Abstract
Minimally invasive devices to detect molecules in dermal interstitial fluid (ISF) are desirable for point-of-care diagnostic and monitoring applications. In this study, we developed a microneedle (MN) patch that collects ISF for on-patch biomarker analysis by surface-enhanced Raman scattering (SERS). The micron-scale MNs create micropores in the skin surface, through which microliter quantities of ISF are collected onto plasmonic paper on the patch backing. The plasmonic paper was prepared by immobilizing poly (styrene sulfonate) (PSS) coated gold nanorods (AuNRs) on a thin strip of filter paper using plasmonic calligraphy. Negatively charged PSS was used to bind positively charged rhodamine 6G (R6G), which served as a model compound, and thereby localize R6G on AuNR surface. R6G bound on the AuNR surface was detected and quantified by acquiring SERS spectra from the plasmonic paper MN patch. This approach was used to measure pharmacokinetic profiles of R6G in ISF and serum from rats in vivo. This proof-of-concept study indicates that a plasmonic paper MN patch has the potential to enable on-patch measurement of molecules in ISF for research and future medical applications.
Keywords: Gold nanorods, interstitial fluid, microneedle patch, pharmacokinetic profile, point-of-care diagnostics and monitoring, surface-enhanced Raman spectroscopy
Point-of-care (POC) diagnostics is a rapidly growing field in the era of decentralized healthcare systems1. Although blood is considered the gold standard for diagnostic assays, blood sampling involves limitations such as difficulty of continuous monitoring due to blood clotting; need for medical professionals to collect blood by venipuncture; apprehension by patients associated with blood draws2 and need for additional processing for red blood cell removal before analysis3.
Interstitial fluid (ISF) is a novel source of biomarkers that fills the spaces between cells in tissues in the body, and complements conventional sources like blood, urine and saliva. ISF has been shown to contain systemic biomarkers and unique biomarkers compared to other body fluids4–6. Also, ISF is a largely colorless fluid devoid of clotting agents and cells, thus simplifying biochemical analysis7. ISF is often collected from the skin, due to easier access compared to internal tissues.
ISF has been relatively unexplored largely due to limitations of sampling techniques such as suction blister8, which requires specialized equipment, takes ~1 h to perform and causes lasting skin wound; reverse iontophoresis9, which is limited to small molecules and requires frequent calibration; and microdialysis10 and open flow microperfusion11, which requires expert personnel to perform minor surgery.
To enable simplified ISF collection, we and others have developed microneedle (MN) patches that can access ISF in a minimally invasive way that is painless, well-tolerated, easy-to-use and effective.12–13 MNs are solid needles measuring hundreds of microns in length and tapering to a sharp tip that are typically assembled in arrays on a patch that can be applied to the skin14–15. MN technology was originally developed for drug delivery into the body. When drug is coated on or encapsulated within MNs, the drug can be released typically by dissolution in the skin, thereby enabling simple administration of a drug that might otherwise require expert injection.
MN technology has been adapted for ISF collection, including suction-based ISF extraction through micropores16–17 hydrogel-forming MNs that swell with ISF in skin18–19, hollow microneedles that collect ISF by diffusion20 or pressure-driven flow21. In these cases, ISF is collected from the MN device and analyzed in a separate instrument, which requires an added step of ISF transfer. Other approaches involve in-situ biomarker detection such as selectively binding biomarkers to MN surfaces22–23, and incorporation of sensors into MNs for in situ analysis of ISF24–25. In these cases, the sensor is inserted into the body, which introduces significant design challenges to address safety/toxicity concerns and avoid sensor fouling.
To overcome limitations of other MN technologies, in this study we developed a MN patch that collects ISF from the skin (thereby avoiding complications of in-dwelling sensors) and allows on-patch detection of biomarkers and other molecules by surface-enhanced Raman spectroscopy (SERS) (thereby avoiding complicating sample transfer from the patch).
We selected surface enhanced Raman scattering (SERS) as a sensing modality for biomarker detection because it is a highly sensitive spectroscopic technique for molecular identification and detection26–27. SERS involves large enhancement of Raman scattering from molecules (analytes) absorbed on or near plasmonic nanostructures30. These plasmonic nanostructures are inert; photostable; optically tunable by changing nanostructure size, shape, composition and environment; and surface-stable for facile surface functionalization with various biological and organic molecules through covalent and non-covalent interactions28–29. These unique properties enable label-free SERS detection and molecular fingerprinting30 for medical diagnostic, environmental monitoring and homeland security applications31. For example, SERS has been used for in-vivo glucose sensing32–33 and for West Nile virus34 and cancer biomarker detection35. We and others have developed low-cost, flexible plasmonic substrates called ‘plasmonic paper’ by immobilizing functionalized plasmonic nanostructures (e.g. gold nanorods (AuNRs)) on filter paper for SERS-based detection36–38. In this study, we integrated plasmonic paper with MN patches to facilitate SERS-based detection of molecules present in ISF, collected by MNs.
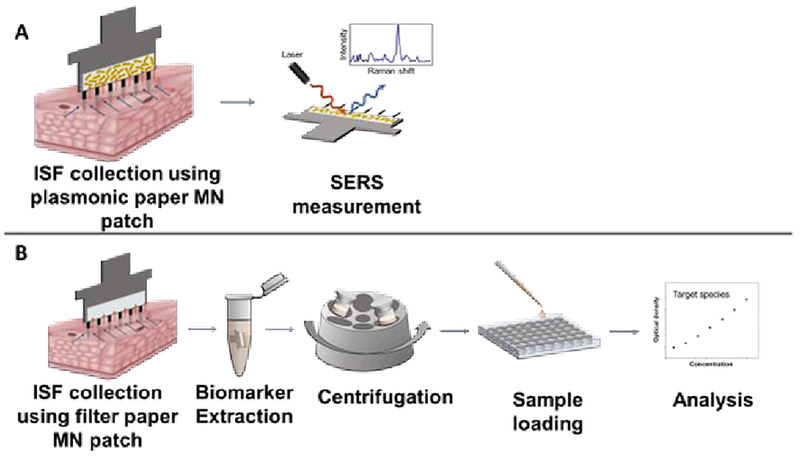
We used R6G as a model compound to demonstrate the proof-of concept. Using a plasmonic paper MN patch for on-patch detection of model molecules in dermal interstitial fluid, we can perform the detection via a two-step process: apply the MN patch to skin and measure concentration directly on the MN patch by SERS (Figure 1A). In contrast, ISF collected using a conventional paper MN patch would require multiple steps for analysis, such as MN patch application to skin, analyte extraction from the paper MN patch by incubation in extraction media (which dilutes the sample) and centrifugation, loading samples into multi-well plates or other substrates for measurement and finally measuring concentration by a suitable analytical method (Figure 1B).
Figure 1.
Detection of molecules in ISF using plasmonic paper MN patch for on-patch detection by SERS versus conventional paper MN patch requiring multiple sample processing steps. (A) After ISF is collected using a plasmonic paper MN patch, analysis can be performed in situ on the plasmonic paper using SERS. (B) After ISF is collected using a conventional paper MN patch, analyte of interest must be eluted and must undergo additional steps for sample processing and analysis.
EXPERIMENTAL SECTION
Cetyltrimethylammonium bromide (CTAB), chloroauric acid (HAuCl4), ascorbic acid, sodium borohydride, poly (styrene sulfonate) (PSS) (Mw = 70,000 g/mol), rat serum, filter paper (Whatman #1) and rhodamine 6G (R6G) were purchased from Sigma Aldrich (St. Louis, MO). Silver nitrate was purchased from VWR International (Radnor, PA). All chemicals were used as received.
Microneedle patch fabrication
MN patches comprising of 9 MNs (650 μm long), each measuring 50 μm × 150 μm in cross section at the base and tapering to a tip of <1 μm radius of curvature were prepared (Tech Etch, Plymouth, MA) and a paper strip (Whatman grade 1 filter paper)_was adhered to the base of each patch without covering the needles, as described previously39.
The MN patches with 2 mm × 7 mm filter paper were used as prepared while the MN patches with 1 mm × 7 mm filter paper were further modified with AuNRs.
Plasmonic paper microneedle patch preparation
AuNRs were synthesized using a seed-mediated approach as previously published40–41. The seed solution was prepared by mixing 0.6 ml of 10 mM ice-cold sodium borohydride solution with 10 ml of CTAB (0.1 M) and HAuCl4 (2.5 × 10−4 M) solution under vigorous stirring. The growth solution was prepared by gently mixing 95 ml of CTAB (0.1 M), 0.5 ml of silver nitrate (10 mM), 4.5 ml of HAuCl4 (10 mM), and 0.55 ml of ascorbic acid (0.1 M). AuNR solution was prepared by adding 0.12 ml of freshly prepared seed solution to the growth solution and left in the dark for 14 h. Prior to use, excess CTAB was removed from the AuNR solution by centrifuging twice at 9300 × g for 10 min in a centrifuge (Eppendorf 5810 R, Hamburg, Germany) and the AuNRs were redispersed in nanopure water (18.2 MΩ cm).
AuNRs were modified with PSS42 and concentrated to form a plasmonic ink43, as described previously. Briefly, 10 ml of PSS (0.2% w/v) in 6 mM NaCl aqueous solution were mixed with 10 ml of twice-centrifuged AuNR solution under vigorous stirring and sonicated for 60 min. Excess PSS solution was removed as supernatant after centrifuging at 9300 × g for 10 min. The recovered pellet of PSS-AuNR was then redispersed in nanopore water to obtain 100 μl of plasmonic ink (200-fold concentration). As described in our previous studies43, the plasmonic ink was then injected into a clean empty ballpoint pen refill (Paper Mate Profile, Oak Brook, IL) and used to “write” PSS-AuNRs onto the 1 mm × 7 mm filter paper. The plasmonic paper was thoroughly rinsed in DI water to remove loosely bound PSS-AuNRs, air-dried and adhered to MN patches to make plasmonic paper MN patches.
ISF collection procedure
A MN patch was inserted into rat skin 5–10 times while pinching the skin with a force of 20–40 N until the filter paper on the MN patch was visually determined to be saturated with ISF. This application force was easily administered by hand and has been shown in other studies of MN patch application not to be associated with pain44–45. The amount of ISF collected was estimated to be 1.1 ± 0.3 μl or 2.0 ± 0.2 μl once the 1 mm × 7 mm or 2 mm × 7 mm filter paper on the MN patch was saturated with ISF, respectively, as described previously46
Characterization
Transmission electron microscopy (TEM) micrographs were obtained using a JEM-2100F field emission instrument (JEOL, Peabody, MA) by drying 2 μl of AuNR solution on a glow discharge-treated carbon-coated grid. Scanning electron microscope (SEM) images were recorded on a gold-sputtered plasmonic paper by using a Nova 2300 Field Emission SEM (FEI, Hillsboro, OR) at an accelerating voltage of 10 kV. UV-Vis extinction spectra were measured using a UV-1800 UV-Vis spectrophotometer (Shimadzu Scientific Instruments Inc., Columbia, MD).
SERS measurements of R6G spiked ISF and serum samples
Raman spectra were collected using an inVia confocal Raman microscope (Renishaw, Gloucestershire, UK) mounted on Leica microscope and controlled with Wire 3.4 software. The 785 nm wavelength diode laser (0.5mW) coupled to a holographic notch filter with a grafting of 1200 lines mm−1 was focused onto the sample using a 20X objective (NA = 0.4) with 10s exposure time., and 1 accumulation was collected per spot. Six spectra were collected from different spots across each substrate, using a motorized XYZ translational stage integral to the microscope.
ISF calibrators were prepared by adding aliquots of R6G stock solution to ISF (extracted from porcine cadaver skin) to create the following concentrations: 0, 0.05, 0.5, 2, 5, 10, 25, 50 and 100 μM R6G in ISF. Serum calibrators were prepared by adding aliquots of R6G stock solution to rat serum (collected from rats in vivo) to create the following concentrations: 0, 0.5, 5, 10, 25, 50 and 100 μM of R6G in Serum. The plasmonic paper was soaked in 150 μL of calibrator for 1 hour, thoroughly rinsed in DI water for 5 min, and air dried prior to collecting SERS spectrum from six different locations across the substrate.
In-vivo study and pharmacokinetic analysis
Procedures were performed on six hairless rats (335–375 gm, female, Charles River Laboratories, Wilmington, MA) continuously anesthetized by isoflurane (Isothesia, Henry Schein Animal Health, Dublin, OH) in 100% oxygen inhalation during drug administration and sample collection. A silicone rubber tube was placed in the right jugular vein and kept locked with sodium heparin (100 U/ml) solution in physiological saline. Care was taken to avoid administration of air bubbles, and blood samples were replaced with an approximately equal volume of heparinized saline. Each rat was infused with 10 mg/ml R6G in sterile water via a 24-gauge angiocatheter in the tail vein at a rate of 0.1 ml/min over 30 min by means of an infusion pump (Harvard Apparatus, Holliston, MA).
Blood samples (≤ 500 μl) were collected in microtainer collection tubes with clot activator (BD Diagnostics, Franklin Lakes, NJ) from the jugular tube once prior to and at 2, 7, 15, 20, 30 and 35 min after the start of R6G infusion. Companion ISF samples were also collected using MN patches with plasmonic paper or bare filter paper (without AuNRs) from the lateral side of the rat at the same time points. At the end of the study, a final blood sample (≤ 1 ml) was collected from each rat before euthanizing by carbon dioxide gas asphyxiation without recovery from isoflurane. These experiments were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee.
Detection of R6G from ISF/serum samples using SERS
Serum was separated from blood samples by centrifuging at 6000 × g for 1.5 min in a centrifuge tube (Eppendorf 5415R, Hamburg, Germany). For SERS-based testing, 1 μl of each serum sample was added on a piece of plasmonic paper and allowed to air dry. The ISF samples collected on plasmonic paper MN patches were tested directly. After rinsing in water, six spectra were collected from different locations across each plasmonic paper substrate to measure the intensity of the 1364 cm−1 Raman band. The corresponding concentration of R6G was determined from a calibration curve.
Detection of R6G from ISF/serum samples using fluorescence spectroscopy
A 1:50 dilution of serum samples was performed by mixing 2 μl of serum with 100 μl of DI water and a 1:50 dilution of ISF samples was performed by extracting ISF from the MN patches by centrifuging at 6000 × g for 1.5 min in 100 μl DI water per MN patch. The R6G concentration in ISF and serum samples was measured against a standard calibration curve of R6G in a 384 well plate (Costar Black Polypropylene, Corning, Corning, NY) using a Synergy H4 hybrid reader (BioTek, Winooski, VT) at an emission wavelength of 516 nm and absorption wavelength of 557 nm.
Determination of R6G in protein-free serum
To determine the extent of binding of R6G to plasma protein, 500 μl of serum sample collected prior to euthanizing the rats was centrifuged in a VivaSpin 500 centrifugal filter (MWCO 30,000; Vivaproducts, Littleton, MA) at 15,000 × g for 5 min. The R6G concentrations in the protein-free filtrate and in the serum prior to centrifugation were measured using the Synergy H4 hybrid reader, as described above.
Statistical analysis
Statistics were calculated using either Origin software (OriginLab, Northampton, MA) or Excel (Microsoft, Redmond, WA). All listed averages represent the arithmetic mean of the samples. Comparisons between individual samples were done using an unpaired t-test. Probability (p) values of <0.05 were considered significant.
RESULTS AND DISCUSSION
Fabrication of plasmonic paper
We employed AuNRs as plasmonic nanostructures for the fabrication of plasmonic paper. AuNRs synthesized by seed-mediated method were found to be 88 ± 7 nm in length and 33 ± 2 nm in width, with an aspect ratio of nearly 3 (Figure 2A). AuNRs were coated with negatively charged PSS to promote binding of positively charged R6G, which is the model compound used in this study. The optical extinction spectra of PSS-AuNRs showed two characteristic peaks at 513 nm and 683 nm, corresponding to the transverse and longitudinal plasmon resonances of the AuNRs (Figure 2B).
Figure 2.
Characterization of AuNRs and plasmonic paper. (A) Representative transmission electron microscopy image of AuNRs dispersed in nanopure water. (B) Representative ultraviolet-visible light extinction spectrum of PSS-AuNRs in nanopure water. (C) Representative scanning electron microscopy image of PSS-AuNRs adsorbed on filter paper after “drawing” by plasmonic calligraphy.
Two hundred-fold concentrated PSS-AuNRs solution was filled in a ball point pen refill and written onto a 1 mm × 7 mm filter paper adhered to a steel MN patch and rinsed with DI water to remove any loosely adhering AuNRs. SEM imaging depicts an evenly speckled surface morphology of the paper (Figure 2C), indicating uniform adsorption of AuNRs to the paper without significant aggregation.
ISF collection using plasmonic paper microneedle patch
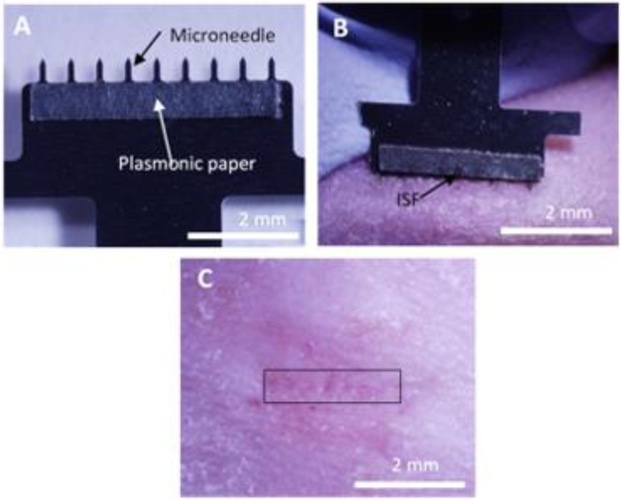
We developed a MN patch to collect ISF from skin. The MN patch was comprised of a stainless-steel array of nine, 650 μm long MNs (used to create micropores in the skin surface) extending from a backing layer (for ease of handling). A strip of plasmonic paper was adhered to one side of the patch backing to create a reservoir calibrated to collect ~1 μl of ISF and bind positively charged R6G (Figure 3A). ISF collection was performed by applying the MN patch to the skin so that the MNs penetrated into the skin surface to create micropores and induce flow of ISF out of the skin and into the paper reservoirs (Figure 3B). MN insertion was repeated at a rate of ~1 insertion per second until ~1 μl of ISF was collected. This process usually required up to 5 MN patch insertions. The MN insertion procedure was well tolerated (Figure 3C) with a very mild, transient erythema observed at the insertion site.
Figure 3.
Collection of ISF using a plasmonic paper microneedle patch. (A) Representative photographic image of a MN patch showing a row of nine MNs measuring 650 μm in length extending from a patch backing with a thin rectangular strip of plasmonic paper adhered to one side. (B) Representative photographic image of the onset of ISF collection after two insertions of a MN patch into the skin of a hairless rat in vivo. The MNs, which are on the lower edge of the patch, are not visible because they are inserted into the skin. ISF is collected in the paper reservoir as observed by wetness of the paper (arrow). (C) Representative photographic image of rat skin 1 min after ISF collection by MN patch. The site of MN insertion has been marked with a rectangular box.
SERS performance of plasmonic paper
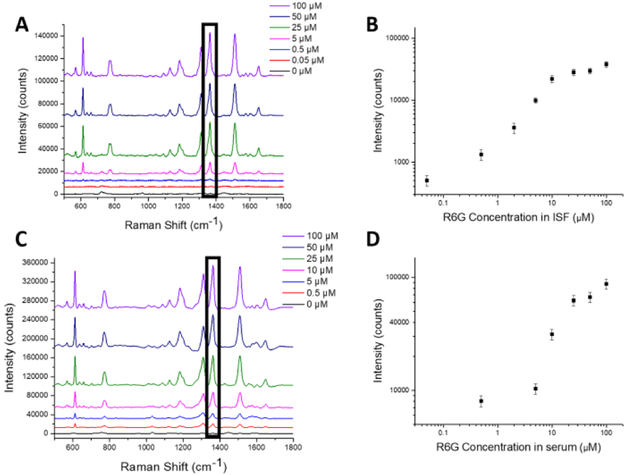
We then turn our attention to the SERS performance of the plasmonic paper using R6G as a model analyte. To acquire a calibration curve, we performed SERS measurements on plasmonic paper incubated with ISF or serum (collected from rats) spiked with a range of concentrations of R6G (Figures 4A and 4C). All measurements were performed after air drying the plasmonic paper. With increasing R6G concentration, the Raman spectra show an increase in intensities of characteristic Raman bands of R6G at 610, 1364 and 1512 cm−1, which are attributed to the C-C-C ring in-plane bending, C-O-C stretching and aromatic C-C stretching modes of R6G47–48.
Figure 4.
Measurement of R6G in plasmonic paper by SERS. Representative SERS spectra obtained from plasmonic paper soaked in different concentrations of R6G in (A) ISF and (C) serum. The Raman spectra show an increase in characteristic Raman peaks for R6G at 610, 1364 and 1512 cm−1 with increase in concentration. The Raman band at 1364 cm−1 (indicated with a black rectangular box) was used to generate calibration curves for R6G concentration in (B) ISF and (D) serum. Data points show mean ± standard deviation from 6 spectra collected from each plasmonic paper substrate.
The intensity of Raman band at 1364 cm−1, which is the most intense Raman band for R6G, was plotted against R6G concentration to generate a calibration curve with good fit for both ISF and serum (R2 = 0.97 and 0.98, respectively) (Figures 4B and 4D). The relative standard deviation is ~15%, which is close to the values observed for commercially available microfabricated SERS substrates49. Both the ISF and serum spectra in absence of R6G showed low Raman counts at 1364 cm−1 suggesting no interference (Figures 4B and 4D). These results indicate that plasmonic paper can serve as a powerful tool to detect and quantify R6G in biological fluids. These calibration curves were used in the subsequent studies to determine the R6G concentration in ISF and serum samples collected in the R6G pharmacokinetic studies presented below.
R6G pharmacokinetics in ISF determined by on-patch SERS measurement using plasmonic paper microneedle patches
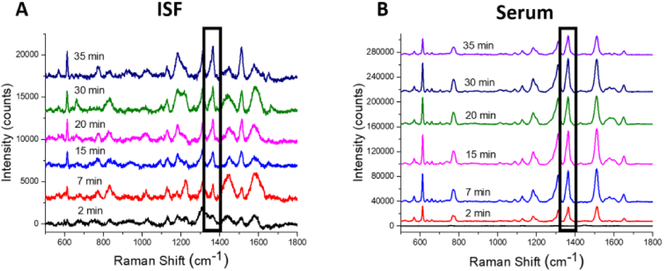
To assess the utility of plasmonic paper MN patches, we studied the pharmacokinetics of R6G in ISF and serum in rats as measured by on-patch SERS after allowing the ISF to air dry. The Raman bands in the spectra obtained from ISF and serum look similar, although the intensity of the bands in ISF is about an order of magnitude lower than in serum, as discussed below (Figure 5).
Figure 5.
Representative spectra from on-patch SERS measurement of samples collected in rats in vivo. (A) ISF was collected on plasmonic paper MN patches. (B) Blood was collected by intravenous catheter, from which serum was isolated and placed on plasmonic paper MN patches.
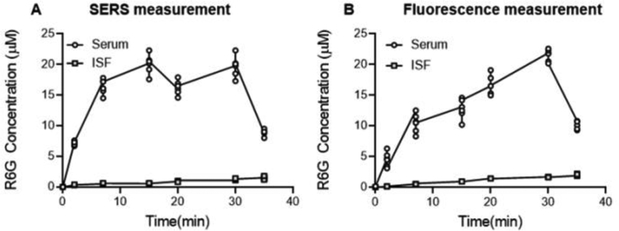
We also performed ISF collection using conventional paper MN patches from which R6G was eluted and measured by fluorescence spectroscopy for comparison. When comparing R6G concentrations by on-patch SERS versus off-patch fluorescence spectroscopy, there were no statistically significant differences in the concentrations measured in ISF (ANOVA, p = 0.61) or in serum samples (ANOVA, p = 0.68) (Figure 6).
Figure 6.
Pharmacokinetics of R6G in ISF and serum. (A) On-patch SERS measurement using plasmonic paper MN patches or (B) fluorescence spectroscopy of R6G eluted from conventional paper MN patches. Data points show mean ± standard deviation (n = 6 rats).
Whether measured by SERS or fluorescence, there are significant differences in the pharmacokinetic profiles in ISF versus serum. In the experiment, R6G was slowly infused intravenously in each animal for 30 min. The resulting R6G concentration profile in serum reflects this, showing an increase in R6G concentration for up to 30 min followed by a sharp decrease in concentration after 30 min in both the measurement techniques (Figure 6). The pharmacokinetic profile in ISF, in contrast, shows a steady increase, without the sudden drop in R6G concentration after 30 min. Moreover, the concentration profile in ISF is an order of magnitude lower than in serum in both the measurement techniques. As discussed below, these differences in the pharmacokinetic profiles may be explained by binding of R6G to plasma proteins, resulting in a steady increase in R6G concentration in ISF.
Binding of R6G to plasma proteins
To understand the lower concentration of R6G seen in ISF compared to serum, we isolated protein-free filtrate from serum samples from the pharmacokinetic study. R6G concentration in serum prior to filtration (9.6 ± 4.2 μM) was dramatically higher than in protein-free serum samples (0.05 ± 0.03 μM) (Student’s t-test, p < 0.008). This indicates that R6G strongly binds to plasma proteins and therefore may not partition well into ISF. This finding is consistent with prior literature, which also reported significant binding of R6G with human serum albumin50. While many plasma proteins are also found in ISF, most of them such as albumin are found at much lower concentration due to capillary membrane and interstitial barriers51–52
ISF is a rich source of biomarkers but it has been relatively unexplored due to lack of good sampling methods. Current methods like suction blister, microdialysis and open flow microperfusion cause significant skin trauma, are time consuming and require expert personnel and equipment to perform8–11. They also require sample processing steps to prepare samples for analysis. This proof of concept study introduces a novel plasmonic paper MN patch for SERS-based detection of molecules in ISF using R6G as a model compound to simulate a biomarker. The plasmonic paper MN patch is minimally invasive, rapid, and simple-to-use. This MN patch also has the capability to perform on-patch SERS-based detection of molecules in ISF using a paper reservoir that captures molecules with functionalized AuNRs. Next steps in this research include optimization of the MN patch for usability, safety and efficacy and development of the sensor for LSPR or SERS-based detection of biomolecules of clinical significance in ISF for future possible medical applications.
The plasmonic paper MN patch involves a simple, low-cost design using readily available materials. The MN array is fabricated from stainless steel sheets by chemical etching, which can be performed in mass production for pennies per array. The paper reservoir is made of conventional filter paper that has high surface area, is low-cost, is biodegradable, is compatible with conventional printing approaches and is commonly used in paper-based sensor devices53–55. The plasmonic calligraphy method controls test domain size in a simple manner by writing with a pen in the desired area. This method also offers the possibility for multiplexed biosensing of multiple biomarkers by simply ‘writing’ different test domains with plasmonic nanostructures functionalized to target different biomarkers of clinical significance. The manufacturing process is scalable due to possibility of inkjet printing of the plasmonic inks onto the paper.
SERS has become a mature analytical technique over the last decades with the development of low-cost, handheld Raman spectrometers. Moreover, development of SERS for medical diagnostic applications has been rapidly increasing56, and SERS has been shown to be useful for glucose sensing in vivo32–33, detection of diseases such as cholera57 and detection for potential exposure to explosives58. Thus, a plasmonic paper MN patch could be a low-cost, portable, miniature diagnostic device suitable for point-of-care treatment in resource-limited environments.
CONCLUSIONS
We developed a plasmonic paper MN patch for on-patch SERS based detection of molecules in ISF, and demonstrated its functionality using R6G as a model analyte. The MN patch consisted of a stainless-steel MN patch that created micropores in skin to access ISF and included a plasmonic paper, as ISF reservoir, which was prepared by immobilizing PSS-AuNRs on a thin strip of filter paper by plasmonic calligraphy. The negatively charged PSS-AuNRs bound to positively charged R6G in ISF, thereby dramatically enhancing the Raman scattering from R6G and thus enabling R6G detection by SERS. Because the plasmonic paper was integrated into the MN patch, it enabled on-patch detection of molecules without additional sample preparation. The utility of this patch was demonstrated by measuring the pharmacokinetic profile of R6G in ISF and serum from rats. This study shows that a plasmonic paper MN patch enables on-patch measurement of molecules in ISF, which can be used for future point-of-care diagnostic applications.
5. ACKNOWLEDGMENT
We thank Donna Bondy for administrative support. This work was supported in part by the National Institutes of Health (R21EB025499 and R01DE027098) and Office of Naval Research (N00014-16-1-3030).
Footnotes
Mark Prausnitz is an inventor of patents that have been or may be licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products, including Micron Biomedical. These potential conflicts of interest have been disclosed and are being managed by Georgia Tech and/or Emory University.
REFERENCES
- 1.Vashist SK; Luppa PB; Yeo LY; Ozcan A; Luong JH, Emerging technologies for next-generation point-of-care testing. Trends in biotechnology 2015, 33 (11), 692–705. [DOI] [PubMed] [Google Scholar]
- 2.Deacon B; Abramowitz J, Fear of needles and vasovagal reactions among phlebotomy patients. J. Anxiety Disord 2006, 20 (7), 946–960. [DOI] [PubMed] [Google Scholar]
- 3.Anderson NL; Anderson NG, The human plasma proteome history, character, and diagnostic prospects. Mol. Cell. Proteomics 2002, 1 (11), 845–867. [DOI] [PubMed] [Google Scholar]
- 4.Niedzwiecki MM; Samant P; Walker DI; Tran V; Jones DP; Prausnitz MR; Miller GW, Human Suction Blister Fluid Composition Determined Using High-Resolution Metabolomics. Anal. Chem 2018, 90 (6), 3786–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran BQ; Miller PR; Taylor RM; Boyd G; Mach PM; Rosenzweig CN; Baca JT; Polsky R; Glaros T, Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J. Proteome Res 2017, 17 (1), 479–485. [DOI] [PubMed] [Google Scholar]
- 6.Celis JE; Gromov P; Cabezón T; Moreira JM; Ambartsumian N; Sandelin K; Rank F; Gromova I, Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment A novel resource for biomarker and therapeutic target discovery. Mol. Cell. Proteomics 2004, 3 (4), 327–344. [DOI] [PubMed] [Google Scholar]
- 7.Kastellorizios M; Burgess DJ, Continuous metabolic monitoring based on multi-analyte biomarkers to predict exhaustion. Sci. Rep 2015, 5, 10603, doi: 10.1038/srep10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiistala U; Mustakallio K, Dermo-epidermal separation with suction: electron microscopic and histochemical study of initial events of blistering on human skin. J. Invest. Dermatol 1967, 48 (5), 466–477. [PubMed] [Google Scholar]
- 9.Sieg A; Guy RH; Delgado-Charro MB, Noninvasive glucose monitoring by reverse iontophoresis in vivo: application of the internal standard concept. Clin. Chem 2004, 50 (8), 1383–1390. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt S; Banks R; Kumar V; Rand KH; Derendorf H, Clinical microdialysis in skin and soft tissues: an update. J. Clin. Pharmacol 2008, 48 (3), 351–364. [DOI] [PubMed] [Google Scholar]
- 11.Ellmerer M; Schaupp L; Brunner GA; Sendlhofer G; Wutte A; Wach P; Pieber TR, Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. American Journal of Physiology-Endocrinology and Metabolism 2000, 278 (2), E352–E356. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RF; Mooney K; Caffarel-Salvador E; Torrisi BM; Eltayib E; McElnay JC, Microneedle-mediated minimally invasive patient monitoring. Ther. Drug Monit 2014, 36 (1), 10–17. [DOI] [PubMed] [Google Scholar]
- 13.El-Laboudi A; Oliver NS; Cass A; Johnston D, Use of microneedle array devices for continuous glucose monitoring: a review. Diabetes Technol. Ther 2013, 15 (1), 101–115. [DOI] [PubMed] [Google Scholar]
- 14.Prausnitz MR, Engineering microneedle patches for vaccination and drug delivery to skin. Annu. Rev. Chem. Biomol. Eng 2017, 8, 177–200. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TT; Park JH, Human studies with microneedles for evaluation of their efficacy and safety. Expert Opin. Drug Delivery 2018, 15 (3), 235–245. [DOI] [PubMed] [Google Scholar]
- 16.Samant PP; Prausnitz MR, Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci 2018, 115, 4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang PM; Cornwell M; Prausnitz MR, Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol. Ther 2005, 7 (1), 131–141. [DOI] [PubMed] [Google Scholar]
- 18.Chang H; Zheng M; Yu X; Than A; Seeni RZ; Kang R; Tian J; Khanh DP; Liu L; Chen P, A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater 2017, 29 (37), doi: 10.1002/adma.201702243. Epub 2017 Jul 17. [DOI] [PubMed] [Google Scholar]
- 19.Caffarel-Salvador E; Brady AJ; Eltayib E; Meng T; Alonso-Vicente A; Gonzalez-Vazquez P; Torrisi BM; Vicente-Perez EM; Mooney K; Jones DS, Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PloS one 2015, 10 (12), e0145644, doi: 10.1371/journal.pone.0145644. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg SK; Potts RO; Ackerman NR; Fermi SJ; Tamada JA; Chase HP, Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care 1999, 22 (10), 1708–1714. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RM; Miller PR; Ebrahimi P; Polsky R; Baca JT, Minimally-invasive, microneedle-array extraction of interstitial fluid for comprehensive biomedical applications: transcriptomics, proteomics, metabolomics, exosome research, and biomarker identification. Laboratory animals 2018, 526–530.. [DOI] [PubMed] [Google Scholar]
- 22.Coffey JW; Meliga SC; Corrie SR; Kendall MA, Dynamic application of microprojection arrays to skin induces circulating protein extravasation for enhanced biomarker capture and detection. Biomater. 2016, 84, 130–143. [DOI] [PubMed] [Google Scholar]
- 23.Muller DA; Corrie SR; Coffey J; Young PR; Kendall MA, Surface modified microprojection arrays for the selective extraction of the dengue virus NS1 protein as a marker for disease. Anal. Chem 2012, 84 (7), 3262–3268. [DOI] [PubMed] [Google Scholar]
- 24.Miller PR; Xiao X; Brener I; Burckel DB; Narayan R; Polsky R, Microneedle‐Based Transdermal Sensor for On‐Chip Potentiometric Determination of K+. Adv. Healthcare Mater 2014, 3 (6), 876–881. [DOI] [PubMed] [Google Scholar]
- 25.Venugopal M; Feuvrel KE; Mongin D; Bambot S; Faupel M; Panangadan A; Talukder A; Pidva R, Clinical evaluation of a novel interstitial fluid sensor system for remote continuous alcohol monitoring. IEEE Sens. J 2008, 8 (1), 71–80. [Google Scholar]
- 26.Stiles PL; Dieringer JA; Shah NC; Van Duyne RP, Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem 2008, 1, 601–626. [DOI] [PubMed] [Google Scholar]
- 27.Kneipp K; Wang Y; Kneipp H; Perelman LT; Itzkan I; Dasari RR; Feld MS, Single molecule detection using surface-enhanced Raman scattering (SERS). Physical review letters 1997, 78 (9), 1667–1670. [Google Scholar]
- 28.Jain PK; Lee KS; El-Sayed IH; El-Sayed MA, Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. The journal of physical chemistry B 2006, 110 (14), 7238–7248. [DOI] [PubMed] [Google Scholar]
- 29.Kelly KL; Coronado E; Zhao LL; Schatz GC, The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. ACS Publications: 2003, 668–677. [Google Scholar]
- 30.Schlücker S, Surface‐Enhanced raman spectroscopy: Concepts and chemical applications. Angewandte Chemie International Edition 2014, 53 (19), 4756–4795. [DOI] [PubMed] [Google Scholar]
- 31.Rosi NL; Mirkin CA, Nanostructures in biodiagnostics. Chemical reviews 2005, 105 (4), 1547–1562. [DOI] [PubMed] [Google Scholar]
- 32.Ma K; Yuen JM; Shah NC; Walsh JT Jr; Glucksberg MR; Van Duyne RP, In vivo, transcutaneous glucose sensing using surface-enhanced spatially offset Raman spectroscopy: multiple rats, improved hypoglycemic accuracy, low incident power, and continuous monitoring for greater than 17 days. Analytical chemistry 2011, 83 (23), 9146–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart DA; Yuen JM; Shah N; Lyandres O; Yonzon CR; Glucksberg MR; Walsh JT; Van Duyne RP, In vivo glucose measurement by surface-enhanced Raman spectroscopy. Analytical chemistry 2006, 78 (20), 7211–7215. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H; Harpster MH; Park HJ; Johnson PA; Wilson WC, Surface-enhanced Raman scattering detection of DNA derived from the West Nile virus genome using magnetic capture of Raman-active gold nanoparticles. Analytical chemistry 2010, 83 (1), 254–260. [DOI] [PubMed] [Google Scholar]
- 35.Wang G; Lipert RJ; Jain M; Kaur S; Chakraboty S; Torres MP; Batra SK; Brand RE; Porter MD, Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Analytical chemistry 2011, 83 (7), 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CH; Tian L; Singamaneni S, based SERS swab for rapid trace detection on real-world surfaces. ACS applied materials & interfaces 2010, 2 (12), 3429–3435. [DOI] [PubMed] [Google Scholar]
- 37.Polavarapu L; Liz-Marzán LM, Towards low-cost flexible substrates for nanoplasmonic sensing. Physical Chemistry Chemical Physics 2013, 15 (15), 5288–5300. [DOI] [PubMed] [Google Scholar]
- 38.Abbas A; Brimer A; Slocik JM; Tian L; Naik RR; Singamaneni S, Multifunctional Analytical Platform on a Paper Strip: Separation, Preconcentration, and Subattomolar Detection. Analytical Chemistry 2013, 85 (8), 3977–3983. [DOI] [PubMed] [Google Scholar]
- 39.Kolluru C; Williams M; Yeh JS; Noel RK; Knaack J; Prausnitz MR, Monitoring drug pharmacokinetics and immunologic biomarkers in dermal interstitial fluid using a microneedle patch. Biomedical microdevices 2019, 21 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orendorff CJ; Murphy CJ, Quantitation of metal content in the silver-assisted growth of gold nanorods. The Journal of Physical Chemistry B 2006, 110 (9), 3990–3994. [DOI] [PubMed] [Google Scholar]
- 41.Huang J; Zhu H; Chen Y; Preston C; Rohrbach K; Cumings J; Hu L, Highly transparent and flexible nanopaper transistors. Acs Nano 2013, 7 (3), 2106–2113. [DOI] [PubMed] [Google Scholar]
- 42.Pastoriza-Santos I; Pérez-Juste J; Liz-Marzán LM, Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chemistry of Materials 2006, 18 (10), 2465–2467. [Google Scholar]
- 43.Tian L; Tadepalli S; Park SH; Liu K-K; Morrissey JJ; Kharasch ED; Naik RR; Singamaneni S, Bioplasmonic calligraphy for multiplexed label-free biodetection. Biosensors and Bioelectronics 2014, 59, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouphael NG; Paine M; Mosley R; Henry S; McAllister DV; Kalluri H; Pewin W; Frew PM; Yu T; Thornburg NJ, The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. The Lancet 2017, 390 (10095), 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arya J; Henry S; Kalluri H; McAllister DV; Pewin WP; Prausnitz MR, Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomater. 2017, 128, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolluru C; Williams M; Chae J; Prausnitz MR, Recruitment and Collection of Dermal Interstitial Fluid Using a Microneedle Patch. Advanced healthcare materials 2019, 1801262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen L; Schatz GC, Resonance Raman scattering of rhodamine 6G as calculated using time-dependent density functional theory. The Journal of Physical Chemistry A 2006, 110 (18), 5973–5977. [DOI] [PubMed] [Google Scholar]
- 48.Hildebrandt P; Stockburger M, Surface-enhanced resonance Raman spectroscopy of Rhodamine 6G adsorbed on colloidal silver. The Journal of Physical Chemistry 1984, 88 (24), 5935–5944. [Google Scholar]
- 49.Hankus ME; Stratis-Cullum DN; Pellegrino PM In Characterization of next-generation commercial surface-enhanced Raman scattering (SERS) substrates, Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XII, International Society for Optics and Photonics: 2011; 80180P, doi: 10.1117/12.886779. [DOI] [Google Scholar]
- 50.Das DK; Mondal T; Mandal AK; Bhattacharyya K, Binding of Organic Dyes with Human Serum Albumin: A Single‐Molecule Study. Chemistry–An Asian Journal 2011, 6 (11), 3097–3103. [DOI] [PubMed] [Google Scholar]
- 51.Fogh-Andersen N; Altura BM; Altura BT; Siggaard-Andersen O, Composition of interstitial fluid. Clin. Chem 1995, 41 (10), 1522–5. [PubMed] [Google Scholar]
- 52.Bent-Hansen L, Whole body capillary exchange of albumin. Acta physiologica scandinavica. Supplementum 1991, 603, 5–10. [PubMed] [Google Scholar]
- 53.Martinez AW; Phillips ST; Nie Z; Cheng C-M; Carrilho E; Wiley BJ; Whitesides GM, Programmable diagnostic devices made from paper and tape. Lab Chip 2010, 10 (19), 2499–2504. [DOI] [PubMed] [Google Scholar]
- 54.Carvalhal RF; Simaão Kfouri M; de Oliveira Piazetta MH; Gobbi AL; Kubota LT, Electrochemical detection in a paper-based separation device. Anal. Chem 2010, 82 (3), 1162–1165. [DOI] [PubMed] [Google Scholar]
- 55.Bracher PJ; Gupta M; Whitesides GM, Patterned paper as a template for the delivery of reactants in the fabrication of planar materials. Soft Matter 2010, 6 (18), 4303–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie W; Schlücker S, Medical applications of surface-enhanced Raman scattering. Physical Chemistry Chemical Physics 2013, 15 (15), 5329–5344. [DOI] [PubMed] [Google Scholar]
- 57.Schmit V; Martoglio R; Carron K, Lab-on-a-bubble surface enhanced Raman indirect immunoassay for cholera. Analytical chemistry 2012, 84 (9), 4233–4236. [DOI] [PubMed] [Google Scholar]
- 58.Botti S; Almaviva S; Cantarini L; Palucci A; Puiu A; Rufoloni A, Trace level detection and identification of nitro‐based explosives by surface‐enhanced Raman spectroscopy. Journal of Raman Spectroscopy 2013, 44 (3), 463–468. [Google Scholar]