Abstract
Background & Aims
Immunotherapies are ineffective against pancreatic cancer. We investigated whether the activity of nuclear factor (NF)κB in pancreatic stromal cells contributes to an environment that suppresses anti-tumor immune response.
Methods
Pancreata of C57BL/6 or Rag1−/− mice were given pancreatic injections of a combination of KrasG12D/+; Trp53 R172H/+; Pdx-1cre (KPC) pancreatic cancer cells and pancreatic stellate cells (PSCs) extracted from C57BL/6 (control) or mice with disruption of the gene encoding the NFkB p50 subunit (Nfkb1 or p50−/− mice). Tumor growth was measured as an endpoint. Other mice were given injections of LLC lung cancer cells or B16-F10 melanoma cells with control or p50−/− fibroblasts. Cytotoxic T cells were depleted from C57BL/6 mice by administration of antibodies against CD8 (anti-CD8), and growth of tumors from KPC cells, with or without control or p50−/− PSCs, was measured. Some mice were given an inhibitor of CXCL12 (AMD3100) and tumor growth was measured. T-cell migration toward cancer cells was measured using the Boyden chamber assay.
Results
C57BL/6 mice co-injected with KPC cells (or LLC or B16-F10 cells) and p50−/− PSCs developed smaller tumors than mice given injections of the cancer cells along with control PSCs. Tumors that formed when KPC cells were injected along with p50−/− PSCs had increased infiltration by activated cytotoxic T cells along with decreased levels of CXCL12, compared to tumors grown from KPC cells injected along with control PSCs. KPC cells, when co-injected with control or p50−/− PSCs, developed the same size tumors when CD8+ T cells were depleted from C57BL/6 mice or in Rag1−/− mice. The CXCL12 inhibitor slowed tumor growth and increased tumor infiltration by cytotoxic T cells. In vitro expression of p50 by PSCs reduced T-cell migration toward and killing of cancer cells. When cultured with cancer cells, control PSCs expressed 10-fold higher levels of CXCL12 than p50−/− PSCs. The CXCL12 inhibitor increased migration of T cells toward KPC cells in culture.
Conclusions
In studies of mice and cell lines, we found that NFκB activity in PSCs promotes tumor growth by increasing expression of CXCL12, which prevents cytotoxic T cells from infiltrating the tumor and killing cancer cells. Strategies to block CXCL12 in pancreatic tumor cells might increase anti-tumor immunity.
Keywords: immune suppression, cytokine, chemokine, CXCR4
Introduction
Cancer development and progression are complex processes that involve changes in normal cells as well as their environment. Both innate and adaptive immune systems are known to protect against tumor formation1, 2. During tumorigenesis, besides developing survival mechanisms to evade senescence and cell death, and to continue proliferating in a harsh environment, a cancer cell evolves various strategies to escape immune attack3. Cancer can manifest itself only when the immune system is no longer able to contain tumor growth and the balance tilts in favor of a progressively growing tumor4–7. However, recent success with immunotherapy in melanoma and in non-small cell lung cancer suggests that even during the stage of immune escape, elements of the anti-tumor immune machinery can be harnessed for therapeutic gains. This is promising but even in immunotherapy-sensitive cancers such as melanoma, the responses to immunotherapy are variable and present in only about 30% of patients. Furthermore, many solid tumors such as pancreatic cancer8, 9 respond poorly to immunotherapy and are thus considered non-immunogenic or “cold”10. A comprehensive understanding of the mechanisms responsible for tumor immune evasion and a search for clinically relevant strategies to harness these signals is needed to usher in the next phase of anti-tumor immunotherapies.
Recent studies have elucidated some of the mechanisms by which cancer cells evade the immune system. For instance, it has been shown that various cytokines and chemokines secreted by cancer cells modulate innate and adaptive immune cells for their benefit10. While many of these effects are directly mediated by cancer cells, some studies suggest that the tumor stroma can also help create and sustain an immunosuppressive environment11. This is of special significance for pancreatic cancer, as its dense stroma has been shown to actively assist enhanced tumor growth12, 13. Understanding the mechanism(s) by which the stroma helps cancer cells to protect themselves from the immune system can help devise strategies for therapeutic gain, either as a stand-alone therapy or to augment the efficacy of immunotherapy. In the current study, we provide evidence that pancreatic cancer uses a desmoplastic stroma to evade the immune system. We further demonstrate that the cancer-associated fibroblasts (CAFs) inhibit cytotoxic T cell infiltration, thereby promoting tumor growth. We also demonstrate that the NFκB pathway mediates the immune-protective effects of CAFs. Our results suggest that stromal cells secrete CXCL12 (SDF-1) in an NFKB1-dependent fashion; which acts as a ‘chemo-repellant’ and prevents CD8+ T cell infiltration. Our observations are further supported by our studies where a pharmacologic inhibitor of CXCL12-CXCR4 interaction enhances CD8+ T cell infiltration and decreases tumor growth. Together, this data suggests that modulation of the stroma either by NFKB1 inhibition in CAFs or by counteracting mediators of its effect such as CXCL12 could emerge as a novel therapeutic strategy for pancreatic cancer.
Material and Methods
Animals and in vivo procedures
All animal experiments were performed in accordance with requirements of the Institutional Animal Care and Use Committee after their review and approval of the protocol. C57BL/6, p50−/− Rag1−/−, athymic nude-Foxn1nu and C57BL/6-Tg (CAG-EGP) Osb/J mice were purchased from The Jackson Laboratories (Bar Harbor, ME). KPC mice, which develop spontaneous tumors by expressing mutant KRAS and P53, were generated by crossing LSL-KrasG12D/+, LSL-Trp53R172H/+ with Pdx-Cre14. Both male and female animals were used and the animals were sex- and age-matched in each experiment. For orthotropic tumor challenge, C57BL/6 mice were administered intra-pancreatic injections of tumor cells derived from KPC mice along with WT and Nfkb1−/−-PSC (p50−/− PSCs) extracted from C57BL/6 and p50−/− mice (1:9 ratio in Matrigel) respectively and mice were sacrificed at 3 weeks. Tumor weight and volume were measured at the indicated time points. To see whether a similar process occur with other cancers, fibroblasts were extracted from lung and dermis of WT and p50−/−mice. LLC (Lewis lung carcinoma) and B16-F10 cells with or without their respective fibroblasts were subcutaneously injected at a ratio of 1:9 in C57BL/6 mice suspended in matrigel. In the CD8 depletion experiment, animals were orthotopically injected with KPC cells alone or along with WT and p50−/−-PSCs. Following this, the mice were randomized and injected intraperitoneally with neutralizing mAbs directed against CD8 (clone YTS169.4; BioXcell) with its corresponding isotype (clone: LTF2; BioXcell) for 4 weeks. Mice were sacrificed after 5 weeks of tumor challenge and the efficiency of CD8-depletion was evaluated in splenic cells by flow cytometry. Additional experiment details are provided in the Supplementary Section.
Results
Loss of NFκB in tumor stroma leads to reduced tumor growth
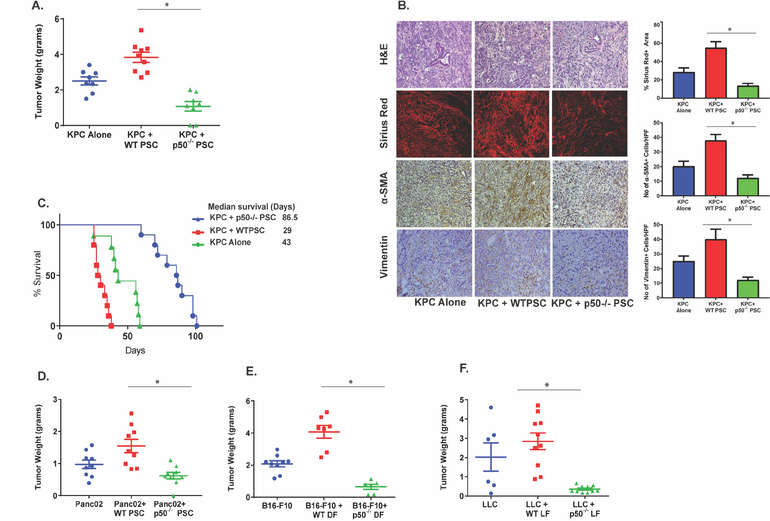
To evaluate the role of NFκB in tumor stroma on tumor progression, we co-injected KPC cancer cells (isolated from cancer developing in KrasG12D/+; Trp53R172H/+; Pdx-1cre mouse model14) with pancreatic stellate cells (PSC) isolated from wild type (C57BL/6, WT) or mice lacking NFκB p50-subunit (also known as NFκb1−/−, p50−/−)15 into the pancreas of a WT mouse. Cancer cells injected without any stromal cells were used as controls. The characterization of the stellate cells isolated from p50−/− mice was performed using methods described by Apte et al16 (Suppl. Figure 1A and B). As seen in Figure 1A, co-injection of KPC cells with WT-PSCs led to increased growth of tumors when compared with KPC cells injected alone. We also observed that the loss of NFKB1 in tumor stroma led to reduced tumor growth as compared to KPC cells injected alone or when injected with WT-PSC (Figure-1A). H&E staining of tumor sections obtained from animals with tumors, with or without NFKB1 depleted in their stroma, revealed that at a global level, the loss of NFKB1 in tumor stroma did not affect the tumor architecture (Figure-1B). However, lack of NFKB1 in the stroma did lead to modulation of stromal composition. For instance, expression of collagen (as measured by Sirius red staining), α-SMA and vimentin decreased when NFKB1 was depleted in the stroma (Figure 1B) whereas the expression of fibronectin was unchanged (Suppl. Figure 1C). These results were corroborated with Western blots, where lack of NFKB1 in the stroma led to decreased expression of α-SMA and vimentin, without change in fibronectin levels (Suppl. Figure 1 C–E). We also used a survival model to evaluate the effect of NFKB1 depletion in tumor stroma on tumor growth. As seen in Figure 1C, genetic deletion of Nfkb1 in tumor stroma led to increased survival. However, it appears that the tumors eventually overcame the lack of NFKB1 in the CAFs and that tumor growth was responsible for the demise of the animals. To evaluate the mechanism by which the cancer cells can eventually overcome the lack of NFκB in stroma, KPC pancreatic cancer cells were co-injected with p50−/− PSCs into the pancreas of EGFP mouse. This was to test the hypothesis that over time, the WT-PSCs from the host are recruited and overcome the effect of absence of NFκB in the stroma, thus providing an additional advantage to the tumors to grow. As seen in Suppl. Figure 1F and G, after a while, NFκB-depleted stroma was replaced by the host stromal cells which are p50+ve as well as GFP+ve and α-SMA+ve. While there may be other mechanisms by which tumor cells are able to overcome absence of NFκB in tumor stroma, recruitment of NFκB1+ve stromal cells contributes to the continued growth of the tumor in this setting. We also compared the viability of WT and p50−/− PSCs in vitro as well as in vivo, as some of the observations could be, at least partially, explained by difference in survival of these PSCs. We observed that the WT and p50−/− PSCs had similar viability, both in vitro (data not shown) as well as in vivo (Suppl. Figure 6). Thus, the effects observed are not due to differences in survival but are rather functional in nature.
Figure. 1. NFKB1 in stroma is required for the growth of cancer.
A. Lack of p50−/− in stromal cells leads to reduced growth of KPC pancreatic tumors. KPC pancreatic cancer cells were either injected alone (KPC) or co-injected with WT-PSC (KPC+WT-PSC) or p50−/−-PSC (KPC+p50−/− PSC) mice in the pancreas of WT-mice and allowed to grow for 30 days (n=at least 8 animals per group). *p<0.05. B. Pancreatic tumor sections harvested from different groups were stained with H&E or Sirius red (Collagen). IHC was performed for α-SMA and Vimentin. Quantification from 10 different fields of examination for 5 different mice is depicted. *p<0.05. C. Lack of NFκB in tumor stroma increases survival of mice with KPC pancreatic cancer. Mice with KPC, KPC+WT-PSC and KPC+p50−/−-PSC tumors were followed until the study end-point was reached. Animal survival was plotted using Kaplan-Meir method. n=at least 10 in each group. D. Lack of p50 in stromal cells leads to reduced growth of another pancreatic cancer cell Panc02. Panc02 cells were either injected alone or co-injected with PSC isolated from WT or p50−/− mice into the pancreas of WT-mice and followed for 30 days. n=at least 9 mice each group. *p<0.05. E. & F. The phenomenon that lack of NFKB1 in tumor stroma reduces tumor growth is observed in Melanoma and Lung cancer as well. E. Melanoma cell line B16-F10 (n=at least 6 mice in each group) or F. Lung cancer cell line LLC (n=at least 6 mice in each group) was injected, either alone, or with dermal or lung fibroblast respectively, from WT or p50−/− mice subcutaneously in a WT-mice. The mice were sacrificed after 30 days and the tumor weights were compared. *p<0.05. See also Suppl. Figure 1
The impact of stromal NFκB depletion on tumor growth was further confirmed using an additional murine pancreatic adenocarcinoma cell line, Panc0217. As seen in Figure 1D, absence of NFKB1 in tumor stroma decreased growth of Panc02 tumors as well. To evaluate whether this phenomenon occurs only in pancreatic cancer or is operative in other cancers as well, we repeated these experiments with melanoma and lung cancer cell lines. B16-F10 melanoma cells or LLC (Lewis lung carcinoma) cells were co-injected subcutaneously with their corresponding (dermal and lung respectively) WT and p50−/− fibroblasts in WT background mice. We observed that the genetic deletion of Nfkb1 in fibroblasts led to reduced melanoma and lung cancer growth (Figure 1E and F), thereby confirming the importance of NFKB1 in tumor stroma for growth of non-pancreatic tumors as well. These data together suggest that NFκB in the stroma promotes cancer growth.
Lack of NFκB in tumor stroma leads to reduced proliferation and increased cell death
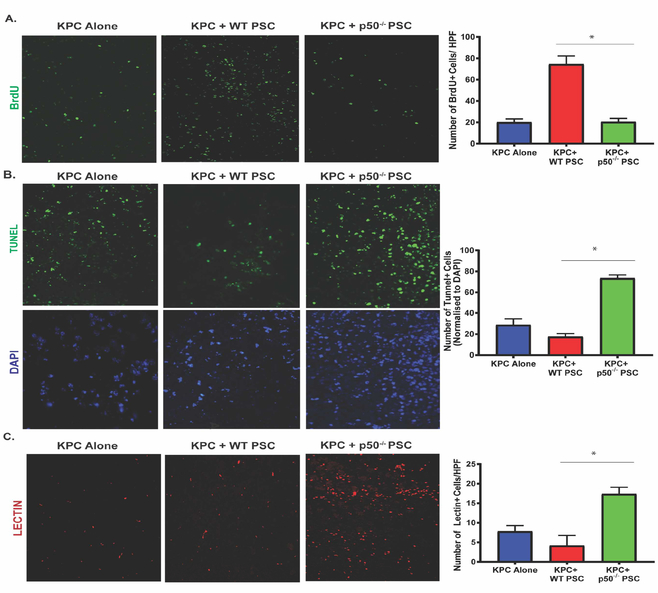
To evaluate the mechanism by which lack of NFκB in the tumor stroma leads to decreased tumor growth, we evaluated proliferation and apoptotic cell death in tumors. Tumor proliferation was measured using BrDU incorporation and Ki67 staining, while apoptotic cell death was measured by TUNEL staining and caspase-3 cleavage. Our results demonstrate that the loss of NFKB1 in the tumor stroma was associated with reduced cell proliferation (Figure 2A, suppl. Figure 2A) as compared with that of tumors with intact NFKB1 in their stroma. The proliferation was decreased to the level observed when KPC cancer cells were injected without PSCs. Loss of NFKB1 in tumor stroma was also associated with increased apoptotic cell death (Figure 2B, suppl. Figure 2C), as compared to tumors with intact stromal NFκB. This was corroborated by the in vitro data (Suppl. Figure 2B), where co-cultured p50−/− PSC with KPC pancreatic cancer cells leads to inhibition of proliferation. This is evidenced by reduced BrDU incorporation, as compared with KPC pancreatic cancer cells cultured alone or co-cultured with WT-PSCs. To evaluate whether angiogenesis may influence tumor growth, we assessed the presence of functional tumor vessels by terminal perfusion with tomato lectin. As seen in Figure 2C, we observed significantly more functional vessels when NFκB was not present in tumor stroma (Figure 2C), when compared with KPC cells injected alone or with WT-PSC.
Figure. 2. Nfkb1 deletion in stroma reduces cellular proliferation and increases cell death and open vessel density in pancreatic tumors.
(A) Representative images and quantification of immunofluorescence evaluation of BrDu incorporation in pancreatic tumors harvested from mice injected with KPC, either alone (KPC) or co-injected with WT (KPC+WT-PSC) or p50−/−-PSC (KPC+p50−/−-PSC) and allowed to grow for 15 days. (B) Representative images and quantification of TUNEL staining in different groups. DAPI is used as a nuclear stain. (C) Representative images and quantification of functional vessels in the study groups, as determined by terminal perfusion with biotin-labelled tomato lectin. Quantification was performed at 20X magnification and is average of 10 random fields per mouse with data from at least 5 animals included. Data is presented mean ± SE; *P< 0.05, non-parametric Mann-Whitney test.
Loss of NFκB in tumor microenvironment leads to high CD8 infiltration
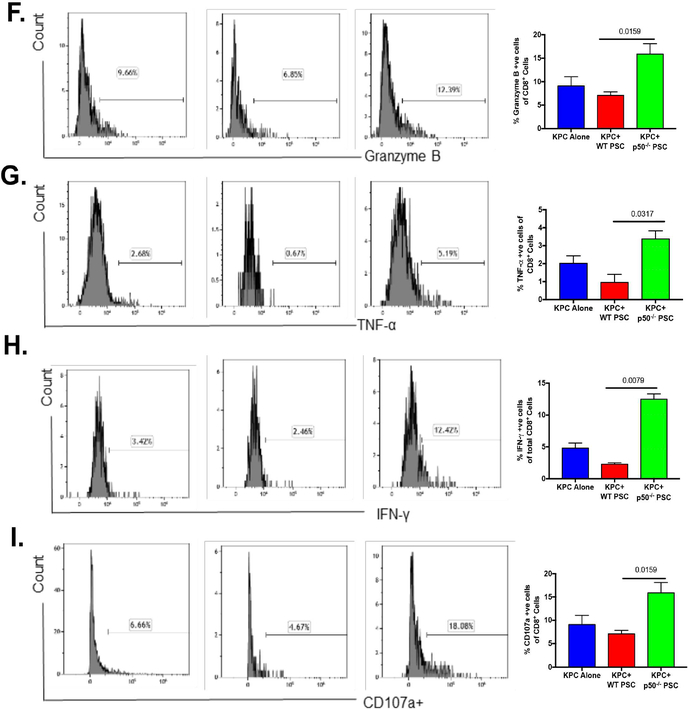
As shown previously, the decreased tumor growth observed when Nfkb1 is genetically deleted in the tumor stroma could be a result of various processes including decreased proliferation and increased cell death. We characterized immune infiltration in the tumors to understand the role of the immune response in increased cell death and decreased tumor growth. In an experiment, similar to the one described in Figure 1A, tumors and spleens were harvested two weeks after tumor implantation, and various immune cell populations were evaluated by flow cytometry. Cells were stained for CD45 along with live/dead staining and the downstream analysis was focused on live CD45+ cells. We observed that, when compared with tumors with NFκB-replete stroma, tumors with Nfκb1 deleted in the stroma had increased leukocytic infiltration (Suppl. Figure 3). We also observed that lack of NFKB1 in the stroma led to increased cytotoxic T cells (Figure 3A) and decreased granulocytic MDSC (Figure 3B) and regulatory T cells (Tregs) (Figure 3C). As seen in Suppl. Figure 3, infiltrating CD4+ T cells, NK cells, NKT cells, monocytic MDSCs, B cells, macrophages, total dendritic cell population, and dendritic type II cells did not change when NFKB1 was lacking in the stroma. However, we did see a small but statistically significant increase in CD11b+, CD103+ migratory dendritic cell population in the tumor when NFKB1 was depleted in the stroma. The changes observed in the splenic immune population when NFKB1 was depleted in the tumor stroma are shown in Suppl. Figure 3. Lack of NFKB1 in tumor stroma leading to increased infiltration of CD8+ cells was further confirmed using immunohistochemistry. As seen in Figure 3D, lack of NFKB1 in the tumor stroma led to increased infiltration with CD8+cells as compared with that in KPC tumors with NFκB intact in the stroma. We also evaluated the expression of immune checkpoint blockade markers as well as cytotoxic T cell activation markers when NFκB was depleted in the stroma. As seen in Figure 3E, lack of NFKB1 in the stroma led to reduced expression of CTLA-4 that did not reach statistical significance, but the expression of PD-1 and TIM-3 did not change significantly with lack of NFκB in the stroma (Suppl. Figure 3). Intriguingly, we observed that in the absence of NFκB in the stroma, the tumor infiltrating cytotoxic T cells were more activated, as evident by the increased granzyme-B, TNF-α, IFN-γ and CD107a expression (Figure 3F–I). Taken together, these results suggest that NFκB in cancer-associated fibroblasts mediates creation of an immunosuppressive environment and excludes cytotoxic T cells from the tumor microenvironment.
Figure. 3. NFKB1 depletion in tumor stroma leads to reversal of immuno-suppressive environment.
KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice (WT), either alone (KPC) or co-injected with WT-PSC (KPC+WT) or p50−/− PSC (KPC+p50−/−). The mice were sacrificed after day and the infiltration of various immune cell populations into the tumors was evaluated. Representative flow cytometry plots are shown and quantification is of data from at least 5 animals in each group. (A) Lack of p50 in stroma leads to marked increase in CD8+ T cell infiltration and decreased (B) granulocytic MDSCs (Ly6G+ and CD11b+) as well as (C) regulatory T cells (CD4+ and FoxP3+ve). (D) IHC was used to confirm the impact of p50 deletion in tumor stroma on CD8+ infiltration. Lack of p50 in tumor stroma also led to decreased (E) CTLA4+ (T cell exhaustion marker) and increased expression of markers for activated T cells including (F) Granzyme B, (G) TNF-α, (H) IFN-γ and (I) CD107a. *p<0.05
Decreased tumor growth observed when NFκB is depleted in stroma is dependent on CD8+ T cells
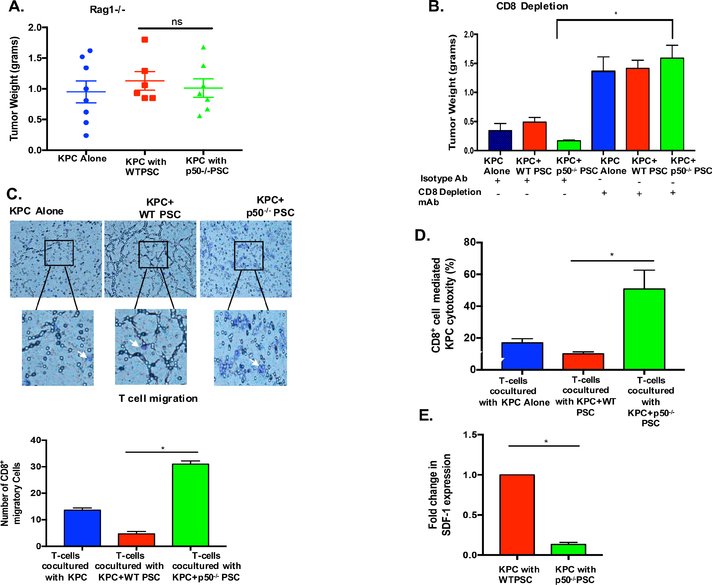
The foregoing experiments demonstrated that genetic deletion of NFKB1 in tumor stroma leads to increased cytotoxic T cell infiltration. Next, we evaluated whether decreased tumor growth observed after depletion of NFκB in stroma is dependent on CD8+ T cells. We used the Rag1−/− mice where, owing to a null mutation in the V(D)J recombination activation gene Rag-1, T cells, B cells18, γδ T cells as well as NKT cells19 are defective. KPC pancreatic cancer cells were co-injected with WT or p50−/− PSC into the pancreas of Rag1−/− mice. As seen in Figure 4A, unlike that observed in WT-mice, lack of NFKB1 in the stroma was unable to inhibit tumors in the pancreas of Rag1−/−, mice, which suggests a role of adaptive immunity in this process. To specifically evaluate the role of CD8+ cells, we used the antibody-mediated CD8+ cell depletion approach. KPC pancreatic cancer cells were co-injected with WT or p50−/− PSC into the pancreas of C57BL/6 mice (Figure 4B). The next day, mice were randomized into either CD8+ cell-depleting antibody or its isotype control antibody groups. KPC pancreatic cancer cells injected without PSCs served as controls. After 4 weeks, the mice were sacrificed and tumor weights were compared between the groups. The efficacy of CD8+-depleting antibody was evaluated by measuring the effect on CD8+ population in the spleen and comparing it with the effect of isotype antibody. As seen in Suppl. Figure 4A, CD8+-depleting antibody markedly depleted the CD8+ levels to ~3% as compared with ~30% in isotype control antibody injection. As seen in Figure 4B, in the absence of CD8+ cells, stromal NFKB1 depletion was unable to inhibit tumor growth, suggesting that CD8+ cytotoxic T cells are required for this phenomenon. Similar results were observed when this experiment was repeated in athymic nude mice, which lack functional B and T cells (Suppl. Figure-4C).
Figure. 4. Lack of NFκB in tumor stroma inhibits growth of tumors in CD8 dependent fashion.
(A) KPC pancreatic cancer cell line was injected into the pancreas of Rag1−/− mice, either alone (KPC) or co-injected with WT-PSC (KPC+WT) or p50−/− PSC (KPC+p50−/−) and allowed to grow for 20 days. In the absence of adaptive immune system, lack of NFKB1 in tumor stroma was unable to inhibit tumor growth. (n=at least 6/group; *p<0.05) (B) KPC pancreatic cancer cell line was injected into the pancreas of C57BL/6 mice, either alone (KPC) or co-injected with WT-PSC (KPC+WT) or p50−/− PSC (KPC+p50−/−). After tumor initiation, mice were randomized into isotype or CD8+ depletion antibody injection. Antibody was administered twice weekly for 21 days. In the absence of CD8+ cells, lack of NFKB1 in the tumor stroma was unable to inhibit tumor growth. (n=at least 8 in each group, *p<0.05). (C) The ability of primed T cells to infiltrate towards cancer cells was evaluated in an assay similar to the Boyden chamber assay. Briefly, T cells were placed in transwells and KPC pancreatic cancer cells were placed in the lower chamber, either alone or in co-culture with WT and p50−/− PSC. Co-culture was continued for 90 minutes, transwell membranes were harvested, stained with crystal violet and T cell migration quantified and compared between various groups. Results represent data from three independent experiments. *p<0.05 (D) The ability of primed T cells, obtained from mice bearing KPC pancreatic tumors to induce cell death in KPC cancer cells, when co-cultured with cancer cells alone or when co-cultured with cancer cells and WT or p50−/− PSCs for 48h, was compared. Presence of p50−/− PSCs markedly increased ability of primed CD8+ cells to kill cancer cells. Results represent data from three independent experiments. *p<0.05. E. CXCL12 (SDF-1) expression levels in WT and p50−/− PSCs, when co-cultured with KPC pancreatic cancer cells in transwell system for 48h. n=3.
To elucidate the mechanism by which NFκB in tumor stroma modulates cytotoxic T cell infiltration and function, we simulated these findings in vitro. First, we evaluated the T cell migration toward cancer cells using an assay similar to the Boyden chamber assay. For this experiment, cancer cells alone or with PSC (from WT or p50−/− animals) were placed in the bottom chamber and the primed T cells (obtained by isolating T cells from the spleen of WT-mice bearing KPC pancreatic tumors) were placed in the top chamber and the migration of T cells toward the cancer cells was evaluated. As seen in Figure 4C, lack of NFκB in the stroma markedly increases the infiltration of T cells, thereby recapitulating the in vivo findings where lack of NFκB in the stroma leads to increased CD8+ infiltration. Additionally, we evaluated the ability of primed T cells to kill cancer cells in the presence of stromal cells. In vivo primed T cells were co-cultured with KPC pancreatic cancer cells or with KPC+WT PSCs or KPC+p50−/− PSCs and the ability of primed T cells to kill cancer cells was measured as described in methods. As seen in Figure-4D, the ability of primed T cells to kill pancreatic cancer cells was significantly augmented when co-cultured with NFKB1 deficient stromal cells. This suggests that NFKB1 in stromal cells not only affects cytotoxic T cell infiltration but also their function. To identify the cytokine by which NFκB depletion in stroma leads to increased T cell infiltration, we co-cultured cancer cells and stellate cells (with or without NFκB depletion) and the expression of cytokine genes was compared between WT and p50−/−-PSCs. As seen in the table shown in Suppl. Figure-4B, when co-cultured with cancer cells, the cytokine expression in WT and p50−/−PSC differed for multiple cytokines. While multiple cytokine collectively may be responsible for the effects observed, we focused our downstream analysis on CXCL12 (SDF-1), a cytokine, which has been shown to play a role in protecting pancreatic cancer cells from T cell, mediated cell death13. Our PCR array results showed that WT-PSCs co-cultured with pancreatic cancer cells expressed CXCL12 ~10 times more than that by p50−/−-PSC. This was confirmed with qPCR, which demonstrated that the CXCL12 expression was dependent on NFKB1 (Figure-4E).
Stromal NFκB-mediated CXCL12 Secretion Promotes Tumor Growth by Excluding Cytotoxic T cells from Tumors
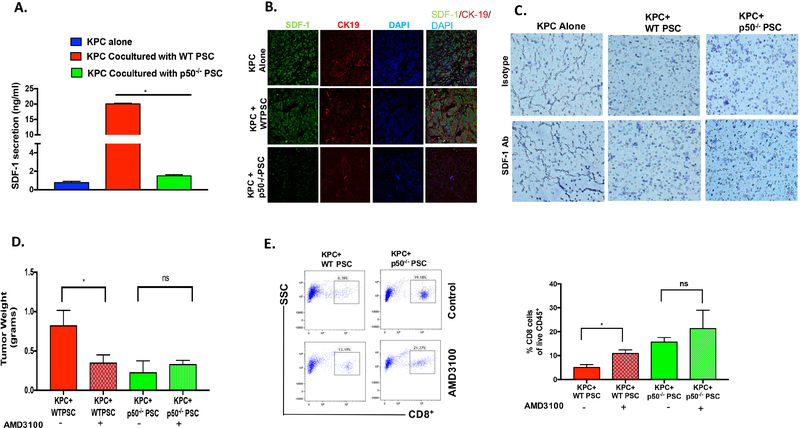
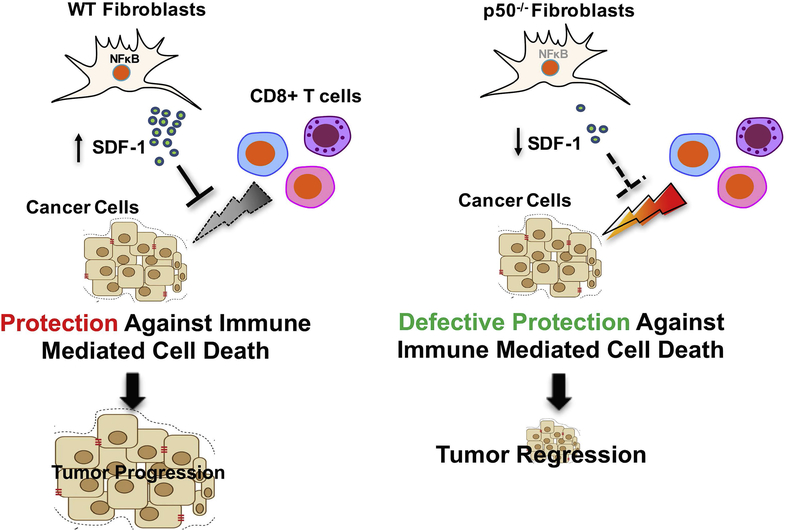
To understand the role of CXCL12 in stromal NFκB-mediated inhibition of T cell infiltration, we first evaluated the impact of lack of NFKB1 on CXCL12 expression from PSC. As seen in Figure 5A, WT-PSCs secrete high amounts of CXCL12, which is markedly more than that secreted by cancer cells alone. Interestingly, lack of NFKB1 in the PSC leads to marked reduction in the CXCL12 secretion (Figure 5A). This was further confirmed in the sections from the tumors in Figure 1. As seen in Figure 5B, lack of NFKB1 in pancreatic cancer leads to marked reduction in the CXCL12 staining, again suggesting that CXCL12 secretion in pancreatic cancer is under control of stromal NFκB. Next, we used our in vitro system to explore the role of stromal CXCL12 on T cell migration. As seen in Figure 5C, antibody-mediated depletion of CXCL12 leads to increased migration of T cells toward KPC and WT-PSC in the lower chamber. The quantification of T cell migration is illustrated in Suppl. Figure-5H. We also evaluated the effect on tumor growth of inhibiting interaction between CXCL12 and its receptor CXCR4 by AMD3100. As seen in Figure 5D, inhibition of CXCL12-CXCR4 interaction by AMD3100 led to decreased tumor growth in animals co-injected with KPC pancreatic cancer cells and WT-PSCs. As expected, lack of NFκB in the tumor stroma led to decreased tumor growth when compared with animals in which NFκB was intact in the stroma. Interestingly, AMD3100 did not change tumor growth when given to mice with tumors lacking NFκB in their tumor stroma. We also observed that interruption of CXCL12-CXCR4 interaction by AMD3100 leads to increased CD8+ in the tumors (Figure 5E). Taken together, these results suggest that NFκB-mediated CXCL12 secretion excludes T cells from the tumor microenvironment and plays a role in NFκB-mediated creation of an immunosuppressive environment. The impact of AMD3100 on infiltration of various immune cell populations into the tumor is demonstrated in Suppl. Figure 5 A–G. The schematic of our findings is shown in Figure 6.
Figure. 5. NFKB1 dependent SDF-1 (CXCL12) Secretion from Stroma Inhibits Cytotoxic T Cell Infiltration and Promotes Tumor Growth.
A. Secretion of SDF-1(CXCL12, ng/ml), as measured by ELISA, from KPC pancreatic cancer cells when cultured alone, or when co-cultured with WT or p50−/− PSCs. *p<0.05. B. Immunofluorescence showing the expression of SDF-1 in KPC, KPC+WT-PSC and KPC+p50−/−-PSC tumors. C. Effect of SDF-1(CXCL12) inhibition (SDF-1/CXCL12 Neutralizing antibody) on CD8+ T cell migration towards KPC pancreatic cancer cells cultured alone or co-cultured with WT or p50−/− PSC in a transwell system. WT and p50−/− PSCs were pretreated with SDF-1/CXCL12 neutralizing antibody for 48 h, followed by addition of KPC cancer cells and T cells. D. KPC cells were injected orthotopically along with WT and p50−/− PSC in WT background mice, on day 2 AMD3100 (SDF-1/CXCL12 inhibitor) was administered intraperitoneally daily for 21 days and tumor weights were measured after 30 days (n=at least 8 in each group, *P<0.05). E. Flow cytometry analysis showed CD8+ cell infiltration in tumors. (n=5/group, *P<0.05).
Figure 6:
Schematic representation summarizing the role of stromal NFκB in pancreatic cancer.
Discussion
While pancreatic cancer is believed to be “cold” or “poorly immunogenic,” the mechanism behind this inability of immune cells to attack and kill pancreatic cancer cells is still unclear. It has been postulated that the mutational burden, which leads to production of neo-antigens, may explain the inability of pancreatic cancer to mount an immune response20. However, mutational burden alone may not explain the whole picture. One of the hallmarks of pancreatic cancer is an intensely desmoplastic stroma that is made up CAFs, matrix proteins and infiltrating immune cells21, 22. While recent studies have implicated the stroma in creating an immune-suppressive environment that makes pancreatic cancer refractory to immunotherapy12, 13, the signaling pathways mediating this phenomenon are not known. Our study not only highlights the role of pancreatic cancer stroma in creating an immunosuppressive environment, but also brings to focus the key role of the stromal NFκB pathway in shielding cancer cells from immune attack by modulating the cytokine milieu, thereby restricting the ability of immune cells to rein in pancreatic cancer. Identification of these immunosuppressive cytokines will provide novel therapeutic targets that could be modulated to increase efficacy of immunotherapy in pancreatic cancer as well as other aggressive malignancies.
Inflammation and inflammatory pathways have been shown to be drivers of tumorigenesis23. While NFκB, a master regulator of inflammation, has been shown to play a critical role in the growth and the ability of pancreatic cancer cells to invade and metastasize24, 25, the role of stromal NFκB in the growth and progression of pancreatic cancer has not been studied. In our studies, we found that the p50 subunit of NFκB complex in stromal cells promotes tumor growth by protecting cancer cells from anti-tumor immune attack mounted by the host. With the depletion of the p50 subunit of NFκB in stroma, we observed an increase in CD8+ cytotoxic T cells. We have also shown that these infiltrating cytotoxic T cells exhibit higher levels of expression of activation markers (IFN-γ, perforin and granzymes) as well as a trend towards decreased expression of exhaustion markers (CTLA-4). Furthermore, genetic deletion of NFκB in tumor stroma not only increases cytotoxic T cells, but also leads to decreased Tregs and MDSCs. This hints at the possibility of a global immunological re-programing by stromal NFκB of the tumor microenvironment and inducing an immune-suppressive state. However, our results indicate that the tumor growth inhibition observed when stromal NFκB was depleted is completely dependent on cytotoxic T cells, suggesting that the NFκB-mediated immunosuppressive program in the stroma is geared towards inhibition of cytotoxic T cell function directly or indirectly. Inhibition may be achieved by modulating other immunosuppressive cells, Tregs and granulocytic MDSCs. This is evident from our studies showing that there was no inhibition of tumor growth when NFκB was depleted in the stroma in Rag1−/− mice, or when antibodies depleted cytotoxic T cells. Thus, we have identified a novel role of stromal NFκB in modulating cytotoxic T cell function and shaping the tumor microenvironment.
Our data suggests a tantalizing possibility that modulation of stroma can convert a non-immunogenic tumor like pancreatic cancer from a ‘cold’ to an ‘inflamed’ phenotype. However, the mechanism by which lack of NFκB in the stroma leads to increased cytotoxic T cell infiltration as well as increased cancer cell death is unknown. Our in vitro studies suggest that NFKB1 in stromal cells not only inhibits T cell migration towards tumor cells, but it also modulates their ability to kill cancer cells. We hypothesized that stromal NFκB was affecting T cell function by modulating secretion of various cytokines, chemokines and immune-inhibitory ligands that sculpt the tumor immune microenvironment. Using a PCR array, we have demonstrated that stromal NFκB modulates several chemokines, which are frequently chemotactic to macrophages, neutrophils or specific subsets of T cells. CXCL12 is one such important cytokine that is classically described as a ‘homing’ cytokine. It binds to the G-protein coupled receptor CXCR4 expressed in a number of cells such as cancer epithelial and immune cells that migrate along a CXCL12 gradient26. However, recent studies have indicated that CXCL12 may confer a tumor protective effect and thus promote tumor growth13, 27–29. Our PCR array analysis, quantitative PCR and ELISA measuring CXCL12 secretion clearly suggest that CXCL12 secretion from the stromal cells was dependent on NFKB1. Using CXCL12-depleting antibody as well as the pharmacologic inhibitor AMD3100, which disrupts interaction of CXCL12 with its receptor CXCR4; we confirmed the role of CXCL12 in preventing T cell infiltration. Remarkably, depleting CXCL12 in vitro promoted T cell infiltration and T cell-induced cancer cell death. Similarly, in animal models, disrupting CXCL12-CXCR4 interaction with AMD3100 increased T cell infiltration as well as reduced tumor growth. This suggest that the effects observed by knocking out NFκB in tumor stroma are, at least partially, mediated through decreased expression of CXCL12. This also suggests that depletion of stromal NFKB1 or attenuation of its downstream signaling (CXCL12) can reverse the immunosuppressive tumor microenvironment and can be used as a strategy to increase efficacy of immunotherapeutic approaches.
While we have shown that lack of NFKB1 in tumor stroma inhibits tumor growth, developing stromal NFκB inhibition as a viable therapeutic strategy for treatment of cancer may require evaluation of multiple avenues. As such, NFκB pathway consists of multiple players that interact synergistically to mediate critical functions30. These components could be putative targets in developing precise strategies to modulate NFκB. Furthermore, the effectors of signaling downstream of NFκB activation could also be considered viable targets. For instance, in the current study we have identified CXCL12 as one of the downstream mediators of NFκB that impede cytotoxic T cell infiltration. Thus, targeting CXCL12 may counteract, at least in part, the immunosuppressive effects of NFκB in the stroma. Given that CXCL12-CXCR4 interaction inhibitors are already in clinical use, this could emerge as a novel therapeutic strategy.
Conclusion
In summary, the current study highlights the role of stromal NFκB pathway in the inhibition of T cell infiltration and immune-mediated cancer cell death. Detailed understanding of the downstream mediators of NFκB pathway such as CXCL12 will provide novel targets, which could possibly be manipulated to convert a ‘cold’, non-immunogenic tumor like pancreatic cancer amenable to immunotherapeutic approaches.
Supplementary Material
Supplementary Figure 1
A. Phase contrast microscopy showing the images of quiescent (flattened rounded morphology with cytoplasmic lipid droplets around nucleus, short arrows) and activated (activated in culture, long and extended morphology without lipid droplets, long arrows) PSCs extracted from C57BL6 and p50−/− mice. B. Immunoblotting showing the expression of alpha-SMA and vimentin in inactivated (inact.) and activated (act.) PSCs extracted from WT and p50−/− mice. As seen PSCs from both WT and p50−/− mice gets activated on ongoing culture. C. Immunohistochemistry represents the expression of fibronectin in tumors from mice where KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone or co-injected with WT or p50−/− PSCs. Immunoblots showing the quantification of fibronectin in the tumors. D. Immunoblots showing the quantification of α-SMA in the tumors. E. Immunoblots showing the quantification of vimentin in the tumors. F. KPC pancreatic cancer cells were co-injected with p50−/− PSCs into the pancreas of GFP mice. Tumors were harvested either at 15 days or at the time of death of mice in a survival study. As seen there is increased staining of p50 in stromal cells at the end of experiment when compared with that in tumors at 15 days. G. This is further corroborated by increased presence of GFP+ve (from the host) stromal cells (α-SMA+ve) at end point when compared with 15 day time point.
Supplementary Figure 2
A. Immunohistochemistry evaluation of Ki67 staining in tumors obtained from mice, where KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone (KPC) or co-injected with WT (KPC + WT PSC) or p50−/− PSCs (KPC + p50−/− PSCs). Quantification performed in 5 animals over 10 fields is demonstrated. *P< 0.05. B. Invitro assay showed decreased proliferation of pancreatic cancer cells when co-cultured with p50−/− PSCs (n=2). C. Immunofluorescence represents cleaved caspase 3 staining in KPC cell alone and when injected with WTPSC and p50−/− PSC.
Supplementary Figure 3
Impact of stromal loss of p50 on immune infiltration in the tumor and the spleen is demonstrated. KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone or co-injected with WT or p50−/− PSCs. Tumors were allowed to grow for 15 days after which animals were sacrificed, tumors harvested and immune cell infiltration studied with flow cytometry. A. Live CD45+ (B) infiltrating CD4+ T cells, (C) NK cells (CD49+), (D) NKT cells (CD49+, CD3+), (E) monocytic MDSCs (Ly6C+), (F) B cells (CD19+), (G) macrophages (F4/80+, MHCII+), (H) total dendritic cell population (CD11c+; MHCII+), (I) migratory dendritic cell population (CD11b+, CD103+), (J) dendritic cell type II (CD11b+, CD11c+), (K) TIM3+ CD8+ T cells and (L) PD1+ CD8+ T cells. The changes observed in the splenic immune population when NFκB1 was depleted in the tumor stroma are depicted in Suppl. Figure 3 M-V.B. Data is presented mean ± SE (n = 5/ group; p values shown).
Supplementary Figure 4
A. Flow cytometry represents the validation of CD8+ depletion by CD8 depleting antibody compared with animals injected with isotype control antibody. B. Loss of p50 in tumor stroma did not affect the tumor growth in athymic nude mice (lacks T-cells). Data is presented mean ± SE (n=10 /group; *P< 0.05). C. Table representing the differential upregulation (as a fold change) of cytokines in WT and p50−/− PSC when cultured with KPC cells.
Supplementary Figure 5 Represents the flow cytometry analysis in tumors from saline and AMD3100 treatment groups. A. Represents % of live CD45+, B. % CD4+, C. % Foxp3+, D. % CD19+, E. CD49b+, F. CD11b+Ly6G+, G. % F4/80+ MHCII+ of live CD45+ cells in tumors injected with KPC alone and along with WT and p50−/− PSC with and without AMD3100 treatment. Data is presented mean ± SE (n =5/group; *P< 0.05)
Supplementary Figure 6: WT PSCs and p50−/− PSCs have similar viability in vivo. WT PSC were labeled by using LuminiCell Tracker™ 540 (green) Cell Labeling Kit (organic fluorescent nanoparticle based) and p50−/− PSC were labeled with LuminiCell Tracker™ 670 (red) Cell Labeling kit respectively. The labeled WT and P50−/− PSC were co-injected with KPC cells (in a 1:9 ratio) into the pancreas of WT (C57BL/6) mouse. Animals were sacrificed after 21 days of co-injection, the tumors harvested, cryo-sectioned and imaged with fluorescent microscope. Five different fields were imaged per mouse and three different tumors (mice) were used per group. The quantitative data, (measured using Image J software) is shown in the bar graph.
Acknowledgement
We thank the University of Miami Histology Core facility, Diabetic Research Institute Flow Cytometery Core Facility, and Mr. Gabriel Gaidosh, UM Bascom Palmer Eye Institute Microscopy Core Facility for valuable assistance in this work.
Grant Support: This work was supported by National Institutes of Health awards CA124723 (Ashok Saluja), CA170946 (Ashok Saluja) and CA184274 (Sulagna Banerjee), DK058694 (Ashok Saluja), DK093047 (Ashok Saluja) and DK111834 (Vikas Dudeja)
Footnotes
Disclosures:
Ashok Saluja: The University of Minnesota has a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. A.S. is the co-founder and the Chief Scientific Officer of this company.
Sulagna Banerjee: S.B. is a compensated consultant with Minneamrita Therapeutics LLC, the licensee of Minnelide, and this relationship is managed by the University of Miami.
Other authors do not have any disclosures to make.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970;13:1–27. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan HS. Role of immunologic disturbance in human oncogenesis: some facts and fancies. Br J Cancer 1971;25:620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 4.Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185–98. [DOI] [PubMed] [Google Scholar]
- 5.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007;121:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol 2006;90:51–81. [DOI] [PubMed] [Google Scholar]
- 8.Weber JS. Current perspectives on immunotherapy. Semin Oncol 2014;41 Suppl 5:S14–29. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrescu DT, Ichim TE, Riordan NH, et al. Immunotherapy for melanoma: current status and perspectives. J Immunother 2010;33:570–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wormann SM, Diakopoulos KN, Lesina M, et al. The immune network in pancreatic cancer development and progression. Oncogene 2014;33:2956–67. [DOI] [PubMed] [Google Scholar]
- 11.Pure E, Lo A. Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors? Cancer Immunol Res 2016;4:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delitto D, Delitto AE, DiVita BB, et al. Human Pancreatic Cancer Cells Induce a MyD88-Dependent Stromal Response to Promote a Tumor-Tolerant Immune Microenvironment. Cancer Res 2017;77:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. [DOI] [PubMed] [Google Scholar]
- 15.Sha WC, Liou HC, Tuomanen EI, et al. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 1995;80:321–30. [DOI] [PubMed] [Google Scholar]
- 16.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 1984;44:717–26. [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson RS, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992;68:869–77. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010;11:197–206. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maitra A, Hruban RH. Pancreatic Cancer. Annu Rev Pathol 2008;3:157–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 2010;18:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura A, McGinn O, Dudeja V, et al. Minnelide effectively eliminates CD133(+) side population in pancreatic cancer. Mol Cancer 2015;14:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clinical Cancer Research 2010;16:2927–2931. [DOI] [PubMed] [Google Scholar]
- 27.Colamussi ML, Secchiero P, Gonelli A, et al. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol 2001;69:263–70. [PubMed] [Google Scholar]
- 28.Righi E, Kashiwagi S, Yuan J, et al. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res 2011;71:5522–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vianello F, Papeta N, Chen T, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol 2006;176:2902–14. [DOI] [PubMed] [Google Scholar]
- 30.Bradford JW, Baldwin AS. IKK/nuclear factor-kappaB and oncogenesis: roles in tumor-initiating cells and in the tumor microenvironment. Adv Cancer Res 2014;121:125–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
A. Phase contrast microscopy showing the images of quiescent (flattened rounded morphology with cytoplasmic lipid droplets around nucleus, short arrows) and activated (activated in culture, long and extended morphology without lipid droplets, long arrows) PSCs extracted from C57BL6 and p50−/− mice. B. Immunoblotting showing the expression of alpha-SMA and vimentin in inactivated (inact.) and activated (act.) PSCs extracted from WT and p50−/− mice. As seen PSCs from both WT and p50−/− mice gets activated on ongoing culture. C. Immunohistochemistry represents the expression of fibronectin in tumors from mice where KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone or co-injected with WT or p50−/− PSCs. Immunoblots showing the quantification of fibronectin in the tumors. D. Immunoblots showing the quantification of α-SMA in the tumors. E. Immunoblots showing the quantification of vimentin in the tumors. F. KPC pancreatic cancer cells were co-injected with p50−/− PSCs into the pancreas of GFP mice. Tumors were harvested either at 15 days or at the time of death of mice in a survival study. As seen there is increased staining of p50 in stromal cells at the end of experiment when compared with that in tumors at 15 days. G. This is further corroborated by increased presence of GFP+ve (from the host) stromal cells (α-SMA+ve) at end point when compared with 15 day time point.
Supplementary Figure 2
A. Immunohistochemistry evaluation of Ki67 staining in tumors obtained from mice, where KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone (KPC) or co-injected with WT (KPC + WT PSC) or p50−/− PSCs (KPC + p50−/− PSCs). Quantification performed in 5 animals over 10 fields is demonstrated. *P< 0.05. B. Invitro assay showed decreased proliferation of pancreatic cancer cells when co-cultured with p50−/− PSCs (n=2). C. Immunofluorescence represents cleaved caspase 3 staining in KPC cell alone and when injected with WTPSC and p50−/− PSC.
Supplementary Figure 3
Impact of stromal loss of p50 on immune infiltration in the tumor and the spleen is demonstrated. KPC pancreatic cancer cells were injected into the pancreas of C57BL/6 mice, either alone or co-injected with WT or p50−/− PSCs. Tumors were allowed to grow for 15 days after which animals were sacrificed, tumors harvested and immune cell infiltration studied with flow cytometry. A. Live CD45+ (B) infiltrating CD4+ T cells, (C) NK cells (CD49+), (D) NKT cells (CD49+, CD3+), (E) monocytic MDSCs (Ly6C+), (F) B cells (CD19+), (G) macrophages (F4/80+, MHCII+), (H) total dendritic cell population (CD11c+; MHCII+), (I) migratory dendritic cell population (CD11b+, CD103+), (J) dendritic cell type II (CD11b+, CD11c+), (K) TIM3+ CD8+ T cells and (L) PD1+ CD8+ T cells. The changes observed in the splenic immune population when NFκB1 was depleted in the tumor stroma are depicted in Suppl. Figure 3 M-V.B. Data is presented mean ± SE (n = 5/ group; p values shown).
Supplementary Figure 4
A. Flow cytometry represents the validation of CD8+ depletion by CD8 depleting antibody compared with animals injected with isotype control antibody. B. Loss of p50 in tumor stroma did not affect the tumor growth in athymic nude mice (lacks T-cells). Data is presented mean ± SE (n=10 /group; *P< 0.05). C. Table representing the differential upregulation (as a fold change) of cytokines in WT and p50−/− PSC when cultured with KPC cells.
Supplementary Figure 5 Represents the flow cytometry analysis in tumors from saline and AMD3100 treatment groups. A. Represents % of live CD45+, B. % CD4+, C. % Foxp3+, D. % CD19+, E. CD49b+, F. CD11b+Ly6G+, G. % F4/80+ MHCII+ of live CD45+ cells in tumors injected with KPC alone and along with WT and p50−/− PSC with and without AMD3100 treatment. Data is presented mean ± SE (n =5/group; *P< 0.05)
Supplementary Figure 6: WT PSCs and p50−/− PSCs have similar viability in vivo. WT PSC were labeled by using LuminiCell Tracker™ 540 (green) Cell Labeling Kit (organic fluorescent nanoparticle based) and p50−/− PSC were labeled with LuminiCell Tracker™ 670 (red) Cell Labeling kit respectively. The labeled WT and P50−/− PSC were co-injected with KPC cells (in a 1:9 ratio) into the pancreas of WT (C57BL/6) mouse. Animals were sacrificed after 21 days of co-injection, the tumors harvested, cryo-sectioned and imaged with fluorescent microscope. Five different fields were imaged per mouse and three different tumors (mice) were used per group. The quantitative data, (measured using Image J software) is shown in the bar graph.