Abstract
Acidic organelles, such as endosomes and lysosomes, store Ca2+ that is released in response to intracellular increases in the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP). In neurons, NAADP and Ca2+ signaling contribute to synaptic plasticity, a process of activity-dependent long-term potentiation (LTP) [or, alternatively, long-term depression (LTD)] of synaptic strength and neuronal transmission that is critical for neuronal function as well as memory formation. We explored the function of and mechanisms regulating acidic Ca2+ store signaling in murine hippocampal neurons. We found that metabotropic glutamate receptor 1 (mGluR1) was coupled to NAADP signaling that elicited Ca2+ release from acidic stores. In turn, this released Ca2+ mediated mGluR1-dependent LTP by transiently inhibiting SK-type K+ channels, possibly through the activation of protein phosphatase 2A. Genetically removing two-pore channels (TPCs), which are endo-lysosomal-specific ion channels, switched the polarity of plasticity from LTP to LTD, indicating the importance of specific receptor-store coupling and providing mechanistic insight into how mGluR1 can produce both synaptic potentiation and synaptic depression. 2
Introduction
Acidic storage vesicles, such as lysosomes and endosomes, have traditionally been viewed as compartments for degradation and recycling of cellular metabolites. However, they have also been recognised as having additional signalling roles, including in intracellular Ca2+ signalling, as through their release of Ca2+ in response to the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP) [1–6]. At present, the functions of NAADP signalling in the central nervous system (CNS) are incompletely understood. NAADP evokes Ca2+ release from brain microsomes [7], and NAADP-binding sites are present throughout the brain [8], suggesting that NAADP is an important signalling molecule in the CNS. Thus far, several key areas in which NAADP signalling plays a role have been identified: (i) in differentiation and growth of [9–11] and augmentation of neurite outgrowth in [12] cortical neurons; (ii) in autophagy in astrocytes, in which NAADP promotes autophagosome formation, which can be reduced in the presence of a dominant negative TPC2, a lysosomal two-pore channel isoform targeted by NAADP [13] or with the NAADP antagonist Ned-19 [14]; and (iii), in regulating neuronal excitability, neurotransmitter release, and synaptic plasticity. The application of membrane-permeable NAADP (NAADP-AM) reportedly causes membrane depolarization in medullary neurons [15] and application of NAADP enhances neurotransmitter release in Aplysia californica [16] and at neuromuscular junctions in frogs [17]. Work from our laboratory has shown that acidic store Ca2+ signalling enhances neurotransmitter release [18] and is essential for dendritic spine growth associated with late-phase long term potentiation (LTP) [19] in hippocampal CA1 pyramidal neurons from rodents.
The activation of various metabotropic receptors reportedly induces NAADP synthesis in non-neuronal cell types [20–24], and the application of glutamate, which can activate metabotropic glutamate receptors (mGluRs), also reportedly stimulates NAADP synthesis in both neurons [25] and astrocytes [14]. However, the specific glutamate receptor subtype(s) responsible for NAADP synthesis has thus far remained elusive. Here, we examined NAADP-mediated changes in neuronal excitability and found that these NAADP-mediated effects are driven by mGluR1 activation. Critically, this pathway led us to identify a unique role for NAADP in the genesis of mGluR1-mediated synaptic potentiation.
Results
NAADP-AM causes membrane depolarization in pyramidal neurons of the hippocampus
NAADP is a charged molecule, making it necessary to bond it to an acetoxymethyl ester group (AM) for it to be membrane permeable [26]. To explore the effect of NAADP on membrane excitability, we introduced this engineered molecule (NAADP-AM) extracellularly, via a glass pipette, ono pyramidal neurons in CA3 and CA1 regions of hippocampal slice cultures. We chose cells that were localized to the surface of the tissue to improve the delivery of NAADP-AM.
Extracellular application of NAADP-AM (pipette concentration of 300 μM) produced transient membrane depolarizations (denoted by ΔVM; Figure 1, B to D) in both CA3 and CA1 pyramidal neurons of the hippocampus. The effect was highly consistent as the application of NAADP-AM at 30-second intervals produced reproducible membrane depolarization. Application of vehicle alone or NAADP alone, in other words the “no AM” group, failed to produce depolarization (Figure 1, B and C).
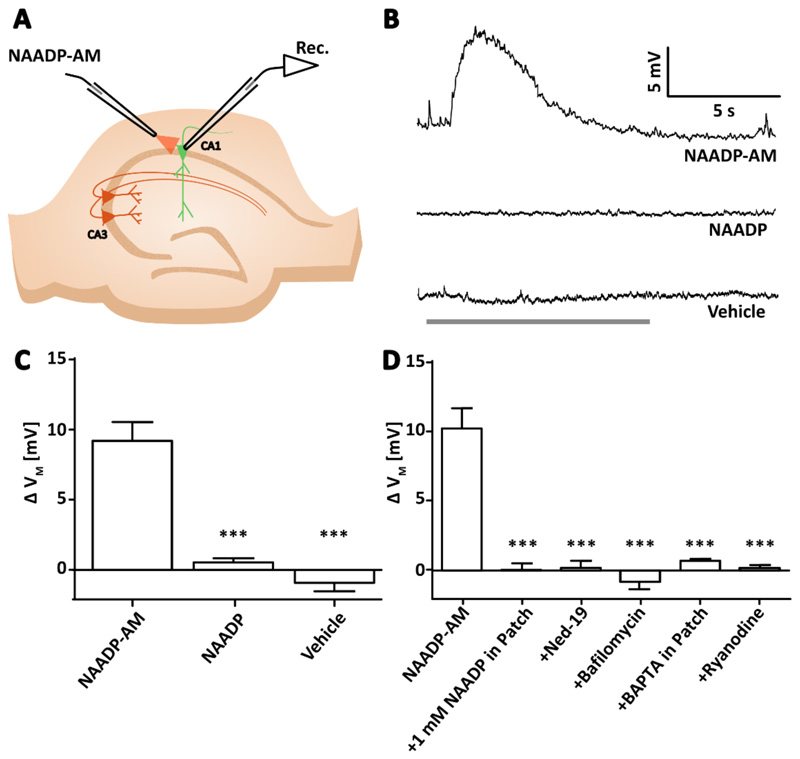
Figure 1. NAADP causes membrane depolarization in pyramidal neurons of the hippocampus in a manner dependent on acidic store signaling, intracellular Ca2+, and RyR.
(A) Diagram showing the experimental configuration to record membrane potential of CA1 or CA3 pyramidal neurons in hippocampal slices whilst NAADP-AM was applied locally. (B) Example voltage traces recorded whilst applying NAADP-AM, NAADP, or vehicle. Arrows indicate the start of delivery and grey bar indicates the total time of application. (C) Transient membrane depolarization (ΔVM) upon application of NAADP-AM (300 μM, n=12 cells), NAADP (300 μM, n=5), or vehicle (n=6). Data are mean ±SEM. (D) Mean ΔVM upon application of NAADP-AM (300 μM, n=11 cells) alone or (left to right) in combination with a desensitizing concentration of NAADP (1 mM) inside the internal solution of the patch pipette (n=6); preincubation with the NAADP antagonist Ned-19 (100 μM, 40 min, n=6); preincubation with the vacuolar H+ ATPase inhibitor bafilomycin (4 μM, 40 min, n=5); with BAPTA (15 mM) inside the internal solution of the patch pipette (n=5); or pre-incubation with ryanodine (30 μM, 40 min, n=4). Significance was assessed with; Data are mean ±SEM; n = single cells. *** P < 0.005 by Kruskal-Wallis and post hoc Dunn’s tests.
To then examine the mechanism by which NAADP-AM produced the membrane depolarization, we performed a series of pharmacological manipulations in combination with the extracellular application of NAADP-AM. A characteristic feature of NAADP signalling in mammals is that at high concentrations NAADP itself can desensitize the NAADP receptor [27]. This feature allows for a specific pharmacological inhibition of NAADP signalling. Desensitization of the NAADP receptor with 1 mM NAADP in the patch pipette abolished responses to NAADP-AM (Figure 1D) as did pharmacological antagonism of the NAADP receptor with Ned-19 [28] (Figure 1D). These data show that NAADP-mediated depolarization depends on canonical mechanisms of NAADP signalling.
We next examined whether an NAADP-mediated increase in intracellular Ca2+ concentration was required for the depolarization we had observed. By introducing the fast Ca2+ chelator BAPTA [1,2-bis (o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid] via the patch pipette (15 mM), we clamped intracellular Ca2+, and depolarization by NAADP-AM was inhibited (Figure 1D). Whereas it was now clear that depolarization was dependent both upon Ca2+ and NAADP, the intracellular source of the Ca2+ required confirmation. NAADP reportedly causes Ca2+ release from acidic stores [29, 30]. Bafilomycin A1 is reported to abrogate endolysosomal Ca2+ release by inhibiting vacuolar H+-ATPases and thereby preventing Ca2+ loading into the store [31, 32]. Pre-incubation with bafilomycin A1 abolished depolarization by NAADP-AM (Figure 1D), suggesting that NAADP-mediated membrane depolarization requires Ca2+ release from an acidic store.
Amplification of acidic store Ca2+ signals by Ca2+-induced Ca2+ release (CICR) from ryanodine receptors (RyR) has been reported in neurons of the medulla [15]; therefore, we examined whether this signalling motif was conserved in pyramidal neurons. Ryanodine inhibits RyRs in the hippocampus at micromolar concentrations [33]. Bath application of ryanodine at 30 μM inhibited NAADP-mediated membrane depolarization (Figure 1D), suggesting that the CICR amplification motif is conserved.
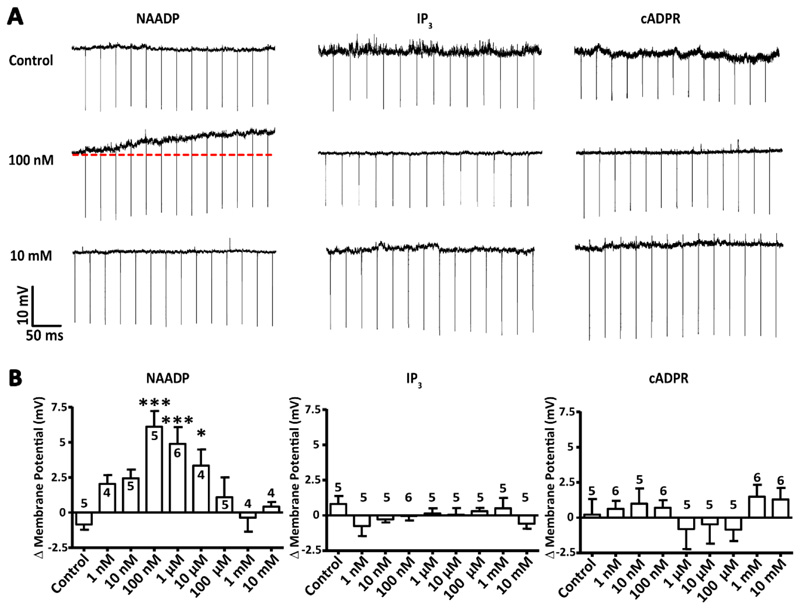
Ca2+-mobilizing second messengers are not generally associated with driving membrane depolarization; thus, we explored whether NAADP is unique in its ability to do so. We dialyzed the known Ca2+-mobilizing second messengers [NAADP, inositol 1,4,5-trisphosphate (IP3), and cyclic adenosine diphosphate ribose (cADPR)] into CA1 pyramidal neurons via a patch pipette and measured membrane potential. We found that only when NAADP was included in the pipette was membrane depolarization observed (Figure 2, A and B). Critically, the magnitude of depolarization generated in response to dialysis with NAADP produced a “bell-shaped” distribution, a hallmark of NAADP signalling where supra-maximal concentrations of NAADP cause inhibition of the associated response [27, 34, 35]. The half-maximal effective concentration (EC50) was determined to be 12.5 nM, and the half maximal inhibitory concentration (IC50) was 7.07 μM. The Hill coefficients were +0.527 for stimulation and -0.927 for inhibition. Furthermore, the maximal ΔVM achieved was at the concentration of 100 nM NAADP, which is within the range previously reported for maximum Ca2+ mobilisation from an acidic store [4, 36]. Neither IP3 nor cADPR, at any concentration, produced significant depolarization (Figure 2B). These results suggest that NAADP is unique in its capacity to produce a membrane depolarization amongst Ca2+-mobilizing second messenger family.
Figure 2. NAADP is unique among second messengers in its ability to depolarize hippocampal pyramidal neurons.
(A) Example voltage traces for dialysis of CA1 pyramidal neurons patched with internal solutions containing various concentrations of the Ca2+- mobilizing second messengers NAADP, IP3 and cADPR. Changes to membrane potential were recorded over time as the second messengers dialysed into the patched cell. (B) Transient membrane depolarization (ΔVM) of the cells described in (A) in response to increasing concentrations of the Ca2+-mobilizing second messengers. Data are mean ± SEM; n = single cells, indicated above each column; n > 4 for all concentrations of second messengers. *** P < 0.005 and * P <0.05 by Kruskal-Wallis and post hoc Dunn’s tests.
Activation of mGluR1 causes NAADP-dependent membrane depolarization
In order that we might begin to understand the significance of the NAADP-mediated depolarization, we sought to identify the mechanism by which NAADP elevations are triggered physiologically. The excitatory neurotransmitter glutamate has previously been shown to generate the synthesis of NAADP in neurons and astrocytes (Pandey, et al. [25], Pereira, et al. [14]), although the specific glutamate receptor subtype(s) were not identified. In a variety of other tissue types, NAADP synthesis is reported to occur following activation of metabotropic receptors [20–23, 37], we therefore looked to metabotropic glutamate receptors as likely candidates. To examine this we pharmacologically isolated the mGluRs with antagonists of ionotropic glutamate and GABA receptors (50 μM AP5, 10 μM NBQX, 100 μM picrotoxin and 2 μM CGP 55845) and delivered patterned electrical stimulation (4x, 20 Hz), known to activate mGluRs in the hippocampus [38], whilst measuring the membrane potential from CA1 pyramidal neurons (Figure 3A). Under these conditions, we observe a depolarization ΔVm of + 2.09 ± 0.15 mV (Figure 3, B and C). To confirm that this was a consequence of mGluR activation the mGluR antagonist LY341495 (100 μM) was added to the bath, at a concentration reported to block all members of the mGluR family [39]. This blocked the electrically-induced membrane depolarization (Figure 3, B and C). Now confident that mGluRs are able to produce depolarization we sought to determine the specific mGluR subtype(s) that mediated the response.
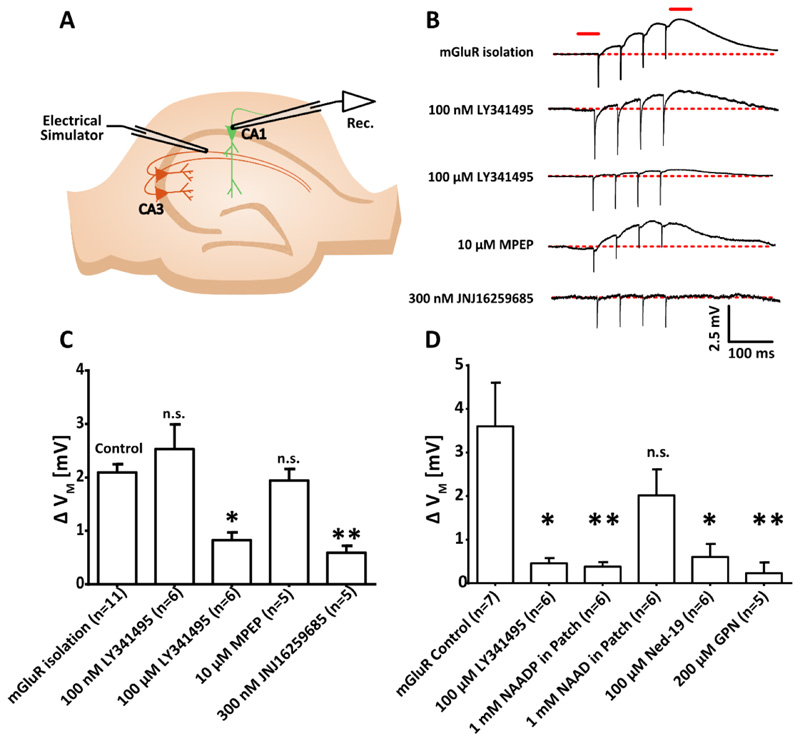
Figure 3. Activation of mGluR1 in CA1 pyramidal neurons causes a membrane depolarization that depends on NAADP signalling and acidic store Ca2+ signalling.
(A) Diagram showing the experimental configuration to record membrane potential of CA1 pyramidal neurons in hippocampal slices whilst mGluRs were pharmacologically isolated (50 μM AP5, 10 μM NBQX, 100 μM picrotoxin, and 2 μM CGP 55845) and electrical stimulation was applied to Schaffer collaterals (4 pulses, 20 Hz), (n=11 cells). (B) Typical voltage recordings from single cells upon electrical stimulation with pharmacological isolation of mGluRs or plus antagonism of group 2 and 3 mGluRs (LY341485 at 100 nM; n=6), panmGluRs (LY341485 at 100 μM; n=6), mGluR5 (MPEP, 10 μM; n=5) or mGluR1 (JNJ16259685, 300 nM; n=5) (top to bottom). The red lines indicate where membrane potentials were compared before and after stimulation. (C and D) Transient membrane depolarization (ΔVM) of CA1 pyramidal neurons after electrical stimulation alone (control; n=7 cells) or with the presence of (C) the mGluR antagonism described in (B), or (D) pan-mGluR antagonist [LY341485, 100 μM; n=6; cells and data set are independent from those in (C)], desensitizing concentration of NAADP inside the internal solution of the patch pipette (1 mM; n=6), NAAD inside the internal solution of the patch pipette (1 mM; n=6), preincubation with the NAADP antagonist Ned-19 (100 μM, 40 min; n=6), or acute administration of the lysosomal disrupting agent GPN (200 μM; n=5). Data are mean ± SEM; n = single cells. ** P < 0.01 and * P < 0.05 by Kruskal-Wallis and post hoc Dunn’s tests.
The mGluR family contains 8 members, divided into three groups based on their pharmacological and functional profiles. Group I (mGluR1, mGluR5), Group II (mGluR2, mGluR3) and Group III (mGluR4, mGluR6, mGluR7, mGluR8) [40, 41]. We systematically isolated members of the mGluR family using a series of mGluR subgroup and/or subtype specific pharmacological antagonists. LY341495 acts as an mGluR Group II and III antagonist at 100 nM, whereas at 100 μM it acts as a pan-mGluR antagonist [39]. The addition of 100 nM LY341495 produced no significant reduction in the depolarization observed upon electrical stimulation), whereas 100 μM LY341495 blocked the membrane depolarization (Figure 3C). Thus, the depolarization is a Group I mGluR-dependent effect. Group 1 contains two mGluR subtypes mGluR1 and mGluR5. MPEP, a selective mGluR5 antagonist [42], did not block the depolarization, whereas JNJ16259685, a selective mGluR1 antagonist [43], abolished the depolarization (Figure 3C). Based on the pharmacological dissection of the response we conclude that of the mGluR family of receptors, only mGluR1 activation produces membrane depolarization in CA1 pyramidal neurons.
We next sought to examine a link between the mGluR1-mediated membrane depolarization, NAADP signalling and acidic store Ca2+ release. We examined their relationship by isolating the mGluR response as described above prior to the introduction of further manipulations. Desensitization of the NAADP receptor with NAADP (1 mM) inside patch pipette abolished mGluR-mediated depolarization, whereas, 1 mM nicotinic acid adenosine dinucleotide (NAAD), an inactive metabolite, had no effect (Figure 3D), suggesting that the action of NAADP is specific. Pre-incubation with the NAADP receptor antagonist Ned-19 also significantly reduced responses to mGluR depolarization, and disruption of the lysosomes with acute application of glycyl-L-phenylalanine 2-naphthylamide (GPN) abolished mGluR-mediated depolarization (Figure 3D). GPN prevents acidic store Ca2+ signalling without compromising cell health [19]; thus, together, these data suggest mGluR membrane depolarization is dependent on NAADP and Ca2+ release from acidic stores.
mGluR1-dependent membrane depolarization does not require dendritic IP3Rs
Our data suggest that NAADP and Ca2+ release from acidic stores drives the membrane depolarization. It therefore seems reasonable that Ca2+ signalling via the acidic store is intimately linked to this process. Several studies report an amplification of acidic store Ca2+ by the ER and we show the RyRs are essential for NAADP-mediated depolarization (Figure 1D). However, we are also mindful that Group 1 mGluRs are thought to be involved in IP3R-mediated Ca2+ release [41], therefore, we needed to explore this relationship in greater detail to better understand these Ca2+ signalling events.
First, we confirmed the requirement of the acidic Ca2+ stores for mGluR1-mediated depolarization by pharmacologically isolating mGluR1s (as previously described) and recording membrane potential whilst glutamate was bath applied (Figure 4, A and B). As expected, acute application of GPN abolished mGluR1-mediated depolarization (Figure 4C). We next wanted to determine whether the mGluR1-mediated depolarization was also dependent on Ca2+ release from the ER. Therefore, we examined whether RyRs or IP3Rs are essential for mGluR1-mediated membrane depolarization. Acute application of desensitizing concentrations of ryanodine prevented mGluR1-mediated depolarization (Figure 4C). In contrast, Xestospongin C, a potent and selective inhibitor of IP3Rs [44], had no effect on the mGluR1-mediated depolarization (Figure 4C).
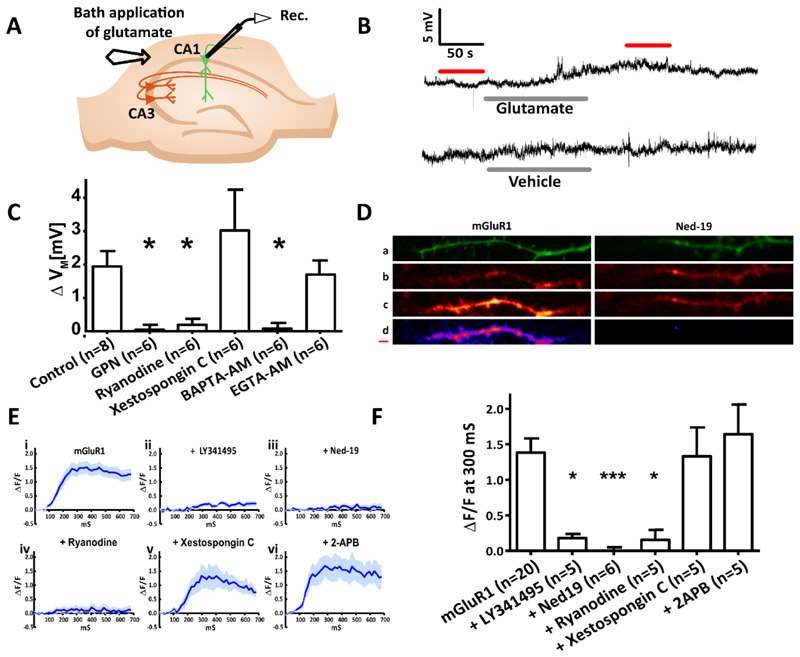
Figure 4. In CA1 pyramidal neurons, mGluR1-dependent membrane depolarization and Ca2+ release require acidic store signalling and Ca2+ release from the ER via ryanodine receptors but not IP3 receptors.
(A) Diagram showing the experimental configuration. The membrane potential of CA1 pyramidal neurons in hippocampal slices was recorded whilst mGluR1 was pharmacologically isolated and extracellular glutamate was applied. (B) Typical voltage recordings recorded upon bath application of glutamate (300 μM, 120 s) or the vehicle. (C) Columns show mean ΔVM of CA1 pyramidal neurons before and after extracellular glutamate application under control conditions (n=8) and in the presence of the lysosomal disrupting agent GPN (200 μM; n=6), ryanodine receptor antagonist ryanodine (40 μM, 15 min; n=6), IP3 receptor antagonist Xestospongin C (2 μM, 15 min; n=6), ‘fast’ Ca2+ chelator BAPTA (20 μM, 15 min; n=6), or the ‘slow’ Ca2+ chelator EGTA (20 μM, 15 min; n=6). (D) Time series images of CA1 neurons filled with Ca2+ indicator OGB-1 (1 mM) were recorded whilst mGluR1 was pharmacologically isolated (50 μM AP5, 10 μM NBQX, 100 μM picrotoxin, and 2 μM CGP 55845) and electrical stimulation was applied (4 pulses, 20 Hz). Images top to bottom: z-stack of the dendritic branch being imaged (green); Ca2+ signal at baseline, before stimulation; Ca2+ signal 300 ms after stimulation; subtraction of Ca2+ at 300 ms from baseline (purple). Scale bar, 0.5 μm. (E) ΔF/F over the imaging time course where mGluR1 was pharmacologically isolated (n=20 cells) in combination with acute application of LY341495 (100 μM, 10 min; n=5), pre-incubation with Ned-19 (100 μM, 1 hour; n=6), or acute application of ryanodine (20 μM, 10 min; n=5), xestospongin C (2 μM, 15 min; n=5), or 2-APB (50 μM, 15 min; n=5). (F) Columns show mean ΔF/F before and after electrical stimulation for each pharmacological manipulation undertaken. Significance was assessed with Kruskal-Wallis and post hoc Dunn’s tests; Error bars denote SEM, n = single cell. Significant differences indicated by asterisks where *** = P < 0.005 ** = P < 0.01 and * = P < 0.05.
Membrane contact sites between the acidic Ca2+ stores and the ER are reported and are suggested to mediate microdomain signalling between these two organelles [45]. To assess whether microdomain Ca2+ signalling is likely to occur with mGluR1-mediated membrane depolarization we compared the action of two Ca2+ chelators: ethylene glycol-bis (β- aminoethyl ether)-N, N, N’, N'-tetraacetic acid (EGTA) and BAPTA. EGTA has an “on” rate for binding Ca2+ of 3-10 μM−1 s−1, whereas BAPTA is several orders of magnitude faster at 100–1000 μM−1 s−1 [46, 47]. Consequently, EGTA is thought not to chelate Ca2+ at a rate fast enough to inhibit microdomain Ca2+ signalling [48], whereas BAPTA can prevent all but elementary Ca2+ signalling events [49]. Bath application of membrane-permeable EGTA (EGTA-AM) did not affect mGluR1-mediated cellular depolarization (Figure 4C), whereas membrane permeable BAPTA (BAPTA-AM) prevented mGluR1-mediated depolarization (Figure 4C). These data suggest that mGlurR1-mediated depolarization is dependent on microdomain Ca2+ signalling.
Dendritic mGluR1-dependent Ca2+ transients require NAADP signalling and RyRs but not IP3Rs
To assess more directly the intracellular Ca2+ signalling events initiated by mGluR1 activation we used confocal microscopy to visualise Ca2+ signals in the dendrites of CA1 pyramidal neurons. Neurons were loaded with the Ca2+ sensitive dye Oregon Green 488 BAPTA-1 (OGB-1) and mGluR1s were pharmacologically isolated as described above.
Electrical stimulation was applied to generate dendritic Ca2+ signals. These Ca2+ signals could be blocked by pharmacologically inhibiting either the NAADP receptor with Ned-19 (Figure 4, D to F) or all members of the mGluR family with LY341495 (Figure 4, E and F), again indicating that mGluR1-mediated Ca2+ signals depend on NAADP signalling. We found that ryanodine abolished mGluR1-dependent Ca2+ signals (Figure 4, E and F), whereas addition of the IP3 receptor antagonists Xestospongin C or 2-APB did not reduce mGluR1 mediated Ca2+ signalling (Figure 4, E and F). Together, these data suggest that mGluR1-mediated Ca2+ signals are dependent on Ca2+ release from both acidic Ca2+ stores and from the ER via RyR, but not IP3Rs. As a positive control, we confirmed that Xestospongin C inhibited IP3R-mediated Ca2+ release by visualising Ca2+ signals in CA1 pyramidal neurons patch clamped with an internal solution containing IP3 under control conditions and after pre-exposure to Xestospongin C (fig. S1, A to C).
mGluR1-dependent depolarization is mediated by the inactivation of SK channels following dephosphorylation
We wished to understand the biophysical basis of the mGluR1-mediated membrane depolarization. A number of studies suggest that Group 1 mGluRs inhibit K+ channels in CA1 pyramidal neurons of the hippocampus [42, 50–57] with Tigaret, et al. [58] providing evidence that mGluR1 activation inhibits SK channels, an important step for the induction of mGluR1-mediated long-term potentiation (LTP) in the hippocampus.
SK channels are present in the dendrites of CA1 pyramidal neurons of the hippocampus [59] where their activation produces action potential after-hyperpolarisation currents (IAHP). SK channels have also been implicated in regulating dendritic excitability [58, 60, 61].
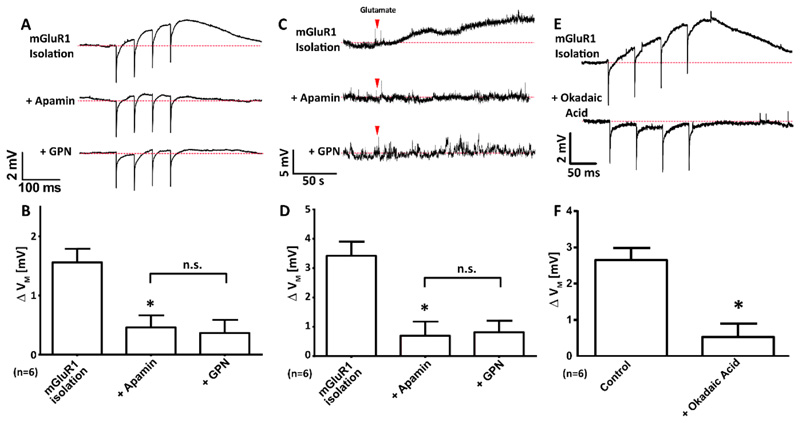
We sought to determine whether the allosteric inhibitor of SK channels, apamin [62] inhibited mGluR1-mediated depolarization. mGluR1s were pharmacologically isolated (as described above) and membrane potential was recorded from CA1 pyramidal neurons while a patterned of electrical stimulation, was delivered. The experiment reveals that synaptic activation of mGluR1 produced membrane depolarization (Figure 5, A and B) and apamin significantly reduced the amplitude of depolarization (Figure 5B). GPN was then introduced to determine whether a common signalling pathway was being utilised. GPN was found to have no further effect on reducing the mGluR1-mediated depolarization (Figure 5B). These data suggest that inhibition of the SK channels by acidic store Ca2+ is the key intermediate step in producing mGluR1-mediated depolarization.
Figure 5. In CA1 pyramidal neurons, mGluR1-dependent depolarization occurs through the inactivation of SK channels by possibly protein phosphatase 2A (PP2A).
(A and B) Representative voltage recordings (A) and mean Δ VM (B) upon electrical stimulation (4x, 20 Hz) of CA1 neurons whilst mGluR1 was pharmacologically isolated, then subsequent addition of apamin (200 nM, 15 min) and finally GPN (200 μM, 10 min); n=6 cells. (C and D) Representative voltage recordings (C) and mean Δ VM (D) upon bath application of glutamate (red arrowhead; 300 μM, 120 s) of CA1 neurons whilst mGluR1 was pharmacologically isolated, then subsequent addition of apamin (200 nM, 15 min) and finally GPN (200 μM, 10 min); n=6 cells. (E and F) Representative voltage recordings (E) and mean Δ VM (F) upon electrical stimulation (4x, 20 Hz) of CA1 neurons whilst mGluR1 was pharmacologically isolated in the absence or presence of okadaic acid (100 nM, 15 min); n=6 cells. Data are means + SEM, each from n = 6 single cells. ** P < 0.01 and * P < 0.05 (relative to mGluR1 isolation-alone condition), and n.s. = no significant difference; by Freidman’s test with post hoc Dunn’s tests (B and D) or a Wilcoxson pair matched signed ranks test (F).
To ensure that the addition of apamin and/or GPN had not interfered with presynaptic glutamate release we repeated this experiment with transient bath application of L-glutamate rather than electrical stimulation. The mGluR1s were again pharmacologically isolated and neurotransmission prevented with TTX. Extracellular glutamate caused membrane depolarization (Figure 5C) and was significantly reduced by the addition of apamin (Figure 5D). Again, we found that application of GPN had no further effect on reducing the amount of mGluR1 depolarization after application of apamin (Figure 5D). Apamin did not affect the resting membrane potential of CA1 neurons of the hippocampus (figure S2, A and B).
SK channels are subject to modulation. Multiple sites for phosphorylation have been reported [63], with SK channels shown to form macromolecular complexes with protein kinase 2 (CK2) and protein phosphatase 2A (PP2A) [64, 65]. Some members of the PP2A family are Ca2+ sensitive [66] and the presence of Ca2+ binding EF-hand domains is noted on regulatory B-type subunits [67]. We therefore used the inhibitor of PP2A (okadaic acid) to determine whether SK channel modulation could affect mGluR1-mediated depolarization. We found that acute application of okadaic acid significantly reduced mGluR1-mediated depolarization (Figure 5, E and F). Therefore, we suggest that activation of mGluR1 and the subsequent Ca2+ signals evoked are key in activating one or more members of the PP2A family.
Finally, it has also been suggested that mGluR1-mediated depolarization may occur via activation of a non-selective cation or TRP channel [68, 69]. To assess this possibility, we used the broad-spectrum antagonists of non-selective cation channels, Flufenamic acid and La3+, to determine whether mGluR1-mediated depolarization could be achieved via these channels. We found that neither had any effect on mGluR1-mediated depolarization (figure S3, A-C).
mGluR1-dependent plasticity requires inhibition of SK channels by acidic store Ca2+
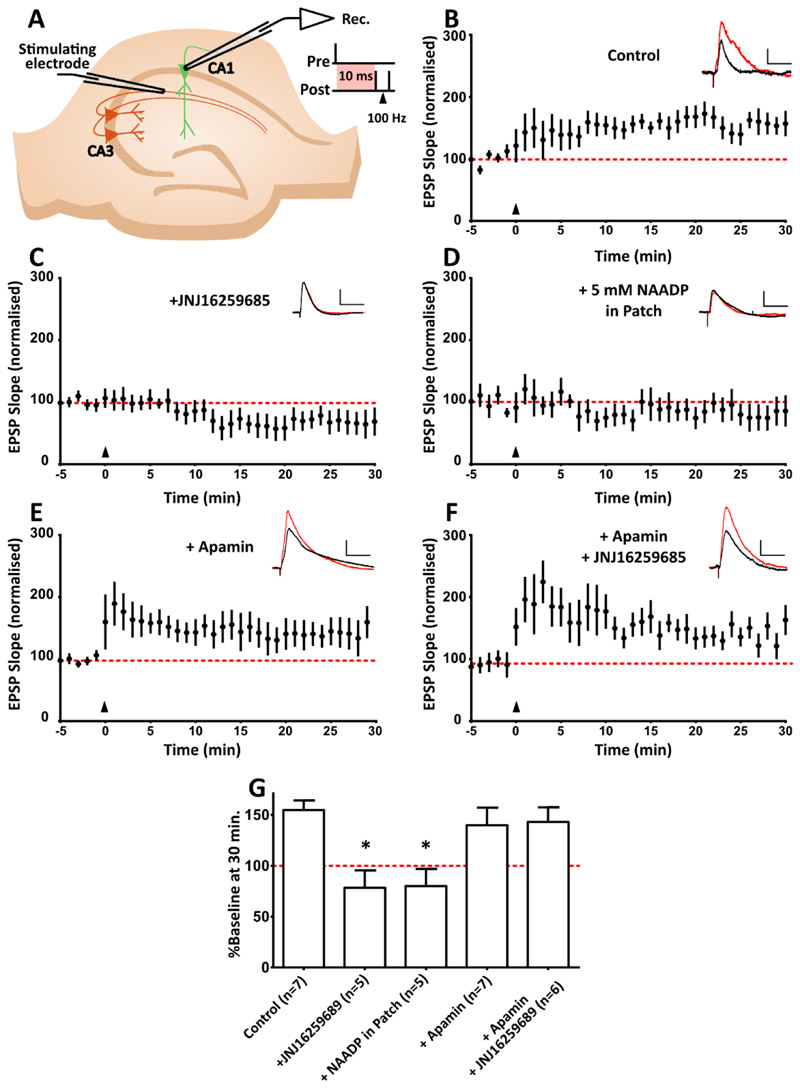
Several studies suggest that hippocampal long-term potentiation can occur following the activation of mGluR1 [58, 70–73]. We wished to determine whether the signalling pathway we describe is required for mGluR1-dependent plasticity. We therefore implemented a spike timing dependent plasticity (STDP) protocol known to produce mGluR1-dependent plasticity [58].
The STDP induction protocol produced LTP of around 150% (Figure 6, A) in CA1 pyramidal neurons. To ensure the protocol induced mGluR1-dependent LTP we repeated the experiment in the presence of an mGluR1 specific antagonist (JNJ16259685), which blocked the LTP (Figure 6, B).
Figure 6. In CA1 pyramidal neurons, mGluR1-dependent synaptic plasticity requires inhibition of SK channels via NAADP signalling.
(A) A causal spike-timing-dependent plasticity protocol was used to induce mGluR1-dependent LTP, in which one causal presynaptic stimulation is paired with two backpropagating action potentials (100 Hz), at a 10 ms interval. The induction protocol is delivered where t = 0, indicated by the black triangles. Example EPSP traces before (black) and after (red) STDP induction are shown at the top right of each graph. Scale bar, 5 mV x 50 ms. This STDP protocol produces LTP lasting at least 30 min (n=7 cells). (B) LTP in the STDP protocol described in (A) with mGluR1-specific antagonism with JNJ16259685 (300 nM; n=5 cells). (C) LTP as described in (A) upon prevention of NAADP/acidic store Ca2+ signalling with a desensitizing concentration of NAADP (5 mM; n=5 cells). (D) Magnitude of LTP upon induction of STDP in the presence of SK channel antagonist apamin (200 nM; n=7 cells). (E) LTP as described in (A) in the presence of apamin and JNJ16259685 (300 nM; n=6 cells). (F) Mean change in synaptic strength at 25-30 min, expressed as a % of the baseline. Data are means ± SEM, n = single cell. * P < 0.05 by Kruskal-Wallis and post hoc Dunn’s tests.
Next, we selectively abolished NAADP signalling with a desensitizing concentration of NAADP [74, 75] in the patch pipette. We found this manipulation prevented LTP (Figure 6, C), indicating that mGluR1-dependent LTP requires NAADP-acidic store Ca2+ signalling.
As the inhibition of SK channels via mGluR1 is thought to be required for mGluR1-mediated LTP, pharmacological inactivation of SK channels with apamin should have no impact on the LTP; SK channels should be inhibited by mGluR1 activation, thereby occluding the apamin’s action. Indeed, we found that apamin alone had no effect on the magnitude of LTP compared to control experiments (Figure 6, D). Critically, we also found that antagonism of mGluR1s with JNJ16259685 while simultaneously inhibiting SK channels with apamin rescues the ability of the STDP protocol to produce mGluR1-dependent plasticity (Figure 6, E). These findings (summarized Figure 6F) indicates that activation of mGluR1 can produce LTP in the hippocampus and that LTP is achieved via the modulation of SK channel activity.
Two-pore channels are essential for mGluR1-dependent membrane depolarization and LTP
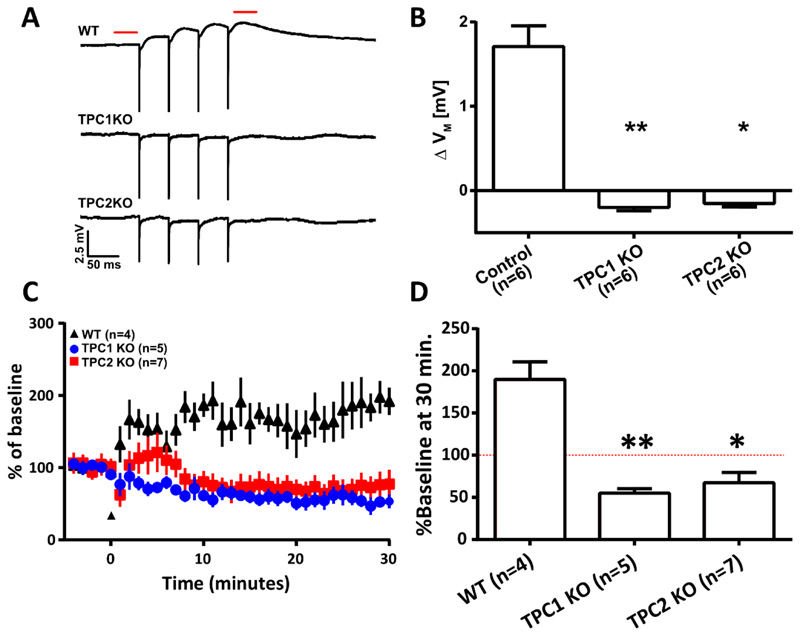
We have shown that NAADP signalling is required for mGluR1-mediated depolarization and LTP. Here we add to the pharmacological evidence by genetically manipulating the signalling pathway. Two-pore channels (TPC1 and TPC2) are localized to acidic Ca2+ stores in mammalian cells and have been shown to be essential for NAADP-mediated Ca2+ release [1–3, 35]. First, we wanted to determine if TPCs were required for NAADP-mediated events in our experimental preparation. Therefore, we dialyzed NAADP into CA1 pyramidal neurons, via a patch pipette at the maximally effective concentration (determined in data shown in Figure 2B), whilst recording membrane potential. NAADP-mediated depolarization was reduced in neurons from Tpc1 -/-and abolished in neurons from Tpc2 -/- (figure S4, A and B).
Next, we explored if either Tpc1 and Tpc2 was required for mGluR1-mediated depolarization and mGluR1-dependent LTP. To achieve this, we first assessed whether mGluR1-dependent depolarization could be produced in CA1 neurons of wild-type (WT) mice. We pharmacologically isolated mGluR1s (as described above) and delivered electrical stimulation confirming that mGluR1 activation produced depolarization (Figure 7, A and B). In contrast, depolarization was not observed in CA1 pyramidal neurons of either Tpc1 -/- or Tpc2 -/- animals.
Figure 7. In CA1 pyramidal neurons, Two-pore channels are required for mGluR1-mediated membrane depolarization and mGluR1-dependent LTP.
(A) Representative average (5 traces) voltage recordings from CA1 neurons in hippocampal slice preparations from wild-type (WT), Tpc1-/-, and Tpc2-/- mice (n=6 mice each). Recordings were obtained upon pharmacological isolation of mGluR1 (50 μM AP5, 10 μM NBQX, 100 nM LY341495, 10 μM MPEP, 100 μM picrotoxin, and 2 μM CGP 55845) and electrical stimulation (4x, 20 Hz) of afferent fibres in stratum radiatum. Dashed red line indicates where membrane potentials were compared before and after stimulation. (B) Columns show mean ΔVM of CA1 pyramidal neurons before and after electrical stimulation described in (A). (C) A causal spike-time-dependent plasticity protocol was used to induce mGluR1-dependent LTP after a baseline of EPSPs were recorded for 5 min (indicated by marker at 0 min) in WT (n=4), Tpc1-/- (n=5) and Tpc2-/- (n=7) animals. One casual presynaptic stimulation is paired with two backpropagating action potentials (100 Hz) at a 10 ms interval. (D) Mean change in synaptic strength at 25-30 min shown/described in (C), expressed as a % of the baseline (red dashed line). Data are means ± SEM, n = single cell. ** P < 0.01 and * P < 0.05 by Kruskal-Wallis and post hoc Dunn’s tests.
Next, we sought to determine whether the TPCs were important for mGluR1-dependent LTP. We confirmed that was is observed in CA1 pyramidal neurons (Figure 7, C and D). Upon assessing LTP in knock-out mice, we were surprised to find that the STDP protocol that had generated LTP instead induced LTD in both Tpc1-/- and Tpc2-/- animals (Figure 7, C and D). It would therefore appear that the change in the Ca2+ signalling profile in these neurons resulted in a switch in the polarity of the plasticity for our STDP stimulus regime.
Discussion
In this work, we show that the metabotropic receptor, mGluR1, is specifically linked to the NAADP signalling cascade, a pathway that uniquely mobilises the release of Ca2+ from acidic organelles such as lysosomes and late endosomes. We found that activation of mGluR1 produced NAADP-dependent Ca2+ release and also membrane depolarization, both of which requiring Ca2+ amplification via the RyRs. As we found cADPR alone was not able to produce depolarization we suggest that mGluR1/NAADP-mediated Ca2+ release from the acidic stores is the critical first step in this signalling pathway. Importantly, the pathway is quite specific, as we find that mGluR5, the second member of Group 1 mGluR group, fails to trigger NAADP-mediated cellular depolarization. We reveal that this pathway is intimately linked to the induction of mGluR1-mediated LTP. The mechanism by which this occurs appears to be a transient modulation of SK channel function following dephosphorylation by PP2A.
In a previous study, we found that acidic stores were essential for the maintenance of spine growth following LTP induction [19]. We now find that in addition to LTP maintenance, acidic stores play a role in the induction of mGluR1-dependent forms of LTP. Whether LTP requires mGluR1; however, depends on the parameters of stimulation, with stronger stimulation protocols capable of bypassing the requirement of mGluRs and, more generally, store Ca2+ release [76–78].
Perhaps the least intuitive of our results is the observation that mGluR1 activation modulates SK channel function to produce depolarization of the membrane. Canonically, SK channels are thought to underlie after-hyperpolarising potentials (AHPs). In this context, the channels are closed and become active as Ca2+ enters neurons following activity. However, it would appear that the regulation of SK channels by Ca2+ is more complex than once thought, with SK channels differentially regulated by Ca2+ from different sources. Ca2+ that enters via R-Type voltage-dependent Ca2+ channels (VDCCs) or N-methyl-D-aspartate receptors (NMDARs) acts to potentiate SK channel opening causing hyperpolarization. In this context, hyperpolarisation helps reinstate the Mg2+ block of NMDARs, reducing the amount of Ca2+ entry and consequently reducing the magnitude of LTP [79, 80]. Conversely, we find that Ca2+ release from acidic stores reduces SK channel activity. We do not fully describe the mechanism as to how this occurs; however, we show that PP2A is required to produce mGluR1-mediated depolarization. PP2A form complexes with SK channels and are only active whilst the channels are in an open state; the result of their activity is to increase the sensitivity of SK channels to Ca2+ [64, 65]. Perhaps this increased sensitivity allows detection of acidic store Ca2+ and in turn reduce SK channel activity. Further work needs to be undertaken to elucidate this signalling mechanism. Additionally, we found apamin had no effect on resting membrane potential of hippocampal neurons. This is consistent with previous findings [81–84] and might be explained by apamin’s allosteric mode of action [62], which perhaps locks SK channels in their current state and thus prevents them from being activated by any source of Ca2+.
Nevertheless, the reduction in SK channel activity is a key step in the induction of metabotropic receptor-dependent forms of LTP [58, 85]. In good agreement with Tigaret et al. [58] we confirm that inhibition of SK channels is essential for mGluR1-mediated LTP, but critically we identify the intermediate step and link mGluR1-mediated depolarization to the NAADP signalling pathway. Underpinning our link to this pathway is the data showing that genetic knock out of either Tpc1 or Tpc2 removes both mGluR1-mediated depolarization and mGluR1-mediated LTP. The importance of both Tpc1 and Tpc2 for mGluR1-mediated LTP may indicate that mGluR1 is interacting with heteromeric complexes of Tpc1 and Tpc2, an arrangement reported in other cell types [86].
Roles for mGluR1 in both long-term depression (LTD) [38, 72, 87–89] and long-term potentiation (LTP) [70, 90–92] have been reported at CA3-CA1 synapses. Whether LTD or LTP is observed seems to be dependent on the stimulation pattern delivered to the neurons, with stronger stimulation regimes generating LTP and weaker ones LTD [72, 76, 93]. Interestingly, mGluR1 can produce both hyperpolarization and depolarization in dopaminergic neurons, with strong stimulation causing depolarization and weak stimulation hyperpolarisation [94]. One clear illustration of the significance of recruiting specific signalling pathways to generate a specific plasticity outcome was seen in our data that showed that when TPCs are genetically removed our LTP induction protocol induced LTD not LTP. Collectively, these data suggest that at least two intracellular signalling pathways can be stimulated by mGluR1 activation and that the pathway engaged, and the consequent polarity of plasticity, is dependent on the stimulation pattern.
The importance of understanding the different mGluR1 signalling pathways is clear when interpreting work in vivo. mGluR1 antagonists impair spatial learning [95] and the deletion of the gene encoding mGluR1 reduces LTP in CA1 region of the hippocampus and impairs context-specific associative learning [96] and spatial memory [97]. Impaired Group 1 mGluR signalling is also implicated in the pathogenesis of Fragile X Syndrome [98, 99] as yet an untreatable disorder, thus the signalling pathway we reveal offers new opportunities towards understanding diverse behavioural phenotypes and routes for novel therapeutic intervention.
Materials and methods
Hippocampal slice preparation
All animal work was carried out in accordance with the Animals (Scientific Procedures) Act, 1986 (UK) and under the project and personal licenses approved by the Home Office (UK). Slice cultures of the hippocampus were prepared from male Wistar rats (P6-8). The hippocampi were isolated in ice-cold Earle’s balanced salt solution (EBSS) with added: HEPES (21 mM), D-(+)-Glucose (27.8 mM) pH adjusted to 7.2 - 7.4 with NaOH and cut into slices of 350 μm thickness with a McIlwain tissue chopper. Slices were placed into Millicell CM culture plate inserts (PTFE filter, pore size 0.4 μm, diam. 12 mm) in a six-well Millicell culture plate (both supplied by Merck Millipore) with 1 mL of culture medium and stored at 34.5°C at 5% CO2. Culture medium composed of 78.8% minimum essential medium (MEM) with GlutaMAX (Gibco), 20% heat-inactivated horse serum, 1% B27 with added CaCl2 (1 mM), HEPES (30 mM,) D-(+)-Glucose (26 mM,) NaHCO3 (5.8 mM) and MgSO4 (2 mM). Culture media was renewed every 3-4 days.
During experiments, slices (10–14 days in vitro [DIV]) were perfused (1–2 mL/min) with heated (32°C–34°C) artificial cerebrospinal fluid (ACSF) which comprised of NaCl (145 mM), KCl (2.5 mM), KH2PO4 (1.2 mM), NaHCO3 (16.0 mM), glucose (11.0 mM), CaCl2 (3.0 mM) and MgCl2(2.0 mM) aerated with 95% O2, 5% CO2.
Electrophysiology
Whole-cell patch clamp recordings were performed on pyramidal neurons from either CA3 or CA1 neurons of hippocampal slice cultures To minimize intracellular dialysis high-resistance patch electrodes (16–20 MΩ) were used where mGluR1 was isolated pharmacologically and all other experiments were undertaken with lower resistance electrodes (5-8 MΩ). Voltage signals were detected using either an Axoclamp 2B (Axon Instruments/Molecular devices) amplifier, signals were digitised using a Digidata 1440A then recorded digitally with WinWCP V4.7.9 (Strathclyde Electrophysiology Software), 50 Hz noise was eliminated with a Hum Bug (Quest Scientific). The internal solution contained (135 mM KGluconate, 10 mM KCl, 10 mM HEPES, 2 mM MgCl2, 2 mM Na2ATP, and 0.4 mM Na3GTP; pH = 7.2–7.4). Pharmacological agents/second messengers added to the internal solution were conjugated to K+ salts, and when present, the KGluconate concentration was reduced by equal molarity in order to maintain osmolarity. Electrical stimulation was applied using a tungsten stimulating electrode with an isolated constant current stimulator (Digitimer Ltd). To produce mGluR1-mediated depolarization the stimulation pattern used consisted of four pulses at 20 Hz. The mGluR1 LTP protocol consisted of causally pairing one pre-synaptic stimulus (to produce a subthreshold excitatory postsynaptic potential (EPSP)) with two backpropagating action potentials (bAPs) (100 Hz), elicited in the postsynaptic neuron via current injection, at a 10 ms interval. This paired induction protocol was repeated 300 times at 5 Hz and delivered within 5 minutes of whole cell breakthrough to prevent dialysis of factors required for mGluR1-dependent LTP.
Ca2+ imaging
Pyramidal neurons in the CA1 region of hippocampal slice cultures, 10-14 D.I.V. were filled with Oregon BAPTA Green 488 (OGB-1) via a patch clamp in whole cell configuration for 1 minute. The patch electrode’s internal solution consisted of: 135 mM K-gluconate, 10 mM KCL, 10 mM HEPES, 1 mM MgCl2, 1 mM OGB-1, 10 mM QX314. Apical dendrites were imaged using confocal laser-scanning microscopy and a 488-nm argon laser whilst an isolated constant current stimulator was used to deliver electrical stimulation (4 pulses, 20 Hz) presynaptic neurons.
Reagents
NAADP-AM & NAADP were synthesised in house [26, 100]). Other drugs were purchased from the following suppliers: Abcam: LY341495, CGP55845, D-AP5, Ned-19, JNJ16259685, MPEP, QX314, TTX. Sigma Aldrich: Ryanodine, bafilomycin A1, picrotoxin, NBQX, nicotinic acid adenine dinucleotide (NAAD) & BAPTA, BAPTA-AM, EGTA-AM. Santa Cruz Biotechnology: GPN. Fisher Scientific: OBG-1. Tocris: U73122, U73343.
Supplementary Material
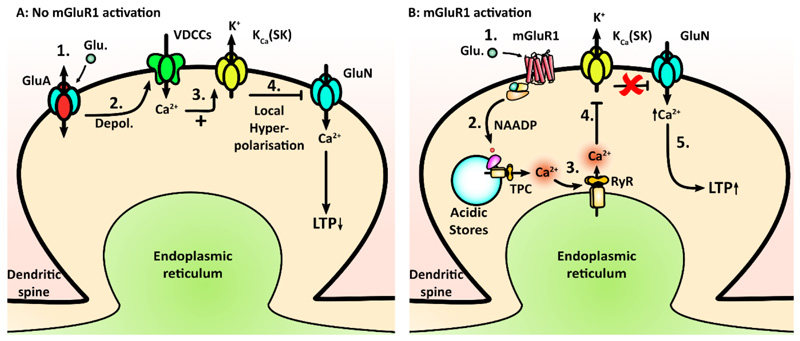
Figure 8. Proposed model for mGluR1-dependent plasticity.
(A) Model of SK channel activation, wherein (i) synaptic glutamate activates GluA receptors to produce (ii) membrane depolarization and (iii) Ca2+ entry via VDCCs. This causes (iv) activation of SK channels and local hyperpolarisation, resulting in inhibition of GluNs by reinstating Mg2+ block, thereby reducing Ca2+ entry through the GluNs and reducing the probability of LTP induction. Where synaptic activity is sufficiently strong, the mGluR1 receptors are recruited. (B) The proposed model for SK channel inhibition mediated by mGluR1 signaling; GluA/VDCC regulation of SK channels is also present but not shown. (i) Glutamate activates mGluR1 receptors and causes (ii) NAADP synthesis, which results in (iii) acidic store Ca2+ release, which is amplified through activation of ryanodine receptors (RyR) in the ER. This somehow inactivates SK channels (iv), which in turn prevents local hyperpolarization and (v) allows greater Ca2+ entry through the GluN receptors, which facilitates the induction of LTP.
Acknowledgements
The authors thank Dr Grant Churchill and Clive Garnham for synthesizing and providing us the NAADP-AM.
Funding: This work was funded by the a Wellcome Trust Senior Investigator Enhancement Grant, “A messenger role for NAADP in the central nervous system” to AG, by an Alison Brading Scholarship from Lady Margaret Hall, Oxford to WJF, and by a grant from the BBSRC to NJE.
Footnotes
Author Contributions: W.J.F. and H.T. performed and analysed the experiments. W.J.F, Z.P, A.F.J. designed the experiments. W.J.F. and N.J.E wrote the manuscript. A.G. and N.J.E supervised the project.
Competing interests: The authors declare the have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
"This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS."
References
- 1.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galione A. NAADP receptors. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004036. a004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 6.Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela J-M. Generation of Specific Ca2+ Signals from Ca2+ Stores and Endocytosis by Differential Coupling to Messengers. Current Biology. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 7.Bak J, White P, Timár G, Missiaen L, Genazzani AA, Galione A. Nicotinic acid adenine dinucleotide phosphate triggers Ca2+ release from brain microsomes. Current Biology. 1999;9:751–754. doi: 10.1016/s0960-9822(99)80335-2. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Churchill GC, Sharp T, Galione A. Widespread distribution of binding sites for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate, in the brain. J Biol Chem. 2000;275:36495–36497. doi: 10.1074/jbc.C000458200. [DOI] [PubMed] [Google Scholar]
- 9.Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZH, Lu YY, Yue J. Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS One. 2013;8:e66077. doi: 10.1371/journal.pone.0066077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao B, Webb SE, Miller AL, Yue J. The role of Ca(2+) signaling on the self-renewal and neural differentiation of embryonic stem cells (ESCs) Cell Calcium. 2016;59:67–74. doi: 10.1016/j.ceca.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S, Dun NJ. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- 13.Pereira GJ, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira GJ, Antonioli M, Hirata H, Ureshino RP, Nascimento AR, Bincoletto C, Vescovo T, Piacentini M, Fimia GM, Smaili SS. Glutamate induces autophagy via the two-pore channels in neural cells. Oncotarget. 2016;5 doi: 10.18632/oncotarget.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brailoiu GC, Brailoiu E, Parkesh R, Galione A, Churchill GC, Patel S, Dun NJ. NAADP-mediated channel "chatter" in neurons of the rat medulla oblongata. Biochem J. 2009;419:91–97. doi: 10.1042/BJ20081138. 92 p following 97. [DOI] [PubMed] [Google Scholar]
- 16.Chameau P, Van de Vrede Y, Fossier P, Baux G. Ryanodine-, IP3- and NAADP-dependent calcium stores control acetylcholine release. Pflugers Arch. 2001;443:289–296. doi: 10.1007/s004240100691. [DOI] [PubMed] [Google Scholar]
- 17.Brailoiu E, Miyamoto MD, Dun NJ. Nicotinic acid adenine dinucleotide phosphate enhances quantal neurosecretion at the frog neuromuscular junction: Possible action on synaptic vesicles in the releasable pool. Molecular Pharmacology. 2001;60:718–724. [PubMed] [Google Scholar]
- 18.McGuinness L, Bardo SJ, Emptage NJ. The lysosome or lysosome-related organelle may serve as a Ca2+ store in the boutons of hippocampal pyramidal cells. Neuropharmacology. 2007;52:126–135. doi: 10.1016/j.neuropharm.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A, Hart ML, Emptage NJ. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron. 2017;93:132–146. doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN. NAADP as a second messenger: neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol. 2007;292:C227–239. doi: 10.1152/ajpcell.00638.2005. [DOI] [PubMed] [Google Scholar]
- 21.Arredouani A, Evans AM, Ma J, Parrington J, Zhu MX, Galione A. An emerging role for NAADP-mediated Ca2+ signaling in the pancreatic beta-cell. Islets. 2010;2:323–330. doi: 10.4161/isl.2.5.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rah SY, Mushtaq M, Nam TS, Kim SH, Kim UH. Generation of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate by CD38 for Ca2+ signaling in interleukin-8-treated lymphokine-activated killer cells. J Biol Chem. 2010;285:21877–21887. doi: 10.1074/jbc.M109.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mushtaq M, Nam TS, Kim UH. Critical role for CD38-mediated Ca2+ signaling in thrombin-induced procoagulant activity of mouse platelets and hemostasis. J Biol Chem. 2011;286:12952–12958. doi: 10.1074/jbc.M110.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosker F, Cheviron N, Yamasaki M, Menteyne A, Lund FE, Moutin MJ, Galione A, Cancela JM. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem. 2010;285:38251–38259. doi: 10.1074/jbc.M110.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey V, Chuang CC, Lewis AM, Aley PK, Brailoiu E, Dun NJ, Churchill GC, Patel S. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem J. 2009;422:503–512. doi: 10.1042/BJ20090194. [DOI] [PubMed] [Google Scholar]
- 26.Parkesh R, Lewis AM, Aley PK, Arredouani A, Rossi S, Tavares R, Vasudevan SR, Rosen D, Galione A, Dowden J, et al. Cell-permeant NAADP: a novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell Calcium. 2008;43:531–538. doi: 10.1016/j.ceca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 28.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP Mobilizes Ca2+ from Reserve Granules, Lysosome-Related Organelles, in Sea Urchin Eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, Galione Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 32.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 33.Emptage N, Bliss TVP, Fine A. Single Synaptic Events Evoke NMDA Receptor–Mediated Release of Calcium from Internal Stores in Hippocampal Dendritic Spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 34.Davis LC, Morgan AJ, Chen JL, Snead CM, Bloor-Young D, Shenderov E, Stanton-Humphreys MN, Conway SJ, Churchill GC, Parrington J, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, et al. Expression of Ca(2)(+)-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guse AH, Ernst IM, Fliegert R. NAADP signaling revisited. Curr Top Med Chem. 2013;13:2978–2990. doi: 10.2174/15680266113136660212. [DOI] [PubMed] [Google Scholar]
- 37.Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela J-M. Generation of Specific Ca2+ Signals from Ca2+ Stores and Endocytosis by Differential Coupling to Messengers. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 38.Fan W, Ster J, Gerber U. Activation conditions for the induction of metabotropic glutamate receptor-dependent long-term depression in hippocampal CA1 pyramidal cells. J Neurosci. 2010;30:1471–1475. doi: 10.1523/JNEUROSCI.5619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 40.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 41.Niswender CM, Conn PJ. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annual review of pharmacology and toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavreysen H, Wouters R, Bischoff F, Nobrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5- trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 45.Levine TP, Patel S. Signalling at membrane contact sites: two membranes come together to handle second messengers. Current opinion in cell biology. 2016;39:77–83. doi: 10.1016/j.ceb.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 47.Naraghi M, Neher E. Linearized Buffered Ca2+ Diffusion in Microdomains and Its Implications for Calculation of [Ca2+] at the Mouth of a Calcium Channel. The Journal of Neuroscience. 1997;17:6961. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dargan SL, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP(3)-evoked Ca(2+) signals. The Journal of Physiology. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horne JH, Meyer T. Elementary calcium-release units induced by inositol trisphosphate. Science. 1997;276:1690–1693. doi: 10.1126/science.276.5319.1690. [DOI] [PubMed] [Google Scholar]
- 50.Charpak S, Gahwiler BH, Do KQ, Knopfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- 51.Desai MA, Conn PJ. Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on pyramidal cells and blockade of synaptic inhibition. J Neurophysiol. 1991;66:40–52. doi: 10.1152/jn.1991.66.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Guerineau NC, Gahwiler BH, Gerber U. Reduction of Resting K+ Current by Metabotropic Glutamate and Muscarinic Receptors in Rat Ca3 Cells - Mediation by G-Proteins. J Physiol-London. 1994;474:27–33. doi: 10.1113/jphysiol.1994.sp019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luthi A, Gahwiler BH, Gerber U. A slowly inactivating potassium current in CA3 pyramidal cells of rat hippocampus in vitro. J Neurosci. 1996;16:586–594. doi: 10.1523/JNEUROSCI.16-02-00586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeshita Y, Harata N, Akaike N. Suppression of K+ conductance by metabotropic glutamate receptor in acutely dissociated large cholinergic neurons of rat caudate putamen. J Neurophysiol. 1996;76:1545–1558. doi: 10.1152/jn.1996.76.3.1545. [DOI] [PubMed] [Google Scholar]
- 55.Wu RL, Barish ME. Modulation of a slowly inactivating potassium current, I(D), by metabotropic glutamate receptor activation in cultured hippocampal pyramidal neurons. J Neurosci. 1999;19:6825–6837. doi: 10.1523/JNEUROSCI.19-16-06825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang SC, Bianchi R, Wong RK. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000;83:2844–2853. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- 57.Ireland DR, Abraham WC. Group I mGluRs increase excitability of hippocampal CA1 pyramidal neurons by a PLC-independent mechanism. J Neurophysiol. 2002;88:107–116. doi: 10.1152/jn.2002.88.1.107. [DOI] [PubMed] [Google Scholar]
- 58.Tigaret CM, Olivo V, Sadowski JH, Ashby MC, Mellor JR. Coordinated activation of distinct Ca(2+) sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity. Nat Commun. 2016;7:10289. doi: 10.1038/ncomms10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen D, Bond CT, Lujan R, Ballesteros-Merino C, Lin MT, Wang K, Klett N, Watanabe M, Shigemoto R, Stackman RW, Jr, et al. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nat Neurosci. 2011;14:744–749. doi: 10.1038/nn.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faber ES. Functions and modulation of neuronal SK channels. Cell Biochem Biophys. 2009;55:127–139. doi: 10.1007/s12013-009-9062-7. [DOI] [PubMed] [Google Scholar]
- 61.Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- 62.Lamy C, Goodchild SJ, Weatherall KL, Jane DE, Liégeois J-F, Seutin V, Marrion NV. Allosteric Block of KCa2 Channels by Apamin. Journal of Biological Chemistry. 2010;285:27067–27077. doi: 10.1074/jbc.M110.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-Conductance, Calcium-Activated Potassium Channels from Mammalian Brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 64.Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, et al. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 65.Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wlodarchak N, Guo F, Satyshur KA, Jiang L, Jeffrey PD, Sun T, Stanevich V, Mumby MC, Xing Y. Structure of the Ca2+-dependent PP2A heterotrimer and insights into Cdc6 dephosphorylation. Cell Res. 2013;23:931–946. doi: 10.1038/cr.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janssens V, Jordens J, Stevens I, Van Hoof C, Martens E, De Smedt H, Engelborghs Y, Waelkens E, Goris J. Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B"/PR72 subunit of protein phosphatase 2A. J Biol Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 68.Congar P, Leinekugel X, BenAri Y, Crepel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. Journal of Neuroscience. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546:655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- 72.Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan W. Group I metabotropic glutamate receptors modulate late phase long-term potentiation in hippocampal CA1 pyramidal neurons: comparison of apical and basal dendrites. Neurosci Lett. 2013;553:132–137. doi: 10.1016/j.neulet.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 74.Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+ J Biol Chem. 1996;271:8513–8516. doi: 10.1074/jbc.271.15.8513. [DOI] [PubMed] [Google Scholar]
- 75.Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J Biol Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- 76.Wilsch VW, Behnisch T, Jager T, Reymann KG, Balschun D. When are class I metabotropic glutamate receptors necessary for long-term potentiation? J Neurosci. 1998;18:6071–6080. doi: 10.1523/JNEUROSCI.18-16-06071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padamsey Z, Tong R, Emptage N. Glutamate is required for depression but not potentiation of long-term presynaptic function. eLife. 2017;6:e29688. doi: 10.7554/eLife.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Padamsey Z, Foster WJ, Emptage NJ. Intracellular Ca(2+) Release and Synaptic Plasticity: A Tale of Many Stores. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2018 doi: 10.1177/1073858418785334. 1073858418785334. [DOI] [PubMed] [Google Scholar]
- 79.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 80.Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 81.Criado-Marrero M, Santini E, Porter JT. Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Frontiers in Behavioral Neuroscience. 2014;8:96. doi: 10.3389/fnbeh.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lappin SC, Dale TJ, Brown JT, Trezise DJ, Davies CH. Activation of SK channels inhibits epileptiform bursting in hippocampal CA3 neurons. Brain Research. 2005;1065:37–46. doi: 10.1016/j.brainres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 83.Lotshaw DP. Effects of K+ channel blockers on K+ channels, membrane potential, and aldosterone secretion in rat adrenal zona glomerulosa cells. Endocrinology. 1997;138:4167–4175. doi: 10.1210/endo.138.10.5463. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki K, Ito KM, Minayoshi Y, Suzuki H, Asano M, Ito K. Modification by charybdotoxin and apamin of spontaneous electrical and mechanical activity of the circular smooth muscle of the guinea-pig stomach. Br J Pharmacol. 1993;109:661–666. doi: 10.1111/j.1476-5381.1993.tb13624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels. Neuron. 2010;68:948–963. doi: 10.1016/j.neuron.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J Biol Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kemp N, McQueen J, Faulkes S, Bashir ZI. Different forms of LTD in the CA1 region of the hippocampus: role of age and stimulus protocol. Eur J Neurosci. 2000;12:360–366. doi: 10.1046/j.1460-9568.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Zhang Z, Shang J, Jiang ZZ, Wang S, Liu Y, Zhang LY. Activation of group I metabotropic glutamate receptors induces long-term depression in the hippocampal CA1 region of adult rats in vitro. Neurosci Res. 2008;62:43–50. doi: 10.1016/j.neures.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- 91.Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raymond CR, Redman SJ. Different calcium sources are narrowly tuned to the induction of different forms of LTP. J Neurophysiol. 2002;88:249–255. doi: 10.1152/jn.2002.88.1.249. [DOI] [PubMed] [Google Scholar]
- 93.Nagaraja RY, Becker A, Reymann KG, Balschun D. Repeated administration of group I mGluR antagonists prevents seizure-induced long-term aberrations in hippocampal synaptic plasticity. Neuropharmacology. 2005;49(Suppl 1):179–187. doi: 10.1016/j.neuropharm.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 94.Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 95.Balschun D, Manahan-Vaughan D, Wagner T, Behnisch T, Reymann KG, Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learning & memory (Cold Spring Harbor, N.Y.) 1999;6:138–152. [PMC free article] [PubMed] [Google Scholar]
- 96.Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 97.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 98.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.