ABSTRACT
Gametogenesis is dependent on intercellular communication facilitated by stable intercellular bridges connecting developing germ cells. During Drosophila oogenesis, intercellular bridges (referred to as ring canals; RCs) have a dynamic actin cytoskeleton that drives their expansion to a diameter of 10 μm. Although multiple proteins have been identified as components of RCs, we lack a basic understanding of how RC proteins interact together to form and regulate the RC cytoskeleton. Thus, here, we optimized a procedure for proximity-dependent biotinylation in live tissue using the APEX enzyme to interrogate the RC interactome. APEX was fused to four different RC components (RC-APEX baits) and 55 unique high-confidence prey were identified. The RC-APEX baits produced almost entirely distinct interactomes that included both known RC proteins and uncharacterized proteins. A proximity ligation assay was used to validate close-proximity interactions between the RC-APEX baits and their respective prey. Furthermore, an RNA interference screen revealed functional roles for several high-confidence prey genes in RC biology. These findings highlight the utility of enzyme-catalyzed proximity labeling for protein interactome analysis in live tissue and expand our understanding of RC biology.
KEY WORDS: Drosophila oogenesis, Ring canal, Actin cytoskeleton, Proximity labeling, APEX, Protein-protein interaction, Mass spectrometry
Summary: We optimize a procedure for enzyme-catalyzed proximity labeling in live Drosophila ovary tissue to interrogate the Drosophila ring canal protein interactome.
INTRODUCTION
Ring canals (RCs) are intercellular bridges connecting developing germline cells during both male and female gametogenesis. Unlike other characterized RCs, Drosophila female germline RCs accumulate an extensive F-actin cytoskeleton that drives their circumferential expansion during development from a ∼1 μm-diameter arrested cleavage furrow to an impressive 10 μm-diameter tunnel. Genetic analyses have led to the identification of key proteins that are involved in the RC structure (for a recent review, see Yamashita, 2018). Interestingly, proper RC development requires the ordered addition and/or activity of key proteins (Cooley, 1998; Haglund et al., 2011; Robinson et al., 1994), highlighting the dynamic and complex nature of the RC interactome. Various RC proteins are also regulated through post-translational modifications, with ubiquitination and phosphorylation being the most well-characterized modifications at RCs to date (Hamada-Kawaguchi et al., 2015; Hudson et al., 2019; Kelso et al., 2002; Kline et al., 2018; Morawe et al., 2011; Yamamoto et al., 2013).
Although it is evident that many RC proteins are regulated in spatially restricted and temporally defined contexts, we still have an incomplete understanding of how RCs are formed and regulated during development. Given their insoluble nature and size heterogeneity, classical biochemical purification and fractionation approaches that rely on abundant starting material to isolate and analyze RC proteins in native conditions are not practical. However, proximity labeling approaches have recently been developed (for reviews, see Chen and Perrimon, 2017; Kim and Roux, 2016) in which an enzyme is fused to a protein or targeted to a subcellular compartment of interest where it can generate reactive ‘handle’ molecules or ‘tags’ (usually biotin based) that covalently interact with proximal proteins in live cells. The resulting biotin-tagged proteins can then be purified by conventional methods and identified by mass spectrometry (MS). Importantly, these new techniques allow for the identification of proteomes and interactomes within the native environment of the cell. Additionally, the use of biotin tags makes the interrogation of insoluble cellular compartments possible because denaturing conditions can be used to solubilize, capture and identify previously inaccessible proteins owing to the strength of biotin-streptavidin noncovalent binding (Kd≈10-15 M; Green, 1975).
The two most common classes of enzyme used for proximity labeling approaches are a bacterial biotin ligase (BirA) and peroxidase-based enzymes [horseradish peroxidase (HRP) and ascorbate peroxidase (APEX)]. BirA (Roux et al., 2012) and its newer, optimized variants (Branon et al., 2018; Kim et al., 2016) have been most commonly used for ‘BioID’ studies of protein-protein interactions (PPIs), whereas HRP- and APEX-based applications have been used almost exclusively to identify subcellular organelle proteomes (for a more comprehensive review, see Kim and Roux, 2016). BioID uses membrane-permeable biotin for the substrate, but the relatively low enzymatic activity of BirA requires labeling reaction times of hours to days and an optimal temperature of 37°C, which is not practical for use in vivo in flies and worms. By contrast, APEX uses a less membrane-permeable substrate, biotin-phenol, but its high enzymatic activity allows for short labeling reaction times (seconds to minutes) at a wider range of temperatures. However, the membrane impermeability of biotin-phenol has posed a significant technical challenge for use of APEX in live tissue.
Here, we report the application of APEX-mediated proximity labeling to the study of PPIs in the highly regulated but largely insoluble F-actin cytoskeleton of Drosophila female RCs. We fused APEX to known RC proteins (‘RC-APEX baits’) to probe their interaction partners and identified high-confidence interactors (‘prey’) either unique to each RC-APEX bait or common to multiple RC-APEX baits. We used a proximity ligation assay and genetic analysis to validate the high-confidence prey. This work demonstrates that APEX-mediated biotinylation can be used to uncover specific PPIs within an intact cytoskeletal structure in living cells.
RESULTS
Use of APEX to identify ring canal protein interactomes in live tissue
To interrogate RC protein interactomes, we made constructs in which APEX was fused to the RC proteins Pavarotti (Pav), Hu li tai shao (Hts)-RC and Kelch, and a substrate-trapping Kelch construct, KREP (Fig. 1A,B), and generated transgenic flies expressing these fusion proteins (RC-APEX baits): Pav::APEX, HtsRC::APEX, APEX::Kelch and APEX::KREP (Fig. 1B).
Fig. 1.

General workflow used to identify RC protein interactomes with APEX. (A) Fluorescence images showing localization of indicated proteins at RCs. Pav was visualized by GFP fluorescence of a (BAC) pav::GFP transgene. HtsRC and Kelch were visualized with anti-HtsRC and anti-Kelch antibodies, respectively. KREP was expressed as UASp-APEX::V5::KREP driven by mat-GAL4 and visualized by anti-V5 immunofluorescence. F-actin was visualized by TRITC-Phalloidin. (B) Scheme of transgenic APEX fusion constructs generated for this study. Cartoons were made based on known structural models of Pav, Kelch and KREP, with APEX positioned according to where it was fused. No structural data are available for HtsRC. (C) (Left) Example workflow of optimized APEX labeling in live ovary tissue with HtsRC::APEX construct. (Right) Overview of streptavidin purification and MS methods used to identify and validate high-confidence prey. Scale bar: 2 μm.
Pav is a kinesin-like protein that is a component of all characterized germline RCs as well as Drosophila somatic cell RCs (Adams et al., 1998; Airoldi et al., 2011; Minestrini et al., 2002). Pav and its known binding partner Tumbleweed (Tum), a Rac GTPase-activating protein (RacGAP), constitute the centralspindlin complex as a heterodimeric tetramer (Adams et al., 1998; Tao et al., 2016). HtsRC is a RC-specific protein required for recruiting the RC actin cytoskeleton (Hudson et al., 2019; Petrella et al., 2007). Kelch is the substrate adaptor component of a Cullin3-RING ubiquitin ligase (CRL3) that targets HtsRC (Hudson and Cooley, 2010; Hudson et al., 2015, 2019). Given that the KREP protein comprises only the substrate-targeting domain of Kelch, it cannot bind to Cullin3 and, hence, cannot ubiquitinate its substrate, HtsRC, which leads to a dominant-negative kelch-like RC phenotype (Hudson and Cooley, 2010; Fig. 1A).
Pav localizes to the outer rim of RCs (Minestrini et al., 2002; Ong and Tan, 2010), whereas HtsRC and Kelch localize to the RC F-actin-rich inner rim (Robinson et al., 1994). Knowing that these RC proteins have discrete ‘sublocalizations’ within the RC as well as unique functions, our goal was to identify the local interactomes of each RC-APEX bait protein within the RC using APEX-mediated proximity biotinylation as the basis for subsequent proteomic analysis (see Fig. 1C for general methods workflow and proteomic approach).
Localization and expression of RC-APEX bait proteins
We matched the expression of the RC-APEX baits as closely as possible to their endogenous levels to minimize mislocalization that could lead to a high background and false-positive results (Hung et al., 2016; Varnaite and MacNeill, 2016). For Pav::APEX, the APEX-coding region was fused to the 3′-end of pav in the context of a bacterial artificial chromosome (BAC) encompassing the pav gene; previous C-terminal protein fusions to Pav did not perturb its function (Goshima and Vale, 2005). To generate HtsRC::APEX, the APEX-coding region was fused to the 3′-end of the ovhts cDNA under control of the otu promoter, which closely matches the expression levels of the endogenous ovhts promoter (Petrella et al., 2007). APEX::kelch and APEX::KREP were put under UASp control and expressed using mat-GAL4 driver, which has been shown previously to be an appropriate GAL4 driver for kelch and KREP constructs (Hudson and Cooley, 2010; Hudson et al., 2015, 2019). We also made a control construct expressing unlocalized APEX by fusing Green Fluorescence Protein (GFP)-Binding Protein (GBP) (Rothbauer et al., 2008) to APEX under UASp control. Epitope tags [FLAG, hemagglutinin (HA), or V5] were included in all five constructs.
Immunofluorescence microscopy revealed that the RC-APEX bait constructs localized properly to RCs (Fig. 2A-D and Fig. S1A-D), and the GBP::APEX control was present throughout the cytoplasm of nurse cells (Fig. 2E-E′ and Fig. S1E-E′). As expected, the APEX::KREP protein induced kelch-like RCs (Fig. 2D″, yellow-boxed inset). Of note, Pav::APEX, HtsRC::APEX and APEX::Kelch all rescued in their respective null mutant backgrounds (data not shown), demonstrating that the RC-APEX fusion constructs were functional. All experiments with APEX::Kelch were done in a kelch mutant background.
Fig. 2.
RC-APEX bait constructs localized to RCs and were expressed at appropriate levels. (A-F) Stage 9 or 10 egg chambers expressing various RC-APEX baits were stained with TRITC-Phalloidin (A″-F″) and epitope tags (A′-F′) to confirm proper RC-APEX bait construct localization to RCs. (G-K) Ovary lysates expressing indicated RC-APEX fusion baits were analyzed by western blotting to assess RC-APEX bait expression levels. (I) Levels of APEX::Kelch, expressed in a kelch mutant background, were comparable with endogenous Kelch. (J) mat-GAL4-driven GBP::APEX had higher expression than (BAC) pav::APEX. (K) mat-GAL4-driven APEX::KREP was the most abundantly expressed RC-APEX bait construct. Scale bar: 50 μm.
We directly compared expression of the RC-APEX constructs with their endogenous counterparts by western blot analysis (Fig. 2G-K). Pav::APEX (Fig. 2G) and HtsRC (Fig. 2H) were expressed at comparable levels to endogenous Pav and HtsRC. APEX::Kelch protein expression was slightly higher than that of endogenous Kelch (Fig. 2I). We also compared expression of different RC-APEX constructs relative to each other using epitope tag antibodies. GBP::APEX expression driven by mat-GAL4 was notably higher compared with Pav::APEX (Fig. 2J). Detection of HtsRC::APEX, APEX::Kelch and APEX::KREP proteins showed that APEX::KREP was the most highly expressed of the three (Fig. 2K), which is consistent with the high expression previously observed with other KREP-derived constructs (Hudson and Cooley, 2010). Overall, expression of the RC-APEX baits was comparable to endogenous levels.
Biotinylation of ring canal proteins by RC-APEX baits and streptavidin purification
Most previous studies using APEX have been in cultured cells that are sufficiently permeable to the biotin-phenol APEX substrate after a 30-min incubation period. The sole Drosophila APEX study (Chen et al., 2015) used dissected Drosophila imaginal discs, salivary glands and larval muscles, all of which were thin enough to allow biotin-phenol entry during the 30-min incubation. However, the egg chamber is within a muscle sheath and basement membrane, and the germline cells are surrounded by a layer of somatic follicle cells. Our initial experiments using the standard APEX labeling protocol developed by the Ting lab (Hung et al., 2016; Rhee et al., 2013) for use in cultured cells were unsuccessful. Suspecting a biotin-phenol permeability issue, we pretreated live egg chambers with a small amount of detergent, which resulted in robust biotin labeling of RCs (Fig. S2).
After optimizing the biotin-phenol labeling protocol (Fig. 1C, left), we were able to achieve consistent and robust RC biotinylation after a 3-min incubation in biotin-phenol, followed by 30 s with H2O2. After quenching the labeling reaction, we used streptavidin-conjugated antibodies to visualize biotinylation by immunofluorescence. The RC-APEX fusions specifically biotinylated RCs in all egg chambers, including stage 6 or 7 (Fig. 3) and stage 9 or 10 (Fig. S3). As expected, Pav::APEX also biotinylated follicle cell RCs (Fig. 3A′, yellow arrows), and GBP::APEX showed cytoplasmic biotin staining (Fig. 3E′).
Fig. 3.
RC-APEX baits biotinylate distinct proteins in live egg chambers. (A-F) Ovaries expressing indicated RC-APEX baits were permeabilized and biotinylated, then egg chambers were fixed and stained with TRITC-Phalloidin (A″-F″) and streptavidin-AF488 (A′-F′) to visualize F-actin and biotin. Representative images are shown of stage 6 or 7 egg chambers. Note the specific biotin signal at RCs for the RC-APEX fusions (A′-D′), the unlocalized cytoplasmic biotin signal for GBP::APEX (E′) and the absence of signal in the no-APEX control (F′). (A′) Yellow arrows denote biotinylated follicle cell RCs in (BAC) pav::GFP. (G-K) APEX-labeled ovaries were lysed in denaturing conditions, biotinylated proteins were captured by magnetic streptavidin beads, and eluates were analyzed by western blotting. (G) Inputs, unbound fractions and eluates were analyzed by western blotting with streptavidin-HRP to confirm that biotinylated proteins were present. (H-K) Western blot analysis was performed on the APEX eluates (labeled on top of lanes) to check for known RC proteins. (H) Pav::APEX protein was abundant in the Pav::APEX eluate. (I) APEX::Kelch was enriched in the APEX::Kelch eluate, whereas a background endogenous Kelch protein band could be detected in all other samples. (J) HtsRC::APEX and endogenous HtsRC were present in the HtsRC::APEX sample, whereas endogenous HtsRC was highly enriched in the APEX::KREP sample. (K) Filamin was observed only in the APEX::KREP sample. Scale bar: 50 μm.
Although treatment with detergent did not affect the structure, stability or apparent composition of RCs (Robinson and Cooley, 1997b), detergent treatment could pose a problem for studying cellular structures that are more labile. Given that APEX was initially developed for use after fixation (Martell et al., 2012), we tested whether APEX remained active in egg chambers after formaldehyde fixation (Fig. S4). APEX retained activity even after a 10-min fixation in 2% paraformaldehyde (Fig. S4B,B″), suggesting that APEX-mediated biotinylation will be broadly useful in fixed tissues. Nonetheless, to avoid potential fixation-induced crosslinks that could obscure peptide identification, we did not use fixative for the following experiments.
To capture biotinylated proteins using streptavidin beads, we needed to verify whether they were solubilized after lysis and present in the soluble fraction after centrifugation and lysate clarification. RCs are known to remain intact after lysis with non-ionic detergents, such as TX-100 (Greenbaum et al., 2007; Robinson and Cooley, 1997b). Therefore, we lysed egg chambers in harsh, denaturing lysis buffer containing 8 M urea using a motorized spinning pestle, and the clarified lysate was incubated with magnetic streptavidin beads (see Materials and Methods for more specific details). The magnetic streptavidin beads were able to capture the biotinylated proteins (Fig. 3G), as shown by the depletion of the streptavidin-HRP signal in the unbound fractions and the strong signal of biotinylated proteins in the eluates. Interestingly, the no-APEX control w1118 sample also showed a smear of biotinylated proteins by western blot (Fig. 3G), possibly because of the presence of endogenously biotinylated proteins or proteins that were biotinylated by other endogenous peroxidases. In any event, the biotin signal was stronger in the eluates for the APEX samples compared with the no-APEX w1118 control.
Before proceeding with MS analysis, we checked for the presence or absence of known RC proteins in the eluates by western blotting (Fig. 3H-K). The results were encouraging. We observed that only the Pav::APEX sample showed enrichment over controls for a Pav protein species (Fig. 3H, Pav::APEX band), which is consistent with Pav being dimeric (Minestrini et al., 2002; Sommi et al., 2010; Tao et al., 2016). Similarly, only the APEX::Kelch sample showed enrichment for a Kelch protein species (Fig. 3I, APEX::Kelch band), which is also consistent with Kelch dimerization via its BTB domain (Hudson and Cooley, 2010; Hudson et al., 2015; Robinson and Cooley, 1997a). APEX::Kelch was expressed in a kelch mutant background, which is why endogenous Kelch was absent from that lane. A faint band corresponding to endogenous Kelch was detectable in the other eluates, probably because of nonspecific binding to the beads. We found that HtsRC was significantly enriched in the APEX::KREP eluate (Fig. 3J), which is consistent with it being the CRL3Kelch substrate (Hudson et al., 2019). We also detected HtsRC and HtsRC::APEX in the HtsRC::APEX eluate, which suggests that HtsRC is able to dimerize. Finally, we checked the eluate samples for the presence of Filamin, a key RC protein. Interestingly, Filamin was only detectable in the APEX::KREP sample (Fig. 3K), suggesting that Filamin is also in close proximity to the Kelch substrate-targeting KREP domain. These results gave us proof of principle that the RC-APEX baits biotinylate unique subsets of proteins, which are probably their native interaction partners at the RC.
Analysis of purified samples by mass spectrometry
To prepare samples for proteomic analysis, we subjected egg chambers from 100 flies (≈200 ovaries) of each genotype to our biotinylation labeling protocol. Two biological replicates were used for each of the six samples (Fig. 1B). Samples were subjected to liquid chromatography (LC)-MS/MS by MS Bioworks. Spectral counts (SpC) were used as a semiquantitative measure of relative protein abundance. See Methods for more detailed information on the MS workflow and proteomic data analysis.
As a quality control check, we assessed the correlation of spectral counts of each identified protein across the biological replicates for each RC-APEX bait (Fig. S5A). Linear regression analysis showed a strong correlation across replicates for each RC-APEX bait, as indicated by the large coefficient of determination (Fig. S5A). To assess how each RC-APEX bait sample differed in total protein identification coverage, we generated density plots of the natural log (ln) of the normalized spectral abundance factor (NSAF) values for all proteins identified by MS (Fig. S5B). The density plots for all four constructs overlapped closely, indicating a similar extent of coverage of spectral counts between the samples, and eliminating any concerns of instrumentation- and sampling-based artifacts or significant deviations in sample complexity or content.
Identification and characterization of RC-APEX high-confidence prey
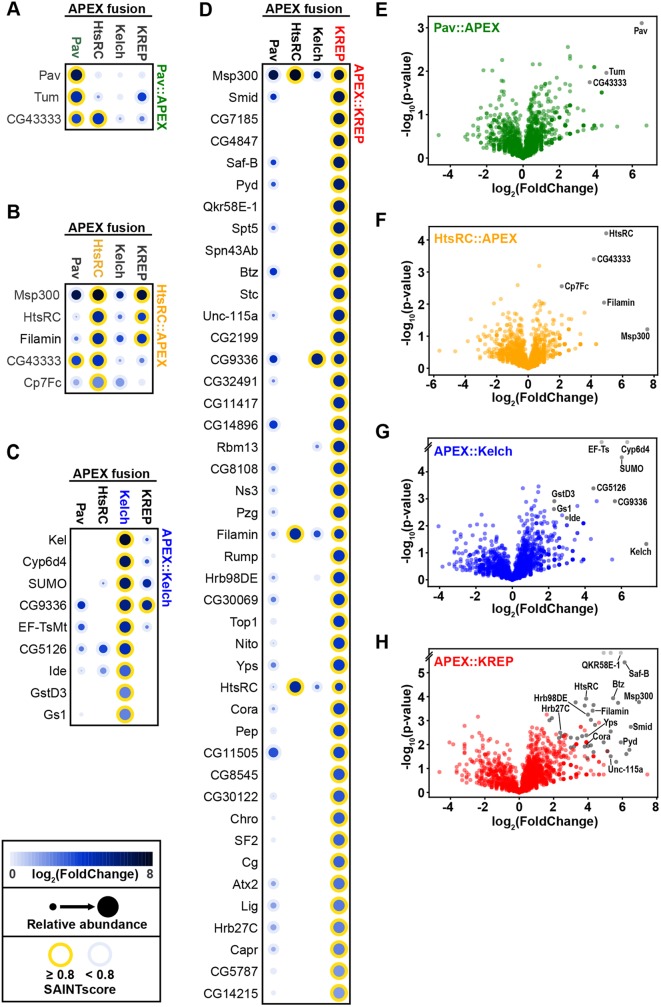
To establish a list of high-confidence prey for each RC-APEX bait from our proteomic data, we used Significance Analysis of INTeractome (SAINT) (Choi et al., 2011; Morris et al., 2014), a common tool used to parse ‘true interactors’ from ‘false interactors’ in affinity purification-MS data sets. We used a SAINT score cut-off of ≥0.8 (corresponding to a 5% false discovery rate), which resulted in 55 high-confidence prey (Fig. 4A, Table S1). Pav::APEX had three high-confidence prey: Pav, Tum and CG43333. HtsRC::APEX had five: Muscle-specific protein 300 kDa (Msp300), Filamin, HtsRC, CG43333 and Chorion protein c at 7F (Cp7Fc). APEX::Kelch and APEX::KREP had nine and 43 high-confidence prey, respectively. Only a few prey (listed in the Venn diagram in Fig. 4A) were shared between the RC-APEX baits.
Fig. 4.
Proteomic analysis reveals RC-APEX baits have unique interactomes that include known RC proteins. (A) Venn diagram showing the extent of overlap of the 55 high-confidence prey identified by the RC-APEX baits. High-confidence prey had a SAINT score ≥0.8, using SAINTexpress analysis. (B) Network map generated in Cytoscape of RC-APEX baits, indicated by colored squares, and their respective prey. The size of the prey gene circle was dependent on its abundance in terms of spectral counts compared with control samples [log2(FoldChange]). Known RC proteins are outlined in black. Dotted lines indicate previously established interactions mined from MIST (Hu et al., 2018).
We used Cytoscape to generate a network map of the RC-APEX baits and prey (Fig. 4B). Known RC proteins were among the high-confidence prey (solid line outlining prey gene circle in Fig. 4B). Consistent with our western blot analysis of RC-APEX bait eluates (Fig. 3), Pav::APEX identified Pav; HtsRC::APEX and APEX::KREP identified HtsRC; APEX::Kelch identified Kelch; and APEX::KREP identified Filamin. Interestingly, Filamin was also identified by HtsRC::APEX, which shows the sensitivity achieved by MS analysis. We mined the Molecular Interaction Search Tool (MIST; Hu et al., 2018), and imported known interactions into the Cytoscape network map (dotted lines connecting prey genes in Fig. 4B). In addition, we searched publicly available expression data at FlyBase (Thurmond et al., 2018) and found that 51 of the 55 identified proteins are expressed in the Drosophila ovary. Taken together, these data indicate that each RC-APEX bait identified unique interactomes that include previously established interactors, new candidate interactors and known RC proteins.
Of the 55 RC-APEX prey genes identified, five were previously known to be involved in RC biology (prey genes with solid outlines in Fig. 4B): pav, tum, hts, cheerio (cher) and kel. That Pav::APEX identified Pav and Tum was reassuring, given that Pav dimerizes (Minestrini et al., 2002; Sommi et al., 2010) and also interacts with Tum (Adams et al., 1998; Tao et al., 2016). Kelch was the top prey for APEX::Kelch, consistent with Kelch dimerizing via its BTB domain (Hudson and Cooley, 2010; Hudson et al., 2015; Robinson and Cooley, 1997a). HtsRC was identified by the CRL3Kelch substrate-trapping APEX::KREP construct, supporting the conclusion that the CRL3Kelch substrate is HtsRC (Hudson et al., 2019). HtsRC::APEX identified HtsRC and Filamin as prey. Although HtsRC and Filamin are known RC proteins that genetically interact (Robinson et al., 1997; Sokol and Cooley, 1999), this is the first evidence of a physical interaction between them. Additionally, the finding that Filamin was a top prey for APEX::KREP suggested that Filamin, HtsRC and KREP are all in close proximity at kelch-like RCs.
For a more global and quantitative visualization of the prey identified by each RC-APEX bait, we generated dot plots using the ProHits-viz tool (Knight et al., 2017) (Fig. 5A-D). The color of each prey ‘dot’ is dependent on the NSAF log2(FoldChange) for each bait compared with the control samples (see key in Fig. 5). The size of each dot is dependent on its relative abundance between bait samples, meaning that the maximum-sized dot for each prey is allocated to the largest SpC value within that bait and the other dots are scaled proportionately. High-confidence prey within each bait have a yellow outline (see key in Fig. 5). This visualization revealed how each RC-APEX bait identified unique sets of prey proteins. For example, APEX::Kelch identified nine high-confidence prey (third column of ‘dots’ all outlined yellow in Fig. 5C) and only one of those prey (CG9336) was shared with another RC-APEX bait (APEX::KREP) (yellow outline around CG9336 dot in fourth column for APEX::KREP in Fig. 5C).
Fig. 5.
Proteomic analysis reveals that RC-APEX baits identified unique prey proteins. (A-D) Dot plots of high-confidence prey identified by Pav::APEX (A), HtsRC::APEX (B), APEX::Kelch (C) and APEX::KREP (D). Dot plots were generated using ProHits-viz (Knight et al., 2017), and high-confidence prey (SAINT score ≥0.8) are outlined in yellow. Each RC-APEX bait identified mostly distinct high-confidence prey. For example, in the APEX::Kelch column (C), only CG9336 was identified by another RC-APEX bait, APEX::KREP. (E-H) Volcano plots for each RC-APEX bait showing statistical significance [-log10(P-value)] and average relative abundance compared with controls [NSAF log2(FoldChange)] of identified proteins across replicate experiments. Notable high-confidence prey are listed and plotted as gray dots. For APEX::Kelch (G) and APEX::KREP (H), the y-axis is broken to allow room for the top-most row of protein prey that had an undefined P-value (e.g. Ef-Ts and Cyp6d4 in APEX::Kelch).
To visualize the quantitative proteomic data relative to statistical significance, we generated volcano plots for each RC-APEX bait (Fig. 5E-H). For each protein identified by MS, a P-value was calculated for its spectral counts within the biological replicates for each RC-APEX bait and control samples. For all identified proteins, the -log10(P-value) was plotted against the NSAF log2(FoldChange) compared with controls for each RC-APEX bait sample. Proteins that showed a high fold-change compared with controls appear on the right side of the plot, whereas proteins that are statistically significant compared with controls (in terms of SpC across replicates) appear towards the top of the plot. Unsurprisingly, proteins in the top right of the plot generally corresponded to our high-confidence prey (gray dots in Fig. 5E-H). For example, the five high-confidence prey identified by HtsRC::APEX were the sole occupants of the upper-right quadrant (gray-labeled dots in Fig. 5F).
Validation of RC-APEX high-confidence prey by proximity ligation assay
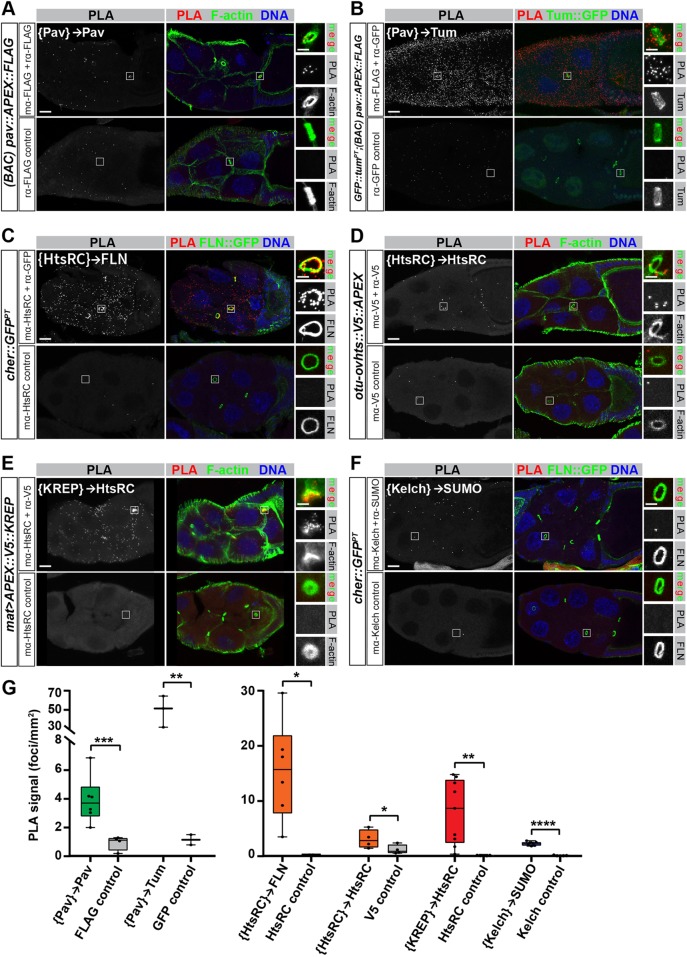
Given that APEX-mediated biotinylation is proximity dependent, the top prey are potential direct interactors or binding partners of the baits. Confirming physical interactions between these protein pairs in their native environments by conventional methods, such as co-immunoprecipitation, was essentially impossible because of the low solubility of the RC cytoskeleton. Therefore, we turned to the Duolink proximity ligation assay (PLA) to test whether RC-APEX baits were in close proximity (<40 nm) to their prey proteins in egg chambers. We were able to test five prey using available antibodies and GFP-tagged stocks. Initial PLA experiments using fixed whole-egg chambers were unsuccessful. Suspecting a penetration issue, we performed the PLA in 20-μm ovary cryosections and succeeded in detecting PLA signals (see Methods for more details).
To test whether Pav dimerization could be detected using the proximity ligation assay, we used egg chambers expressing the pav::APEX::FLAG BAC transgene. The presence of the FLAG epitope in the fusion protein allowed us to use FLAG antibodies generated in two species (mouse and rabbit) to target the same FLAG antigen. The PLA assay confirmed that Pav interacts with itself, as indicated by fluorescent foci throughout egg chambers and at RCs (Fig. 6A, top panel) compared with the negative control sample in which one of the antibodies was omitted (Fig. 6A, bottom panel). We detected an interaction between Pav and Tum using egg chambers expressing a GFP::Tum protein trap and the pav::APEX::FLAG BAC transgene. Fluorescence microscopy showed a striking accumulation of PLA foci throughout the entire egg chamber and at the RCs (Fig. 6B, top panel), whereas the negative control was devoid of PLA signal (Fig. 6B, bottom panel). Quantification of PLA foci showed that there were significantly more foci per egg chamber unit area in the {Pav}→Pav and {Pav}→Tum samples compared with their respective negative controls (Fig. 6G, left graph). These results indicate that the proximity ligation assay can detect true PPIs at their native environment in tissue, and validates Pav and Tum as Pav::APEX prey.
Fig. 6.
In situ proximity ligation assay confirms close-proximity interactions between RC-APEX bait proteins and respective prey. (A-F) Proximity ligation assays were performed on cryosectioned ovary tissues of the indicated genotypes to test for close-proximity interactions between different protein pairs, listed in white text in the top left of each panel (with brackets around the RC-APEX bait protein and an arrow pointing to the prey protein). A positive PPI was indicated by the presence of PLA signal (left panels), which was visualized by fluorescent probes that bind to the PLA DNA product. Antibodies used for each reaction are indicated on the left, and the bottom panels served as negative controls because one antibody was omitted. Boxed insets contain RCs showing F-actin, GFP::Tum, or Filamin::GFP (FLN::GFP) patterns, as indicated in the panel labels. The protein pairs tested exhibited a distinct RC signal, with the exception of the {Kelch}→SUMO (F) interaction, which was mostly dispersed throughout the cytoplasm. (G) Positive PLA foci in each egg chamber imaged were counted in FIJI using thresholding and particle analysis, and graphed as a function of egg chamber unit area. Student's t-test was used to compare the number of PLA foci per unit area in experimental samples versus controls. *P≤0.1; **P≤0.05; ***P≤0.001; ****P≤0.0001. Scale bars: 20 μm (main panel) and 5 μm (insets).
We also tested the previously unknown potential interaction between HtsRC and Filamin in egg chambers expressing a cher::GFP protein trap using rabbit anti-GFP and mouse anti-HtsRC antibodies. Compared with the negative control, there were abundant PLA foci throughout the egg chamber as well as a strong signal at RCs (Fig. 6C). Similarly, we tested potential oligomerization of HtsRC in egg chambers expressing the otu-ovhts::V5::APEX transgene, which allowed us to use mouse and rabbit antibodies targeting the same V5 antigen. We detected PLA foci throughout the egg chamber and at RCs (Fig. 6D). Quantification of PLA foci per egg chamber unit area showed that the {HtsRC}→Filamin and {HtsRC}→HtsRC samples had significantly more foci than their respective controls (Fig. 6G, right graph). These results validate our proteomic results, and support the conclusion that HtsRC can dimerize or oligomerize as well as interact directly with Filamin, both in the cytosol and at RCs.
HtsRC was a high-confidence prey for the substrate-trapping APEX::KREP construct, which was expected based on previous work (Hudson et al., 2019). We validated this result in egg chambers expressing APEX::V5::KREP using mouse anti-HtsRC antibodies and rabbit anti-V5 antibodies. As expected, we observed strong PLA signal throughout the cytoplasm and at RCs (Fig. 6E) with essentially no foci in the negative control (Fig. 6E, bottom panel; quantified in Fig. 6G, right). These results provide proof of principle that APEX fusion constructs can be used to identify substrates of E3 ubiquitin ligases in live tissue.
Finally, we tested an unexpected interaction between SUMO (encoded by the smt3 gene) and Kelch using Drosophila SUMO rabbit antibodies (Abgent) and mouse anti-Kelch antibodies. Excitingly, we observed PLA foci throughout the cytoplasm of egg chambers in the experimental sample but not the negative control (Fig. 6F; quantification in Fig. 6G, right). The results suggest that Kelch is in close proximity to the SUMO protein in the cytoplasm of egg chambers, but not at RCs. Future experiments are needed to test whether Kelch is covalently SUMOylated or interacting with SUMO, perhaps through its predicted SUMO interaction motif (Zhao et al., 2014).
As an additional control, we used the PLA to test interactions between RC-APEX bait proteins and proteins that were not identified as prey. Specifically, we tested for interactions between Pav and Kelch (Fig. S6A), Kelch and Filamin (Fig. S6B), Pav and HtsRC (Fig. S6C), and HtsRC and Kelch (Fig. S6D). All interactions tested were negative, and quantification revealed no significant enrichment of PLA foci in the experimental samples compared with their respective negative controls (Fig. S6E). These results were all consistent with our RC-APEX bait and prey protein pairs and support our conclusion that the PLA was faithfully recapitulating real PPIs.
Identification of novel ring canal regulators through an RNAi screen of RC-APEX prey genes
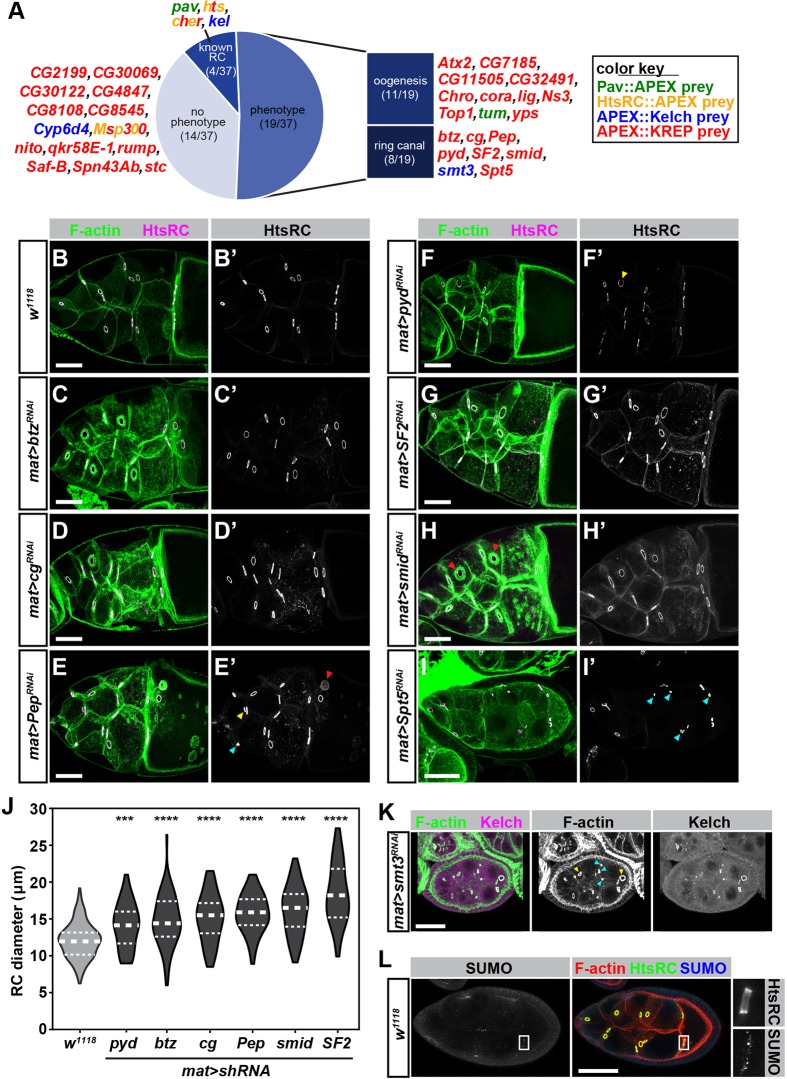
To test genetically whether the RC-APEX preys were involved in RC biology, we performed an RNA interference (RNAi) screen using stocks from the Transgenic RNAi Project (TRiP). We screened 33 prey gene small hairpin (sh)RNA lines using the mat-GAL4 driver for germline-specific expression. Of the 33 genes, 19 had a noticeable phenotype; eight phenotypes were RC specific and 11 had general oogenesis defects (Fig. 7A). Fourteen genes showed no phenotype, which could be because: (1) the RNAi line did not work; (2) the gene functions in early oogenesis before the mat-GAL4 driver is expressed; or (3) knocking down the gene has no deleterious effects on the egg chamber.
Fig. 7.
RNAi screen reveals that prey genes are involved in RC biology. (A) Summary of results from RNAi screening of 33 high-confidence prey [with each prey color coded to indicate its respective RC-APEX bait(s)]. pav, hts, cher and kel are included for 37 genes in total. Of the 33 prey genes screened, 19 had a phenotype, eight of which were RC specific. (B-I) Egg chambers of indicated genotypes were stained with TRITC-Phalloidin and HtsRC (B′-I′) to reveal RC size and morphology. Red arrows indicate abnormal F-actin structures. Yellow and blue arrows indicate deformed and collapsed RCs, respectively. (J) RC diameters of the indicated genotypes were measured in FIJI using HtsRC staining as a RC marker, and violin plots were used to represent the data. Thick-dashed white lines show median RC diameter, and thinner upper and lower white lines denote the upper and lower quartiles, respectively. One-way ANOVA was used to compare the mean RC diameter between the RNAi lines and w1118 control. ***P≤0.0001; ****P≤0.00001. (K) RNAi-mediated knockdown of smt3 caused collapsed and deformed RCs, marked by blue and yellow arrows, respectively. (L) Some RCs were positive for SUMO staining (inset). Scale bars: 20 μm in L, 25 μm in K and 50 μm in B-I.
Six of the eight RC-specific phenotypes [barentsz (btz), combgap (cg), Protein on ecdysone puffs (Pep), polychaetoid (pyd), Splicing factor 2 (SF2) and smallminded (smid)] were significantly enlarged RCs (Fig. 7B-H; RC diameter quantified in Fig. 7J). RNAi-mediated knockdown of Pep also led to abnormal F-actin structures as well as deformed and collapsed RCs (represented by red, yellow, and blue arrows in Fig. 7E′, respectively). Knockdown of pyd also resulted in deformed RCs (yellow arrows in Fig. 7F′), whereas smid knockdown caused abnormal excess cortical F-actin surrounding RCs in stage 10 egg chambers (red arrows in Fig. 7H). Spt5 knockdown led to egg chamber degeneration in mid-to-late-staged egg chambers (Fig. 7I) and collapsed RCs (blue arrows in Fig. 7I′). Finally, RNAi-mediated knockdown of smt3 (the Drosophila SUMO gene) led to egg chamber arrest as well as deformed and collapsed RCs (Fig. 7K, yellow and blue arrows). To examine a possible role for SUMO in RC biology, we stained egg chambers with anti-SUMO antibodies and found occasional RC localization (Fig. 7L, boxed insets).
Interestingly, none of the eight genes with RC-specific phenotypes were known previously to have functions related to RCs. We were particularly surprised to see that five of these genes (btz, cg, Pep, SF2 and Spt5) had established functions involving DNA and/or RNA binding, processing or regulation, suggesting possible ‘new’ roles in RC biology. In summary, this preliminary RNAi screen revealed that prey genes are involved in RC biology, which provides additional support that the RC-APEX baits identified physiologically relevant protein interactors.
DISCUSSION
APEX-mediated proximity labeling can be used to identify protein-protein interactions in live tissue
Since its introduction in 2013, APEX-mediated localized biotinylation has been used successfully in several studies to identify organellar or cellular proteomes, with most of the work done in cultured cells. Three studies reported APEX-mediated labeling in live tissue. Two of these studies were performed in Caenorhabditis elegans in a mutant background that compromised cuticle integrity, which allowed for successful delivery of the biotin-phenol substrate into the worm tissue (Reinke et al., 2017a,b). The third labeled the mitochondrial proteome in dissected Drosophila imaginal discs, salivary glands and larval muscles (Chen et al., 2015). These tissues are relatively thin, which permits sufficient entry of biotin-phenol into the cells without notable intervention. Here, we adapted the use of APEX-mediated proximity labeling to thicker tissue by using digitonin as a means to sufficiently permeabilize cell membranes and allow biotin-phenol substrate entry. Our approach could be adapted to other organisms and tissues, live or formaldehyde fixed, to enable the more widespread use of APEX in vivo.
Of note, the Ting lab recently engineered more catalytically active mutants of the BirA enzyme used in BioID approaches, referred to as TurboID and miniTurbo (Branon et al., 2018). Essentially, these mutants combine the catalytic efficiency of APEX (i.e. a 10-min labeling reaction time) with the ease-of-use of BioID (i.e. straightforward biotin delivery to cells or tissues of interest). However, when TurboID was used in flies and worms, biotin supplementation took hours or days, which limits its potential for capturing more dynamic processes that require fine temporal control and resolution of the labeling reaction. In these cases, our APEX protocol could be used with dissected tissue to achieve robust biotinylation on a faster (seconds to minutes) timescale. Further adaptation and optimization of these proximity labeling approaches will undoubtedly open the door to proteomic experiments in living organisms.
RC-APEX baits identified known binding partners as well as potential new interactors
We set out to test the feasibility of using APEX in vivo to identify PPIs as opposed to whole-organelle or whole-cell proteomes. Although several RC proteins have been characterized, a full understanding of F-actin regulation in RCs requires more complete information about PPIs in the structure. Given that biotinylation of nearby proteins is dependent on their proximity to the APEX enzyme, we envisioned that the RC-APEX bait proteins could identify new binding partners. Our results were promising. We were especially excited that the two top prey of Pav::APEX were Pav and Tum, because identifying the Centralspindlin complex (Tao et al., 2016) provided a powerful positive control and a compelling proof of principle that the RC-APEX baits were capturing real protein interactors of the respective RC bait proteins. Our results also indicated that APEX fused to different domains within proteins can identify domain-specific interactors. APEX fused to the Kelch N terminus, which is the site of Kelch homodimerization through its BTB domain (Canning et al., 2013; Errington et al., 2012; Ji and Privé, 2013), labeled distinct proteins compared with APEX::KREP, which contains just the substrate-binding domain of Kelch. Kelch itself was a top prey for APEX::Kelch, whereas HtsRC was a top prey for APEX::KREP. Surprisingly, APEX::Kelch did not identify Cullin3, a known binding partner of the Kelch BTB domain (Canning et al., 2013; Errington et al., 2012; Ji and Privé, 2013; Xu et al., 2003), as a top prey. This might be because of the transient nature of the Kelch-Cullin3 interaction or the relatively low abundance of Cullin3 present at RCs compared with its strong cytoplasmic localization (Hudson and Cooley, 2010; Hudson et al., 2015).
Identification of E3 ubiquitin ligase substrates is challenging because substrates are short-lived and their interactions with the ligase are transient. Those challenges, coupled with the insolubility of the RC actin cytoskeleton, made identifying the CRL3Kelch RC substrate particularly hard. We recently identified HtsRC as the substrate of CRL3Kelch with other methods (Hudson et al., 2019), and of the 40 prey identified by APEX::KREP in this study, only one, HtsRC, contains the known Kelch-binding motif (PEAEQ) (Schumacher et al., 2014). Thus, our work suggests that APEX is a valuable new tool for identifying E3 ubiquitin ligase substrates, particularly in previously inaccessible contexts like in live tissue, or within insoluble cellular compartments, such as the cytoskeleton. In fact, ours is the first study of its kind to use APEX in vivo to identify an E3 ubiquitin ligase substrate. The advent of substrate-trapping constructs that can be fused to APEX and expressed in model organisms could lead to future breakthroughs for substrate identification in tissue-specific and developmental contexts, which will help move the E3 ubiquitin ligase field into physiologically relevant models.
The APEX::KREP bait identified a surprisingly large number of high-confidence prey proteins compared with the other three baits. This could be because of its abundance both in the cytoplasm and RCs, leading to more efficient biotinylation of proteins. Given that expression of KREP leads to a dominant-negative phenotype of kelch-like RCs, it is also possible that protein complexes were caught or trapped in the RC cytoskeleton, which could have facilitated their biotinylation. Interestingly, many APEX::KREP prey genes are involved in RNA binding or processing as well as ribonucleoprotein complexes. RNPs are transported along microtubules, through RCs from the nurse cells into the oocyte (Mische et al., 2007); thus, they might indeed have been trapped. However, RNAi-mediated knockdown of genes for several of these RNP-associated APEX::KREP prey genes led to RC-specific phenotypes, suggesting a role for mRNA regulation and RNP complexes in RC biology. In any event, the smaller number of proteins identified with HtsRC, Pav and Kelch baits compared with KREP suggests that we succeeded in finding specific interactors for these proteins within intact, morphologically normal RCs.
Proximity labeling revealed new insights into the ring canal substructure
RCs are massive structures that undergo dramatic and dynamic structural and possibly compositional changes during egg chamber development. Given the complex nature of the RC cytoskeleton (e.g. F-actin-dependent membrane and RC expansion, actin recruitment, F-actin crosslinking and bundling, and cytoskeleton disassembly in the RC lumen), it would make sense that different subcomplexes exist in RCs to carry out these processes. Evidence already exists for distinct domains within the RC. HtsRC, Kelch and F-actin localize to the inner rim of RCs (Robinson et al., 1994), Pav localizes to the outer rim (Minestrini et al., 2002; Ong and Tan, 2010), and Filamin is present throughout the entire RC cytoskeleton. However, a more finely resolved picture of RC protein complex localization is emerging from our localized biotinylation results.
Our data showed that different RC-APEX bait constructs identified unique subsets of RC proteins suggesting that there are subcomplexes within the RC that orchestrate mechanical functions, such as nucleating actin filament assembly at the membrane to expand the RC diameter or controlling HtsRC levels to maintain an open RC lumen. Of particular interest is the finding that HtsRC::APEX identified Filamin and HtsRC as top prey. Given that localization of HtsRC to RCs is genetically dependent on the cher gene (Robinson et al., 1997; Sokol and Cooley, 1999), we can now speculate that Filamin directly recruits HtsRC to the RC, where HtsRC functions to recruit the abundant F-actin cytoskeleton (Hudson et al., 2019). This also suggests that HtsRC has the ability to dimerize or oligomerize, possibly through its C-terminal coiled-coil domain (predicted using Phyre2; Kelley et al., 2015). It will be interesting to test whether the oligomerization status of HtsRC differs based on its localization in the cytoplasm or at RCs and whether this affects its actin-regulating activity.
It is also possible that many of the RC-APEX bait-prey interactions we identified occurred in the cytoplasm rather than the RC. The proximity ligation assay showed RC-specific signals for most of the interactions tested, but there were also positive foci present in the cytoplasm. Cytoplasmic interactions could be important for assembling protein complexes destined for RCs; for example, HtsRC could bind to Filamin in the cytoplasm before localizing to RCs. We expect that additional analysis of the RC-APEX prey will lead to new insights into RC formation, maintenance and growth during development.
MATERIALS AND METHODS
Drosophila husbandry
Drosophila were maintained at 25°C on standard fly food medium. Before ovary dissections, females were fattened on wet yeast paste overnight at 25°C. See Table S2 for a detailed list of the fly stocks used in this study.
Molecular cloning and Drosophila transgenesis
(BAC) pav::HA::APEX2::FLAG
APEX2 was recombineered into a BAC containing the pav gene at its C terminus (BAC ID 322-102N3) to create pav::HA::APEX2::FLAG. This BAC contains the entire pav locus on a 21-kb genomic fragment (chr3L:4,229,286…4,250,505, FlyBase release 6). Briefly, we used a two-step BAC recombineering protocol to first insert a Kanamycin resistance cassette (Wang et al., 2006), which was subsequently replaced by HA::APEX2::FLAG through streptomycin selection. The HA::APEX2::FLAG sequence was made through PCR amplification of APEX2 from pcDNA3-APEX2-NES (Addgene Plasmid #49386) with primers designed to add an N-terminal HA epitope tag sequence and a C-terminal FLAG epitope tag sequence. The APEX2 sequence contained a C-terminal nuclear export signal (NES), which was incorporated in an attempt to keep Pav::APEX localized exclusively to RCs. The final plasmid was injected into BL#24872 into the attP3B site on chr2L at Rainbow Transgenic Flies, Inc.
otu-ovhts::V5::APEX1
The V5::APEX1 coding sequence was amplified from pcDNA3-mito-V5::APEX1 (Rhee et al., 2013; Addgene Plasmid #42607) using primers designed to create a V5::APEX1 fragment flanked by 3′ XhoI and 5′ NotI restriction sites. pCOH-ovhts::GFP (Petrella et al., 2007) was digested with XhoI and NotI to excise GFP and the V5::APEX1 fragment was ligated into the plasmid in-frame and in place of GFP. Transgenic flies were generated via P-element-mediated insertion at GenetiVision.
UASp-APEX2::V5::kelch
The APEX2 coding sequence was amplified from pcDNA3-APEX2-NES (Addgene Plasmid #49386) and the entire kelch coding sequence was amplified with primers designed to contain an N-terminal V5 tag sequence. Through overlap-extension PCR, the APEX2::V5::kelch coding sequence was assembled with flanking attB1 and attB2 sites and recombined first into pDONR201 and then into pPW-attB (Table S2) in BP Clonase II and LR Clonase II Gateway recombination reactions, respectively. The pPW-APEX2::V5::kelch plasmid was injected into a strain carrying the attP2 phiC31 landing site on chr3L at Rainbow Transgenic Flies.
UASp-APEX2::V5::KREP
The APEX2 coding sequence was amplified from pcDNA3-APEX2-NES (Addgene Plasmid #49386) and the KREP coding sequence of kelch was amplified with primers designed to contain an N-terminal V5 tag sequence. Through overlap-extension PCR, the APEX2::V5::KREP coding sequence was assembled with flanking attB1 and attB2 sites and recombined first into pDONR201 and then into pPW-attB (Table S2) in BP Clonase II and LR Clonase II Gateway recombination reactions, respectively. The pPW-APEX2::V5::KREP plasmid was injected into a strain carrying the attP2 phiC31 landing site on chr3L at Rainbow Transgenic Flies.
UASp-GBP::FLAG::APEX2
The GBP (Rothbauer et al., 2008) coding sequence was amplified with primers designed to contain 3′ and 5′ SfiI restriction sites. The FLAG::APEX2 sequence was amplified from pcDNA3-APEX2-NES (Addgene Plasmid #49386) with primers designed to contain a 3′ SfiI site and a 5′ BamHI site. The fragments were digested with their respective restriction enzymes and ligated into a pUASp-attB plasmid modified to contain a polylinker sequence downstream of UASp site and P-element transposons. Transgenic flies were generated via P-element-mediated insertion at Rainbow Transgenic Flies.
Tissue fixation, fluorescence microscopy and imaging
See Table S2 for more information regarding the antibodies and reagents used in this study. Ovaries were dissected in ionically matched Drosophila saline (IMADS) buffer (Singleton and Woodruff, 1994) and fixed for 10 min in either 6% formaldehyde, 75 mM KCl, 25 mM NaCl, 3 mM MgCl2, and 17 mM potassium phosphate, pH 6.8 (Verheyen and Cooley, 1994) or 4% formaldehyde in PBT [PBS with 0.3% Triton X-100 and 0.5% bovine serum albumin (BSA)]. Fixed tissue was washed in PBT and incubated with the indicated primary antibodies in PBT for ∼1-2 h at room temperature or overnight at 4°C. Following washes with PBT, tissue was incubated with secondary antibodies conjugated to Alexa Fluor and DAPI in PBT for ∼1-2 h at room temperature. F-actin was labeled with Phalloidin conjugated to Tetramethylrhodamine (TRITC). Samples were washed in PBT and mounted on slides in ProLong Diamond (Thermo Fisher Scientific). Samples were imaged with a Leica SP8 laser scanning confocal microscope using a 40×1.3 NA oil-immersion objective lens or a Zeiss Axiovert 200 m equipped with either a CARV II spinning disc confocal imager or a CrEST X-light spinning disc system and a Photometrics CoolSNAP HQ2 camera using a 40×1.2 NA water-immersion objective.
Western blot analysis of ovary lysates
Ovary lysates were generated by homogenizing dissected ovaries in SDS-PAGE sample buffer. One ovary equivalent was loaded per lane and separated by SDS-PAGE. Proteins were transferred to a nitrocellulose membrane, stained with amido black to visualize total protein, blocked in 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 30 min at room temperature, and probed with the indicated primary antibodies for ∼2 h at room temperature in 5% milk/TBST. After washing the blots three times in TBST for 5 min, the blots were incubated with HRP-conjugated secondary antibodies for ∼1 h at room temperature followed by enhanced chemiluminescence (ECL) development for 5 min. A CCD camera was used for imaging. See Table S2 for more information on the antibodies used in this study.
Proteomic analysis of APEX samples
In vivo biotinylation of proteins using RC-APEX baits
Before dissection, female flies were fattened overnight on wet yeast paste at 25°C in a vial with males. For small-scale pilot experiments or experiments in which fluorescence microscopy was the final experimental readout, ovaries from 10-20 flies were used for biotin-phenol proximity labeling. For large-scale proteomic experiments, three batches of labeling were performed with ovaries from 30-35 flies in each batch, yielding a total of 100 flies used per sample. Ovaries were dissected in 1× PBS, pH 8.0 and gently pipetted up and down ∼ten times to splay open the ovaries. The pH of all solutions was kept above 7.5 to prevent precipitation of the biotin-phenol. Egg chambers were incubated in 500 μl biotin-phenol labeling solution (1 mM biotin-phenol, 0.5% digitonin, 1× PBS pH 8.0) for 3 min with rotation at room temperature. To initiate APEX-mediated biotinylation, H2O2 was added to a final concentration of 0.05 mM and the solution was gently inverted to mix for 30 s. After 30 s, 10 mM NaN3 was added to quench the reaction. Egg chambers were washed 3× in 500 μl quenching buffer (10 mM NaN3 in 1x PBS, pH 8.0). For every labeling reaction performed, egg chambers were always fixed and analyzed by fluorescence microscopy to ensure that the labeling reaction was successful (i.e. that RCs were biotinylated). Standard fixation and fluorescence protocols were used, as described earlier. To visualize biotin signal in egg chambers, fixed and permeabilized egg chambers were incubated with streptavidin-AF488 or streptavidin-AF568 at a 1:500 dilution in PBT for ∼1 h at room temperature. For large-scale labeling reactions, a very small amount of egg chambers was removed after the last wash in quenching buffer and analyzed by fluorescence microscopy. Immediately after the last wash, the remaining quenching buffer was removed from the egg chambers, and the tissue was frozen at -80°C.
Purification of biotinylated proteins from ovary lysates
Labeled and quenched egg chambers were thawed on ice and the tissue from the three batches of labeling was combined into one tube for each sample. Tissue was ground in 100-200 μl urea lysis buffer (8 M urea, 10 mM Tris pH 8.0, 50 mM NaPi, 300 mM NaCl, 5 μg/ml each of chymostatin, leupeptin, antipain and pepstatin, 10 mM NaN3, 1 μM bortezomib, 10 mM N-ethylmaleimide, 5 mM Trolox, 10 mM sodium ascorbate) with a mechanical homogenizer. After grinding, additional lysis buffer was added to a final volume of 1 ml and the lysate was incubated for an additional 30 min at room temperature to ensure sufficient lysis and solubilization. The crude lysate was centrifuged at 13,200 rpm (16,100 g) for 10 min at room temperature, and the clarified lysate was transferred to a new tube with 500 μl pre-equilibrated magnetic streptavidin beads and incubated for 1 h at room temperature with rotation. The unbound fraction was removed from the beads and the beads were washed three times in 1 ml urea wash buffer (2 M urea, 10 mM Tris pH 8.0, 50 mM NaPi, 300 mM NaCl, 5 μg/ml each of chymostatin, leupeptin, antipain, pepstatin, 10 mM NaN3, 1 μM bortezomib, 10 mM N-ethylmaleimide, 5 mM Trolox, 10 mM sodium ascorbate). To analyze the purified fraction by western blotting, 100 μl bead/wash solution was removed during the last wash (i.e. 10% of bead fraction). Beads were eluted with vigorous mixing at 95°C in 50 μl elution buffer [SDS sample buffer (0.1 M Tris pH 8.0, 4% SDS, 0.01% Bromophenol Blue, 5% β-mercaptoethanol) with 10 mM biotin]. Following the last wash, the remaining wash solution was removed from the rest of the beads and the beads were frozen immediately at -80°C or on dry ice. Beads were shipped on dry ice overnight to MS Bioworks for MS analysis using their IP-works platform.
Sample preparation for mass spectrometry
Proteins were eluted from streptavidin beads by incubation with 1.5× lithium dodecyl sulfate (LDS) buffer for 15 min at 100°C. Eluted proteins were separated from the beads by centrifugation and magnetic separation. Then, 50% of each eluate was processed by SDS-PAGE with a 10% Bis-Tris NuPAGE gel using the MES buffer system (Invitrogen). The gel lanes were excised, sliced into ten equal-sized fragments, and subjected to in-gel trypsin digestion using a robot (ProGest, DigiLab). For the in-gel trypsin digestion, gel slices were washed with 25 mM ammonium bicarbonate followed by acetonitrile, reduced with 10 mM dithiothreitol at 60°C, alkylated with 50 mM iodoacetamide at room temperature, digested with sequencing grade trypsin (Promega) at 37°C for 4 h, and quenched with formic acid. The resultant supernatant was analyzed by MS.
LC-MS/MS data acquisition and processing
Half of each digested sample was analyzed by nano LC-MS/MS with a Waters NanoAcquity HPLC system interfaced to a Thermo Fisher Scientific Q Exactive. Peptides were loaded on a trapping column and eluted over a 75-μm analytical column at a flow rate of 350 nl/min. Both columns were packed with Luna C18 resin (Phenomenex). The MS instrument was operated in data-dependent mode with the Orbitrap operating at 780,000 FWHM for MS and 17,500 FWHM for MS/MS. The 15 most abundant ions were selected for MS/MS. Data were searched using a local copy of Mascot (Version 2.6.0; Matrix Science) using the UniProt Drosophila melanogaster database with common laboratory contaminants added as well as custom protein sequences for HtsRC that included known single nucleotide polymorphisms (SNPs) and an N-terminal cleavage site at V693, and the APEX1 and APEX2 protein sequences. Trypsin was selected as the enzyme and two missed cleavages were allowed. Cysteine carbamidomethylation was selected as a fixed modification. Methionine oxidation, N-terminal acetylation, N-terminal pyroglutamylation, asparagine or glutamine deamidation, and tyrosine biotinylation by biotin-tyramide (chemical formula: C18H23N3O3S) were selected as variable modifications. A peptide mass tolerance of 10 ppm and a fragment mass tolerance of 0.02 Da were used. The resultant Mascot DAT files were parsed using Scaffold (Version 4.8.4; Proteome Software) for validation, filtering, and to create a nonredundant protein list per sample. Experiment-wide grouping with binary peptide-protein weights was selected as the Protein Grouping Strategy. Peptide and Protein Thresholds were set to 95% and 99%, respectively, with a 1 peptide minimum. These settings resulted in a 0.0% peptide false discovery rate (FDR) and a 0.4% protein FDR as measured by Decoy matches. The Scaffold Samples report was exported as an Excel spreadsheet. Contaminant proteins were removed from the spreadsheet data set. Spectral peptide matches for the Ovhts polyprotein encoded by the ovhts transcript were manually parsed into its two corresponding protein products: HtsF (AA 1-658) and HtsRC (AA 659-1156). Thus, spectral counts were manually entered into the spreadsheet for the HtsF and HtsRC proteins across each sample.
Data analysis using SAINT
The Automated Processing of SAINT Templated Layouts (APOSTL) (Kuenzi et al., 2016) tool was used to preprocess the MS data to generate corresponding data files that were compatible with analysis by SAINTexpress (Teo et al., 2014). The resultant SAINTexpress data set was processed further using APOSTL; namely, normalized spectral abundance factor (NSAF) values were calculated for each prey based on its respective SpC observed in each bait as well as the log2 fold-change compared with control [log2(FoldChange)].
Interactome network visualization with Cytoscape
Cytoscape was used to display the ‘interactomes’ of the RC-APEX baits and their respective high-confidence prey. RC-APEX baits and their prey genes were indicated by colored squares and circles, respectively. The size of the prey gene circle was scaled based on its average abundance compared with control samples [log2(FoldChange) NSAF]. Known RC genes were manually denoted with a solid outline. The Molecular Interaction Search Tool (MIST) (Hu et al., 2018) was used to search for previously established interactions between all high-confidence prey genes, and positive interactions were indicated with a dotted line.
Generation of dot plots and heatmaps with ProHits-viz
The ProHits-viz tool (Knight et al., 2017) was used to generate dot plots to visualize proteomics data for any high-confidence prey across all four RC-APEX bait samples. The color of each prey ‘dot’ was dependent on the NSAF log2(FoldChange) for each bait compared with the control samples. The size of each dot was dependent on its relative abundance between bait samples, meaning that the maximum-sized dot for each prey was allocated to the largest SpC value within that bait and the other dots were scaled proportionately. High-confidence prey within each bait had a yellow outline, corresponding to a SAINT score ≥0.8, whereas prey with a SAINT score <0.8 for that bait had a faint outline.
Generation of volcano plots
Volcano plots were generated for each RC-APEX to visualize the quantitative proteomic data relative to their statistical significance. For each protein identified by MS, a P-value was calculated for its spectral counts within the biological replicates for each RC-APEX bait and control samples. For all identified proteins, the -log10(P-value) was plotted against the NSAF log2(FoldChange) compared with controls for each RC-APEX bait sample.
Validation of RC-APEX high-confidence prey using proximity ligation assay
Ovaries from ∼50 flies were dissected and fixed in 6% formaldehyde, 75 mM KCl, 25 mM NaCl, 3 mM MgCl2 and 17 mM potassium phosphate, pH 6.8 with heptane for 8 min. After washing three times for 10 min in PBT, the tissue was incubated with 500 μl 30% sucrose solution with gentle rotation for 30 min, at which point the ovaries settled to the bottom of the tube. Tissue was rinsed with fresh sucrose solution and transferred to an Eppendorf tube cap, where it was fully submerged in cold optimal cutting temperature (OCT) compound. After a 1-2 h incubation, the tissue was embedded in a cryoblock mold in OCT by incubating on dry ice for 10 min. Cryoblocks were stored at -80°C until sectioning. Before sectioning, blocks were equilibrated at -20°C for 5 min. Then, 20-μm slices were sectioned, transferred to Superfrost Plus charged slides (Fisher Scientific), and stored at -80°C until future use. For the PLA, the Duolink® In Situ Red Starter Kit Mouse/Rabbit (Sigma-Aldrich) was used with minor protocol modifications. Slides containing ovary cryosections were equilibrated at room temperature for 5 min, then blocked for 1 h in PLA blocking buffer (1× PBS, 0.3% Triton X-100, 1% BSA). An Aqua Hold II pen was used to form hydrophobic barriers around tissue on the slide to contain the small volumes of solutions used in this protocol. Slides were placed in a humidity chamber and tissue was incubated with 40 μl primary antibody solution and FITC-Phalloidin in PLA blocking buffer for 2 h at room temperature. See Table S2 for a list of antibodies and their concentrations used in this assay. Tissue was washed twice for 5 min with PLA blocking buffer and incubated in 40 μl PLA probe solution (8 μl PLUS probe, 8 μl MINUS probe, 24 μl antibody diluent) for 1.5 h at 37°C in the humidity chamber. Slides were washed twice for 5 min in wash buffer A then incubated for 45 min in the humidity chamber at 37°C in 40 μl ligation solution (8 μl ligation buffer, 31 μl ddH2O, 1 μl ligase). Slides were washed twice for 5 min in wash buffer A and incubated for 2 h in the humidity chamber at 37°C in 40 μl amplification solution (8 μl amplification buffer, 31.5 μl ddH2O, 0.5 μl polymerase) supplemented with FITC-Phalloidin. Slides were washed twice for 10 min in wash buffer B, rinsed for 1 min with 0.01× wash buffer B, and mounted in 30 μl PLA mounting medium overnight in the humidity chamber at 4°C. Slides were imaged on a Leica SP8 laser scanning confocal microscope using a 40×1.3 NA oil-immersion objective lens and all laser settings were equal between each slide and its respective control to allow for quantitative comparison of PLA foci. Positive PLA foci were counted in FIJI using the Auto-thresholding and Particle Analysis tools, with the minimum area of a foci set to 2 μm2. Egg chamber area was calculated in FIJI using the Freehand and Area Measure tools.
Validation of prey genes by RNAi screening
See Table S2 for a list of fly stocks used for the RNAi screen. Ovaries were dissected from fattened females, fixed and stained with TRITC-Phalloidin and anti-HtsRC antibodies to check for any abnormalities in egg chamber development or RC morphology.
Supplementary Material
Acknowledgements
We thank Peter McLean for help with the (BAC) pav::APEX transgene. We thank Tian Xu (Yale University, Westlake University) for providing access to the Leica SP8 confocal microscope. We are grateful to Ellen LeMosy (Augusta University) as well as members of the Weatherbee lab (Yale University) for help with the cryosectioning protocol. We thank Alice Ting for helpful comments and access to protocols for APEX-mediated biotinylation. We are grateful to Michael Ford and the MS Bioworks team for their services. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank the TRiP at Harvard Medical School for providing transgenic RNAi fly stocks used in this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.M.M., L.C.; Methodology: K.M.M., R.M.S., R.S.K., L.C.; Investigation: K.M.M., R.M.S., R.S.K., L.C.; Resources: K.M.M., R.M.S., R.S.K., L.C.; Writing - original draft: K.M.M.; Writing - review & editing: K.M.M., R.M.S., R.S.K., L.C.; Visualization: K.M.M., R.M.S., L.C.; Supervision: L.C.; Funding acquisition: L.C.
Funding
K.M.M. was a Cold Spring Harbor Proteomics Course participant. K.M.M. and R.S.K. were supported in part by a National Institute of General Medical Sciences National Institutes of Health training grant T32 GM007223 and a Gruber Science Fellowship. This work was funded by National Institutes of Health grants R01 GM043301 and RC1 GM091791. Deposited in PMC for release after 12 months.
Data availability
A Scaffold file containing all proteomics data, including peptide spectral matches (PSM), is available from the Dryad Digital Repository (Mannix et al., 2019): dryad.9p9c594.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.176644.supplemental
References
- Adams R. R., Tavares A. A. M., Salzberg A., Bellen H. J. and Glover D. M. (1998). Pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12, 1483-1494. 10.1101/gad.12.10.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi S. J., McLean P. F., Shimada Y. and Cooley L. (2011). Intercellular protein movement in syncytial Drosophila follicle cells. J. Cell Sci. 124, 4077-4086. 10.1242/jcs.090456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branon T. C., Bosch J. A., Sanchez A. D., Udeshi N. D., Svinkina T., Carr S. A., Feldman J. L., Perrimon N. and Ting A. Y. (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880-887. 10.1038/nbt.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning P., Cooper C. D. O., Krojer T., Murray J. W., Pike A. C. W., Chaikuad A., Keates T., Thangaratnarajah C., Hojzan V., Ayinampudi V. et al. (2013). Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J. Biol. Chem. 288, 7803-7814. 10.1074/jbc.M112.437996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-L. and Perrimon N. (2017). Proximity-dependent labeling methods for proteomic profiling in living cells: Proximity-dependent labeling methods. Wiley Interdiscip. Rev. Dev. Biol. 6.4, e272 10.1002/wdev.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-L., Hu Y., Udeshi N. D., Lau T. Y., Wirtz-Peitz F., He L., Ting A. Y., Carr S. A. and Perrimon N. (2015). Proteomic mapping in live Drosophila tissues using an engineered ascorbate peroxidase. Proc. Natl. Acad. Sci. USA 112, 12093-12098. 10.1073/pnas.1515623112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Larsen B., Lin Z.-Y., Breitkreutz A., Mellacheruvu D., Fermin D., Qin Z. S., Tyers M., Gingras A.-C. and Nesvizhskii A. I. (2011). SAINT: probabilistic scoring of affinity purification–mass spectrometry data. Nat. Methods 8, 70-73. 10.1038/nmeth.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L. (1998). Drosophila ring canal growth requires Src and Tec kinases. Cell 93, 913-915. 10.1016/S0092-8674(00)81196-4 [DOI] [PubMed] [Google Scholar]
- Errington W. J., Khan M. Q., Bueler S. A., Rubinstein J. L., Chakrabartty A. and Prive G. G. (2012). Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure 20, 1141-1153. 10.1016/j.str.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Goshima G. and Vale R. D. (2005). Cell cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells. Mol. Biol. Cell 16, 3896-3907. 10.1091/mbc.e05-02-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. (1975). “Avidin”. Advances in Protein Chemistry, Vol. 29, pp. 85-133. Academic Press. [DOI] [PubMed] [Google Scholar]
- Greenbaum M. P., Ma L. and Matzuk M. M. (2007). Conversion of midbodies into germ cell intercellular bridges. Dev. Biol. 305, 389-396. 10.1016/j.ydbio.2007.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K., Nezis I. P. and Stenmark H. (2011). Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun. Integr. Biol. 4, 1-9. 10.4161/cib.4.1.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N., Nishida Y. and Yamamoto D. (2015). Btk29A-mediated tyrosine phosphorylation of armadillo/β-catenin promotes ring canal growth in drosophila oogenesis. PLOS ONE 10, e0121484 10.1371/journal.pone.0121484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Vinayagam A., Nand A., Comjean A., Chung V., Hao T., Mohr S. E. and Perrimon N. (2018). Molecular Interaction Search Tool (MIST): an integrated resource for mining gene and protein interaction data. Nucleic Acids Res. 46, D567-D574. 10.1093/nar/gkx1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. M. and Cooley L. (2010). Drosophila Kelch functions with Cullin-3 to organize the ring canal actin cytoskeleton. J. Cell Biol. 188, 29-37. 10.1083/jcb.200909017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. M., Mannix K. M. and Cooley L. (2015). Actin Cytoskeletal organization in drosophila germline ring canals depends on Kelch function in a Cullin-RING E3 ligase. Genetics 201, 1117-1131. 10.1534/genetics.115.181289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. M., Mannix K. M., Gerdes J. A., Kottemann M. C. and Cooley L. (2019). Targeted substrate degradation by Kelch controls the actin cytoskeleton during ring canal expansion. Development 146, dev169219 10.1242/dev.169219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V., Udeshi N. D., Lam S. S., Loh K. H., Cox K. J., Pedram K., Carr S. A. and Ting A. Y. (2016). Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 11, 456-475. 10.1038/nprot.2016.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji A. X. and Privé G. G. (2013). Crystal structure of KLHL3 in complex with Cullin3. PLoS ONE 8, e60445 10.1371/journal.pone.0060445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. A., Mezulis S., Yates C. M., Wass M. N. and Sternberg M. J. E. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845-858. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso R. J., Hudson A. M. and Cooley L. (2002). Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J. Cell Biol. 156, 703-713. 10.1083/jcb.200110063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. I. and Roux K. J. (2016). Filling the void: proximity-based labeling of proteins in living cells. Trends Cell Biol. 26, 804-817. 10.1016/j.tcb.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. I., Jensen S. C., Noble K. A., Kc B., Roux K. H., Motamedchaboki K. and Roux K. J. (2016). An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 27, 1188-1196. 10.1091/mbc.E15-12-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A., Curry T. and Lewellyn L. (2018). The Misshapen kinase regulates the size and stability of the germline ring canals in the Drosophila egg chamber. Dev. Biol. 440, 99-112. 10.1016/j.ydbio.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. D. R., Choi H., Gupta G. D., Pelletier L., Raught B., Nesvizhskii A. I. and Gingras A.-C. (2017). ProHits-viz: a suite of web tools for visualizing interaction proteomics data. Nat. Methods 14, 645-646. 10.1038/nmeth.4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzi B. M., Borne A. L., Li J., Haura E. B., Eschrich S. A., Koomen J. M., Rix U. and Stewart P. A. (2016). APOSTL: an interactive galaxy pipeline for reproducible analysis of affinity proteomics data. J. Proteome Res. 15, 4747-4754. 10.1021/acs.jproteome.6b00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix K. M., Starble R. M., Kaufman R. S. and Cooley L. (2019). Data from: Proximity labeling reveals novel interactomes in live Drosophila tissue. Dryad Digital Repository. 10.5061/dryad.9p9c594 [DOI] [PMC free article] [PubMed]
- Martell J. D., Deerinck T. J., Sancak Y., Poulos T. L., Mootha V. K., Sosinsky G. E., Ellisman M. H. and Ting A. Y. (2012). Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat. Biotechnol. 30, 1143-1148. 10.1038/nbt.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minestrini G., Mathe E. and Glover D. M. (2002). Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J. Cell Sci. 115, 725-736. [DOI] [PubMed] [Google Scholar]
- Mische S., Li M., Serr M., Hays T. S. and Wente S. (2007). Direct observation of regulated ribonu- cleoprotein transport across the nurse cell/oocyte boundary. Mol. Biol. Cell 18, 2254-2263. 10.1091/mbc.e06-10-0959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawe T., Honemann-Capito M., von Stein W. and Wodarz A. (2011). Loss of the extraproteasomal ubiquitin receptor Rings lost impairs ring canal growth in Drosophila oogenesis. J. Cell Biol. 193, 71-80. 10.1083/jcb.201009142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. H., Knudsen G. M., Verschueren E., Johnson J. R., Cimermancic P., Greninger A. L. and Pico A. R. (2014). Affinity purification–mass spectrometry and network analysis to understand protein-protein interactions. Nat. Protoc. 9, 2539-2554. 10.1038/nprot.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. and Tan C. (2010). Germline cyst formation and incomplete cytokinesis during Drosophila melanogaster oogenesis. Dev. Biol. 337, 84-98. 10.1016/j.ydbio.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Petrella L. N., Smith-Leiker T. and Cooley L. (2007). The Ovhts polyprotein is cleaved to produce fusome and ring canal proteins required for Drosophila oogenesis. Development 134, 703-712. 10.1242/dev.02766 [DOI] [PubMed] [Google Scholar]
- Reinke A. W., Balla K. M., Bennett E. J. and Troemel E. R. (2017a). Identification of microsporidia host- exposed proteins reveals a repertoire of rapidly evolving proteins. Nat. Commun. 8 10.1038/ncomms14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A. W., Mak R., Troemel E. R. and Bennett E. J. (2017b). In vivo mapping of tissue- and subcellular- specific proteomes in Caenorhabditis elegans. Sci. Adv. 3, e1602426 10.1126/sciadv.1602426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H.-W., Zou P., Udeshi N. D., Martell J. D., Mootha V. K., Carr S. A. and Ting A. Y. (2013). Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328-1331. 10.1126/science.1230593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. N. and Cooley L. (1997a). Drosophila kelch is an oligomeric ring canal actin organizer. J. Cell Biol. 138, 799-810. 10.1083/jcb.138.4.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. N. and Cooley L. (1997b). Examination of the function of two kelch proteins generated by stop codon suppression. Development 124, 1405-1417. [DOI] [PubMed] [Google Scholar]
- Robinson D. N., Cant K. and Cooley L. (1994). Morphogenesis of Drosophila ovarian ring canals. Development 120, 2015-2025. [DOI] [PubMed] [Google Scholar]
- Robinson D. N., Smith-Leiker T. A., Sokol N. S., Hudson A. M. and Cooley L. (1997). Formation of the Drosophila ovarian ring canal inner rim depends on cheerio. Genetics 145, 1063-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbauer U., Zolghadr K., Muyldermans S., Schepers A., Cardoso M. C. and Leonhardt H. (2008). A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol. Cell. Proteomics 7, 282-289. 10.1074/mcp.M700342-MCP200 [DOI] [PubMed] [Google Scholar]
- Roux K. J., Kim D. I., Raida M. and Burke B. (2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801-810. 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher F.-R., Sorrell F. J., Alessi D. R., Bullock A. N. and Kurz T. (2014). Structural and biochemical characterization of the KLHL3-WNK kinase interaction important in blood pressure regulation. Biochem. J. 460, 237-246. 10.1042/BJ20140153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton K. and Woodruff R. I. (1994). The osmolarity of adult Drosophila hemolymph and its effect on oocyte-nurse cell electrical polarity. Dev. Biol. 161, 154-167. 10.1006/dbio.1994.1017 [DOI] [PubMed] [Google Scholar]
- Sokol N. S. and Cooley L. (1999). Drosophila filamin encoded by the cheerio locus is a component of ovarian ring canals. Curr. Biol. 9, 1221-1230. 10.1016/S0960-9822(99)80502-8 [DOI] [PubMed] [Google Scholar]
- Sommi P., Ananthakrishnan R., Cheerambathur D. K., Kwon M., Morales-Mulia S., Brust-Mascher I. and Mogilner A. (2010). A mitotic kinesin-6, Pav-KLP, mediates interdependent cortical reorganization and spindle dynamics in Drosophila embryos. J. Cell Sci. 123, 1862-1872. 10.1242/jcs.064048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Fasulo B., Warecki B. and Sullivan W. (2016). Tum/RacGAP functions as a switch activating the Pav/kinesin-6 motor. Nat. Commun. 7, 11182 10.1038/ncomms11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo G., Liu G., Zhang J., Nesvizhskii A. I., Gingras A.-C. and Choi H. (2014). SAINTexpress: Improvements and additional features in Significance Analysis of INTeractome software. J. Proteomics 100, 37-43. 10.1016/j.jprot.2013.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J., Goodman J. L., Kaufman T. C., Strelets V. B., Calvi B. R., Millburn G., Antonazzo G., Attrill H., Marygold S. J., Trovisco V. et al. (2018). FlyBase 2.0: the next generation. Nucleic Acids Res. 47, D759-d765. 10.1093/nar/gky1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnaite R. and MacNeill S. A. (2016). Meet the neighbors: mapping local protein interactomes by proximity- dependent labeling with BioID. Proteomics 16, 2503-2518. 10.1002/pmic.201600123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen E. and Cooley L. (1994). Looking at oogenesis. Methods Cell Biol. 44, 545-561. 10.1016/S0091-679X(08)60931-0 [DOI] [PubMed] [Google Scholar]
- Wang J., Sarov M., Rientjes J., Hu J., Hollak H., Kranz H., Xie Y., Stewart A. F. and Zhang Y. (2006). An improved recombineering approach by adding RecA to Λ Red recombination. Mol. Biotechnol. 32, 43-53. 10.1385/MB:32:1:043 [DOI] [PubMed] [Google Scholar]
- Xu L., Wei Y., Reboul J., Vaglio P., Shin T. H., Vidal M., Elledge S. J. and Harper J. W. (2003). BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425, 316-321. 10.1038/nature01985 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Bayat V., Bellen H. J. and Tan C. (2013). Protein phosphatase 1ß limits ring canal constriction during Drosophila germline Cyst formation. PLoS ONE 8, e70502 10.1371/journal.pone.0070502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y. M. (2018). Subcellular specialization and organelle behavior in germ cells. Genetics 208, 19-51. 10.1534/genetics.117.300184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Xie Y., Zheng Y., Jiang S., Liu W., Mu W., Liu Z., Zhao Y., Xue Y., and Ren J., (2014). GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res. 42, W325-W330. 10.1093/nar/gku383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.