ABSTRACT
Although K+ channels are important in mediating the driving force for colonic ion transport, their role in small intestinal transport is poorly understood. To investigate this, small intestinal short circuit currents (Isc) and HCO3− secretion were measured in mice, and intracellular pH (pHi) was measured in small intestinal epithelial SCBN cells. The expression and location of Kv subtypes were verified by RT-PCR, western blotting and immunohistochemistry. Diabetic mice were also used to investigate the role of Kv subtypes in regulating intestinal glucose absorption. We found that KV7.1 is not involved in duodenal ion transport, while KCa3.1 selectively regulates duodenal Isc and HCO3− secretion in a Ca2+-mediated but not cAMP-mediated manner. Blockade of KCa3.1 increased the rate of HCO3− fluxes via cystic fibrosis transmembrane conductance regulator (CFTR) channels in SCBN cells. Jejunal Isc was significantly stimulated by glucose, but markedly inhibited by 4-aminopyridine (4-AP) and tetraethylammonium (TEA). Moreover, both Kv1.1 and Kv1.3 were expressed in jejunal mucosae. Finally, 4-AP significantly attenuated weight gain of normal and diabetic mice, and both 4-AP and TEA significantly lowered blood glucose of diabetic mice. This study not only examines the contribution of various K+ channel subtypes to small intestinal epithelial ion transport and glucose absorption, but also proposes a novel concept for developing specific K+ channel blockers to reduce weight gain and lower blood glucose in diabetes mellitus.
KEY WORDS: Small intestine, Kv channel subtypes, Glucose absorption, Anion secretion, Diabetes mellitus
Summary: This study examines the different functions of various K+ channel subtypes in small intestinal epithelial, which proposes a novel concept for the development of weight-loss drugs and hypoglycemic drugs.
INTRODUCTION
The small intestinal epithelium has two important physiological functions: the absorption of nutrients and the secretion of ions. Both processes are associated with electrogenic ion transport across the plasma membrane of intestinal epithelial cells (IECs) (Heitzmann and Warth, 2008). The absorption of most nutrients in the small intestine, such as glucose (Wright et al., 1997), amino acids (Stevens, 1992) and long-chain free fatty acids (Berk and Stump, 1999; Berk et al., 1997; Stremmel, 1988), is usually associated with electrogenic activity. For example, glucose uptake in the jejunum is driven by the transmembrane Na+ gradient and the membrane potential. The major route for entry of dietary glucose from the jejunal lumen into enterocytes is Na+/glucose cotransport via Na+/glucose co-transporter 1 (SGLT1) (Gorboulev et al., 2012). Although the membrane potential of IECs is important in regulating the activity of SGLT1, the membrane potential itself is maintained by plasma membrane ion channels and transporters. While the Na+/glucose cotransporter has been extensively studied in jejunal glucose absorption, little is currently known regarding their regulation by plasma membrane ion channels, such as K+ channels in IECs.
The small intestinal epithelium also secretes and reabsorbs electrolytes such as Cl− and HCO3−. Intestinal Cl− secretion plays an important role in regulating the amount of fluid in the intestinal lumen, while HCO3− secretion in the upper GI tract is crucial to protecting the vulnerable epithelium against gastric acid and pepsin (Barrett, 1997; Bedine, 2000). Indeed, the importance of duodenal mucosal HCO3− secretion can be readily demonstrated in patients with duodenal ulcers, in whom acid-stimulated HCO3− secretion is attenuated by 41% compared to healthy subjects (Allen and Flemström, 2005; Allen et al., 1993; Flemström and Isenberg, 2001; Isenberg et al., 1987). It has become widely accepted that epithelial transport relies on the membrane potential, which provides the driving force necessary for movement of nutrients and electrolytes across the plasma membrane. Growing evidence indicates that epithelial K+ channels play an important role in maintaining this driving force as well as stabilizing the membrane potential (Warth, 2003).
K+ channels are ubiquitously expressed in almost all excitable or non-excitable cells, including IECs. There are several subtypes of K+ channels already described in IECs, including Kv1.1 (KCNA1), Kv1.3 (KCNA3), KCa3.1 (KCNN4) and Kv7.1 (KCNQ1) (Heitzmann and Warth, 2008). These channels are implicated in a variety of cellular functions, such as ion transport, volume regulation, cell migration, wound healing, proliferation, apoptosis and carcinogenesis (Heitzmann and Warth, 2008). Although it is known that K+ channels are important in stabilizing the membrane potential and mediating the driving force for electrogenic ion transport in colonic epithelial cells, their role in regulating epithelial transport remains to be elucidated in the small intestine. Therefore, the aims of the present study are to identify if K+ channels subtypes, Kv1.1, Kv1.3, KCa3.1 and Kv7.1 are functionally expressed in the small intestinal epithelium; and if so, whether they are involved in controlling physiological functions. Here, we describe various roles for K+ channel subtypes in small intestinal epithelial transport, specifically in regulating duodenal anion secretion and jejunal glucose absorption. Our study also provides incentive for developing specific blockers for K+ channel subtypes as novel therapeutic agents to modulate intestinal epithelial secretion, reduce weight gain and improve glycemic control in diabetes mellitus.
RESULTS
Role of KV7.1 (KCNQ1) in duodenal anion secretion
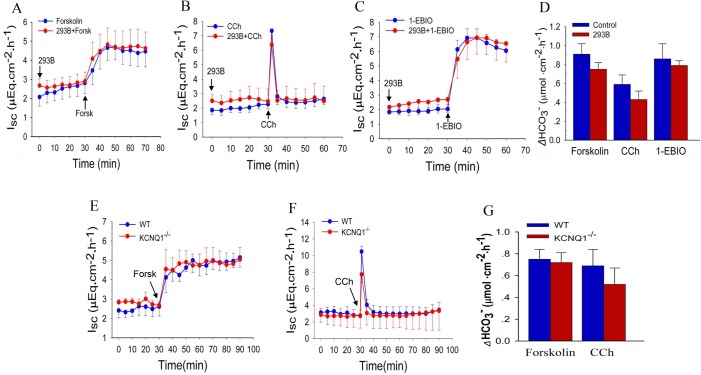
Since KV7.1 channels have been shown to play roles in regulating the secretion of gastric acid and jejunal Cl− (Dong et al., 2006), we began by testing whether KV7.1 channels were functionally involved in duodenal anion secretion. We chose three different agents for pharmacological stimulation of epithelial anion secretion: (1) forskolin, through triggering the cAMP pathway; (2) carbachol (CCh), by mobilizing intracellular Ca2+; (3) 1-ethyl-2-benzimidazolinone (1-EBIO), which activates the KCa channel by increasing its Ca2+ sensitivity. When introduced to the serosal side of duodenal tissue from C57BL/6J mice, forskolin (10 μM), CCh (100 μM), and 1-EBIO (1 mM) all induced significant intestinal short circuit current (Isc) and HCO3− secretion (Fig. 1A–D). The net peak HCO3− secretion (Fig. 1D) was calculated as the peak HCO3− secretion at 10 min minus the basal value. Addition of a selective KV7.1 blocker, chromanol 293B (10 μM), to both sides did not alter the time courses of these responses. Furthermore, we did not find any difference in forskolin- and CCh-induced duodenal Isc (Fig. 1E,F) and net peak HCO3− secretion (Fig. 1G) between KV7.1−/− mice and wild-type mice. Therefore, consistent with Liao's previous report (Buresi et al., 2002), we confirmed that KV7.1 channels are not involved in Cl− and HCO3− secretion mediated by cAMP and Ca2+ signaling in the duodenum.
Fig. 1.
No involvement of Kv7.1 (KCNQ1) subtype in duodenal ion transport in mice. Forskolin (A), CCh (B) and 1-EBIO (C) stimulated duodenal Isc and net peak HCO3− secretion (D) in the absence or the presence of 293B in C57BL/6J WT mice. 293B (10 μM) and 1-EBIO (1 mM) were added to both sides, but forskolin (10 μM) and CCh (100 μM) were added to the serosal sides at the times indicated. Forskolin- (E) and CCh- (F) stimulated mucosal Isc and net peak HCO3− secretion (G) in KV7.1−/− mice and WT mice. Forskolin (10 μM) and CCh (100 μM) were added to the serosal sides at the times indicated. Values are means±s.e.m.; n=5–7 tissues in each series; Student's t-test, no statistical significances were identified between each group.
Specific role of KCa3.1 (KCNN4) channels in Ca2+-mediated duodenal anion secretion
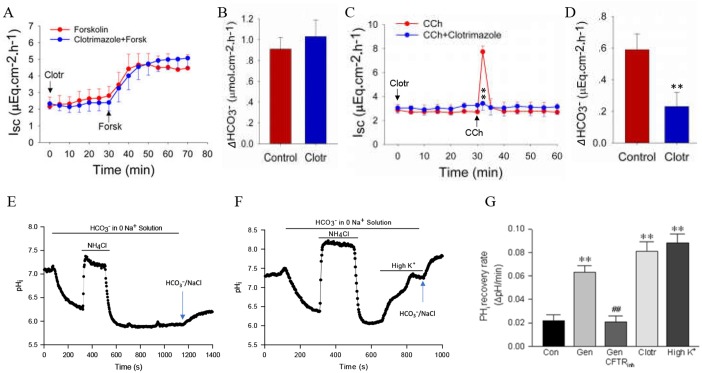
Although we previously demonstrated the important role of KCa3.1 channels in regulating duodenal Cl− and HCO3− secretion in mice (Cheng, 2012), it is unclear whether KCa3.1 channels are specifically activated through the Ca2+ pathway for this process. Therefore, both cAMP and Ca2+ pathways were examined in the present study. Selective blockade of KCa3.1 with clotrimazole (30 μM) did not alter the time course of forskolin-induced duodenal Isc and net peak HCO3− secretion in wild-type mice (Fig. 2A,B), excluding the non-selectivity of clotrimazole for the cAMP signaling pathway. To confirm this notion, we further examined the effect of clotrimazole on CCh-induced duodenal Isc and net peak HCO3− secretion in KV7.1−/− mice, to exclude the possible involvement of KV7.1 channels in duodenal Cl− and HCO3− secretion. We found that clotrimazole (30 μM) significantly attenuated the time course of CCh-induced duodenal Isc and net peak HCO3− secretion in these mice (Fig. 2C,D). By combining selective pharmacological blockade and genetic knockout mice, we confirm that KCa3.1 channels are involved in regulating Ca2+- but not cAMP-mediated duodenal anion secretion.
Fig. 2.
Important role of Ca2+-mediated KCa3.1 (KCNN4) subtype in duodenal ion transports. (A,B) Forskolin-stimulated duodenal Isc and net peak HCO3− secretion in the presence of clotrimazole in WT mice. (C,D) CCh-stimulated duodenal Isc and net peak HCO3− secretion in the presence of clotrimazole in KV7.1−/− mice. Clotrimazole (30 μM) was added to both sides, but forskolin (10 μM) and CCh (100 μM) were added to the serosal sides at the time indicated. **P<0.01 versus control; n=5–6 tissues in each series. (E) Control time course of pHi changes induced by NH4Cl (30 mM) in Na+-free/HCO3− solution, in which pHi first increases and then decreases after washout. The cells remained acidic, with relatively stable pHi, which began to recover after addback of Na+/HCO3− solution (the last peak). (F) High K+-induced HCO3− fluxes through CFTR channels. The time course of pHi changes in cells was similar to the control in E except high K+ was added to the cells acidified in HCO3−/0Na+ solution as indicated. (G) Summary data showing the effects of genistein (Gen, 30 μM), CFTRinh-173 (10 μM), clotrimazole (Clotr, 30 μM), and high K+ (80 mM) on HCO3− fluxes in SCBN cells. Student's t-test, **P<0.01 versus control (Con), and ##P<0.01 versus Gen, n=40–50 cells in each series.
Since the cystic fibrosis transmembrane conductance regulator (CFTR) channel has been characterized in SCBN cells and they are often used to study epithelial anion secretion (Buresi et al., 2001, 2005; Vallon et al., 2005), we chose this cell line to test the role of CFTR channels in HCO3− entry. SCBN cells were treated with NH4Cl in Na+-free/HCO3− solution, which initially caused an increase in pHi due to entry of the weak base NH3, followed by a decrease in pHi when NH4+ was washed from the extracellular solution. Subsequently, pHi gradually recovered due to likely entry of HCO3− through CFTR channels, and addback of Na+/HCO3− solution resulted in further recovery of pHi through both CFTR and Na+-dependent mechanisms of HCO3− entry (Fig. 2E). The recovery rate of pHi was calculated to represent HCO3− entry via CFTR channels after NH4+ washout with Na+-free/HCO3− solution. Indeed, the pHi recovery rate was increased by the CFTR activator genistein (30 μM) (Tuo et al., 2009) and significantly inhibited by the CFTR blocker CFTRinh-172 (10 μM) (Fig. 2G). These data provide strong evidence for the important role of CFTR channels in HCO3− entry in SCBN cells (Buresi et al., 2001). When high K+ (80 mM) was added to induce membrane depolarization in SCBN cells that were acidified in Na+-free/HCO3− solution, a quick initial pHi recovery was observed, with further recovery after addback of Na+/HCO3− solution (Fig. 2F,G). Similar experiments were performed with clotrimazole (30 μM), which also increased the pHi recovery rate in Na+-free/HCO3− solution (Fig. 2G). These results not only provide further evidence to support our previous finding that KCa3.1 channels are important for HCO3− secretion, but also elucidate the underlying mechanisms of KCa3.1-mediated HCO3− entry through CFTR channels in SCBN cells (Buresi et al., 2001).
Glucose induces jejunal Isc via the Na+/glucose co-transporter
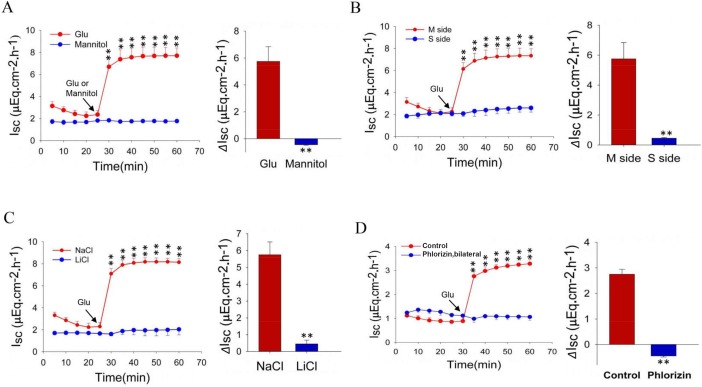
Since it is well known that jejunal active glucose absorption is through mucosal SGLT1, we conducted Ussing chamber experiments to record jejunal Isc in wild-type mice. First, when glucose (25 mM) was added to the mucosal side of jejunal tissue, it induced marked Isc while mannitol (25 mM), a similar organic compound, also derived from sugar, did not (Fig. 3A). Second, mucosal addition of glucose induced marked Isc, but not serosal addition (Fig. 3B). Third, mucosal addition of glucose induced marked Isc in the presence of NaCl, but not in the presence of LiCl (Na+-free substitute) (Fig. 3C). Finally, glucose-induced Isc in the presence of NaCl was abolished by phlorizin (1 mM), a specific inhibitor of SGLT1 (Fig. 3D). Together, these results strongly suggest that jejunal mucosal Na+/glucose co-transporter (i.e. SGLT1) mediates this glucose-induced intestinal Isc.
Fig. 3.
The time courses and net peaks of jejunal glucose absorption under various conditions. (A) Comparison of jejunal Isc induced by mucosal addition of glucose (Glu) or mannitol in the presence of extracellular Na+ (140 mM). (B) Comparison of jejunal Isc induced by mucosal (M side) or serosal (S side) addition of glucose in the presence of extracellular Na+. (C) Comparison of jejunal Isc induced by M side addition of glucose in the presence of NaCl or LiCl (the absence of extracellular Na+). (D) Inhibitory effect of phlorizin (1 mM) on jejunal Isc induced by mucosal addition of glucose in the presence of extracellular Na+. Glucose or mannitol (25 mM for both) was added at the time indicated. These tests were conducted in WT mice. Values are means±s.e.m.; Student's t-test, **P<0.01 versus control, n=4–5 tissues in each series.
Roles of K+ channel subtypes in the regulation of jejunal glucose absorption
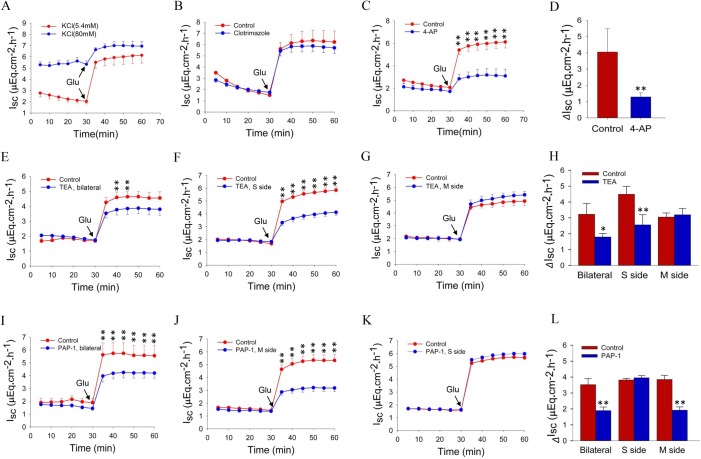
To test the general roles of membrane potential and K+ channels in the regulation of nutrient absorption, high K+ (80 mM) was added bilaterally to Ussing chambers to inhibit all plasma membrane K+ channels. As shown in Fig. 4A, high K+ not only raised the baseline jejunal Isc but also inhibited the glucose-induced Isc, indicating the critical role of K+ channels in the regulation of nutrient absorption. Since KCa3.1 channels are important in intestinal Isc and HCO3− secretion, we tested if KCa3.1 plays a role in regulating jejunal glucose absorption; however, clotrimazole (30 μM) did not alter glucose-induced jejunal Isc (Fig. 4B). We then tested 4-aminopyridine (4-AP) (1 mM), a broad-spectrum blocker of Kv channels (Castle et al., 2003), and found it abolished Isc (Fig. 4C,D). Thus, we focused on Kv channels and their potential role in the regulation of jejunal glucose absorption, using the selective KV1.1 inhibitor tetraethylammonium (TEA) (Castle et al., 2003). We found that glucose-induced jejunal Isc is significantly attenuated with bilateral addition of TEA (0.5 mM) (Fig. 4E), and specifically only when added to the serosal side, but not the mucosal side (Fig. 4F,G). This suggests the functional expression of KV1.1 is polarized (Fig. 4H), and these findings overall reveal a role for serosal KV1.1 channels in the regulation of jejunal glucose absorption.
Fig. 4.
Effects of selective blockers for Kv channel subtypes on time courses and net peaks of jejunal glucose absorption. (A,B) Glucose-induced jejunal Isc after bilateral addition of high K+ (80 mM) or clotrimazole (30 μM). (C,D) Effect of addition of 4-AP (1 mM) to both sides on time courses and net peaks of jejunal glucose absorption. (E–G) Glucose-induced Isc after bilateral, serosal or mucosal addition of TEA (0.5 mM). (H) Effect of TEA on net peak of jejunal glucose absorption after bilateral, serosal or mucosal addition. (I–K) Glucose-induced jejunal Isc after bilateral, mucosal or serosal addition of PAP-1 (0.5 μM). (L) Effect of PAP-1 on net peak of jejunal glucose absorption after bilateral, mucosal or serosal addition. Glucose (25 mM) was added at the time indicated after pretreatment with the selective Kv subtype blockers. Values are means±s.e.m.; Student's t-test, *P<0.05, **P<0.01 versus control, n=5–7 tissues in each series.
Next, we shifted our focus to Kv1.3 channels. As shown in Fig. 4I, bilateral addition of 5-(4-phenoxybutoxy) psoralen (PAP-1) (0.1 μM) significantly attenuated glucose-induced Isc. To determine if functional expression of Kv1.3 is polarized, PAP-1 was selectively added to either side of epithelial cells, and glucose-induced Isc was only attenuated when PAP-1 was added to the mucosal side rather than the serosal side (Fig. 4J,K), in contrast to TEA. Fig. 4L summarizes the effect of PAP-1 on the net peak jejunal glucose absorption after either mucosal, serosal or bilateral addition. These findings highlight the potential role of mucosal Kv1.3 channels in the regulation of jejunal glucose absorption.
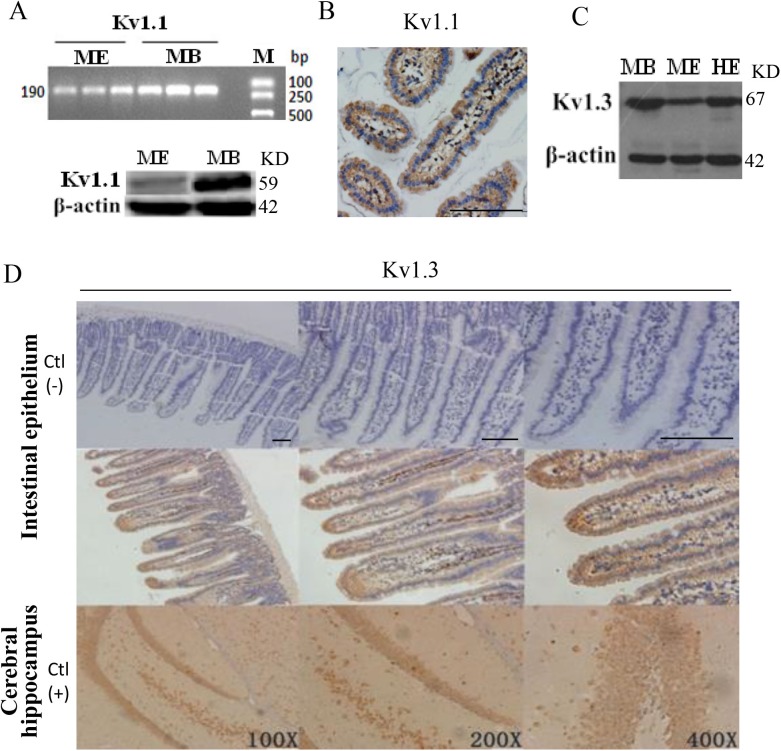
Expression and location of Kv1.1 and Kv1.3 channels in jejunal epithelium
Although both Kv1.1 and Kv1.3 channels have been identified in the brain and other tissues, their expression and localization are not well characterized in the jejunal intestinal epithelium. We used PCR analysis to detect mRNA expression of Kv1.1 transcripts in mouse jejunal epithelium (ME), while mouse brain (MB) served as a positive control (Fig. 5A). Western blot analysis was performed and detected the protein expression of Kv1.1 in both ME and MB by anti-KV1.1 antibody (Fig. 5A). Immunohistochemistry analysis further identified the expression and location of Kv1.1 channels in epithelial cells (Fig. 5B). Similarly, Kv1.3 protein expression was also detected by western blot analysis in MB, ME and human jejunal epithelium (HE) (Fig. 5C), and immunohistochemistry analysis identified the expression and location of Kv1.3 channels in mouse epithelial cells and mouse cerebral hippocampal neurons (Fig. 5D). No staining was observed on mouse epithelial cells without primary anti-Kv1.1 and anti-Kv1.3 antibodies (Fig. 5D), suggesting the observed results are specific to KV1.1 and KV1.3 channels.
Fig. 5.
Expression and location of Kv1.1 and Kv1.3 in jejunal epithelium. (A) Kv1.1 expression of mRNA (upper) and protein (lower) in mouse jejunal epithelium (ME) and mouse brain (MB) as a positive control. M, marker; KD, molecular weight. (B) Expression and localization of Kv1.1 protein in epithelial cells of mouse jejuna. (C) Protein expression of Kv1.3 in MB as a positive control, in ME and human jejunal epithelium (HE). (D) Expression and localization of Kv1.3 protein in epithelial cells of mouse jejunum and mouse cerebral hippocampus. Upper panels: immunohistochemistry staining of mouse jejunal epithelium without primary antibody as a negative control. Middle panels: immunohistochemistry staining of the epithelial cells in mouse jejunal epithelium with primary anti-Kv1.3 antibody. Lower panels: immunohistochemistry staining of the hippocampal neurons in mouse brain with primary anti-Kv1.3 antibody as a positive control. These are representative of three independent experiments with similar results. Scale bars: 200 μm, 100 μm, 50 μm.
Roles of Kv channel subtypes in the regulation of body weight and blood glucose in mice
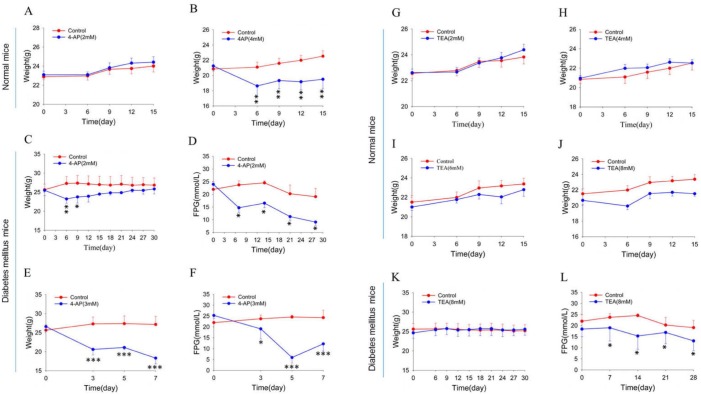
Since our in vitro Ussing chamber experiments showed that Kv channels are important in the regulation of intestinal glucose absorption, we further examined their roles in vivo. First, body weight in wild-type mice were measured and compared between the control group given water only and the experimental groups given water containing 4-AP (2 and 4 mM) for 2 weeks. 4-AP at 4 mM significantly decreased body weight of wild-type mice by 20%, but no difference was observed at 2 mM (Fig. 6A,B). Second, in a mouse model of diabetes mellitus, 4-AP caused weight loss (Fig. 6C,E) and reduced blood glucose (Fig. 6D,F) in a dose-dependent manner. Interestingly, at 2–8 mM TEA did not significantly alter body weight in wild-type mice (Fig. 6G–J), but in diabetic mice, 8 mM TEA lowered blood glucose (Fig. 6L) without any effect on body weight (Fig. 6K). Taken together, our data suggest that Kv channels, especially the Kv1.1 subtype, may be involved in global control of body weight and blood glucose through regulating intestinal glucose absorption.
Fig. 6.
Effects of 4-AP and TEA on time courses of body weight in normal mice and measurement of body weight and blood glucose in diabetic mice. (A,B) Effect of 4-AP (2 and 4 mM) on body weight in normal mice. (C,D) Effect of 4-AP (2 mM) on body weight and fasting plasma glucose (FPG) in diabetic mice. (E,F) Effect of 4-AP (3 mM) on body weight and FPG in diabetic mice. (G–J) Effect of TEA (2–8 mM) on body weight in normal mice. (K,L) Effect of TEA (8 mM) on body weight and FPG in diabetic mice. Mice were given drinking water only (control) or drinking water containing different concentrations of 4-AP or TEA. Values are means±s.e.m.; Student's t-test, *P<0.05, **P<0.01, ***P<0.001, n=6–7 mice in each series.
DISCUSSION
Although the expression of K+ channel subtypes has been identified in GI epithelium, their physiologic function in the small intestine is poorly understood. In the present study, using a combination of pharmacologic blockers and genetic knockout mice for K+ channel subtypes we reveal that: (1) KCa3.1 channels may specifically regulate Ca2+-mediated intestinal anion secretion, but not glucose absorption; (2) Kv1.1 and Kv1.3 channels may play important roles in regulating intestinal glucose absorption; (3) Kv channel subtypes may regulate body weight and blood glucose through modulation of glucose absorption in both normal and diabetic mice. Therefore, our results suggest that various subtypes of K+ channels play different roles in the regulation of small intestinal ion and glucose transport.
It is generally accepted that K+ channels are important in stabilizing the membrane potential and mediating the driving force for electrogenic ion transport in colonic epithelium (Heitzmann and Warth, 2008). However, their expression and function in small intestinal epithelium are not well understood, and an even greater mystery is the involvement of different K+ channel subtypes in regulating ion and glucose transport. In a previous study (Cheng, 2012), we identified the expression of KCa3.1 (KCNN4) in murine duodenal mucosae and characterized their role in regulating duodenal epithelial anion secretion. Although Matos et al. also confirmed the important role of KCa3.1 in mouse colonic Cl− secretion (Matos et al., 2007), the detailed mechanisms remain unclear for how KCa3.1 channels regulate intestinal epithelial anion secretion, and almost nothing is known about KCa3.1-mediated duodenal HCO3− secretion. Therefore, we aimed to elucidate the underlying cellular mechanisms. It has been reported that KCa3.1 activation induces membrane hyperpolarization (when Em becomes more negative inside of the plasma membrane) that likely provides the driving force for anion efflux out of colonic epithelial cells (Matos et al., 2007). Indeed, we measured HCO3− flux through IECs and demonstrated for the first time that high K+ induced membrane depolarization (when Em becomes less negative inside of the plasma membrane) and increased the driving force for HCO3− influx into IECs. Similarly, clotrimazole increased the driving force for HCO3− influx into IECs likely via blockade of KCa3.1 to induce membrane depolarization. Consistent with this, clotrimazole selectively inhibited Ca2+-mediated duodenal HCO3− secretion in vitro. In the present study, we not only provided new evidence to support the notion that KCa3.1 channels hold a critical role in regulating duodenal epithelial anion secretion, but also elucidated the underlying mechanism that Ca2+ activation of KCa3.1 channels generates the driving force for HCO3− secretion (Fig. 7A). Our findings agree with those from Flores's report that small intestine obtained from KCNN4 null mouse completely lack Cl− secretion in response to Ca2+ mobilizing agonists, indicating the crucial role of KCa3.1 in Ca2+-mediated epithelial ion transport (Flores et al., 2007). Thus, KCa3.1 may represent a novel pharmacological target that could be exploited to manipulate small intestinal anion secretion in general and to specifically augment HCO3− secretion to protect duodenal mucosa against acid-induced injury.
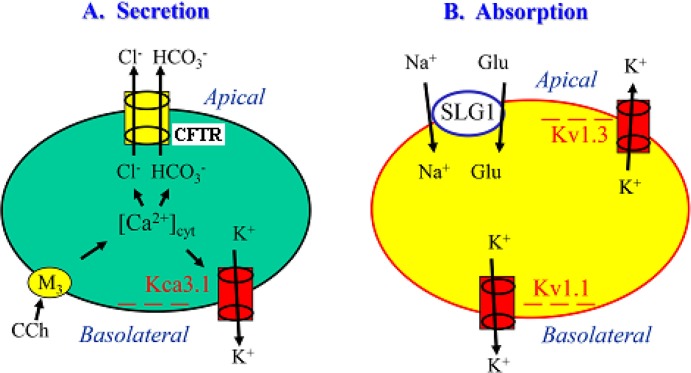
Fig. 7.
Schematic diagram showing the different possible roles of various Kv channel subtypes in the regulation of ion and glucose transports through IECs. (A) CCh activation of M3 receptors elicits [Ca2+]cyt signaling in IECs, which activates the KCa3.1 subtype on the basolateral side of IECs, leading to membrane hyperpolarization to provide a driving force for transepithelial Cl− and HCO3− flux through CFTR channels. (B) Glucose is absorbed from the intestinal lumen into IECs through SLGT1. This process is likely regulated by Kv1.3 channels functionally expressed on the apical side and by Kv1.1 channels functionally expressed on the basolateral side. Blockade of these Kv subtypes cause membrane depolarization, which would inhibit anion secretion and Na+-dependent glucose absorption by reducing the driving force for these electrogenic ion transports in IECs. − − −, membrane hyperpolarization.
We also tested the possible involvement of other K+ channel subtypes, such as Kv7.1 (KCNQ1), since their roles in the regulation of intestinal ion transports have been controversial. Liao et al. previously reported that Kv7.1 channels were not essential for activating colonic epithelial Cl− secretion (Buresi et al., 2002), but Matos et al. found that Kv7.1 channels drove colonic Cl− secretion (Matos et al., 2007). However, only pharmacological blockers of Kv7.1 were used in those studies. By combining selective pharmacological blockers and KV7.1 knockout mice, we not only excluded the involvement of Kv7.1 in small intestinal anion secretion, but further confirmed the importance of KCa3.1. Our results obtained in KV7.1 knockout mice differ from Vallon et al., who observed a decrease in forskolin-induced jejunal Cl− secretion in KV7.1 knockout mice (Vallon et al., 2005). However, we consistently found no difference in forskolin-, 1-EBIO- and CCh-induced duodenal anion secretion using pharmacological blockers in both wild-type and knockout mice. The discrepancy between Vallon et al. and our study is likely due to the segment differences (duodenum versus jejunum), which requires further investigation.
Glucose is mainly imported into IECs through the SGLT1 located on the apical membrane in the small intestine (Wright et al., 1997; Stevens, 1992). In our Ussing chamber study, the glucose-induced intestinal Isc occurred specifically in the presence of both Na+ and glucose on the mucosal side of the small intestine, which was abolished by selective inhibition of SGLT1. This co-transporter is driven by the transmembrane Na+ gradient and the electrical potential difference (Em). Since the negative Em inside IECs is a major driving force for movement of Na+ into the cell, membrane depolarization due to blockade of K+ channels may reduce the driving force for Na+, and subsequently inhibit Na+-dependent glucose absorption in IECs. K+ channels are presumably involved by repolarizing the cell membrane, which is critical in stabilizing the driving force for electrogenic Na+-coupled glucose transport. Indeed, we found that high K+ markedly inhibited glucose-induced intestinal Isc, indicating the negative Em generated by K+ channels is essential for Na+-coupled glucose absorption. Interestingly, we show that although KCa3.1 plays an essential role in Ca2+-mediated intestinal anion secretion, it is not involved in the regulation of intestinal glucose absorption. Consistent with this, we did not detect any glucose-induced changes in cytosolic Ca2+ concentrations in IECs (data not shown), excluding the role of these Ca2+-activated KCa channels in glucose absorption.
Although IECs express multiple Kv channel subtypes, such as Kv1.1, Kv1.3 and Kv7.1, their function in intestinal glucose transport are unclear except for the Kv7.1 subtype, which was previously reported to contribute to electrogenic Na+-coupled glucose transport in the jejunum (Dong et al., 2006). In the present study, we investigated the role of other Kv channel subtypes and identified the expression and localization of Kv1.1 and Kv1.3 channels in IECs of the small intestine in humans and mice. We demonstrated for the first time that both Kv1.1 and Kv1.3 channels were functionally expressed on the serosal and mucosal side, respectively, and that they are involved in the modulation of intestinal glucose absorption (Fig. 7B). Although the concentrations of K+ channel subtype blockers in the present study were higher than their EC50 values usually measured from cultured single cells, it is worth noting that we used primary intestinal tissues and whole-animal models. Under these more physiological conditions, it is much more complicated than cultured single cells, with varying factors such as higher cellular density and irregular drug access to the channels, etc. In view of this, the final verdict on Kv1.1 and Kv1.3 channels in regulating intestinal glucose absorption would require confirmation pending availability of their genetic knockout mice.
Kv1.1 and Kv1.3 subtypes are functionally expressed not only in IECs but also in smooth muscle cells of mesenteric arteries. The membrane depolarization in IECs induced by Kv channel blockers decreases the driving force that is required for Na+-driven glucose absorption in the small intestine. Moreover, membrane depolarization in smooth muscle cells would cause vasoconstriction of mesenteric arteries and limit the distribution of absorbed nutrients to other tissues for storage (McDaniel et al., 2001). Restriction of both nutrient absorption and distribution would reduce glucose intake and subsequent weight gain. Indeed, we found that 4-AP significantly attenuated the weight gain of wild-type and diabetic mice, and both 4-AP and TEA significantly lowered the blood glucose of diabetic mice. Therefore, Kv1.1 and Kv1.3 channels may play important roles in controlling glucose intake and weight gain. This study provides a new concept for developing specific blockers for K+ channel subtypes in the digestive system as novel therapeutic agents to reduce weight gain and to improve blood glucose control in diabetes mellitus.
MATERIALS AND METHODS
Ussing chamber experiments in vitro
This study was approved by the Committee on Investigations Involving Animal Subjects, Army Medical University. Experiments were performed with male 8–10-week-old male C57BL/6J mice and KV7.1 knockout mice (Kv7.1−/−), generated as previously described (Casimiro et al., 2001). Littermates (Kv7.1+/+) were used as wild-type controls.
Mice were anesthetized by i.p. injection of Hypnorm/Midazolam cocktail (25% Hypnorm plus 25% Midazolam) at a dose of 10 mg/kg. The duodenum and proximal jejunum were removed from C57BL/6J, Kv7.1−/−, or Kv7.1+/+ mice and immediately placed in ice-cold iso-osmolar mannitol with 10 μM indomethacin. The duodenal tissue from each animal was stripped of seromuscular layers, divided and mounted in three chambers (window area, 0.1 cm2). Experiments were performed under continuous short-circuited conditions (Voltage-Current Clamp, VCC 600; Physiologic Instruments, San Diego, USA). Luminal pH was maintained at 7.40 by continuous infusion of 5 mM HCl under automatic control of a pH-stat system (ETS 822; Radiometer America, Westlake, USA). The volume of HCl titrated was recorded in real time, then quantified as the steady-state rate of H+ equivalents required per hour for neutralization. The rate of HCO3− secretion was calculated and normalized to tissue surface area (µmol cm−2 h−1). Measurements were taken at 5-min intervals and averaged for consecutive 5- or 10-min periods. The short-circuit current (Isc) was measured in microamperes and converted into µEq cm−2 h−1 (Dong et al., 2005). Basal parameters were initially recorded for the first 30 min, then with the addition of inhibitors for another 30 min, as dictated by the experimental design. Drugs were then added to the serosal side, the mucosal side or both, and electrophysiological parameters and bicarbonate secretion were measured for 60 min. As shown in our previous publications, in control experiments, addition of 10 µl vehicle (DMSO or distilled water) to both sides of the duodenal tissue in 3 ml chambers did not alter Isc or HCO3− secretion, which were sustained during the 90 min experimental period.
The mucosal solution used in Ussing chamber experiments contained (in mM): 140 Na+, 5.4 K+, 1.2 Ca2+, 1.2 Mg2+, 120 Cl−, 25 gluconate and 10 mannitol. The serosal solution contained (in mM): 140 Na+, 5.4 K+, 1.2 Ca2+, 1.2 Mg2+, 120 Cl−, 25 HCO3−, 1.4 HPO, 2.4 H2PO4, 10 glucose and 0.01 indomethacin. The osmolality for both solutions were ∼285 mOsmol/kg H2O. In Na+-free solutions, Na+ was replaced with Li+.
Epithelial cell culture
SCBN is a non-transformed duodenal epithelial crypt cell line of canine origin. As described previously (Pang et al., 1996; Buresi et al., 2001), cells in flasks were fed with fresh DMEM supplemented with 10% fetal bovine serum, l-glutamine, and streptomycin every 2–3 days (Buret and Lin, 2008). Cells of passages 23–33 were grown to confluence (∼5 days) in 75-cm2 flasks (Corning, USA), then replated onto 12-mm round coverslips (Warner Instruments Inc., Hamden, USA) and incubated for at least 24 h before use in pHi measurements.
Measurement of HCO3− fluxes in SCBN cells
SCBN cells were used for pHi measurements as previously described (Negulescu and Machen, 1990). Briefly, cells plated on coverslips were incubated with 2 μM 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), AM in physiological salt solution, for 30 min at room temperature then washed for 30 min. The ratio of BCECF fluorescence with excitation at 495 and 440 nm (F495/440) was captured using an intensified charge-coupled device camera and a MetaFluor imaging system. The NaCl/HCO3− solutions contained (in mM): 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 1 MgSO4, 1 CaCl2 and 10 glucose, equilibrated with 5% CO2/95% O2 (pH 7.4). In Na+-free (Na+-free/HCO3−) solutions, Na+ was replaced with N-methyl-d-glucamine. The solutions were osmotically balanced with LiCl to ∼285 mOsmol/kg H2O and pH was adjusted to 7.4 with HCl.
RT-PCR analysis
A RT-PCR analysis of mouse duodenal mucosae and brain was applied as previously described (Cheng, 2012). Briefly, total RNA from C57BL/6J mice duodenal mucosae and brain were isolated with TRIzol reagent (Invitrogen, Carlsbad, USA). Total RNA was converted into cDNA with reverse transcriptase (Takara, Japan). Primers were synthesized by Invitrogen. Each cDNA sample was prepared using AMV RT and random primers (Takara, Japan). Mice Kv1.1-specific sense and antisense primers (GenBank accession no. NM_010595.3) were 5′-AAGCTCTTACCCCTGCACTG-3′ and 5′-AACGGGTCTTAGCATTGGGG-3′. Mouse GAPDH sense and antisense primers as described by Sharkey, K. A. et al. were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ (Vallon et al., 2005). The samples were amplified in an automated thermal cycler (GeneAmp 2400, Applied Biosystems). The products were electrophoresed on a 1.5% agarose gel TAE buffer, stained with ethidium bromide (0.5 µg ml−1), then photographed under UV light.
Western blot analysis
A western blot analysis of mouse duodenal mucosae and intestinal epithelial cells was conducted as previously described (Cheng, 2012). PVDF membranes (Millipore, Billerica, USA) with resolved proteins (50 µg) were incubated at 4°C overnight with anti-Kv1.1 (1:1000, sc-11184, Santa Cruz Biotechnology), anti-Kv1.3 (1:500, sc-398855, Santa Cruz Biotechnology), or anti-GAPDH antibodies (1:5000, Ambion, Austin, USA). After washing with PBS plus 1% Tween (PBST), the secondary antibody (rabbit anti-goat or anti-mouse, 1:1000, both from ZSGB-BIO, China) was applied to the membranes for 1 h at room temperature. Membranes were then treated with a chemiluminescent solution (Fivephoton Biochemicals, San Diego, USA) and captured on X-ray film. Densitometric analysis of the blots was performed using an AlphaImager digital imaging system (Alpha Innotech, San Leandro, USA).
Immunohistochemistry
Immunohistochemistry was carried out as previously described (Liao et al., 2005). Briefly, dewaxed and rehydrated slides with small intestinal tissue from male C57BL/6J mice were blocked in goat serum for 1 h at room temperate, then incubated with anti-Kv1.1 (1:200) and anti-Kv1.3 (1:200) antibodies at 4°C overnight. The primary antibodies were detected with biotinylated rabbit anti-goat or goat anti-mouse IgG (1:5000, Vector Laboratories, Burlingame, USA) secondary antibodies for 1 h at room temperature. Immunoreactivity was detected using a horseradish peroxidase (3′-,3′-diaminobenzidine) kit (BioGenex, San Francisco, USA) followed by counterstaining with hematoxylin, dehydration and mounting.
Determination of mouse body weight and streptozotocin-induced mouse model of diabetics
Male 8–10-week-old C57BL/6J mice were housed in an environmentally controlled facility with 12-h light/12-h dark cycles, and body weight was measured by scale every 3 days in the morning. Male C57BL/6J mice were fed with high-fat and high-glucose diets containing 18% lard oil, 20% sugar and 3% yolk. After 8 weeks, they were injected intraperitoneally with 35 mg/kg/day streptozotocin (STZ) for 7 consecutive days. Age-matched control mice received an equal volume of vehicle. After the seventh STZ injection, blood glucose levels were measured, and mice with blood glucose levels over 11.1 mmol/l were used for experiments.
Chemicals
Carbachol (CCh, AchR activator), forskolin (Adenylate Cyclase activator), clotrimazole (KCa3.1 blocker), D-glucose and D-mannitol, TEA (Kv1.1 inhibitor), 4-AP (Kv blocker), PAP-1 (Kv1.3 inhibitor) and indomethacin were purchased from Sigma Chemical. 1-ethyl-2-benzimidazolinone (1-EBIO, Adenylate Cyclase activator) was from Tocris (Ellisville, USA). BCECF, AM was from Invitrogen.
Statistical analysis
Results are expressed as mean±s.e.m. (standard error of the mean). Differences between means were considered to be statistically significant if P<0.05, using Student's t-test for paired or unpaired values, or analysis of variance, as appropriate.
Acknowledgements
We would like to thank the staff of the Department of Gastroenterology of Xinqiao Hospital for their assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.D.; Methodology: C.D.; Software: S.C.; Validation: H.W.; Investigation: L.C.; Resources: L.L.; Data curation: H.G., B.T.; Writing - original draft: H.D.; Visualization: B.T.; Supervision: H.D.; Project administration: H.D.; Funding acquisition: H.D.
Funding
These studies were supported by research grants from the National Key Research and Development Program of China (No. 2016YFC1302200 to H.D.) and the National Natural Science Foundation of China (No. 81570477 and 31371167 to H.D.).
References
- Allen A. and Flemström G. (2005). Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 288, C1-C19. 10.1152/ajpcell.00102.2004 [DOI] [PubMed] [Google Scholar]
- Allen A., Flemstrom G., Garner A. and Kivilaakso E. (1993). Gastroduodenal mucosal protection. Physiol. Rev. 73, 823-857. 10.1152/physrev.1993.73.4.823 [DOI] [PubMed] [Google Scholar]
- Barrett K. E. (1997). Integrated regulation of intestinal epithelial transport: intercellular and intracellular pathways. Am. J. Physiol. 272, C1069-C1076. 10.1152/ajpcell.1997.272.4.C1069 [DOI] [PubMed] [Google Scholar]
- Bedine M. S. (2000). Textbook of gastroenterology. Gastroenterology 118, 984-985. 10.1016/S0016-5085(00)70191-0 [DOI] [PubMed] [Google Scholar]
- Berk P. D. and Stump D. D. (1999). Mechanisms of cellular uptake of long chain free fatty acids. Mol. Cell. Biochem. 192, 17-31. 10.1023/A:1006832001033 [DOI] [PubMed] [Google Scholar]
- Berk P. D., Zhou S.-L., Kiang C.-L., Stump D., Bradbury M. and Isola L. M. (1997). Uptake of long chain free fatty acids is selectively up-regulated in adipocytes of Zucker rats with genetic obesity and non-insulin-dependent diabetes mellitus. J. Biol. Chem. 272, 8830-8835. 10.1074/jbc.272.13.8830 [DOI] [PubMed] [Google Scholar]
- Buresi M. C., Schleihauf E., Vergnolle N., Buret A., Wallace J. L., Hollenberg M. D. and Macnaughton W. K. (2001). Protease-activated receptor-1 stimulates Ca(2+)-dependent Cl(−) secretion in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G323-G332. 10.1152/ajpgi.2001.281.2.G323 [DOI] [PubMed] [Google Scholar]
- Buresi M. C., Buret A. G., Hollenberg M. D. and MacNaughton W. K. (2002). Activation of proteinase-activated receptor 1 stimulates epithelial chloride secretion through a unique MAP kinase- and cyclo-oxygenase-dependent pathway. FASEB J. 16, 1515-1525. 10.1096/fj.02-0039com [DOI] [PubMed] [Google Scholar]
- Buresi M. C., Vergnolle N., Sharkey K. A., Keenan C. M., Andrade-Gordon P., Cirino G., Cirillo D., Hollenberg M. D. and Macnaughton W. K. (2005). Activation of proteinase-activated receptor-1 inhibits neurally evoked chloride secretion in the mouse colon in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G337-G345. 10.1152/ajpgi.00112.2004 [DOI] [PubMed] [Google Scholar]
- Buret A. and Lin Y.-C. (2008). Genotypic characterization of an epithelial cell line for the study of parasite-epithelial interactions. J. Parasitol. 94, 545-548. 10.1645/GE-1395.1 [DOI] [PubMed] [Google Scholar]
- Casimiro M. C., Knollmann B. C., Ebert S. N., Vary J. C., Greene A. E., Franz M. R., Grinberg A., Huang S. P. and Pfeifer K. (2001). Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc. Natl. Acad. Sci. USA 98, 2526-2531. 10.1073/pnas.041398998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Wickenden A. D. and Zou A. (2003). Electrophysiological analysis of heterologously expressed Kv and SK/IK potassium channels. Curr Protoc Pharmacol 20, 11.5.1-11.5.27. 10.1002/0471141755.ph1105s20 [DOI] [PubMed] [Google Scholar]
- Cheng S. X. (2012). Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G60-G70. 10.1152/ajpgi.00425.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Sellers Z. M., Smith A., Chow J. Y. C. and Barrett K. E. (2005). Na(+)/Ca(2+) exchange regulates Ca(2+)-dependent duodenal mucosal ion transport and HCO(3)(−) secretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G457-G465. 10.1152/ajpgi.00381.2004 [DOI] [PubMed] [Google Scholar]
- Dong H., Smith A., Hovaida M. and Chow J. Y. (2006). Role of Ca2+-activated K+ channels in duodenal mucosal ion transport and bicarbonate secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G1120-G1128. 10.1152/ajpgi.00566.2005 [DOI] [PubMed] [Google Scholar]
- Flemström G. and Isenberg J. I. (2001). Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol. Sci. 16, 23-28. 10.1152/physiologyonline.2001.16.1.23 [DOI] [PubMed] [Google Scholar]
- Flores C. A., Melvin J. E., Figueroa C. D. and Sepúlveda F. V. (2007). Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+channel Kcnn4. J. Physiol. 583, 705-717. 10.1113/jphysiol.2007.134387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorboulev V., Schurmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R. et al. (2012). Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187-196. 10.2337/db11-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D. and Warth R. (2008). Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol. Rev. 88, 1119-1182. 10.1152/physrev.00020.2007 [DOI] [PubMed] [Google Scholar]
- Isenberg J. I., Selling J. A., Hogan D. L. and Koss M. A. (1987). Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N. Engl. J. Med. 316, 374-379. 10.1056/NEJM198702123160704 [DOI] [PubMed] [Google Scholar]
- Liao T., Wang L., Halm S. T., Lu L., Fyffe R. E. W. and Halm D. R. (2005). K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am. J. Physiol. Cell Physiol. 289, C564-C575. 10.1152/ajpcell.00561.2004 [DOI] [PubMed] [Google Scholar]
- Matos J. E., Sausbier M., Beranek G., Sausbier U., Ruth P. and Leipziger J. (2007). Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl− secretion. Acta Physiol. (Oxf.) 189, 251-258. 10.1111/j.1748-1716.2006.01646.x [DOI] [PubMed] [Google Scholar]
- Mcdaniel S. S., Platoshyn O., Yu Y., Sweeney M., Miriel V. A., Golovina V. A., Krick S., Lapp B. R., Wang J.-Y. and Yuan J. X.-J. (2001). Anorexic effect of K channel blockade in mesenteric arterial smooth muscle and intestinal epithelial cells. J. Appl. Physiol. 91, 2322-2333. 10.1152/jappl.2001.91.5.2322 [DOI] [PubMed] [Google Scholar]
- Negulescu P. A. and Machen T. E. (1990). Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 192, 38-81. 10.1016/0076-6879(90)92062-I [DOI] [PubMed] [Google Scholar]
- Pang G., Buret A., O'loughlin E., Smith A., Batey R. and Clancy R. (1996). Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology 111, 8-18. 10.1053/gast.1996.v111.pm8698229 [DOI] [PubMed] [Google Scholar]
- Stevens B. R. (1992). Vertebrate intestine apical membrane mechanisms of organic nutrient transport. Am. J. Physiol. 263, R458-R463. 10.1152/ajpregu.1992.263.3.R458 [DOI] [PubMed] [Google Scholar]
- Stremmel W. (1988). Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J. Clin. Invest. 82, 2001-2010. 10.1172/JCI113820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo B., Wen G. and Seidler U. (2009). Differential activation of the HCO3− conductance through the cystic fibrosis transmembrane conductance regulator anion channel by genistein and forskolin in murine duodenum. Br. J. Pharmacol. 158, 1313-1321. 10.1111/j.1476-5381.2009.00398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V., Grahammer F., Volkl H., Sandu C. D., Richter K., Rexhepaj R., Gerlach U., Rong Q., Pfeifer K. and Lang F. (2005). KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc. Natl. Acad. Sci. USA 102, 17864-17869. 10.1073/pnas.0505860102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth R. (2003). Potassium channels in epithelial transport. Pflugers Arch. 446, 505-513. 10.1007/s00424-003-1075-2 [DOI] [PubMed] [Google Scholar]
- Wright E. M., Hirsch J. R., Loo D. D. and Zampighi G. A. (1997). Regulation of Na+/glucose cotransporters. J. Exp. Biol. 200, 287-293. [DOI] [PubMed] [Google Scholar]