Abstract
Lifestyle or age-related risk factors over-activate the inflammation that triggers acute heart failure (HF)-related mortality following myocardial infarction (MI). Post-MI activated leukocytes express formyl peptide receptor 2 (FPR2) that is essential for inflammation-resolution and in cardiac healing. However, the role of FPR2 in acute HF is incomplete and remain of interest. Here, we aimed to determine whether pharmacological inhibition of FPR2 perturb leukocyte trafficking in acute HF. Male C57BL/6 (8 to 12 weeks) mice were subjected to acute HF (MI-d1) using permanent coronary artery ligation that develops irreversible acute and chronic heart failure. FPR2 antagonist WRW4 (1μg/kg/day) was subcutaneously injected 3 hr post-MI maintaining saline-injected MI-controls. Leukocytes were quantitated using flow cytometry, and acute decompensated HF was confirmed using echocardiography and histology. FPR2 inhibition decreased the expression of FPR2 in the LV and spleen tissues. Administration of WRW4 inhibitor to mice primed immature and inactive neutrophils infiltration Ly6Gint and intensified the Ccl2 expression compared to MI-control in the infarcted LV post-MI. Leukocyte profiling revealed an overall decrease in monocytes (23.3±2%) in WRW4-injected mice compared with MI-control (49.1±2%) in infarcted LV. FPR2 inhibition increased F4/80+/Ly6Chi pro-inflammatory macrophages (14.8±2%) compared with MI-control (10±1%) with increased transcripts of pro-inflammatory markers TNF-α and IL-1β, and decreased Arg-1 expression in the infarcted LV compared to MI-controls is suggestive of the impaired acute inflammatory response. Inhibition of FPR2 using WRW4 also disturbed splenocardiac leukocytes recruitment by priming immature neutrophils leading to the onset of incomplete resolution signaling in acute decompensated HF post-MI.
Keywords: Formyl peptide receptor, inflammation, leukocytes, myocardial infarction, resolution of inflammation
Introduction
After myocardial infarction (MI), acutely decompensated heart failure (HF) remains a frequent cause of progressive chronic HF, disability, congestive edema, and death worldwide (1). In acute HF, inflammation is a prerequisite for myocardial healing, but it can paradoxically extend tissue injury if remain uncontrolled that lead to a pathological remodeling process; hence it needs to be optimally balanced in acute HF. Therefore, for optimal cardiac healing a synchrony between inflammation and resolution phase with mature scar formation is necessary to return cardiac homeostasis that can lasts from few days to weeks and encompasses the reparative phase. Therefore, leukocytes ‘get-in’ and ‘get-out’ signal has to be balanced that determines onset and termination of inflammation. On time leukocytes activation with temporal and spatial inter-organ coordination between injured heart and spleen during an inflammation-resolution phase is required for optimal cardiac healing (2, 3).
The G-protein–coupled formyl peptide receptors (FPRs) plays an important role in controlled recruitment of immune cells to the site of injury (4). Mouse has a family of 8 FPRs, functionally FPR1 and FPR2 are present on immune cells and share a high degree of amino acid sequence, showing similarity particularly at the cytosolic (signal transducing) domains and the functional repertoire induced by these two receptors is almost identical. Despite these signaling similarities, there are some fundamental differences between these two receptors, that leads to different agonists that some of them bind specifically to FPR2 or FPR1. Secondly, FPR1 plays a major role in anti-bacterial inflammation and metastasis of malignant glioma cells while FPR2 interact with a number of endogenous chemotactic agonist peptides produced by the host in defense and immune responses triggering both proinflammatory and anti-inflammatory responses (5, 6). Since FPR2 serve as a ligand for multiple bioactive lipids, peptides, and mediates proresolution by efficient coordination of leukocytes in spleen and left ventricle (7, 8). FPR2 is also widely expressed on different cell types and emerged as a central check point in inflammatory and resolving processes and adaptive immunity although the precise role in acute HF is not well understood (9–11). In the context of cardiac healing, the variable function of FPR2 is dependent on the nearby bioactive lipids/peptides as available ligands, in a time-dependent manner (12). Neutrophil-platelet interaction and in-vitro peritonitis study emphasise the role of FPR2 in promoting an inflammatory response (13, 14). However, a diversified function for FPR2 in non-resolving inflammation relevant to the splenocardiac nexus in cardiac injury remains of interest. In acute decompensated HF, particularly after occlusion of non-perfused cardiac injury, the FPR2 is activated by bioactive lipids such as aspirin-triggered lipoxin A4, resolvin D1, allowing a resolution of inflammation and return to homeostasis (7, 8).
To determine how FPR2 inactivation impacts the initial acute inflammatory response in acute HF, we pharmacologically blocked FPR2 using selective antagonist WRW4. We tested whether FPR2 impedes leukocyte phenotypes and kinetics in acute decompensated HF. Our findings revealed that FPR2 inhibition impaired leukocyte trafficking, by decreasing leukocytes infiltrating in the infarcted LV and spleen. FPR2 antagonist WRW4 primed immature and inactive neutrophils infiltration Ly6Gint that enhanced Ccl2 expression compared to acute HF controls. Also, inhibition of FPR2 increased F4/80+/Ly6Chi, a set of pro-inflammatory macrophages in LV indicating amplified non-resolving milieu of innate immune cells. After FPR2 inhibition using WRW4, the intensified pro-inflammatory leukocytes were validated by an increase in TNF-α and IL-1β, with a decrease in Arg-1 expression suggestive of non-resolving or immune suppressive inflammation in acute HF. Thus, the presented report suggests that, FPR2 inhibition using WRW4 distrupt leukocyte ‘get-in signal’ by engaging immature post-MI neutrophils leading to the onset of incomplete resolution signaling in acute decompensated HF.
Methods
Animal care compliance
All animal procedures were conducted according to the “Guide for the Care and Use of Laboratory Animals” (8th Edition. 2011), AVMA Guidelines for the Euthanasia of Animals: (2013 Edition) and were approved by the Institutional Animal Care and Use Committees at the University of Alabama, Birmingham, USA.
Coronary ligation surgery and WRW4 injection
C57BL/6J mice of 8–12 weeks old were obtained from Jackson Laboratory (Bar Harbor, Maine, USA) and were maintained under constant temperature (19.8–22.2°C). The mice were given free access to water and standard lab diet. Mice were subjected to permanent coronary artery ligation as described previously (15). Vehicle or WRW4-(Trp-Arg-Trp-Trp-Trp-Trp-NH2) (Torcis, catalog no. 557–55-2), is a selective antagonist for Formyl Peptide Receptor 2 (FPR2) and was dissolved in PBS (1μg/kg/body weight) and injected subcutaneously and then experiments were performed 24 hr post-MI. The mice were divided into 3 groups- (1) Group-1 as a control group with no surgery (day 0: no-MI control), (2) Group-2 as MI-saline group having MI surgery with vehicle treatment (MI-d1 post 24 hr), (3) Group-3 was administered post MI 3 hr with WRW4 and evaluated 24hr post-MI (MI-d1+WRW4). To induce MI, mice were subjected to the surgical ligation of the left anterior descending coronary artery. In brief, the mice were anesthetized with 2% isoflurane, and the left anterior descending coronary artery was permanently ligated using nylon 8–0 sutures (ARO Surgical Instruments Corporation, CA, USA) in minimally invasive surgery. Before MI surgery buprenorphine (0.1 mg/kg; Intraperitonal (IP) and post-MI carprofen (5 mg/kg; subcutaneous (SQ)) were administered to reduce pain (16).
Echocardiography
For echocardiographic acquisition, mice were anesthetized using 1.5–2.0% isoflurane in a 100% oxygen mix. Images were acquired using the VEVO 3100 with probe MX400 and an axial resolution of 30 μm in the vivo imaging system. Increased heart rates (heart rate > 400 beats per minute) were maintained during the acquisition to achieve physiological measurements and short (M-mode), and long axis (B-mode) images of hearts were obtained as discussed in our paper (15). Echocardiography was performed before necropsy for d0 control mice as well as MI-d1 control and post-MI-d1 WRW4-injected mice. Three images were obtained for each variable from consecutive cardiac cycles. Ultrasound operator was blinded to study groups to exclude bias in the comparison of MI-controls versus FPR2 inhibitor (15).
Necropsy
No-MI naïve control (d0), MI-d1 control and post-MI-d1+WRW4 treated mice were anesthetized under 2% isoflurane anesthesia in 100% oxygen mix. To collect plasma, heparin (4 IU/g; I.P.) injection was used. The lungs and left and right ventricles were collected, weighed and processed as previously described (15). The spleen was dissected by making an incision in the left of the peritoneal wall. The spleen was divided into two halves, and the broad and concave portion was fixed in 10% zinc formalin for IHC, and the rest of the spleen was snap-frozen for biochemical and molecular analysis (8, 15).
LV histology and immunohistochemistry
For histological measurements, left ventricle (LV) transverse and spleen sections were embedded in paraffin and sectioned. Paraffin-embedded sections were de-paraffinized in citrisolv and rehydrated through graded ethanol. Hematoxylin followed by eosin was used for H&E staining. For neutrophil staining, the de-paraffinized sections are subjected to heat-mediated antigen retrieval to expose antigen epitopes (Target Retrieval Solution, Dako S1699) using a pressure cooker (BioSB Tinto Retriever). Sections were then blocked with normal rabbit or goat serum as per antibody and incubated with rat anti-mouse neutrophils (CL 8993AP, clone 7 1:50; Cedarlane). Neutrophils staining were followed with the Vectastain Elite ABC kit (Vector). The slides were mounted using permount and allowed to dry for use in image analysis (8).
LV confocal microscopy
The de-paraffinized sections were fixed using 4% paraformaldehyde and. permeated using 0.1% Triton. The tissues were blocked for 1hr in 10% goat serum. Subsequently, tissues were incubated with FPR2 (M-73) antibody (Santa Cruz, sc-66901) overnight. Alexa 555-labeled anti-rabbit antibody (Molecular Probes, A21422) and Wheat germ agglutinin (WGA) (W6748, 1:1000; Molecular. Probes) was added to sections for 60 min, followed by Hoechst staining for 5 min. The stained sections were washed with PBS and mounted with anti-fade and acquired using confocal microscopy (17).
Flow cytometry analysis for LV and spleen
Single mononuclear cells were isolated from LV and spleen from post-MI-d1 control and post-MI-d1 WRW4 and were analyzed by flow cytometry with slight modification (18). The cell count for LV mononuclear cells or splenocytes was adjusted to ~1–2 million cells/stain. Isolated cell suspensions were finally suspended in 200 μl of 1:500 Fc block and incubated for 10 min on ice. The LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit (Thermo Fischer Scientific, L34961) is used to determine the viability of cells before the fixation. A cocktail of fluorophore-labeled monoclonal antibodies in 2X concentration was added for 30 min on ice as appropriate for each study. We used CD45-PE (BD Biosciences,103114), CD11b-APC (BD Biosciences, 553312) F4/80-Percp (Thermo Fischer Scientific, 45–4801-821), Ly6C-FITC (BD Biosciences), Ly6G-pacific blue (BioLegend, 127612) in a cocktail. All population is primarily gated using CD45+ markers for hematopoietic cells. Further, the neutrophils were defined as CD11b+/Ly6G+cells. Activated macrophages were defined as the cells having dual expression CD11b and F4/80 surface marker. The macrophages (F4/80+) were also classified as M1 (classically activated macro-phages) and M2 (alternatively activated macrophages) based on Ly6Chi and Ly6Clo respectively. Detailed gating strategy is provided in supplementary figure 1. Data were acquired on BDTM LSRII Flow Cytometer and analyzed with FlowJo software, version 7.6.3 as described previously (19).
RNA isolation and quantitative real time-PCR
Post-necropsy, samples were processed for RNA extraction as described previously (8). For qPCR, reverse transcription was performed with 2.0 μg of total RNA from LV using SuperScript® VILO cDNA Synthesis kit (Invitrogen, CA, USA). Quantitative PCR for the FPR2 (Mm00484464)), Ccl2 (Mm00441242), Ptgs1(Mm00477214_m1), Ptgs-2 (Mm00478374_m1), Alox12 (Mm00478374_m1), Alox15 (Mm00507789_m1), Alox5 (Mm01182747_m1), Tnf-α (Mm00443258), IL-1β (Mm00434228), Il10 (Mm01329362), Mrc1 (Mm01288386), Arg1(Mm00475988), and Ym1(Mm00657889) genes were performed using TaqMan probes, as done previously.(20) Gene expression was normalized with hypoxanthine phosphoribosyltransferase-1 (Hprt-1) (Mm03024075_m1) as the housekeeping gene. The results were reported as 2^−ΔCT. All the experiments were performed in duplicate with n= 5–6 per group (19).
Statistical analysis
Data are expressed as mean per group and SEM. Statistical analyses were performed using GraphPad Prism 7. Analysis of variance (ANOVA), followed by Newman–Keuls post-hoc test, was done for multiple comparisons between no-MI naïve control, MI-d1 control, MI+WRW4.
Results
Coronary ligation induced cardiac injury with profound pathological remodeling
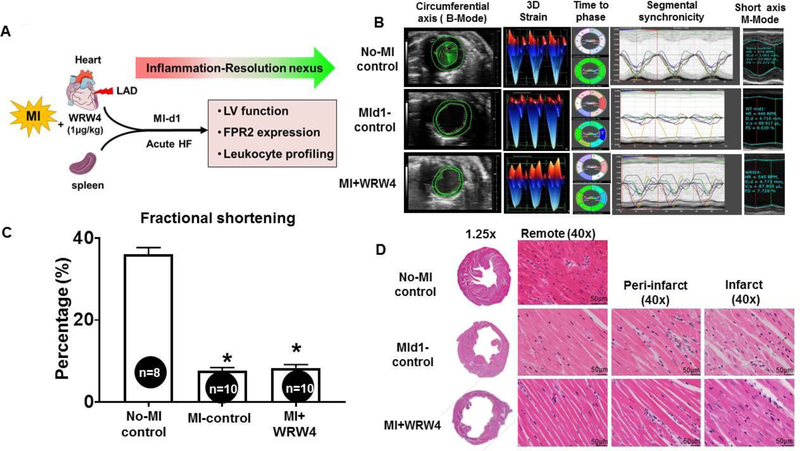
The current study was designed to evaluate how FPR2 inhibition impacted leukocyte ‘get-in’ signal post-MI (Figure 1A). To determine the impact of WRW4 on FPR2 signaling in acute HF post-MI, the LV function was measured using echocardiography. The ultrasound images of heart were acquired in no-MI naïve control, MI-control (MI-d1; 24h) and MI+WRW4-injected mice. Speckle tracking-based blinded analyses revealed that MI-control mice and MI+WRW4 had impaired strain (short green vector) with intense cardiac dysfunction from remote anterior base compared with no-MI naive control. Representative M-mode images confirm the loss of posterior wall thickness suggestive of anterolateral infarct and LV dysfunction due to coronary ligation in MI-control and MI+WRW4 mice (Figure 1B). Echocardiography data displayed a decrease in fractional shortening (Figure 1C and Table 1 A) in MI-control (8±1%), MI+WRW4 (8±1%) compared with no-MI control mice (36.1±1.6%). Hematoxylin and eosin staining of LV in MI-control and MI+WRW4 mice showed obvious necrosis and disorganized structure in the infarcted area compared with no-MI control mice with notable changes in gravimetric parameters (Figure 1D and Table 1B). Our results confirmed that the coronary ligation in mice induced profound cardiac dysfunction in MI-control and MI+WRW4 treated mice suggestive of acute decompensated HF.
Figure 1. FPR2-antagonist WRW4 leads to LV dysfunction post-MI.
A. An experimental design indicating WRW4 injection strategy in coronary artery ligation model with time points. B. Speckle tracking-based LV function analyses using echocardiography. C. Bar graph representing percentage fractional shortening at d1 in MI-control and WRW4-injected mice post-MI compared to no-MI naïve controls. D. Hematoxylin and eosin images of no-MI, MI-control, and MI+WRW4-injected mice LV (remote area, peri-infarct, and infarct) post-MI. Images are taken at 40X; scale=50μM. *p<0.05 vs no-MI controls; values are means ±SEM; n=6–8 mice/group.
Table 1.
Echocardiography and necropsy parameters at day 1 post-MI compared to naïve controls (no-MI).
| Parameters | No-MI control (n=8) | MI-day 1 (n=10) | MI-day 1+WRW4 (n=10) |
|---|---|---|---|
| Heart rate (bpm) | 446±12 | 481±17 | 540±10*$ |
| EDD (mm) | 3.61±0.07 | 4.64±0.10* | 4.67±0.08* |
| ESD (mm) | 2.31±0.10 | 4.33±0.11* | 4.28±0.08* |
| Fractional shortening (%) | 36±2 | 8 ±1* | 8±1* |
| PWTd (mm) | 0.66±0.04 | 0.48±0.02* | 0.55±0.03* |
| PWTs (mm) | 1.09±0.03 | 0.52±0.02* | 0.64±0.03*$ |
| Necropsy parameters | |||
| Body weight (g) | 26±1 | 24±1 | 26±0.4 |
| LV (mg) | 80±3 | 88±2 | 91±2* |
| LV/BW (mg/g) | 3.2±0.1 | 3.7±0.1* | 3.5±0.1* |
| Right ventricle (mg) | 20±1 | 21±1 | 21±1 |
| Spleen (mg) | 77±5 | 61±4* | 71±3*$ |
| LV / Tibia (mg/mm) | 4.8±0.2 | 5.2±0.1* | 5.2±0.1* |
| Tibia (mm) | 17±0.1 | 17±0.1 | 17±0.1 |
Values are mean± SEM; n indicates sample size. bpm, beats per minute; EDD, end-diastolic dimension; ESD, end-systolic dimension, mm, millimeter; g, gram; mg, milligram
p< 0.05 vs. No-MI control
p< 0.05 vs. MI-d1 control with respective time point.
FPR2 inhibition using WRW4 upregulated monocyte marker CCL2 along with LOXs and COX-1 post-MI
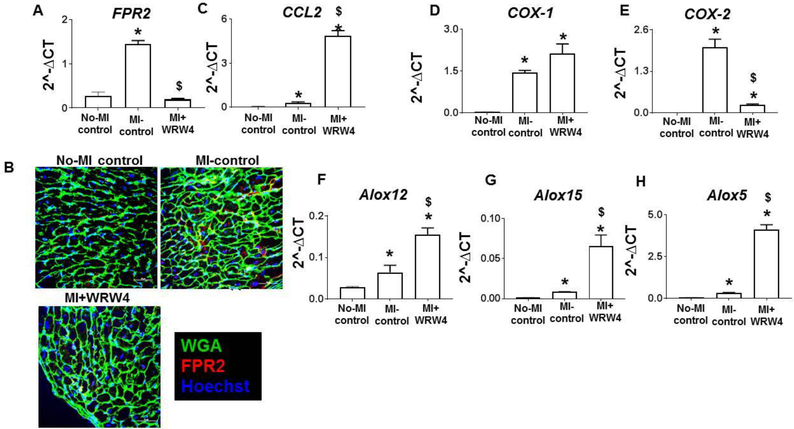
Since, FPR2 plays and important role in both innate and adaptive response, we further examined how FPR2 inhibition would impact chemotactic response to leukocytes after cardiac injury. FPR2, is a competitive receptor that is also activated by bioactive lipid biomolecule to offer cardio-protection with clearance of neutrophils (7). Here, first, we validated whether 1μg/kg dose is enough to inhibit FPR2. For this, we measured mRNA and did immunofluorescence to validate FPR2 inhibition post-MI (Figure 2A and B). Increase in chemotactic response in LV post-MI is an obvious phenomenon due to cardiac injury. However, an increased chemotactic response to the chemokine CCL2 was observed in LV infarct of MI+WRW4 mice (Figure 2C). The cardiac healing using FPR2 bioactive ligand molecules is essential in response to cardiac injury or infection (7, 8, 21). We further validated how FPR2 inhibition would impact FPR2 ligand biosynthesizing enzymes such as cyclooxygenase (COX) and lipoxygenase (LOXs) that uses arachidonic acid as a substrate. Thus, we analyzed the LV mRNA expression of the COXs (−1 and −2) and LOXs (−15, −12 and-5). Post-MI, WRW4 treatment differentially impacted COX-1 and COX-2 in LV infarct. WRW4 increased COX-1 expression (1.5 fold; p<0.05) while decreased in COX-2 expression was observed (−10 fold, p<0.05) compared with MI-control in acute HF (Figure 2D and E). WRW4 increased LV mRNA expression of Alox12 (2.7 fold; p<0.05), Alox15 (7.7 fold; p<0.05) and Alox5 (12.9 fold; p<0.05) post-MI compare with MI-control (Figure. 2F–H). These results indicate that FPR2 inhibition increased LV chemotaxis rendering over activated CCL2 response post-MI, which may result in a feed-forward uncontrolled increase in COX-1 and LOXs expression, in LV post-MI.
Figure 2. WRW4 upregulated monocyte marker ccl2 along with LOXs with COX-1 post-MI.
A. Bar graph representing mRNA expression of FPR2 normalized with HPRT-1 in LV. B. representative LV images indicating FPR2 (red) expression with wheat germ agglutinin (WGA) and hoechst (blue) from MI-control and MI+RvD1-injected mice (scale bars: 20 μm). Bar graph representing mRNA expression of C. CCL2 D. COX-1 E. COX-2 F. ALOX12 G. ALOX15 H. ALOX5 in LV normalized with HPRT-1. *p<0.05 versus no-MI. $p < 0.05 versus post MI-D1 group. Values are means ±SEM; n=5 mice/group.
FPR2 inhibition using WRW4 activated pro-inflammatory macrophages in the infarcted LV post-MI
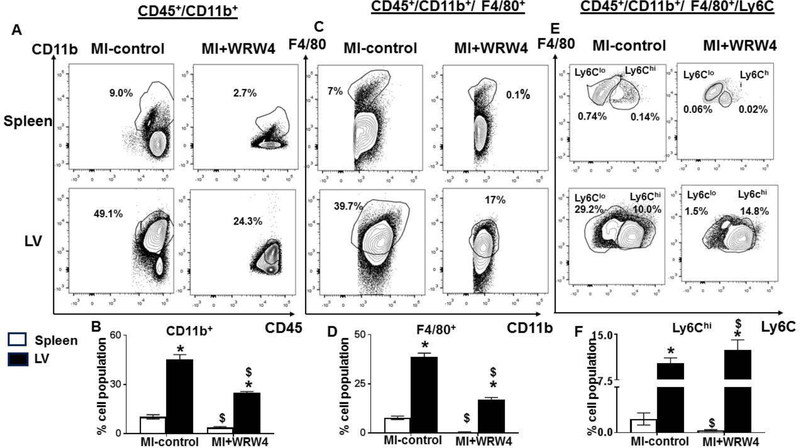
Splenic neutrophils and monocytes infiltrate heart post-MI in the infarcted area to facilitate healing and myocardium (2). We applied quantitative flow cytometry-based approach that defined the impact of FPR2 inhibition on leukocyte profiling in LV and spleen post-MI in acute HF. After mononuclear cell isolation, the cell viability was assessed by using LIVE/DEAD™ - fixable blue dead cell stain kit. WRW4 injected mice demonstrated a significant decrease in CD45+CD11b+ (monocytes) cells in LV (23.3±2%) and spleen (3.8±0.5%) compared with MI-control which showed 49.1±2% and 10±1.3% population respectively (Figure. 3A–B). Total population of macrophages (F4/80+) was decreased in WRW4-injected mice in spleen (0.45 ±0.2% vs 7.8 ±1%) and LV (17±1% vs 38.6 ± 2%) compared to MI-control (Figure. 3C–D). Despite the decrease in total macrophages in LV, FPR2 inhibition using WRW4 showed increase in macrophages percentage F4/80+/Ly6Chi (14.8±2%) in LV indicative of proinflammatory leukocyte profile compared with MI-control (10±1%) (Figure. 3E–F). Thus, FPR2 inhibition impaired leukocyte trafficking (‘get-in’ signal) by decreasing the density of infiltrating leukocytes in the spleen and infarcted LV in acute HF post-MI.
Figure 3. FPR2 inhibition using WRW4 activated F4/80+/Ly6Chi in the infarcted LV with a decrease in CD11b+ and F4/80+ population post-MI.
A. Representative FACs counter plots are identifying spleen and LV CD45+/CD11b+population in MI+WRW4–injected mice compared with MI-control mice. B. Bar graph displaying CD45+/CD11b+ population in spleen and LV. C. Representative counter plots showing CD45+/CD11b+/F4/80+population in spleen and LV in MI+WRW4-injected mice compared with no-MI control. D. Bar graph displaying CD45+/CD11b+/F4/80+population in spleen and LV. E. Representative FACs counter plots identifying spleen and LV CD45+/CD11b+/F4/80+/Ly6C population in MI+WRW4–injected mice compared with MI-control mice. F. Bar graph displaying CD45+/CD11b+/F4/80+/Ly6Chi population in spleen and LV. *p<0.05 versus spleen; $ p<0.05 respective-MI-control. Values are means ±SEM; n=4 mice/group.
FPR2 inhibition using WRW4 primed immature and inactive neutrophils infiltration
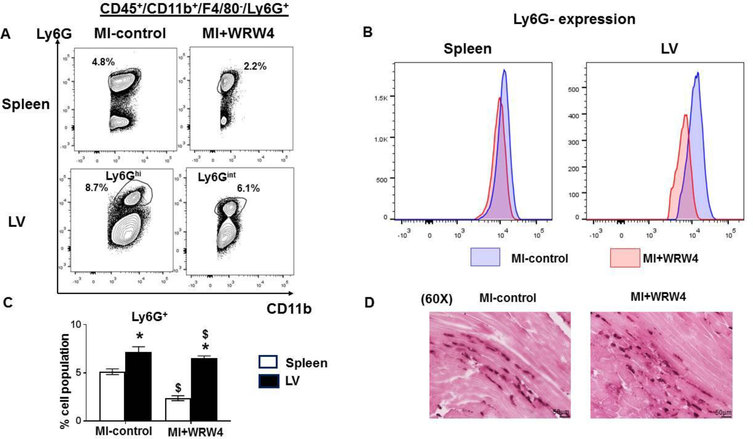
Neutrophils are first phagocytic non-specific responder at the site of cardiac injury and respond to diverse chemo attractants including FPR2 receptor and leukotrienes (22). Intriguingly, flowcytometry-based quantitation of leukocytes from spleen and LV displayed lower neutrophils in the spleen of WRW4-injected mice (2.4±0.3%) compared with MI-control (5.1±0.3%) in acute HF. However, there was no difference observed in percentage of neutrophils population in the LV. Interestingly, LV revealed two types of the Ly6G population; MI-control group displayed Ly6Ghi while WRW4 treated group exhibited a Ly6G population having intermittent expression termed as Ly6Gint neutrophils (Figure 4A–D). Studies have reported that immature neutrophils express intermediate Ly6G levels which are inactive and dysfunctional (23). Thus, WRW4-mediated FPR2 inhibition primed immature and inactive neutrophils in cardiac injury leading to non-resolving inflammation.
Figure 4. WRW4 dysregulated neutrophils clearance post-MI.
A. Representative flow cytometry (FACs) counterplots showing spleen and LV neutrophils population (CD45+/CD11b+/F4/80−/Ly6G+) population in MI-control and MI+WRW4-injected mice post-MI. B. Representative spleen and LV FACs histogram displaying Ly6G expression. C. Bar graphs shows percentage of Ly6G+ population in LV and spleen post-MI. D. Representative neutrophil immunohistochemistry images of LV transverse section of MI-control and WRW4-injected mice post-MI (Magnification 40X, scale = 50 μm). *p<0.05 vs spleen; $ p<0.05 respective MI-control. Values are means ±SEM; n=4 mice/group.
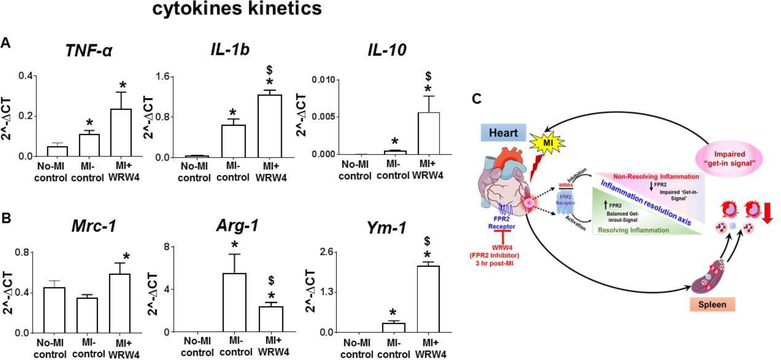
WRW4-mediated FPR2 inhibition dysregulated cytokine-chemokine kinetics post-MI in acute HF
Since, FPR2 inhibition using WRW4 decreased over all leukocytes and activated immature neutrophils in acute HF, we quantified mRNA levels of chemokines markers (proinflammatory and proresolving) TNF-α, IL-6, IL-10, Mrc-1/CD206, Arg-1, and Ym-1. Administration of WRW4 in acute HF increased transcripts of pro-inflammatory markers TNF-α and IL-1β indicative of non-resolving or amplified acute inflammation. However, we also observed potential increase in Arg1, IL-10, and Ym-1 (p<0.05) in infarcted LV of WRW4-injected mice compared to MI-controls with no change in MRC-1/CD206 expression. These results indicated an imbalance of pro-inflammatory and pro-resolving cytokines-chemokines expression suggestive of dysregulated innate response by FPR2 inhibition in acute HF.
Discussion
Chronic and non-resolving inflammation is a critical component in the progression of HF post-MI. In response to cardiac injury splenic leukocytes, diversity plays a critical role in resolution of post-MI inflammation (2). On time, activated leukocytes ‘arrival’ or ‘get-in signal’ and ‘departure’ or ‘get-out’ signal is essential post-MI cardiac healing (24). Formyl peptide receptor 2 (FPR2), is known to mediate both pro-resolving and inflammatory responses by modulating myeloid cells kinetics in multiple organs including lacrimal and salivary glands by binding to the respective receptors (9, 25, 26). Our previous studies defined that proresolving RvD1 and 15-epi-LXA4 ligands activates FPR2 in order to resolve inflammation in cardiac healing post-MI (7, 8). The current report investigated how FPR2 inhibition impacted ‘get-in’ signals of leukocytes in acute HF. Our study defined the critical role of FPR2 in acute HF since the post-MI inhibition; 1) primed immature and inactive neutrophils (Ly6Gint) infiltration (over activated ‘get-in’ signal); 2) depleted over all leukocytes population in LV and spleen; 3) increased F4/80+/Ly6Chi pro-inflammatory macrophages in acute HF (delayed ‘get-out signal’). Thus, inhibition of FPR2 altered leukocyte ‘get-in’ signal thereby eliciting a non-resolving response in acute HF post-MI.
Cells producing chemokines act on chemokine receptors (mostly GPCRs) and initiate a chemotactic response which is required to initiate an acute inflammatory response in HF essential for cardiac healing (27). The chemotactic response depends on the number of chemokines produced and their gradient and also on the expression levels of their receptors (28). FPR2 is a chemoattractant GPCR, expressed by human monocytes/macrophages and neutrophil and is stimulated by lipids and peptides (29, 30). FPR2 is a dual-edge sword and its activation can either lead to pro-inflammatory or proresolving response (31). FPR2 is activated in response to cardiac injuries such as MI and both spleen and LV coordinate to resolve inflammation (15). After cardiac injury, neutrophils are the first key responders (32, 33), this FPR2 being competitive receptor is highly expressed in the splenic leukocytes including neutrophils compared with LV in acute HF. After cardiac injury, splenic monocyte/macrophages and neutrophils are vital for clearing the debris and interact with macrophages to resolve inflammation to return tissue to homeostasis. The inhibition of FPR2, using the WRW4 enhanced the unaltered neutrophil response in acute HF indicating no change in neutrophil population. However, the neutrophils appeared to be immature revealing Ly6Gint expression indicative of impaired of ‘get-in’ signal that altered or delayed initiation of the acute inflammatory response. Deniset et al. discovered that immediate presence of neutrophils in spleen help to eradicate infection carrying bacteria and with a possibility that the circulating neutrophils that enter the red pulp to mature and perform a necessary function indicating that neutrophil presence in the spleen are critical for innate immune response; likewise dysfunctional FPR2 aggravate sepsis and myocardial dysfunction (21, 23). Our study outcome aligns with former reports, indicating the presence of immature neutrophils blunting ‘get-in’ signal of leukocytes. A recent study in FPR2 null mice demonstrated that Fpr2 is required for SCF/c-Kit-mediated Linc-Kit+Sca-1+ cell proliferation indicating an FPR2 role for the development of myeloid lineage (9). Thus, our study using a pharmacological inhibitor of FPR2 highlighted the critical role of FPR2 in leukocytes activation and redirecting neutrophils toward the site of injury in acute HF.
The absence of FPR2 promotes chronic inflammation that impacts neutrophil response and maturation (34). FPR2 is also essential in myeloid cell development and mediating leukocyte recruitment (10). In contrast, our study has shown that FPR2 inhibition intensified CCL2, which is a signal for enhanced leukocytes mobilization but we observed overall decrease in monocytes and macrophages in acute HF which indicated initiation failure of innate immune response. FPRs are ubiquitously expressed, but FPR2 inhibition decreased overall leukocytes, and neutrophils antecede monocyte/macrophage in acute HF indicated FPR2 control leukocyte kinetics in acute HF (32). FPR2 inactivation increased proinflammatory (F4/80+/Ly6Chi) macrophage infiltration which can be correlated with the gain in the chemokine CCL2. FPR2 can impact both pro-inflammatory and pro-resolving response depending up on the agonist (7, 8, 35, 36). Thus, FPR agonist/antagonist dependent activation of polarized macrophages that explain the leukocyte phenotype heterogeneity. Our study has provided evidence that inhibition of FPR2 using WRW4 boosted pro-inflammatory markers TNF-α and IL-6 with a compensatory increase in Arg-1, IL-10, and Ym-1 in acute HF indicative of overactivated inflammatory microenvironment. Initiation of the acute inflammatory response is essential for cardiac healing in an optimal manner (2, 15), but FPR2 inhibition led to an imbalance of chemokine signaling which failed to activate a required initial inflammatory response in acute HF.
Our study provided a critical insight with FPR2 inactivation that delayed initiation of splenocardiac acute inflammatory response which is mandatory for cardiac healing. Thus, FPR2 play agonist/antagonist dependent switch controlling the ‘get-in’ signal in acute HF. In summary, our results revealed evidence that timely activation of FPR2 is important to resolve post-MI inflammation. Pharmacological inhibition of FPR2 alters ‘get-in’ signaling of leukocyte leading to non-resolving inflammation in acute HF. Our study has a limitation that we used a single dose of FPR2 antagonist WRW4 to inactivate FPR2 for 24 hr, moreover the LV dysfunction difference was insignificant. Thus, long-term studies are warranted to precisely monitor leukocyte ‘get-in’ and ‘get-out’ signal and phenotypic leukocyte polarization in context of FPR2 signaling.
Figure 5. WRW4 modulated chemokine kinetics post-MI in acute HF.
A. Bar graph representing mRNA expression of A. Tnf-α, IL-1β, and IL-10 B. Mrc-1, Arg-1 and Ym-1 in LV-infarct post-MI. C. Schematic representation of inflammation-resolution axis depicting the role of WRW4 and non-resolving mechanism in acute HF. *p<0.05 versus no-MI control post-MI. Values are means ±SEM; n=4 mice/group.
Acknowledgement
This work was supported by National Institutes of Health (HL132989) and The University of Alabama at Birmingham (UAB) Pittman scholar award to G.V.H., American Heart Association postdoctoral fellowship (POST31000008) to VK
This work was supported in part by National Institutes of Health [AT006704 and HL132989] and The University of Alabama at Birmingham (UAB) Pittman scholar award to G.V.H., American Heart Association postdoctoral fellowship [POST31000008] to V.K.
Footnotes
Conflict of interest - none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Degoricija V, Trbusic M, Potocnjak I, Radulovic B, Teresak SD, Pregartner G, Berghold A, Tiran B, Frank S. Acute Heart Failure developed as worsening of Chronic Heart Failure is associated with increased mortality compared to de novo cases. Sci Rep. 2018;8(1):9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halade GV, Norris PC, Kain V, Serhan CN, Ingle KA. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal. 2018;11(520). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21(2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He H-Q, Liao D, Wang Z-G, Wang Z-L, Zhou H-C, Wang M-W, Ye RD. Functional characterization of three mouse formyl peptide receptors. Molecular pharmacology. 2013;83(2):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y-H, Le Y, Gong W, Proost P, Van Damme J, Murphy WJ, Wang JM. Bacterial Lipopolysaccharide Selectively Up-Regulates the Function of the Chemotactic Peptide Receptor Formyl Peptide Receptor 2 in Murine Microglial Cells. The Journal of Immunology. 2002;168(1):434–442. [DOI] [PubMed] [Google Scholar]
- 6.Iribarren P, Zhou Y, Hu J, Le Y, Wang JM. Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in alzheimer disease. Immunologic Research. 2005;31(3):165–176. [DOI] [PubMed] [Google Scholar]
- 7.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kain V, Liu F, Kozlovskaya V, Ingle KA, Bolisetty S, Agarwal A, Khedkar S, Prabhu SD, Kharlampieva E, Halade GV. Resolution Agonist 15-epi-Lipoxin A4 Programs Early Activation of Resolving Phase in Post-Myocardial Infarction Healing. Sci Rep. 2017;7(1):9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Singh VK, Tang P, Bao Z, He T, Xiang Y, Gong W, Yoshimura T, Le Y, Tessarollo L, Chen X, Wang JM. Deficiency in Fpr2 results in reduced number of Lin(−)c-Kit(+)Sca1(+) myeloid progenitor cells. J Biol Chem. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, Yoshimura T, Tessarollo L, Wang JM. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184(7):3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CS, Baker OJ. The G-Protein-Coupled Receptor ALX/Fpr2 Regulates Adaptive Immune Responses in Mouse Submandibular Glands. Am J Pathol. 2018;188(7):1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, Levy BD. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J Immunol. 2018;200(8):2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessi MC, Cenac N, Si-Tahar M, Riteau B. FPR2: A Novel Promising Target for the Treatment of Influenza. Front Microbiol. 2017;8:1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, Gavins FN. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation. 2016;133(22):2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halade GV, Kain V, Ingle KA. Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am J Physiol Heart Circ Physiol. 2018;314(2):H255–h267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey ML, Bolli R, Canty JM Jr., Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol. 2018;314(4):H812–h838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadapalli JK, Wright GM, Kain V, Sherwani MA, Yusuf N, Halade GV. Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters inflammation-resolution program in the myocardium. Am J Physiol Heart Circ Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114(2):266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY). 2016;8(11):2611–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging. 2016;8(11):2611–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbetti T, Coldewey SM, Chen J, McArthur S, le Faouder P, Cenac N, Flower RJ, Thiemermann C, Perretti M. Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc Natl Acad Sci U S A. 2014;111(52):18685–18690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deniset JF, Surewaard BG, Lee WY, Kubes P. Splenic Ly6G(high) mature and Ly6G(int) immature neutrophils contribute to eradication of S. pneumoniae. J Exp Med. 2017;214(5):1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourki B, Halade G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 2017;31(10):4226–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson JW, Leigh NJ, Mellas RE, McCall AD, Aguirre A, Baker OJ. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol. 2014;306(2):C178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin CX, Finlayson SB, Al-Sharea A, Tate M, De Blasio MJ, Deo M, Rosli S, Prakoso D, Thomas CJ, Kiriazis H, Gould E, Yang YH, Morand EF, Perretti M, Murphy AJ, Du XJ, Gao XM, Ritchie RH. Author Correction: Endogenous Annexin-A1 Regulates Haematopoietic Stem Cell Mobilisation and Inflammatory Response Post Myocardial Infarction in Mice In Vivo. Sci Rep. 2018;8(1):7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel J, Channon KM, McNeill E. The downstream regulation of chemokine receptor signalling: implications for atherosclerosis. Mediators of inflammation. 2013;2013:459520–459520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harbor perspectives in biology;7(5):a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. [DOI] [PubMed] [Google Scholar]
- 30.Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14(4):7193–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corminboeuf O, Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem. 2015;58(2):537–559. [DOI] [PubMed] [Google Scholar]
- 32.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 2014;109(6):444. [DOI] [PubMed] [Google Scholar]
- 33.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A, Gao JL, Murphy PM, Wang JM. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep. 2012;2:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99(3):1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao JL, Murphy PM, Oppenheim JJ, Wang JM. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21(2):RC123. [DOI] [PMC free article] [PubMed] [Google Scholar]