Abstract
Biosensors on the membrane of the vascular endothelium are responsible for sensing mechanical and chemical signals in the blood. Transduction of these stimuli into intracellular signaling cascades regulate cellular processes including ion transport, gene expression, cell proliferation, and/or cell death. The primary cilium is a well-known biosensor of shear stress but its role in sensing extracellular pH change has never been examined. As a cellular extension into the immediate microenvironment, the cilium could be a prospective sensor for changes in pH and regulator of acid response in cells. We aim to test our hypothesis that the primary cilium plays the role of an acid sensor in cells using vascular endothelial and embryonic fibroblast cells as in vitro models. We measure changes in cellular pH using pH-sensitive 2′,7′-biscarboxyethy1-5,6-carboxyfluorescein acetoxy-methylester (BCECF) fluorescence and mitogen-activated protein kinase (MAPK) activity to quantify responses to both extracellular pH (pHo) and intracellular pH (pHi) changes. Our studies show that changes in pHo affect pHi in both wild-type and cilia-less Tg737 cells and that the kinetics of the pHi response are similar in both cells. Acidic pHo or pHi was observed to change the length of primary cilia in wild-type cells while the cilia in Tg737 remained absent. Vascular endothelial cells respond to acidic pH through activation of ERK1/2 and p38-mediated signaling pathways. The cilia-less Tg737 cells exhibit delayed responsiveness to pHo dependent and independent pHi acidification as depicted in the phosphorylation profile of ERK1/2 and p38. Otherwise, intracellular pH homeostatic response to acidic pHo is similar between wild-type and Tg737 cells, indicating that the primary cilia may not be the sole sensor for physiological pH changes. These endothelial cells respond to pH changes with a predominantly K+-dependent pHi recovery mechanism, regardless of ciliary presence or absence.
Keywords: acidosis, ERK1/2, p38, pH, primary cilia
1. Introduction
The normal blood pH level is tightly maintained between 7.35 and 7.45 by the renal and respiratory systems along with buffering mediators in the blood. Lowering of blood pH < 7.35 or acidosis causes several symptoms such as drowsiness, exhaustion, and arrhythmia depending on the type of acidosis. More dramatic changes in pH will induce cytotoxicity and neuronal cell death. The vascular endothelium, which also regulates physical dynamics of blood such as local blood flow and pressure, is best suited to measure local circulating blood pH. Any pH sensor in the body needs to be responsive to extracellular pH fluctuations and regulate downstream mechanisms for homeostatic adaptation. Such adaptation mechanisms include local activation of ion transporters and modulation of channel activity to balance the intracellular ionic gradient, while global regulation is achieved by adjusting the ventilation or renal excretion.
Several studies have shown that mitogen-activated protein kinases (MAPKs) are activated by changes in pH [1,2]. The three known MAPKs, extracellular signal-regulated kinase (ERK1/2 aka p32/p44), p38, and JNK1/2, respond to a variety of environmental stimuli to mediate gene expression, ion transport, cell proliferation, and/or apoptosis [3]. ERK1/2 phosphorylation regulates acid-stimulated vacuolar H+-ATPase and Na+/H+ exchanger (NHE) activation [1,4]. When intracellular pH becomes acidic, ERK1/2 activation acts in parallel with Pyk2 kinase to increase NHE3 activity [5,6]. The MAPK, p38 is activated by various extracellular stress stimuli such as UV light, heat, inflammatory cytokines, and pH changes. Depending on the initial stimuli, substrates of activated p38 include transcription factors and the MAP kinase-activated protein kinase 2 (MK2). MK2 subsequently activates various small heat shock protein 27 (HSP27), lymphocyte-specific protein 1 (LSP1), cAMP response element-binding protein (CREB) among others [7,8].
In the present study, we explore if the primary cilia, distinct from motile cilia in the brain ventricles or Hensen’s node, have a role in acid pH sensation in endothelial cells. Our interest arises from the fact that the primary cilium, a solitary extension of the cell, has been implicated in the sensation of mechanical forces and chemical cues [9,10,11,12]. With the localization of various ion channels, G protein-coupled receptors and receptor-cytoskeletal proteins in the ciliary membrane, the primary cilium is a candidate biosensor that responds to a variety of stimuli [13]. Numerous studies have shown that cilia regulate cytosolic calcium influx and intracellular calcium release upon application of shear stress [12,14,15,16]. The response to blood flow-induced shear stress is very important in the regulation of blood pressure, vascular tone, and vasodilation [13]. As a cellular structure that protrudes out to the vascular lumen and remains in contact with the extracellular milieu, the cilia are poised to be a sensory extension. A recent study on Zebrafish showed localization of acid-sensing ion channels (ASICs), which are proton-gated cation channels, in the cilia of the non-sensory olfactory cell [17]. ASICs are Na+ channels activated by external protons and exhibit a rapid response to reduction in extracellular pH (pHo) below pH 6.9 [18]. Another Na+ channel, the alpha-epithelial sodium channel, has been immunodetected in the cilia and are regulated by flow as well as acidic pHo through Na+ gating phenomenon of self-inhibition [19,20,21].
A study by Banizs et al. on cilia-less Tg737 mice shows that Tg737 mice have lower intrinsic buffering power when challenged with a weak acid NH4+ compared to wild-type mice [22]. This indicates that primary cilia might be involved in either sensing pHo change or regulating intracellular pH (pHi) in response to pHo changes through ciliary ion transport activity. With the evidence that pH sensitive channels are selectively localized in the cilia of the non-sensory olfactory epithelium [17] and the cilium is known as a sensory organelle of the extracellular milieu [9,12,23,24], we hypothesize that primary cilia could function as pH sensors. We, therefore, examine the role of the primary cilia in acid-activation of MAPK signaling pathways in endothelial cells. We compare the acid response of cilia-less Tg737 endothelial cells to their wild-type counterparts to examine a possible pH sensing role of the primary cilia.
2. Materials and Methods
2.1. Cell Culture
Previously isolated and characterized vascular endothelial cells (Tg737+/+ and Tg737−/−) were used for the study [23,25]. These cell lines were generated from the same littermates of Tg737+/− mice with Balb/C background. The Tg737 gene encodes for polaris, a structural protein for cilia [26]. These endothelial cells were also immortalized from mice carrying the simian virus-40 (SV40) gene. The promoter of SV40 is regulated by temperature and IFN-γ. As such, cells were grown under permissive conditions in the presence of 0.75 μg/L IFN-γ at 33 °C express SV40 large T antigen regardless of the status of their confluence. The permissive conditions allow cells to hyper-proliferate. When switched to non-permissive conditions in the absence of IFN-γ at 37 °C, the endothelial cells completely shut down the SV40 gene. Cells under the non-permissive conditions are readily differentiated [23,25].
These cells express common markers for endothelial cells, including eNOS, ICAM-2 (CD102), PECAM-1 (CD31), VE-cadherin (CD144), readily responding to acetylcholine, forming endothelial barrier integrity and having functional intracellular calcium signaling, focal adhesion kinase, calmodulin, Akt/PKB, protein kinase C and eNOS activity [23,25,27]. Aside from abnormal mechanosensory function due to lacking primary cilia, the Tg737 cilia-less cells also have abnormal cell division [28,29].
Three days prior to experiments, cells were cultured under sterile conditions and maintained at 37 °C in a 5% CO2 incubator. Cells were kept in Dulbecco’s Modification of Eagle’s Medium (DMEM), media with 4.5 g/L glucose, l-glutamate, and sodium pyruvate (Corning Cellgro) containing 2% fetal bovine serum (FBS) and 5% penicillin/streptomycin. DMEM with 2% FBS is a low serum condition that promotes ciliation [30]. For NIH3T3 fibroblast cells, growth media consisting of 10% bovine calf serum (BCS) and 5% penicillin/streptomycin in DMEM was used. Cells were grown on poly-l-lysine coated cover glass and incubated with low serum media (2% BCS, 5% penicillin/streptomycin and DMEM) to promote ciliation. To investigate Hedgehog (Hh) signaling in various pHo, purmorphamine (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 10 μM was used as a positive control. Purmorphamine was added and incubated for 1 h with the cells to induce Hh activation.
2.2. Decreased Extracellular pH (pHo)
Physiological saline solution (PSS; Table 1) was adjusted to pHo 5.5, 6.0, 6.5, and 7.0 from pH 7.4 (control) using 100 mM HCl. For immunoblot, each 35-mm dish was exposed to media of a given pH for 10 min. Control cells underwent similar treatment with vehicle. Cells were trypsinized and 106 cells transferred into 100 µL 2× Laemelli Sample Buffer (BioRad, Hercules, CA, USA) containing β-mercaptoethanol. Samples were sonicated and heated at 100 °C for 5 min. For tracings of pHi measurement, BCECF-AM-loaded cells were exposed to media of each pHo, one at a time for 10 min, sequentially from pHo 7.4 to 5.5. In all our experiments, we maintained our solution osmolality between 290–300 mOsm/L.
Table 1.
Composition of solutions used in the NH4Cl pre-pulse. Solution names are listed along the first row, and the composition of each is shown in each column. Components of each solution are in mM. HEPES = (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); NMDG = N-methyl-D-glucamine.
| Solution | PSS | NH4Cl | 0K+/0Na+ | 5K+/0Na+ |
|---|---|---|---|---|
| CaCl2 | 1.8 | 1.8 | 1.8 | 1.8 |
| MgSO4 | 0.8 | 0.8 | 0.8 | 0.8 |
| Glucose | 5.5 | 5.5 | 5.5 | 5.5 |
| HEPES | 10 | 10 | 10 | 10 |
| NaCl | 135 | 0 | 0 | 0 |
| KCl | 5 | 0 | 0 | 5 |
| NH4Cl | 0 | 20 | 0 | 0 |
| NMDG | 0 | 120 | 140 | 135 |
2.3. Decreased Intracellular pH (pHi)
The NH4Cl pulse was used to alter pHi using a series of solutions, as previously described [1] and shown in Table 1. All solutions were adjusted to pHo 7.4 and maintained at 37 °C with osmolality between 290–300 mOsm/L. PSS was added to the cells for 5 min, then aspirated. The NH4Cl solution (20 mM NH4Cl) was then added to cells until intracellular pH stabilized, then aspirated. The cells were immediately incubated in 0K+/0Na+ solution, causing dissociation of intracellular NH4, releasing protons into the cytosol, thus decreasing pHi. pHi recovery was accomplished by adding 5K+/0Na+ solution (5 mM KCl) to the cells and incubation in PSS.
2.4. Intracellular pH Measurement
Intracellular pH was measured with 2′,7′-biscarboxyethy1-5,6-carboxyfluorescein acetoxy-methylester (BCECF-AM; Molecular Probes, #B1150, Invitrogen, Eugene, OR, USA). Wild-type and Tg737 cells were incubated with 5 μM BCECF-AM for 15 min at 37 °C. Images were acquired with a Nikon Eclipse Ti-E inverted microscope using 40× objective and NIS-Elements imaging software (version 4.30, Melville, NY, USA, 2016). Intracellular pH measurements were recorded with emission intensity at wavelength 535 nm. The ratio of emission intensity was determined through excitation wavelengths of pH-dependent 490 nm and an isosbestic point 440 nm. BCECF fluorescence ratio intensity was calibrated to represent intracellular pH using H+ ionophore nigericin-containing solutions (Sigma-Aldrich, #N7143). This calibration was performed at the end of each experiment. 20 μM nigericin was used to equilibrate pHo and pHi to pH values of 5.5, 6.0, 6.5, 7.0 and 7.4. Once the 490/440 ratio for each calibration pH value was obtained, the ratio values were fitted to a sigmoidal plot. Subsequent experimental ratios were converted to the pH values.
2.5. Immunoblot
All extracellular and intracellular pH manipulations were performed in the same manner for both pH measurement and Western blot analysis. 35 mm dishes were each lysed at different steps of decreased pHo or pHi with NH4Cl pre-pulse. Control cells underwent similar treatment with vehicle. Immunoblot of the lysates was used to analyze the phosphorylation of p38 and ERK1/2 in response to decreased pHo or during the different steps of the NH4Cl pre-pulse. Blots were probed for β-actin to confirm equal protein loading. Membranes were blocked for 1 h then incubated with primary antibody for 2 h. Primary antibodies include: anti-ERK1/2 (Cell Signaling, #9101), anti-phospho-ERK1/2 (Cell Signaling, #9102), anti-p38 (Abcam, #ab7952), anti-phospho-p38 (Abcam, #ab45381), or anti-β-actin (CellBioLabs, #AKR-002). Cells were rinsed 3× for 10 min then incubated in secondary antibody for 1 h. Secondary antibodies include anti-mouse IgG, HRP-linked (Cell Signaling Technologies, #7076) or anti-rabbit IgG, HRP-linked (Cell Signaling Technologies, #7074). After rinsing three times for 10 min each, membranes were visualized using SuperSignaling West Pico Luminol Enhancers solution (Thermo Scientific, #1859675) and detected with the ChemiDoc from BioRad. Images were acquired and analyzed using ImageLab3.0 software.
2.6. Primary Cilia Immunostaining
Cells were grown to confluence on coverslips according to the cell culture conditions mentioned above. The cells were then exposed to media with pH of 5.5, 6.0, 6.5, 7.0 and 7.4 (control) for 5 min. The cells were fixed using 4% paraformaldehyde and 2% sucrose in PBS for 10 min and permeabilized for 5 min in 10% Triton X-100. The cells were incubated with Gli antibody (1:200 dilution in PBS, Abcam, Cambridge, MA, USA) for 16 h at 4 °C, acetylated-α-tubulin (1:10,000 dilution in PBS, Sigma Aldrich, St. Louis, MO, USA) for 1 h at 27 °C followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody then Texas-red conjugated anti-rabbit antibody (1:1000 dilution in PBS, Vector Labs Burlingame, CA) for 1 h at 27 °C. Slides were mounted with DAPI hard set mounting media (Southern Biotech, Birmingham, AL, USA). Images were acquired using a Nikon Eclipse Ti-E inverted microscope with the NIS-Elements imaging software (version 4.30) in 100× magnification fields with z-stack slices of 0.25 μm. Flat cilia defined by consistent length in four z-slices were measured [31]. The majority of our cilia were flattened on the slide surface, and cilia were measured in three-dimensionally (3D). To ensure cilia flattened on the surface, we included a 3D movie in the supplement (Supplement Movie). We used Nikon NIS-Elements for Advanced Research software to capture and measure all cilia length in 3D. This software package included pre-programmed length analysis through iterations of automatic object recognition followed by image scanning and segmentation, optical flow and 3D object reconstruction. The single-particle tracking was activated only when cilia length was less than 1 μm, especially in Tg737 cells. In such cases (wild-type and Tg737 cells), cells with less than 1 μm length of cilia were denoted as non-ciliated cells.
Length measurements of 150 primary cilia was randomly selected using NIS-Elements. To obtain number of cells possessing cilia, six random 100× fields were scanned and a maximum intensity projection created for each field. The total number of cilia and nuclei, as a representation of cell number, was used to calculate ciliated cell percentage. Statistical analysis was performed on Prism GraphPad 8.1.2 software (GraphPad, San Diego, CA, USA).
2.7. Scanning Electron Microscopy
Cells were fixed with 2.5% paraformaldehyde/glutaraldehyde in sodium cacodylate buffer for 1 h at 27 °C. Samples were post-fixed with 1% aqueous osmium tetroxide solution. Dehydration was done using ethanol solutions. Samples were further dried with a 2-h incubation in 50% hexamethyldisilazane (HMDS)-ethyl alcohol mixture, followed by two 30-min incubations in 100% HMDS. Micrographs were obtained and analyzed using a Hitachi HD-2300 scanning electron microscope (SEM) [24].
2.8. Data Analysis
The rate of pHi changes is denoted as a rate constant of ΔpHi and expressed as dpH/dt (ΔpHi units/min). Because the ΔpHi is defined as rate constant of pHi decreased with respect to time, this pHi was not necessarily decreased at a constant speed. In other words, the changes in pHi could speed up and slow down during the period of measurement. We, therefore, looked at a second order kinetics of these acceleration and deceleration events. The second order kinetics were determined through the tangential rates in the changes of rate constant of ΔpHi using Microsoft Excel software (version 15.32). The mathematical expression to calculate the change of function at the time is as follows:
All the data shown are mean ± SEM from at least three independent experiments. Data was analyzed using ANOVA test followed by Tukey post-hoc test for multiple groups with p < 0.05 being considered as significant. Analysis of data was performed with Prism GraphPad 7 software (GraphPad, San Diego, CA, USA).
3. Results
3.1. Intracellular Acidosis in Response to Decreasing pHo in Wild-Type and Tg737 Cells
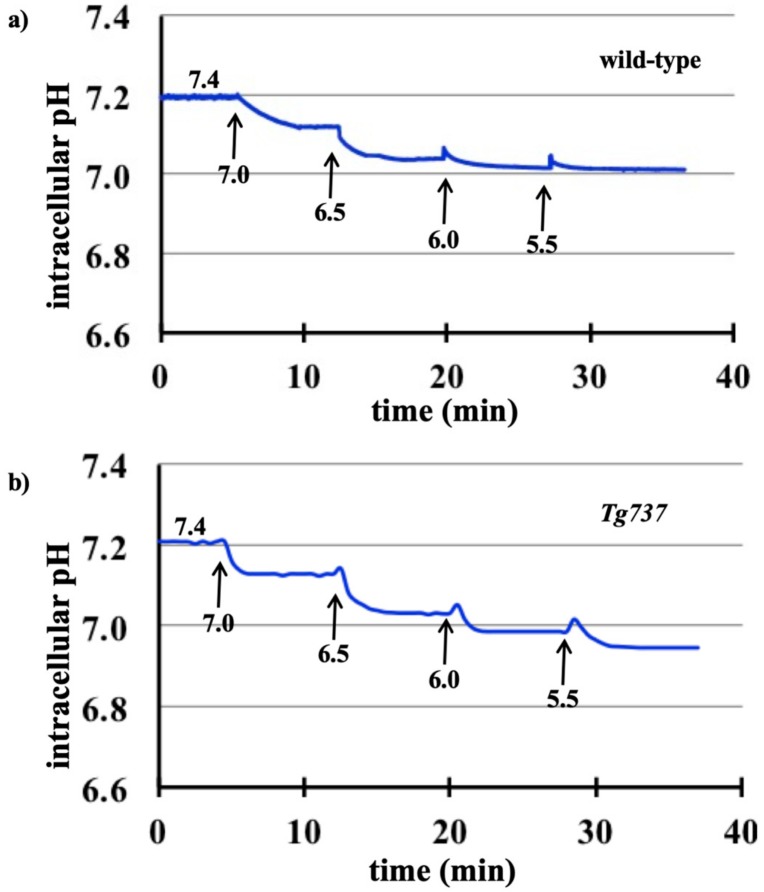
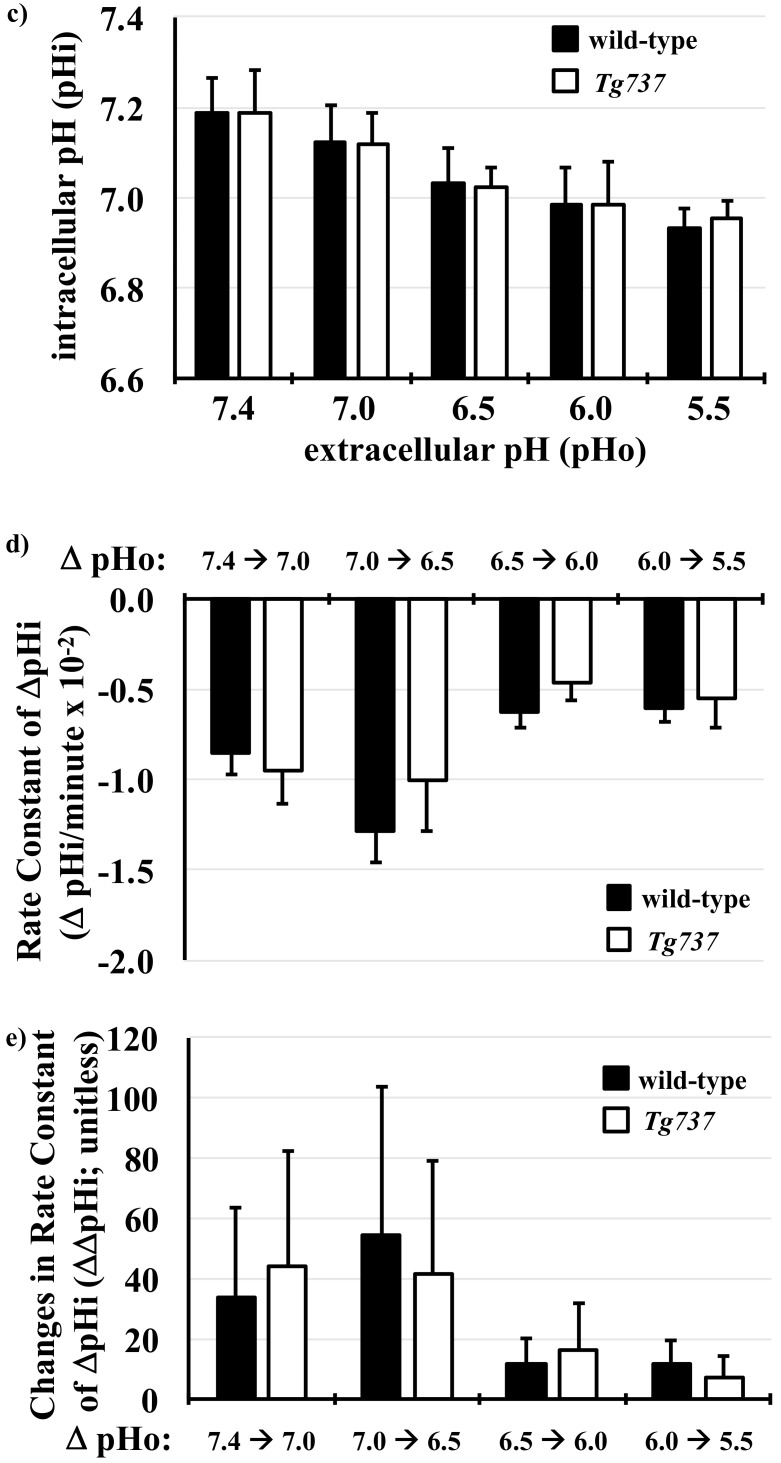
Incubation of cells in PSS of decreasing extracellular pH (pHo) from 7.4 to 5.5 acidified the intracellular environment in both wild-type (Figure 1a) and cilia-less Tg737 cells (Figure 1b). The acidic pHo mediated decrease in pHi was similar in both wild-type and Tg737 cells (Figure 1c). The rate of pHi changes (ΔpHi) was not significantly different between wild-type and Tg737 cells (Figure 1d). The negative values of ΔpHi indicated that the pHi was decreased in acidified media. There was no significant different between wild-type and Tg737 cells in the uniformity of or changes in ΔpHi (ΔΔpHi; Figure 1e). The positive values of ΔΔpHi indicated that the ΔpHi was predominantly involved in acceleration to decrease pHi.
Figure 1.
Decreased intracellular pH in response to acidic extracellular pH in wild-type and Tg737 cells. (a,b) Representative tracings of changes in intracellular pHi when wild-type and Tg737 cells are exposed to media of decreasing extracellular pH (pHo) from 7.4 to 5.5. (c) As the pHo is decreased, both cell lines show a similar decrease in their pHi. (d) The rate constant pHi changes (ΔpHi) in response step changes in pHo are also similar in both cell lines. A negative value indicates a decrease in pHi. (e) Changes in ΔpHi (ΔΔpHi) are normalized to identify variability within ΔpHi in response to step changes in pHo. No variation is observed if there is no acceleration or deceleration (first order kinetic or ΔΔpHi = 0). Highest variation (ΔΔpHi = 100) indicates that an alternate acceleration-deceleration pattern occurred (if acceleration = deceleration, then ΔpHi = 0).
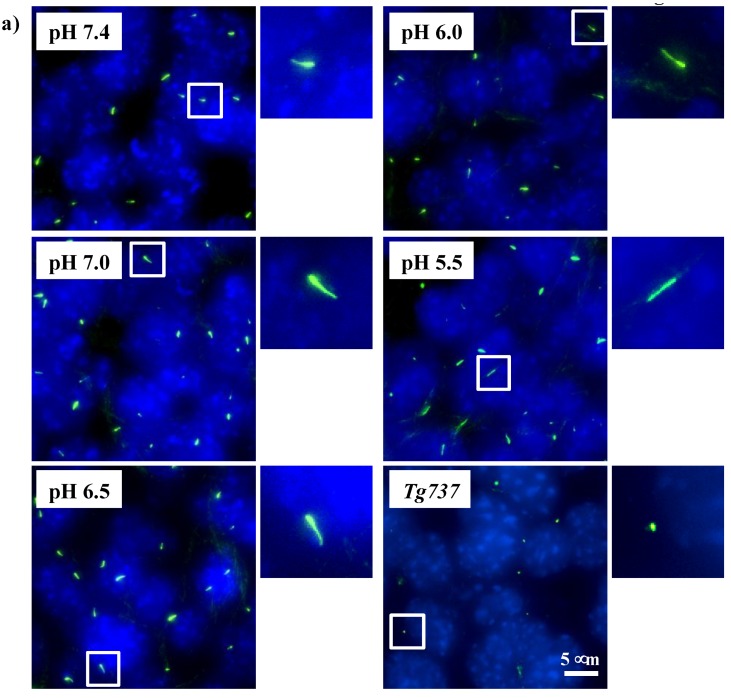
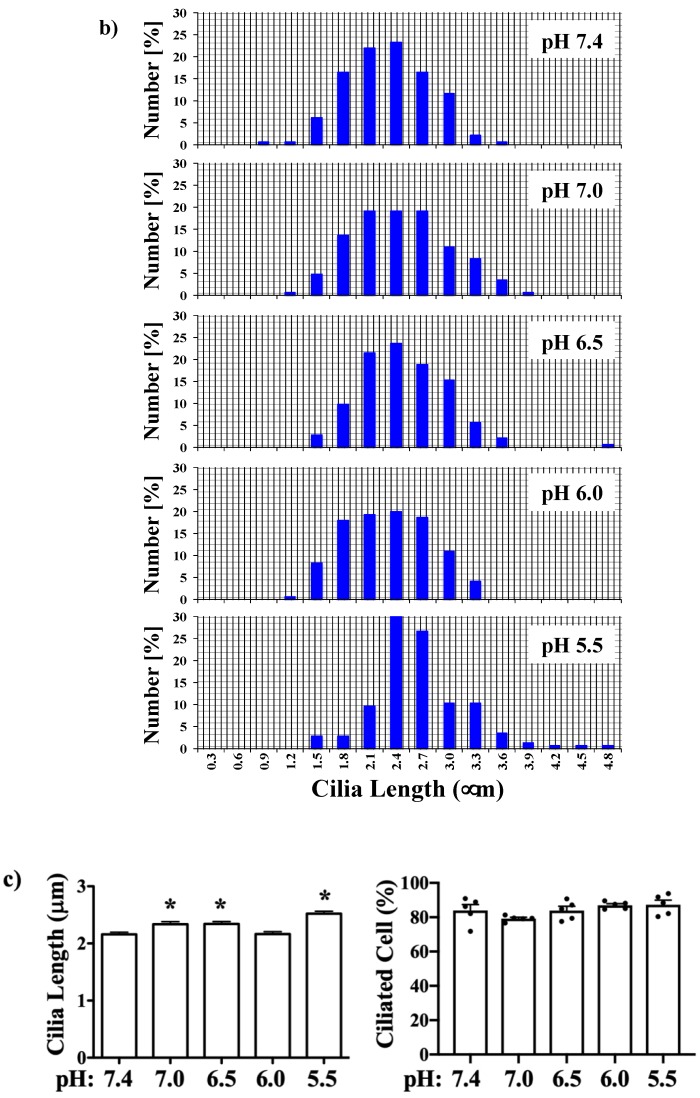
To validate that primary cilia remained intact and structurally stable in acidified media, endothelial cilia were examined with ciliary marker acetylated-α-tubulin (Figure 2a). To examine the effect of extracellular pH on the length of primary cilia, the distribution of cilia length as well as ciliation frequency is tabulated in the bar graph (Figure 2b). Compared to control at pH 7.4, a small but significant increase in cilia length was observed at pHo of 7.0, 6.5 and 5.5 while there were no significant differences observed in ciliation frequency (Figure 2c). There were no apparent differences in the cilia formation at different extracellular pH levels. Approximately 80–85% of wild-type cells were ciliated in acidified media, as well as at pH 7.4. For cilia-less Tg737 cells, the only representative image at pH 7.4 is shown with no further apparent differences in acidified media. There were no cilia length increase in Tg737 cells at various pHi.
Figure 2.
Immunofluorescence staining to study effects of pHo on primary cilia. (a) Cells (wild-type and Tg737) were stained with ciliary marker (acetylated-α-tubulin; green) and nucleus marker (DAPI; blue). Representative images are shown for wild-type cells at different pHo and Tg737 at pHo 7.4. White boxes show enlargement of the images to depict the presence of primary cilia. (b) The lengths of primary cilia from 50 cells were measured from each preparation (N = 3) and illustrated in the bar graph to depict length distribution within each pHo. (c) Cilia length was averaged from 150 cells (N = 3; each with 50 randomly selected cells) and the number of cells possessing cilia represented as a percentage with each point representing an individual experimental datapoint. * indicates a significant difference to control pHo 7.4.
Most importantly, the data shows that wild-type cells continued to possess primary cilia without any structural aberrations in an acidic environment. Further validation with another ciliated cell line, NIH3T3, was conducted to observe changes in ciliary length or ciliation frequency when challenged with acidified media (Supplement Figure S1–S3). Lower acidic media was able to significantly increase ciliary length at pHo of 6.5 and 5.5 while ciliation frequency remained unchanged at lower pHo. Among the many signaling activities related to the primary cilia, Hedgehog signaling (Hh) is unique in translocating activated receptors and proteins to the cilia [32]. To observe changes in functional role of the primary cilia under our experimental conditions, we used purmophamine to activate the Hh pathway. After observing no effect of Hh activation on ciliary length or ciliation frequency of the cells, we studied if acidified media induced Hh signaling, as shown by Gli translocation to the ciliary tip in NIH3T3 cells (Supplement Figure S3). There is also no apparent structural defect in cilia following a decrease in intracellular pH (Supplement Figure S4).
3.2. MAPK Activation in Response to Decreasing pHo in Wild-Type and Tg737 Cells
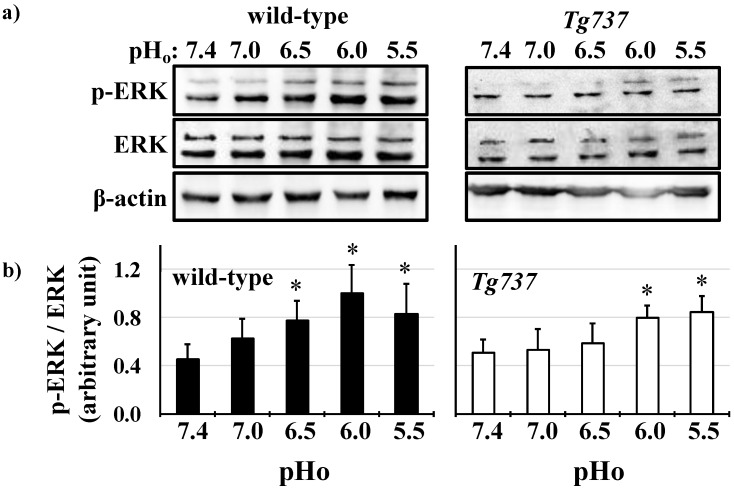
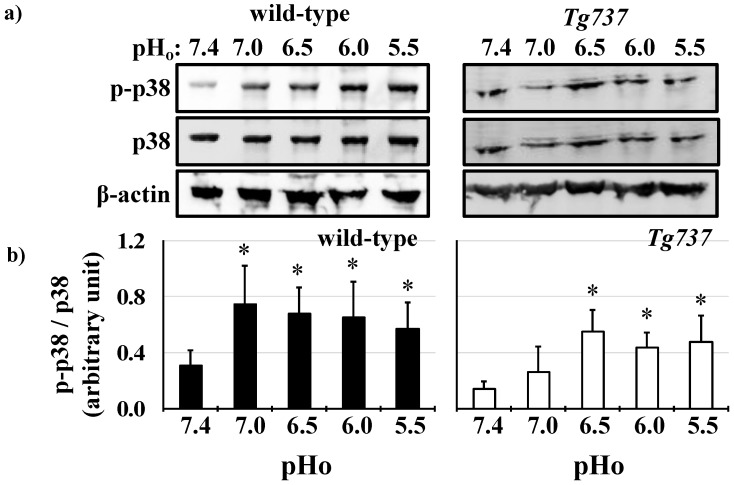
Both wild-type and Tg737 cells exhibited MAPK phosphorylation in response to decreased pHo. Significant increase of ERK1/2 phosphorylation occurred at pHo 6.5 in wild-type cells (178% increase versus control pHo 7.4) and persisted at pHo 6.0 and pHo 5.5 (227% and 189% increase in ERK1/2 phosphorylation, respectively versus control pHo 7.4) (Figure 3a). ERK1/2 phosphorylation occurred at pHo 6.0 in Tg737 cells (140% increase versus control pHo 7.4) (Figure 3b). Another MAPK, p38 was phosphorylated at pHo 7.0 in wild-type cells (242% increase versus control pHo 7.4) and persisted at pHo 6.0 and pHo 5.5 (227% and 189% increase in p38 phosphorylation, respectively versus control pHo 7.4) (Figure 4a). p38 phosphorylation occurred at pHo 6.5 in Tg737 cells (393% increase versus control pHo 7.4) and persisted through pHo 5.5 (321% and 360% increase in p38 phosphorylation at pHo 6.0 and 5.5, respectively, versus control pHo 7.4) (Figure 4b). Tg737 cells required higher acidic conditions to increase MAPK phosphorylation than the wild-type cells. There did not seem to be a significant impairment in cilia-less Tg737 cells’ ability to sense and respond to decreased pHo, but we find a lower pH threshold for activation of MAPK in Tg737 compared to wild-type cells.
Figure 3.
Decreasing extracellular pH increases ERK1/2 phosphorylation in wild-type and Tg737 cells. (a) Representative immunoblots show ERK1/2 phosphorylation in wild-type and Tg737 cells as they are exposed to media of decreasing extracellular pH (pHo) from 7.4 to 5.5. (b) Bar graph shows the mean p-ERK/ERK, where wild-type cells phosphorylate ERK1/2 at pH of 6.5 while the same level of phosphorylation occurs at a lower pH of 6.0. * indicates p < 0.05 as compared to control pHo 7.4; N = 10 for wild-type cells and N = 10 for Tg737 cells.
Figure 4.
p38 phosphorylation by decreased extracellular pH in wild-type and Tg737 cells. (a) Representative immunoblots show p38 phosphorylation in wild-type and Tg737 cells as they are exposed to media of decreasing extracellular pH (pHo) from 7.4 to 5.5. (b) Bar graph shows mean p-p38/p38. Significant increase in p-38 phosphorylation occurs when pH changes from 74. to 7.0. But in Tg737 cells the pH had to drop to 6.5 before significant changes in p38 phosphorylation was observed. * indicates p < 0.05 as compared to control pHo 7.4; N = 8 for wild-type cells and N = 8 for Tg737 cells.
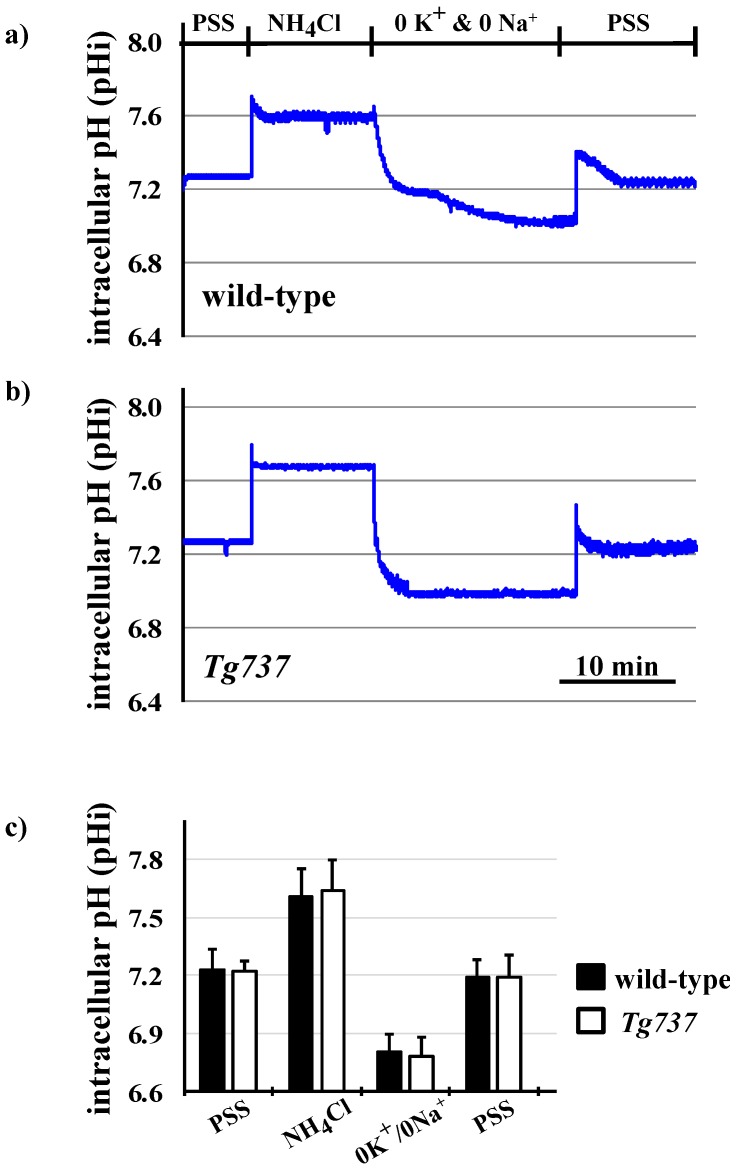
3.3. NH4Cl Pre-Pulse Induces Intracellular Acidosis in Wild-Type and Tg737 Cells
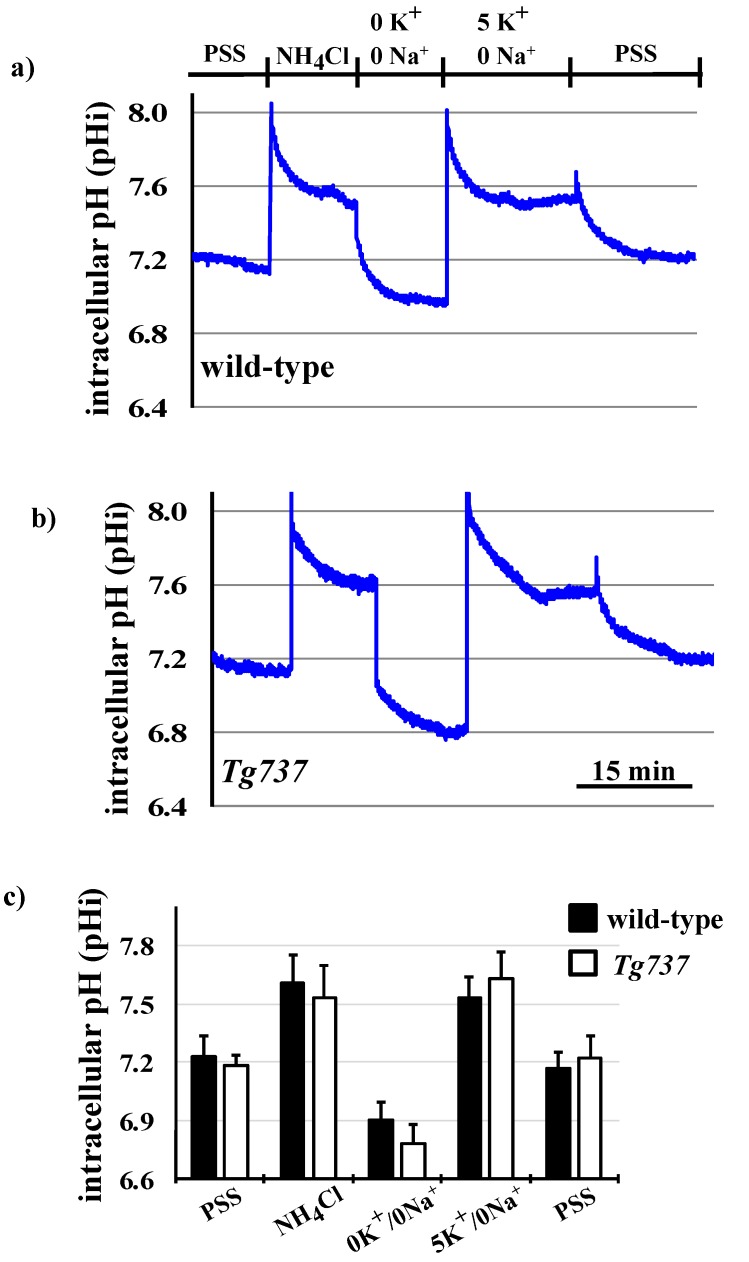
To eliminate the effect of pHo and observe intracellularly restricted pH acidosis NH4Cl pre-pulse was used to lower pHi. Changes in pHi during the NH4Cl pre-pulse using Na+- and K+-containing PSS for pHi recovery are depicted in representative tracings (Figure 5a,b of wild-type and Tg737 cells, respectively) and data is summarized in Figure 5c. In wild-type cells, NH4Cl caused an increase in intracellular pH (0.014 ± 0.002 pH units/min); in Tg737 cells, NH4Cl also increased the pHi (0.019 ± 0.003 pH units/min). In the absence of sodium and potassium, the pHi decreased in both cell types (0.028 ± 0.004 pH units/min in wild-type cells and 0.020 ± 0.003 pH units/min in Tg737 cells, from NH4Cl conditions). pHi recovery occurred upon addition of the Na+- and K+-containing PSS solution, at a rate of 0.006 ± 0.0001 pH units/min in wild-type cells, and more significantly in Tg737 cells at a rate of 0.031 ± 0.003 pH units/min, from 0K+/0Na+ conditions. Because rates did not increase during incubation in the 0K+/0Na+ solution, Na+- or K+-dependent transport is likely responsible for endothelial cells’ recovery from decreased pHi. Another inference is that Na+- and K+-independent transport may not be present or activated by intracellular acidosis, in vascular endothelial cells.
Figure 5.
Changes in pHi during the NH4Cl pre-pulse in wild-type and Tg737 cells. (a,b) Representative tracings of changes in intracellular pH when wild-type and Tg737 cells are exposed to solutions of the NH4Cl pre-pulse in Na+- and K+-devoid solution for pHi recovery. (c) Bar graph shows the summary of mean changes in pHi recovery in wild-type and Tg737 cells. N = 5 for wild-type cells and N = 3 for Tg737 cells.
To validate that primary cilia remained intact and structurally stable after intracellular acidification independent of pHo, endothelial cilia were examined with ciliary marker acetylated-α-tubulin (Supplement Figure S5). To examine the effect of pHi acidification on the length of primary cilia, the distribution of cilia length as well as ciliation frequency is tabulated in the bar graph (Supplement Figure S6). Compared to control at pHi 7.4, a small but significant decrease in cilia length was observed at pHi of 7.0 while there were no significant differences observed in ciliation frequency. There were no apparent differences in the cilia formation between pHi 7.4 and 7.0. Approximately 80–85% of wild-type cells were ciliated. There were no cilia length recovery in Tg737 cells at pHi of 7.0.
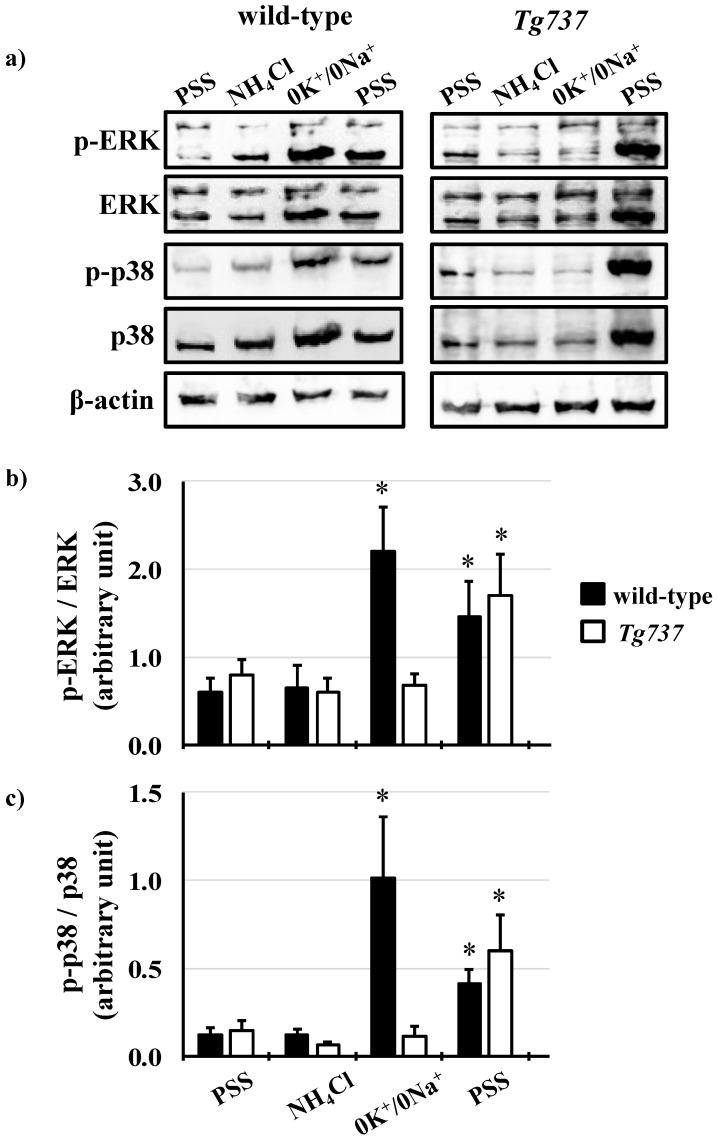
3.4. Intracellular Acidosis Activates MAPK Signaling Pathways
MAPK phosphorylation during the NH4Cl pre-pulse was similar in both cell types, with slight variations (Figure 6a). ERK1/2 phosphorylation at acid pHi during the 0K+/0Na+ step of the NH4Cl pre-pulse occurred in only wild-type cells (3.7-fold increase in ERK1/2 phosphorylation versus control conditions) (Figure 6b). ERK1/2 phosphorylation was observed during pHi recovery in both wild-type and Tg737 cells (2.1-fold and 2.3-fold respective increases in phosphorylation versus control conditions). p38 phosphorylation pattern varied between the wild type and cilia-less Tg737 as well. In wild-type cells, p38 phosphorylation occurred at low pHi during the 0K+/0Na+ step of the NH4Cl pre-pulse (10-fold increase in phosphorylation versus control conditions); in Tg737 cells, p38 phosphorylation was dampened, occurring only during pHi recovery (4.0-fold increase in p38 phosphorylation versus control conditions) (Figure 6c).
Figure 6.
ERK1/2 and p38 phosphorylation during the NH4Cl pre-pulse in wild-type and Tg737 cells. (a) Representative immunoblots show ERK1/2 and p38 phosphorylation in wild-type and Tg737 cells as they are exposed to different solutions during the NH4Cl pre-pulse. (b,c) Bar graphs show mean p-ERK/ERK and p-p38/p38. * indicates p < 0.05 as compared to control conditions; for ERK1/2 N = 6 for wild-type cells and N = 5 for Tg737 cells; for p38 N = 5 for wild-type cells and N = 5 for Tg737 cells.
3.5. Effects of K+ on pHi Recovery and on MAPK Phosphorylation during the NH4Cl Pre-Pulse in Wild-Type and Tg737 Cells
Potassium-containing 5K+/0Na+ solution could be used to study K+-dependent pHi recovery [1]. When the NH4Cl pre-pulse was expanded to include potassium-containing 5K+/0Na+ solution in between the 0K+/0Na+ and PSS solutions during pHi recovery, the pHi increased very rapidly in wild-type cells and Tg737 cells, highlighting the importance of K+-dependent transporter activity during pHi recovery in vascular endothelial cells, shown in representative tracings in Figure 7a,b, and summarized in Figure 7c. Addition of potassium-containing 5K+/0Na+ solution drove the pHi up in both cell types (0.030 ± 0.003 pH units/min in wild-type cells and 0.035 ± 0.003 pH units in Tg737 cells, versus 0K+/0Na+ conditions). Interestingly, addition of PSS at the end of the pulse returned the pHi to a normal level by driving the pHi down at a rate of 0.036 ± 0.005 pH units/min in wild-type cells and 0.035 ± 0.006 pH units/min in Tg737 cells, from 5K+/0Na+ conditions.
Figure 7.
Changes in pHi during the NH4Cl pre-pulse in wild-type and Tg737 cells. (a,b) Representative tracings of changes in intracellular pH when wild-type and Tg737 cells are exposed to solutions of the NH4Cl pre-pulse including the 5 mM K+ solution during pHi recovery of wild-type and Tg737 cells. (c) Bar graph shows the summary of pHi recovery data from pre-pulse tracings in wild-type and Tg737 cells. N = 5 for wild-type cells and N = 5 for Tg737 cells with the 5K+/0Na+ solution.
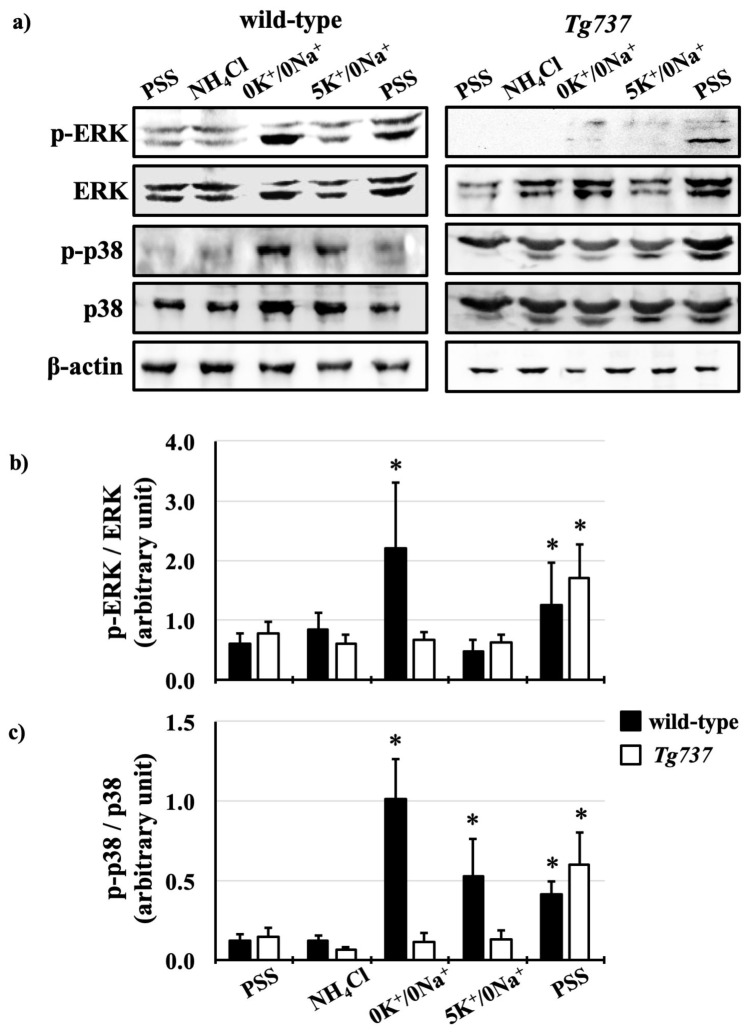
Addition of potassium-containing 5K+/0Na+ solution during pHi recovery also influenced MAPK phosphorylation, more specifically p38 phosphorylation (Figure 8). In wild-type cells, p38 phosphorylation coincided with ERK1/2 phosphorylation at acidified pHi, during the 0K+/0Na+ step of the NH4Cl pre-pulse (10-fold increase in phosphorylation versus control conditions), and p38 phosphorylation persisted through pHi recovery using the 5K+/0Na+ solution (5.5-fold increase in phosphorylation versus control conditions). In Tg737 cells, p38 phosphorylation occurred later during pHi recovery with the PSS solution (4.0-fold increase in phosphorylation versus control conditions). Both ERK1/2 and p38 phosphorylation were absent in Tg737 cells as compared to wild-type cells, where significant increases in MAPK phosphorylation occurred after inducing intracellular acidosis.
Figure 8.
Effect of K+ on ERK1/2 and p38 phosphorylation during the NH4Cl pre-pulse in wild-type and Tg737 cells. (a) Representative immunoblots show ERK1/2 and p38 phosphorylation in wild-type and Tg737 cells as they are exposed to different solutions during the NH4Cl pre-pulse. (b,c) Bar graphs show mean p-ERK/ERK and p-p38/p38, respectively. * denotes p < 0.05 as compared to control conditions. For ERK1/2, N = 8 for wild-type cells and N = 8 for Tg737 cells; for p38, N = 7 for wild-type cells and N = 5 for Tg737 cells.
4. Discussion
In the present studies, we assessed the role of primary cilia in pH sensing of vascular endothelial cells. We compared intracellular responses in wild-type and cilia-less Tg737 mutant cells against extracellular pH changes and obtained three main results. First, intracellular pH homeostasis was not significantly different between the wild-type and Tg737 cells. Second, phosphorylation of two mitogen-activated kinases, p38 and ERK1/2, were increased by lowering extracellular pH in both the wild-type and the Tg737 cells, but for Tg737 cells the same extent of phosphorylation needed a stronger acidic condition. Third, when the cells were exposed to 0K+/0Na+ and 5K+/0Na+ solutions after the NH4Cl solution, the phosphorylation of p38 and ERK1/2 was enhanced only in the wild-type cells.
Wild-type and Tg737 cells have similar responses to decreased extracellular pH (pHo), including decreased intracellular pH (pHi) relative to drops in pHo and acute ERK1/2 and p38 phosphorylation at pHo < 6.0 (Figure 1, Figure 2, Figure 3 and Figure 4). Diminished MAPK phosphorylation was observed in Tg737 cells compared to wild-type cells at certain pHo. A significant finding from these experiments was that MAPK phosphorylation in vascular endothelial cells when exposed to low pHo may be associated to the cilia or there could be inherited machinery differences between wild-type and Tg737 cells in terms of ERK1/2 and p38 phosphorylation.
An acidic environment increased the length of primary cilia in wild-type cells, whereas isolated acidification of intracellular pH decreased cilia length. The Tg737 cells remained cilia-less under all conditions. Similar to endothelial cells, the NIH3T3 fibroblast cells also presented with longer cilia when challenged with an acidic environment. The physiological significance of this cilia length changes is not clear at present. However, it has been speculated that cilia length could be used as a cellular marker in response to injury or environmental insults [33,34,35]. SEM micrographs showed no structural defects in the cilia after exposure to acidified media. With the aim of finding any functional effects of acidified media in the cilium, we looked at possible acidic pHo induced Hh signaling but found no unexpected activation of Hh.
The next set of experiments were designed to bypass extracellular pH sensing by lowering intracellular pH only (Figure 5, Figure 6, Figure 7 and Figure 8). In terms of pHi acidification, NH4Cl pre-pulse procedure lowered control and Tg737 cell pHi in the same manner. Tg737 cells showed no ERK1/2 or p38 phosphorylation in response to decreased intracellular pH, compared to wild-type cells, consistent with a lower pH requirement as seen in pHo induced intracellular acidification. Phosphorylation of p38 during K+-mediated pHi recovery is absent in Tg737 cells but this does not produce any differences in pHi recovery pattern in comparison to wild-type cells. From our findings we conclude that endothelial cilia are unlikely to serve as the only acid sensing organelle but may be involved in buffering capacity of cytosolic pH in vascular endothelial cells. The significance in this could mean that ciliopathy (abnormal cilia) may have very little direct role in the physiological acid-base imbalance.
Acid-induced MAPK activation has been observed in the renal epithelial cells [36]. Our studies show that acid activation of MAPK, p38, is relevant in endothelial cells and may be involved in the regulation of acid-mediated transport. Consistent with our observation, Flacke et al. showed that Wistar rat coronary endothelial cells exposed to acidosis (pH 6.4) led to a transient activation of p38 and Akt kinases, which are essential for protection against apoptosis [37].
The importance of K+ channels in pHi recovery in vascular endothelial cells has been highlighted in this study, with profound increases in intracellular pH upon addition of K+-containing solution following intracellular acidosis. Our data on K+-dependent pHi recovery indicates that K+-transporters are primarily activated by low pHi, which could include K+-channels, Na+/K+ pumps, and/or Na+/K+/2Cl− cotransporters. Studies have shown that ATP-sensitive K+ channels are activated directly by intracellular but not by extracellular acidosis. This has been established in rat basilar artery where pHi-acidification mediated dilation was blocked by glibenclamide, an inhibitor of ATP-sensitive potassium channels [38]. Future studies will be needed to determine the precise potassium channel, pump, or transporter responsible for K+-dependent pHi recovery in vascular endothelial cells.
A single, universal pH/acid sensor in the cardiovascular and renal systems that regulates MAPK pathways and ion transport has yet to be identified, but there are several possible candidates. These putative pH sensors expressed by vascular endothelial cells will lie upstream of acid-activated ERK1/2 and p38, and may include epidermal growth factor receptor (EGFR) [4], an acid-sensing ion channel (ASIC) [39], or a G-protein coupled receptor. The GPCR GPR4 is known to be acid-activated [40,41] and regulates potassium-driven transport to maintain pH [42]. GPR4-null mice have minor defects in renal acid excretion and mild metabolic acidosis. GPR4 deficiency also affects the quality of small blood vessels during angiogenesis [43]. In vascular endothelial cells, acidosis activation of GPR4 stimulates inflammatory responses [43]. With p38 being the notorious inflammatory MAPK [3] and its activation being clear in response to acid pH, GPR4 would be a promising pH sensor in vascular endothelial cells in the regulation of p38-mediated signaling pathways described here.
Other good candidates for a pH sensor in vascular endothelial cells are acid-sensing ion channels (ASICs), which are ligand-gated and amiloride-sensitive cation channels activated by extracellular H+ [44]. ASICs are members of the degenerin/epithelial sodium channel (DEG/ENaC) superfamily and contain an acidic pocket responsible for acid-dependent gating of sodium and calcium, albeit to a lesser degree. ASICs are expressed in the central and peripheral nervous system, including afferent tissues such as skin, cardiovascular system, muscle, joint, teeth, vestibular, and visceral cells [45].
With the primary cilium being established as a sensor of different mechanical and biochemical cues, we test whether the primary cilium has a role in sensing and transducing pH changes. We compare acidosis response using vascular endothelial cells as an in vitro model compared to cilia-less Tg737 cells. Our study on non-motile primary cilia may not be extrapolated to the motile cilia in developing nodes or in the brain ventricles. Nonetheless, our data shows that acid-activation of p38- and ERK1/2-mediated signaling pathways regulate ion transport to maintain acid-base homeostasis in endothelia. We showed that pHi recovery after an NH4Cl pre-pulse in vascular endothelial cells is predominantly a K+-dependent process. We also observed that a more acidic pHo was needed to induce MAPK phosphorylation in cilia-less Tg737 cells compared to wild-type cell. NH4Cl pre-pulse is a technique used to create pHo-independent pHi acidification, in this scenario pHi recovery was seen to be delayed in Tg737 cells. Therefore, we conclude that the primary cilium, a known cardiovascular mechanosensor [23,46,47] and chemoreceptor [24,48,49] is not the sole sensor for acid sensation but does influence the pH threshold for MAPK kinase phosphorylation. Future studies to examine the identity of the acid sensors distributed in the cilia might be able to detail the nuanced role of primary cilia or Tg737 deletion that might affect the buffering capacity of the Tg737 mice model.
Acknowledgments
Authors thank Maki Takahashi for her technical support, and the laboratory of Khaled Elsaid for technical assistance with cell maintenance. We thank Juan Codina, Thomas D. DuBose, Jr., and Snezana Petrovic at Wake Forest School of Medicine for their knowledge and technical expertise.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/8/7/704/s1, Supplement Movie: 3D reconstruction of z-stack (0.25 μm slices) from NIH3T3 cells highlighting the cilia (green), Gli (red) and nucleus (blue).Figure S1: NIH3T3 were stained with ciliary marker (acetylated-α-tubulin; green), Gli (red) and nucleus marker (DAPI; blue). Figure S2: The lengths of primary cilia from 150 NIH3T3 cells were measured from each preparation (N = 3; each 50 randomly selected cilia). Figure S3: (a) Cilium length of NIH3T3 cells before and after Hh activation or different acidic pHo exposures was averaged. (b) The percentage of cells with cilia is shown. (c) The percentage of cells is shown with Gli localization to the cilia. Figure S4: Electron micrographs of endothelial cells at pH 7.4 (top) and pH 5.5 (bottom). Figure S5: Endothelial cells were stained with ciliary marker (acetylated-α-tubulin; green) and nucleus marker (DAPI; blue). Figure S6: The lengths of primary cilia from 150 endothelial cells were measured from each preparation before and after NH4Cl pre-pulse in 0 Na+/K+ solution (N = 3; each 50 randomly selected cilia).
Author Contributions
K.F.A. performed and analyzed data on the endothelial cells and R.T.S. independently confirmed the results and analyses. K.F.A., R.T.S. and S.M.N. contributed in writing the manuscript. All authors read and approved the final manuscript.
Funding
The project was funded in part by Congressionally Directed Medical Research Program PR130153 and the National Institutes of Health HL131577. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fisher K.D., Codina J., Petrovic S., DuBose T.D., Jr. Pyk2 regulates H+-ATPase-mediated proton secretion in the outer medullary collecting duct via an ERK1/2 signaling pathway. Am. J. Physiol. Ren. Physiol. 2012;303:F1353–F1362. doi: 10.1152/ajprenal.00008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S., Sato S., Yang X., Preisig P.A., Alpern R.J. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J. Clin. Investig. 2004;114:1782–1789. doi: 10.1172/JCI200418046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 4.Coaxum S.D., Blanton M.G., Joyner A., Akter T., Bell P.D., Luttrell L.M., Raymond J.R., Lee M.H., Blichmann P.A., Garnovskaya M.N., et al. Epidermal growth factor-induced proliferation of collecting duct cells from oak ridge polycystic kidney mice involves activation of Na+/H+ exchanger. Am. J. Physiol. Cell Physiol. 2014;307:C554–C560. doi: 10.1152/ajpcell.00188.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skelton L.A., Boron W.F. Effect of acute acid-base disturbances on the phosphorylation of phospholipase c-gamma1 and ERK1/2 in the renal proximal tubule. Physiol. Rep. 2015;3:e12280. doi: 10.14814/phy2.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preisig P.A. The acid-activated signaling pathway: Starting with pyk2 and ending with increased NHE3 activity. Kidney Int. 2007;72:1324–1329. doi: 10.1038/sj.ki.5002543. [DOI] [PubMed] [Google Scholar]
- 7.Zarubin T., Han J. Activation and signaling of the p38 map kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 8.Xue L., Lucocq J.M. Low extracellular pH induces activation of ERK 2, JNK, and p38 in a431 and swiss 3t3 cells. Biochem. Biophys. Res. Commun. 1997;241:236–242. doi: 10.1006/bbrc.1997.7759. [DOI] [PubMed] [Google Scholar]
- 9.Jin X., Mohieldin A.M., Muntean B.S., Green J.A., Shah J.V., Mykytyn K., Nauli S.M. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell. Mol. Life Sci. CMLS. 2014;71:2165–2178. doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon R.Y., Temiyasathit S., Tummala P., Quah C.C., Jacobs C.R. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic amp in bone cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010;24:2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K.L., Guevarra M.D., Nguyen A.M., Chua M.C., Wang Y., Jacobs C.R. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masyuk A.I., Masyuk T.V., Splinter P.L., Huang B.Q., Stroope A.J., LaRusso N.F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and camp signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto K., Ando J. New molecular mechanisms for cardiovascular disease:Blood flow sensing mechanism in vascular endothelial cells. J. Pharm. Sci. 2011;116:323–331. doi: 10.1254/jphs.10R29FM. [DOI] [PubMed] [Google Scholar]
- 14.Rohatgi R., Battini L., Kim P., Israeli S., Wilson P.D., Gusella G.L., Satlin L.M. Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2008;294:F890–F899. doi: 10.1152/ajprenal.00341.2007. [DOI] [PubMed] [Google Scholar]
- 15.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E., Lu W., Brown E.M., Quinn S.J., et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 16.Praetorius H.A., Spring K.R. Bending the mdck cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 17.Vina E., Parisi V., Abbate F., Cabo R., Guerrera M.C., Laura R., Quiros L.M., Perez-Varela J.C., Cobo T., Germana A., et al. Acid-sensing ion channel 2 (asic2) is selectively localized in the cilia of the non-sensory olfactory epithelium of adult zebrafish. Histochem. Cell Biol. 2015;143:59–68. doi: 10.1007/s00418-014-1264-4. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 19.Collier D.M., Snyder P.M. Extracellular protons regulate human enac by modulating Na+ self-inhibition. J. Biol. Chem. 2009;284:792–798. doi: 10.1074/jbc.M806954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raychowdhury M.K., McLaughlin M., Ramos A.J., Montalbetti N., Bouley R., Ausiello D.A., Cantiello H.F. Characterization of single channel currents from primary cilia of renal epithelial cells. J. Biol. Chem. USA. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 21.Satlin L.M., Sheng S., Woda C.B., Kleyman T.R. Epithelial Na+ channels are regulated by flow. Am. J. Physiol. Ren. Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 22.Banizs B., Komlosi P., Bevensee M.O., Schwiebert E.M., Bell P.D., Yoder B.K. Altered pH(i) regulation and Na+/HCO3− transporter activity in choroid plexus of cilia-defective tg737(ORPK) mutant mouse. Am. J. Physiol. Cell Physiol. 2007;292:C1409–C1416. doi: 10.1152/ajpcell.00408.2006. [DOI] [PubMed] [Google Scholar]
- 23.Nauli S.M., Kawanabe Y., Kaminski J.J., Pearce W.J., Ingber D.E., Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul-Majeed S., Nauli S.M. Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension. 2011;58:325–331. doi: 10.1161/HYPERTENSIONAHA.111.172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AbouAlaiwi W.A., Takahashi M., Mell B.R., Jones T.J., Ratnam S., Kolb R.J., Nauli S.M. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoder B.K., Tousson A., Millican L., Wu J.H., Bugg C.E., Jr., Schafer J.A., Balkovetz D.F. Polaris, a protein disrupted in ORPK mutant mice, is required for assembly of renal cilium. Am. J. Physiol Ren. Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 27.Jones T.J., Adapala R.K., Geldenhuys W.J., Bursley C., AbouAlaiwi W.A., Nauli S.M., Thodeti C.K. Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J. Cell Physiol. 2012;227:70–76. doi: 10.1002/jcp.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aboualaiwi W.A., Muntean B.S., Ratnam S., Joe B., Liu L., Booth R.L., Rodriguez I., Herbert B.S., Bacallao R.L., Fruttiger M., et al. Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation. Circulation. 2014;129:660–672. doi: 10.1161/CIRCULATIONAHA.113.005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AbouAlaiwi W.A., Ratnam S., Booth R.L., Shah J.V., Nauli S.M. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum. Mol. Genet. 2011;20:354–367. doi: 10.1093/hmg/ddq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauli S.M., Jin X., AbouAlaiwi W.A., El-Jouni W., Su X., Zhou J. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013;525:1–20. doi: 10.1016/B978-0-12-397944-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dummer A., Poelma C., DeRuiter M.C., Goumans M.-J.T.H., Hierck B.P. Measuring the primary cilium length: Improved method for unbiased high-throughput analysis. Cilia. 2016;5:7. doi: 10.1186/s13630-016-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tukachinsky H., Lopez L.V., Salic A. A mechanism for vertebrate hedgehog signaling: Recruitment to cilia and dissociation of sufu-gli protein complexes. J. Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodromou N.V., Thompson C.L., Osborn D.P., Cogger K.F., Ashworth R., Knight M.M., Beales P.L., Chapple J.P. Heat shock induces rapid resorption of primary cilia. J. Cell Sci. 2012;125:4297–4305. doi: 10.1242/jcs.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verghese E., Zhuang J., Saiti D., Ricardo S.D., Deane J.A. In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol. Int. 2011;35:909–913. doi: 10.1042/CBI20090154. [DOI] [PubMed] [Google Scholar]
- 35.Verghese E., Weidenfeld R., Bertram J.F., Ricardo S.D., Deane J.A. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol. Dial. Transpl. 2008;23:834–841. doi: 10.1093/ndt/gfm743. [DOI] [PubMed] [Google Scholar]
- 36.Brown D., Wagner C.A. Molecular mechanisms of acid-base sensing by the kidney. J. Am. Soc. Nephrol. 2012;23:774–780. doi: 10.1681/ASN.2012010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flacke J.P., Kumar S., Kostin S., Reusch H.P., Ladilov Y. Acidic preconditioning protects endothelial cells against apoptosis through p38- and AKT-dependent bcl-xl overexpression. Apoptosis. 2009;14:90–96. doi: 10.1007/s10495-008-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santa N., Kitazono T., Ago T., Ooboshi H., Kamouchi M., Wakisaka M., Ibayashi S., Iida M. ATP-sensitive potassium channels mediate dilatation of basilar artery in response to intracellular acidification in vivo. Stroke. 2003;34:1276–1280. doi: 10.1161/01.STR.0000068171.01248.97. [DOI] [PubMed] [Google Scholar]
- 39.Abboud F.M., Benson C.J. Asics and cardiovascular homeostasis. Neuropharmacology. 2015;94:87–98. doi: 10.1016/j.neuropharm.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen A., Dong L., Leffler N.R., Asch A.S., Witte O.N., Yang L.V. Activation of gpr4 by acidosis increases endothelial cell adhesion through the CAMP/EPAC pathway. PLoS ONE. 2011;6:e27586. doi: 10.1371/journal.pone.0027586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X., Yang L.V., Tiegs B.C., Arend L.J., McGraw D.W., Penn R.B., Petrovic S. Deletion of the pH sensor gpr4 decreases renal acid excretion. J. Am. Soc. Nephrol. 2010;21:1745–1755. doi: 10.1681/ASN.2009050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codina J., Opyd T.S., Powell Z.B., Furdui C.M., Petrovic S., Penn R.B., DuBose T.D., Jr. pH-dependent regulation of the alpha-subunit of H+-K+-ATPase (HKalpha2) Am. J. Physiol. Ren. Physiol. 2011;301:F536–F543. doi: 10.1152/ajprenal.00220.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong L., Li Z., Leffler N.R., Asch A.S., Chi J.T., Yang L.V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE. 2013;8:e61991. doi: 10.1371/journal.pone.0061991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S., Cheng X.Y., Wang F., Liu C.F. Acid-sensing ion channels: Potential therapeutic targets for neurologic diseases. Transl. Neurodegener. 2015;4:10. doi: 10.1186/s40035-015-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C.C., Wong C.W. Neurosensory mechanotransduction through acid-sensing ion channels. J. Cell Mol. Med. 2013;17:337–349. doi: 10.1111/jcmm.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedrozo Z., Criollo A., Battiprolu P.K., Morales C.R., Contreras-Ferrat A., Fernandez C., Jiang N., Luo X., Caplan M.J., Somlo S., et al. Polycystin-1 is a cardiomyocyte mechanosensor that governs l-type Ca2+ channel protein stability. Circulation. 2015;131:2131–2142. doi: 10.1161/CIRCULATIONAHA.114.013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharif-Naeini R., Folgering J.H., Bichet D., Duprat F., Lauritzen I., Arhatte M., Jodar M., Dedman A., Chatelain F.C., Schulte U., et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 48.Kathem S.H., Mohieldin A.M., Nauli S.M. The roles of primary cilia in polycystic kidney disease. AIMS Mol. Sci. 2014;1:27–46. doi: 10.3934/molsci.2013.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Upadhyay V.S., Muntean B.S., Kathem S.H., Hwang J.J., Aboualaiwi W.A., Nauli S.M. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front. Physiol. 2014;5:72. doi: 10.3389/fphys.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.