Abstract
Objective:
We sought to characterize the United States nationwide temporal trends in recanalization therapy utilization for ischemic stroke among patients with and without cancer.
Methods:
We identified all acute ischemic stroke hospitalizations in the National Inpatient Sample from January 1, 1998-September 30, 2015. The primary exposure was solid or hematologic cancer. The primary outcome was use of intravenous thrombolysis. The secondary outcome was use of endovascular therapy.
Results:
Among 9,508,804 acute ischemic stroke hospitalizations, 503,510 (5.3%) involved cancer patients. Intravenous thrombolysis use among ischemic stroke patients with cancer increased from 0.01% (95% CI, 0.00–0.02%) in 1998 to 4.91% (95% CI, 4.33–5.48%) in 2015; while intravenous thrombolysis use among ischemic stroke patients without cancer increased from 0.02% (95% CI, 0.01–0.02%) in 1998 to 7.22% (95% CI, 6.98–7.45%) in 2015. The demographic- and comorbidity-adjusted odds ratio/year of receiving intravenous thrombolysis was similar in patients with cancer (1.21; 95% CI, 1.20–1.23) versus those without (1.20; 95% CI, 1.19–1.21). Endovascular therapy use among ischemic stroke patients with cancer increased from 0.05% (95% CI, 0.02–0.07%) in 2006 to 1.90% (95% CI, 1.49–2.31%) in 2015; while endovascular therapy use among ischemic stroke patients without cancer increased from 0.09% (95% CI, 0.00–0.18%) in 2006 to 1.88% (95% CI, 1.68–2.09%) in 2015.
Conclusions:
Among 9.5 million acute ischemic stroke hospitalizations, patients with cancer received intravenous thrombolysis about two-thirds as often as patients without cancer. This difference persisted over time despite increased utilization in both groups. Endovascular therapy utilization was similar between cancer and non-cancer acute ischemic stroke patients.
Keywords: cancer, ischemic stroke, thrombolysis, endovascular therapy, recanalization therapy, oncology
Introduction
Acute ischemic stroke (AIS) is associated with substantial morbidity, particularly when caused by a large vessel occlusion.(1) Two proven treatments for AIS are intravenous tissue plasminogen activator (IV-tPA) and endovascular therapy (EVT).(2) IV-tPA was first shown to be beneficial in AIS in 1995,(3) while the first positive AIS EVT trial was in 2014.(4) These acute recanalization therapies significantly increase the odds of a good neurological outcome at 3 months, especially if recanalization is achieved.(5, 6) Consequently, utilization of these therapies has gradually increased over time, contributing, in part, to lower rates of death and disability from AIS.(7, 8)
Like stroke, cancer is a common and often morbid disease. In the United States, two of every five persons are expected to develop cancer in their lifetime.(9) These individuals face an increased risk of stroke,(10) especially in the first 6 months after cancer diagnosis.(11) Cancer-mediated hypercoagulability is presumed to account for much of this increased stroke risk. Potential mechanisms of cancer-mediated hypercoagulability include circulating procoagulant microparticles, platelet overactivation, tissue factor overexpression, coagulation factor derangements, and neutrophil extracellular trap formation.(12–15) Prothrombotic effects of chemotherapy and reductions in antithrombotic use may also contribute to the link between cancer and AIS.(16) Furthermore, 5–10% of patients with AIS have comorbid cancer and this frequency is expected to increase with continued advances in cancer survival.(16–18)
Most clinical trials evaluating recanalization therapies for AIS excluded patients with cancer, and therefore the effectiveness of these therapies in the cancer population is uncertain. Nevertheless, single-center case series and claims-based cohort studies suggest that selective use of these therapies in cancer patients who otherwise meet eligibility criteria may be safe.(19–21) Furthermore, neither the American Heart Association/American Stroke Association nor the United States Food and Drug Administration, per the product label for Alteplase, consider active cancer to be an absolute contraindication for IV-tPA or EVT for AIS.(22, 23) Therefore, these recanalization therapies are presumably being used in some cancer patients with AIS; however, the frequency of their use is uncertain. We sought to characterize the United States nationwide temporal trends in the use of IV-tPA and EVT among AIS patients with and without cancer. Because of limited data supporting the use of IV-tPA in the cancer population, as well as presumed increased contraindications, such as chemotherapy-induced thrombocytopenia,(24–26) our prespecified hypothesis was that there would be differences in utilization rates between cancer and non-cancer patients and that these disparities would widen over time.
Methods
Design
We used inpatient discharge data from the 1998 through 2015 releases of the Healthcare Cost and Utilization Project’s (HCUP) National Inpatient Sample (NIS).(27) The United States NIS is a nationally representative deidentified dataset that is funded by the Agency for Healthcare Research and Quality (AHRQ), and includes data from a 20% stratified probability sample of community hospitals participating in HCUP. Each discharge record includes 1 primary diagnosis code, up to 29 secondary diagnosis codes, 1 primary procedure code, and up to 14 secondary procedure codes, all of which are assigned according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system. The Weill Cornell Medicine institutional review board approved the analysis of these data and waived the need for informed consent. We adhered to recommended guidelines for best research practices when using the NIS.(28) The data used in this analysis includes restricted NIS claims data and therefore cannot be shared directly with other investigators because of the terms of the data use agreement. However, investigators can obtain access to these data by application to the AHRQ.
Population
We identified all hospitalizations for AIS from January 1, 1998 through September 30, 2015, defined by the presence of ICD-9-CM diagnosis codes 433.xx, 434.xx, or 436 in any position in the absence of codes for trauma or a code for rehabilitation in the primary position. This validated diagnosis code algorithm was previously shown to have a positive predictive value greater than 90%.(29) We did not evaluate hospitalizations after September 30, 2015 due to the adoption of ICD-10 in the United States after that date.
Measurements
The primary exposure was the presence of comorbid systemic cancer. Our approach to identifying systemic cancer has been previously described, and consisted of a composite of solid tumors without metastases (140.xx-190.xx, 193.xx-195.xx, 209.00–209.30), hematologic tumors without metastases (200.xx-208.xx, 238.7x), and metastatic solid or hematologic tumors (196.xx-198.xx, 209.7x).(20) Patients with primary brain tumors were excluded. The primary outcome was the use of IV-tPA, which was defined as the presence of ICD-9-CM procedure code 99.10; and the secondary outcome was the use of EVT, which was defined as the presence of ICD-9-CM procedure code 39.74. Data were also collected on patients’ demographics and relevant comorbidities.
Statistical Analysis
Descriptive statistics were used to evaluate patient characteristics. Depending on data distribution, continuous variables were compared with Student’s t-test or the Wilcoxon rank sum test and categorical variables were compared with the chi-square test or Fisher’s exact test. Sampling weights provided by the NIS were used to obtain United States national estimates for all outcomes. We used the updated “trend” weights for the period between 1998–2011 and the original discharge weights for 2012–2015 (in order to account for the NIS sampling redesign in 2012). Annual rates of acute recanalization therapy use were calculated among AIS hospitalizations involving patients with and without cancer. Because a procedure code for EVT first became available in 2006, we do not report EVT utilization rates before then. Variance-weighted linear least squares regression was used to test for temporal trends. All trend analyses were two-tailed and used an alpha error of 0.05.
To evaluate the associations between time and our outcomes, we also created multivariable logistic regression models adjusted for age, sex, race/ethnicity, and stroke risk factors that included the year of hospitalization as a covariate. Subgroup analyses were performed among patients with different cancer subtypes and among patients treated at teaching hospitals. For the cancer subtype analyses, patients could have both non-metastatic solid tumors and non-metastatic hematologic tumors; however, if patients had diagnosis codes for both non-metastatic tumors and metastatic tumors, they were assigned to the metastatic group. Statistical analyses were performed using Stata (version 14.0, College Station, TX).
Results
Patient Characteristics
Among 9,508,804 hospitalizations for AIS in the United States between January 1, 1998 and September 30, 2015, 503,510 (5.3%) involved patients with diagnoses of comorbid cancer. Approximately 65% of these patients had non-metastatic solid or hematologic cancers while the remainder had metastatic cancers. Compared to patients without cancer, patients with cancer were more often older, male, white, and had chronic obstructive pulmonary disease but less often had diabetes mellitus, hypertension, and coronary artery disease (Table 1). Among patients with cancer, those who received recanalization therapies were generally younger and had more stroke risk factors (Table 2).
Table 1.
Baseline Characteristics of Patients with Hospitalization Events for Acute Ischemic Stroke, Stratified by the Presence of Cancer, 1998–2015
| Patient Characteristics* | Cancer (n=503,510) |
No Cancer (n=9,005,294) |
|---|---|---|
| Age, mean (SE) | 72.8 (0.1) | 71.3 (0.1) |
| Female sex | 240,310 (47.7) | 4,893,444 (54.3) |
| Race/Ethnicity | ||
| White | 324,017 (77.1) | 5,272,625 (71.6) |
| Black | 54,956 (13.1) | 1,171,122 (15.9) |
| Hispanic | 22,159 (5.3) | 527,778 (7.2) |
| Asian | 9,335 (2.2) | 185,495 (2.5) |
| Native American | 1,303 (0.3) | 30,180 (0.4) |
| Other | 8,704 (2.1) | 173,718 (2.4) |
| Stroke Risk Factors | ||
| Hypertension | 224,172 (44.5) | 5,087,769 (56.5) |
| Diabetes mellitus | 115,982 (23.0) | 2,842,414 (31.6) |
| Atrial fibrillation | 107,733 (21.4) | 1,989,726 (22.1) |
| COPD | 106,416 (21.1) | 1,373,119 (15.2) |
| Coronary artery disease | 103,705 (20.6) | 2,198,233 (24.4) |
| Tobacco use | 98,187 (19.5) | 1,670,867 (18.6) |
| Congestive heart failure | 77,034 (15.3) | 1,458,986 (16.2) |
| Acute myocardial infarction | 51,932 (10.3) | 904,969 (10.0) |
| Renal disease | 48,750 (9.7) | 804,300 (8.9) |
| Peripheral vascular disease | 36,385 (7.2) | 744,751 (8.3) |
| Liver disease | 8,614 (1.7) | 100,043 (1.1) |
| Charlson comorbidity score, mean (SE) | 3.4 (0.01) | 2.2 (0.004) |
Abbreviations: SE, standard error; COPD, chronic obstructive pulmonary disease.
All values are presented as No. (%) unless otherwise specified. Percentages may not add up to 100 because of rounding.
Table 2.
Baseline Characteristics of Patients with Cancer and Acute Ischemic Stroke Hospitalizations, Stratified by Use of Acute Recanalization Therapies, 1998–2015
| Patient Characteristics*† | Endovascular Therapy (n=2,206) |
Intravenous Thrombolysis (n=10,315) |
No Recanalization Therapy (n=491,856) |
|---|---|---|---|
| Age, mean (SE) | 68.3 (0.7) | 72.1 (0.3) | 72.8 (0.1) |
| Female sex | 1,136 (51.5) | 5,019 (48.7) | 234,632 (47.7) |
| Race/Ethnicity | |||
| White | 1,454 (75.8) | 7,065 (77.1) | 316,022 (77.1) |
| Black | 158 (8.3) | 1,138 (12.4) | 53,730 (13.1) |
| Hispanic | 145 (7.6) | 491 (5.4) | 21,574 (5.3) |
| Asian | 75 (3.9) | 244 (2.7) | 9,064 (2.2) |
| Native American | 0 (0) | 18 (0.2) | 1,271 (0.3) |
| Other | 85 (4.4) | 202 (2.2) | 8,449 (2.1) |
| Stroke risk factors | |||
| Hypertension | 1,116 (50.6) | 5,240 (50.8) | 218,254 (44.4) |
| Atrial fibrillation | 757 (34.3) | 3,313 (32.1) | 103,945 (21.1) |
| Tobacco use | 623 (28.2) | 2,731 (26.5) | 95,040 (19.3) |
| Diabetes mellitus | 516 (23.4) | 2,557 (24.8) | 113,135 (23.0) |
| COPD | 498 (22.6) | 1,994 (19.3) | 104,104 (21.2) |
| Coronary artery disease | 464 (21) | 2,386 (23.1) | 101,048 (20.5) |
| Congestive heart failure | 350 (15.9) | 1,732 (16.8) | 75,098 (15.3) |
| Acute myocardial infarction | 313 (14.2) | 1,107 (10.7) | 50,616 (10.3) |
| Peripheral vascular disease | 246 (11.2) | 891 (8.6) | 35,355 (7.2) |
| Renal disease | 218 (9.9) | 1,344 (13) | 47,271 (9.6) |
| Liver disease | 25 (1.1) | 215 (2.1) | 12,338 (2.5) |
| Charlson comorbidity score, mean (SE) | 3.9 (0.06) | 3.6 (0.03) | 3.4 (0.01) |
| Cancer type‡ | |||
| Non-metastatic solid tumors | 995 (45.1) | 4,783 (46.4) | 198,318 (40.3) |
| Non-metastatic hematologic tumors | 576 (26.1) | 3,271 (31.7) | 123,481 (25.1) |
| Metastatic solid/hematologic tumors | 681 (30.9) | 2,391 (23.2) | 173,921 (35.4) |
Abbreviations: SE, standard error; COPD, chronic obstructive pulmonary disease.
All values are presented as No. (%) unless otherwise specified. Percentages may not add up to 100 because of rounding.
Some patients were treated with both endovascular therapy and intravenous thrombolysis. Endovascular therapy data is only provided from 2006 onward because that is when its procedure code first became available.
Numbers add up to more than the total denominator and percentages add up to more than 100 because some patients had both non-metastatic solid tumors and non-metastatic hematologic tumors. Patients who had diagnosis codes for metastatic and non-metastatic tumors were assigned to the metastatic group.
Intravenous Thrombolysis Utilization
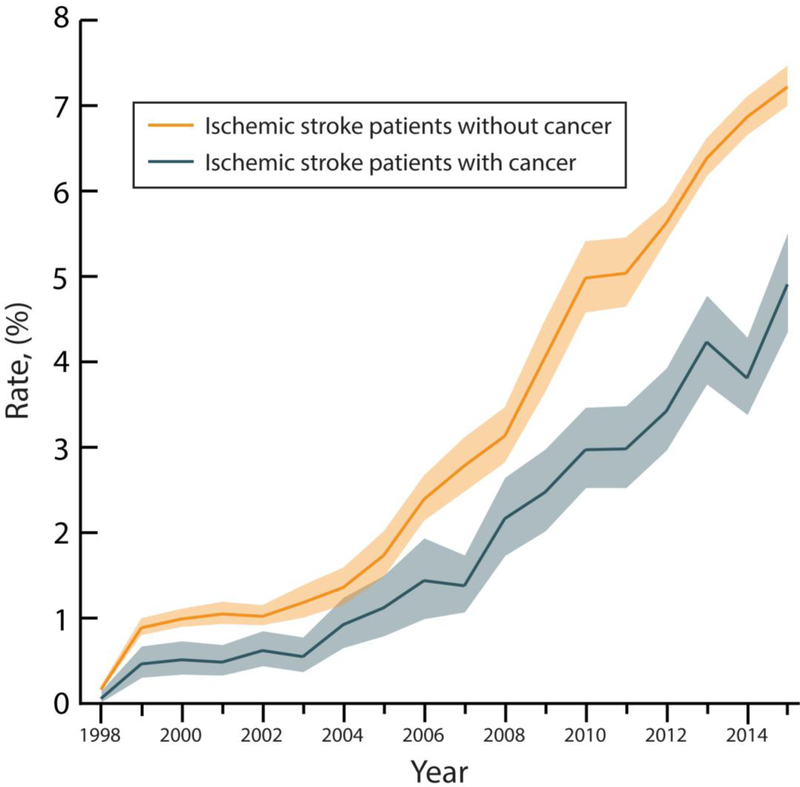
Overall rates of IV-tPA use throughout the study period were 2.0% (95% confidence interval [CI], 1.9–2.1%) among AIS patients with cancer versus 3.2% (95% CI, 3.1–3.3%) among AIS patients without cancer. The rate of IV-tPA use among AIS patients with cancer increased from 0.01% (95% CI, 0.00–0.02%) in 1998 to 4.91% (95% CI, 4.33–5.48%) in 2015 (p<0.001 for trend); while the rate of IV-tPA use among AIS patients without cancer increased from 0.02% (95% CI, 0.01–0.02%) in 1998 to 7.22% (95% CI, 6.98–7.45%) in 2015 (p<0.001 for trend) (Figure 1). In multivariable analysis, the demographic- and comorbidity-adjusted odds ratio per year of receiving IV-tPA was similar in patients with cancer (1.21; 95% CI, 1.20–1.23) versus those without (1.20; 95% CI, 1.19–1.21).
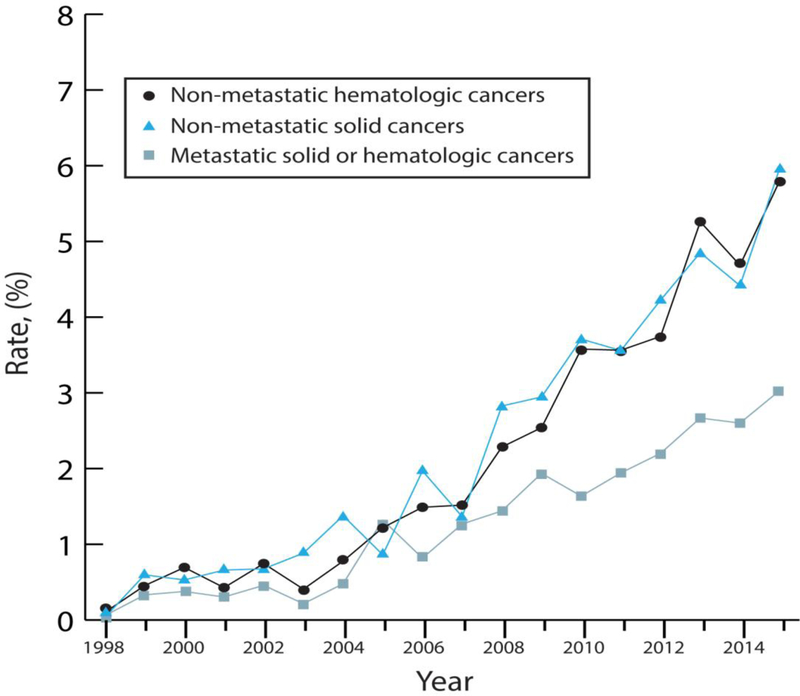
Figure 1.
A and B. Intravenous Thrombolysis Use Among Hospitalizations for Ischemic Stroke in the National Inpatient Sample, 1998–2015.
A. Annual rates of intravenous thrombolysis use among hospitalizations for acute ischemic stroke in the National Inpatient Sample from 1998–2015. Data is stratified by the presence of comorbid cancer. The lighter shade sections of the curves denote 95% confidence intervals.
B. Annual rates of intravenous thrombolysis use among hospitalizations for acute ischemic stroke in patients with cancer in the National Inpatient Sample from 1998–2015. Data is stratified by cancer type, including patients with non-metastatic hematologic cancers, non-metastatic solid cancers, and metastatic hematologic or solid cancers.
Endovascular Therapy Utilization
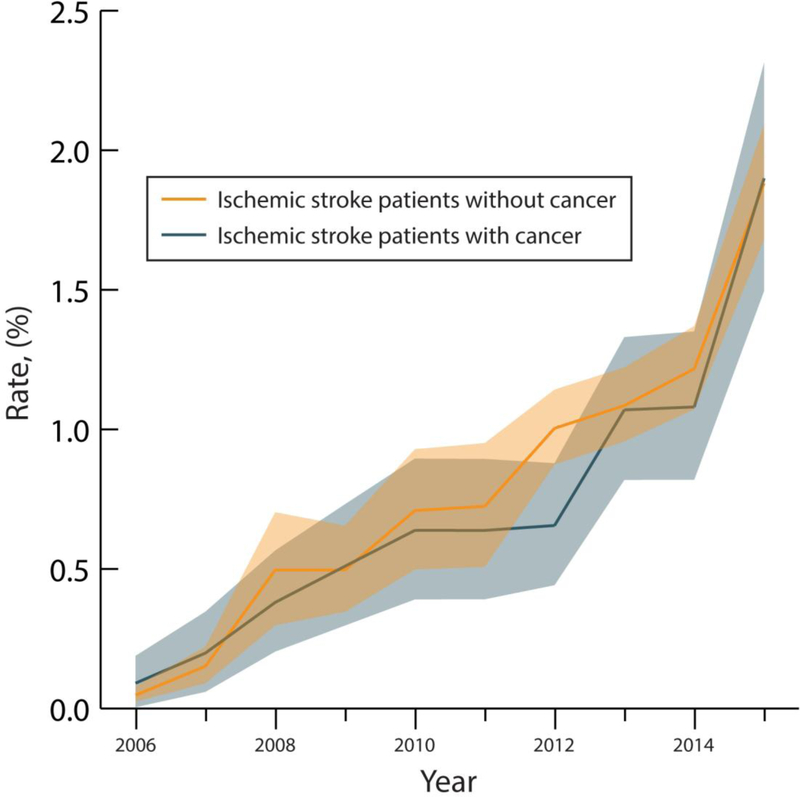
From January 1, 2006 through September 30, 2015, overall rates of EVT use for AIS were 0.7% (95% CI, 0.7–0.8%) among patients with cancer versus 0.8% (95% CI, 0.7–0.9%) among patients without cancer. The rate of EVT use among AIS patients with cancer increased from 0.05% (95% CI, 0.02–0.07%) in 2006 to 1.90% (95% CI, 1.49–2.31%) in 2015 (p<0.001 for trend); while the rate of EVT use among AIS patients without cancer increased from 0.09% (95% CI, 0.00–0.18%) in 2006 to 1.88% (95% CI, 1.68–2.09%) in 2015 (p<0.001 for trend) (Figure 2). In multivariable analysis, the demographic- and comorbidity-adjusted odds ratio per year of receiving EVT was similar in patients with cancer (1.20; 95% CI, 1.18–1.22) versus those without (OR, 1.19; 95% CI, 1.18–1.20).
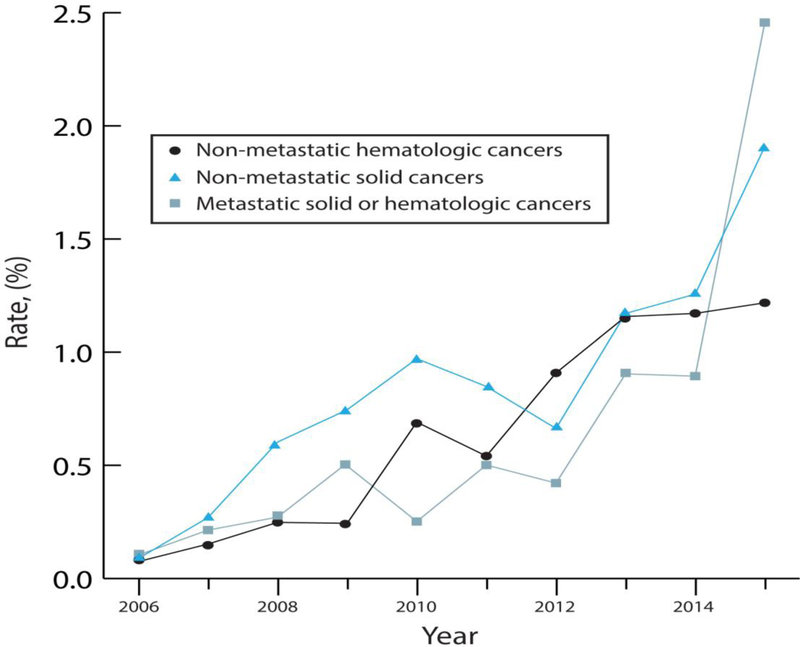
Figure 2.
A and B Endovascular Therapy Use Among Hospitalizations for Ischemic Stroke in the National Inpatient Sample, 2006–2015.
A. Annual rates of endovascular therapy use among hospitalizations for acute ischemic stroke in the National Inpatient Sample from 2006–2015. Data is stratified by the presence of comorbid cancer. The lighter shade sections of the curves denote 95% confidence intervals.
B. Annual rates of endovascular therapy use among hospitalizations for acute ischemic stroke in patients with cancer in the National Inpatient Sample from 2006–2015. Data is stratified by cancer type, including patients with non-metastatic hematologic cancers, non-metastatic solid cancers, and metastatic hematologic or solid cancers.
Subgroup Analyses
Rates of IV-tPA use increased over time in all cancer patients regardless of cancer subtype (p<0.001 for trends), but were higher in AIS patients with non-metastatic solid or hematologic cancers than in those with metastatic cancers. Similarly, rates of EVT use also increased over time in all cancer patients regardless of cancer subtype (p<0.001 for trends), but were higher throughout the study period in AIS patients with non-metastatic solid or hematologic cancers than in those with metastatic cancers.
Rates of IV-tPA use were higher in teaching hospitals than in non-teaching hospitals (p<0.001); however, the difference in utilization rates between cancer (2.6%; 95% CI, 2.4–2.8%) and non-cancer patients (4.4%; 95% CI, 4.2–4.6%) persisted. Rates of EVT use were also higher in teaching hospitals than in non-teaching hospitals (p<0.001); however, unlike with IV-tPA, utilization rates were similar between cancer (1.0%; 95% CI, 0.9–1.2) and non-cancer patients (1.2%; 95% CI, 1.1–1.3%) at teaching hospitals.
Discussion
In a retrospective analysis of a large nationwide sample of inpatient hospitalizations from 1998–2015, we found that cancer patients with AIS received IV-tPA about two-thirds as often as non-cancer patients with AIS. Contrary to our prespecified hypothesis, this difference in AIS IV-tPA use between cancer and non-cancer patients did not widen over time, as utilization rates increased about 20% per year in both groups. IV-tPA utilization rates were highest in patients without metastatic cancer and at teaching hospitals. In contrast, rates of EVT use for AIS were similar between cancer and non-cancer patients throughout the study period, and EVT utilization rates increased about 20% per year in both groups. EVT utilization rates were highest in patients without metastatic cancer and at teaching hospitals.
Several retrospective studies have evaluated IV-tPA and EVT use in cancer patients with AIS. These analyses concentrated on the risk of hemorrhagic complications and death, and most were small, single-center case series that lacked control groups.(19–21, 30, 31) These studies suggested that careful use of acute recanalization therapies in select cancer patients with AIS might be safe, as hemorrhage rates were similar to those from large randomized trials of predominantly non-cancer AIS patients.(3, 32) Our study, in contrast, was focused on temporal trends and disparities in stroke recanalization therapies between the cancer and non-cancer populations. As hypothesized, IV-tPA use was less frequent in AIS patients with cancer versus those without, although EVT use was surprisingly similar between groups. In this claims-based study, which lacked granular clinical data, we were unable to determine the reason(s) for this discrepancy; however, one possible explanation would be that compared to non-cancer patients, cancer patients might have had higher rates of exclusions for IV-tPA but not for endovascular therapy. For instance, thrombocytopenia, recent major surgery, intracranial lesions, and anticoagulant use are all exclusions for IV-tPA, as well as common scenarios in patients with cancer, but they generally do not exclude a patient from receiving EVT.(16, 33)
Alternative potential explanations for the discrepancy in utilization of IV-tPA and EVT include possible differences in physician factors, such as the goals of care of providers, or patient factors, such as premorbid functional status, perceived survival, stroke severity, stroke mechanisms, and access to stroke care between the cancer and non-cancer groups. Future prospective studies with detailed case ascertainment will be necessary to determine why cancer patients are one-third less likely to receive IV-tPA, but as likely to receive EVT, for AIS, than patients without cancer. The difference in IV-tPA use for AIS between cancer and non-cancer patients did not widen over the 17-year study period, and by 2015, nearly 5% of cancer patients with AIS were receiving thrombolysis treatment. It is important that patients with cancer have sufficient access to cutting-edge stroke care and treatments as they currently make up 5–10% of all stroke cases, and this co-prevalence is expected to increase with advances in cancer screening, molecular diagnostics, and targeted chemotherapy, including immunotherapy. In the United States, two-thirds of patients with cancer already survive more than 5 years from their diagnosis.(17)
This study has several limitations. First, it was a retrospective analysis of claims data, and therefore lacked information on patients’ premorbid functional status and eligibility for recanalization therapies, cancer stage and treatments, stroke severity and mechanisms, presence of large vessel occlusions, time to treatment, devices used for EVT, and the attitudes of treating clinicians. Second, because of our reliance on diagnosis and procedure codes, there may have been misclassification of stroke diagnoses and treatments received. We attempted to mitigate this concern by using previously validated algorithms for claims data.(29) Third, this study was restricted to patients in the United States and therefore its results may not generalize to other populations. Fourth, NIS captures hospitalization events, not individual patients; therefore, multiple observed events could have occurred in a single patient, which could have affected the precision of our estimated outcome rates.
In summary, from 1998–2015, patients with cancer in the United States were one-third less likely to receive IV-tPA for AIS as compared to patients without cancer. This difference in treatment rates persisted throughout the study period, although utilization rates increased by about 20% each year, and in 2015, at the end of study, nearly 5% of cancer patients with AIS received IV-tPA. Furthermore, patients with metastatic cancer were less likely to receive IV-tPA than those with non-metastatic solid or hematologic cancers. In contrast, patients with cancer were as likely to receive EVT for AIS as compared to patients without cancer, and utilization rates also increased by about 20% each year in both groups. Additionally, similar to IV-tPA utilization, patients with metastatic cancer were less likely to receive EVT than those with non-metastatic solid or hematologic cancers. Although frequently performed in clinical practice—as indicated in this analysis—future prospective studies should evaluate the safety and efficacy of AIS recanalization therapies in patients with cancer, particularly among those with metastatic cancer, who appear to have the highest risks of stroke.(10, 34)
Acknowledgments
Sources of Funding: This research was supported by National Institutes of Health grant K23NS091395 (Navi) and the Florence Gould Endowment for Discovery in Stroke (Navi).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests
The authors declare that they have no conflict of interest.
Contributor Information
Abhinaba Chatterjee, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY, 646-962-8284.
Alexander E. Merkler, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Department of Neurology, Weill Cornell Medicine, New York, NY, 646-962-8263.
Santosh B. Murthy, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Department of Neurology, Weill Cornell Medicine, New York, NY, 212-746-2596.
Jaclyn E. Burch, Department of Neurology, Weill Cornell Medicine, New York, NY, 212-746-0225.
Monica L. Chen, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY, 646-962-8284.
Gino Gialdini, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY, 646-962-8284.
Hooman Kamel, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Department of Neurology, Weill Cornell Medicine, New York, NY, 212-746-0225.
Karla V. Ballman, Department of Healthcare Policy and Research Department of Medicine, Weill Cornell Medicine, New York, NY, 646-962-8023.
Babak B. Navi, Clinical and Translational Neuroscience Unit, Feil Family Brain and Mind Research Institute, Department of Neurology, Weill Cornell Medicine, New York, NY, 212-746-0225.
References
- 1.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009;40:3834–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jauch EC, Saver JL, Adams HP Jr., et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Neurological Disorders and Stroke rt-TPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 5.Yeo LL, Paliwal P, Teoh HL, et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol 2013;70:353–358. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, van der Lugt A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 7.Menon BK, Saver JL, Goyal M, et al. Trends in endovascular therapy and clinical outcomes within the nationwide Get With The Guidelines-Stroke registry. Stroke 2015;46:989–995. [DOI] [PubMed] [Google Scholar]
- 8.Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 10.Navi BB, Howard G, Howard VJ, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology 2018;90:e2025–e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotiou D, Sergentanis TN, Papageorgiou L, et al. Longer procoagulant phospholipid-dependent clotting time, lower endogenous thrombin potential and higher tissue factor pathway inhibitor concentrations are associated with increased VTE occurrence in patients with newly diagnosed multiple myeloma: results of the prospective ROADMAP-MM-CAT study. Blood Cancer J 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrigos K, Grapsa D, Sangare R, et al. Prospective Assessment of Clinical Risk Factors and Biomarkers of Hypercoagulability for the Identification of Patients with Lung Adenocarcinoma at Risk for Cancer-Associated Thrombosis: The Observational ROADMAP-CAT Study. Oncologist 2018;23:1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thalin C, Demers M, Blomgren B, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res 2016;139:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navi BB, Iadecola C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann Neurol 2018;83:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henley SJ, Singh SD, King J, et al. Invasive cancer incidence and survival--United States, 2011. Morb Mortal Wkly Rep 2015;64:237–242. [PMC free article] [PubMed] [Google Scholar]
- 18.Sanossian N, Djabiras C, Mack WJ, et al. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis 2013;22:1146–1150. [DOI] [PubMed] [Google Scholar]
- 19.Graber JJ, Nayak L, DeAngelis LM. Use of recombinant tissue plasminogen activator in cancer patients with acute stroke. J Neurooncol 2012;107:571–573. [DOI] [PubMed] [Google Scholar]
- 20.Murthy SB, Karanth S, Shah S, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 2013;44:3573–3576. [DOI] [PubMed] [Google Scholar]
- 21.Casado-Naranjo I, Calle ML, Falcon A, et al. Intravenous thrombolysis for acute stroke in patients with cancer. J Neurol Neurosurg Psychiatry 2011;82:1404–1405. [DOI] [PubMed] [Google Scholar]
- 22.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020–3035. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food & Drug Administration. Drug Approval Package: Alteplase Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/alteplase_toc.cfm. Accessed June 5, 2018.
- 24.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004;109:3122–3131. [DOI] [PubMed] [Google Scholar]
- 25.Hitron A, Steinke D, Sutphin S, et al. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract 2011;17:312–319. [DOI] [PubMed] [Google Scholar]
- 26.Navi BB, Marshall RS, Bobrow D, et al. Enoxaparin vs Aspirin in Patients With Cancer and Ischemic Stroke: The TEACH Pilot Randomized Clinical Trial. JAMA Neurol 2018;75:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality. NIS Overview. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed June 5th, 2018.
- 28.Khera R, Krumholz HM. With Great Power Comes Great Responsibility: Big Data Research From the National Inpatient Sample. Circ Cardiovasc Qual Outcomes 2017;10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 30.Masrur S, Abdullah AR, Smith EE, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis 2011;20:124–130. [DOI] [PubMed] [Google Scholar]
- 31.Merkler AE, Marcus JR, Gupta A, et al. Endovascular therapy for acute stroke in patients with cancer. Neurohospitalist 2014;4:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 33.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016;47:581–641. [DOI] [PubMed] [Google Scholar]
- 34.Navi BB, Reiner AS, Kamel H, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol 2017;70:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]