Abstract
Background: Topical application of tacrolimus (FK506) was effective in treating atopic dermatitis (AD); however, the therapeutic efficiency is hampered by its poor penetration into the skin and local side effects of transient irritation symptoms with a burning sensation, a feeling of warmth or heat. Menthol and camphor have been widely used in topical compound formulations for adjunctive pharmacotherapy for antipruritics and analgesics owing to their cool nature, and both present skin penetration enhancing effects. Moreover, they can form a liquid eutectic oil to solubilize hydrophobic drugs.
Purpose: Taking advantages of menthol/camphor eutectic (MCE), this work aims to integrate FK506 into MCE to construct a microemulsion system, i.e., FK506 MCE ME, which simultaneously enhances the percutaneous delivery and treatment efficacy, while reduces the side effects of FK506.
Methods: The formulation of FK506 MCE ME was optimized and characterized. Different formulations containing FK506 were topically administered to treat 1–chloro–2, 4–dinitrobenzene (DNCB)-induced murine AD.
Results: MCE solubilized FK506. FK506 in MCE ME penetrated skin in vitro more than in the commercial ointment, and MCE predominantly exerted the enhancing effects in MCE ME. FK506 MCE ME or FK506 MCE ME gel had greater effects on clinical symptoms, histological analysis, and IgE than did commercial FK506. The anti-pruritic and down-regulation of substance P effects of MCE ME vehicle mitigated the side effects of FK506 application.
Conclusion: MCE ME presented the excellent properties of simultaneously enhancing the percutaneous delivery and treatment efficacy, while reducing the side effects of FK506 for AD. Therefore, MCE ME is a promising nanoscale system for FK506 to effectively treating AD with low irritation and high medication adherence.
Chemical compounds studied in this article: Tacrolimus (PubChem CID: 445643); menthol (PubChem CID: 1254); camphor (PubChem CID: 2537)
Keywords: tacrolimus, microemulsion, menthol, camphor, eutectic, atopic dermatitis, percutaneous delivery
Introduction
Atopic dermatitis (AD) is a chronically relapsing allergic inflammatory disease influenced by complex interactions between immunological and environmental factors. Its prevalence ranges from 10% to 20% in children and 1% to 3% in adults.1,2 The International Study of Asthma and Allergies in Childhood (ISAAC) has reported that higher prevalence occurred in affluent European and Australasian populations with rising eczema burden.3,4 AD patients typically suffer from chronic or recurrent inflammation, pruritus, and plaques,5 and the onset of itching and subsequent scratching and intractable dermatitis is amplified by the itch–scratch cycle. Anti pruritic treatment is important for AD treatment. AD pathology develops with elevated levels of blood IgE and skin substance P (SP), and SP leads to itching.
Tacrolimus (FK506), one of topical calcineurin inhibitors (TCIs), inhibits the skin inflammation by inhibiting the enzyme calcineurin phosphatase.6,7 The commercial formulations of FK506, 0.03% (w/w) and 0.1% (w/w) ointment, were approved by Food and Drug administration (FDA) in the United States for topical application for moderate-to-severe AD in children and adults, respectively.8 Nevertheless, our previous research revealed that the retention of FK506 from the commercial ointment in the target skin site is deficient owing to the poor solubility, high molecular weight of drug, and the oil vehicle of ointment.9,10 Various nanocarriers including microemulsion (ME), ethosomes, nanoparticles, and lipid nanoparticles enhance drug penetration and target the skin,11–14 and ME offers greater properties than other nanocarriers with high drug-solubilizing capacity, long-term stability, and easy scale-up.15,16 Moreover, topical application of FK506 can evoke skin irritations of transient burning sensation at the beginning of therapy. The burning sensation is a feeling of warmth or heat and stinging itch with subsequent pain, erythema, and pruritus in the applied skin.17–19 The transient irritations mainly associated with the drug characteristic of TCIs.19 TCIs can activate the transient receptor potential A1 (TRPA1) channel followed by SP release, which induces the initial side effects of burning and itching during the therapy with FK506,20–22 and itch-triggered scratching immediately leads to symptom exacerbation, and pruritus–scratch–pruritus easily turns into a vicious circle. These irritations of FK506 in AD patients often lead to treatment discontinuation. However, efficient AD treatment requires a long-term intermittent treatment of topical FK506 ointment.23 Therefore, relieving thermal discomfort, suppressing SP release, and antipruritic treatment are important for preventing the withdrawn therapy with topical FK506 for AD patients. The present study aims at fabricating a compound topical formulation for FK506 percutaneous delivery system to treat AD while simultaneously enhancing its skin retention and treatment efficacy and reducing its transient skin irritations.
Menthol, a natural cyclic terpene alcohol of peppermint, is widely used in dermatology for adjunctive pharmacotherapy in antipruritic, antiseptic, analgesic, and cooling formulations.24,25 And it shows penetration-enhancing capacity with low dermal irritation and systemic toxicity.26 As an agonist of transient receptor potential member 8 (TRPM8) ion channels, menthol activates the TRPM8 primary cold sensors in the skin to exert cool sensation lasting up to 70 mins and thereby providing relief for itching.27,28 Camphor, another natural ingredient of plant origin, often accompanies menthol in dermatological antipruritic and analgesic preparations for synergetic effects. Menthol and camphor lotion are applied to relieve hydroxyethyl starch–induced pruritus, offering an alternative therapy for this refractory disease,29 and both menthol and camphor are collected in the guidelines for the management of chronic pruritius.30 Interestingly, they can form menthol/camphor eutectic (MCE) oil solution after being mixed,29,31 and the MCE is anticipated to solubilize water-insoluble drug and to decrease the volatility of menthol and camphor. Gohel et al reported using MCE as the penetration enhancer for captopril.32In our preliminary experiment, MCE solubilizes FK506 to 136 mg/mL.
Based on the abovementioned characteristics of FK506, menthol, camphor, and MCE, we hypothesize that MCE can be used as the oil phase to develop FK506 MCE ME, which can promote the retention of FK506 in the skin, suppress its transient skin pruritus, and then facilitate its anti-AD efficacy. In this study, we fabricated 0.1% (w/w) FK506 MCE ME and MCE ME gel. The penetration of FK506 from different formulations through the rat skin in vitro was performed with Franz diffusion cell method. The safety of MCE ME to immortalized human keratinocytes (HaCaT) was evaluated. AD model was constructed with BALB/c mice treated with 1–chloro–2,4–dinitrobenzene (DNCB). The anti-AD and anti-pruritus efficacy was evaluated by the dermatitis score, transepidermal water loss (TEWL), histological analysis, IgE, scratch frequency, and SP.
Materials and methods
Materials
Menthol was purchased from Tianmu Pharmaceutical Group Limited (Anhui, China). Camphor was gifted from Baiyunshan Chem-Pharm (Guangzhou, China). Transcutol P was purchased from Gattefosse (Saint-Priest, France). Cremphor EL was gifted from BASF (Ludwigshafen, Germany). Carbopol®940 was purchased from Jianglai Reagent Company (Shanghai, China). Tacrolimus (FK506) was purchased from Teva Czech Industries (S.R.O. Ostravska 29305, 74770 Opava-Komarov, Czech Republic). FK506 commercial ointment (Protopic®, 0.1%, w/w) was obtained from Astellas Toyama Co., Ltd. (Toyama, Japan). DNCB was purchased from Sigma-Aldrich (St Louis, MO, USA). Propylene carbonate (PC) was purchased from Aladdin (Shanghai, China). FBS and DMEM were purchased from Gibco (Gaithersburg, MD, USA). Other reagents were of analytical grade.
HaCaT were obtained from CCTCC (Wuhan, China). Sprague Dawley rats (male, 180–220 g) and BALB/c mice (male, 18–20 g) were purchased from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). Animals were raised under specific pathogen-free condition with humidity (40–70%) and temperature (20–26°C) on 12-hr light/dark cycles and provided with food and water ad libitum. All work undertaken with animals were in accordance with the Principles of Laboratory Animal Care and Use in Research published by the Chinese Ministry of Health, and the protocols were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. Chemical compounds studied in this article: Tacrolimus (PubChem CID: 445643); menthol (PubChem CID: 1254); camphor (PubChem CID: 2537)
Preparation and characterization of MCE ME and MCE ME gel
Based on our preliminary experiments, MCE was chosen as the oil phase, Cremophor EL as the surfactant, transcutol P as the co-surfactant, and deionized water as the aqueous phase. With the aim to fabricate thermodynamically stable eutectic, the ratio of menthol/camphor varied with 1/1, 2/1, 3/1, and 4/1 (w/w). MCE was prepared by grinding at room temperature. The surfactant and co-surfactant were mixed at the ratio of 1/1, 2/1, 3/1, respectively. MCE was then added to the mixture of surfactant and co-surfactant at the ratio of 1/9, 2/8, 3/7, 4/6, 5/5, 6/4, 7/3, 8/2, and 9/1, respectively. The concentrated solution was prepared by mixing MCE, Cremophor EL, with Transcutol P at the ratios as above mentioned, and FK506 was dissolved in the concentrated solution. The deionized water was titrated dropwise into the mixture under continuous stirring for 30 mins at 25°C. The desired ME was evaluated based on its transparent appearance, low viscosity, and single phase. Carbopol®940 dissolved in deionized water (0.5%, w/w) was used as a thickening agent for preparing MCE ME gel. Concentrated MCE ME and Carbopol®940 solution were mixed and the pH value was adjusted to ~7.0 with triethanolamine for gelation. MCE ME vehicle without FK506 and oil-free solution containing FK506 (0.1%, w/v) and the same surfactant and co-surfactant as ME but without MCE were also prepared by mixing the corresponding components, respectively, and stirring at room temperature for 30 mins.
The average droplet size and polydispersity index (PDI) of MCE ME vehicle and MCE ME containing FK506 were detected with dynamic light scattering (Malvern Instruments Nano ZS90, Worcestershire, UK). Its morphology was imaged with atomic force microscope (AFM, Dimension FastSca AFM system, Bruker, Switzerland).
Skin penetration of FK506 MCE ME and MCE ME gel in vitro
Sprague Dawley rats were anesthetized with urethane (20%, w/w) and sacrificed by cervical dislocation. The hair on the abdomen was carefully shaved off using a razor, and the shaved skin was separated from other subcutaneous tissues surgically and wetted with normal saline (NS), then stored at −20°C until use. Franz diffusion cell (TK-12A, Kaikai Technology, Ltd, Shanghai, China), with a permeated area of 3.14 cm2 and receptor volume of 7.5 mL, was used to perform the penetration study in vitro. The skin was sandwiched between the donor and receptor cells with the stratum corneum (SC) facing the donor compartment. Each receptor compartment was filled with 7.5 mL NS containing 25% (v/v) ethanol as the receiving medium with respect to the sink conditions,9 stirred at 300 rpm, and maintained at 37°C ±0.5°C in a circulating water bath. Then, 500 μL MCE ME vehicle and FK506-containing formulations (0.1%, w/v) including PC solution (PC is the solvent to dissolve FK506 in the commercial ointment), oil-free solution, MCE ME, 0.5g MCE ME gel, and the commercial ointment were positioned into the donor cell, respectively (n=6). At the scheduled time of 12,14, 16, 19, 22, and 24 hrs, 1 mL receiving medium was collected from the receiving cell, and equal volume of fresh medium was immediately supplemented to the cell. After 24 hrs, the permeated skin with an area of 3.14 cm2 was cleaned and cut into pieces. One milliliter methanol was added along with sonication for 30 mins to extract FK506 retention in the skin and then centrifuged at 5000 rpm for 5 mins. The supernatant, filtered with a 0.22 μm microporous nylon membrane, was detected with FK506 by HPLC.
MTT assay
The safety of MCE ME vehicle was tested with HaCaT. HaCaT cells were cultivated into 96-well plates (Corning Inc., Corning, NY, USA) at a density of 8×103cells per well and incubated overnight in the moist atmosphere of 95% air with 5% CO2 at 37°C (Forma 3111, Thermo Fisher Scientific, Waltham, MA, USA). MCE ME vehicle was diluted with DMEM medium to 0.7 mg/mL, 1 mg/mL, and 1.3 mg/mL, respectively, and added to the well. The cells were incubated with MCE ME vehicle for 12 hrs and 24 hrs, respectively. Then, 0.5 mg/mL MTT mixed with DMEM without FBS was added into the wells and incubated for 4 hrs at 37°C. After removing the MTT medium from the wells, 150 mL dimethyl sulfoxide (DMSO) was added to solubilize formazan and the 96-well plates were vibrated for 15 mins shielded from light. The absorbance was detected at 490 nm with an ELISA microplate reader (Synergy H1, BioTek Instruments, Inc., Winooski, VT, USA). Relative cell viability was calculated with the absorbance of test wells divided by the absorbance of control wells.
Establishment of DNCB-induced AD-like mice model
BABL/c mice (male, 18–20 g) were used to construct AD model with DNCB. The hair on the dorsal skin was shaved and the skin recovered for 24 hrs. Briefly, DNCB was solubilized in acetone/olive oil/alcohol (2/1/1, v/v/v) to prepare 0.5% (w/v) and 1.0% (w/v) DNCB solution, respectively. The mouse was sensitized by 200 μL of 0.5% (w/v) DNCB onto the shaved dorsal skin and 20 μL onto the right ear, respectively. The sensitization was performed daily for three consecutive days. After the sensitization, 100 μL and 10 μL of 1.0% (w/v) DNCB were repeatedly applied to the sensitized dorsal skin and the right ear, respectively, on days 4, 7, 11, 14, 17, 20, 23, 26, and 28 for challenge. The control group was treated with NS instead of DNCB.33
AD-like mice treatment
The animals were randomly divided into 8 groups (6 mice per group) as follows: 1) control: healthy mice with shaved dorsal region; 2) DNCB: AD-induced mice without drug treatment; 3) DNCB + PC: AD-induced mice treated with PC containing 0.1% (w/v) FK506; 4) DNCB + vehicle: AD-induced mice treated with MCE ME vehicle without FK506; 5) DNCB + oil free: AD-induced mice treated with oil free containing 0.1% (w/v) FK506; 6) DNCB + MCE ME: AD-induced mice treated with MCE ME containing 0.1% (w/v) FK506; 7) DNCB + MCE ME gel: AD-induced mice treated with MCE ME gel containing 0.1% (w/w) FK506; and 8) DNCB + ointment: AD-induced mice treated with the commercial ointment containing 0.1% (w/w) FK506. Each mouse in groups (2)–(8) was sensitized with 0.5% (w/w) DNCB and challenged with 1.0% (w/w) DNCB as described in the section “Establishment of DNCB-induced AD-like mice model.“ For the DNCB group, the sensitized and challenged mice were treated with NS; and for group (3)–(7), the sensitized and challenged mice were treated with different formulations containing 0.1% (w/v) FK506 of 100 μL (μg) on the shaved dorsal area and 10 μL (μg) on the right ear on days 6, 8, 10, 13, 16, 19, 22, 25, and 27, respectively.
At the end of the experiments, mice were weighted and the thickness of the treated ear of each mouse was measured with a digimatic micrometer (CD–6 CSX, Mitutoyo Corporation, Kawasaki, Japan). After mice were sacrificed, the treated dorsal skin underwent histopathological and immunohistochemical analysis. Spleen was weighted to calculate the spleen index (spleen weight/body weight). Blood samples were collected to measure the total serum IgE by ELISA.
Evaluation of dermatitis severity
Clinical dermatitis severity was visualized and scored on days 4, 10, 16, 22, and 28 based on 4 clinical symptoms of AD including erythema/hemorrhage, scarring/dryness, edema, and excoriation/erosion. Briefly, each clinical symptom was scored independently in accordance with severity as follows: 0, none; 1, slight; 2, moderate; 3, marked. The final dermatitis score was calculated by the sum of four severity scores above. Moreover, the scratch frequency was recorded for 30 mins following the different formulations treated on days 4, 16, and 28. In brief, mice generally scratched their ears and dorsal skin with their paws for about 1 s, which was considered as one episode of scratching.
Measurement of TEWL
An S/N SWL5141 device (Delfin Technologies, Horn, Netherlands) was used to evaluate the dorsal skin barrier of the mice. TEWL was measured on days 0, 4,10, 16, 22, and 28 under the standard conditions (25 ± 5°C, 55 ± 5% RH).
Histopathological studies
The treated dorsal skin tissue specimens of each mouse were fixed in 10% (w/v) paraformaldehyde in 0.1 M PBS (pH 7.4), embedded in paraffin wax, and serially sectioned at 6 –8 μm. The sections were stained with HE and toluidine blue (TB) in accordance with the standard procedures to assess the inflammation and to observe the histological alterations in the skin. Inflammatory cells, mast cells, and the thickness of SC were observed with light microscopy at 100× magnification, and cells were counted in a high-power field (HPF) at 200×magnifiation.
Measurement of total serum IgE by ELISA
Blood samples were centrifuged at 2000 rpm for 30 mins at 4°C, and then serum was collected and stored at −80°C for further investigation. Total IgE concentration in mouse serum was measured via enzyme-linked immunosorbent assay (ELISA) kit (Abcam ab157718, Cambridge, MA, USA) according to the instructions of the manufacturer.
Substance P
The dorsal skin tissue was post fixed in 4% paraformaldehyde at 4°C for 4 hrs and cryprotected in phosphate-buffered 30% sucrose at 4°C overnight. Following post fixation, the skin was embedded in optimal cutting temperature (OCT) compound, frozen, and cut with a cryostat microtome (CM 1900, LECIA, Wetzlar, Germany) vertically to the surface with the width of 8 μm, and then mounted onto microscope slides for staining. The sections were washed with PBS solution containing 0.3% (v/v) Tween 20 for 5 mins and 3 times, fixed with 4% (w/v) paraformaldehyde in PBS for 1 hr, blocked with PBS containing 10% (v/v) goat serum, and 0.03% (v/v) Triton for 2 hrs at room temperature, and then incubated with primary antibody overnight at 4°C. Primary antibodies were diluted in blocking solution: anti-SP (1/100, Abcam ab14184); negative control slides were also conducted by incubated with blocking solution. Next, the sections were washed with PBS solution containing 0.3% (v/v) Tween 20 for 5 mins 3 times. The sections were incubated with secondary antibodies (1/200, Abcam ab150116) in blocking solution for 1 hr and washed with PBS solution containing 0.3% (v/v) Tween 20 for 5 mins 3 times. Microscopic observation was done with a Zeiss microscopic system (LSM710, Zeiss Microsystems, Oberkochen, Germany).
Statistical analysis
All experimental data were analyzed by one-way ANOVA, followed by the least significant difference (LSD) test as post hoc analysis (SPSS version 19.0; SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± SD.
Results and discussion
MCE ME formulation and characterization
As a widely used drug for treating AD, FK506’s efficacy was limited by its poor aqueous solubility (4–12 μg/mL) and high molecular weight (MW 803.5 Da),34,35 which resulted in poor penetration into the skin. Menthol and camphor are two natural ingredients extensively applied in dermatology for antipruritic treatment and they can form eutectic (MCE). In our study, MCE formed at the ratio of 2/1 and 3/1 (menthol/camphor, w/w), while MCE did not form at the ratio less than 2/1 or more than 4/1. The MCE prepared by menthol and camphor at the ratio of 3/1 can solubilize FK506 to 136.21±5.92 mg/mL. We innovatively chose MCE (menthol/camphor, 3/1, w/w) as the oil phase of FK506 ME. Moreover, our previous report demonstrated that transcutol P and Cremophor EL possessed good solubilizing capacity on FK506, and Lee et al had certified that transcutol P possessed penetration-enhancing effect, nontoxic property, and biocompatibility with the skin on FK506.36 Therefore, Cremophor EL and transcutol P were chosen as the surfactant and co-surfactant, respectively, in FK506 MCE ME formulation.
When the ratio of MCE/mixed surfactant was above 2/3 and cremophor EL/transcutol P was 3/1, the formulation was turbid, inhomogeneous, and thermodynamic unstable. Considering the probable irritation of surfactant and co-surfactant to the skin, the content of cremophor EL and transcutol P in the formulation should be decreased as much as possible. Therefore, the optimal formulation was composed of the ratio of MCE/mixed surfactant as 3/7, and cremophor EL/transcutol P as 2/1. Taken together, the optimal MCE ME formulation consisted of 8.6% MCE (menthol of 6.45% and camphor 2.15%, w/w) as oil phase, 13.3% (w/w) Cremophor E and 6.7% (w/w) Transcutol P as mixed surfactants, and 70.4% (w/w) distilled water as aqueous phase. In this MCE ME, FK506 can be solubilized more than 10 mg/mL. FK506 MCE ME gel was formulated by 0.5% (w/v) Carbopol®940 as aqueous phase, while the ratios of other phases remained the same as in FK506 MCE ME.
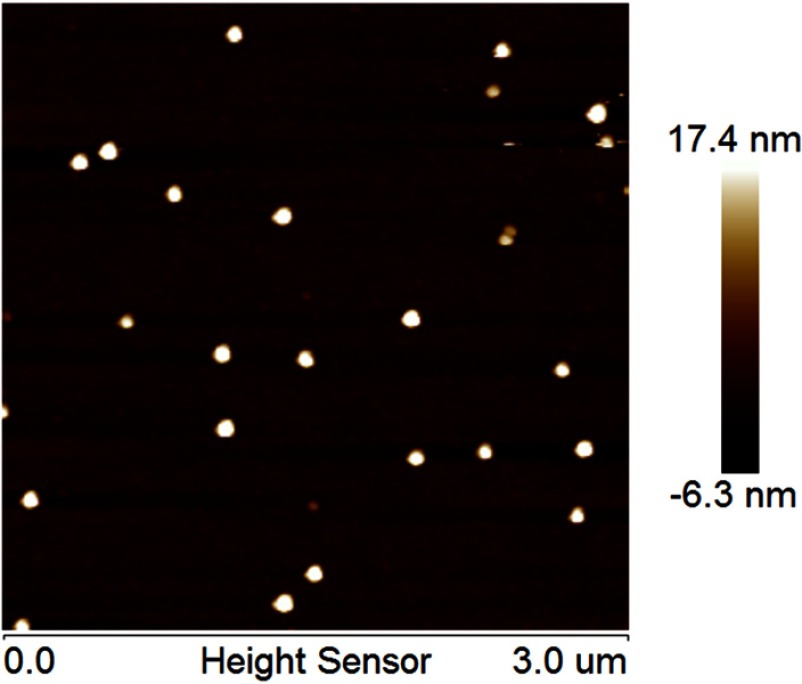
The average droplet size and PDI of FK506 MCE ME determined by dynamic light scattering were 26.59±0.24 nm and 0.084±0.012, respectively. FK506 MCE ME morphology was imaged by AFM. As shown in Figure 1, FK506 MCE ME presented small, uniform vesicles, and the “nanosize” was confirmed to be ~27nm, which was consistent with the result from the dynamic light scattering determination.
Figure 1.
The AFM image of FK506 MCE ME.
Abbreviations: AFM, atomic force microscope; FK506, tacrolimus; FK506 MCE ME, tacrolimus loaded microemulsion based on menthol/camphor eutectic.
Skin penetration of FK506 MCE ME and MCE ME gel in vitro
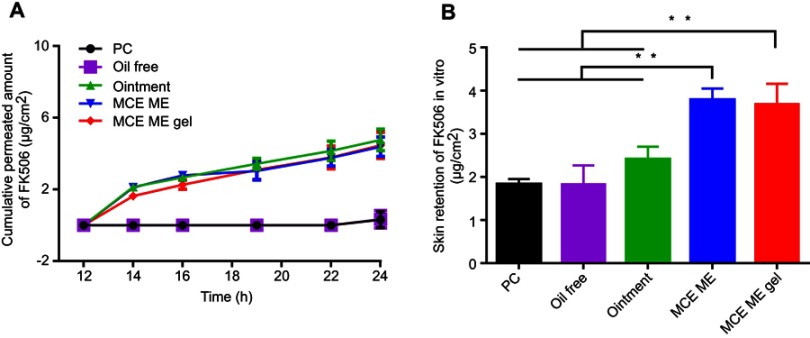
In order to assess the skin permeability of FK506 from different formulations, the cumulative permeated amount of FK506 was plotted against time, and the retention of FK506 in the skin after 24-hr penetration is shown in Figure 2A and B, respectively. The flux of FK506 among different formulations was calculated by the slope of the profile of FK506 cumulative permeated amount to time (Table 1). The PC group and the oil-free group contained FK506 (0.1%, w/v) and presented the similar permeability of FK506 with negligible penetration at each time point; the 24-hr cumulative permeated amount and flux from the PC group and the oil-free group were 0.32±0.04 μg·cm−2 and 0.34±0.05 μg·cm−2, 0.10 μg·cm−2·hr−1and 0.12 μg·cm−2·hr−1, respectively, and the retention of FK506 in the skin was 1.83±0.44 μg·cm−2 and 1.83±0.12 μg·cm−2, respectively. FK506 (0.1%, w/v) can be completely dissolved in PC or oil-free solution; however, PC or oil-free solution cannot efficiently enhance FK506 permeability.
Figure 2.
In vitro skin penetration of FK506 from different formulations (A) and skin retention of FK506 after 24 h penetration (B).
Note: **P<0.001.
Abbreviations: FK506, tacrolimus; PC, The solution of 0.1% tacrolimus (FK506,w/v) dissolved in propylene carbonate; Oil free, 0.1% (w/v) FK506 solution containing the same surfactant and cosurfactant as microemulsion but without menthol/camphor eutectic; Ointment, 0.1%(w/w) FK506 commercial ointment; MCE ME, 0.1% (w/v) FK506 microemulsion based on menthol/camphor eutectic; MCE ME gel, 0.1% (w/w) FK506 microemulsion gel based on menthol/camphor eutectic; h, hours; SD, standard deviation.
Table 1.
Cumulative permeated amount, flux, and retention of FK506 from different formulations after 24-hr penetration in vitro (mean ± SD, n=6)
| Permeated amount (μg· cm−2) | Flux (μg· cm−2· hr−1) | Retention (μg· cm−2) | |
|---|---|---|---|
| PC | 0.32±0.04 | 0.10 | 1.83±0.44 |
| Oil free | 0.34±0.05 | 0.12 | 1.83±0.12 |
| Ointment | 4.76±0.60** | 0.28** | 2.41±0.29 |
| MCE ME | 4.39±0.54** | 0.28** | 3.69±0.47++ |
| MCE ME gel | 4.46±0.73** | 0.29** | 3.79±0.26++ |
Notes: **P<0.001 in comparison with PC or oil free; ++P<0.001 in comparison with PC, oil free, or ointment. Data represented mean ± SD, n=6/group.
Abbreviations: FK506, tacrolimus; PC, the solution of 0.1% tacrolimus (FK506,w/v) dissolved in propylene carbonate; oil free, 0.1% (w/v) FK506 solution containing the same surfactant and cosurfactant as microemulsion but without menthol/camphor eutectic; ointment, 0.1% (w/w) FK506 commercial ointment; MCE ME, 0.1% (w/v) FK506 microemulsion based on menthol/camphor eutectic; MCE ME gel, 0.1% (w/w) FK506 microemulsion gel based on menthol/camphor eutectic.
The 24-hr cumulative permeated amount of FK506 from commercial ointment, MCE ME, or MCE gel was similar (4.76±0.60 μg·cm−2 vs 4.39±0.54 μg·cm−2 vs 4.46±0.73 μg·cm−2); any of them was higher than that of the PC group or the oil-free group (P<0.001); while FK506 retention in the skin (2.41±0.29 μg·cm−2) for commercial ointment presented no significant difference in comparison with that of the PC group or the oil-free group. However, FK506 retention in the skin for MCE with 3.69±0.47 μg·cm−2 and MCE ME gel with 3.79±0.26 μg·cm−2 presented higher values than that of the PC group or the oil-free group (P<0.001), even higher than that of commercial ointment (P<0.001). The results indicated that MCE in MCE ME predominantly exerted enhancing FK506 penetration through and into the skin, and MCE ME and MCE ME gel facilitated FK506 skin retention in comparison with the commercial ointment. Therefore, MCE ME and MCE ME gel could increase FK506 retention in the target skin site. The Carbomer 940® gelatinized MCE ME to MCE ME gel and did not affect the penetration of FK506.
MTT assay for cytotoxicity
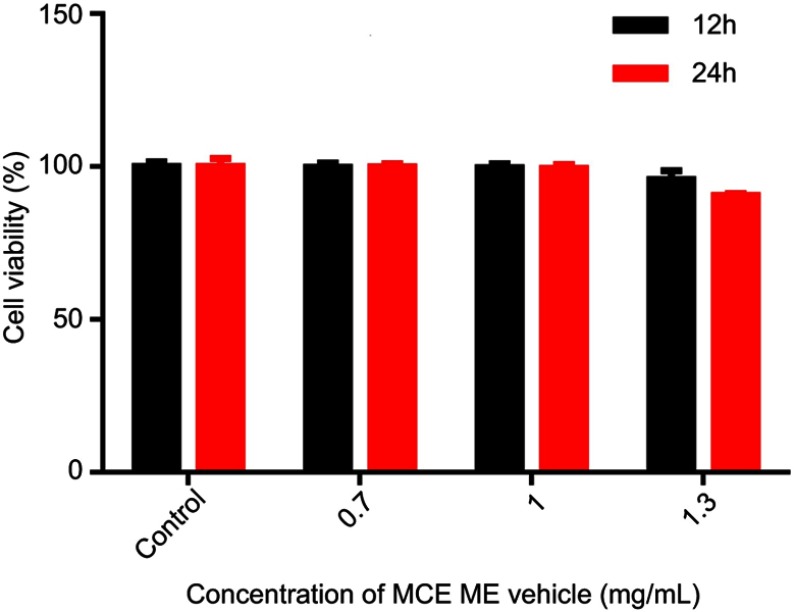
The MCE ME vehicle was diluted 0.7 mg/mL, 1 mg/mL, and 1.3 mg/mL, respectively, and incubated with HaCaT cells for 12 and 24 hrs for MTT assay. As shown in Figure 3, the HaCaT cell viability was over 99% when the concentration of the vehicle was 0.7 mg/mL and 1 mg/ml incubated for 12 and 24 hrs, with no significant difference in comparison with the control group. Even if the concentration of the vehicle was increased to 1.3 mg/mL, the cell viability was still higher than 90%, indicating that MCE ME was safe to HaCaT cells.
Figure 3.
Cell viability of HaCaT cells after incubation with MCE ME vehicle of different concentrations for 12 h and 24 h, respectively.
Abbreviations: HaCaT, immortal human keratinocytes; MCE, ME vehicle; Microemulsion based on menthol/camphor eutectic without FK506; SD, standard deviation.
Evaluation of MCE ME and MCE ME gel on ameliorating AD-like skin symptoms
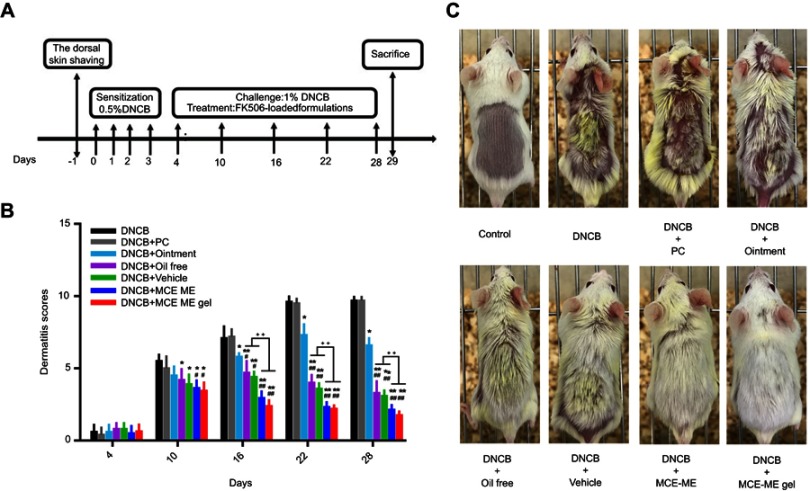
An AD-like model was constructed and treated (Figure 4A), and Figure 4B shows the dermatitis scores of different groups throughout the treatment. After 0.5% (w/v) DNCB was applied to sensitize the shaved dorsal skin for three consecutive days, the skin appeared slightly dry in comparison with the control group. With the further challenge of 1.0% (w/v) DNCB, severe AD-like skin lesions, including erythema/hemorrhage, edema, and scaling/dryness, were constantly aggravated on the dorsal skin of the DNCB group and persisted throughout the experiment. In comparison with the DNCB group, the atopic skin symptoms of the mice treated with PC group were not ameliorated throughout the treatment. PC, the solvent used to dissolve FK506 in the commercial ointment, has been reported to irritate skin to counteract the treatment of FK506 on AD.37 However, treatment with other formulations except the commercial ointment inhibited these atopic skin symptoms since day 10, and MCE ME and MCE ME gel took better effects than the vehicle and oil free (P<0.05). The commercial ointment could not take effect against AD in short-term administration. Interestingly, on day 16, MCE ME vehicle ameliorated the DNCB-induced skin similar to the oil-free group (P>0.05), and both of them were greater than the commercial ointment (P<0.05), which indicated that the vehicle containing MCE could decrease AD symptoms and the lower concentration of surfactant and co-surfactant in MCE ME did not irritate the AD-like skin. The commercial ointment irritates skin, resulting in erythema, dryness, and itching in AD patients.22 Moreover, we observed that it is difficult for the commercial ointment with sticky ointment to spread out on the impaired skin during the treatment. The dermatitis scores in DNCB+MCE ME or DNCB + MCE ME gel groups decreased since day 16 compared with all other groups (P<0.001). The ameliorating effects of MCE ME or MCE ME gel on AD-like skin might be owing to the combination effects of FK506 with MCE. On day 28, the dorsal skin of DNCB+MCE ME or DNCB + MCE ME gel group recovered and new hair grew (Figure 4C), while AD skin symptoms in DNCB + ointment were inhibited indistinctively and dryness was still very severe. FK506 MCE ME and MCE ME gel based on the MCE ameliorated exerted AD-like skin in synergy.
Figure 4.
Schematic diagram of the experimental procedure (A); effects of different formulations containing 0.1% FK506 on dermatitis scores throughout the experiment (B); and clinical features of AD-like skin lesions in the end of experiment (C).
Notes: *P<0.05 in comparison with DNCB; **P<0.001 in comparison with DNCB; #P<0.05 in comparison with DNCB + Ointment; ##P<0.001 in comparison with DNCB + Ointment; ++P<0.001.
Abbreviations: AD, atopic dermatitis; DNCB, 1–chloro–2,4–dinitrobenzene; FK506, tacrolimus; Control, healthy mice with shaved dorsal region; DNCB, AD-induced mice without drug treatment; DNCB + PC, AD-induced mice treated with propylene carbonate containing 0.1% (w/v) FK506; DNCB + Ointment; AD-induced mice treated with commercial ointment containing 0.1% (w/w) FK506; DNCB + Oil free, AD-induced mice treated with Oil free containing 0.1% (w/v) FK506; DNCB + Vehicle, AD-induced mice treated with microemulsion based on menthol/camphor eutectic vehicle without FK506; DNCB + MCE ME, AD-induced mice treated with microemulsion based on menthol/camphor eutectic containing 0.1% (w/v) FK506; DNCB + MCE ME gel, AD-induced mice treated with microemulsion based on menthol/camphor eutectic gel containing 0.1% (w/w) FK506; SD, standard deviation.
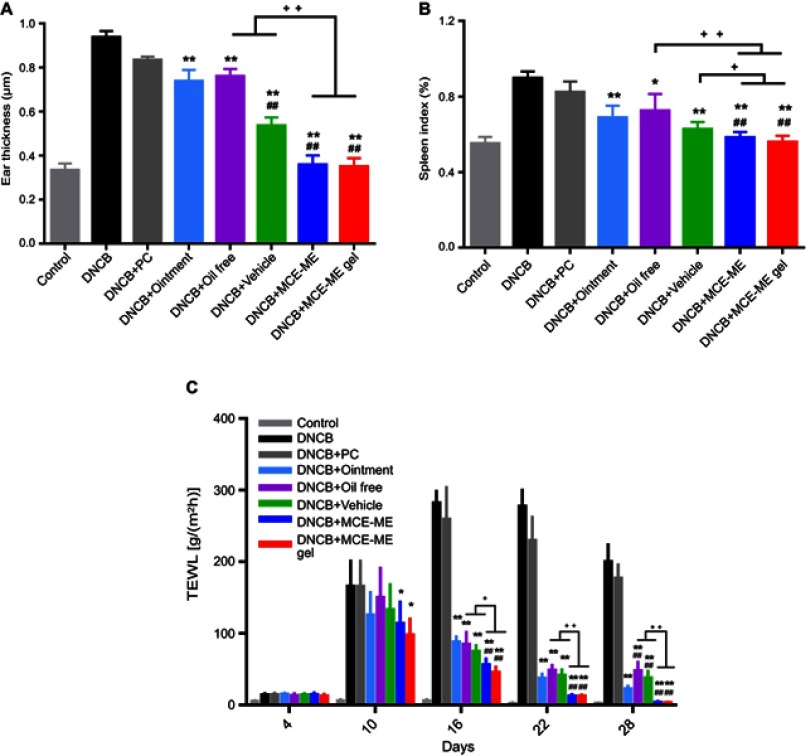
Effects of MCE ME and MCE ME gel on the ear thickness, spleen index, and TEWL
The ear swelling was represented with the ear thickness to evaluate the severity of AD inflammation. As shown in Figure 5A, the ear thickness of DNCB-induced AD mouse was 0.94±0.03 μm; it is thicker than that of the control group with 0.34±0.03 μm (P<0.001). After DNCB-induced AD mouse was treated with different formulations, the ear swelling was decreased except DNCB+PC (0.84±0.01 μm). The ear thickness of DNCB+Oil free and DNCB+Ointment treated was 0.76±0.03 μm and 0.74±0.05 μm, respectively, and DNCB+vehicle (0.54±0.04 μm) was thinner than DNCB + oil free or DNCB + ointment group (P<0.001), suggesting that the MCE as the oil phase exerted anti-inflammation effect over FK506 in commercial ointment. After treated with MCE ME and MCE ME gel, the ear thickness was further decreased to 0.36±0.04 μm and 0.35±0.04 μm (P<0.001), respectively, which confirmed that MCE ME and MCE ME gel caused a greater reduction in edema.
Figure 5.
The ear thickness (A), spleen index (B) on day 28, and TEWL (C) of the mice treated with different formulations throughout the experiment.
Notes: *P<0.05 in comparison with DNCB; **P<0.001 in comparison with DNCB; #P<0.05 in comparison with DNCB + Ointment; ##P<0.001 in comparison with DNCB +Ointment; +P<0.05; ++P<0.001.
Abbreviations: AD, atopic dermatitis; DNCB, 1–chloro–2,4–dinitrobenzene; FK506, tacrolimus; TEWL, transdermal water loss; Control, healthy mice with shaved dorsal region; DNCB, AD-induced mice without drug treatment; DNCB + PC, AD-induced mice treated with propylene carbonate containing 0.1% (w/v) FK506; DNCB + Ointment: AD-induced mice treated with commercial ointment containing 0.1% (w/w) FK506; DNCB + Oil free, AD-induced mice treated with Oil free containing 0.1% (w/v) FK506; DNCB + Vehicle; AD-induced mice treated with microemulsion based on menthol/camphor eutectic vehicle without FK506; DNCB + MCE ME, AD-induced mice treated with microemulsion based on menthol/camphor eutectic containing 0.1% (w/v) FK506; DNCB + MCE ME gel, AD-induced mice treated with microemulsion based on menthol/camphor eutectic gel containing 0.1% (w/w) FK506; SD, standard deviation.
Similar results were obtained with the spleen index as shown in Figure 5B, which was calculated by weight of spleen/weight of body ×100%, reflecting the abnormal immune activation in the AD mouse model. The spleen index of the control mice was 0.55±0.03%, and it increased to 0.90±0.12% after DNCB induced. After the DNCB-induced mice were treated with different formulations, PC could not apparently decrease the rise of spleen index (0.83±0.05%). The efficacy of DNCB + oil free (0.73±0.09%) and DNCB + vehicle (0.63±0.04%) was approximate to that of DNCB + ointment (0.69±0.06%) (P>0.05); however, DNCB + MCE ME (0.58±0.03%) or DNCB + MCE ME gel (0.56±0.03%) was better than that of DNCB + ointment (P<0.001). The results further confirmed that the MCE can suppress the abnormal immune activation in AD-induced mice and facilitate the therapy of FK506 against AD.
AD, as an immune-mediated skin barrier function disorder, is characterized by relapsing eczema with intense pruritus over dry skin lesions, accompanied by increased TEWL.38 As shown in Figure 5C, the TEWL of the intact skin of mice in the control was 5.44±0.78 g/(m2hr). After the mouse was induced by DNCB, TEWL increased and reached the maximum of 282.40±16.07 g/(m2hr) on day 16, and remained at 200.40±23.30 g/(m2hr) on day 28, which indicated that DNCB-induced AD impaired the mouse skin barrier. In comparison with DNCB-induced mice, TEWL was slightly decreased after treated with PC (P>0.05). However, MCE ME and MCE ME gel treatment decreased TEWL since day 10 (P<0.05), and the other treatments since day 16 (P<0.001) . Moreover, TEWL of MCE ME or MCE ME gel group was less than that of the commercial ointment, oil free, or vehicle since day 10 and throughout the treatment, and it recovered to 4.02±1.02 g/(m2hr) and 3.52±0.56 g/(m2hr) on day 28 and was similar to the control group (P>0.05).
Taken together, MCE in MCE ME and MCE ME gel can effectively alleviate the abnormal immunity activation of AD and facilitate the skin barrier recovery.
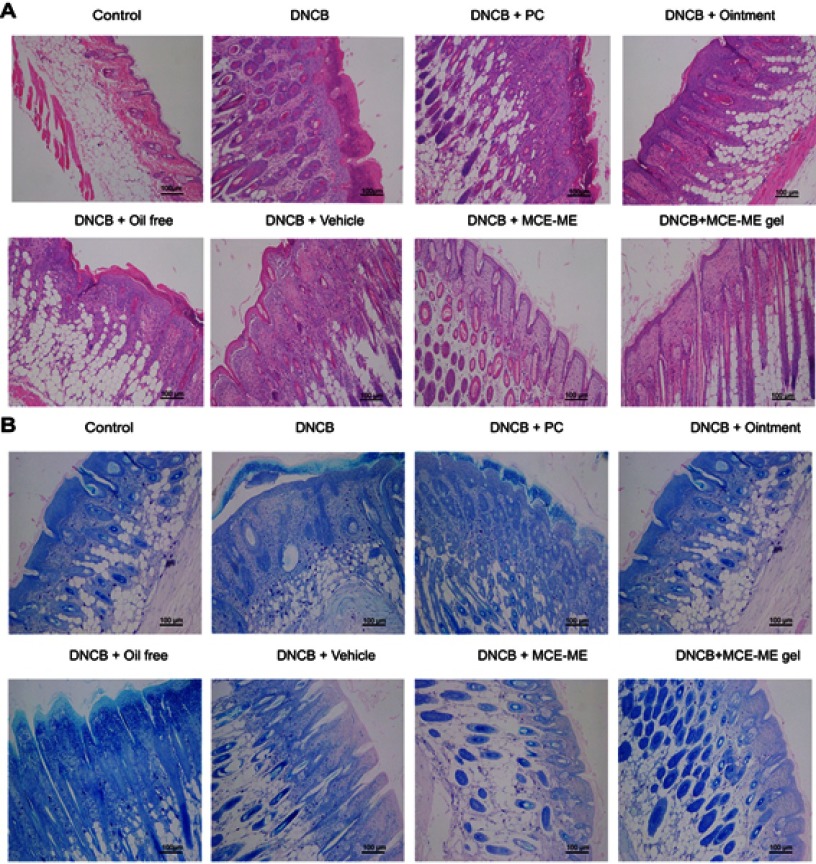
Histopathological analysis
HE and TB staining of the AD-like dorsal skin sections induced by DNCB was visualized to further evaluate the effects of MCE ME and MCE ME gel on the infiltration of lymphocytes and mast cells. Phagocytic cells infiltrate the epidermis first, and Langerhans cells then shift to lymph nodes and perform antigen presentation that can produce various inflammatory cytokine and mobilize mast cell.39 In HE-stained skin tissues (Figure 6A), the skin was thickened in DNCB-induced AD with epidermis hyperkeratosis and hypertrophy. After being treated with PC, oil free, Vehicle, or the commercial ointment, the skin thickness was moderately decreased, while it was attenuated by MCE ME and MCE ME gel administration. In the TB-stained skin tissues (Figure 6B), the number of mast cells increased in the SC and even infiltrated the epidermis and dermis of the DNCB-induced group in comparison with that of the control group. DNCB + PC or DNCB + oil free slightly decreased the mast cell number and infiltration, and DNCB + vehicle or DNCB + ointment was greater than DNCB + PC or DNCB + oil free. However, DNCB + MCE ME and DNCB + MCE ME gel groups showed a significant decrease. HE and TB demonstrated that the MCE played the crucial adjuvant role to FK506 against the inflammation histopathology of AD.
Figure 6.
Histological features of the skin treated with different formulations in AD mouse model. The sections were stained with hematoxylin and eosin (A) and toluidine blue (B), respectively.
Abbreviations: AD, atopic dermatitis; DNCB, 1–chloro–2,4–dinitrobenzene; FK506, tacrolimus; Control, healthy mice with shaved dorsal region; DNCB, AD-induced mice without drug treatment; DNCB + PC, AD-induced mice treated with propylene carbonate containing 0.1% (w/v) FK506; DNCB + Ointment; AD-induced mice treated with commercial ointment containing 0.1% (w/w) FK506; DNCB + Oil free; AD-induced mice treated with Oil free containing 0.1% (w/v) FK506; DNCB + Vehicle, AD-induced mice treated with microemulsion based on menthol/camphor eutectic vehicle without FK506; DNCB + MCE ME, AD-induced mice treated with microemulsion based on menthol/camphor eutectic containing 0.1% (w/v) FK506; DNCB + MCE ME gel, AD-induced mice treated with microemulsion based on menthol/camphor eutectic gel containing 0.1% (w/w) FK506.
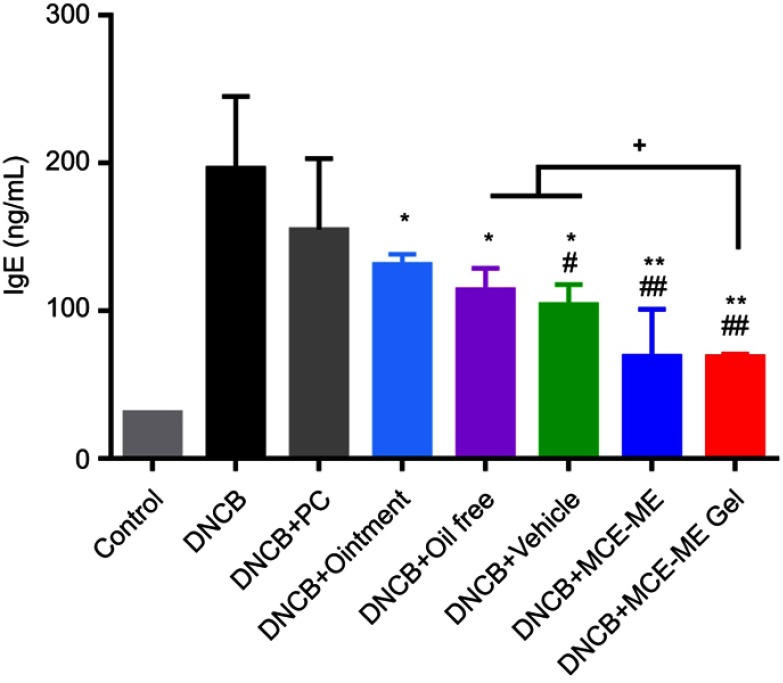
Effects of MCE ME and MCE ME gel on serum total IgE
AD is an IgE-mediated hypersensitive skin disease and the increase of serum total IgE level is a typical characteristic in DNCB-induced BALB/c mice with AD-like skin lesion.40,41 As shown in Figure 7, the total serum IgE increased to 196.54±48.57 ng/mL after inducing DNCB and 6.3-fold of that of the control group. However, after the DNCB-induced mice were treated with different formulations, the total serum IgE significantly decreased to 114.43±14.55 ng/mL for oil free (P<0.05), 104.33±13.64 ng/mL for vehicle (P<0.05), 131.61±6.85 ng/mL for the commercial ointment (P<0.05), 69.50±31.60 ng/mL for MCE ME (P<0.001), and 69.17±1.78 ng/mL for MCE ME gel (P<0.001), while it slightly decreased to 155.15±48.08 ng/mL for PC (P>0.05). Therefore, MCE facilitates the decrease of the pathological marker protein IgE in AD, and MCE ME and MCE ME gel are greater than the commercial ointment (P<0.05).
Figure 7.
Effects of different formulations on serum total IgE.
Notes: *P<0.05 in comparison with DNCB; **P<0.001 in comparison with DNCB; #P<0.05 in comparison with DNCB + Ointment; ##P<0.001 in comparison with DNCB +Ointment; +P<0.05; ++P<0.001.
Abbreviations: AD, atopic dermatitis; DNCB, 1–chloro–2,4–dinitrobenzene; FK506, tacrolimus; Control, healthy mice with shaved dorsal region; DNCB, AD-induced mice without drug treatment; DNCB + PC, AD-induced mice treated with propylene carbonate containing 0.1% (w/v) FK506; DNCB + Ointment: AD-induced mice treated with commercial ointment containing 0.1% (w/w) FK506; DNCB + Oil free, AD-induced mice treated with Oil free containing 0.1% (w/v) FK506; DNCB + Vehicle, AD-induced mice treated with microemulsion based on menthol/camphor eutectic vehicle without FK506; DNCB + MCE ME, AD-induced mice treated with microemulsion based on menthol/camphor eutectic containing 0.1% (w/v) FK506; DNCB + MCE ME gel, AD-induced mice treated with microemulsion based on menthol/camphor eutectic gel containing 0.1% (w/w) FK506; SD, standard deviation.
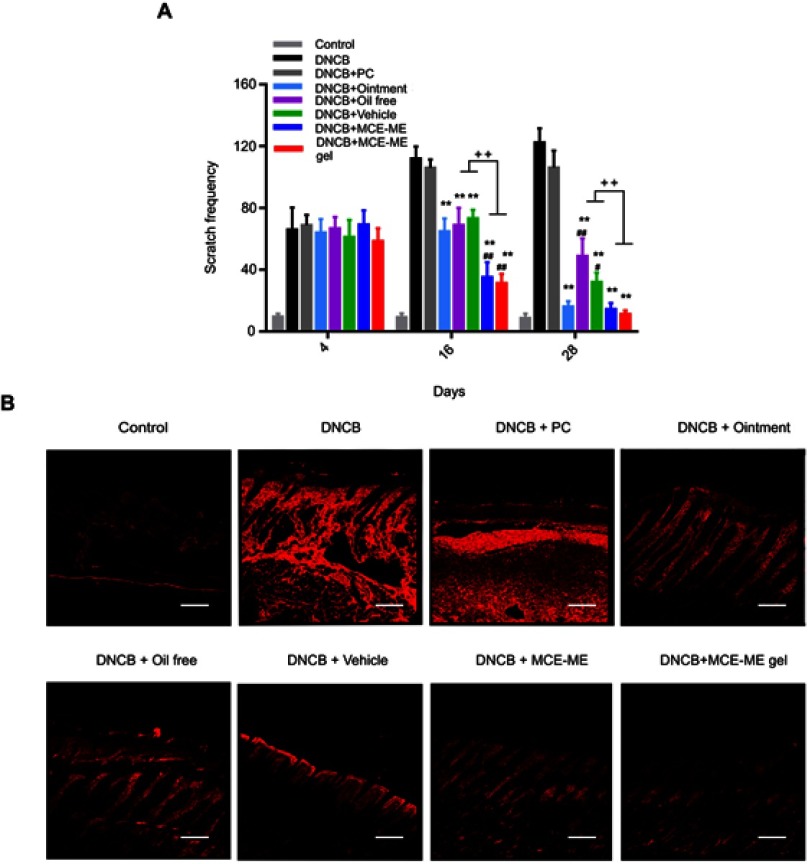
Decreasing the scratch frequency and down regulation of SP
The initial side effects of burning and itch during FK506 therapy in AD triggered scratch immediately resulting in symptom exacerbation, and pruritus–scratch–pruritus easily turned into a vicious circle.42,43 SP is an endogenous neuropeptide that belongs to the tachykinin family and it is one of the critical factors that induces itch in topical therapy of FK506.44,45 We evaluated if the MCE ME relieved the side effects of topical FK506 therapy by observing the response of mouse upon administration, recording scratch behavior, and measuring SP expression.
After the different formulations loading FK506 were topically administered onto the DNCB-induced skin lesion on day 4, 16, and 28, respectively, the behavior of mice was recorded. On day 4, the mice behaved normally because the skin was just sensitized without being challenged, they tolerated the administrations. On day 16, the mice presented the most marked AD symptoms, and the mice treated with the commercial ointment and PC behaved with trembling and screaming, which indicated that the transient burning and stinging sensation occurred when the commercial ointment and PC were applied onto the skin with marked AD symptoms. However, the mice in other groups treated with formulations containing MCE behaved quietly. The scratch behavior and SP expression are shown in Figure 8. As shown in Figure 8A, the scratch behavior occurred in the DNCB-induced mice and deteriorated with time. However, the treatment with different formulations except PC effectively decreased the scratch frequency after persisting treatment till day 28, and MCE ME and MCE ME gel even reduced the scratch frequency since day 16 in comparison with DNCB + ointment, DNCB + vehicle, or DNCB + oil free (P<0.001). On day 28, treatment with the commercial ointment presented a similar effect on reducing the scratch frequency to MCE ME or MCE ME gel. These results demonstrated that MCE ME and MCE ME gel exerted anti-pruritic effect faster than the commercial ointment, and the commercial ointment was effective on anti-pruritus of AD when it was used long term.
Figure 8.
Inhibition effects of different formulations on scratch frequency (A) and substance P expression (B).
Notes: *P<0.05 in comparison with DNCB; **P<0.001 in comparison with DNCB; #P<0.05 in comparison with DNCB + Ointment; ##P<0.001 in comparison with DNCB +Ointment; +P<0.05; ++P<0.001.
Abbreviations: AD, atopic dermatitis; DNCB, 1–chloro–2,4–dinitrobenzene; FK506, tacrolimus; Control, healthy mice with shaved dorsal region; DNCB, AD-induced mice without drug treatment; DNCB + PC, AD-induced mice treated with propylene carbonate containing 0.1% (w/v) FK506; DNCB + Ointment: AD-induced mice treated with commercial ointment containing 0.1% (w/w) FK506; DNCB + Oil free, AD-induced mice treated with Oil free containing 0.1% (w/v) FK506; DNCB + Vehicle, AD-induced mice treated with microemulsion based on menthol/camphor eutectic vehicle without FK506; DNCB + MCE ME, AD-induced mice treated with microemulsion based on menthol/camphor eutectic containing 0.1% (w/v) FK506; DNCB + MCE ME gel, AD-induced mice treated with microemulsion based on menthol/camphor eutectic gel containing 0.1% (w/w) FK506; SD, standard deviation.
SP expression in the skin after 28 days’ treatment was visualized by immunofluorescent staining (Figure 8B). The high expression of SP was observed in DNCB and DNCB + PC groups, which is consistent with the induced itch and frequent scratch in these two groups. SP expression was moderately down regulated after oil-free and the commercial ointment treatment, indicating that FK506 in oil-free and the commercial ointment was effective in down regulating SP expression and anti-pruritus. Interestingly, vehicle presented, with significance, down regulated SP expression in comparison with DNCB or DNCB + PC group, and even better than the commercial ointment. It demonstrated that MCE in vehicle can inhibit SP expression, and SP expression down regulation contributes to itch relief and scratch decrease. MCE ME and MCE ME gel further down regulated SP expression in comparison with vehicle. Menthol in MCE can activate TRPM8 channels, the primary cold sensors extensively distributed in the skin, to generate cooling sensation and then relieve the itch and break the itch–scratch cycle.46,47,48 Our work first reported that MCE based on menthol blended with camphor could downregulate the SP expression and inhibit SP-mediated pruritus or other unpleasant sensations; it will thus help to reduce the topical side effects of calcineurin inhibitor FK506 and consequently alleviate AD. Taken together with the enhancing percutaneous and anti-AD efficacy of MCE in conjunction with FK506, MCE may reduce the dose of FK506 for anti-AD. However, further studies should be performed to confirm this hypothesis.
Conclusion
FK506 is an effective topical drug for AD treatment. However, its efficiency is hampered by poor penetration into the skin and the onset of transient irritation symptoms in the applied skin. These challenges can be addressed by novel drug delivery systems. In this study, we developed a ME system for FK506 based on MCE, ie, FK506 MCE ME. The optimum MCE ME was demonstrated to have low cytotoxicity and enhance FK506 penetration into the skin in comparison with the commercial ointment. The treatment efficiency of FK506 MCE ME or FK506 MCE ME gel on DNCB-induced AD-like skin was greater than that of the commercial ointment in terms of ameliorating AD-like skin symptoms, reducing the ear thickness and spleen index, restoring the skin barrier, decreasing the serum total IgE and the scratch frequency, and down regulating SP expression. MCE ME vehicle also presented significant anti-pruritic efficiency to ameliorate AD symptoms. Therefore, it can improve medication adherence with FK506 in patients with AD. Overall, MCE ME presented excellent properties of simultaneously enhancing percutaneous delivery and treatment efficacy and reducing the side effects of FK506 for AD. Therefore, it is a promising nanoscale system of FK506 for the effective treatment of AD.
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (No. 81073066 and No. 81473358) and Science and Technology Planning Project of Guangdong Province, China (No. 2017A020211020).
Abbreviation list
AD, atopic dermatitis; MCE, menthol/camphor eutectic; ME, microemulsion; FK506, tacrolimus; CLSM, confocal laser scanning microscope; DNCB, 1–chloro–2,4–dinitrobenzene; PC, propylene carbonate; HaCaT, immortalized human keratinocytes; TB, toluidine blue; HPF, high-power field; TEWL, transepidermal water loss; SP, Substance P; LSD, least significant difference test; SD, Sprague Dawley; SC, stratum corneum; Th2, T helper 2.
Disclosure
All authors report grants from National Natural Science Foundation of China (No. 81073066 and No. 81473358) and grants from Science and Technology Planning Project of Guangdong Province, China (No. 2017A020211020), during the conduct of the study. All authors have a patent pending of the preparation process and compositions for treating skin immune diseases. The authors report no other conflicts of interest in this work.
References
- 1.Dubrac S, Schmuth M, Ebner S. Atopic dermatitis: the role of langerhans cells in disease pathogenesis. Immuno Cell Biol. 2010;88(4):400–409. doi: 10.1038/icb.2010.33 [DOI] [PubMed] [Google Scholar]
- 2.Heratizadeh A, Werfel T, Wollenberg A, et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Iimmunol. 2017;140(3):845–853. doi: 10.1016/j.jaci.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 3.Alyoussef A. Blocking TGF-beta type 1 receptor partially reversed skin tissue damage in experimentally induced atopic dermatitis in mice. Cytokine. 2018;106:45–53. doi: 10.1016/j.cyto.2018.02.025 [DOI] [PubMed] [Google Scholar]
- 4.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J Allergy Clin Immunol. 2009;124(6):1251–1258. doi: 10.1016/j.jaci.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 5.Mesquita Kde C, Igreja AC, Costa IM. Atopic dermatitis and vitamin D: facts and controversies. An Bras Dermatol. 2013;88(6):945–953. doi: 10.1590/abd1806-4841.20132660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pople PV, Singh KK. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis. Int J Pharm. 2010;398(1–2):165–178. doi: 10.1016/j.ijpharm.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Schauber J, Weisenseel P, Ruzicka T. Topical treatment of perianal eczema with tacrolimus 0.1%. Br J Dermatol. 2009;16(6):1384–1386. doi: 10.1111/j.1365-2133.2009.09345.x [DOI] [PubMed] [Google Scholar]
- 8.Czarnecka-Operacz M, Jenerowicz D. Topical calcineurin inhibitors in the treatment of atopic dermatitis - an update on safety issues. J Dtsch Dermatol Ges. 2012;10(3):167–172. doi: 10.1111/j.1610-0387.2011.07791.x [DOI] [PubMed] [Google Scholar]
- 9.Pan W, Qin M, Zhang G, et al. Combination of hydrotropic nicotinamide with nanoparticles for enhancing tacrolimus percutaneous delivery. Int J Nanomedicine. 2016;11:4037–4050. doi: 10.2147/IJN.S108545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan T, Pan J, Long Y, et al. Dual roles of TPGS based microemulsion for tacrolimus: enhancing the percutaneous delivery and anti-psoriatic efficacy. Int J Pharm. 2017;528(1–2):511–523. doi: 10.1016/j.ijpharm.2017.06.050 [DOI] [PubMed] [Google Scholar]
- 11.Andrade LM, Silva LAD, Krawczyk-Santos AP, et al. Improved tacrolimus skin permeation by co-encapsulation with clobetasol in lipid nanoparticles: study of drug effects in lipid matrix by electron paramagnetic resonance. Eur J Pharm Biopharm. 2017;119:142–149. doi: 10.1016/j.ejpb.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 12.Li G, Fan Y, Fan C, et al. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82(1):49–57. doi: 10.1016/j.ejpb.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 13.Tessema EN, Gebre-Mariam T, Paulos G, Wohlrab J, Neubert RHH. Delivery of oat-derived phytoceramides into the stratum corneum of the skin using nanocarriers: formulation, characterization and in vitro and ex-vivo penetration studies. Eur J Pharm Biopharm. 2018;127:260–269. doi: 10.1016/j.ejpb.2018.02.037 [DOI] [PubMed] [Google Scholar]
- 14.Zhuo F, Abourehab MAS, Hussain Z. Hyaluronic acid decorated tacrolimus-loaded nanoparticles: efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr Polym. 2018;197:478–489. doi: 10.1016/j.carbpol.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 15.Kiselmann C, Dobler D, Schmidts T, et al. Development of a skin-friendly microemulsion for dermal allergen-speicific immunotherapy. Int J Pharm. 2018;550(1–2):463–469. doi: 10.1016/j.ijpharm.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Michniak-Kohn BB. Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole. Int J Pharm. 2018;536(1):345–352. doi: 10.1016/j.ijpharm.2017.11.041 [DOI] [PubMed] [Google Scholar]
- 17.Kirsner RS, Heffernan MP, Antaya R. Safety and efficacy of tacrolimus ointment versus pimecrolimus cream in the treatment of patients with atopic dermatitis previously treated with corticosteroids. Acta Derm Venereol. 2010;90(1):58–64. doi: 10.2340/00015555-0748 [DOI] [PubMed] [Google Scholar]
- 18.Pople PV, Singh KK. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis-part II: in vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Int J Pharm. 2012;434(1–2):70–79. doi: 10.1016/j.ijpharm.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka M, Yokota S, Iwao Y, Noguchi S, Itai S. Development and evaluation of a tacrolimus cream formulation using a binary solvent system. Int J Pharm. 2014;464(1–2):19–26. doi: 10.1016/j.ijpharm.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 20.Kita T, Uchida K, Kato K, Suzuki Y, Tominaga M, Yamazaki J. FK506 (tacrolimus) causes pain sensation through the activation of transient receptor potential ankyrin 1 (TRPA1) channels. J Physiol Sci. 2019;69(2):305–316.T. doi: 10.1007/s12576-018-0647-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stander S, Stander H, Seeliger S, Luger TA, Steinhoff M. Topical pimecrolimus and tacrolimus transiently induce neuropeptide release and mast cell degranulation in murine skin. Br J Dermatol. 2007;156(5):1020–1026. doi: 10.1111/j.1365-2133.2007.07813.x [DOI] [PubMed] [Google Scholar]
- 22.Wong LS, Otsuka A, Yamamoto Y, et al. TRPA1 channel participates in tacrolimus-induced pruritus in a chronic contact hypersensitivity murine model. J Dermatol Sci. 2018;89(2):207–209. doi: 10.1016/j.jdermsci.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuki M, Morimoto H, Nakagawa H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: review on safety and benefits. J Dermatol. 2018;45(8):936–942. doi: 10.1111/1346-8138.14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamatou GP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. doi: 10.1016/j.phytochem.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 25.Klein AH, Sawyer CM, Takechi K, et al. Topical hindpaw application of L-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons. Neuroscience. 2012;219:234–242. doi: 10.1016/j.neuroscience.2012.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xavier-Junior FH, Vauthier C, Morais AR, Alencar EN, Egito ES. Microemulsion systems containing bioactive natural oils: an overview on the state of the art. Drug Dev Ind Pharm. 2017;43(5):700–714. doi: 10.1080/03639045.2016.1235186 [DOI] [PubMed] [Google Scholar]
- 27.Lucaciu OC, Connell GP. Itch sensation through transient receptor potential channels: a systematic review and relevance to manual therapy. J Manipulative Physiol Ther. 2013;36(6):385–393. doi: 10.1016/j.jmpt.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 28.Mizumura K, Koda H. Potentiation and suppression of the histamine response by raising and lowering the temperature in canine visceral polymodal receptors in vitro. Neurosci Lett. 1999;266:9–12. [DOI] [PubMed] [Google Scholar]
- 29.Haught JM, Jukic DM, English JC 3rd. Hydroxyethyl starch-induced pruritus relieved by a combination of menthol and camphor. J Am Acad Dermatol. 2008;59(1):151–153. doi: 10.1016/j.jaad.2008.03.034 [DOI] [PubMed] [Google Scholar]
- 30.Metz M, Staubach P. Itch management: topical agents. Curr Probl Dermatol. 2016;50:40–45. doi: 10.1159/000446040 [DOI] [PubMed] [Google Scholar]
- 31.Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol. 2007;57(5):873–878. doi: 10.1016/j.jaad.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 32.Gohel MC, Nagori SA. Resolving issues of content uniformity and low permeability using eutectic blend of camphor and menthol. Indian J Pharm Sci. 2009;71(6):622–629. doi: 10.4103/0250-474X.59543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu K, Wang Y, Wan T, et al. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose. Int J Nanomedicine. 2017;13:129–142. doi: 10.2147/IJN.S150319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SaviĆ V, TodosijeviĆ M, IliĆ T, et al. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: structure characterization and in vitro/in vivo performances. Int J Pharm. 2017;529(1–2):491–505. doi: 10.1016/j.ijpharm.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 35.Patel P, Patel H, Panchal S, Mehta T. Formulation strategies for drug delivery of tacrolimus: an overview. Int J Pharm Investig. 2012;2(4):169–175. doi: 10.4103/2230-973X.106981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SG, Kang JB, Kim SR, et al. Enhanced topical delivery of tacrolimus by a carbomer hydrogel formulation with transcutol P. Drug Dev Ind Pharm. 2016;42(10):1636–1642. doi: 10.3109/03639045.2016.1160107 [DOI] [PubMed] [Google Scholar]
- 37.Papier A, Strowd LC. Atopic dermatitis: a review of topical nonsteroid therapy. Drugs Context. 2018;7:212521. doi: 10.7573/dic.212521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi YY, Kim MH, Lee H, et al. Cynanchum atratum inhibits the development of atopic dermatitis in 2,4-dinitrochlorobenzene-induced mice. Biomed Phaemacother. 2017;90:321–327. doi: 10.1016/j.biopha.2017.03.065 [DOI] [PubMed] [Google Scholar]
- 39.Ibaraki H, Kanazawa T, Takashima Y, Okada H, Seta Y. Transdermal anti-nuclear kappaB siRNA therapy for atopic dermatitis using a combination of two kinds of functional oligopeptide. Int J Pharm. 2018;542(1–2):213–220. doi: 10.1016/j.ijpharm.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 40.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X [DOI] [PubMed] [Google Scholar]
- 41.Chan CC, Liou CJ, Xu PY, Set AL. Effect of dehydroepiandrosterone on atopic dermatitis-like skin lesions induced by 1-chloro-2,4-dinitrobenzene in mouse. JJ Dermatol Sci. 2013;72(2):149–157. doi: 10.1016/j.jdermsci.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 42.Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. 2010;65(7):805–821. doi: 10.1111/j.1398-9995.2010.01995.x [DOI] [PubMed] [Google Scholar]
- 43.Yosipovitch G, Gold LF, Lebwohl MG, Silverberg JI, Tallman AM, Zane LT. Early relief of pruritus in atopic dermatitis with crisaborole ointment, a non-steroidal, phosphodiesterase 4 inhibitor. Acta Derm Venereol. 2018;98(5):484–489. doi: 10.2340/00015555-2893 [DOI] [PubMed] [Google Scholar]
- 44.Inagaki N, Shiraishi N, Igeta K. Net AL. Depletion of substance P, a mechanism for inhibition of mouse scratching behavior by tacrolimus. Eur J Pharmacol. 2010;626(2–3):283–289. [DOI] [PubMed] [Google Scholar]
- 45.Maeno H, Kiyama H, Tohyama M. Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. BrainRes Mol Brain Res. 1993;18(1–2):43–58. doi: 10.1016/0169-328X(93)90172-L [DOI] [PubMed] [Google Scholar]
- 46.Andersen HH, Melholt C, Hilborg SD, et al. Antipruritic effect of cold-induced and transient receptor potential-agonist-induced counter-irritation on histaminergic itch in humans. Acta Derm Venereol. 2017;97(1):63–67. doi: 10.2340/00015555-2447 [DOI] [PubMed] [Google Scholar]
- 47.Palkar R, Ongun S, Catich E, et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J Invest Dermatol. 2018;138(6):1391–1399. doi: 10.1016/j.jid.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez de Vega MJ, Gómez-Monterrey I, Ferrer-Montiel A, González-Muňiz R. Transient receptor potential melastatin 8 channel (TRPM8) modulation: cool entryway for treating pain and cancer. J Med Chem. 2016;59(22):10006–10029. doi: 10.1021/acs.jmedchem.6b00305 [DOI] [PubMed] [Google Scholar]