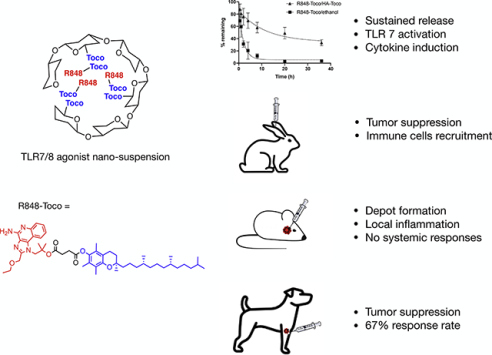
Abstract
The toll-like receptor 7 and 8 (TLR7/8) agonist Resiquimod (R848) has been recognized as a promising immunostimulator for the treatment of cutaneous cancers in multiple clinical trials. However, systemic administration of R848 often results in strong immune-related toxicities while having limited therapeutic effects to the tumor. In the present study, a prodrug-based nanocarrier delivery system was developed that exhibited high therapeutic efficiency. R848 was conjugated to α-tocopherol to constitute an R848-Toco prodrug, followed by formulating with a tocopherol-modified hyaluronic acid (HA-Toco) as a polymeric nano-suspension. In vitro evaluation showed that the delivery system prolonged the release kinetics while maintaining TLR agonist activities. When administered subcutaneously, the nano-suspension formed a depot at the injection site, inducing localized immune responses without systemic expansion. This formulation also suppressed tumor growth and recruited immune cells to the tumor in a murine model of head and neck cancer. In a pilot canine clinical trial of spontaneous mast cell tumors, the treatment led to a 67% response rate (3 partial remissions and 1 complete remission).
Keywords: Toll-like receptor agonists, nanotherapeutic formulation, depot effect, sustained delivery, cancer immunotherapy
Graphical Abstract
1. Introduction
The innate immune system recognizes conserved features of viral and microbial pathogens through pattern-recognition receptors (PRRs) [1]. Activation of these receptors leads to complex signaling cascades culminating in an immune response, which can involve components of both innate and adaptive immune systems. Toll-like receptors (TLRs) are a family of PRRs capable of inducing the secretion of pro-inflammatory cytokines that promote innate immune responses. To date, ten functional TLRs have been identified in humans [2]. TLR7 and TLR8 are endosomal transmembrane receptors that recognize viral single-strand RNA and bacterial DNA. The activation of TLR7 and TLR8 induces the MyD88 immune signaling pathway and secretion of transcriptional activator NF-κB [3]. NF-κB activates immune cells including dendritic cells (DCs), monocytes and macrophages, leading to the secretion of pro-inflammatory cytokines including IFN-γ, TNF-α, IL-6, −8 and −12 [4]. The production of these cytokines enhances T helper-1 (Th-1) biased immune responses and can lead to the expansion of antigen-specific cytotoxic T-cells, both of which are important to tumorspecific T cell responses and inhibition of tumor growth [5].
Imidazoquinolines (IMQs) are a group of synthetic small molecules that can activate TLR7, TLR8, or both. The TLR7 agonist imiquimod has been licensed as a topical treatment (Aldara™) of genital warts and superficial basal cell carcinoma for over 20 years [6]. The TLR7/8 dual agonist resiquimod (R848), a more potent agonist than imiquimod, has been in clinical trials for over a decade for similar antiviral and anti-tumoral indications. R848 recently received orphan designation in Europe for cutaneous T-cell lymphoma [7]. Despite the promising activity IMQs have shown as topical anticancer immunotherapeutics, systemic administration of these compounds has not been approved in any country. R848 have a low molecular weight (314 Da), and upon subcutaneous or intratumoral injection, it distributes systemically into the circulation and induces whole body inflammation, causing severe immune-related toxicities including lymphopenia, anemia, and flu-like symptoms [8,9]. Only a small portion of the dose can be delivered to the desired treatment site after circulation, resulting in limited therapeutic effects to solid tumors [10].
Consequently, methods to localize the delivery of R848 would effectively reduce the undesirable adverse effects. Various nanotherapeutic formulations have shown promise in preclinical testing, such as the conjugation with antigens or peptides or the encapsulation into lipid-based formulations [11–13]. Natural copolymers such as hyaluronic acid (HA) are commonly applied for the delivery of tumor-targeting compounds. HA is a natural endogenous mucopolysaccharide and is the major component of extracellular matrices. HA-based polymers are highly water soluble, biocompatible, biodegradable, and have specific interaction with CD44 receptors expressed on tumor cells, resulting in enhanced targeting ability of the delivery system [14]. HA can be either the polymeric vehicle to load the drug or be directly conjugated to many small molecule drugs through the carboxylic handles, after partial oxidation, or via a linker. Although some success have been proved for both methods, the application of HA-conjugated drugs can be restricted by harsh synthetic conditions that degrade HA, low drug loading, and the loss of immune-activation efficacy of the drug or the receptor-mediated drug targeting of HA [15,16]. Using slightly modified HA as a nanocarrier is a more versatile approach of achieving high drug loading without compromising the characteristics of both HA and the drug. In this study, a novel delivery system of R848 has been prepared. R848 was covalently linked to α-tocopherol (vitamin E) to form an R848-α-tocopherol conjugate (R848-Toco), producing a larger lipophilic molecule that can release free R848 molecules in aqueous conditions. To overcome the solubility limitation of this lipophilic prodrug, a modified HA polymer was applied as the nanocarrier to obtain an aqueous-based formulation. This nano-sized suspension was evaluated in vitro and in vivo for its controlled and localized delivery and immunotherapeutic efficiency.
2. Materials and methods
2.1. Materials
Resiquimod (R848), HEK-Blue cell lines and QUANTI-blue™ were purchased from Invivogen (San Diego, CA, USA). Hyaluronic acid (32 kDa) was purchased from Contipro C (Dolni Dobrouc, Czech Republic). Reagents, including (+)-α-tocopherol, (+)-α-tocopherol succinate, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), triethylamine (TEA), N,N’-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP), N-(tert-butoxycarbonyl)glycine (Boc-Gly-OH), trifluoroacetic acid (TFA), tetrabutylammonium hydroxide (TBA-OH), 1-hydroxybenzotriazole hydrate (HOBt·H2O), anhydrous dichloromethane (CH2Cl2), dimethylformamide (DMF), silica gel 60 and thin-layer chromatography plates were purchased from Sigma Aldrich (St. Louis, MO, USA). Dialysis devices were purchased from Repligen (Boston, MA, USA). Solvents for column chromatography, high-performance liquid chromatography (HPLC) grade solvents, sterile water for injection (WFI) and cell culture supplies were purchased from Fisher Scientific (Hampton, NH, USA). Zorbax C8 columns were purchased from Agilent (Santa Clara, CA, USA). Alexa Fluor® antibodies were purchased from BioLegend (San Diego, CA, USA).
2.2. Animal studies
Animal studies were performed under the supervision of the University of Kansas Animal Care Unit and Institutional Animal Care and Use Committee. Rabbit studies were conducted by HylaPharm LLC, and histopathology tissue examinations were performed by Kanas State Veterinary Hospital. Canine biological samples were donated by local veterinary practices or collected under an approved protocol. The pilot canine study was conducted in a multi-site investigational trial at regional veterinary hospitals with owner informed consent.
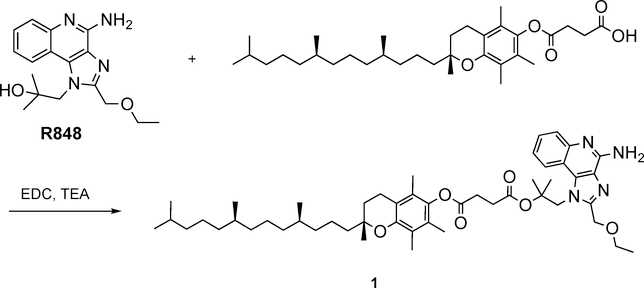
2.3. Preparation and characterization of the R848-Toco conjugate (1)
R848 (100 mg, 0.32 mmol) was conjugated to (+)-α-tocopherol succinate (253 mg, 0.47 mmol) in the presence of EDC·HCl (75 mg, 0.48 mmol) and TEA (0.2 mL) in CH2Cl2 (10 mL) under a nitrogen atmosphere and at ambient temperature (ca. 20 °C) for 15 h. The mixture was then purified by silica gel chromatography using hexane - ethyl acetate as eluent solvents (product eluted at 70% ethyl acetate) and dried in vacuo to provide a clear pale-yellow viscous oil product 1 (177 mg, 67%). Purity was 99.4% by HPLC analysis (LC-2010C HT, Shimadzu, Kyoto, Japan) with a UV detector (254 nm) and a C8 column (4.6 μm × 50 mm, 5 μm), thermostatic at 45 °C. Mobile phases were A: water/0.1% TFA and B: acetonitrile/0.1% TFA with a gradient elution (20–95% B) over 8 min at a flow rate of 1.0 mL/min. Lipophilicity (cLogP) has been calculated using ChemDraw Ultra 18.1 software (Cambridge, MA, USA). 1H NMR (400 MHz, Chloroform-d) δ 8.20 (d, J = 8.3 Hz, 1H), 8.00 (dd, J = 8.4, 1.4 Hz, 1H), 7.54 (dddd, J = 39.6, 8.3, 7.0, 1.3 Hz, 2H), 4.89 (s, 2H), 4.77 (s, 2H), 3.62 (q, J = 7.0 Hz, 4H), 3.11 (t, J = 6.9 Hz, 2H), 2.58 (t, J = 6.8 Hz, 2H), 2.07 (d, J = 7.8 Hz, 6H), 2.02 (s, 3H), 1.77 (ddq, J = 20.0, 13.2, 6.8 Hz, 2H), 0.94 – 0.73 (m, 13H). Chemical formula: C50H74N4O6. ESI-MS: M+H+ = 827.5608, found 827.5660.
2.4. Preparation and characterization of HA-Toco
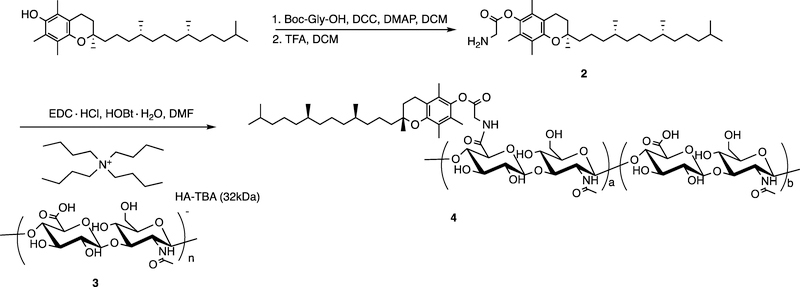
2.4.1. Preparation of Glycine-α-Tocopherol (2)
A mixture of Boc-Gly-OH (0.89 g, 5.11 mmol), (+)-α-tocopherol (2.20 g, 5.11 mmol), DCC (1.05 g, 5.11 mmol), and DMAP (64 mg, 0.52 mmol) in CH2Cl2 (20 mL) was stirred at ca. 20 °C overnight. The reaction mixture was then cooled down to −20 °C and filtered to remove unwanted precipitate. Filtrate was then purified by silica gel chromatography using hexane-ethyl acetate as eluent solvents (product eluted at 5% ethyl acetate) and dried in vacuo to provide colorless oil (2.89 g, 96%). The oil was dissolved in CH2Cl2 (15 mL), and a TFA solution in CH2Cl2 (50% v/v, 5 mL) was added dropwise. The mixture was stirred at 0 °C for 0.5 h and then warmed to room temperature (ca. 20 °C) and stirred for an additional 1 h, followed by evaporation in vacuo to remove the solvent and TFA. The gray solid was purified via silica gel chromatography using hexane-ethyl acetate as eluent solvents (product eluted at 5% ethyl acetate) and dried in vacuo to provide a white solid as the product 2 (14.24 g, 60%). 1H NMR (400 MHz, DMSO-d6) δ 8.44 (s, 2H), 4.26 (s, 2H), 2.57 (t, J = 6.9 Hz, 2H), 2.02 (s, 3H), 1.96 (s, 3H), 1.94 (s, 3H), 1.81–1.72 (m, 2H), 1.56–1.44 (m, 3H), 1.40 (t, J = 8.7 Hz, 4H), 1.31–1.16 (m,11H), 1.16–0.99 (m, 6H), 0.83 (dd, J = 9.1, 6.5 Hz, 12H). Chemical formula: C31H53NO3. ESI-MS: M+H+ = 487.4025, found 487.4106.
2.4.2. Preparation of hyaluronan-tetrabutylammonium salt (HA-TBA, 3)
Hyaluronic acid sodium salt (32 kDa, 10 g) was dissolved in deionized H2O (200 mL) and stirred for 1 h, followed by the addition of Dowex AG 50W-X8 resin (30 g), and the mixture was stirred for 12 h at ca. 20 °C. The resin was removed via filtration, and the filtrate was titrated with TBA-OH (1M, aqueous solution) to adjust pH to 8–9. The water solution was lyophilized to obtain a pale-yellow powdered solid 3 (6.7g, 64%).
2.4.3. Preparation of HA-Tocopherol (4)
A solution of 3 (200 mg, 0.32 mmol) in DMF (15 mL) was stirred for 1 h at ca. 20 °C. DMF solutions of 2 (19 mg, 0.03 mmol, 10mg/mL), EDC·HCl (153 mg, 0.80 mmol, 10mg/mL), and HOBt·H2O (74 mg, 0.48 mmol, 20mg/mL) were added subsequently to the reaction mixture, which was stirred at ca. 20 °C for 12 h. The reaction mixture was dialyzed using dialysis tubing (MWCO 10,000 kDa) against 50% EtOH/H2O for 12 h, sodium chloride solution (150 mM) for 12 h, followed by three water changes over another 48 h. The dialyzed mixture was lyophilized to yield a white cotton-like polymer 4 (139 mg, 69%). The degree of substitution (SD) of tocopherol molecules on HA was calculated as around 7% on molar basis by comparing the intensity ratio between the representative peaks of N-acetyl peak of HA (1.8 ppm) and methyl groups of tocopherol (0.8 ppm) in 1H NMR spectrum (Figure S1).
2.5. Preparation of agonist/HA-Toco nano-suspension
The agonist-loaded nano-emulsion was prepared by an emulsification-solvent evaporation method [17]. An aqueous solution of 4 (10 mg/mL, 3 mL) was added dropwise to an ethanol solution of R848 or 1 (10 mg/mL, 2 mL) while stirring, and the mixture was stirred at 200 rpm for 10 min. The emulsion was then dried using a Centrivap concentrator (Labconco, Kansas City, MO, USA) to obtain a pale-yellow transparent solid, which was rehydrated with sterile water (2 mL) for injection over 12 h to form a white emulsion. The particle size was measured by Dynamic Light Scattering (DLS) using a ZetaPALS (Brookhaven Instruments Corp., ZetaPALS, Holtsville, New York) at 25 °C. Samples were diluted in 1x PBS to a concentration of 0.05 mg/mL and filtered through a 0.45 μm.
To determine the drug concentration in the formulation, a sample was diluted 1:100 v/v in acetone and sonicated for 10 min. The mixture was centrifuged (12,000 × g, 5 min) and the supernatant was measured by HPLC (LC-2010C HT, Shimadzu, Kyoto, Japan) with a UV detector (254 nm) and a C8 column (4.6 um × 150 mm, 5 μm), thermostated at 45 °C. The mobile phases were A: 0.05% TFA/water and B: 0.05% TFA/acetonitrile with a gradient elution (20–100% B) over 13 min at a flow rate of 1.0 mL/min. The percentage of encapsulation efficiency (%EE) was calculated using the following formula:
2.6. In vitro release kinetics
Solutions (1 mL) of free R848 in ethanol, R848/HA-Toco in water, with a concentration of 1 mg/mL R848 were dialyzed using SnakeSkin® dialysis tubing (MWCO 3500 kDa) against 50 mL of phosphate buffered saline release medium (PBS, 10 mM sodium phosphate, 137 mM NaCl, pH 7.4) at 37 °C. At predetermined time points, 1-mL aliquots of release medium were sampled and replaced with an equal volume of fresh medium. Samples were kept frozen (−20 °C) until HPLC analysis. Formulations for the determination of percentage of cumulated drug released (%CDR) include:
where Pt = percentage of released drug at time t; Pt-1 = percentage of released drug at the time point previous to t.
Solutions (1 mL) of R848-Toco in ethanol, and R848-Toco/HA-Toco in water, with a concentration of 0.5 mg/mL were dialyzed using Float-A-Lyzer G2® dialysis devices (MWCO 3.5 − 5 kDa) against 500 mL of PBS (10 mM sodium phosphate, 137 mM NaCl, pH 7.4) at 37 °C. At predetermined time points, solutions in release cartridges were removed for weight and density determination, and 10 μL aliquots were sampled before the remaining solution was put back. Samples containing HA-Toco were sonicated and centrifuged as mentioned above. Formulations for the determination of percentage of remaining drug include:
where
2.7. Hydrolysis
R848-Toco (0.1 mg) was dissolved in 1 mL of DMSO, and the solution was added into 9 mL of hydrolysis media (PBS, pH 7.4). The solution was then rapidly mixed and filtered through a 0.22-μm filter. A sample (50 μL) was taken immediately for the determination of initial concentrations of the analytes. The filtrate was then incubated at 37 °C under agitation (250 rpm). At predetermined time points, 50 μL of the solution was sampled and analyzed immediately by HPLC. The mobile phases were A: 100% water with 10 mM ammonium acetate; B: 100% acetonitrile with 10 mM ammonium acetate and C: 100% acetonitrile with 0.1% TFA. The mobile phase eluded at 1.0 mL/min beginning at 70% solvent A and 30% solvent B, increasing to 100% solvent B from 0 to 6 min. From 6.5 to 9 min, 100% solvent C was applied. From 9.5 to 12.5 min, the mobile phase was re-equilibrated to the initial composition of 70% solvent A and 30% solvent B.
2.8. In vitro TLR7 reporter assay
The TLR7 activity of R848-Toco, the formulation and its components were assessed using HEK-293 cells co-transfected with the human TLR7 (hTLR7) gene and an inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene along with the corresponding null control line (HEK-Blue™ hTLR7 and Null-1k cells, Invivogen). Cells were maintained in DMEM media with selective antibiotics according to the manufacturer protocol [18]. HEK-Blue™ cells were seeded into 96 well plates at a density of 220,000 cells/mL with 180 μL/well of culture medium. Stock solutions in DMSO were first serially diluted to seven concentrations (DMSO solutions further diluted into H2O). Each well was stimulated with 20 μL of agonists or agonist-free controls (equivalent concentration of HA-Toco, D-α-Tocopherol succinate, PBS or 100 μM DMSO) at the determined final concentrations in 5 replicates. After 16 h of incubation at 37 °C in a 5% CO2 atmosphere, 20 μL of the supernatant from each well was sampled and spiked into 180 μL of QUANTI-blue™ solution followed by incubation for 1 h at 37 °C. Relative TLR7 activity was then analyzed by UV-Vis at 637 nm using a microplate UV reader (SpectraMAX GeminiXS, Molecular Devices, San Jose, CA, USA). For each compound and concentration, a corresponding sample was applied to the HEK-Blue™ Null-1k cell line. The TLR7 agonist activity was determined after averaging across hTLR7 replications and subtracting the averaged Null response.
2.9. In vitro cytokine secretion from canine peripheral blood mononuclear cells (PBMCs) following agonist stimulation
Peripheral blood from healthy canine donors of 7 – 10 years age (similar to the age of typical canines with cancer [19]) was obtained with appropriate informed consent of the owner. Blood (8 mL per tube) was drawn via sterile venipuncture into a BD Vacutainer cell preparation tube containing sodium heparin (BD Biosciences). PBMCs were isolated within one hour following procurement by density gradient centrifugation according to the manufacturer protocol and resuspended to 3×106 cells/mL in sterile RPMI 1640 medium with L-glutamine (Gibco) supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin (Corning) and 10% (v/v) heat-inactivated Fetalgro (RMBIO). Corning 3917, 96-well tissue culture treated plates were seeded with the PBMC suspension (180 μL/well), 20 μL of the serially diluted R848 samples were added in triplicate and HA-Toco vehicle in duplicate to yield the desired concentrations in a 1% (v/v) DMSO cell suspension. Samples (n = 6) and standards (n = 3) were thawed and plated onto a Maxisorp flat-bottom, 384-well plate (Thermo Scientific) using a BioTek Precision XS pipetting system (BioTek Instruments, Winooski, VT, USA). Sample cytokine concentrations were determined by interpolation of a four-parameter logistic curve of the recombinant standards using GraphPad Prism 6. Cells were incubated at 37 °C in 5% CO2/3% O2 for 15 hours after which supernatants were removed and frozen at −80 °C. Cytokine secretion levels were measured by ELISA (Duoset, R&D Systems, Minneapolis, MN, USA) according to the manufacturer protocol.
2.10. Rabbit subcutaneous injections and quantification of plasma TNF-α secretion
New Zealand White rabbits (3 – 4 kg) were anaesthetized using 5 % isoflurane and injected subcutaneously (s.c.) with 5 mg of R848 in 250 μl of vehicle into the ipsilateral ear. R848-Toco/HA-Toco was formulated as described above, and the R848 parent compound was formulated in 0.5 % polysorbate 80 (w/v) in water. For histopathology, punch biopsies were taken 24 hours post injection, fixed in formalin, and analyzed by a board-certified veterinary pathologist (Kanas State Veterinary Hospital, Topeka, KS, USA). In separate animals, blood samples were taken pre-injection and at 0.5, 1, 2, 5, and 24 h post-injection time points from the contra-lateral ear (marginal vein, 22 ga). Whole blood was centrifuged at 1500 × g for 5 min and the serum were collected for cytokine evaluation. All samples and controls were prepared, and serum cytokine levels were measured as described in section 2.9.
2.11. In vivo anti-tumor activity assay
2.11.1. Tumor cell model
AT84 cells were derived from a spontaneous squamous cell carcinoma in the oral mucosa of a C3H mouse and were gifted by Aldo Venuti (Regina Elena National Cancer Institute, Rome, Italy) [20]. Cells tested negative for interspecies contamination (species: mouse(+), rat(−), human(−), Chinese hamster(−), African green monkey(−); Idexx BioResearch), negative for rodent pathogens (Idexx BioResearch, 21 pathogen IMPACT I PCR profile), and negative for Mycoplasm contamination prior to animal studies (Lonza, MycoAlert test kit). Idexx CellCheck STR (short tandem repeat) profile: MCA-4–2: 20.3, 21.3; MCA-5–5: 15; MCA-6–4: 18, 19; MCA-6–7: 12; MCA-9–2: 15; MCA-12–1: 16; MCA-15–3: 25.3, 26.3; MCA-18–3: 16; MCA-X-1: 26, 27. Cells were cultured in RPMI-1640 media (Gibco) supplemented with 10% FBS (Corning), and 100 U/mL penicillin / 100 μg/mL streptomycin (HyClone) in a humidified incubator at 37°C and 5% CO2.
2.11.2. Mice
Wildtype C3H mice (Charles River Strain 025, 6–8 weeks old) were used for in vivo tumor studies. Both male and female mice were used in the studies. Results are presented with both sexes as a single group since no significant differences were found in growth rates or results between sexes.
2.11.3. Tumor model for efficacy
Mice were anesthetized using 5% isoflurane in O2 for 5 min. One million AT84 cells in 50 μL of PBS were injected subcutaneously into the floor of the mouth using an extra oral route to obtain orthotopic allograft tumors [21]. Treatment began when tumors reached 50mm3, generally between days 4 and 7. Under isoflurane anesthesia, 50 μL of R848-Toco/HA-Toco (25 μg on R848 basis) was injected intratumorally on the first two days of three consecutive weeks. The vehicle control was 50 μL of HA-Toco (16.7 mg/mL) and was injected on the same schedule. Tumor size was calculated as tumor volume (mm3) = 4π / 3 × (width / 2)2 × length / 2, where length is the longer of two perpendicular dimensions [22,23].
2.11.4. Tumor model for immune marker analysis
Orthotopic allografts were generated as in the efficacy studies. When tumors reached 200–400 mm3 (about 2 weeks post-cell injection), R848-Toco/HA-Toco (25 μg on R848 basis) in 50 μL volume was injected intratumorally. After 24 h, tumors were dissected and embedded in OCT medium (Fisher Scientific), followed by storage at −80 °C. Tumors were sectioned (10 μm) with a cryotome. Sections were fixed for 2 × 10 min with acetone and washed with PBS. Primary antibodies were diluted to 5 μg/mL in blocking buffer (5% goat serum in PBS) and incubated overnight at 4 °C. Antibodies used were Alexa Fluor® 488 anti-CD8a, Alexa Fluor® 594 anti-CD11b, and Alexa Fluor® 647 anti-CD11c (BioLegend). After antibody staining, sections were stained with DAPI (0.5 μg/mL in PBS; Invitrogen) for 10 minutes, mounted in SouthernBiotech™ Fluoromount-G™ Slide Mounting Medium and stored in the dark at 4 °C. Images were acquired using an Olympus IX-81 inverted epifluorescence microscope at 10x magnification. and images were captured with a Hamamatsu EM-CDD Digital Camera. The entire tumor section was imaged. Many 10x images were montaged together using SlideBook 6 to view the whole section.
2.12. Preclinical canine trial
An open-label, compassionate-care veterinary pilot clinical trial in companion dogs with naturally-occurring mast cell tumors was conducted by multiple veterinary clinics in the Kansas City area. The trial contained a total of 6 canine patients, including two Labrador Retrievers (8 and 12.5-year old), an Italian Greyhound (13-year old), two American Staffordshire Terriers (7 and 9-year old), and a Boxer (7-year old). The diagnostics were obtained via either histopathology or cytology. The patients were treated at 3-week intervals for up to 4 injections of R848-Toco/HA-Toco at a dose level ranging from 0.07 to 1.36 mg on R848 basis. Complete blood counts and clinical chemistry were performed prior to the first treatment and 1-week post each subsequent injection. Published data were used for the untreated control arm [24].
2.13. Statistical analysis
The statistical analyses are shown in figure legends and were completed using GraphPad Prism 8 software. The analysis of the pilot study was conducted using the internet version of the Evidence-based Clinical Decision Support Tool and Calculator for Medical Professionals [25].
3. Results and discussion
3.1. Design and characterization of R848-Toco/HA-Toco as a nano-suspension
Nanoformulations and depots are useful strategies for localized targeted delivery and sustained release [26]. Compared to conjugation of the parent drug directly to the polymer, prodrug synthesis followed by polymer encapsulation has more versatility, such as low steric hindrance, easy purification and quantification of the prodrug [27]. In this study, R848 was prepared as a prodrug by conjugation to α-tocopherol (Toco). α-Tocopherol, a constituent of vitamin E, is a hydrophobic compound that itself has been investigated as an anti-cancer drug, as it shows anti-neoplastic activity with no toxicity to normal cells [28,29]. The R848-Toco prodrug had increased lipophilicity (cLogP = 14.8896), compared to the parent drug (cLogP = 1.843), and could be easily encapsulated into the hydrophobic polymeric nanocarriers because of favorable hydrophobic interactions.
HA-Toco was prepared by conjugating tocopherol to HA through a glycine linker, yielding a polymer that easily associates with R848-Toco under aqueous conditions. The substitution degree (SD) of tocopherol molecules to HA was around 7% on molar basis. This low SD (< 25%) suggests that the tocopherol modification would not largely affect the characteristics and delivery performance of native HA [14]. HA itself undergoes some self-association to form a loose gel-like network, with a particle size of about 12.2 nm for 30 kDa HA [30]. With the hydrophilic HA backbone and the hydrophobic tocopherol, HA-Toco self-aggregates to form nanoparticles in aqueous solution. This self-association resulted in a nearly 30-fold size increase (HA-Toco particle size = 353 ± 18 nm (Table 1)), which is consistent with previous published results (350–400 nm) [29].
Table 1.
Particle sizes and polydispersity index (PDI). a
| Sample | Z-average (d. nm) | PDI |
|---|---|---|
| HA-Toco | 353 ± 18 | 0.149 ± 0.012 |
| R848-Toco/HA-Toco | 501 ± 33 | 0.091 ± 0.008 |
The intensity-averaged hydrodynamic radius was reported. PDI is indicated in parentheses. Data are shown as mean ± standard deviation of 3 separately prepared batches.
Rehydration of the solid nanoparticles yielded a stable homogenous nano-suspension that was readily injectable. R848-Toco in the formulated sample was close to the targeted concentration, with the encapsulation efficiency as 94.2 ± 8.9%. The particle size of R848-Toco/HA-Toco was measured as 525 ± 8 nm (Table 1, Figure S2), which was larger than the vehicle HA-Toco (353 ± 18 nm), suggesting that the R848-Toco conjugates were embedded into the hydrophobic inner core of HA-Toco polymeric nanoparticles (Figure S3). A homogenous nanoparticle population was indicated by a polydispersity index (PDI) of less than 0.2. All sizing measurements were taken from three separately prepared samples, demonstrating consistency of the batch-to-batch quantification.
3.2. In vitro release kinetics
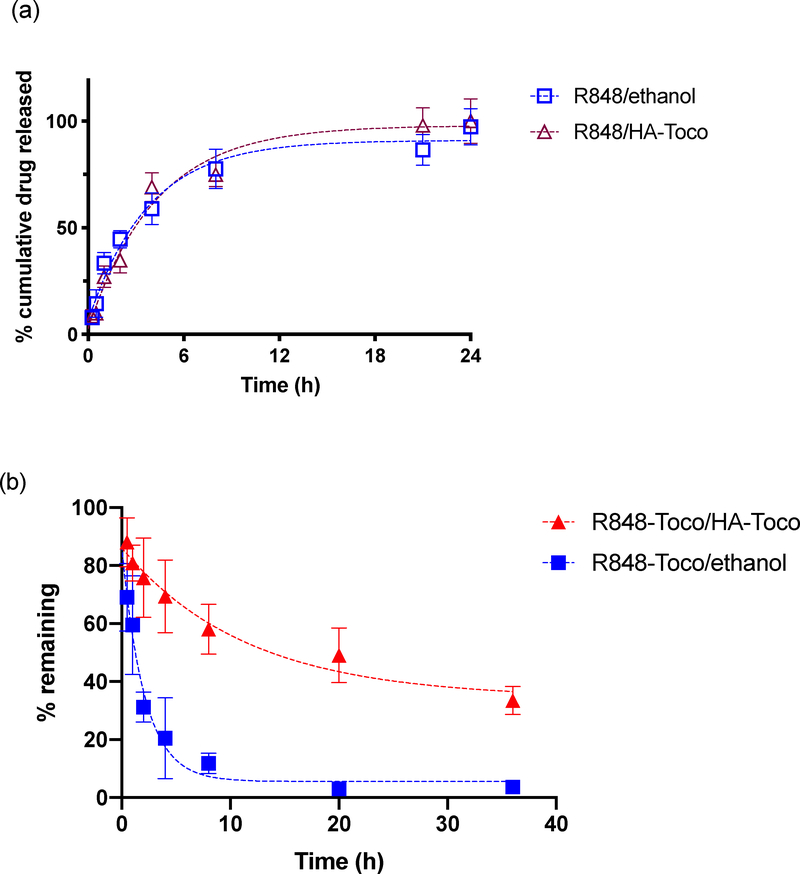
The in vitro release profiles of R848 and R848-Toco from the HA-Toco were studied using a dialysis method. Similar release kinetics were observed whether R848 was formulated with or without HA-Toco (t1/2, release = 2.5 h), and in both conditions nearly 100% of the agonist was released over 24 hours (Figure 1a). Thus, although HA-Toco could formulate the poorly soluble R848 parent drug, it did not provide any prolonged release.
Figure 1.
a) Cumulative release profile of unformulated R848 and R848/HA-Toco in PBS. Aliquots were sampled from the release medium. b) fraction remaining of unformulated R848-Toco and R848-Toco/HA-Toco in PBS. Aliquots were sampled from the release cartridge. Values are means ± SD, n = 3. Half-life: R848 (both conditions): 2 h; R848-Toco/ethanol: 1.5 h; R848-Toco/HA-Toco: 8 h. Half-life was estimated by one phase exponential decay model using GraphPad Prism 8.
Sustained release was observed when the R848-Toco prodrug was formulated with HA-Toco (Figure 1b). Since R848-Toco has low water solubility (< 20 μg/mL), the analyte in the release medium was below the detection limit of HPLC analysis, even when excessive amount of drug was loaded into the dialysis cartridge. To maintain sink conditions and avoid the analytical limitation, drug remaining in the dialysis cartridge was measured at regular intervals as an indirect measurement of drug release. Using this sampling strategy, samples with low initial concentrations can be applied, preventing potential aggregation of the poor water-soluble prodrug before it releases into the medium. The nanoformulation released about 60% of the loaded prodrug over 36 hours, and the half-life was 8 hours. The unformulated R848-Toco, delivered by ethanol, showed burst release within the first five hours and a 1.5 hour half-life. Since HA-Toco provided a prolonged release of R848-Toco but had little effect on the release of the parent R848, the interaction between the conjugate R848-Toco and the vehicle HA-Toco was most likely due to the lipophilic association between tocopherol molecules on the prodrug and the nanocarrier.
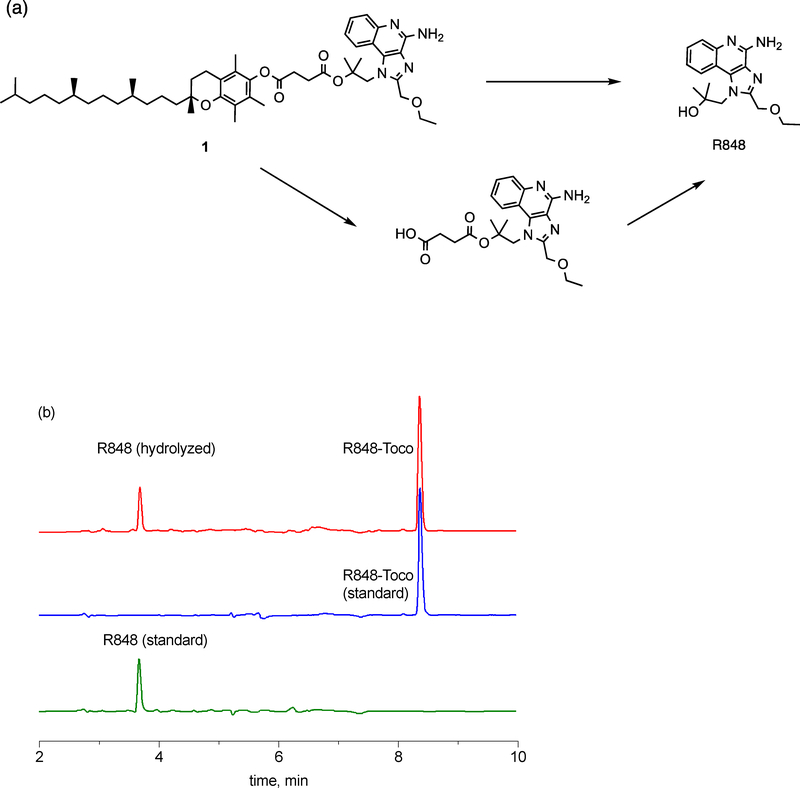
In this aqueous nanoformulation, the parent R848 was released from the prodrug, with no presence of undesired intermediates or degradants. R848 and α-tocopherol were conjugated using a succinate linker, with the advantage that the parent R848 can be released either directly by the cleavage of the ester bond adjacent to R848 or indirectly through R848-succinate, by cleavage of the ester bond adjacent to the α-tocopherol molecule. The released R848-succinate is then hydrolyzed to form R848 (Figure 2a). During the initial eight hours of hydrolysis study, 72% of the prodrug was hydrolyzed to free R848, as HPLC analysis showed the presence of R848-Toco and free R848 in the media (Figure 2b). Since no peak of the R848-succinate has been found, hydrolysis of the ester bond adjacent to R848 appeared to be the dominant release mechanism. Markovic et al. observed that enhanced polarity of the C-O2 bond of the ester could improve the hydration kinetics for a prodrug [32]. The parent R848 is more polar than the α-tocopherol, making the ester bond closer to R848 more labile to cleave. Alternatively, it is also possible that any R848-succinate rapidly hydrolyzed to R848 before it could be detected. Water exclusion by the bulky lipophilic α-tocopherol is another possible reason for the less favorable hydrolysis site closer to the lipid. A similar hydrolysis pattern was shown by Fu et al., as their prodrugs using succinate as the linker hydrolyzed to release the free parent drug, and the hydrolysis was favored by decreased steric hindrance and increased polarity [33].
Figure 2.
(a) Schematic illustration of proposed R848-Toco hydrolysis. (b) HPLC chromatogram of the hydrolysis media. The parent R848 was the only observed hydrolyzed product (red). Standards were measured to confirm the peak (blue: R848-Toco; green: R848). Traces were subtracted from blanks, and the raw data was replotted for better visualization.
3.3. In vitro TLR7 activity assay
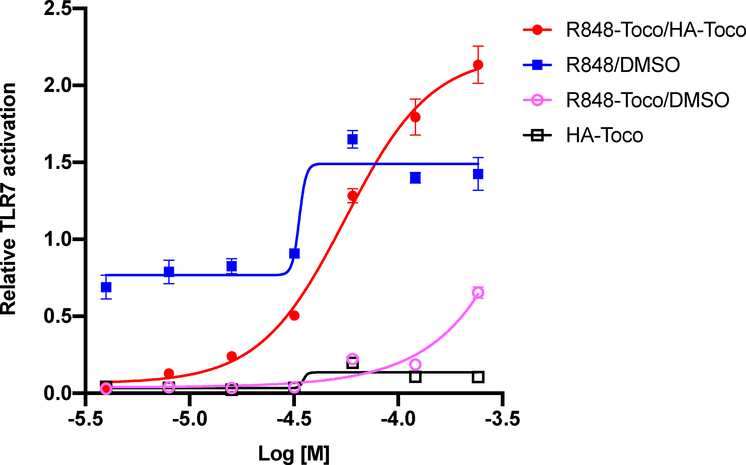
TLR agonist activity was measured in a dose-response study using HEK-293 cells expressing both hTLR7 and a SEAP reporter gene under the control of a NF-κB induced promoter [18]. The Null1-k cell line, expressing the NF-κB/SEAP reporter system, was used as a control. Activation levels were calculated as the difference between the average readings of the TLR7 cell line and the Null1-k cell line, to account for non-TLR7 related induction of NF-κB. As shown in Figure 3, the known agonist R848 induced strong dose-dependent activation as expected, and R848-Toco/HA-Toco was more active than free R848 at higher screened concentrations [34]. TLR7 agonists have been reported to be toxic at high concentrations in transformed cells lines, whereas the sustained release of the conjugate may prevent some toxicity [35,36]. At lower concentrations, R848-Toco/HA-Toco showed weaker TLR7 activity than free R848, although this might be due to the incomplete release within 16 h of exposure. The unformulated R848-Toco was much less active, due to the poor solubility as observed in the kinetics studies, while formulating with HA-Toco improved the solubility and prevented R848-Toco aggregation in the aqueous solution. Non-conjugated Toco-succinate and blank HA-Toco vehicle did not induce any activation. Therefore, this study confirmed that R848-Toco, formulated with HA-Toco as an emulsion, maintained its TLR agonist activity. Furthermore, tocopherol and tocopherol succinate, which have anti-inflammatory properties and are known to inhibit NF-κB activation at higher concentrations had no effect on agonist activity in the conjugate or over the concentration range screened [37,38].
Figure 3.
Relative TLR7 activation. R848-Toco/HA-Toco EC50 = 56 ± 1.1 μM, n = 5.
3.4. In vitro cytokine secretion assay
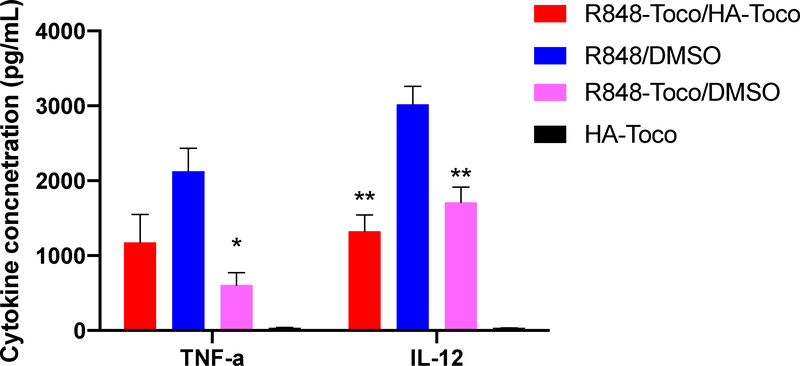
As a TLR7/8 agonist, R848 binds to endosomal TLR7 and 8 receptors located on antigen presenting cells (APCs). The activation of APCs produces pro-inflammatory cytokines including TNF-α and IL-12. These cytokines, together with stimulated APCs that take up tumor antigens, enhance the activation of anti-tumor Th-1 immune responses that involve both CD4+ T helper cells and CD8+ cytotoxic T cells [4]. To evaluate the Th-1 associated cytokine production in vitro, isolated canine PBMCs were treated with R848, formulated or unformulated R848-Toco, and the secretion of TNF-α and IL-12 were measured by ELISA as markers of generalized inflammatory response. Canines were chosen as a model over mice because of the greater genetic similarity to humans and more similar immune systems [39]. Strong secretion of TNF-α was observed for free R848 and R848-Toco/HA-Toco, and to a lesser extent for unformulated R848-Toco. IL-12 levels were highest in free R848 samples, with a lower secretion in R848-Toco/HA-Toco and unformulated R848-Toco groups. HA-Toco alone did not produce any observable secretion of either TNF-α or IL-12. Taken together, R848-Toco retained its ability to induce the selected Th-1 related cytokines in canine PBMCs. Similar to the TLR7 reporter cell line, the cytokine induction levels were slightly lower than parent R848 drug, potentially due to incomplete release of free R848 during the time frame.
3.5. Depot effect of R848-Toco/HA-Toco
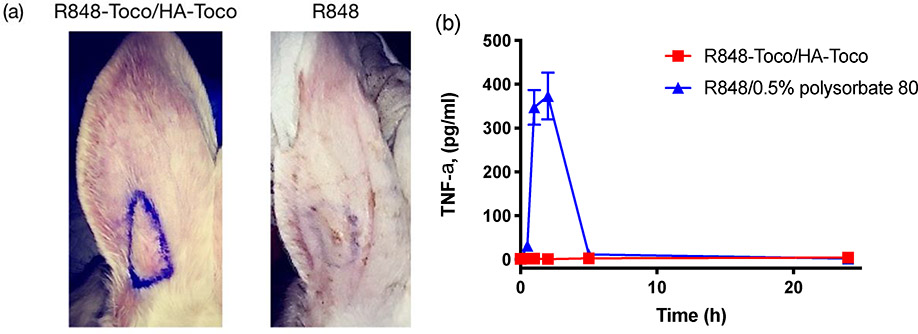
We hypothesized that a local subcutaneous injection of the R848-Toco/HA-Toco nano-emulsion would form a depot at the site of injection and release the loaded agonist in a sustained fashion, producing localized immune stimulation while minimizing the systemic off-target effects. To test the localization effect, an ear inflammation test was conducted in healthy rabbits. R848-Toco/HA-Toco and unformulated R848 were injected subcutaneously into rabbit ears, and local inflammation and systemic TNF-α responses were observed. The solubility of free R848 in water is lower than 1 mg/mL, and thus polysorbate 80 was added as 0.5% into the aqueous solution of R848 to provide a higher concentration for the treatments, allowing a maximum dosing of 5 mg per rabbit. Further, 5 mg in a rabbit is approximately equivalent to 25 μg in a mouse, which was used in previous efficacy studies of TLR7/8 agonists [40,41]. Unformulated R848-Toco was not tested in this study due to the lack of TLR7 activity (Figure 3) and the formation of low-concentration suspension with large aggregates due to poor aqueous solubility. The nano-emulsion provoked warmth and swelling at the injection site over seven to ten days (Figure 5a), and histopathological examination of ear tissue confirmed local macrophage infiltration. Systemic TNF-α was not elevated in the plasma, indicating that the response was localized to the injection site during the time frame (Figure 5b). In contrast, free R848 caused no local reaction, based on visual observation and histopathological examination, yet a significant elevation of plasma TNF-α levels occurred within two hours post-treatment. R848-Toco/HA-Toco induced a localized immune response at the injection site while minimizing the rapid clearance and subsequent systemic activity, suggesting that the formulation may be applicable for local treatments such as intratumoral injections. Pharmacokinetics is a critical measurement of the drug dispersion in vivo, but in a trial performed in mice, due to the low dosage level the drug concentration was below the detection limit and no LC chromatogram could be obtained (data not shown).
Figure 5.
(a) Local response at the injection site of rabbit ears seven days post-treatment. The left showed sustained swelling and warmth after injection of nano-suspension, while the right showed minimum change after injection of free R848. Injection points were marked in purple. (b) Plasma TNF-α levels (mean ± SEM, n = 4). No TNF-α secretion was observed for plasma of rabbits injected with R848-Toco/HA-Toco.
3.6. In vivo anti-tumor efficacy
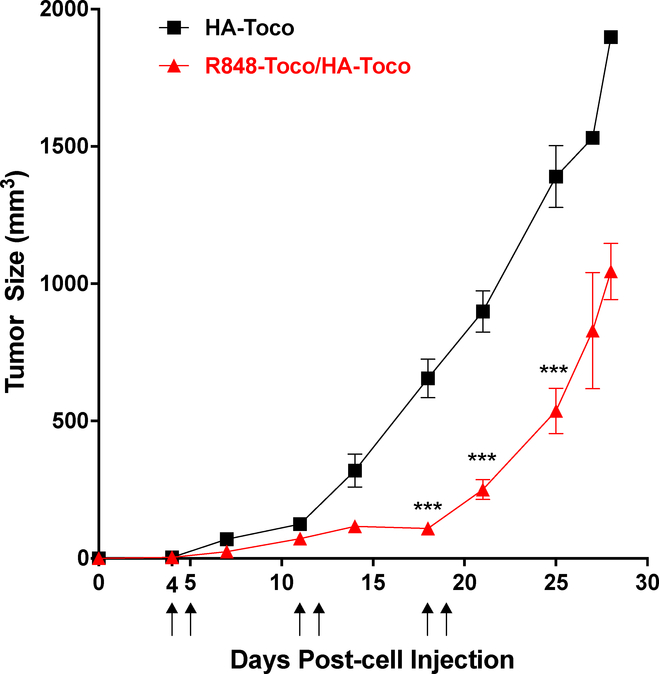
The anti-tumor efficacy of the R848-Toco nano-emulsion was next evaluated in an immune-competent murine allograph model of head and neck cancer. The AT84 cell line was derived from a spontaneous oral squamous cell carcinoma (OSCC) of a C3H mouse and display several key similarities to the human disease including local invasiveness and lung metastases [20,21]. Before studies were performed, the cell line was confirmed to be free of mouse pathogens and other microbial contaminants that could induce an immunological response to the tumor cell injections. C3H mice with OSCC allografts were treated with R848-Toco/HA-Toco or the vehicle HA-Toco for the first two days of three consecutive weeks. Consecutive treatment days have shown antitumor effects for other intratumoral TLR7 agonists [42], and three treatment weeks demonstrated a robust infiltration of immune cells. The dose of 25 μg was based on previous studies of TLR7/8 agonists [40,41]. Since the unformulated R848 shows rapid release profiles (Figure 1a) and no localized immune effects (Figure 5), and the unformulated R848-Toco has minimum TLR7 activity (Figure 3) and forms suspension with large aggregates, neither is suitable for intratumoral injection and was not tested in this study. The R848-Toco/HA-Toco significantly suppressed the tumor growth compared to HA-Toco vehicle over the time course (Figure 6). Tumor growth recovered after the treatment ended, indicating that either an increased dose or treatment time may be required to achieve a more durable response. Alternatively, R848-Toco may be combined with other immunotherapies that prevent immunosuppression. For example, the TLR9 agonist CpG injected intratumorally has minimum activity as a monotherapy, but demonstrated a significant enhancement of survival time in clinical trials for metastatic melanoma [43].
Figure 6.
R848-Toco/HA-Toco shows efficacy in OSCC allografts. Mice with OSCC allograft tumors began treatment when turmos reached 50 mm3 (generally days 4 to 7) and were treated on the first 2 days of 3 consecutive weeks (marked by arrows). R848-Toco/HA-Toco significantly suppresses tumor growth compared to the vehicle (two-way ANOVA: p<0.001 for treatment, time, and interaction; Bonferroni posttest *** p < 0.001; n = 6–8).
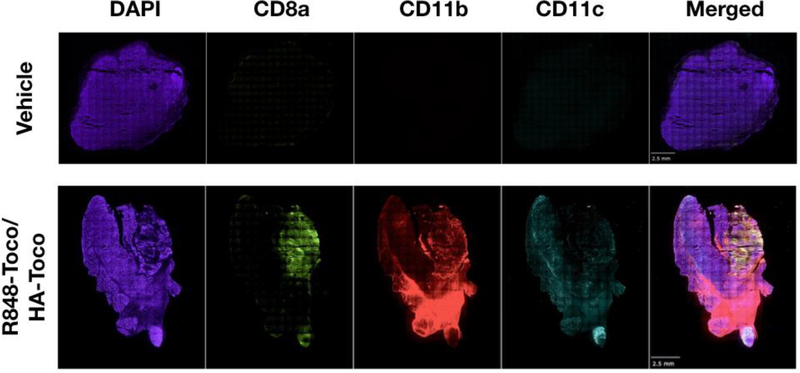
To determine whether R848-Toco/HA-Toco induced immune cells infiltration that may contribute to the anti-tumor efficacy and similarly to the inflammatory responses in rabbit (Figure 5), immunohistochemistry was performed on excised tumors. AT84 allografts of 200–400 mm3 were injected with R848-Toco/HA-Toco or vehicle (HA-Toco) 6 times as in efficacy studies. Forty-eight hours after final injection, tumors were removed, sectioned, and stained with fluorescently labeled antibodies of selected immunological markers (CD8a, CD11c, CD11b). R848-Toco/HA-Toco generated an increase in marker staining in allografts (Figure 7). CD8a is marker for activated cytotoxic T cells, CD11b is a marker for macrophages, and CD11c is primarily a marker for DCs, but may also be expressed on T cells and macrophages. These immune cells are important toward generating robust antitumoral T cell responses [44,45]. Macrophages are more associated with a T helper cell response, while DCs activate cytotoxic CD8a+ T cells. It is important to only compare antibody staining between treatments and not between different antibodies, as different antibodies have different affinities to their respective antigens. Therefore, these results are qualitative only, not quantitative. RNA sequencing studies are currently underway to make more quantitative comparisons. The recruitment of immune cells after R848-Toco/HA-Toco injection suggests immune activation at the injection site.
Figure 7.
R848-Toco recruits immune cells to tumors. Immune cell markers, CD8a, CD11d, and CD11c, are increased in AT84 tumors after 6 treatments of intratumoral Res-Toco/HA-Toco (25 μg on R848 basis). Representative images are shown from 3 independent experiments using an Olympus IX-81 inverted epifluorescence microscope. The entire tumor section is viewed as a montage of 10x magnification images. Scale bar is 2.5 mm.
3.7. Pilot canine trial
Mast cell cancer is one of the most common neoplasms in canines. Treatment typically includes surgical removal of the tumor, but it has a high propensity for recurrence even if clean margins are achieved at the surgery. The only FDA-approved veterinary medication for the treatment of mast cell tumors is the kinase inhibitor toceranib phosphate (Palladia®). In a double-blind, randomized clinical study of toceranib phosphate in mast cell tumors, the response rate was 37.2% in 86 canine patients (7 complete response and 25 partial response) versus 7.9% (5 partial response) in 63 placebo-treated dogs [24].
The proof-of-concept trial treated a total of 6 pet dogs with recurrent and/or inoperable mast cell tumors. Three of six had partial response (50%); one of six had complete response (17%); another two had stable disease (17%) and progressive disease (17%), respectively. All 6 dogs have completed their treatments. The authors recognize that this is a small pilot study. A multi-center, placebo-controlled, double-blind, randomized clinical study will be conducted in the future in 50–100 pet dogs with spontaneous mast cell tumors, guided by the information collected from the pilot trial.
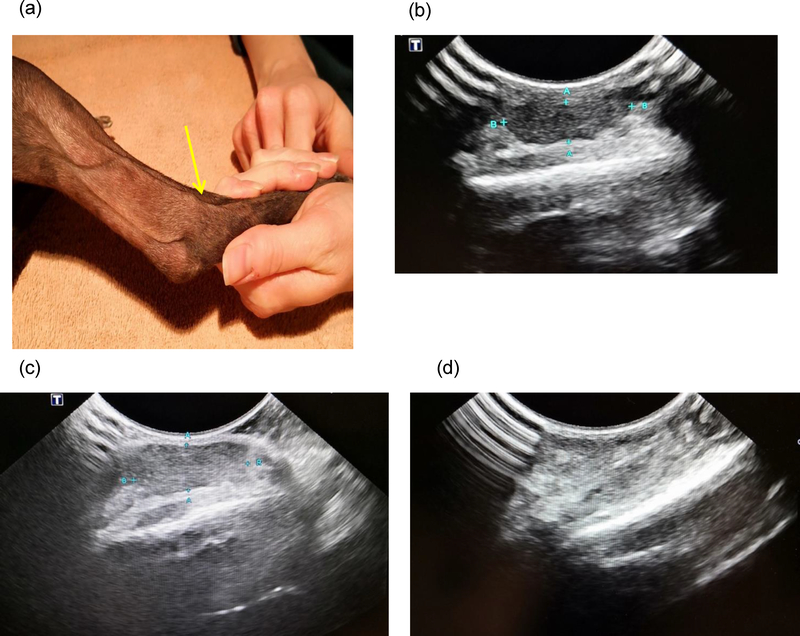
In the complete responder, a firm tumor was located over the lateral right hock of the dog (Figure 8a). The mass was inoperable without amputation due to its location. The dog had previously been treated with toceranib phosphate but discontinued due to side effects and lack of effect. The dog received a total of 2 nano-emulsion injections intralesionally at a dose of 0.34 mg on a R848 basis per injection 3 weeks apart. The administration of the medication was performed via ultrasound guidance. The tumor measured 0.49 cm by 1.58 cm prior to the first injection (Figure 8b). Three weeks later, the pet was rechecked, and the tumor size of the mass was about the same as prior to the first injection (Figure 8c). We administered the second treatment intratumorally to the center of the mass tumor. Three weeks after the second treatment, the dog was presented for a physical exam, during which no gross evidence of the tumor was found, and ultrasound of the right hock area revealed no tumor (Figure 8d). All prestudies and post treatment blood work results were unremarkable. The tumor remained in complete remission at the one-month follow-up.
Figure 8.
A mast cell tumor of a 13-year old Italian Greyhound went into complete remission after two injections. (a) location of the tumor in the lateral right hock, indicated by the yellow arrow; (b) prior to the first injection, tumor indicated by the blue mark; (c) prior to the second injection; and (d) 3 weeks after the second injection.
The three partial responders demonstrated similar remissions and clinical behaviors. These findings will be discussed as a group. At the beginning of the study, all three dogs have had tumors smaller than 2.5 cm in the longest dimension. All three pets responded after the first treatment indicating by tumor shrinkage in at least one dimension. For example, on the day of the second treatments (approximately 3 weeks after the first treatment), the percent reduction in the length of the masses was 57% (from 0.7 cm to 0.3 cm), 50% (from 2.4 cm to 1.2 cm) and 25% (from 1.6 cm to 1.2 cm), respectively, compared to the dimensions on the day of the first treatment. All three dogs have completed four required injections of the trial. Their response status remained as partial remission at the end of the study. Complete blood counts with differentials and clinical chemistry tests (liver enzymes, BUN/creatinine etc.) revealed no significant differences between pre-study and post-treatment results.
The other two pets had either stable or progressive disease at the end of the trial. The dog with stable disease had a recurrent, large (longer than 9.0 cm in the longest dimension) mast cell tumor in her left armpit. The tumor did not increase in size during the treatments. The dog with progressive disease had multiple tumor burdens (a total of 7 individual, medium to large sized masses). Unfortunately, he did not respond to the therapy after completing all four injections. Both dogs had no reportable side effects during the treatment and their bloodwork was unremarkable.
The statistics of the responses were analyzed using the Internet version of the Evidence-based Clinical Decision Support Tool and Calculator for Medical Professionals to determine the minimum number of subjects to achieve adequate study power. In two independent study groups, a published placebo group [20] and our TLR7 conjugate study group, to achieve a statistically different response in 7.9% of placebo treated (result reported in the placebo study) and in 66.7% of TLR7 conjugate treated patients (result obtained in the pilot study) with an enrollment ratio of 10.5 (number of dogs in each group: placebo=63 and TLR7=6), with a Type I error rate of 0.05 (indicating a 5% probability that a significant difference is actually due to chance and is not a true different), and with a Type II error rate of 80% (indicating a 80% probability that a significant difference is not missed), the minimally required number of participants in the placebo and the TLR7 groups are 32 and 3, respectively. This requirement has been met in the pilot study, which contained 6 patients in the TLR7 group. The published placebo study contained 63 patients, also sufficing the validity of the comparison.
4. Conclusions
The prodrug of TLR 7/8 agonist, R848, was developed as an injectable aqueous nanotherapeutic suspension that provided a sustained release of the loaded drug. The injection of this nano-suspension appeared to form a depot at the injection site, and induce localized immune responses such as macrophage recruitments, without having systemic effects. The agonist activity of R848-Toco was not affected by the formulation with HA-Toco, as assessed by hTLR7 activation. The canine PBMCs showed an induction of IL-12 and TNF-α, consistent with a Th-1 type response. Moreover, the depot injection of R848-Toco/HA-Toco significantly suppressed tumor growth, indicating that this formulation may be feasible for intratumoral administration. A pilot study conducted in 6 pet dogs with naturally occurring mast cell tumors showed a response rate as 67%, with 1 complete responder and 3 partial responders. Additional clinical trials in other types of common canine cancers, such as oral melanoma, are in progress.
Supplementary Material
Figure 4.
Cytokine secretion levels in canine PBMCs. All R848 formulations increase TNFα and IL-12 from baseline, but differ from each other. Graph shows mean ± SEM. One-way AVOVA for TNFα: p<0.01, Tukey’s multiple comparisons test *p<0.05 vs R848/DMSO, n=3. One-way AVOVA for IL-12: p<0.0001, Tukey’s multiple comparisons test **p<0.01 vs R848/DMSO, n=3.
Scheme 1.
Synthesis of R848-Toco (1) through ester condensation using EDC and TEA.
Scheme 2.
Synthesis of HA-Tocopherol.
Acknowledgement
This research was supported by generous gifts from the Brandmeyer family to the University of Kansas Department of Pharmaceutical Chemistry, and The National Cancer Institute (NCI R01 1R01CA173292-01). The authors would like to thank the attending veterinarians, Drs. Abby Faerber, Sally Barchman, Laura Grigsby, Donald Dinges, and Alicia Bangert for their expertise and excellent veterinary care.
Footnotes
Conflict of Interest
Shuang Cai and Chad Groer are employees of HylaPharm. Daniel J. Aires and Laird Forrest have a financial interest in HylaPharm.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bochud P-Y, Bochud M, Telenti A, Calandra T, Innate immunogenetics: a tool for exploring new frontiers of host defence., Lancet. Infect. Dis 7 (2007) 531–42. doi: 10.1016/S1473-3099(07)70185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kumar H, Kawai T, Akira S, Toll-like receptors and innate immunity, Biochem. Biophys. Res. Commun 388 (2009) 621–625. doi: 10.1016/J.BBRC.2009.08.062. [DOI] [PubMed] [Google Scholar]
- [3].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat. Immunol 11 (2010) 373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [4].Smits ELJM, Cools N, Lion E, Van Camp K, Ponsaerts P, Berneman ZN, Van Tendeloo VFI, The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells, Cancer Immunol. Immunother 59 (2010) 35–46. doi: 10.1007/s00262-009-0721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Netea MG, Van Der Meer JWM, Sutmuller RP, Adema GJ, Kullberg B-J, From the Th1/Th2 Paradigm towards a Toll-Like Receptor/T-Helper Bias, Antimicrob. Agents Chemother 49 (2005) 3991–3996. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perry CM, Lamb HM, Topical Imiquimod, Drugs. 58 (1999) 375–390. doi: 10.2165/00003495-199958020-00017. [DOI] [PubMed] [Google Scholar]
- [7].European Medicines Agency, Orphan designation EU/3/16/1653, (2016). https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3161653.
- [8].Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S, Vasilakos JP, Gorski KS, Miller JS, First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer, Clin. Cancer Res 13 (2007) 7119–7125. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- [9].Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD, Vaccine adjuvant activity of 3m-052: An imidazoquinoline designed for local activity without systemic cytokine induction, Vaccine. 29 (2011) 5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- [10].Dovedi SJ, Melis MHM, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J, Illidge TM, Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma., Blood. 121 (2013) 251–9. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- [11].van Aalst S, Jansen MAA, Ludwig IS, van der Zee R, van Eden W, Broere F, Routing dependent immune responses after experimental R848-adjuvated vaccination, Vaccine. 36 (2018) 1405–1413. doi: 10.1016/J.VACCINE.2018.01.077. [DOI] [PubMed] [Google Scholar]
- [12].Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPMM, Kishimoto TK, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPMM, Kishimoto TK, Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release, Vaccine. 32 (2014) 2882–2895. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fox CB, Orr MT, Van Hoeven N, Parker SC, Mikasa TJT, Phan T, Beebe EA, Nana GI, Joshi SW, Tomai MA, Elvecrog J, Fouts TR, Reed SG, Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: A formulation approach, J. Control. Release 244 (2016) 98–107. doi: 10.1016/j.jconrel.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, Lee MY, Hoffman AS, Hahn SK, Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives, J. Control. Release 141 (2010) 2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- [15].Yoo E, Salyer ACD, Brush MJH, Li Y, Trautman KL, Shukla NM, De Beuckelaer A, Lienenklaus S, Deswarte K, Lambrecht BN, De Geest BG, David SA, Hyaluronic Acid Conjugates of TLR7/8 Agonists for Targeted Delivery to Secondary Lymphoid Tissue, Bioconjug. Chem 29 (2018) 2741–2754. doi: 10.1021/acs.bioconjchem.8b00386. [DOI] [PubMed] [Google Scholar]
- [16].Kenne L, Gohil S, Nilsson EM, Karlsson A, Ericsson D, Helander Kenne A, Nord LI, Modification and cross-linking parameters in hyaluronic acid hydrogels—Definitions and analytical methods, Carbohydr. Polym 91 (2013) 410–418. doi: 10.1016/J.CARBPOL.2012.08.066. [DOI] [PubMed] [Google Scholar]
- [17].Bouchemal K, Briançon S, Perrier E, Fessi H, Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation, Int. J. Pharm 280 (2004) 241–251. doi: 10.1016/J.IJPHARM.2004.05.016. [DOI] [PubMed] [Google Scholar]
- [18].Invivogen, HEK-Blue TLR7 cells, (2018). https://www.invivogen.com/hek-blue-tlr7.
- [19].MacEwen EG, Spontaneous tumors in dogs and cats: Models for the study of cancer biology and treatment, Cancer Metastasis Rev. 9 (1990) 125–136. doi: 10.1007/BF00046339. [DOI] [PubMed] [Google Scholar]
- [20].Paolini F, Massa S, Manni I, Franconi R, Venuti A, Immunotherapy in new preclinical models of HPV-associated oral cancers., Hum. Vaccin. Immunother 9 (2013) 534–43. doi: 10.4161/HV.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hier MP, Black MJ, Shenouda G, Sadeghi N, Karp SE, A murine model for the immunotherapy of head and neck squamous cell carcinoma, Laryngoscope. 105 (1995) 1077–1080. doi: 10.1288/00005537-199510000-00013. [DOI] [PubMed] [Google Scholar]
- [22].Euhus DM, Hudd C, Laregina MC, Johnson FE, Tumor measurement in the nude mouse, J. Surg. Oncol 31 (1986) 229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- [23].Tomayko MM, Reynolds CP, Determination of subcutaneous tumor size in athymic (nude) mice, Cancer Chemother. Pharmacol 24 (1989) 148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- [24].London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein MK, Hintermeister JG, Bergman PJ, Couto GC, Mauldin GN, Michels GM, Multi-center, Placebo-controlled, Double-blind, Randomized Study of Oral Toceranib Phosphate (SU11654), a Receptor Tyrosine Kinase Inhibitor, for the Treatment of Dogs with Recurrent (Either Local or Distant) Mast Cell Tumor Following Surgical Excision, Clin. Cancer Res 15 (2009) 3856 LP–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- [25].Sample Size Calculator, (n.d.). https://clincalc.com/stats/samplesize.aspx (accessed May 9, 2019).
- [26].Singh Y, Palombo M, Sinko P, Recent Trends in Targeted Anticancer Prodrug and Conjugate Design, Curr. Med. Chem 15 (2008) 1802–1826. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Delplace V, Couvreur P, Nicolas J, Recent trends in the design of anticancer polymer prodrug nanocarriers, Polym. Chem 5 (2014) 1529–1544. doi: 10.1039/C3PY01384G. [DOI] [Google Scholar]
- [28].Prasad KN, Kumar B, Yan X-D, Hanson AJ, Cole WC, α-Tocopheryl Succinate, the Most Effective Form of Vitamin E for Adjuvant Cancer Treatment: A Review, J. Am. Coll. Nutr 22 (2003) 108–117. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- [29].Duhem N, Danhier F, Préat V, Vitamin E-based nanomedicines for anti-cancer drug delivery, J. Control. Release 182 (2014) 33–44. doi: 10.1016/J.JCONREL.2014.03.009. [DOI] [PubMed] [Google Scholar]
- [30].Kuehl C, Zhang T, Kaminskas LM, Porter CJH, Davies NM, Forrest L, Berkland C, Hyaluronic Acid Molecular Weight Determines Lung Clearance and Biodistribution after Instillation, Mol. Pharm. Pharm 13 (2016) 1904–1914. doi: 10.1021/acs.molpharmaceut.6b00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, Kwon IC, Jeong SY, Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: synthesis, characterization, and in vivo biodistribution, J. Mater. Chem 19 (2009) 4102. doi: 10.1039/b900456d. [DOI] [Google Scholar]
- [32].Markovic BD, Dobricic VD, Vladimirov SM, Cudina OA, Savic VM, Karljikovic-Rajic KD, Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters-An In Vitro Model for Prodrug Activation, Molecules. 16 (2011) 2658–2671. doi: 10.3390/molecules16032658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fu Q, Wang Y, Ma Y, Zhang D, Fallon JK, Yang X, Liu D, He Z, Liu F, Programmed Hydrolysis in Designing Paclitaxel Prodrug for Nanocarrier Assembly, Sci. Rep 5 (2015) 12023. doi: 10.1038/srep12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA, Structure-Activity Relationships in Human Toll-Like Receptor 7-Active Imidazoquinoline Analogues, J. Med. Chem 53 (2010) 4450–4465. doi: 10.1021/jm100358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hotz C, Treinies M, Mottas I, Rötzer LC, Oberson A, Spagnuolo L, Perdicchio M, Spinetti T, Herbst T, Bourquin C, Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses, Oncoimmunology. 5 (2016) e1232219. doi: 10.1080/2162402X.2016.1232219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singh M, Khong H, Dai Z, Huang X-F, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW, Effective Innate and Adaptive Antimelanoma Immunity through Localized TLR7/8 Activation, J. Immunol. 193 (2014) 4722–4731. doi: 10.4049/JIMMUNOL.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nakamura T, Goto M, Matsumoto A, Tanaka I, Inhibition of NF- κB transcriptional activity by α-tocopheryl succinate, BioFactors. 7 (1998) 21–30. doi: 10.1002/biof.5520070104. [DOI] [PubMed] [Google Scholar]
- [38].Suzuki YJ, Packer L, Inhibition of NF-κB Activation by Vitamin E Derivatives, Biochem. Biophys. Res. Commun. 193 (1993) 277–283. doi: 10.1006/bbrc.1993.1620. [DOI] [PubMed] [Google Scholar]
- [39].Felsburg PJ, Overview of immune system development in the dog: comparison with humans, Hum. Exp. Toxicol 21 (2002) 487–492. doi: 10.1191/0960327102ht286oa. [DOI] [PubMed] [Google Scholar]
- [40].Fakhari A, Nugent S, Elvecrog J, Vasilakos J, Corcoran M, Tilahun A, Siebenaler K, Sun J, Subramony JA, Schwarz A, Thermosensitive Gel–Based Formulation for Intratumoral Delivery of Toll-Like Receptor 7/8 Dual Agonist, MEDI9197, J. Pharm. Sci 106 (2017) 2037–2045. doi: 10.1016/J.XPHS.2017.04.041. [DOI] [PubMed] [Google Scholar]
- [41].Nie Y, Yang D, Trivett A, Han Z, Xin H, Chen X, Oppenheim JJ, Development of a Curative Therapeutic Vaccine (TheraVac) for the Treatment of Large Established Tumors, Sci. Rep 7 (2017) 14186. doi: 10.1038/s41598-017-14655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EEW, Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer, JCI Insight. 2 (2017). doi: 10.1172/JCI.INSIGHT.93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A, Britten CM, Smolle J, Koller S, Mauch C, Tantcheva-Poor I, Grabbe S, Loquai C, Esser S, Franckson T, Schneeberger A, Haarmann C, Krieg AM, Stingl G, Wagner SN, Phase II Trial of a Toll-Like Receptor 9–Activating Oligonucleotide in Patients With Metastatic Melanoma, J. Clin. Oncol 24 (2006) 5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- [44].Qualai J, Li L-X, Cantero J, Tarrats A, Fernández MA, Sumoy L, Rodolosse A, McSorley SJ, Genescà M, Expression of CD11c Is Associated with Unconventional Activated T Cell Subsets with High Migratory Potential, PLoS One. 11 (2016) e0154253. doi: 10.1371/journal.pone.0154253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B, Increase of Regulatory T Cells in the Peripheral Blood of Cancer Patients, Clin. Cancer Res 9 (2003) 606 LP–612. http://clincancerres.aacrjournals.org/content/9/2/606.abstract. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.