Abstract
Intranasal delivery of oxytocin (Oxt) has been identified as a potential therapeutic to target human conditions characterized by social deficits, yet the ability of this administrative route to deliver to the brain is unconfirmed. Oxt knockout (Oxt KO) and wildtype C57BL/6J male mice received Oxt (12 μg total amount) either by nasal or intraperitoneal administration. Oxt concentrations were monitored for 2 hours after administration in circulation via a jugular vein catheter and in the brain by two intracerebral microdialysis probes. Group sizes varied from 4 to 7 mice (n = 22 total). We document for the first time that Oxt applied to the nasal mucosa after nasal administration is delivered to the extracellular fluid in the brain. After nasal application, Oxt concentrations in circulation and in the extracellular fluid of the amygdala and, to an extent, the dorsal hippocampus, rose within the first 30 minutes and remained elevated for the subsequent hour. These findings were confirmed in an Oxt KO mouse line, establishing that the circulating and brain Oxt elevations derive from the administered dose. Interestingly, the pharmacokinetics of Oxt were slightly biased to the brain after nasal administration and to the periphery following intraperitoneal injection. No change in vasopressin levels was detected. These findings have stimulating implications for the interpretation of various behavioral and physiological effects described in animal and human studies after nasal administration of Oxt and provide the pharmacokinetics necessary to develop this drug delivery route for therapeutic purposes.
Keywords: Oxytocin, Pharmacokinetics, Nasal administration, Central Nervous System, Cerebrospinal fluid, Circulatory System
Graphical Abstract
The hormone oxytocin (Oxt) promotes social cognition and behavior in neurotypical adult humans and numerous mammalian species [1, 2]. Administering intranasal Oxt to healthy adult humans affects trust [3, 4], recognition of emotional facial expressions [5, 6], social memory [7], and pair-bonding related behaviors [8, 9]. These results led to its consideration as a therapeutic to target human conditions characterized by deficits in social functioning, especially autism spectrum disorder and schizophrenia [10–12]. Notably, there are currently 63 clinical trials on Oxt-related effects on the central nervous system (CNS) listed by the ClinicalTrials.gov registry (National Institutes of Health, USA). Still, there is an open discussion regarding the efficiency of nasal Oxt both in terms of a delivery route to the CNS and as a therapeutic, most recently highlighted in a series of reviews and correspondences [13–18].
One significant conundrum has been how to deliver Oxt to the brain without resorting to invasive procedures, due to poor blood-brain barrier permeability [19] and the brief half-life of peripherally-administered Oxt [20, 21]. Intranasal administration of Oxt has become the primary non-invasive delivery route, from basic research to experimental clinical trials. This “direct nose-to-brain” transport is anticipated to avoid first-pass metabolism, bypass the blood-brain barrier, produce a fast onset of therapeutic effects, and prove valuable to treat CNS diseases. Recently, a series of studies profiled the pharmacokinetics of Oxt after its nasal application [22–28]. These studies all reported significant increases in plasma and cerebrospinal fluid (CSF) Oxt levels after treatment. While the pharmacokinetics of plasma Oxt seem to be rather consistent across these studies, peaking within the first 15-30 minutes and persisting for 75-90 minutes, the rise in CSF Oxt occurs at shifted time points (15-30 minutes: ref. [22, 23, 28]; 60 minutes: ref. [26]; 75 minutes: ref. [24]). Further, except for one of these studies [22], the concentration of Oxt in the extracellular fluid (ECF) in the brain was not measured. Without such information, it is difficult to determine the bioavailability of Oxt throughout the brain. Key factors regarding the administration of Oxt (e.g., amount and method) and sampling procedures (e.g., target location and time course) may contribute to the variance observed in the pharmacokinetics, which highlights the need to establish standards for nasal Oxt administration. Despite this new knowledge, the ultimate question regarding the access of Oxt to the brain following nasal application remains unanswered, as all samples measured included endogenous Oxt concentrations in addition to the synthetic Oxt administered. It is even possible that nasal administration of Oxt could indirectly stimulate increased endogenous Oxt release within the brain. Such information is vital to inform the efficacy, tolerability, and safety of intranasally administered Oxt in human populations.
Therefore, the present study was designed to focus on the pharmacokinetics of Oxt centrally and peripherally following nasal or a systemic (e.g., intraperitoneal) administration. Intraperitoneal administration was selected as a reference, alternate administrative route of Oxt as many animal studies used to guide human clinical studies have used, and continue to use this route [29–31]. We utilized an established rodent paradigm to measure Oxt following nasal or intraperitoneal administration with frequent and concurrent sampling of blood and ECF in different brain regions [22]. The experiments were conducted with wildtype and Oxt knockout (Oxt KO) mice that were implanted with a jugular vein catheter and two intracerebral microdialysis probes, targeting the amygdala and hippocampus, so that samples could be collected repeatedly over a 2.5-hour period. We included an intraperitoneal dosing to allow for a comparison of the kinetics between nasal and an alternative peripheral administrative route.
METHODS AND MATERIALS
Subjects
All breeding, housing, and experimental methods were conducted according to US National Institutes of Health guidelines for animal research and were approved by the National Institute of Mental Health Animal Care and Use Committee. Adult Oxt KO (8 for nasal and 6 for i.p. administration) and wildtype C57BL/6J (7 for nasal and 6 for i.p. administration) male mice were used for experiments. Generation of mice with targeted disruption of the Oxt gene was previously described [32]. These Oxt KO mice had elimination of the neurophysin-encoding second and last exons of the Oxt gene, resulting in an absence of endogenous Oxt production. Genotyping was conducted by PCR assay. All mice were housed singly during experiments and maintained on a 12-h light cycle (lights off at 1500 h) with ad libitum access to food and water.
Surgical Procedures
The day prior to the experiment, mice were anesthetized with tribromoethanol (Avertin), fitted with a jugular vein catheter, and implanted with self-constructed, linear microdialysis probes (molecular cut-off: 18kDa; 1 mm membrane) within the left amygdala (coordinates from bregma: anterior-posterior: −1.06, dor sal-ventral: −5.75, medial-lateral: 1.75) and right dorsal hippocampus (anterior-posterior: −1.42, dorsal-ventral: −2.75, medial-lateral: −1.60). The catheter was filled with heparinized saline to prevent blood clotting in the catheter tubing and guided subcutaneously to the nape of the neck and exteriorized.
For the jugular vein catheterization, the catheter tubing was filled with a heparinized ticarcillin solution (33 mg of ticarcillin and 0.3 mg of heparin per 10 ml of sterile saline). A 2 cm long midscapular incision was made and a second shallow 1 cm incision was made from the right clavicle upward. A pair of fine surgical forceps was threaded subcutaneously from front to back incision, then the catheter was guided through this path until 2 cm of the tip was exteriorized from the front incision. Superficial connective and adipose tissues were gently dissected away from the right jugular vein, and a sterile plastic bar was placed underneath the vein to isolate it from the body cavity. Two loose open suture knots were made around the vein and catheter. A 20-gauge insertion needle was used to pierce the vein between the sutures and guide the catheter into the vein. Blood was drawn to confirm placement, sutures were tightened to secure the catheter, incision sites were closed, and 0.1 ml of the heparinized ticarcillin solution was injected to maintain catheter patency.
Microdialysis probe construction was previously described [33]. Briefly, the active area of the dialysis membrane was designed to be 1.0 mm with a molecular weight cutoff of 18 kDa. Probes were perfused continuously at 1.0 μl/min with an artificial cerebrospinal fluid (aCSF) solution using a gas-tight Hamilton syringe connected to an automatic micropump (World Precision Instruments, Sarasota, FL). The surgical site was prepared by making a 1 mm incision to expose the crown of the skull, leveling the head position using bregma and lambda as reference points, and two small holes were drilled for probe implantation. Probes were placed into the left amygdala and right dorsal hippocampus, which were histologically verified upon completion of the collection of samples. All ‘missed’ or ‘partial hits’ were excluded from the data analyses.
Experimental Procedures
The next day, the catheter was flushed with 0.1 ml heparinized ticarcillin solution, and both microdialysis probes were perfused (1.0 μl/min, aCSF) for 60 minutes without sampling. Baseline samples were collected from both probes and the catheter. The dialysate was collected for 30 minutes into 5 μl of 0.1 N HCl then stored immediately on dry ice. Blood samples were taken 10 minutes after the beginning of the 30-min dialysis period and stored briefly at 4°C in a vial containing EDTA (4 μ1/100 μl blood). Oxt was administered either via nasal application (1 μg/μl; 2 × 6 μl saline) or intraperitoneal injection (12 pg/0.1 ml saline). Briefly, for nasal administration, the solution was pipetted bilaterally on the rhinarium, i.e., the glabrous skin around the nostrils, and allowed to diffuse in the squamous epithelium. Following administration, 4 additional dialysate and blood samples were collected every 30 minutes, as described above. Dialysates were evaporated via vacuum concentration without heat and stored at −80°C until assayed. Blood was double centrifuged at 6000 rpm for 15 minutes at 4°C and stored at −80°C until assayed.
In vitro recovery of microdialysates
In vitro recovery of microdialysis probes was determined by placing probes into aCSF containing 1 ng Oxt/mL. for 30 minutes (n = 5).
ELISA and Radioimmunoassay
Unextracted dialysates were processed as full dialysate samples (30 μl) via radioimmunoassay (RIAgnosis, Munich), which has previously been validated for use in mice [22]. The Oxt radioimmunoassay is highly sensitive (minimum detection is 0.1 pg/sample) and selective (no significant cross-reactivity with related compounds). Extracted plasma samples were assayed for Oxt and vasopressin (Avp) using Oxt (Assay Designs Inc., Ann Arbor, MI) and Avp (Assay Designs Inc.) ELISAs, respectively. Extractions were conducted according to the recommended protocols for these assays. Briefly, plasma samples plus an equal volume of 0.1% trifluoroacetic acid were eluted through equilibrated 200 mg C18 Sep-Pak columns. The eluents were then evaporated using a vacuum concentrator. Samples were then assayed in duplicate according to both of the Oxt and Avp ELISA protocols. The detection limits were 11.7 pg/ml for Oxt and 3.39 pg/ml for Avp. The intra-assay and inter-assay CV (coefficients of variation) for each were less than was 2.08% and 1.80%, respectively.
Statistical Analysis
Statistical analyses were performed on data that were expressed as means ± s.e.m’s using IBM SPSS Statistics 19 (SPSS, an IBM Company, Chicago, IL). Repeated measures ANOVAs were used to evaluate the effect of time on peptide concentration, and the bonferroni post-hoc test was used if any main effects reached statistical significance (p < 0.05). In addition, pharmacokinetic measurements were calculated and are reported in Table 1. Specifically, we evaluated the Cmax (maximum concentration observed), T½ (half-life), area under the curve with respect to ground (AUCg or area under the curve with respect to zero), and area under the curve with respect to increase (AUCI or area under the curve with respect to the value at 24 h separation).
Table 1.
Oxytocin pharmacokinetic parameters
| Condition | Sampled Fluid | N | Cmax | AUCG | AUCI | T1/2 |
|---|---|---|---|---|---|---|
| Nasal – WT | Amygdala ECF | 7 | 5.74 ± 1.38a | 249 ± 69 | 206 ± 45 | 25.9 ± 8.6 |
| Hippocampus ECF | 6 | 2.70 ± 1.24 | 173 ± 114 | 85.4 ± 53.4 | 14.6 ± 4.2 | |
| Plasma | 8 | 398± 101 | 23,600 ± 4880a | 17,300 ± 5260 | 25.8 ± 6.0 | |
| Nasal – Oxt KO | Amygdala ECF | 6 | 3.84 ± 1.26ab | 266. ± 113 | 254 ± 113 | 39.6 ± 16.1 |
| Hippocampus ECF | 5 | 3.27 ± 1.19 | 197 ± 96.3 | 185 ± 96 | 18.5 ± 5.3 | |
| Plasma | 7 | 131 ± 29 | 7920 ± 1280b | 6120± 1280 | 32.4 ± 9.1 | |
| IP – WT | Amygdala ECF | 5 | 1.25 ± 0.37b | 61.5 ± 36.9 | 37.9 ± 12.1 | 18.9 ± 4.7 |
| Hippocampus ECF | 5 | 2.23 ± 1.07 | 151 ± 89 | 134 ± 84 | 20.1 ± 4.5 | |
| Plasma | 4 | 291 ± 42 | 18,000 ± 987a | 9900 ± 2150 | 32.0 ± 9.1 | |
| IP – Oxt KO | Amygdala ECF | 4 | 2.31 ± 1.60ab | 101 ± 51 | 89.1 ± 51.3 | 14.7 ± 1.7 |
| Hippocampus ECF | 4 | 1.94 ± 0.85 | 98.7 ± 38.8 | 86.7 ± 38.8 | 18.8 ± 1.9 | |
| Plasma | 6 | 255 ± 44 | 14,900 ± 1930b | 12,000 ± 1950 | 34.5 ± 8.9 |
Note. Letters indicate significant group differences of a parameter within a given sampled fluid (e.g., value a is different from value b). Cmax (pg/sample) is the maximum concentration measured during the 2 hours after oxytocin administration. Area under curve-ground (AUCG; pg*30 min/sample) = area under the curve with respect to zero, and AUC-increase (AUCI; pg*30 min/sample) = area under curve with respect to the value at baseline. T/12 (half-life; min) is the time until samples drop to half of Cmax. Abbreviations: WT, wildtype; Oxt KO, oxytocin knockout; IP, intraperitoneal; ECF, extracellular fluid.
RESULTS
Oxt was detectable in all microdialysates and plasma samples from wildtype and Oxt knockout mice, with the exception of baseline and some latter recovery samples in knockout mice. The in vitro recovery of synthetic Oxt in dialysates collected over a 30-minute periods averaged 4.58%.
Nasal Oxt in Normal Mice
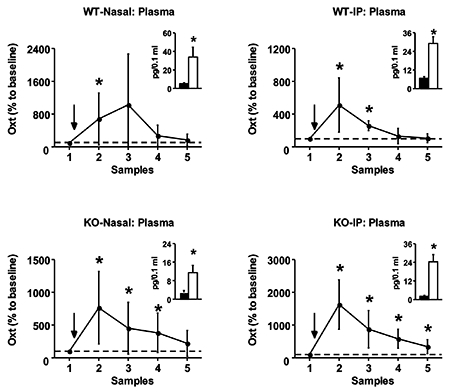
Wildtype C57BL/6J male mice were administered Oxt through the nasal cavity, which resulted in a concurrent rise in Oxt concentrations in circulation and in the brain (Figure 1A–C). Specifically, Oxt concentrations in the ECF of the amygdala spiked 30 minutes after nasal Oxt administration, remaining significantly above baseline for an hour (F4, 24 = 6.33, p < .001). Similarly, Oxt levels rose three times above baseline in the hippocampus during the first 30 minutes following nasal administration, though this was not statically significant presumably due to high variance (F4, 24 = 1.82, p = 0.16). Plasma Oxt concentrations also rose within 30 minutes of treatment and remained significantly elevated for an hour (F4, 28 = 5.48, p < .005). Plasma Avp did not vary as a result of the nasal Oxt administration (F4, 20 = 0.43, p = 0.78).
Figure 1.
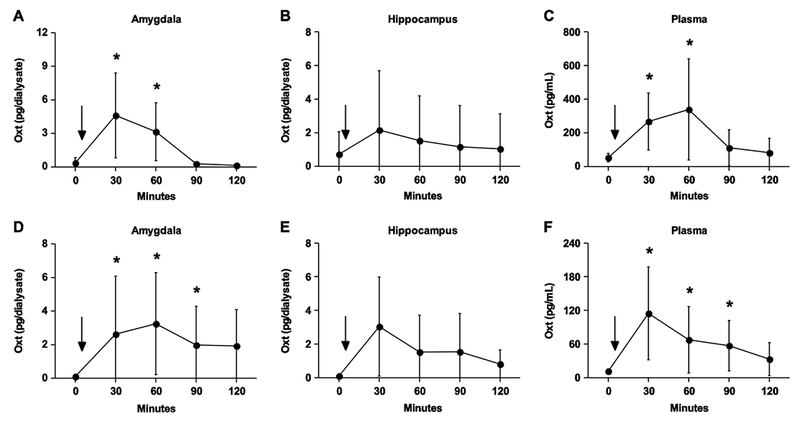
Nasal administration of Oxt elevates Oxt in the circulatory and central nervous systems in normal (A-C) and Oxt knockout (D-F) mice. (A) In the amygdala, Oxt concentrations peak 30 minutes after nasal administration and remain elevated for an hour. (B) No statistically significant change in Oxt concentration was observed in the hippocampus. (C) Plasma Oxt spikes 30 minutes after nasal administration and persists for an hour. (D) Oxt concentrations are detectable 30 minutes after nasal application and remain above baseline for 90 minutes within the amygdala (E) Oxt concentrations are detected in the hippocampus after nasal administration, though statistically this effect was only a trend (p = 0.06) (F) The plasma Oxt profile mirrors that in the amygdala, increasing for 90 minutes after the nasal administration. Asterisk denotes a significant difference between the value at that time point and the baseline value by a post-hoc analysis following a significant main effect detected in the repeated-measures ANOVA (p < 0.05). Data are expressed as mean ± SEM
Nasal Oxt in Oxt KO Mice
Oxt was also administered nasally to Oxt KO mice, a genetic mouse with deletions of the neurophysin-encoding second and last exons of the Oxt gene to eliminate endogenous Oxt production. Oxt was undetectable in the brain (0.1 pg/sample minimal detection limit) or circulation (11.7 pg/mL) of these mice at baseline. Strikingly, Oxt concentrations were observed in the amygdala (F4, 20 = 3.22, p < .05) and hippocampus (F4, 16 = 2.72, p = 0.06) 30 minutes after nasal administration of Oxt (Figure 1D–F). These detectable concentrations remained for a total of 90 minutes. In addition, Oxt was detected in circulation for 90 minutes after nasal administration, spiking in the first 30 minutes (F4, 24 = 3.82, p < .05). While Avp was measureable in plasma at baseline, no change in concentration was observed after nasal Oxt administration (F4, 24 = 1.41, p = 0.36).
Intraperitoneal Oxt in Normal Mice
For comparative purposes, Oxt was systemically administered, via intraperitoneal injection, into wildtype C57BL/6J mice (Figure 2A–C). As with nasal administration, Oxt concentrations rose in the brain, significantly in the amygdala (F4, 16 = 5.37, p < .01) but not in the hippocampus (F4, 16 = 1.98, p = 0.15). Oxt concentrations spiked in the amygdala 30 minutes after administration and remained elevated for a hour. The time course for the Oxt rise in the brain was mirrored in the blood, a spike within 30 minutes that lasted for an hour (F4, 12 = 16.34, p < .001). Plasma Avp remained unaffected (F4, 12 = 0.69, p = 0.62).
Figure 2.
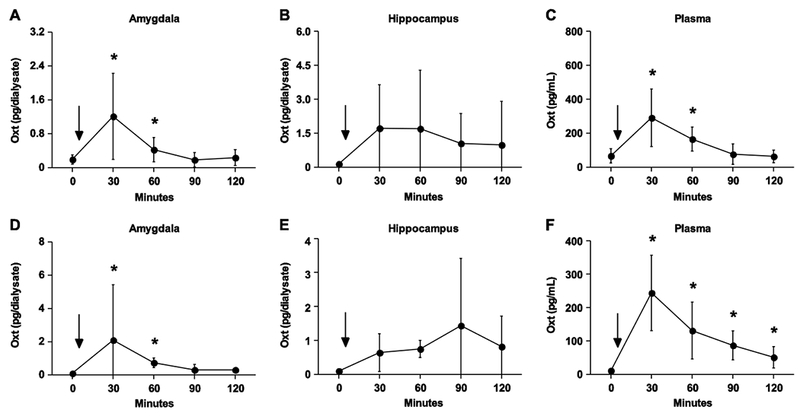
Intraperitoneal injections of Oxt elevate concentrations in the blood and brain of normal (A-C) and Oxt knockout (D-F) mice. (A) Oxt rises sharply 30 minutes after injections in the amygdala, remaining elevated for a total of an hour. (B) No statistically significant change in Oxt concentration was observed in the hippocampus. (C) For an hour, intraperitoneal injections increase Oxt concentrations in circulation. (D) Oxt concentrations are detectable 30 minutes after nasal application and remain above baseline for an hour within the amygdala but (E) do not elevate significantly above baseline values in the hippocampus (F) Plasma Oxt concentrations remain elevated for the extent of the 2 hour observation period. Asterisk denotes a significant difference between the value at that time point and the baseline value by a postdoc analysis following a significant main effect detected in the repeated-measures ANOVA (p < 0.05). Data are expressed as mean ± SEM.
Intraperitoneal Oxt in Oxt KO Mice
Oxt was detectable in the brain and circulation after an intraperitoneal injection in Oxt KO mice (Figure 2D–F). Oxt concentrations were elevated for an hour after intraperitoneal administration, with a spike in the first 30 minutes, in the amygdala (F4, 12 = 3.18, p < .05). Oxt was detectable in the hippocampus after intraperitoneal administration, but these levels did not reach statistical significance (F4, 12 = 1.04, p = 0.43). Intraperitoneal administration of Oxt in Oxt KO mice yielded the most persistent elevation of Oxt concentrations, remaining significantly above baseline for the complete two-hour monitoring period (F4, 20 = 10.60, p < .001). Plasma Avp remained unaffected (F4, 20 = 0.33, p = 0.86).
Pharmacokinetics Across Genotype and Administrative Route
In addition to characterizing the pharmacokinetic profile of nasal and intraperitoneal administration of Oxt in normal and Oxt KO mice, we compared the kinetic parameters across genotype and administrative route (Table 1). Most parameters were comparable across these conditions. However, the maximum concentration (Cmax) of Oxt in the amygdala following nasal administration was significantly higher than after an intraperitoneal injection in normal mice (F3, 21 = 3.16, p < .05). In addition, AUCG was significantly higher in wildtype mice compared to Oxt KO mice regardless of the administrative route (F3, 24 = 4.30, p < .05). This genotypic difference is likely due to the fact that wildtype, but not Oxt KO, mice produce endogenous Oxt, as AUCG reflects the total hormonal output from endogenous and synthetic concentrations. No pharmacokinetic parameters varied as a function of genotype or administrative route in the hippocampus for Oxt (F3, 16 < 0.92, p > .45) or plasma for Avp (F3, 16 < 0.62, p > .61).
DISCUSSION
Numerous studies have speculated on the potential use of nasal administration of Oxt as a therapeutic treatment for human conditions characterized by deficits in social functioning, especially autism spectrum disorder and schizophrenia (e.g., [10–12]). With dozens of clinical trials currently underway to evaluate this hypothesis, only a handful of studies have focused on the pharmacokinetics of this administrative route. One of the significant questions still to be determined is if nasal administration provides a non-invasive route for delivery of Oxt to the brain. Here, we provide a detailed pharmacokinetic examination of Oxt concurrently in the circulatory system and CNS of mice after nasal administration. Most significantly, we utilized Oxt knockout mice to monitor the distribution of nasally delivered Oxt in the absence of endogenous production of Oxt to validate the source of the detectable Oxt. Our study demonstrates for the first time that nasal administration of Oxt permeates into specific areas of the brain. Furthermore, nasal administration yields a higher peak in central Oxt concentrations compared to intraperitoneal injections in wildtype mice. By contrast, we observed a prolonged return to baseline in plasma Oxt concentrations from intraperitoneal injections compared to nasal administration, an effect that was even greater in Oxt KO mice. Together, this suggests that nasal administration of Oxt may be a preferred route to target Oxt to the CNS, while intraperitoneal injections provide a systemic targeting alternative.
The pharmacokinetics of Oxt in brain ECF and plasma after nasal and intraperitoneal administration in wildtype mice have been previously documented [22]. Indeed, the concentrations versus time profiles reported by Neumann et al. [22] and in the current study are nearly indistinguishable, demonstrating reproducibility. Nasal and intraperitoneal administration of Oxt significantly increases the brain ECF concentrations and plasma for an hour, with peak concentration occurring between 30-60 minutes. One distinction between the two administrative routes is in the peak concentration (Cmax) within the brain ECF. Specifically, nasal administration (Cmax = 19.01 ± 4.60 pg/mL or 4.16 ± 1.00 ng/mL adjusted for recovery rate) of Oxt yields a significantly higher Cmax in the amygdala than an intraperitoneal injection (Cmax = 4.17 ± 1.23 pg/mL or 0.91 ± 0.27 ng/mL adjusted for recovery rate) in wildtype mice. This suggests that nasal administration may be a more efficient route to deliver Oxt to the brain than a systemic injection. However, a recent study with macaques would suggest that intranasal administration of oxytocin does not offer an advantage to intravenous administration in introducing oxytocin into the CSF in the cisterna magna [28]. This could constitute as species difference between macaques and mice in the efficacy of nasal administration of oxytocin, as there are certain differences in nasal anatomy. However, it could also be a difference in the accumulation of oxytocin in the extracellular fluid within brain regions and CSF in the cisterna magna, as there were even distinctions found in oxytocin concentrations post-administration between the amygdala and hippocampus in this study. Entry of most proteins to the brain via the bloodstream is significantly impaired due to the blood-brain barrier [34]. Several have speculated that peptides administered through the nasal cavity have a greater accessibility to the brain. In practice, it has been documented that 2×10−6 of the total Oxt from an intravenous injection [20] and 5×10−6 of the total Oxt from nasal administration reaches the CNS [13]. Studies have used autoradiography to measure permeability and distribution of radio-labelled peptides in the brain [35, 36], recording that the radioactivity is found in the brain as a gradient, with the highest concentrations found in regions in closest proximity to the nose (i.e., olfactory bulb). Unfortunately, these studies did not distinguish between intact peptide, metabolites, and free label. Still, the fact that Oxt Cmax is higher following nasal compared to systemic administration suggests that Oxt from the nasal cavity more appreciably enters the brain. It is interesting to note that while Oxt concentrations were elevated in both the amygdala and hippocampus, only the increase in the amygdala was statistically above baseline concentrations. Therefore, it is possible there is a gradient in how Oxt defuses throughout the brain, consistent with previous modeling for other peptides after nasal administration [37]. In addition, increasing group sizes (currently 47 mice) may affect statistical outcomes in the hippocampus.
Furthermore, both nasal and intraperitoneal administrations of Oxt yield detectable concentrations of Oxt in brains of Oxt KO mice, both in the amygdala and hippocampus. The primary significance of this result is that there is no endogenous production of Oxt from these mice, establishing that the source of Oxt measured in the brain tissue originates solely from the administration. Thus, Oxt from the nasal cavity enters the brain. There are at least two possible routes a drug can be transported between the nasal cavity and brain: blood vessels after absorption and directly through the olfactory membrane [38]. Focusing on the olfactory membrane pathway, the most important factor limiting nasal absorption of polar peptides, such as Oxt, is low membrane permeability. As a result, polar peptides are limited in passing the nasal membrane; however, low amounts will pass via an endocytotic transport process [39, 40]. Still, drugs can be transported to the olfactory bulb through olfactory neurons by extracellular diffusion or extracellular convection (bulk flow). Lochhead and colleagues [37] calculated the time required for a peptide to travel from the nasal cavity to the brain using each of these three mechanistic possibilities: 0.74-2.7 hours via intracellular axonal transport, 0.73-2.3 hours via extracellular diffusion, and 0.33 hours via extracellular convection. In the current study, the Cmax of Oxt occurred between 30-60 minutes after nasal administration, occurring within the range of each of these delivery mechanisms. While the precise pathways and mechanisms Oxt travels from the nasal cavity to the brain remains an open question, our data confirm that this passage occurs. In addition, it is possible that nasal administration of Oxt is absorbed into and transported through the bloodstream to the brain, though as mentioned previously, only a small amount will permeate the blood-brain barrier. In fact, from the Oxt KO data, we calculate that the blood-brain barrier permeability, calculated as log(Cbrain/Cblood) or logBB, for nasal and intraperitoneal administration of Oxt is −1.09 and −2.37, respectively. Compounds with logBB < −1.0 are considered to be poorly distributed to the brain [41]. Still, this pathway is plausible as both nasal and systemic administrative routes, which readily deliver Oxt into circulation, result in detectable concentrations of Oxt in the brain.
Intriguingly, the pharmacokinetic profiles of these drug delivery routes in the Oxt KO mice are different. While both nasal and intraperitoneal administration of Oxt delivers Oxt to the circulatory system and CNS, Oxt concentrations persist longer in the brain after nasal administration, and in the blood after intraperitoneal administration. Specifically, Oxt concentrations in the amygdala return to baseline slower after nasal administration (between 90120 minutes) compared to intraperitoneal injection (between 60-90 minutes). By contrast, plasma Oxt concentrations return to baseline faster after nasal administration (between 90-120 minutes) than intraperitoneal injection (exceeding 120 minutes). The variance in the return to baseline in the brain and blood between nasal and intraperitoneal administration could reflect distinctions in drug distribution and ultimate bioavailability of Oxt for various tissue types. The clearance of Oxt in plasma after both administrative routes is consistent with a two-compartmental system, corresponding to the plasma volume and the extravascular fluid volume [42]. After nasal or intraperitoneal administration, the plasma concentration is expected to rise rapidly (e.g., after entry of inhaled oxytocin into the blood via the lungs) with a second slow component of entry from extravascular fluid; the resulting plasma concentrations are cleared rapidly via the kidneys and liver [43]. Brain levels, assuming the penetrate via the blood, will rise following entry at the high concentrations achieved initially, but remain elevated due to slow clearance. It is plausible that a greater distinction, particularly with the true peak concentration and associated time course, could be established between the two administrative routes with a more refined timescale. Some technical considerations of the microdialysis method should be noted. First, it is not conceivable that a microdialysis probe can be inserted into the brain of a small animal without significant damage to blood vessels, impairing the local integrity of the blood-brain barrier. Second, the concentrations measured in microdialysates are not measures of total extracellular concentration – rather, they need to be corrected for the recovery of peptides by microdialysis. The recovery rate for peptides is usually low, and varies between 2% and 20% depending on the probes used, flow rate, and other conditions.
While nasal administration has become the primary non-invasive method to deliver Oxt in basic research and experimental clinical trials, a major critique of the field has been that no information was available regarding the pharmacokinetics of this drug delivery route until quite recently. In fact, until just a few years ago, studies administering Oxt relied on the pharmacokinetic properties of vasopressin administration [44]. The new knowledge learned from our work and works completed by others should influence the anticipated time course and dosages used for nasal administration of Oxt. To this end, investigations already attempted to standardize nasal application of Oxt for mice and rats [22], nonhuman primates [23], and humans [45]. These studies all reported increased Oxt concentrations in the circulatory system and CNS after nasal administration [22–27]. Specifically, the pharmacokinetics of Oxt in circulation after nasal application are comparable across these studies, peaking within the first 15-30 minutes and returning to baseline between 75-90 minutes. However, the rise in CSF Oxt occurs at shifted time points, highlighting the fact that the administration of Oxt (e.g., amount and method) and sampling procedures (e.g., target location) may contribute to the variance observed in the pharmacokinetics. Further, this work shows that the pharmacokinetics of Oxt after nasal administration in plasma do not mirror concentrations in CSF or brain ECF. One factor to note is that peak concentration of Oxt detected in plasma after administration will be depend on sampling intervals. As the half-life of Oxt in plasma is estimated to be about two minutes, shorter sample intervals may provide greater accuracy in true Cmax detection. Thus, there should be reservations to utilizing peripheral measures of Oxt as a reflection of CNS concentrations. We anticipate that as more is learned about the pharmacokinetics of nasal administration of Oxt, a better understanding will be realized for the sizeable and ever-growing amount of data cultivated using this administrative route.
ACKNOWLEDGEMENTS AND DISCLOSURES
We thank Drs. Sarah Williams and Adi Cymerblit-Sabba for their comments on a previous version of this manuscript and Emily Shepard for assistance with mouse colony maintenance and genotyping. Jarrett Fastman, Michael Granovetter and Matthew Vincent engaged in useful discussions. This research was supported by the intramural research program of the NIMH (ZIA-MH-002498-24).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
Conflict of intrest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- [1].Evans SL, Dal Monte O, Noble P, Averbeck BB, Intranasal oxytocin effects on social cognition: A critique, Brain Res 1580 (2014) 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Quintana DS, Alvares GA, Hickie IB, Guastella AJ, Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model, Neurosci. Biobehav. Rev 49 (2015) 182–192. [DOI] [PubMed] [Google Scholar]
- [3].Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E, Oxytocin shapes the neural circuitry of trust and trust adaptation in humans, Neuron 58 (2008) 639–650. [DOI] [PubMed] [Google Scholar]
- [4].Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, Oxytocin increases trust in humans, Nature 435 (2005) 673–676. [DOI] [PubMed] [Google Scholar]
- [5].Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ, Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers, J Psychopharmacol 23 (2009) 241–248. [DOI] [PubMed] [Google Scholar]
- [6].Marsh AA, Yu HH, Pine DS, Blair RJ, Oxytocin improves specific recognition of positive facial expressions, Psychopharmacology 209 (2010) 225–232. [DOI] [PubMed] [Google Scholar]
- [7].Rimmele U, Hediger K, Heinrichs M, Klaver P, Oxytocin makes a face in memory familiar, J Neurosci 29 (2009) 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, Hurlemann R, Oxytocin modulates social distance between males and females, J Neurosci 32 (2012) 16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R, Oxytocin enhances brain reward system responses in men viewing the face of their female partner, Proc Natl Acad Sci U S A 110 (2013) 20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS, A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder, Psychoneuroendocrinology 34 (2009) 917–923. [DOI] [PubMed] [Google Scholar]
- [11].Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A, Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients, Biol Psychiatry 68 (2010) 678–680. [DOI] [PubMed] [Google Scholar]
- [12].Okamoto Y, Ishitobi M, Wada Y, Kosaka H, The potential of nasal oxytocin administration for remediation of autism spectrum disorders, CNS Neurol Disord Drug Targets (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leng G, Ludwig M, Intranasal oxytocin: Myths and delusions, Biol Psychiatry 79 (2016) 243–250. [DOI] [PubMed] [Google Scholar]
- [14].Walum H, Waldman ID, Young LJ, Statistical and methodological considerations for the interpretation of intranasal oxytocin studies, Biol Psychiatry 79 (2016) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Quintana DS, Woolley JD, Intranasal oxytocin mechanisms can be better understood, but its effects on social cognition and behavior are not to be sniffed at, Biol Psychiatry 79 (2016) e49–50. [DOI] [PubMed] [Google Scholar]
- [16].Leng G, Ludwig M, Reply to: Intranasal oxytocin mechanisms can be better understood, but its effects on social cognition and behavior are not to be sniffed at, Biol Psychiatry 79 (2016) e51–52. [DOI] [PubMed] [Google Scholar]
- [17].Leng G, Ludwig M, Reply to: Improving research standards to restore trust in intranasal oxytocin, Biol Psychiatry 79 (2016) e55–56. [DOI] [PubMed] [Google Scholar]
- [18].Carson DS, Yuan H, Labuschagne I, Improving research standards to restore trust in intranasal oxytocin, Biol Psychiatry 79 (2016) e53–54. [DOI] [PubMed] [Google Scholar]
- [19].Kendrick KM, Keverne EB, Hinton MR, Goode JA, Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep, Brain Res. Bull 26 (1991) 803–807. [DOI] [PubMed] [Google Scholar]
- [20].Mens WB, Witter A, van Wimersma Greidanus TB, Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): Half-times of disappearance of these neuropeptides from CSF, Brain Res 262 (1983) 143–149. [DOI] [PubMed] [Google Scholar]
- [21].Lauson HD, Metabolism of the neurohypophysis hormones, in: Greop RO, Astwood EB, Knobi E, Sawyer WH (Eds.), Handbook of Physiology, Section Endocrinology, American Physiological Society, Washington DC, 1974, pp. 287–393. [Google Scholar]
- [22].Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R, Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice, Psychoneuroendocrinology 38 (2013) 1985–1993. [DOI] [PubMed] [Google Scholar]
- [23].Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, Roberts JA, Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques, Psychoneuroendocrinology 66 (2016) 185–194. [DOI] [PubMed] [Google Scholar]
- [24].Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R, Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans, Scientific reports 3 (2013) 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB, CSF and blood oxytocin concentration changes following intranasal delivery in macaque, PLoS One 9 (2014) e103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA, Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques, Psychoneuroendocrinology 45 (2014) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML, Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatto), Proc Natl Acad Sci U S A 109 (2012) 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB, Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay, Mol Psychiatry 23 (2018) 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bowen MT, McGregor IS, Oxytocin and vasopressin modulate the social response to threat: A preclinical study, The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 17 (2014) 1621–1633. [DOI] [PubMed] [Google Scholar]
- [30].Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS, Prosocial effects of oxytocin in two mouse models of autism spectrum disorders, Neuropharmacology 72 (2013) 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS, Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats, PLoS ONE 6 (2011) e27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Young WS 3rd, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI, Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition, J Neuroendocrinol 8 (1996) 847–853. [DOI] [PubMed] [Google Scholar]
- [33].Curtis JT, Stowe JR, Wang Z, Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles, Neuroscience 118 (2003) 1165–1173. [DOI] [PubMed] [Google Scholar]
- [34].Pardridge WM, Peptide drug delivery to the brain, Raven Press, New York, 1991. [Google Scholar]
- [35].Thorne RG, Pronk GJ, Padmanabhan V, Frey WH, 2nd, Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration, Neuroscience 127 (2004) 481–496. [DOI] [PubMed] [Google Scholar]
- [36].Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH, 2nd, Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis, J Neuroimmunol 151 (2004) 66–77. [DOI] [PubMed] [Google Scholar]
- [37].Lochhead JJ, Thorne RG, Intranasal delivery of biologics to the central nervous system, Adv Drug Deliv Rev 64 (2012) 614–628. [DOI] [PubMed] [Google Scholar]
- [38].Illum L, Nasal drug delivery-possibilities, problems and solutions, J Control Release 87 (2003)187–198. [DOI] [PubMed] [Google Scholar]
- [39].Grass GM, Robinson JR, Mechanisms of corneal drug penetration. II: Ultrastructural analysis of potential pathways for drug movement, J. Pharm. Sci 77 (1988) 15–23. [DOI] [PubMed] [Google Scholar]
- [40].Inagaki M, Sakakura Y, Itoh H, Ukai K, Miyoshi Y, Macromolecular permeability of the tight junction of the human nasal mucosa, Rhinology 23 (1985) 213–221. [PubMed] [Google Scholar]
- [41].Abraham MH, Takacs-Novak K, Mitchell RC, On the partition of ampholytes: Application to blood-brain distribution, J. Pharm. Sci 86 (1997) 310–315. [DOI] [PubMed] [Google Scholar]
- [42].Leng G, Sabatier N, Measuring oxytocin and vasopressin: Bioassays, immunoassays, and random numbers, J Neuroendocrinol 28 (2016) doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ginsburg M, Smith MW, The fate of oxytocin in male and female rats, Br J Pharmacol Chemother 14 (1959) 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL, Sniffing neuropeptides: A transnasal approach to the human brain, Nat Neurosci 5 (2002) 514–516. [DOI] [PubMed] [Google Scholar]
- [45].Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB, Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research, Psychoneuroendocrinology 38 (2013) 612–625. [DOI] [PubMed] [Google Scholar]


