinase that controls numerous signaling pathways like inflammation, neuronal polarity, and cell membrane signaling [132,133,134]. GSK-3β downregulates β-catenin cytosolic stabilization and nuclear migration. Dickkopf (DKK) and soluble frizzled-related proteins (SFRP) are also WNT inhibitors and interact with FZD, LRP5, and LRP6 [135,136,137].
4.3. WNT and Inflammation in Cancer
Positive interplay between WNT/β-catenin and NF-κB has been observed [138]. The activation of the WNT/β-catenin leads to stimulate IκB-α (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) degradation and then NF-κB stimulation [139]. Stimulation of the target gene, CRD-BP (coding region determinant-binding protein, an RNA-binding protein), by activated β-catenin stabilizes mRNA of βTrCP (B-transducin repeat-containing protein) [140]. In colon cancer, the activation of both βTrCP and CRD-BP is correlated with the stimulation of the β-catenin and NF-κB, leading to proliferation and metastasis [140,141]. In breast cancer, TLR3 activation stimulates β-catenin, leading to overstimulation of the NF-κB pathway [142]. Moreover, the β-catenin and NF-κB pathways stimulate each other in diffuse large B-cell lymphomas [143]. The WNT/β-catenin pathway activates COX-2, which then enhances the inflammatory response [144]. E-cadherin and GSK-3β are downregulated in melanoma cells by β-catenin pathway [145]. Concomitant GSK-3β and E-cadherin inhibition with cytoplasmic accumulation of β-catenin leads to NF-κB-dependent iNOS expression in hepatic cells [146]. The WNT/β-catenin signaling stimulates its target TNFRSF19 in colon cancer, that leads to activation of NF-κB signaling [147]. Nevertheless, the observed synergistic interaction between β-catenin and NF-κB depends on the β-catenin-TCF/LEF link [148].
NF-κB overexpression inactivates GSK-3β whereas it stimulates β-catenin signaling [149,150]. GSK-3β activation leads to the downregulation of TNF-α-induced NF-κB activation in carcinoma cells [149]. IκB is stabilized by GSK-3β activation, resulting in the downregulation of the NF-κB pathway [150]. NF-κB signaling can modulate the WNT/β-catenin pathway by IKKα (IκB Kinase-α) use [151] and RelA [152]. IKKα stimulates β-catenin signaling while IKKβ inhibits β-catenin pathway [153]. IKKα activates the β-catenin/TCF/LEF link [154]. The stimulation of IKKα leads to the cytoplasmic accumulation of β-catenin resulting in GSK3-β and APC inactivation [151].
4.4. WNT and Oxidative Stress in Cancer
The over-activated PI3K/Akt pathway observed in the cancer process is stimulated by ROS production [155,156]. PTEN is the main inhibitor of the PI3K/Akt pathway [118]. NADPH oxidase and superoxide dismutase oxidize PTEN to inhibit it. Downregulation of PTEN leads to an increase in Akt activity, which enhances the phosphorylation of GSK-3β. Thus, GSK-3β inhibited by Akt does not bind β-catenin. Inactivation of PTEN stimulates Akt and β-catenin [157]. Moreover, ROS production participates in the stabilization of HIF-1α thereby activating glycolytic enzymes [68,155]. The WNT/β-catenin pathway stimulates HIF-1α by activating the PI3K/Akt pathway [18]. Although this mechanism remains unclear, recent studies have presented that ROS production stimulates the WNT/β-catenin pathway [158]. In parallel, Akt [159] and c-Myc [160] enhance ROS production.
5. Interplay between NSAIDs - PPARγ and the WNT/β-Catenin Pathway in Cancers
5.1. PPAR Gamma and the WNT/β-Catenin Pathway
The role of PPARγ agonists remains unclear in cancer cells, even if their role is well understood in the regulation of differentiation and stemness programs [161]. In physiological cells, PPARγ inhibits tumorigenesis and WNT signaling through the target of phosphorylated β-catenin at the proteasome through a process that involves its catenin-binding domain within PPARγ. Nevertheless, oncogenic β-catenin counteracts proteasomal degradation by downregulating PPARγ expression, that needs its TCF/LEF binding domain [162]. In adipocyte cells, PPARγ leads to increased differentiation and a reduction in proliferation by targeting the WNT/β-catenin pathway. PPARγ binds with GSK3-β to activate the differentiation factor C/EBPα, leading to adiponectin production [74,163]. PPARγ stimulation downregulates β-catenin at both the mRNA and protein levels to induce differentiation [164]. In metastatic prostate cancer LnCaP cells, PPARγ inhibits the WNT pathway by affecting phosphorylated β-catenin in the proteasome [162,165]. In colorectal and gastric cancer cells, PPARγ inhibits β-catenin signaling, cytoplasmic localization and target effectors, leading to the control of numerous genes, including telomerase reverse transcriptase and Sox9, both of which are implicated in cell differentiation and the survival phenomenon [166,167,168]. PPARγ agonists, by decreasing the WNT/β-catenin pathway, may be utilized in association with other drugs, including inhibitors of tyrosine kinases [169], Akt [170], and MAPK cascades, to maximize the anti-tumor and pro-differentiating effect.
5.2. NSAIDs and the WNT/β-Catenin Pathway
NSAIDs downregulate the activity of COX-2 and thereby inhibit the synthesis of prostaglandins (PGE2) and then the WNT pathway [171]. Recent studies have observed that NSAIDS can have an anti-tumor effect, having revealed a chemo preventive effect against colon cancer [172,173,174]. The possible cellular pathway underlying the chemo preventive effect of NSAIDs involves the induction of cell-cycle arrest, apoptosis, and angiogenesis inhibition [173,174].
Several studies have also shown that NSAIDs can inhibit the canonical WNT/β-catenin pathway [22]. Both aspirin and indomethacin downregulate the transcriptional activity of β-catenin/TCF-responsive genes [175]. NSAIDs diminishes nuclear β-catenin levels and leads to the degradation of β-catenin [176]. The NSAIDs, including sulindac, exisulind, and celecoxib, inhibit β-catenin levels and then decrease the transcriptional activity of the β-catenin/TCF/LEF complex [177]. Celecoxib directly decreases cancer cell growth by downregulating the expression of the WNT/β-catenin signaling pathway [178] and by inducing the degradation of the TCF7L2 [179]. Sulindac also inhibits the WNT/β-catenin pathway by downregulating nuclear β-catenin localization and β-catenin/TCF target gene transcription [180]. Colon cancer therapy with celecoxib is associated with an inhibition of the canonical WNT/β-catenin pathway [181,182]. Celecoxib inhibits the activity of the complex TCF/LEF and thus the activity of cyclin D1, suggesting that this component inhibits the expression of WNT/β-catenin target genes [182]. Aspirin decreases glioma cell proliferation and invasion by inhibiting β-catenin/TCF transcription [183]. It also stops glioma cells cycle at the G0/G1 phase and inhibits invasion and tumor growth by downregulating β-catenin/TCF activity [183,184].
6. Circadian clock: An Interesting Pathway in Cancer Development
6.1. Circadian Clock
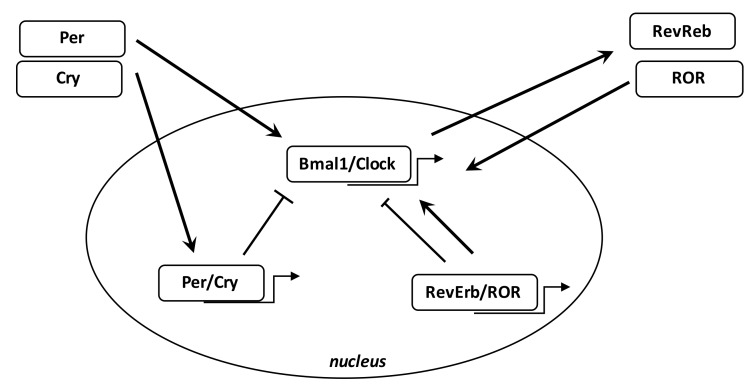
Numerous biological phenomena in the body are regulated by the circadian “clock” (circadian locomotors output cycles kaput). The circadian clock is in the hypothalamic suprachiasmatic nucleus (SCN). CRs are endogenous and entrainable free-running periods that last approximately 24 h. Numerous transcription factors can regulate CRs. These are called circadian locomotor output cycles kaput (Clock), brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1 (Bmal1), Period 1 (Per1), Period 2 (Per2), Period 3 (Per3), and Cryptochrome (Cry 1 and Cry 2) [185,186] (Figure 3). These factors are controlled by positive and negative self-regulation mediated by CRs [187,188]. Clock and Bmal1 heterodimerize and thus enhance the transcription of Per1, Per2, Cry1, and Cry2 [189]. The Per/Cry heterodimer can downregulate its stimulation through negative feedback. It migrates back to the nucleus to directly inhibit the Clock/Bmal1 complex and then repress its own transcription [189]. The Clock/Bmal1 heterodimer also stimulates the transcription of retinoic acid-related orphan nuclear receptors, Rev-Erbs, and retinoid-related orphan receptors (RORs). By a positive feedback, RORs stimulate Bmal1 transcription, while Rev-Erbs inhibit their transcription by a negative feedback [189].
Figure 3.
Circadian clock genes. The clock consists of a stimulatory circle, with the Bmal1/Clock heterodimer activating the transcription of Per and Cry genes, and an inhibitory feedback circle with the Per/Cry heterodimer translocating to the nucleus and repressing the transcription of the Clock and Bmal1 genes. An additional circle involves the RORs and RevErbs factors with a positive feedback by ROR and a negative feedback by RevErbs.
6.2. Circadian Clock Disruption in Cancers
Epidemiological and fundamental evidence supports the idea of linking circadian disruption with cancer [29]. DNA repair, apoptosis and cell cycle regulation follow circadian rhythms in humans [190]. Disruption of the CRs is correlated with dysregulation in cell proliferation and thus the initiation of cancer [191]. Clock/Bmal1, Per1 and Per2 maintain the rhythmic pattern of cell proliferation and repair of DNA damage [192,193]. Bmal1 overexpression has been observed in cell growth of NIH 3T3 cells [194]. Metastatic cancers present high levels of Clock or Bmal1 genes [195,196]. Clock overexpression is often associated with cell proliferation in colorectal carcinoma cells [197]. Bmal1 upregulation is found in certain types of pleural mesothelioma while Bmal1 knockdown is associated with reduced cell growth and induced apoptosis [198]. Bmal1 is considered an attractive target in leukemia cells [199].
7. Circadian Clock Disruption Enhances Both Inflammation and Oxidative Stress
7.1. Circadian Clock and Inflammation
Melatonin has been used in the treatment of chronic bowel inflammation resulting in decreasing inflammation through inhibition of COX-2 and iNOS [200]. Moreover, melatonin can act on iNOS and COX-2 by inhibiting p52 acetylation and transactivation [201]. Melatonin inhibits NF-κB and COX-2 in murine macrophage-like cells [202]. An anti-inflammatory response of melatonin has been observed through a decrease in NF-κB activity [203]. Melatonin downregulates the nuclear translocation of NF-κB, leading to an enhancement of anti-cancer effects in lung cancer [204].
7.2. Circadian Clock and Oxidative Stress
Recent studies have indicated that the hypoxic response in cancer could be directly controlled by the circadian rhythm Clock/Bmal1 [205]. In a similar way, blood oxygen levels present daily rhythms influenced by clock genes [206]. Metabolic dysregulation in cancers may result in disruption of Bmal1 in a hypoxic-dependent way [207]. Considerable evidence connects circadian disruption with hormone-dependent diseases, like breast and prostate cancers. One of the main factors is melatonin, a hormone produced by the pineal gland in a circadian manner to control sleep [208]. In the mitochondria, melatonin is linked to the regulation of oxidative stress [209]. Melatonin increases glutathione peroxidase and glutathione reductase activities [210]. Moreover, melatonin directly regulates the mitochondrial respiratory chain, which modulates ATP production [209]. Furthermore, alteration of melatonin secretion by sleep disruption could enhance ROS and RNS production [211].
8. WNT, NSAIDs, and PPAR Gamma with Circadian Clocks
8.1. The WNT/β-Catenin Pathway and the Circadian Clock
The WNT/β-catenin pathway is the downstream target of the RORs control factors and has several putative Bmal1 clock-binding sites within its promoter [212] (Figure 4). Through such relationships, circadian genes can control the progression of the cell cycle by the WNT signaling [213]. The WNT pathway can be inhibited by a Bmal1 knockdown [214]. Levels of WNT-related genes in wild-type mice are higher than those observed in Bmal1 knockdown mice [215,216]. Cell proliferation and cell cycle progression are controlled by Bmal1 through the activation of the canonical WNT/β-catenin pathway [217]. Bmal1 enhances β-catenin transcription, inhibits the degradation of β-catenin and downregulates GSK-3β activity [218]. Per2 degradation induced by β-catenin increases circadian disruption in the intestinal mucosa of ApcMin/+ mice [219].
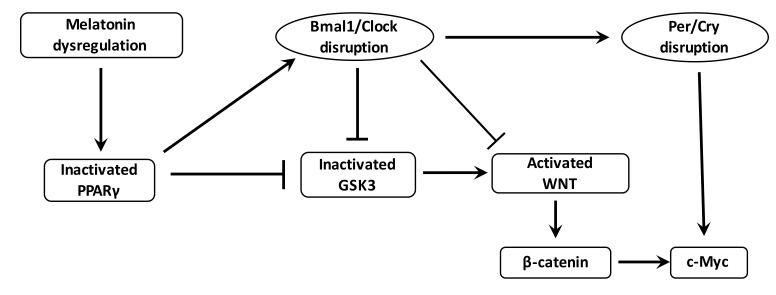
Figure 4.
Interactions between PPARγ, WNT pathway and circadian rhythms in cancer. Dysregulation of melatonin and nocturin decreases the expression of PPARγ in cancer. Decreased PPARγ dysregulates the Bmal1/Clock heterodimer. Decreased PPARγ expression directly activates the formation of the heterodimer Bmal1/Clock and β-catenin cytosolic accumulation but inhibits the activity of GSK3, the main inhibitor of the WNT/β-catenin pathway. Bmal1/Clock knockout also decreases GSK3 activity and activates the WNT/β-catenin pathway and its downstream gene c-Myc through the stimulation of the heterodimer Per/Cry. The activation of the WNT/β-catenin pathway by the cytosolic accumulation of the β-catenin and the activation of c-Myc lead to cancer initiation (oxidative stress and chronic inflammation).
In physiological circumstances, the core circadian genes work in accurate feedback circles and keep the molecular clockworks in the SCN. They permit the regulation of peripheral clocks [187,188]. Per1 and Per2 maintain cell circadian rhythms and control cell-related gene activity, including c-Myc, so as to sustain the physiologic cell cycle [220,221]. mRNAs and proteins levels of circadian genes oscillate throughout the 24-hour period [187].
8.2. NSAIDs and the Circadian Clock
Few studies have investigated the role of NSAIDs regarding the circadian clock. Nevertheless, Kowanko et al. observed that pain reported in rheumatoid arthritis after a twice a day therapy by flurbiprofen may be more efficient than four times daily flurbiprogen, and that regimen without an evening dose was the least efficient of three twice-daily therapies tested. Moreover, their results showed that morning stiffness in rheumatoid arthritis was not only the result of nocturnal inactivity but also a response to an appropriately timed medication [222]. Moreover, patients with indomethacin and ketoprofen have shown a reduction in neurological and gastro-intestinal side effects when these products are ingested once-daily in the evening rather than in the morning [223].
8.3. PPARγ and the Circadian Clock
PPARγ acts directly with the core clock genes and presents diurnal variations in liver and blood vessels [30,224] (Figure 4). In mice, dysregulation in diurnal rhythms are induced by the inhibition of PPARγ [225]. PPARγ agonists regulate Bmal1 and thus the heterodimer Clock/Bmal1 formation [30,226], and can interact with Rev-Erb [227]. Downregulation of the clock-controlled gene Nocturin inhibits PPARγ oscillations in the liver of mice fed on a high-fat diet. In physiological circumstances, nocturin interacts with PPARγ to enhance its transcriptional activity [228]. PPARγ diminution alters the circadian function of 15-Deoxy-D 12,14-prostaglandin J2 (15-PGJ2) [225]. The associate of PPARγ, RXR, interacts with Clock protein in a ligand-dependent manner and then blocks Clock/Bmal1 heterodimer formation and transcriptional activity [229]. PPARγ binds to the mammalian clock to control metabolic metabolism [229]. Circadian metabolism is controlled by PPARγ in a direct manner [225]. Retinoic acid receptor-related orphan receptor gamma t (ROR gammat) is considered a major transcriptional factor for Th17 cell differentiation [230,231]. Th17 cells represent another subset of CD4+ T cells and selectively produce interleukin (IL)-17. PPARγ can influence the function of Th cell clones [232]. PPARγ agonists decrease Th17 differentiation by inhibiting ROR gammat induction [233,234,235]. CD4+ T cells fail to express ROR gammat under the action of PPARγ agonists [233].
9. Relevance of “Chronotherapy” in Cancer Clinical Therapy
The numerous interactions between clock dysregulation and cancer underline the interest of circadian therapeutic actions [29]. The temporal peak of cell activity could be targeted by pharmacological drugs used at an optimal time of day. Few studies have shown the potential role of WNT and PPAR gamma with circadian clocks in cancer development. Nevertheless, interest in the association between PPAR gamma agonists and melatonin in cancer therapy is not new [236]. In cultured cells, the addition of melatonin with a PPAR gamma agonist (such as troglitazone) is associated with a significant reduction in cell numbers [237]. Moreover, other studies have shown a potent apoptotic effect of a combination of melatonin with PPAR gamma agonists in breast cancer cells [238,239]. In parallel, recent studies have shown that melatonin could inhibit WNT pathway expression [240,241].
In mouse ovaries, melatonin administration protects against ROS production and mitochondrial damage [242]. In colorectal cancer, the combination of 5-fluorouracil and melatonin is correlated with the downregulation of cell proliferation by suppressing of the PI3K/Akt pathway, NF-κB pathway and nitric oxide synthase signaling [243]. Moreover, melatonin inhibits GSK3-β to stop invasion in breast cancer cells [244]. The association between carcinogenesis and the circadian clock remains complex and difficult to unravel. Strong evidence shows the involvement of the circadian clock in cancer development. Numerous molecular pathways are dynamically circadian, such as the WNT/β-catenin pathway and PPAR gamma. Thus, the time at which these pathways are targeted may be critical. NSAIDs, by acting as PPAR gamma agonists and focusing on the WNT/β-catenin pathway, should be utilized in concordance with the circadian clock genes, and therefore administered at the optimum time of day. Further studies should focus on the importance of the day/night cycle in cancer therapy and the circadian profiles of cancer cells.
10. Conclusions
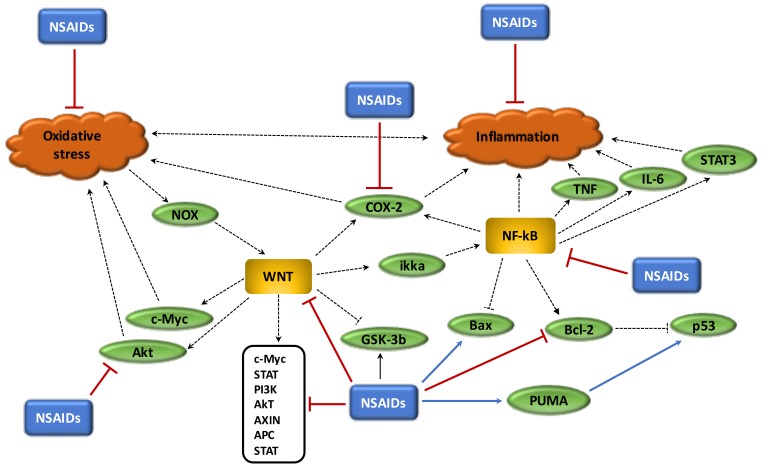
Chronic inflammation, oxidative stress and the disruption of circadian rhythms are important factors in the cancer process and are enhanced by overstimulation of the WNT/β-catenin pathway. In cancers, the WNT/β-catenin pathway is generally activated whereas PPARγ is decreased. These two signaling pathways act in opposing manners and this could explain their unidirectional profile observed in cancers. The use of NSAIDs, which act as PPARγ agonists, could be interesting in the reduction of both chronic inflammation and oxidative stress and in the control of circadian rhythms by inhibiting the WNT/β-catenin pathway (Figure 5). Due to the considerable impact of cancers on mortality and morbidity rates worldwide, it would appear of the utmost importance to better understand the action of NSAIDs in cancers (Table 1), and particularly their role in the inhibition of the major signaling system known as the WNT/β-catenin pathway.
Figure 5.
Beneficial role of NSAIDs in cancer process. (1) NSAIDs reduce oxidative stress; (2) NSAIDs reduce chronic inflammation; (3) NSAIDs inhibit Akt pathway activity; (4) NSAIDs downregulate WNT pathway and its target genes, inhibit Bcl-2, activate PUMA to stimulate p53 and activate GSK-3beta; (5) NSAIDs inhibit NF-κB and COX-2.
Table 1.
Differential effects of NSAIDs in tumors.
| NSAIDs | Target | Target Function | Interaction | References |
|---|---|---|---|---|
| Aspirin | PGE2 | Immune system attenuation | Inhibition of PGE2 synthesis | [245] |
| Aspirin | Platelets | Reduction cell activity | Inhibition of COX | [246] |
| Aspirin | Genetic mutations | Tumorigenesis inhibition | Downregulation of gene mutation accumulation | [247] |
| Aspirin | WNT pathway | Inhibition of cell proliferation and invasion | Inhibition of β-catenin accumulation | [183] |
| Aspirin | WNT pathway | Tumor suppressor | Inhibition of COX | [39] |
| Aspirin | WNT pathway | Arrest G0/G1 phase | Inhibition of β-catenin/TCF | [183,184] |
| Indomethacin | T-cell therapy | Tumorigenesis inhibition | Downregulation of cellular drug resistance | [246] |
| Sulindac | WNT pathway | Inhibition of invasion and cell growth | Phosphorylation of Akt signaling | [50,51] |
| Aspirin and indomethacin | WNT pathway | Tumorigenesis inhibition | Inhibition of β-catenin and TCF/LEF | [175] |
| celecoxib | WNT pathway | Inhibition cancer cell growth | Inhibition of WNT | [178] |
| celecoxib | WNT pathway | Inhibition cancer cell growth | Inhibition of TCF/LEF | [179] |
| Sulindac | WNT pathway | Tumorigenesis inhibition | Inhibition of β-catenin accumulation | [180] |
| celecoxib | WNT pathway | Tumorigenesis inhibition | Inhibition of cyclin D1 | [181,182] |
PGE2: prostaglandin E2, COX: cyclooxygenase, TCF/LEF: T-cell factor/lymphoid enhancer-binding factor.
Abbreviations
| APC | Adenomatous polyposis coli |
| βTrCP | Beta-transducin repeat-containing protein |
| Bmal1 | Brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1 |
| Clock | Circadian locomotor output cycles kaput |
| CRD-BP | Coding Region Determinant-Binding Protein, an RNA-binding protein |
| Cry | Cryptochrome |
| CRs | circadian rhythms |
| CK1 | casein kinase 1 |
| COX-2 | Cyclooxygenase-2 |
| FZD | Frizzled |
| GSK-3β | Glycogen synthase kinase-3β |
| IκB-α | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| LRP 5/6 | Low-density lipoprotein receptor-related protein 5/6 |
| MAPK | Mitogen-activated protein kinases |
| NF-κB | nuclear factor κB |
| NOX | NADPH oxidase |
| Per | Period |
| NSAID | nonsteroidal anti-inflammatory drug |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PI3K-Akt | Phosphatidylinositol 3-kinase-protein kinase B |
| RORs | retinoid-related orphan receptors |
| ROS | reactive oxygen species |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TNF-α | tumor necrosis factor alpha |
Author Contributions
All listed authors have made substantial and intellectual contributions to this work and have approved it for publication.
Funding
This research received no external funding
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- 1.Ames B.N., Gold L.S. Animal cancer tests and cancer prevention. J. Natl. Cancer Inst. Monogr. 1992;12:125–132. [PubMed] [Google Scholar]
- 2.Guyton K.Z., Kensler T.W. Oxidative mechanisms in carcinogenesis. Br. Med. Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 3.Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990;50:5127–5135. [PubMed] [Google Scholar]
- 4.Bartsch H., Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov S.I., Karin M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonda T.A., Tu S., Wang T.C. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 8.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A. Cancer: Inflammation by remote control. Nature. 2005;435:752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 10.Chung P.-Y., Lam P.-L., Zhou Y.-Y., Gasparello J., Finotti A., Chilin A., Marzaro G., Gambari R., Bian Z.-X., Kwok W.-M., et al. Targeting DNA Binding for NF-κB as an Anticancer Approach in Hepatocellular Carcinoma. Cells. 2018;7:177. doi: 10.3390/cells7100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallée A., Lecarpentier Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018;9:745. doi: 10.3389/fimmu.2018.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol. Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Shacter E., Beecham E.J., Covey J.M., Kohn K.W., Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- 15.Yang D., Kim J. Mitochondrial Retrograde Signalling and Metabolic Alterations in the Tumour Microenvironment. Cells. 2019;8:275. doi: 10.3390/cells8030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 17.Onyido E.K., Sweeney E., Nateri A.S. Wnt-signalling pathways and microRNAs network in carcinogenesis: Experimental and bioinformatics approaches. Mol. Cancer. 2016;15:56. doi: 10.1186/s12943-016-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallée A., Guillevin R., Vallée J.-N. Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas. Rev. Neurosci. 2017;29:71–91. doi: 10.1515/revneuro-2017-0032. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J., Otto F., Baron J.A., Brown P.H., Burn J., Greenwald P., Jankowski J., La Vecchia C., Meyskens F., Senn H.J., et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell P.M., Wilson M., Price J.F., Belch J.F.F., Meade T.W., Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 21.Schreinemachers D.M., Everson R.B. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.-N. Opposite Interplay Between the Canonical WNT/β-Catenin Pathway and PPAR Gamma: A Potential Therapeutic Target in Gliomas. Neurosci. Bull. 2018;34:573–588. doi: 10.1007/s12264-018-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Algra A.M., Rothwell P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Chen F., Shang L. Advances in antitumor effects of NSAIDs. Cancer Manag. Res. 2018;10:4631–4640. doi: 10.2147/CMAR.S175212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 26.Sgarbi G., Gorini G., Liuzzi F., Solaini G., Baracca A. Hypoxia and IF1 Expression Promote ROS Decrease in Cancer Cells. Cells. 2018;7:64. doi: 10.3390/cells7070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters J.M., Shah Y.M., Gonzalez F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandera Merchan B., Tinahones F.J., Macías-González M. Commonalities in the Association between PPARG and Vitamin D Related with Obesity and Carcinogenesis. PPAR Res. 2016;2016 doi: 10.1155/2016/2308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masri S., Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018;24:1795–1803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N., Yang G., Jia Z., Zhang H., Aoyagi T., Soodvilai S., Symons J.D., Schnermann J.B., Gonzalez F.J., Litwin S.E., et al. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.-N. Thermodynamics in Neurodegenerative Diseases: Interplay Between Canonical WNT/Beta-Catenin Pathway-PPAR Gamma, Energy Metabolism and Circadian Rhythms. Neuromol. Med. 2018;20:174–204. doi: 10.1007/s12017-018-8486-x. [DOI] [PubMed] [Google Scholar]
- 32.Gala M.K., Chan A.T. Molecular pathways: Aspirin and Wnt signaling-a molecularly targeted approach to cancer prevention and treatment. Clin. Cancer Res. 2015;21:1543–1548. doi: 10.1158/1078-0432.CCR-14-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y., Liu Y., Zhang C., Su Z.-Y., Li W., Huang M.-T., Kong A.-N. The epigenetic effects of aspirin: The modification of histone H3 lysine 27 acetylation in the prevention of colon carcinogenesis in azoxymethane and dextran sulfate sodium-treated CF-1 mice. Carcinogenesis. 2016;37:616–624. doi: 10.1093/carcin/bgw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allaj V., Guo C., Nie D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013;3:8. doi: 10.1186/2045-3701-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh N., Chaki R., Mandal V., Mandal S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010;62:233–244. doi: 10.1016/S1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 36.Bank A., Yu J., Zhang L. NSAIDs downregulate Bcl-X(L) and dissociate BAX and Bcl-X(L) to induce apoptosis in colon cancer cells. Nutr. Cancer. 2008;60(Suppl. 1):98–103. doi: 10.1080/01635580802381261. [DOI] [PubMed] [Google Scholar]
- 37.Raza H., John A., Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011;668:15–24. doi: 10.1016/j.ejphar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Liu H.-F., Hsiao P.-W., Chao J.-I. Celecoxib induces p53-PUMA pathway for apoptosis in human colorectal cancer cells. Chem. Biol. Interact. 2008;176:48–57. doi: 10.1016/j.cbi.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Benamouzig R., Uzzan B., Martin A., Deyra J., Little J., Girard B., Chaussade S., APACC Study Group Cyclooxygenase-2 expression and recurrence of colorectal adenomas: Effect of aspirin chemoprevention. Gut. 2010;59:622–629. doi: 10.1136/gut.2008.175406. [DOI] [PubMed] [Google Scholar]
- 40.Willoughby D.A., Moore A.R., Colville-Nash P.R. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet. 2000;355:646–648. doi: 10.1016/S0140-6736(99)12031-2. [DOI] [PubMed] [Google Scholar]
- 41.Chan A.T., Ogino S., Fuchs C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 42.Xu J., Yin Z., Gao W., Liu L., Wang R., Huang P., Yin Y., Liu P., Yu R., Shu Y. Meta-analysis on the association between nonsteroidal anti-inflammatory drug use and lung cancer risk. Clin. Lung Cancer. 2012;13:44–51. doi: 10.1016/j.cllc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Kim B.H., Kim C.I., Chang H.S., Choe M.S., Jung H.R., Kim D.Y., Park C.H. Cyclooxygenase-2 overexpression in chronic inflammation associated with benign prostatic hyperplasia: Is it related to apoptosis and angiogenesis of prostate cancer? Korean J. Urol. 2011;52:253–259. doi: 10.4111/kju.2011.52.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X.-L., Su B.-S., Sun R.-Q., Zhang J., Wang Y.-L. Relationship between expression and distribution of cyclooxygenase-2 and bcl-2 in human gastric adenocarcinoma. World J. Gastroenterol. 2005;11:1228–1231. doi: 10.3748/wjg.v11.i8.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung E., McArthur D., Morris A., Williams N. Cyclooxygenase-2 inhibition prevents migration of colorectal cancer cells to extracellular matrix by down-regulation of matrix metalloproteinase-2 expression. Dis. Colon Rectum. 2008;51:342–347. doi: 10.1007/s10350-007-9120-4. [DOI] [PubMed] [Google Scholar]
- 46.Li H., Zhu F., Boardman L.A., Wang L., Oi N., Liu K., Li X., Fu Y., Limburg P.J., Bode A.M., et al. Aspirin Prevents Colorectal Cancer by Normalizing EGFR Expression. EBioMedicine. 2015;2:447–455. doi: 10.1016/j.ebiom.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakravarti A., Dicker A., Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: A review of preclinical and correlative clinical data. Int. J. Radiat. Oncol. 2004;58:927–931. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- 48.Mellinghoff I.K., Wang M.Y., Vivanco I., Haas-Kogan D.A., Zhu S., Dia E.Q., Lu K.V., Yoshimoto K., Huang J.H.Y., Chute D.J., et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 49.Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H.-C., Park I.-C., Park M.-J., An S., Woo S.-H., Jin H.-O., Chung H.Y., Lee S.-J., Gwak H.-S., Hong Y.-J., et al. Sulindac and its metabolites inhibit invasion of glioblastoma cells via down-regulation of Akt/PKB and MMP-2. J. Cell. Biochem. 2005;94:597–610. doi: 10.1002/jcb.20312. [DOI] [PubMed] [Google Scholar]
- 51.Paw I., Carpenter R.C., Watabe K., Debinski W., Lo H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362:1–7. doi: 10.1016/j.canlet.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ranjbar M.M., Assadolahi V., Yazdani M., Nikaein D., Rashidieh B. Virtual Dual inhibition of COX-2/5-LOX enzymes based on binding properties of alpha-amyrins, the anti-inflammatory compound as a promising anti-cancer drug. EXCLI J. 2016;15:238–245. doi: 10.17179/excli2016-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallée A., Lévy B.L., Blacher J. Interplay between the renin-angiotensin system, the canonical WNT/β-catenin pathway and PPARγ in hypertension. Curr. Hypertens. Rep. 2018;20:62. doi: 10.1007/s11906-018-0860-4. [DOI] [PubMed] [Google Scholar]
- 54.Vallée A., Vallée J.-N., Lecarpentier Y. PPARγ agonists: Potential treatment for autism spectrum disorder by inhibiting the canonical WNT/β-catenin pathway. Mol. Psychiatry. 2018;1:643–652. doi: 10.1038/s41380-018-0131-4. [DOI] [PubMed] [Google Scholar]
- 55.McGettigan P., Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: Systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098. doi: 10.1371/journal.pmed.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grösch S., Maier T.J., Schiffmann S., Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J. Natl. Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 57.Hau P., Kunz-Schughart L., Bogdahn U., Baumgart U., Hirschmann B., Weimann E., Muhleisen H., Ruemmele P., Steinbrecher A., Reichle A. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—A phase II study. Oncology. 2007;73:21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- 58.Ghanghas P., Jain S., Rana C., Sanyal S.N. Chemopreventive action of non-steroidal anti-inflammatory drugs on the inflammatory pathways in colon cancer. Biomed. Pharmacother. 2016;78:239–247. doi: 10.1016/j.biopha.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Tinsley H.N., Gary B.D., Keeton A.B., Lu W., Li Y., Piazza G.A. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin-mediated transcription in human breast tumor cells. Cancer Prev. Res. 2011;4:1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tinsley H.N., Grizzle W.E., Abadi A., Keeton A., Zhu B., Xi Y., Piazza G.A. Prospects for Chemoprevention of Colorectal Neoplasia. Springer; Berlin/Heidelberg, Germany: 2013. New NSAID targets and derivatives for colorectal cancer chemoprevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilroy D.W., Colville-Nash P.R. New insights into the role of COX 2 in inflammation. J. Mol. Med. 2000;78:121–129. doi: 10.1007/s001090000094. [DOI] [PubMed] [Google Scholar]
- 62.Puhl A.C., Milton F.A., Cvoro A., Sieglaff D.H., Campos J.C.L., Bernardes A., Filgueira C.S., Lindemann J.L., Deng T., Neves F.A.R., et al. Mechanisms of peroxisome proliferator activated receptor γ regulation by non-steroidal anti-inflammatory drugs. Nucl. Recept. Signal. 2015;13:e004. doi: 10.1621/nrs.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elbrecht A., Chen Y., Cullinan C.A., Hayes N., Leibowitz M.D., Moller D.E., Berger J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem. Biophys. Res. Commun. 1996;224:431–437. doi: 10.1006/bbrc.1996.1044. [DOI] [PubMed] [Google Scholar]
- 65.Fajas L., Auboeuf D., Raspé E., Schoonjans K., Lefebvre A.M., Saladin R., Najib J., Laville M., Fruchart J.C., Deeb S., et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 66.Lecarpentier Y., Claes V., Hébert J.-L. PPARs, Cardiovascular Metabolism, and Function: Near- or Far-from-Equilibrium Pathways. PPAR Res. 2010;2010 doi: 10.1155/2010/783273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricote M., Glass C.K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.-N. Thermodynamics in Gliomas: Interactions between the Canonical WNT/Beta-Catenin Pathway and PPAR Gamma. Front. Physiol. 2017;8:352. doi: 10.3389/fphys.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanquicett C., Roman J., Hart C.M. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008;6:25–34. [PMC free article] [PubMed] [Google Scholar]
- 70.Elrod H.A., Sun S.-Y. PPARgamma and Apoptosis in Cancer. PPAR Res. 2008;2008:704165. doi: 10.1155/2008/704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson J.A., Gale E.A.M. Diabetes, insulin use, and cancer risk: Are observational studies part of the solution-or part of the problem? Diabetes. 2010;59:1129–1131. doi: 10.2337/db10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galli A., Mello T., Ceni E., Surrenti E., Surrenti C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin. Investig. Drugs. 2006;15:1039–1049. doi: 10.1517/13543784.15.9.1039. [DOI] [PubMed] [Google Scholar]
- 73.Colca J.R., Tanis S.P., McDonald W.G., Kletzien R.F. Insulin sensitizers in 2013: New insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin. Investig. Drugs. 2014;23:1–7. doi: 10.1517/13543784.2013.839659. [DOI] [PubMed] [Google Scholar]
- 74.Ohta T., Elnemr A., Yamamoto M., Ninomiya I., Fushida S., Nishimura G.-I., Fujimura T., Kitagawa H., Kayahara M., Shimizu K., et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, modulates the E-cadherin/beta-catenin system in a human pancreatic cancer cell line, BxPC-3. Int. J. Oncol. 2002;21:37–42. [PubMed] [Google Scholar]
- 75.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 76.Home P.D., Pocock S.J., Beck-Nielsen H., Curtis P.S., Gomis R., Hanefeld M., Jones N.P., Komajda M., McMurray J.J.V., RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 77.Hernandez A.V., Usmani A., Rajamanickam A., Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: A meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am. J. Cardiovasc. Drugs. 2011;11:115–128. doi: 10.2165/11587580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.Dormandy J.A., Charbonnel B., Eckland D.J.A., Erdmann E., Massi-Benedetti M., Moules I.K., Skene A.M., Tan M.H., Lefèbvre P.J., Murray G.D., et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 79.Goltsman I., Khoury E.E., Winaver J., Abassi Z. Does Thiazolidinedione therapy exacerbate fluid retention in congestive heart failure? Pharmacol. Ther. 2016;168:75–97. doi: 10.1016/j.pharmthera.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Horita S., Nakamura M., Satoh N., Suzuki M., Seki G. Thiazolidinediones and Edema: Recent Advances in the Pathogenesis of Thiazolidinediones-Induced Renal Sodium Retention. PPAR Res. 2015;2015:646423. doi: 10.1155/2015/646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anuja K., Roy S., Ghosh C., Gupta P., Bhattacharjee S., Banerjee B. Prolonged inflammatory microenvironment is crucial for pro-neoplastic growth and genome instability: A detailed review. Inflamm. Res. 2017;66:119–128. doi: 10.1007/s00011-016-0985-3. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y., Antony S., Meitzler J.L., Doroshow J.H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164–173. doi: 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kundu J.K., Surh Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Rakoff-Nahoum S. Why cancer and inflammation? Yale J. Biol. Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 86.Roessner A., Kuester D., Malfertheiner P., Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 87.Rouse J., Jackson S.P. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- 88.Itzkowitz S.H., Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 89.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 90.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 91.Lin W.-W., Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charles K.A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R.G., Kollias G., Smyth J.F., et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Investig. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szlosarek P., Charles K.A., Balkwill F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Tang Q., Li J., Zhu H., Li P., Zou Z., Xiao Y. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediat. Inflamm. 2013;2013:713859. doi: 10.1155/2013/713859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sobolewski C., Cerella C., Dicato M., Ghibelli L., Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 97.Dvorak H.F. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am. J. Pathol. 2003;162:1747–1757. doi: 10.1016/S0002-9440(10)64309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bubici C., Papa S., Pham C.G., Zazzeroni F., Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol. Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- 99.Herrero R., Castellsagué X., Pawlita M., Lissowska J., Kee F., Balaram P., Rajkumar T., Sridhar H., Rose B., Pintos J., et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J. Natl. Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 100.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 101.Kim Y.-H., Woo K.J., Lim J.H., Kim S., Lee T.J., Jung E.M., Lee J.-M., Park J.-W., Kwon T.K. 8-Hydroxyquinoline inhibits iNOS expression and nitric oxide production by down-regulating LPS-induced activity of NF-kappaB and C/EBPbeta in Raw 264.7 cells. Biochem. Biophys. Res. Commun. 2005;329:591–597. doi: 10.1016/j.bbrc.2005.01.159. [DOI] [PubMed] [Google Scholar]
- 102.Duracková Z. Some current insights into oxidative stress. Physiol. Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 103.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999;57:231–245. doi: 10.1016/S0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 104.Poyton R.O., Ball K.A., Castello P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 106.Weyemi U., Lagente-Chevallier O., Boufraqech M., Prenois F., Courtin F., Caillou B., Talbot M., Dardalhon M., Al Ghuzlan A., Bidart J.-M., et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Debnath T., Kim D.H., Lim B.O. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18:7253–7270. doi: 10.3390/molecules18067253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giudice A., Montella M. Activation of the Nrf2-ARE signaling pathway: A promising strategy in cancer prevention. BioEssays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 109.Khor T.O., Huang M.-T., Prawan A., Liu Y., Hao X., Yu S., Cheung W.K.L., Chan J.Y., Reddy B.S., Yang C.S., et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev. Res. 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin M., Zhai X., Wang G., Tian X., Gao D., Shi L., Wu H., Fan Q., Peng J., Liu K., et al. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase II detoxification gene expression via activation of the PI3K and PKC signaling pathways. J. Pharmacol. Sci. 2015;127:203–210. doi: 10.1016/j.jphs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 111.Dey A., Lakshmanan J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013;4:1148–1184. doi: 10.1039/c3fo30317a. [DOI] [PubMed] [Google Scholar]
- 112.Padhi S., Saha A., Kar M., Ghosh C., Adhya A., Baisakh M., Mohapatra N., Venkatesan S., Hande M.P., Banerjee B. Clinico-Pathological Correlation of β-Catenin and Telomere Dysfunction in Head and Neck Squamous Cell Carcinoma Patients. J. Cancer. 2015;6:192–202. doi: 10.7150/jca.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park J.-H., Mangal D., Frey A.J., Harvey R.G., Blair I.A., Penning T.M. Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2’-deoxyguanosine formation by the aldo-keto reductase product benzo[a]pyrene-7,8-dione. J. Biol. Chem. 2009;284:29725–29734. doi: 10.1074/jbc.M109.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simon A.R., Rai U., Fanburg B.L., Cochran B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 115.Clerkin J.S., Naughton R., Quiney C., Cotter T.G. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 2008;266:30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 116.Rusyn I., Rose M.L., Bojes H.K., Thurman R.G. Novel role of oxidants in the molecular mechanism of action of peroxisome proliferators. Antioxid. Redox Signal. 2000;2:607–621. doi: 10.1089/15230860050192350. [DOI] [PubMed] [Google Scholar]
- 117.Manning B.D., Cantley L.C. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Plas D.R., Thompson C.B. Akt-dependent transformation: There is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 119.Jimeno A., Gordon M., Chugh R., Messersmith W., Mendelson D., Dupont J., Stagg R., Kapoun A.M., Xu L., Uttamsingh S., et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 120.Le P.N., McDermott J.D., Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le P.N., Keysar S.B., Miller B., Eagles J.R., Chimed T.-S., Reisinger J., Gomez K.E., Nieto C., Jackson B.C., Somerset H.L., et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol. Carcinog. 2019;58:398–410. doi: 10.1002/mc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeng C.-M., Chen Z., Fu L. Frizzled Receptors as Potential Therapeutic Targets in Human Cancers. Int. J. Mol. Sci. 2018;19:1543. doi: 10.3390/ijms19051543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferri M., Liscio P., Carotti A., Asciutti S., Sardella R., Macchiarulo A., Camaioni E. Targeting Wnt-driven cancers: Discovery of novel tankyrase inhibitors. Eur. J. Med. Chem. 2017;142:506–522. doi: 10.1016/j.ejmech.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 124.Wiese M., Walther N., Diederichs C., Schill F., Monecke S., Salinas G., Sturm D., Pfister S.M., Dressel R., Johnsen S.A., et al. The β-catenin/CBP-antagonist ICG-001 inhibits pediatric glioma tumorigenicity in a Wnt-independent manner. Oncotarget. 2017;8:27300–27313. doi: 10.18632/oncotarget.15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Proffitt K.D., Madan B., Ke Z., Pendharkar V., Ding L., Lee M.A., Hannoush R.N., Virshup D.M. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 126.Loh K.M., van Amerongen R., Nusse R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev. Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 127.Oren O., Smith B.D. Eliminating Cancer Stem Cells by Targeting Embryonic Signaling Pathways. Stem Cell Rev. 2017;13:17–23. doi: 10.1007/s12015-016-9691-3. [DOI] [PubMed] [Google Scholar]
- 128.Ferrer-Lorente R., Bejar M.T., Tous M., Vilahur G., Badimon L. Systems biology approach to identify alterations in the stem cell reservoir of subcutaneous adipose tissue in a rat model of diabetes: Effects on differentiation potential and function. Diabetologia. 2014;57:246–256. doi: 10.1007/s00125-013-3081-z. [DOI] [PubMed] [Google Scholar]
- 129.Borrell-Pagès M., Romero J.C., Juan-Babot O., Badimon L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur. Heart J. 2011;32:2841–2850. doi: 10.1093/eurheartj/ehr062. [DOI] [PubMed] [Google Scholar]
- 130.Escate R., Padro T., Borrell-Pages M., Suades R., Aledo R., Mata P., Badimon L. Macrophages of genetically characterized familial hypercholesterolaemia patients show up-regulation of LDL-receptor-related proteins. J. Cell. Mol. Med. 2017;21:487–499. doi: 10.1111/jcmm.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ye B., Hou N., Xiao L., Xu Y., Boyer J., Xu H., Li F. APC controls asymmetric Wnt/β-catenin signaling and cardiomyocyte proliferation gradient in the heart. J. Mol. Cell. Cardiol. 2015;89:287–296. doi: 10.1016/j.yjmcc.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ambacher K.K., Pitzul K.B., Karajgikar M., Hamilton A., Ferguson S.S., Cregan S.P. The JNK-and AKT/GSK3β-Signaling Pathways Converge to Regulate Puma Induction and Neuronal Apoptosis Induced by Trophic Factor Deprivation. PLoS ONE. 2012;7:e46885. doi: 10.1371/journal.pone.0046885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hur E.-M., Zhou F.-Q. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu D., Pan W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kerekes K., Bányai L., Patthy L. Wnts grasp the WIF domain of Wnt Inhibitory Factor 1 at two distinct binding sites. FEBS Lett. 2015;589:3044–3051. doi: 10.1016/j.febslet.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 136.Tao H., Yang J.-J., Shi K.-H., Li J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism. 2016;65:30–40. doi: 10.1016/j.metabol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 137.Ueland T., Caidahl K., Askevold E.T., Karlsson T., Hartford M., Aukrust P. Secreted Frizzled-Related Protein 3 (sFRP3) in acute coronary syndromes. Int. J. Cardiol. 2015;190:217–219. doi: 10.1016/j.ijcard.2015.03.401. [DOI] [PubMed] [Google Scholar]
- 138.Ma B., Hottiger M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spiegelman V.S., Slaga T.J., Pagano M., Minamoto T., Ronai Z., Fuchs S.Y. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell. 2000;5:877–882. doi: 10.1016/S1097-2765(00)80327-5. [DOI] [PubMed] [Google Scholar]
- 140.Noubissi F.K., Elcheva I., Bhatia N., Shakoori A., Ougolkov A., Liu J., Minamoto T., Ross J., Fuchs S.Y., Spiegelman V.S. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- 141.Ougolkov A., Zhang B., Yamashita K., Bilim V., Mai M., Fuchs S.Y., Minamoto T. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J. Natl. Cancer Inst. 2004;96:1161–1170. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 142.Jia D., Yang W., Li L., Liu H., Tan Y., Ooi S., Chi L., Filion L.G., Figeys D., Wang L. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015;22:298–310. doi: 10.1038/cdd.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vallée A., Lecarpentier Y., Vallée J.-N. Hypothesis of Opposite Interplay Between the Canonical WNT/beta-catenin Pathway and PPAR Gamma in Primary Central Nervous System Lymphomas. Curr. Issues Mol. Biol. 2019;31:1–20. doi: 10.21775/cimb.031.001. [DOI] [PubMed] [Google Scholar]
- 144.Phelps R.A., Broadbent T.J., Stafforini D.M., Jones D.A. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 145.Kuphal S., Poser I., Jobin C., Hellerbrand C., Bosserhoff A.K. Loss of E-cadherin leads to upregulation of NFkappaB activity in malignant melanoma. Oncogene. 2004;23:8509–8519. doi: 10.1038/sj.onc.1207831. [DOI] [PubMed] [Google Scholar]
- 146.Bandino A., Compagnone A., Bravoco V., Cravanzola C., Lomartire A., Rossetto C., Novo E., Cannito S., Valfrè di Bonzo L., Zamara E., et al. Beta-catenin triggers nuclear factor kappaB-dependent up-regulation of hepatocyte inducible nitric oxide synthase. Int. J. Biochem. Cell Biol. 2008;40:1861–1871. doi: 10.1016/j.biocel.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 147.Schön S., Flierman I., Ofner A., Stahringer A., Holdt L.M., Kolligs F.T., Herbst A. β-catenin regulates NF-κB activity via TNFRSF19 in colorectal cancer cells. Int. J. Cancer. 2014;135:1800–1811. doi: 10.1002/ijc.28839. [DOI] [PubMed] [Google Scholar]
- 148.Ma B., Zhong L., van Blitterswijk C.A., Post J.N., Karperien M. T cell factor 4 is a pro-catabolic and apoptotic factor in human articular chondrocytes by potentiating nuclear factor κB signaling. J. Biol. Chem. 2013;288:17552–17558. doi: 10.1074/jbc.M113.453985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saegusa M., Hashimura M., Kuwata T., Hamano M., Okayasu I. Crosstalk between NF-kappaB/p65 and beta-catenin/TCF4/p300 signalling pathways through alterations in GSK-3beta expression during trans-differentiation of endometrial carcinoma cells. J. Pathol. 2007;213:35–45. doi: 10.1002/path.2198. [DOI] [PubMed] [Google Scholar]
- 150.Buss H., Dörrie A., Schmitz M.L., Frank R., Livingstone M., Resch K., Kracht M. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J. Biol. Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- 151.Carayol N., Wang C.-Y. IKKalpha stabilizes cytosolic beta-catenin by inhibiting both canonical and non-canonical degradation pathways. Cell. Signal. 2006;18:1941–1946. doi: 10.1016/j.cellsig.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 152.Schwitalla S., Fingerle A.A., Cammareri P., Nebelsiek T., Göktuna S.I., Ziegler P.K., Canli O., Heijmans J., Huels D.J., Moreaux G., et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 153.Lamberti C., Lin K.M., Yamamoto Y., Verma U., Verma I.M., Byers S., Gaynor R.B. Regulation of beta-catenin function by the IkappaB kinases. J. Biol. Chem. 2001;276:42276–42286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- 154.Albanese C., Wu K., D’Amico M., Jarrett C., Joyce D., Hughes J., Hulit J., Sakamaki T., Fu M., Ben-Ze’ev A., et al. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol. Biol. Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiao R., Liu Y., Gao H., Xiao J., So K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016;44:463–488. doi: 10.1142/S0192415X16500269. [DOI] [PubMed] [Google Scholar]
- 156.Shankar E., Weis M.C., Avva J., Shukla S., Shukla M., Sreenath S.N., Gupta S. Complex Systems Biology Approach in Connecting PI3K-Akt and NF-κB Pathways in Prostate Cancer. Cells. 2019;8:201. doi: 10.3390/cells8030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Covey T.M., Edes K., Coombs G.S., Virshup D.M., Fitzpatrick F.A. Alkylation of the tumor suppressor PTEN activates Akt and β-catenin signaling: A mechanism linking inflammation and oxidative stress with cancer. PLoS ONE. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang D.-Y., Pan Y., Zhang C., Yan B.-X., Yu S.-S., Wu D.-L., Shi M.-M., Shi K., Cai X.-X., Zhou S.-S., et al. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol. Cell. Biochem. 2013;374:13–20. doi: 10.1007/s11010-012-1498-1. [DOI] [PubMed] [Google Scholar]
- 159.Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R.S., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vafa O., Wade M., Kern S., Beeche M., Pandita T.K., Hampton G.M., Wahl G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 161.Novellasdemunt L., Antas P., Li V.S.W. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am. J. Physiol. Cell Physiol. 2015;309:C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu J., Wang H., Zuo Y., Farmer S.R. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol. Cell. Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Piwien-Pilipuk G., Van Mater D., Ross S.E., MacDougald O.A., Schwartz J. Growth hormone regulates phosphorylation and function of CCAAT/enhancer-binding protein beta by modulating Akt and glycogen synthase kinase-3. J. Biol. Chem. 2001;276:19664–19671. doi: 10.1074/jbc.M010193200. [DOI] [PubMed] [Google Scholar]
- 164.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 165.Liu J.-J., Dai X.-J., Xu Y., Liu P.-Q., Zhang Y., Liu X.-D., Fang Z.-G., Lin D.-J., Xiao R.-Z., Huang R.-W., et al. Inhibition of lymphoma cell proliferation by peroxisomal proliferator-activated receptor-γ ligands via Wnt signaling pathway. Cell Biochem. Biophys. 2012;62:19–27. doi: 10.1007/s12013-011-9253-x. [DOI] [PubMed] [Google Scholar]
- 166.Guo F., Ren X., Dong Y., Hu X., Xu D., Zhou H., Meng F., Tian W., Zhao Y. Constitutive expression of PPARγ inhibits proliferation and migration of gastric cancer cells and down-regulates Wnt/β-Catenin signaling pathway downstream target genes TERT and ENAH. Gene. 2016;584:31–37. doi: 10.1016/j.gene.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 167.Panza A., Pazienza V., Ripoli M., Benegiamo G., Gentile A., Valvano M.R., Augello B., Merla G., Prattichizzo C., Tavano F., et al. Interplay between SOX9, β-catenin and PPARγ activation in colorectal cancer. Biochim. Biophys. Acta. 2013;1833:1853–1865. doi: 10.1016/j.bbamcr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Ren X., Zheng D., Guo F., Liu J., Zhang B., Li H., Tian W. PPARγ suppressed Wnt/β-catenin signaling pathway and its downstream effector SOX9 expression in gastric cancer cells. Med. Oncol. 2015;32:91. doi: 10.1007/s12032-015-0536-8. [DOI] [PubMed] [Google Scholar]
- 169.Prost S., Relouzat F., Spentchian M., Ouzegdouh Y., Saliba J., Massonnet G., Beressi J.-P., Verhoeyen E., Raggueneau V., Maneglier B., et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525:380–383. doi: 10.1038/nature15248. [DOI] [PubMed] [Google Scholar]
- 170.Liu L., Yang Z., Xu Y., Li J., Xu D., Zhang L., Sun J., Xia S., Zou F., Liu Y. Inhibition of oxidative stress-elicited AKT activation facilitates PPARγ agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells. PLoS ONE. 2013;8:e73038. doi: 10.1371/journal.pone.0073038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu M., Guan J., Li C., Gunter S., Nusrat L., Ng S., Dhand K., Morshead C., Kim A., Das S. Aberrantly activated Cox-2 and Wnt signaling interact to maintain cancer stem cells in glioblastoma. Oncotarget. 2017;8:82217–82230. doi: 10.18632/oncotarget.19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thun M.J., Namboodiri M.M., Heath C.W. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 173.Thun M.J., Henley S.J., Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 174.Chan T.A. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3:166–174. doi: 10.1016/S1470-2045(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 175.Dihlmann S., Siermann A., von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20:645–653. doi: 10.1038/sj.onc.1204123. [DOI] [PubMed] [Google Scholar]
- 176.Gurpinar E., Grizzle W.E., Piazza G.A. NSAIDs inhibit tumorigenesis, but how? Clin. Cancer Res. 2014;20:1104–1113. doi: 10.1158/1078-0432.CCR-13-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thompson W.J., Piazza G.A., Li H., Liu L., Fetter J., Zhu B., Sperl G., Ahnen D., Pamukcu R. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 178.Nagano A., Arioka M., Takahashi-Yanaga F., Matsuzaki E., Sasaguri T. Celecoxib inhibits osteoblast maturation by suppressing the expression of Wnt target genes. J. Pharmacol. Sci. 2017;133:18–24. doi: 10.1016/j.jphs.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 179.Takahashi-Yanaga F., Yoshihara T., Jingushi K., Miwa Y., Morimoto S., Hirata M., Sasaguri T. Celecoxib-induced degradation of T-cell factors-1 and -4 in human colon cancer cells. Biochem. Biophys. Res. Commun. 2008;377:1185–1190. doi: 10.1016/j.bbrc.2008.10.115. [DOI] [PubMed] [Google Scholar]
- 180.Boon E.M.J., Keller J.J., Wormhoudt T.A.M., Giardiello F.M., Offerhaus G.J.A., van der Neut R., Pals S.T. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br. J. Cancer. 2004;90:224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maier T.J., Janssen A., Schmidt R., Geisslinger G., Grösch S. Targeting the beta-catenin/APC pathway: A novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. FASEB J. 2005;19:1353–1355. doi: 10.1096/fj.04-3274fje. [DOI] [PubMed] [Google Scholar]
- 182.Sakoguchi-Okada N., Takahashi-Yanaga F., Fukada K., Shiraishi F., Taba Y., Miwa Y., Morimoto S., Iida M., Sasaguri T. Celecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cells. Biochem. Pharmacol. 2007;73:1318–1329. doi: 10.1016/j.bcp.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 183.Lan F., Yue X., Han L., Shi Z., Yang Y., Pu P., Yao Z., Kang C. Genome-wide identification of TCF7L2/TCF4 target miRNAs reveals a role for miR-21 in Wnt-driven epithelial cancer. Int. J. Oncol. 2012;40:519–526. doi: 10.3892/ijo.2011.1215. [DOI] [PubMed] [Google Scholar]
- 184.Liu X., Wang L., Zhao S., Ji X., Luo Y., Ling F. β-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastma cells. Med. Oncol. 2011;28:608–614. doi: 10.1007/s12032-010-9476-5. [DOI] [PubMed] [Google Scholar]
- 185.Hogenesch J.B., Gu Y.Z., Jain S., Bradfield C.A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., Weitz C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 187.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 188.Schibler U., Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/S0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 189.Ko C.H., Takahashi J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 190.Truong K.K., Lam M.T., Grandner M.A., Sassoon C.S., Malhotra A. Timing Matters: Circadian Rhythm in Sepsis, Obstructive Lung Disease, Obstructive Sleep Apnea, and Cancer. Ann. Am. Thorac. Soc. 2016;13:1144–1154. doi: 10.1513/AnnalsATS.201602-125FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Savvidis C., Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol. Med. 2012;18:1249–1260. doi: 10.2119/molmed.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gu X., Xing L., Shi G., Liu Z., Wang X., Qu Z., Wu X., Dong Z., Gao X., Liu G., et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012;19:397–405. doi: 10.1038/cdd.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gery S., Komatsu N., Baldjyan L., Yu A., Koo D., Koeffler H.P. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 194.Lévi F., Zidani R., Misset J.L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350:681–686. doi: 10.1016/S0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- 195.Coudert B., Focan C., Genet D., Giacchetti S., Cvickovic F., Zambelli A., Fillet G., Chollet P., Amoroso D., Van Der Auwera J., et al. A randomized multicenter study of optimal circadian time of vinorelbine combined with chronomodulated 5-fluorouracil in pretreated metastatic breast cancer patients: EORTC trial 05971. Chronobiol. Int. 2008;25:680–696. doi: 10.1080/07420520802384036. [DOI] [PubMed] [Google Scholar]
- 196.Gallion H.H., Brunetto V.L., Cibull M., Lentz S.S., Reid G., Soper J.T., Burger R.A., Andersen W., Gynecologic Oncology Group Study Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003;21:3808–3813. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 197.Bertheault-Cvitkovic F., Lévi F., Soussan S., Brienza S., Adam R., Itzhaki M., Misset J.L., Bismuth H. Circadian rhythm-modulated chemotherapy with high-dose 5-fluorouracil: A pilot study in patients with pancreatic adenocarcinoma. Eur. J. Cancer. 1993;29:1851–1854. doi: 10.1016/0959-8049(93)90536-O. [DOI] [PubMed] [Google Scholar]
- 198.Giacchetti S., Bjarnason G., Garufi C., Genet D., Iacobelli S., Tampellini M., Smaaland R., Focan C., Coudert B., Humblet Y., et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: The European Organisation for Research and Treatment of Cancer Chronotherapy Group. J. Clin. Oncol. 2006;24:3562–3569. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 199.Puram R.V., Kowalczyk M.S., de Boer C.G., Schneider R.K., Miller P.G., McConkey M., Tothova Z., Tejero H., Heckl D., Järås M., et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165:303–316. doi: 10.1016/j.cell.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dong W.-G., Mei Q., Yu J.-P., Xu J.-M., Xiang L., Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J. Gastroenterol. 2003;9:1307–1311. doi: 10.3748/wjg.v9.i6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Deng W.-G., Tang S.-T., Tseng H.-P., Wu K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 2006;108:518–524. doi: 10.1182/blood-2005-09-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Murakami Y., Yuhara K., Takada N., Arai T., Tsuda S., Takamatsu S., Machino M., Fujisawa S. Effect of melatonin on cyclooxygenase-2 expression and nuclear factor-kappa B activation in RAW264.7 macrophage-like cells stimulated with fimbriae of Porphyromonas gingivalis. In Vivo. 2011;25:641–647. [PubMed] [Google Scholar]
- 203.Huang S.-H., Cao X., Wei W. Melatonin decreases TLR3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected RAW264.7 macrophages. J. Pineal Res. 2008;45:93–100. doi: 10.1111/j.1600-079X.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- 204.Lu J.-J., Fu L., Tang Z., Zhang C., Qin L., Wang J., Yu Z., Shi D., Xiao X., Xie F., et al. Melatonin inhibits AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 2016;7:2985–3001. doi: 10.18632/oncotarget.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu Y., Tang D., Liu N., Xiong W., Huang H., Li Y., Ma Z., Zhao H., Chen P., Qi X., et al. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017;25:73–85. doi: 10.1016/j.cmet.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 206.Adamovich Y., Ladeuix B., Golik M., Koeners M.P., Asher G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017;25:93–101. doi: 10.1016/j.cmet.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 207.Peek C.B., Levine D.C., Cedernaes J., Taguchi A., Kobayashi Y., Tsai S.J., Bonar N.A., McNulty M.R., Ramsey K.M., Bass J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017;25:86–92. doi: 10.1016/j.cmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zawilska J.B., Skene D.J., Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009;61:383–410. doi: 10.1016/S1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- 209.Reiter R.J., Tan D.X., Manchester L.C., Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 210.León J., Acuña-Castroviejo D., Escames G., Tan D.-X., Reiter R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 211.Proietti S., Cucina A., Minini M., Bizzarri M. Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 2017;74:4015–4025. doi: 10.1007/s00018-017-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen T.L. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone. 2004;35:83–95. doi: 10.1016/j.bone.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 213.Soták M., Sumová A., Pácha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann. Med. 2014;46:221–232. doi: 10.3109/07853890.2014.892296. [DOI] [PubMed] [Google Scholar]
- 214.Guo B., Chatterjee S., Li L., Kim J.M., Lee J., Yechoor V.K., Minze L.J., Hsueh W., Ma K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26:3453–3463. doi: 10.1096/fj.12-205781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yasuniwa Y., Izumi H., Wang K.-Y., Shimajiri S., Sasaguri Y., Kawai K., Kasai H., Shimada T., Miyake K., Kashiwagi E., et al. Circadian disruption accelerates tumor growth and angio/stromagenesis through a Wnt signaling pathway. PLoS ONE. 2010;5:e15330. doi: 10.1371/journal.pone.0015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Janich P., Pascual G., Merlos-Suárez A., Batlle E., Ripperger J., Albrecht U., Cheng H.-Y.M., Obrietan K., Di Croce L., Benitah S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 217.Lin F., Chen Y., Li X., Zhao Q., Tan Z. Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem. Funct. 2013;31:166–172. doi: 10.1002/cbf.2871. [DOI] [PubMed] [Google Scholar]
- 218.Sahar S., Sassone-Corsi P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 219.Yang X., Wood P.A., Ansell C.M., Ohmori M., Oh E.-Y., Xiong Y., Berger F.G., Peña M.M.O., Hrushesky W.J.M. Beta-catenin induces beta-TrCP-mediated PER2 degradation altering circadian clock gene expression in intestinal mucosa of ApcMin/+ mice. J. Biochem. 2009;145:289–297. doi: 10.1093/jb/mvn167. [DOI] [PubMed] [Google Scholar]
- 220.Duffield G.E., Best J.D., Meurers B.H., Bittner A., Loros J.J., Dunlap J.C. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr. Biol. 2002;12:551–557. doi: 10.1016/S0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 221.Sancar A., Lindsey-Boltz L.A., Unsal-Kaçmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 222.Kowanko I.C., Pownall R., Knapp M.S., Swannell A.J., Mahoney P.G. Circadian variations in the signs and symptoms of rheumatoid arthritis and in the therapeutic effectiveness of flurbiprofen at different times of day. Br. J. Clin. Pharmacol. 1981;11:477–484. doi: 10.1111/j.1365-2125.1981.tb01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Haus E., Sackett-Lundeen L., Smolensky M.H. Rheumatoid arthritis: Circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull. NYU Hosp. Jt. Dis. 2012;70(Suppl. 1):3–10. [PubMed] [Google Scholar]
- 224.Yang X., Downes M., Yu R.T., Bookout A.L., He W., Straume M., Mangelsdorf D.J., Evans R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 225.Yang G., Jia Z., Aoyagi T., McClain D., Mortensen R.M., Yang T. Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS ONE. 2012;7:e38117. doi: 10.1371/journal.pone.0038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang H.-M., Zhao Y.-X., Zhang S., Liu G.-D., Kang W.-Y., Tang H.-D., Ding J.-Q., Chen S.-D. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J. Alzheimers Dis. 2010;20:1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 227.Fontaine C., Dubois G., Duguay Y., Helledie T., Vu-Dac N., Gervois P., Soncin F., Mandrup S., Fruchart J.-C., Fruchart-Najib J., et al. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J. Biol. Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 228.Green C.B., Douris N., Kojima S., Strayer C.A., Fogerty J., Lourim D., Keller S.R., Besharse J.C. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2007;104:9888–9893. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen L., Yang G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014;2014:653017. doi: 10.1155/2014/653017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 231.Manel N., Unutmaz D., Littman D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Clark R.B., Bishop-Bailey D., Estrada-Hernandez T., Hla T., Puddington L., Padula S.J. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J. Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 233.Klotz L., Burgdorf S., Dani I., Saijo K., Flossdorf J., Hucke S., Alferink J., Nowak N., Novak N., Beyer M., et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li W., Zhang Z., Zhang K., Xue Z., Li Y., Zhang Z., Zhang L., Gu C., Zhang Q., Hao J., et al. Arctigenin Suppress Th17 Cells and Ameliorates Experimental Autoimmune Encephalomyelitis Through AMPK and PPAR-γ/ROR-γt Signaling. Mol. Neurobiol. 2016;53:5356–5366. doi: 10.1007/s12035-015-9462-1. [DOI] [PubMed] [Google Scholar]
- 235.Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J. Exp. Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Korkmaz A., Tamura H., Manchester L.C., Ogden G.B., Tan D.-X., Reiter R.J. Combination of melatonin and a peroxisome proliferator-activated receptor-gamma agonist induces apoptosis in a breast cancer cell line. J. Pineal Res. 2009;46:115–116. doi: 10.1111/j.1600-079X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 237.Hansen M.B., Nielsen S.E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 238.Crowe D.L., Chandraratna R.A.S. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6:R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Eck-Enriquez K., Kiefer T.L., Spriggs L.L., Hill S.M. Pathways through which a regimen of melatonin and retinoic acid induces apoptosis in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 2000;61:229–239. doi: 10.1023/A:1006442017658. [DOI] [PubMed] [Google Scholar]
- 240.Yu N., Sun Y.-T., Su X.-M., He M., Dai B., Kang J. Melatonin attenuates TGFβ1-induced epithelial-mesenchymal transition in lung alveolar epithelial cells. Mol. Med. Rep. 2016;14:5567–5572. doi: 10.3892/mmr.2016.5950. [DOI] [PubMed] [Google Scholar]
- 241.Wu S.-M., Lin W.-Y., Shen C.-C., Pan H.-C., Keh-Bin W., Chen Y.-C., Jan Y.-J., Lai D.-W., Tang S.-C., Tien H.-R., et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPβ and NFκB cleavage. J. Pineal Res. 2016;60:142–154. doi: 10.1111/jpi.12295. [DOI] [PubMed] [Google Scholar]
- 242.Barberino R.S., Menezes V.G., Ribeiro A.E.A.S., Palheta R.C., Jiang X., Smitz J.E.J., Matos M.H.T. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol. Reprod. 2017;96:1244–1255. doi: 10.1093/biolre/iox053. [DOI] [PubMed] [Google Scholar]
- 243.Gao Y., Xiao X., Zhang C., Yu W., Guo W., Zhang Z., Li Z., Feng X., Hao J., Zhang K., et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. Pineal Res. 2017;62:e12380. doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 244.Mao L., Dauchy R.T., Blask D.E., Slakey L.M., Xiang S., Yuan L., Dauchy E.M., Shan B., Brainard G.C., Hanifin J.P., et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol. Endocrinol. 2012;26:1808–1820. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zelenay S., van der Veen A.G., Böttcher J.P., Snelgrove K.J., Rogers N., Acton S.E., Chakravarty P., Girotti M.R., Marais R., Quezada S.A., et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rachidi S., Metelli A., Riesenberg B., Wu B.X., Nelson M.H., Wallace C., Paulos C.M., Rubinstein M.P., Garrett-Mayer E., Hennig M., et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci. Immunol. 2017;2:eaai7911. doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kostadinov R.L., Kuhner M.K., Li X., Sanchez C.A., Galipeau P.C., Paulson T.G., Sather C.L., Srivastava A., Odze R.D., Blount P.L., et al. NSAIDs modulate clonal evolution in Barrett’s esophagus. PLoS Genet. 2013;9:e1003553. doi: 10.1371/journal.pgen.1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]